Introduction
Hypertension is a major public health issue,
affecting >1.3 billion people worldwide (1) and >90% of patients with
hypertension exhibit essential hypertension (EH) (2). EH is a multifactorial chronic disease
that is influenced by genetic factors, obesity, stress, diet and
other factors (3-6).
The primary regions affected by hypertension are the heart, brain,
kidneys and blood vessels (7).
Effective and stable control of blood pressure is urgently required
for public health. Hypertension is primarily treated with drugs
(8), which have certain
disadvantages, including poor patient compliance (9), adverse reactions (10) and unstable curative effects
(11).
Numerous studies have demonstrated that acupuncture
lowers blood pressure (12-14).
Acupuncture has been practiced in China for >3,000 years and
spread to Europe and North America between the 16 and 19th
centuries (15). Huang Di Nei Jing
(Huangdi's Inner Classic), an ancient Chinese medical book,
recorded the use of acupuncture treatment for hypertension 2,000
years ago (16). Acupuncture
treatment has been reported to have a therapeutic effect on various
diseases, particularly hypertension (17,18).
Previous studies have demonstrated that reinforcing and reducing
manipulation was able to decrease blood pressure via multiple
biological processes, including upregulation of nitric oxide
synthase and cyclic guanosine monophosphate in arterial tissues
(19), the brain network (20) and cell signal transduction pathways
(21). Positron emission tomography
functional imaging the brains of rats who had receiving twirling
reinforcing and reducing manipulation indicated that these
acupuncture manipulations had central effects on mechanisms such as
cerebral glucose metabolism (22).
Different acupuncture manipulations activate different brain areas
and these areas produce neurotransmitters that regulate blood
pressure (22). These results
indicated that the brain exhibited core response to acupuncture
manipulations and that neural protein expression, including the
expression of related neurotransmitters, was impacted. These
neurotransmitters acted on target organs to reduce blood pressure.
A previous proteomics study demonstrated that the expression of
various proteins other than neurotransmitters was altered in rat
medullas following acupuncture treatment (23). Furthermore, a previous study
reported that acupuncture affects multiple systems and targets,
including the renin-angiotensin-aldosterone system, the
neuroendocrine system, the vascular endothelium and oxidative
stress (24). The cross-effect of
these targets may be the mechanism involved in lowering blood
pressure (25).
Twirling reinforcing manipulation, twirling reducing
manipulation and electroacupuncture are different acupuncture
techniques and may produce effects on the brain (26). The hypothalamus serves an important
role in the development and maintenance of hypertension (27). Taichong (LR3) is located between the
first and the second metatarsal bone of dorsal foot (28) and is the most commonly used
acupuncture point in acupuncture for hypertension (29). The current study hypothesized that
electroacupuncture, twirling reinforcing manipulation and twirling
reducing manipulation may activate the hypothalamus to release
neurotransmitters by influencing protein expression profile, thus
regulating blood pressure and that different acupuncture
manipulations have different protein expression patterns, leading
to differences in the effects on blood pressure. Spontaneously
hypertensive rats (SHRs) were established as an essential
hypertension animal model by inbreeding of Wistar-Kyoto (WKY) rats,
as previously described (30). The
aim of the current study was to determine the potential
antihypertensive effect of different acupuncture manipulations in
lowering blood pressure.
Materials and methods
Animals
All animal experimental procedures were conducted in
accordance with the World Health Organization's International
Guiding Principles for Biomedical Research Involving Animals
(31) and were approved by the
Animal Care and Use Committee of Beijing University of Chinese
Medicine, Beijing, China (permit no. BUCM-3-2016090301-3003). All
rats were housed with 4 individuals per clean cage, with free
access to water and food on a 12 h light/dark cycle at a humidity
of 50-60% and at a temperature of 18-22˚C during the process. A
total of 32 SHR and 8 WKY male rats (age, 9 weeks; weight, 215±20
g) were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. SHRs were randomly divided into the model (M),
twirling reinforcing manipulation (TRFM), twirling reducing
manipulation (TRDM) and electroacupuncture (EA) groups (n=8/group).
In order to establish the model male WKY rats with continuous
systolic blood pressures of 150-175 mmHg were mated with female WKY
rats with systolic blood pressures of 130-140 mmHg by Beijing Vital
River Laboratory Animal Technology Co., Ltd. Offspring with
systolic blood pressures >150 mmHg were obtained. Rats with high
blood pressure were selected for inbreeding and the stable
inheritance of hypertension was obtained by selective inbreeding
for 20 generations and the SHR model was established (30,32).
WKY rats were used as the normotensive control group of SHR and the
control strain of hypertensive rats, as previously described
(33).
Sample preparation
All acupuncture manipulations were performed
following 1 week of adaptation. The blood pressure of the WKY rats
was relatively stable. SHRs were easily irritated and hyperactive;
however, they were relatively quiet in the morning. The systolic
blood pressure in the caudal artery was measured by two experienced
technicians at a controlled temperature of (20±2˚C). Each rat was
gently placed in a restrainer and its tail was fixed using the
rat-tail fixing facility. The ventral portion of each rat was
placed on a heat pad, while the blood pressure measurement cuffs
were put in place. Once a batch of 2 rats was in place, calm rats
were preheated at 36˚C for 10 min, after which their systolic blood
pressure was measured with a non-invasive blood pressure instrument
(BP-6; Chengdu TME Technology Co., Ltd.) and recorded by the pulse
recording sensor while the rats were quiet and conscious. Each rat
was measured three times and the mean value of the three times was
taken as the systolic pressure. The systolic blood pressure of the
rats was measured between 8:00 and 12:00 a.m. every two days
between day 1 (one day prior the acupuncture experiment) and day
15. Acupuncture at Taichong (LR3) was performed between 2:00 and
4:00 p.m. every day for 2 weeks. The 8 WKY (group WKY) and 8 SHRs
(group M) did not undergo acupuncture; however, they were handled
and held for 20 min in the same restrainer. Acupuncture on the SHRs
in group TRFM, TRDM and EA was performed according to previous
published studies (22,33). Briefly, acupuncture needles were
inserted to LR3 loci with respective acupuncture manipulations. For
TRFM, the manipulation was performed with the forward thumb
exerting heavy force, while the backward thumb exerted light force;
meanwhile, for TRDM, the manipulation was conducted with the
forward thumb exerting light fore with the backward thumb exerting
with heavy force. The frequency and angle used were the same as a
previous study (34). EA was
conducted according to another previous study (35). The rats were treated once per day
for 14 days. The same acupuncturist performed all acupuncture and
sham treatments.
Hypothalami were collected for proteomics analysis.
All experimental rats were sacrificed following anesthesia with an
intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg).
The anesthetic regimen resulted in adequate anesthesia (Pinna
reflex and pedal reflex were tested.) in all rats within 10 min
(36,37). Rats were sacrificed by cervical
dislocation (38). The hypothalamus
was isolated from each rat brain using tweezers and transferred to
liquid nitrogen for preservation.
Protein extraction and digestion
Hypothalami were homogenized three times at speed 6
for 40 sec in 8 M urea supplemented with cOmplete™ protease
inhibitor cocktail (Roche Diagnostics) using a FastPrep instrument
(MP Biochemicals, Inc.; Thermo Fisher Scientific, Inc.). The
supernatant containing total protein was collected following
centrifugation at 13,000 x g for 30 min at 4˚C. Concentrations were
determined using a BCA assay kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. A total of 150 µg of
protein from each sample was reduced with 10 mM
Tris-(2-carboxyethyl) phosphine (Thermo Fisher Scientific, Inc.) at
37˚C for 60 min and alkylated with 40 mM iodoacetamide (cat. no.
A3221; Sigma-Aldrich; Merck, KGaA) in the dark at room temperature
for 30 min. Protein samples were collected using acetone
precipitation. A volume of 400 µl acetone was added to each sample
which was then stored at -20˚C for 2 h. Proteins was collected by
centrifugation at 16,000 x g at 4˚C for 20 min. Then the protein
samples were digested with trypsin (Promega Corporation) at 37˚C
overnight. The peptides were desalted on Sep-Pak tC18 cartridges
(Waters Corporation), according to the manufacturer's protocol.
Label-free proteomics analysis
Tryptic peptides were analyzed on a Q-Exactive mass
spectrometer (Thermo Fisher Scientific, Inc.) coupled to an
Easy-nLC 1200 nanoflow liquid chromatography system (Thermo Fisher
Scientific, Inc.). The dried peptides were redissolved with Solvent
A [2% acetonitrile (ACN) and 0.1% trifluoroacetic acid (TFA; cat.
no. 28903; Thermo Fisher Scientific, Inc.) in water] and 2 µg was
loaded onto a C18 column (1.9 µm; 75x25 cm; Thermo Fisher
Scientific, Inc.) and separated with a gradient of 5-38% Solvent B
(80% ACN and 0.1% formic acid at a flow rate of 300 nl/min. The
data-dependent acquisition mode was used for the mass spectrometry
(MS) for 120 min at room temperature. Full mass scans (350-1800
m/z) were acquired at a resolution of 70,000 and the top 20 most
abundant precursor ions were selected for higher-energy collisional
dissociation fragmentation at a resolution of 17,500. The dynamic
exclusion was 18 sec.
Proteome Discoverer software (version 2.2; Thermo
Fisher Scientific, Inc.) was used for protein identification
against the Rattus norvegicus UniProt database (https://www.uniprot.org/uniprot/?query=reviewed:yes%20taxonomy:10114;
28-08-2018). Cysteine reduced with iodoacetamide was set as the
static modification and the oxidation of methionine and acetylation
of the protein N-terminus were set as the dynamic modifications.
The maximum missed cleavage sites and the precursor mass tolerance
were set to 2 and 10 ppm, respectively. The cutoff of the global
false discovery rate was set to 0.01 at the peptide level.
Parallel reaction monitoring (PRM)-MS
validation
Protein abundance differences obtained in the
label-free proteomics analysis were confirmed using PRM assays.
Independent retention time peptides (Biognosys AG) were added to
the samples, according to the manufacturer's protocol. The
scheduled PRM assays were conducted on the Q-Exactive HF coupled
Easy-nLC 1,200 nanoflow liquid chromatography system (Thermo Fisher
Scientific, Inc.). SpectroDive 9 software (version SW-3002;
Biognosys AC) was used to develop the inclusion list (Table SI) and perform data analysis using
the default parameters.
Statistical analysis
Mixed ANOVA and the Bonferroni's post-hoc test was
performed to detect differences in blood pressure. Label-free and
PRM data were analyzed using one-way ANOVA followed Tukey's
post-hoc test. The Venn diagram was drawn using R version 3.6.3
(VennDiagram package). Gene ontology (GO) annotation was performed
using Blast2GO® (www.blast2go.com) and GOATOOLS (v0.6.10; https://github.com/tanghaibao/goatools/releases/tag/v0.6.10)
was used to run the GO enrichment analysis. P-values were assessed
using Fisher's exact test and adjusted following the
Benjamini-Hochberg method. Qiagen Ingenuity Pathway Analysis (IPA)
software (version 2019; QIAGEN, Inc.) was used for the function and
network analyses of the differentially expressed proteins (DEPs,
P<0.05; fold change ≥1.3). A z-score algorithm was used to
predict the function of the DEPs. A size of 35 focus molecules,
both direct and indirect relationships with Fisher's exact test
were applied to run the network analysis (score=-logP-value).
One-way ANOVA and Bonferroni's post-hoc test was performed for PRM
data analysis. Graphs of PRM data were prepared using GraphPad
Prism (version 6; GraphPad Software, Inc.). All statistical
analysis was performed using SPSS (version 22; IBM Corp.)
Results
Multiple acupuncture manipulations
lower blood pressure in SHRs
To study the antihypertensive effects of different
acupuncture manipulations, TRFM, TRDM and EA was performed on SHRs.
The systolic blood pressure of all experimental rats was measured
regularly (Table I). The results
demonstrated that there was no significant difference in the blood
pressure measurements between the acupuncture-treated groups (TRDM,
TRFM and EA) and group M (all, P>0.05) on day 0 (one day prior
to acupuncture manipulation). However, the blood pressure
measurements of the M, TRDM, TRFM and EA groups were significantly
higher compared with group WKY at all time points (all P<0.05).
The blood pressure measurements of the TRDM, TRFM and EA groups
decreased significantly compared with the M group on days 8, 13 and
15 (all, P<0.05). All three acupuncture manipulation groups
exhibited attenuated blood pressures following 15 days of
treatment; however, the blood pressure measurements of the TRDM,
TRFM and EA groups were reduced by varying degrees. The blood
pressure measurements of the TRDM group were lower compared with
the TRFM and EA groups on day 15; however, the differences were not
significant (both, P>0.05). These results indicated that the
acupuncture manipulations had a positive effect in lowering the
blood pressure measurements of the SHR groups, with TRDM possibly
being the most effective treatment. The blood pressure measurements
of the TRDM group declined over time and there was a significant
difference at day 15 vs. day 3(P<0.05). There was a slight
decrease in the blood pressure measurements in the TRDM and EA
groups; however, P>0.05 in all cases (all the others days vs.
day 0). These data indicated that TRDM, TRFM and EA lowered the
blood pressures of the SHRs by varying degrees. Furthermore, the
data revealed an instant and long-term protective effect of TRDM
and an instant effect of TRFM and EA in SHRs.
 | Table ISystolic blood pressure measurements
in the rats. |
Table I
Systolic blood pressure measurements
in the rats.
| Group | Day 0 | Day 3 | Day 8 | Day 13 | Day 15 |
|---|
| WKY | 110.12±1.46 | 111.62±2.07 | 112.75±2.38 | 112.25±3.24 | 112.50±3.66 |
| M |
165.13±1.46a |
165.25±2.25a |
170.38±2.00a,c,d |
173.38±3.34a,c,d |
174.00±2.73a,c,d |
| TRFM |
164.63±2.20a |
164.50±1.93a |
163.63±3.38a,b |
162.62±3.74a,b |
162.50±3.36a,b |
| TRDM |
164.88±2.70a |
163.75±2.60a |
161.38±3.54a,b |
159.88±2.53a-c |
158.13±3.04a-d |
| EA |
165.00±2.00a |
163.88±3.76a |
163.38±3.02a,b |
162.75±3.77a,b |
162.25±4.83a,b |
Analyses of the proteomics data
To investigate the effect of acupuncture treatment
on the central nervous system, proteomics analysis on the
hypothalami of the SHRs was performed. Hypothalami from all rats
were subjected to proteomics analysis through a label-free
technique to obtain profiling data. The results identified 4,483
proteins (Fig. 1A, Table SII). Following this, four
comparisons (M/WKY, TRFM/M, EA/M and TRDM/M) were performed to
identify altered proteins. A total of 241 (M/WKY), 61 (TRFM/M), 117
(EA/M) and 86 (TRDM/M) proteins were identified as DEPs (P<0.05;
fold change ≥1.3). Further comparisons were performed to
investigate the differences and overlaps in the DEPs induced by the
TRDM, TRFM and EA manipulations via Venn analysis (Fig. 1B), indicating the presence of
numerous rescue effect-related proteins. GO enrichment analysis of
DEPs in the M group compared with the WKY group was performed
(Fig. 1C). The dysregulated
proteins in groups TRFM, EA and TRDM were compared with the M
group. Following this, GO enrichment analyses of these proteins was
conducted. Extracellular exosome, Golgi apparatus and endoplasmic
reticulum membrane were among the top 5 most highly enriched
(P<0.05) terms in all of the GO enrichment analyses (Fig. 1C and D), indicating that hypertension and
acupuncture manipulations affected the expression of secreted
proteins in the hypothalami of the SHRs. Furthermore, other cell
component terms, including symmetric synapses, and biological
process terms, including endosome organization, were also
significantly enriched (Table
SIII). In total 11 neuron development and
neuritogenesis-related proteins were identified: A2MG, NENF, ACY2,
DVL1, DYR1A, FNTA, KCJ10, KDIS, COLI, RAB10 and SNX1 (Table SIII). In summary, these results may
elucidate the effect of acupuncture manipulation on the central
nervous system in the SHRs.
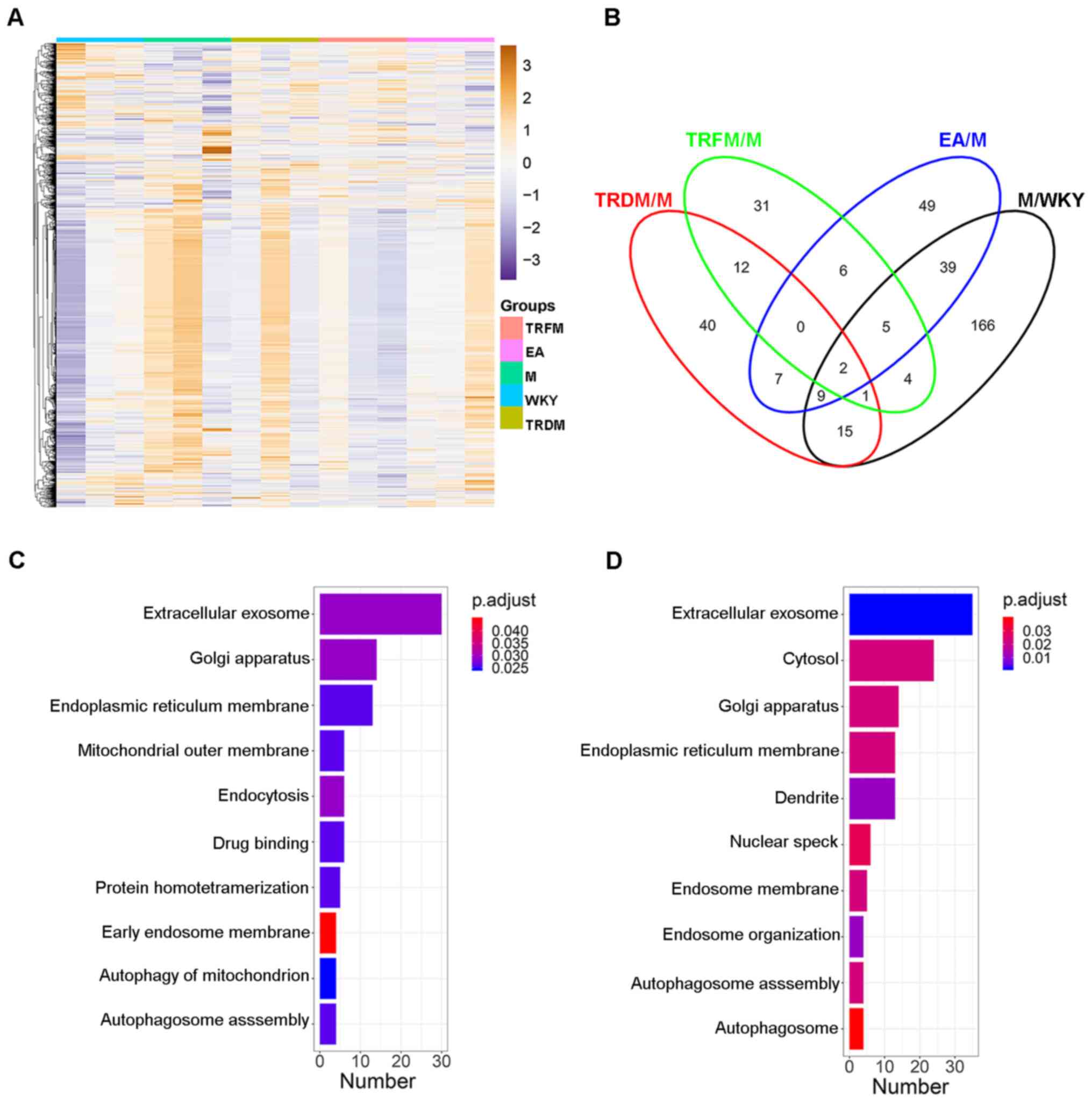 | Figure 1Bioinformatics analyses of label-free
proteomics data. (A) Heatmap of differentially expressed proteins
in the four comparison groups (M/WKY, TRFM/M, EA/M, TRDM/M). (B)
Venn diagram of the comparison groups. (C) Top 10 GO terms in the
GO enrichment analysis of DEPs in the M groups compared with the
WKY group. (D) Top 10 GO terms in the GO enrichment analysis of all
DEPs in the acupuncture groups (TRDM, TRFM and EA) compared with
the M group. M, model; WKY, Wistar-Kyoto; TRFM, twirling
reinforcing manipulation; EA, electroacupuncture; TRDM, twirling
reducing manipulation; GO, gene ontology; DEPs, differentially
expressed proteins. |
IPA function and network analysis of
the DEPs associated with acupuncture manipulation
The function and network of all the dysregulated
proteins in the acupuncture-treated SHRs were evaluated using IPA
software. The acupuncture manipulation-affected genes were
significantly associated with multiple physiological processes
(Fig. 2A), including ‘increased
neuronal development activity’ and ‘decreased neurological disease
tremor and movement disorder activity’ (Fig. 2B, Table
SIV). The top 5 activated functions (‘cytoplasm organization’,
‘cytoskeleton organization’, ‘microtubule dynamics’, ‘neuron
development’ and ‘neuritogenesis’) are presented. Considering these
functions contained numerous overlapping genes, the functions were
merged to produce a broader view of the genes involved.
Furthermore, to examine the networks associated with acupuncture
manipulation, IPA network analysis of the dysregulated genes in the
hypothalami of the TRDM, TRFM and EA groups was performed. The
analysis identified two networks: Cellular Assembly and
Organization, Nervous System Development and Function, Neurological
Disease (CNN) with 24 focus genes (Fig.
2C, Table SIV) and Cellular
Assembly and Organization, Cellular Function and Maintenance,
Molecular Transport (CCM) with 19 focus genes (Fig. 2D, Table
SIV) were identified. The CNN network was modulated by c-Jun,
insulin, p38 mitogen-activated protein kinase, PI3K complex and
cytokine, while the CCM network was modulated by Akt, AMPK, MTORC,
PP2A, mTOR and STAT. Networks including ‘Psychological Disorders’,
‘Nervous System Development and Function’ and ‘Neurological
Disease’ were assessed (Table SV).
Multiple disease-related functions and networks affected by
acupuncture treatments were identified.
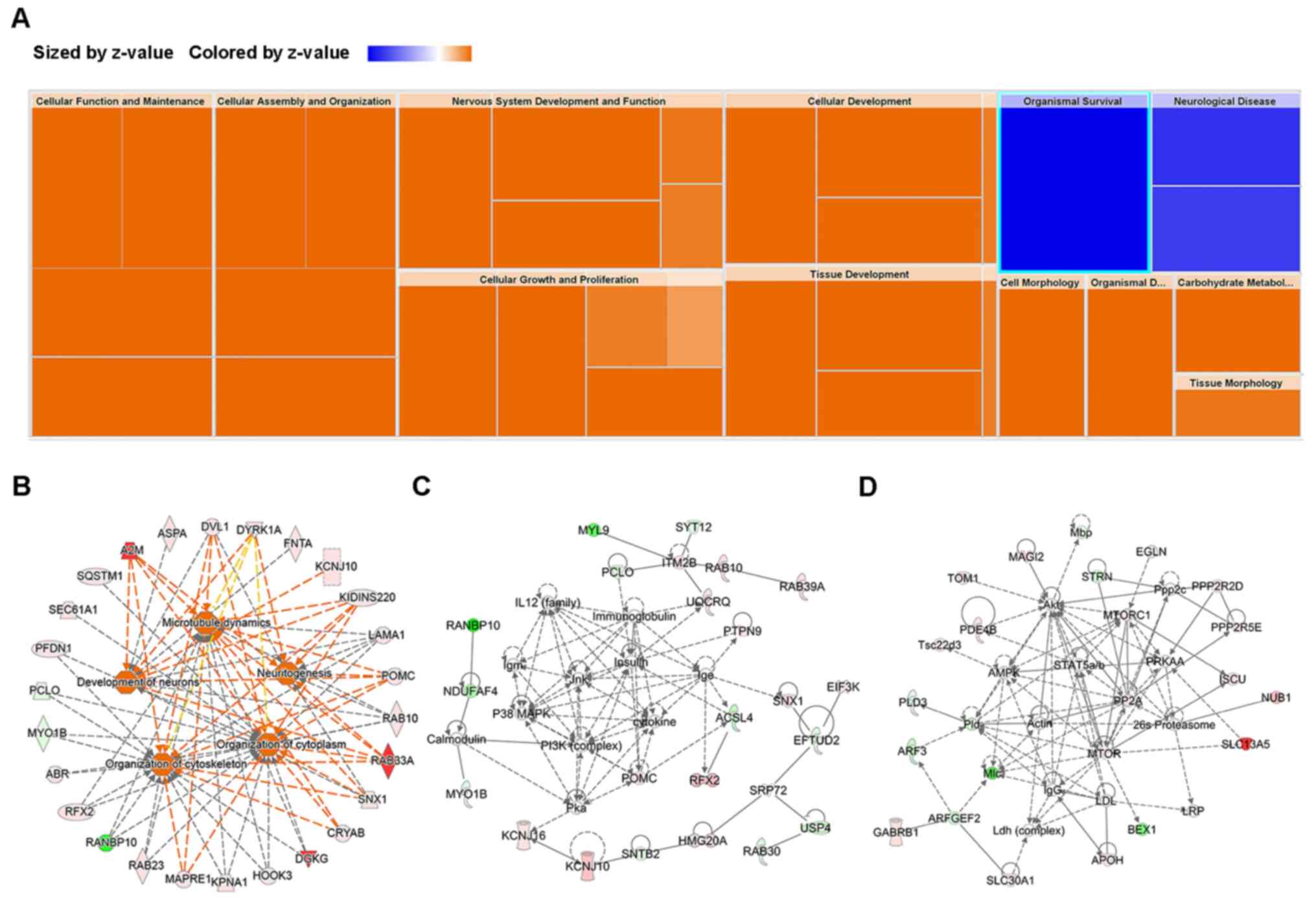 | Figure 2IPA analyses of the differentially
expressed proteins. (A) Heatmap of the function and disease
analysis of all differentially expressed genes (TRFM/M, EA/M,
TRDM/M). Colors were defined by the z-scores, with orange
representing upregulation and blue downregulation. Size was defined
by the-log(P-value). (B) The increased effects on the cytoplasm
organization, cytoskeleton organization, microtubule dynamics,
neuron development and neuritogenesis, the red symbols indicate
upregulated genes and green symbols downregulated genes. (C) An IPA
network involved in cell morphology (Cell Morphology, Digestive
System Development and Function, RNA Post-Transcriptional
Modification; (D) An IPA network involved in nervous system
(Nervous System Development and Function, Neurological Disease,
Tissue Morphology). TRFM, twirling reinforcing manipulation; M,
mode; EA, electroacupuncture; TRDM, twirling reducing manipulation.
Further details of specific genes may be found in Table SIV. |
Validation of the dysregulated
proteins via PRM
Several proteins, including pre-mRNA-processing
factor 6 (PRP6), heme binding protein 2 isoform CRA b (D3ZHC4),
glucose-fructose oxidoreductase domain-containing 1 (D3ZR63), cell
adhesion molecule L1-like (M0RC17), protein O-linked-mannose
β-1,4-N-acetylglucosaminyltransferase 2 (PMGT2), RAB10 member RAS
oncogene family (Q5RKJ9), dual specificity
tyrosine-phosphorylation-regulated kinase 1A (DYR1A), neudesin
(NENF), sorting nexin-1 (SNX1), phosphoserine phosphatase (SERB)
and NADH dehydrogenase 1 α-subcomplex assembly factor 4 (NDUF4)
selected from the GO enrichment and IPA analyses were subjected to
validation by PRM (Fig. 3). D3ZHC4,
MORC17, PMGT2, Q6IUR5, Q9NQR8 and Q5M819 in the hypothalami of the
M group were significantly downregulated compared with the WKY
group. EA manipulation rescued the expression of D3ZHC4, MORC17,
PMGT2, Q6IUR5 and Q9NQR8 to varying extents. The TRFM group rescued
Q6IUR5 and Q5M819. Furthermore, Q63470 was significantly
upregulated in the M group compared with the WKY group and
significantly downregulated in the TRFM and EA groups compared with
Group M. However, NENF, a neurotrophic factor in neurons (39), was significantly upregulated in both
TRFM and EA group compared with M group, indicating that
acupuncture manipulation had an effect on the central nervous
system.
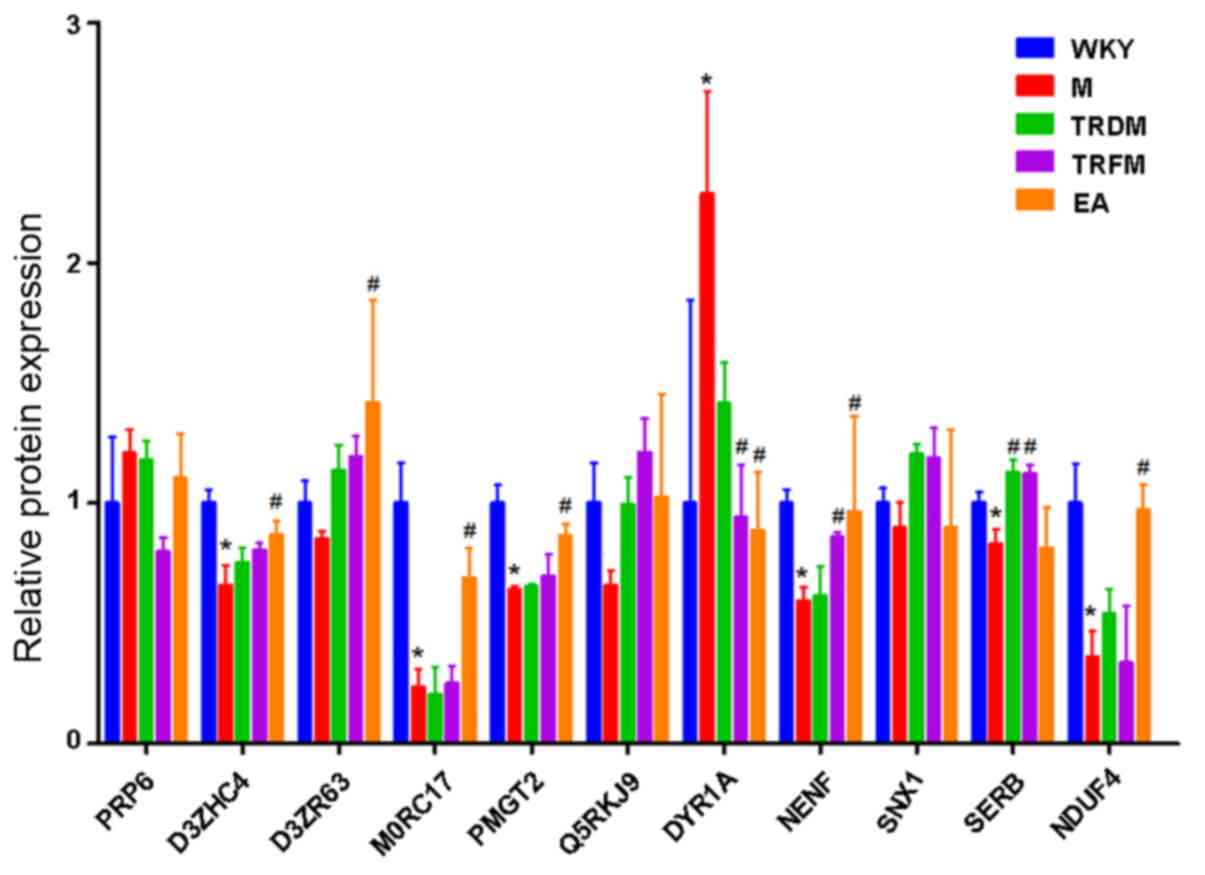 | Figure 3Protein expression profile of the
protein candidates was validated by parallel reaction monitoring.
*P<0.05 vs. the WKY group. #P<0.05 vs.
the M group. WKY, Wistar-Kyoto; M, model; TRDM, twirling reducing
manipulation; TRFM, twirling reinforcing manipulation; EA,
electroacupuncture; PRP6, pre-mRNA-splicing factor 6; D3ZHC4, Heme
binding protein 2 (Predicted), isoform CRA_b; D3ZR63,
glucose-fructose oxidoreductase domain-containing 1; M0RC17, cell
adhesion molecule L1-like, PMGT2, protein O-linked-mannose
β-1,4-N-acetylglucosaminyltransferase 2; Q5RKJ9, RAB10 member RAS
oncogene family; DYR1A, dual specificity
tyrosine-phosphorylation-regulated kinase 1A; NENF, neudesin
neurotrophic factor; SNX1, sorting nexin-1; SERB, phosphoserine
phosphatase; NDUF4, NADH dehydrogenase [ubiquinone] 1 α subcomplex
assembly factor 4. |
Discussion
Numerous experimental and case studies have
indicated that acupuncture manipulation lowered the blood pressure
measurements of SHRs and patients with hypertension (23,40,41)
and LR3 was the most frequently used location in clinical trials
(42,43). In previous studies, TRDM, TRFM and
EA at the LR3 location lowered the blood pressure measurements of
SHRs and affected vascular smooth muscle through a central
mechanism involving activated key brain regions (35,44).
Several studies have demonstrated that LR3 affects biological
processes via the hypothalamus (5-47).
Acupuncture manipulation on LR3 has been reported to inhibit the
hyperactive hypothalamic-pituitary-adrenal axis in depression
treatments (45). Luo et al
(46) demonstrated that needling at
LR3 may reduce blood pressure in renal canaliculi by altering the
number of cells in the hypothalamus and the expression of glucose
transporter 1. The twirling reinforcing-reducing manipulation
(TRRM) treatment in LR3 significantly lowered the blood pressure
and protected hypothalamic neurons by positively regulating the
expression of the hypothalamic renin-angiotensin system (RAS)
components of SHRs, which may be one of the central mechanisms by
which TRRM induces antihypertensive effects (47).
In the present study, the antihypertensive effects
of three acupuncture manipulations (TRDM, TRFM and EA) on LR3 were
compared. To further reveal the central mechanism underlying the
antihypertensive effect of acupuncture treatments, proteomics
analysis was performed to examine the protein expression in the
hypothalamus, followed by a PRM experiment for validation. The
results demonstrated that different acupuncture treatments lowered
blood pressure to varying degrees and multiple dysregulated
proteins and potential networks associated with acupuncture stimuli
were identified.
A series of blood pressure tests demonstrated that
all three acupuncture manipulations used in the current study
exhibited antihypertensive effects to varying degrees. The blood
pressure measurements of the SHRs constantly rose compared with WKY
rats. As indicated in Table I, TRFM
and EA stimuli of SHRs reduced the blood pressure in comparison to
that of the M group from day 8. A continuous decline trend in the
blood pressure of TRDM group was identified and the blood pressure
measurements in the TRDM group were the lowest of all groups (M, EA
and TRFM) at the end of the experiment, indicating that stimulating
LR3 by TRDM exhibited the highest efficacy. The decreased blood
pressure by acupuncture manipulations varied with the acupuncture
methods and, for the LR3 loci, TRDM was the most effective in
reducing blood pressure. Furthermore, the LI11 (Quchi), GB20
(Fengchi), ST36 (Zusanli), ST40 (Fenglong), LI4 (Hegu) and ST9
(Renying) points have been used in the clinical treatment for
hypertension (48,49). Further studies are required to
investigate the antihypertensive effects of different acupuncture
manipulations on different acupuncture points.
The central nervous system, particularly the
hypothalamus, influences the regulation of the cardiovascular
system and the hypertension development (27). Gene expression, including expression
of mRNAs and microRNAs, in response to acupuncture therapy has been
previously studied (28,50). Furthermore, it has been hypothesized
that protein expression levels are partially associated with
transcript levels (51). A previous
study has indicated that acupuncture manipulation at LR3 influences
cerebral glucose metabolism in multiple brain regions, including
the cerebellum, hypothalamus, midbrain and hippocampus (34). To further investigate the effect of
acupuncture manipulation at LR3 on hypothalamus, the protein
expression profiles in the hypothalamus were examined and GO and
IPA were used for data mining. The results demonstrated that
numerous secreted proteins participated in the antihypertensive
mechanism in the hypothalamus and that certain secreted proteins
(Table SV). The results also
revealed that NENF, was downregulated in the hypothalamus of the
SHRs compared with the WKY rats and that SHRs receiving acupuncture
treatments exhibited reversal of the downregulation of NENF. NENF,
a secreted protein, serves a role in the development of obesity,
diabetes and melanoma (52-55),
and may therefore represent a potential drug target. While it has
been demonstrated that the neurotrophic mechanism of NENF is
dependent on heme binding activity, the exact pathway remains to be
elucidated (54). A previous study
reported that NENF is associated with protein deglycase DJ-1 and
PTEN-induced kinase 1 (PINK1) and that this association may promote
neurotrophic activity and neuronal survival (55). Additionally, NENF has been reported
to serve as an anorexigenic factor in the hypothalamus (56). Due to this, NENF may represent a
potential target for drugs to treat hypertension. Furthermore, 10
more neuron development- and neuritogenesis-related proteins were
identified; A2MG, ACY2, DVL1, DYR1A, FNTA, KCJ10, KDIS, COLI, RAB10
and SNX1 (Table SIII). Further
research is required to address the association between NENF and
these proteins, with regards to neurotrophic activity. In future
studies the expression of DJ-1 and PINK1 will be analyzed in the
hypothalami of acupuncture-treated SHRs to evaluate the
antihypertensive effect of hypothalamic DJ-1 and PINK1. To fully
elucidate the mechanism of the antihypertensive effect of
acupuncture manipulation, further research on protein expression
analysis in the hypothalamus and other brain regions is
required.
In conclusion, the results of the current study
demonstrated that TRDM, TRFM and EA manipulations at the LR3 point
reduced blood pressure measurements and that TRDM was the most
effective of the techniques used. The hypothalamic protein
expression profiles of the SHRs were altered following acupuncture
therapy. Furthermore, multiple networks and proteins, particularly
NENF, were identified and they may represent potential
antihypertensive drug targets.
Supplementary Material
Inclusion list in parallel-reaction
monitoring. CS, charges. NE, collision energy.
Protein identification &
quantification. WKY, Wistar-Kyoto; M, model; TRFM, twirling
reinforcing manipulation; TRDM, twirling reducing manipulation; EA,
electroacupuncture.
GO enrichment of DEPs. GO, Gene
Ontology; DEP, differentially expressed proteins.
IPA analysis of DEPs. IPA, Ingenuity
pathway analysis; DEPs, differentially expressed proteins.
Gene name table.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by Chinese Nature
Science Foundation Grants (grant no. 81774413).
Availability of data and materials
All the thermo raw files of proteomics analysis can
be obtained from iProx database (https://www.iprox.org/page/HMV006.html, accession
number IPX0001978001). Additional data is available from the
corresponding author on reasonable request.
Authors' contributions
JRL wrote the paper and was responsible for the
design and running of the main experiments. QGL was involved in the
design of the study. JJW and XDZ measured the blood pressure of
rats. XMH, TXZ and JS performed acupuncture manipulations. ZJ, KP
and KML analyzed the data. All authors read and approved the final
maunscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Use Committee of Beijing University of Chinese
Medicine, Beijing, China (permit no. BUCM-3-2016090301-3003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bloch MJ: Worldwide prevalence of
hypertension exceeds 1.3 billion. J Am Soc Hypertens. 10:753–754.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Messerli FH, Williams B and Ritz E:
Essential hypertension. Lancet. 370:591–603. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garfinkle MA: Salt and essential
hypertension: Pathophysiology and implications for treatment. J Am
Soc Hypertens. 11:385–391. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sousa AC, Mendonça MI, Pereira A, Gouveia
S, Freitas AI, Guerra G, Rodrigues M, Henriques E, Freitas S,
Borges S, et al: Synergistic association of genetic variants with
environmental risk factors in susceptibility to essential
hypertension. Genet Test Mol Biomarkers. 21:625–631.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wrzosek M, Sokal M, Sawicka A, Wlodarczyk
M, Glowala M, Wrzosek M, Kosior M, Talalaj M, Biecek P and Nowicka
G: Impact of obesity and nitric oxide synthase gene G894T
polymorphism on essential hypertension. J Physiol Pharmacol.
66:681–689. 2015.PubMed/NCBI
|
|
6
|
Fonkoue IT, Wang M and Carter JR:
Sympathetic neural reactivity to mental stress in offspring of
hypertensive parents: 20 Years revisited. Am J Physiol Heart Circ
Physiol. 311:H426–H432. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mensah GA, Croft JB and Giles WH: The
heart, kidney, and brain as target organs in hypertension. Curr
Probl Cardiol. 28:156–193. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Azizi M, Rossignol P and Hulot JS:
Emerging drug classes and their potential use in hypertension.
Hypertension. 74:1075–1083. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gniwa Omezzine R, Akkara A, Abdelkafi
Koubaa A, Belguith Sriha A, Rdissi A and Amamou K: Predictors of
poor adherence to hypertension treatment. Tunis Med. 97:564–571.
2019.PubMed/NCBI
|
|
10
|
Nagayama T, Nishida M, Hizue M, Ogino Y
and Fujiyoshi M: Adverse drug reactions for medicines newly
approved in japan from 1999 to 2013: Hypertension and hypotension.
Basic Clin Pharmacol Toxicol. 118:306–312. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, Zhang L, Jian L, Li L and Niu S:
Curative effect of amlodipine combined with enalapril in curing
hypertension of the aged. Pak J Pharm Sci. 28 (2 Suppl):S693–S696.
2015.PubMed/NCBI
|
|
12
|
Flachskampf FA, Gallasch J, Gefeller O,
Gan J, Mao J, Pfahlberg AB, Wortmann A, Klinghammer L, Pflederer W
and Daniel WG: Randomized trial of acupuncture to lower blood
pressure. Circulation. 115:3121–3129. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Turnbull F and Patel A: Acupuncture for
blood pressure lowering: Needling the truth. Circulation.
115:3048–3049. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tjen-A-Looi SC and Longhurst CA: John C.
Longhurst, MD, PhD (1947-2018). A pioneer in acupuncture
hypertension research. Am J Physiol Heart Circ Physiol.
314:H1153–H1154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhuang Y, Xing JJ, Li J, Zeng BY and Liang
FR: History of acupuncture research. Int Rev Neurobiol. 111:1–23.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
He JL: Preliminary discussion on the
theory of body physiognomy in the Huangdineijing (Huangdi's Inner
Classic). Zhonghua Yi Shi Za Zhi. 39:189–192. 2009.PubMed/NCBI(In Chinese).
|
|
17
|
Zhu DC, Leng C, Xiong J and Ye WG:
Thermosensitive moxibustion induces a better therapeutic effect in
the treatment of facial paralysis patients. Zhen Ci Yan Jiu.
43:666–669. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Xu L, Jing L, He K, Wang JL and Wang Y:
Treatment of knee osteoarthritis with acupuncture and moxibustion:
A randomized controlled trial. Zhongguo Zhen Jiu. 33:871–876.
2013.PubMed/NCBI(In Chinese).
|
|
19
|
Liu W, Zhu LQ, Chen SS, Lu SC, Tang J and
Liu QG: Effect of twirling-reinforcing-reducing needling
manipulations on contents of serum acetylcholine and arterial NOS
and cGMP in stress-induced hypertension rats. Zhen Ci Yan Jiu.
40:136–140. 2015.PubMed/NCBI(In Chinese).
|
|
20
|
Chen H, Dai J, Zhang X, Wang K, Huang S,
Cao Q, Wang H, Liang Y, Shi C, Li M, et al: Hypothalamus-related
resting brain network underlying short-term acupuncture treatment
in primary hypertension. Evid Based Complement Alternat Med.
2013(808971)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cheng L, Li P, Tjen-A-Looi SC and
Longhurst JC: What do we understand from clinical and mechanistic
studies on acupuncture treatment for hypertension? Chin Med.
10(36)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Wang Y, He K, Peng C, Wu P, Li C and
Lai X: Effect of acupuncture at LR3 on cerebral glucose metabolism
in a rat model of hypertension: A 18F-FDG-PET study.
Evid Based Complement Alternat Med. 2018(5712857)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lai X, Wang J, Nabar NR, Pan S, Tang C,
Huang Y, Hao M, Yang Z, Ma C, Zhang J, et al: Proteomic response to
acupuncture treatment in spontaneously hypertensive rats. PLoS One.
7(e44216)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li J, Sun M, Ye J, Li Y, Jin R, Zheng H
and Liang F: The mechanism of acupuncture in treating essential
hypertension: A narrative review. Int J Hypertens.
2019(8676490)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Santisteban MM, Kim S, Pepine CJ and
Raizada MK: Brain-gut-bone marrow axis: Implications for
hypertension and related therapeutics. Circ Res. 118:1327–1336.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Feng XF and Ma TM: Compared with twirling
and rotating acupuncture manipulations in similarities and
differences. J Liaoning Univ Traditi Chin Med. 12(No. 10)2010.(In
Chinese).
|
|
27
|
Khor S and Cai D: Hypothalamic and
inflammatory basis of hypertension. Clin Sci (Lond). 131:211–223.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma SM, Yang JW, Tu JF, Yang NN, Du YZ,
Wang XR, Wang L, Huang J and Liu CZ: Gene-level regulation of
acupuncture therapy in spontaneously hypertensive rats: A whole
transcriptome analysis. Evid Based Complement Alternat Med.
2019(9541079)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang JW, Ye Y, Wang XR, Li F, Xiao LY, Shi
GX and Liu CZ: Acupuncture attenuates renal sympathetic activity
and blood pressure via beta-adrenergic receptors in spontaneously
hypertensive rats. Neural Plast. 2017(8696402)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Okamoto K and Aoki K: Development of a
strain of spontaneously hypertensive rats. Jpn Circ J. 27:282–23.
1963.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jones-Bolin S: Guidelines for the care and
use of laboratory animals in biomedical research. Curr Protoc
Pharmacol. 59:A.4B.1–A.4B.9. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Conn PM (ed): Animal models for the study
of human disease. 2nd edition. Academic Press, pp19, 1177,
2017.
|
|
33
|
Okamoto K, Tabei R, Fukushima M, Nosaka S
and Yamori Y: Further observations of the development of a strain
of spontaneously hypertensive rats. Jpn Circ J. 30:703–716.
1966.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guo Q, Liu Q, Sun D and Nie B: Twirling
reinforcing-reducing manipulation-central mechanism underlying
antihypertensive effect on spontaneous hypertension in rats. J
Tradit Chin Med. 38:391–398. 2018.PubMed/NCBI
|
|
35
|
Guo Y, Xie X, Guo C, Wang Z and Liu Q:
Effect of electro-acupuncture on gene expression in heart of rats
with stress-induced pre-hypertension based on gene chip technology.
J Tradit Chin Med. 35:285–294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yi-Ming W, Shu H, Miao CY, Shen FM, Jiang
YY and Su DF: Asynchronism of the recovery of baroreflex
sensitivity, blood pressure, and consciousness from anesthesia in
rats. J Cardiovasc Pharmacol. 43:1–7. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Redfors B, Shao Y and Omerovic E:
Influence of anesthetic agent, depth of anesthesia and body
temperature on cardiovascular functional parameters in the rat. Lab
Anim. 48:6–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiang B, Zhong P, Fang L, Wu X, Song Y and
Yuan H: miR-183 inhibits microglia activation and expression of
inflammatory factors in rats with cerebral ischemia reperfusion via
NF-κB signaling pathway. Exp Ther Med. 18:2540–2546.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Moutaoufik MT, Malty R, Amin S, Zhang Q,
Phanse S, Gagarinova A, Zilocchi M, Hoell L, Minic Z, Gagarinova M,
et al: Rewiring of the human mitochondrial interactome during
neuronal reprogramming reveals regulators of the respirasome and
neurogenesis. iScience. 19:1114–1132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chiu YJ, Chi A and Reid IA: Cardiovascular
and endocrine effects of acupuncture in hypertensive patients. Clin
Exp Hypertens. 19:1047–1063. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang J, Xiong X and Liu W: Acupuncture for
essential hypertension. Int J Cardiol. 169:317–326. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao XF, Hu HT, Li JS, Shang HC, Zheng HZ,
Niu JF, Shi XM and Wang S: Is acupuncture effective for
hypertension? A systematic review and meta-analysis. PLoS One.
10(e0127019)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Y, Zheng Y, Qu S, Zhang J, Zhong Z,
Zhang J, Huang H, Li M, Xu Y, Chen J, et al: Cerebral targeting of
acupuncture at combined acupoints in treating essential
hypertension: An Rs-fMRI study and curative effect evidence. Evid
Based Complement Alternat Med. 2016(5392954)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Qiulei G: Twirling reinforcing-reducing
manipulation on upoints in treating essential hypertension: An
Rs-fMRn spontaneous hypertension in rats. J Tradit Chin Med.
38:391–398. 2018.
|
|
45
|
Le JJ, Yi T, Qi L, Li J, Shao L and Dong
JC: Electroacupuncture regulate hypothalamic-pituitary-adrenal axis
and enhance hippocampal serotonin system in a rat model of
depression. Neurosci Lett. 615:66–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Luo X, Huang J, Yu J and nTang C: Effect
of Taichong (LR 3) acupuncture in spontaneously hypertensive rats.
J Tradit Chin Med. 39:74–80. 2019.PubMed/NCBI
|
|
47
|
Amlie-Lefond C, Sébire G and Fullerton HJ:
Recent developments in childhood arterial ischaemic stroke. Lancet
Neurol. 7:425–435. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang L, Shen P and Wang S: Acupuncture
treatment for hypertension: A case study. Acupunct Med. 32:73–76.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee H, Kim SY, Park J, Kim YJ, Lee H and
Park HJ: Acupuncture for lowering blood pressure: Systematic review
and meta-analysis. Am J Hypertens. 22:122–128. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang JY, Li H, Ma CM, Wang JL, Lai XS and
Zhou SF: MicroRNA profiling response to acupuncture therapy in
spontaneously hypertensive rats. Evid Based Complement Alternat
Med. 2015(204367)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu Y, Beyer A and Aebersold R: On the
dependency of cellular protein levels on mRNA abundance. Cell.
165:535–550. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kratochvilova H, Lacinova Z, Klouckova J,
Kavalkova P, Cinkajzlova A, Trachta P, Krizova J, Benes M,
Dolezalova K, Fried M, et al: Neudesin in obesity and type 2
diabetes mellitus: The effect of acute fasting and weight reducing
interventions. Diabetes Metab Syndr Obes. 12:423–430.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Polkowska A, Pasierowska IE, Pasławska M,
Pawluczuk E and Bossowski A: Assessment of serum concentrations of
adropin, afamin, and neudesin in children with type 1 diabetes.
Biomed Res Int. 2019(6128410)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ortega-Bernal D, La Rosa CHG,
Arechaga-Ocampo E, Alvarez-Avitia MA, Moreno NS and Rangel-Escareño
C: A meta-analysis of transcriptome datasets characterizes
malignant transformation from melanocytes and nevi to melanoma.
Oncol Lett. 16:1899–1911. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kimura I, Nakayama Y, Zhao Y, Konishi M
and Itoh N: Neurotrophic effects of neudesin in the central nervous
system. Front Neurosci. 7(111)2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Byerly MS, Swanson RD, Semsarzadeh NN,
McCulloh PS, Kwon K, Aja S, Moran TH, Wong GW and Blackshaw S:
Identification of hypothalamic neuron-derived neurotrophic factor
as a novel factor modulating appetite. Am J Physiol Regul Integr
Comp Physiol. 304:R1085–R1095. 2013.PubMed/NCBI View Article : Google Scholar
|

















