Introduction
Autophagy, which is a highly conserved evolutionary
process, degrades harmful protein aggregates and intracellular
toxins to provide nutrients, such as amino acids, nucleotides and
fatty acids, that aid in sustaining ATP production in cells
(1,2). Prostate cancer (PCa) has been revealed
to be one of the most common tumors in men worldwide (3). Emerging evidence has indicated that
autophagy may promote tumor progression and resistance to treatment
in advanced PCa (4-6).
Multiple autophagy regulators, such as sequestosome-1 (SQSTM1/p62)
(7,8) and autophagy related 7 (ATG7) (9), have been demonstrated to serve key
roles in PCa progression. For example, increased ATG7 expression
and decreased PTEN levels have been revealed to drive PCa growth
(9). Xenophagy is a type of
selective autophagy that is associated with the removal of
intracellular pathogens (10,11).
Of note, xenophagy may also affect tumor progression and treatment
(12-14).
For example, Helicobacter pylori- or Epstein-Barr
virus-induced xenophagy has been associated with gastric
tumorigenesis (14). However, the
role of xenophagy in PCa remains largely elusive.
Calcium-binding and coiled-coil domain 2 (CALCOCO2;
also referred to as NDP52) is considered to be the most important
known xenophagy receptor (15).
Xenophagy has been indicated to protect the mammalian cytosol
against bacterial infection (15,16).
CALCOCO2 has been revealed to interact with microtubule-associated
proteins 1A/1B light chain 3 (MAP1LC3)A, MAP1LC3B and/or
γ-aminobutyric acid receptor-associated protein (GABARAP)-like
(GABARAPL) 2 to regulate bacterial targeting via autophagosomes,
and with MAP3LC3C to regulate pathogen-containing autophagosome
maturation (17,18). Moreover, a previous study has
demonstrated that CALCOCO2 binding to galectin-8 activated
antibacterial autophagy to suppress the proliferation of infecting
pathogens (19). CALCOCO2 may also
bind to influenza virus protein PB1-F2 to modulate the innate
immune response (20). Of note, a
number of studies have indicated that CALCOCO2 may be associated
with human cancer progression by interacting with tumor-related
pathways, such as IκB kinase and the NF-κB pathway (20). Using a CALCOCO2 interacting protein
dataset, which was downloaded from The National Center for
Biotechnology Information, the present study revealed that CALCOCO2
was associated with ‘autophagosome assembly’, ‘nucleophagy’ and
‘nucleic acid metabolic process’. Exploring the roles of CALCOCO2
in PCa may provide a potential biomarker for cancer treatment.
The purpose of the present study was to explore the
potential roles of CALCOCO2 in PCa. Loss-of-function assays were
performed to detect the effect of CALCOCO2 on PCa cell
proliferation, apoptosis and colony-forming ability. Moreover,
bioinformatics analysis was performed to identify the mechanisms of
function of CALCOCO2 in PCa. The results of the current study
demonstrated that CALCOCO2 may be of value as a novel therapeutic
and prognostic target for PCa.
Materials and methods
Cell culture
293T, PC-3 and DU145 cells were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were verified using
DNA-fingerprinting and mycoplasma, isozyme and cell vitality
detection (data not shown). The cells were maintained in RPMI-1640
medium supplemented with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Thermo Fisher
Scientific, Inc.). Cultures were maintained at 37˚C in a 5%
CO2 cell incubator.
Lentiviral constructs and
infection
Human lentivirus-short hairpin (sh)CALCOCO2 and
lentivirus-shControl (shCtrl) sequences were designed and purchased
from Shanghai GeneChem Co., Ltd. Recombinant lentiviral vectors
carrying CALCOCO2 shRNA were constructed using standard molecular
techniques according to our previous study (21). Briefly, 293T cells were infected
with 1,200 ng pCV146-Luc-Puromycin-shCALCOCO2 or shCtrl vector, 900
ng pHelper 1.0 vector (Shanghai GeneChem Co., Ltd.) and 600 ng
pHelper 2.0 vector (Shanghai GeneChem Co., Ltd.) using 7.5 µl
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) to
generate stably transfected cells. A total of 4 h after
transfection, Opti-modified Eagle's medium (Opti-MEM; cat. no.
31985062; Thermo Fisher Scientific, Inc.) was changed to RPMI-1640
medium containing 10% FBS (Cytiva) and were cultured at 37˚C in 5%
CO2. After 24 h, concentrated lentiviruses were
collected. Concentrated lentiviruses were transfected into DU145
and PC-3 cells at a MOI of 40 in serum-free RPMI-1640 medium. The
supernatant was replaced with complete culture medium (RPMI-1640
medium containing 10% FBS) after 24 h. Following 48 h the infection
efficiency of CALCOCO2 shRNA was detected via reverse
transcription-quantitative PCR (RT-qPCR) analysis. The CALCOCO2
shRNA sequence was
3'-CCGGGAGCTGCTTCAACTGAAAGAACTCGAGTTCTTTCAGTTGAAGCAGCTCTTTTT-5',
and the shCtrl sequence was
5'-CCGGTCTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAAGATTTTTG-3'.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from PCa
cell lines. RNA (1 µg) was reverse transcribed into complementary
DNA according to the protocol of PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd.). The reaction conditions for the RT were
as follows: 25˚C for 5 min, 50˚C for 25 min and 75˚C for 10 min.
qPCR was performed using the iQ SYBR®-Green Supermix and
ABI Prism 7900 platform (both from Bio-Rad Laboratories, Inc.),
according to the manufacturer's protocol. The PCR cycling
conditions were as follows: 50˚C for 2 min, followed by 95˚C for 10
min; 40 cycles of 95˚C for 15 sec; and 60˚C for 1 min. GAPDH was
used as an internal control. The 2-ΔΔCq method was used
to calculate the relative expression level and GAPDH was used as
the internal reference gene (22).
The primers used were as follows: CALCOCO2 forward,
5'-TGAAGGAGGCGCAAGACAAAA-3' and reverse,
5'-CATCTGCTGTTGCTCCAAGGT-3'; GAPDH forward,
5'-TGACTTCAACAGCGACACCCA-3' and reverse,
5'-CACCCTGTTGCTGTAGCCAAA-3'. All primers were synthesized by
Shanghai GeneChem Co., Ltd. Each sample was run in triplicate to
ensure quantitative accuracy.
Western blotting
Following transfection for 48 h, DU145 and PC-3 cell
lysates were prepared using RIPA lysis buffer and protease cocktail
inhibitor I (Merck KGaA). After quantification with a BCA assay kit
(Thermo Fisher Scientific, Inc.), 10 µg proteins per lane were
separated using 10% SDS-PAGE and subsequently transferred to a PVDF
membrane. After blocking in 5% non-fat milk at room temperature for
1 h, the membranes were washed with TBS + 0.1% Tween-20. Membranes
were incubated with primary antibodies against cyclin-E1 (1:1,000;
cat. no. 4129; Cell Signaling Technology, Inc.); p53 (1:1,000; cat.
no. 2527; Cell Signaling Technology, Inc.), GAPDH (1:1,000; cat.
no. sc-32233; Santa Cruz Biotechnology, Inc.) at 4˚C overnight,
followed by incubation with HRP-conjugated anti-rabbit IgG
(1:2,000; cat. no. 7074) or HRP-conjugated anti-mouse IgG (1:2,000;
cat. no. 7076; both from Cell Signaling Technology, Inc.) secondary
antibodies for 1 h at room temperature. Protein bands were
visualized using Pierce ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). The protein band intensity was analyzed
using ImageJ software, v1.41 (National Institutes of Health).
Plate analysis using the Celigo
adherent cell cytometry system
The Celigo adherent cell cytometry system (Nexcelom
Bioscience LLC) was used for rapid quantification of cellular
fluorescence expression, as previously described (23). Following CALCOCO2 knockdown, PC-3
and DU145 cells were seeded into 96-well plates (1.5x103
cells/well) and cultured at 37˚C in a 5% CO2 cell
incubator for 5 days. Cell counting was performed using the Celigo
Imaging Cytometer and the captured cell images were analyzed using
Celigo software version 1.0 (both from Nexcelom Bioscience
LLC).
Cell apoptosis assay
A total of 1x105 shCALCOCO2 and shCtrl
stably transfected PC-3 and DU145 cells were harvested and washed
with PBS three times. Cells were assayed with an Annexin V-APC
Apoptosis Detection kit (cat. no. 88-8007-74; eBioscience; Thermo
Fisher Scientific, Inc.) and were analyzed using a flow cytometer
(BD FASCCalibur; BD Biosciences). Furthermore, Annexin V-FITC/PI
staining was also performed using flow cytometry (cat. no.
88-8005-72; eBioscience; Thermo Fisher Scientific, Inc.). Briefly,
1x105 shCALCOCO2 and shCtrl PC-3 and DU145 cells were
collected and washed with ice-cold PBS. Subsequently, the cells
were resuspended in 300 µl binding buffer, which was included in
the Annexin V-FITC/PI kit. The cells were incubated with 5 µl
Annexin V-FITC and 5 µl propidium iodide (PI) in the dark for 15
min at room temperature. Finally, the cells were analyzed via flow
cytometry (BD FASCCalibur; BD Biosciences). The total percentage of
apoptotic cells was defined as the sum of both early apoptosis
(Annexin V-FITC positive, PI negative) and late apoptosis (Annexin
V-FITC PI positive), top and bottom right quadrants in flow
cytometric dot plots, respectively.
Colony formation assays
shCALCOCO2 and shCtrl stably transfected PC-3 and
DU145 cells were seeded into six-well plates with a density of 500
cells/well. After 10 days in a 37˚C incubator, cell colonies
(>50 cells) were counted via staining with 0.5% crystal violet
solution (Sigma-Aldrich; Merck KGaA) for 30 sec at room temperature
following fixation with 10% formaldehyde for 5 min at room
temperature. The stained colonies were observed under a light
microscope at a magnification of x20 (Nikon Corporation) and
counted using ImageJ software (version 4.0; National Institutes of
Health). Triplicate wells were measured for each treatment
group.
Gene Ontology (GO) and pathway
enrichment analysis of differentially expressed genes
GO analysis and Kyoto Encyclopedia of Genes and
Genomes pathway analysis (24) were
performed. The Database for Annotation, Visualization and
Integrated Discovery (david.abcc.ncifcrf.gov), was used to integrate
functional genomic annotations (25). P<0.05 was considered to indicate
a statistically significant difference.
Integration of the protein-protein
interaction (PPI) network
The Search Tool for the Retrieval of Interacting
Genes/Proteins version 10.0 (STRING; string-db.org)
was used for the exploration of potential interactions among genes
(26). A PPI score of >0.4 was
considered significant. The PPI networks were visualized using
Cytoscape software version 3.6.1 (http://www.cytoscape.org) (27). P<0.05 was considered to indicate
a statistically significant difference.
Public datasets analysis
The present study analyzed CALCOCO2 protein levels
in PCa tissues using a public dataset, which was derived from The
Human Protein Atlas (HPA; https://www.proteinatlas.org/) (28). HPA, as a database containing images
from immunohistochemical-based tissue microarrays (46 normal human
tissues and 20 types of human cancer) for 11,250 human proteins,
was accessed to analyze CALCOCO2 protein expression in PCa tissues
and non-cancerous prostate tissues, and the images and expression
levels of CALCOCO2 were downloaded from the website following
guidelines (https://www.proteinatlas.org/ENSG00000136436-CALCOCO2/pathology/prostate+cancer).
To examine the effect of CALCOCO2 on PCa cell survival, the
gene-level differential dependency scores (https://depmap.org/rnai/index) were investigated to
assess the association of CALCOCO2 with tumor survival (29). The CALCOCO2 interacting protein map
was directly downloaded from NCBI (https://www.ncbi.nlm.nih.gov/gene/10241).
Statistical analysis
The differences between two groups in terms of gene
expression, cell apoptosis, colony number and migrating cell number
were evaluated using unpaired Student's t-test. All statistical
analyses were performed using GraphPad Prism software v5.0
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
CALCOCO2 knockdown inhibits the
proliferation of PCa cells
DepMap database analysis indicated that CALCOCO2
knockdown suppressed the proliferation of androgen
receptor-dependent LNCaP cells (Fig.
S1). In order to evaluate the effect of CALCOCO2 on PCa
progression, specific shRNAs were designed to target CALCOCO2 in
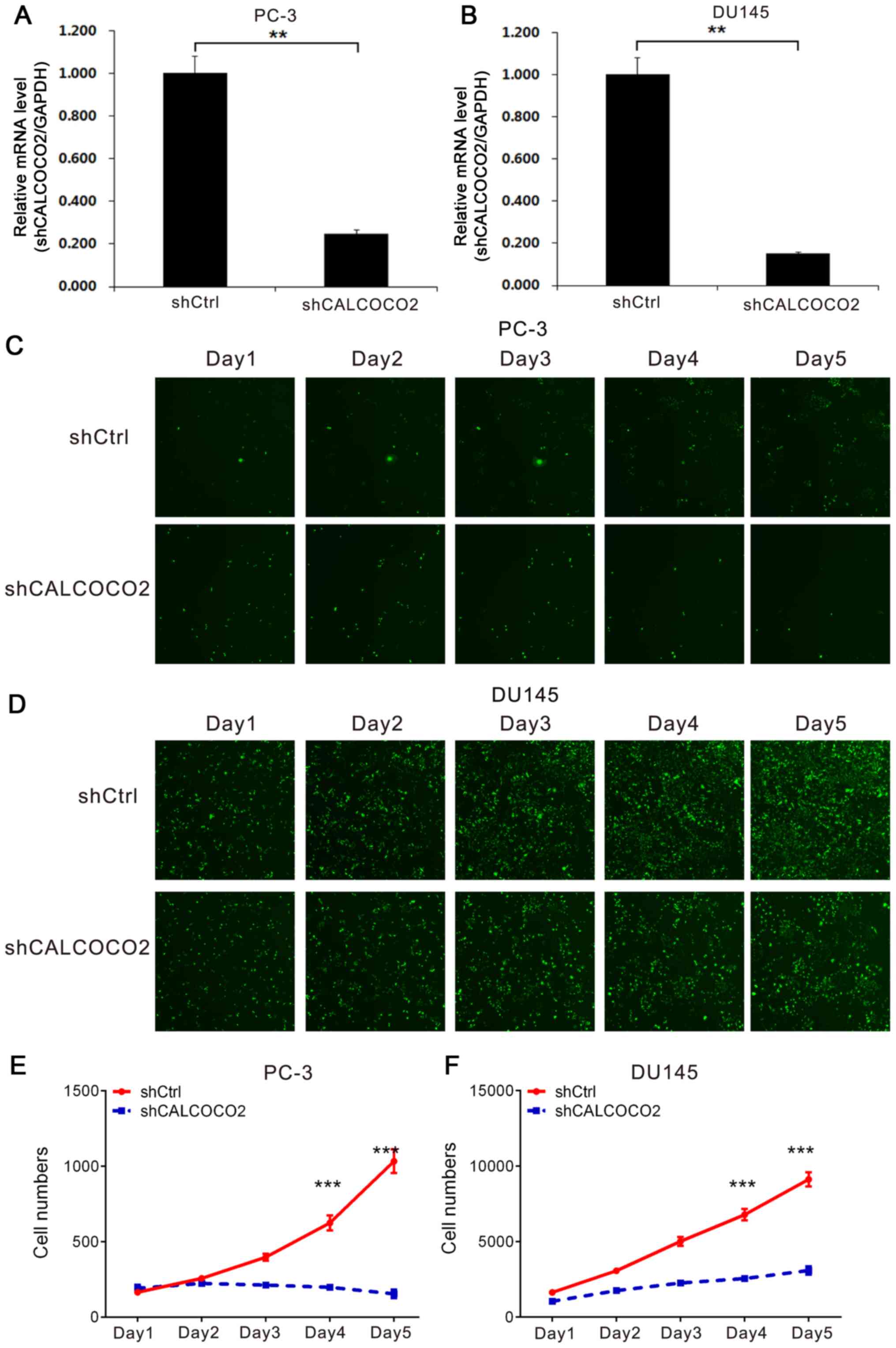
the PCa cell lines PC-3 and DU145. As presented in Fig. 1A and B, PC-3 and DU145 cells transfected with
shCALCOCO2 lentiviral plasmid exhibited significantly lower
CALCOCO2 expression levels compared with those transfected with
shCtrl lentiviral plasmid.
Subsequently, the role of CALCOCO2 in the regulation
of PCa cell proliferation was examined using the Celigo Cell
Counting assay. Cell counting revealed that the PCa cell
proliferation rate was significantly lower in shCALCOCO2 PC-3
(Fig. 1C and E) and DU145 (Fig. 1D and F) cells compared with the shCtrl
groups.
Knockdown of CALCOCO2 suppresses PCa
cell colony formation
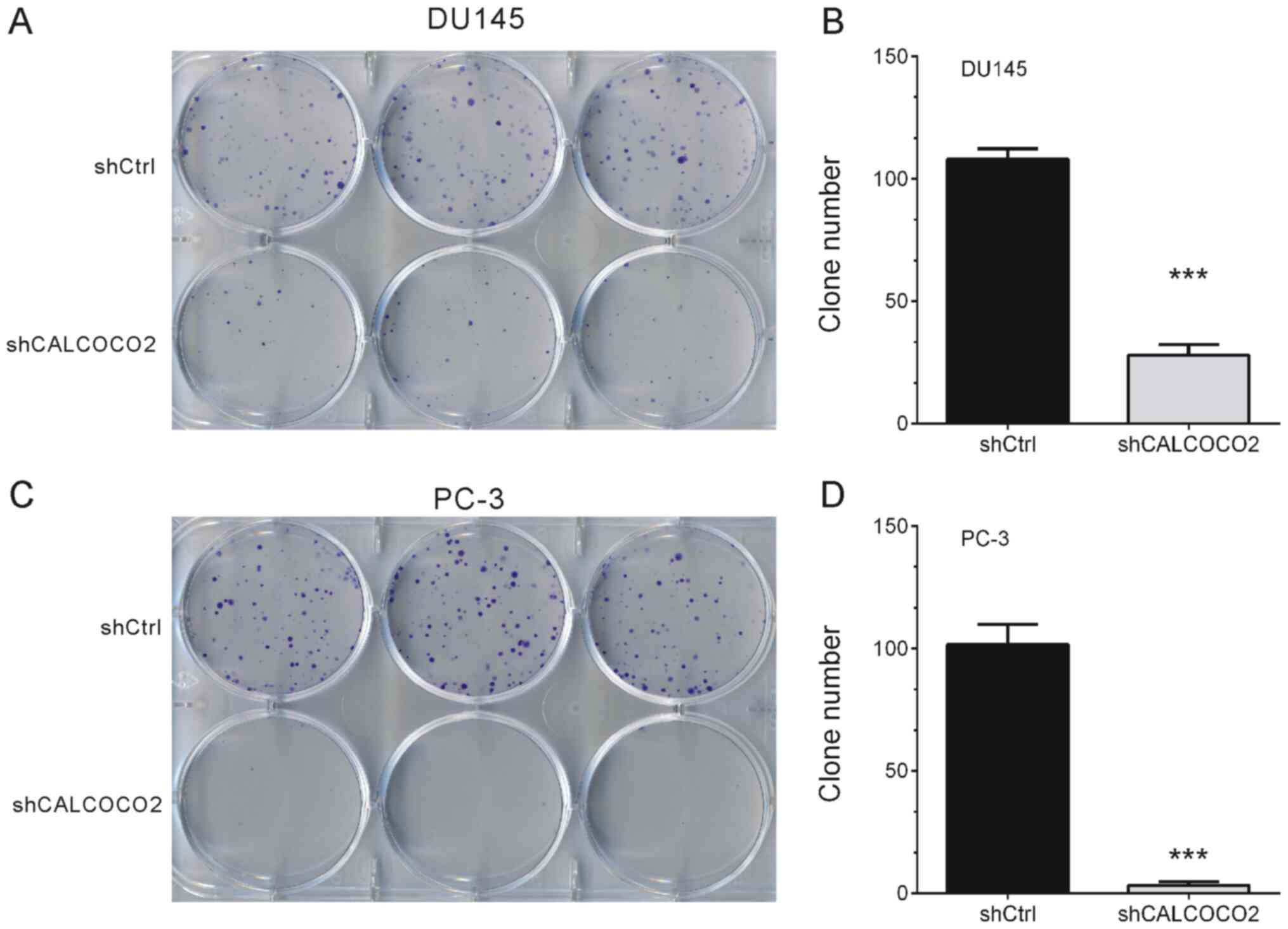
The effects of CALCOCO2 knockdown on DU145 and PC-3
cell tumorigenesis potential were examined in vitro by
assessing colony formation. As indicated in Fig. 2, knockdown of CALCOCO2 resulted in
significant alterations in the ability of PCa cells to form
colonies. Compared with a mean of ~100 colonies in shCtrl DU145 and
PC-3 cells, CALCOCO2 knockdown significantly suppressed PCa cell
colony formation to a mean of 28 colonies in DU145 cells (Fig. 2A and B) and 3 colonies in PC-3 cells (Fig. 2C and D).
Knockdown of CALCOCO2 promotes
apoptosis of PCa cells
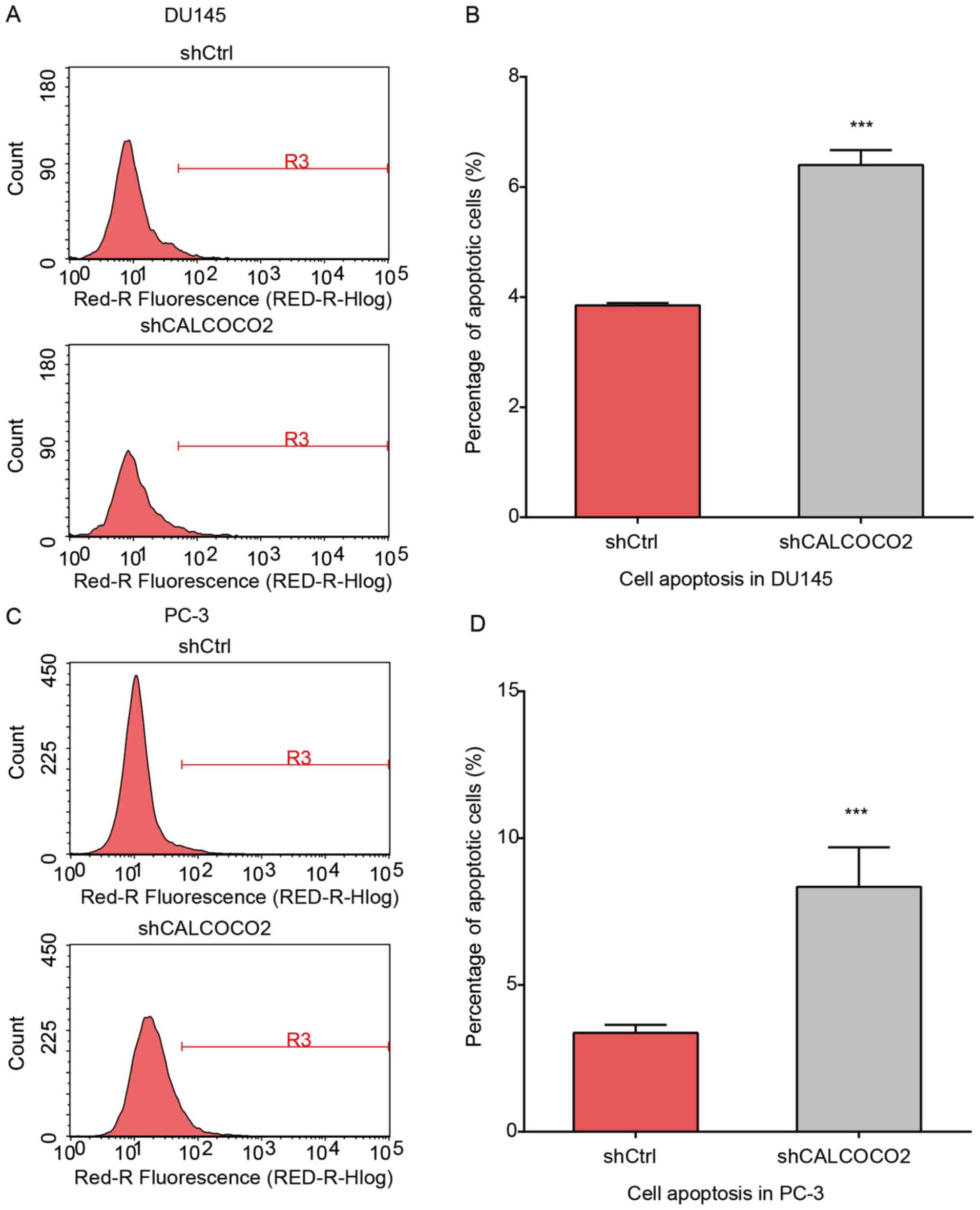
The present study examined the apoptosis of PCa
cells using Annexin V staining following CALCOCO2 knockdown. As
demonstrated in Fig. 3, the
percentage of apoptotic cells in the shCALCOCO2 group was
significantly increased compared with shCtrl cells both in the
DU145 (Fig. 3A and B) and PC-3 (Fig. 3C and D) cell lines.
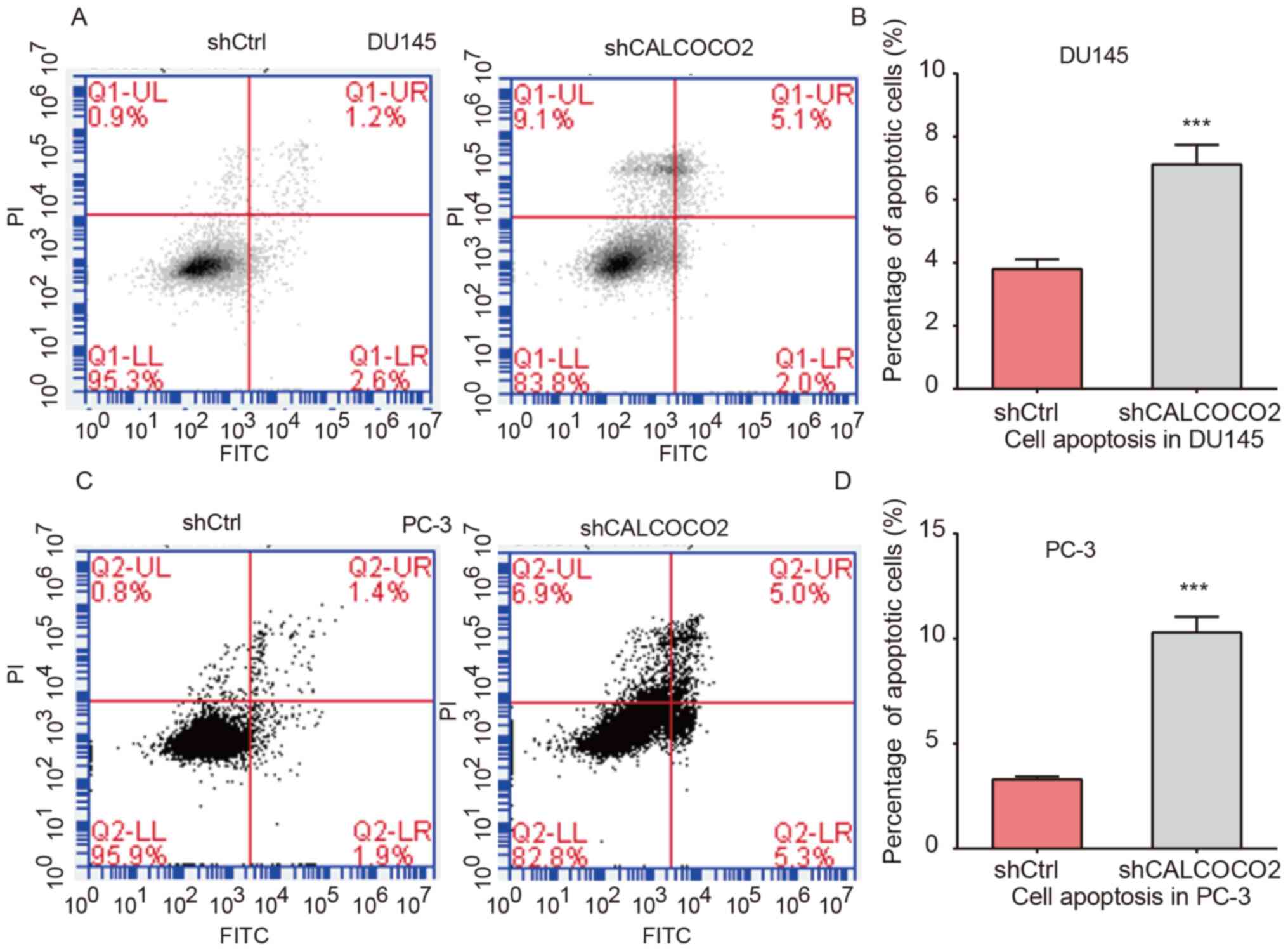
In order to further investigate the
apoptosis-inducing effects of CALCOCO2 on PCa cells, DU145 and PC-3
cells following CALCOCO2 knockdown were stained with Annexin V and
PI, and subsequently analyzed using flow cytometry. The early and
late apoptotic rates of shCALCOCO2 DU145 (Fig. 4A and B) and PC-3 (Fig. 4C and D) cells were significantly increased
compared with the control groups. The combined quantification of
the early and late apoptotic rates is presented in Fig. 4B and D.
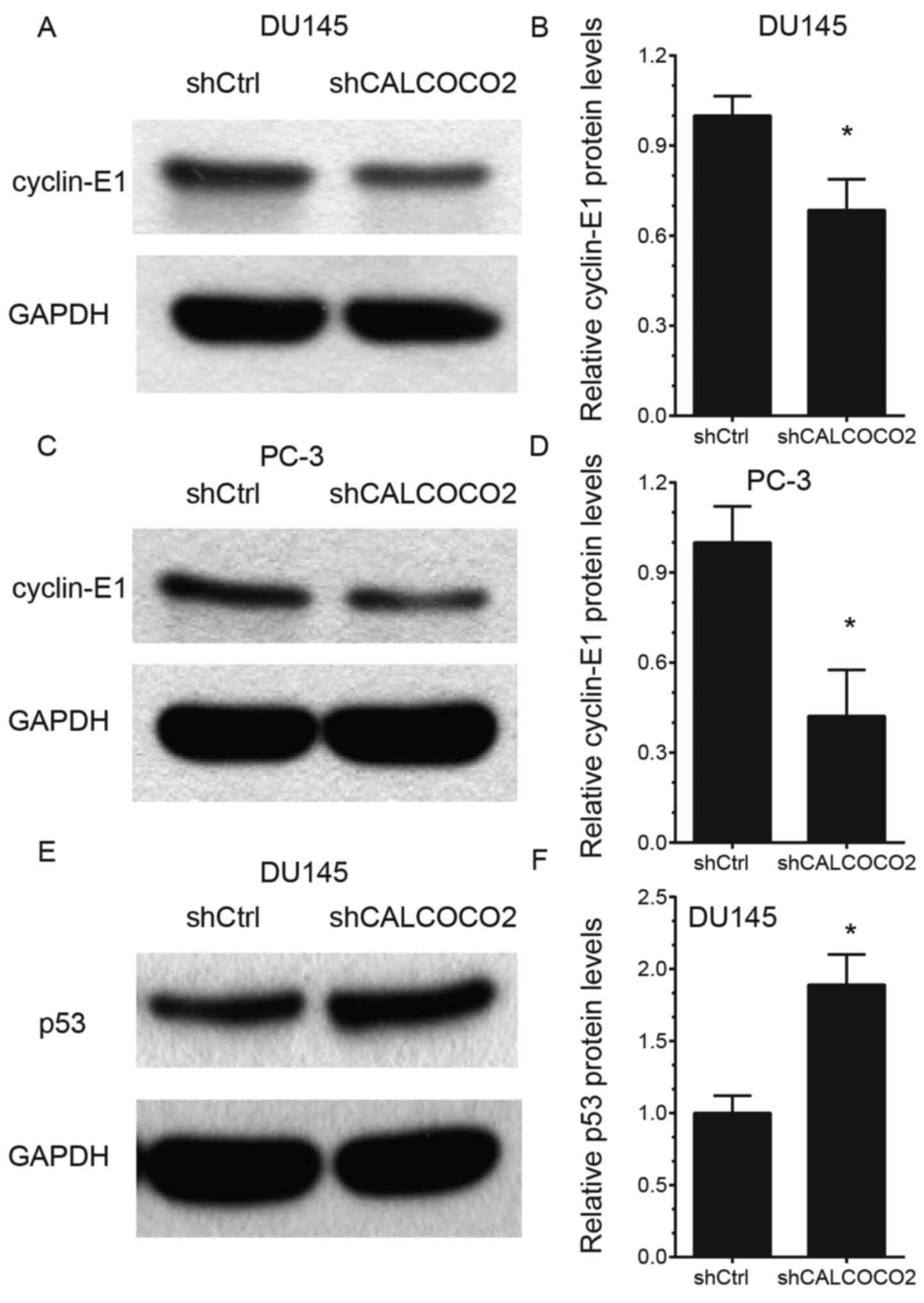
Knockdown of CALCOCO2 suppresses
cyclin-E1 and promotes p53 expression in PCa cells
In order to elucidate the potential mechanisms of
action of CALCOCO2 in the regulation of PCa cell proliferation and
apoptosis, the effects of CALCOCO2 knockdown on cyclin-E1 and p53
expression, which are considered to be key regulators of cancer
growth (30,31), were detected. The results
demonstrated that knockdown of CALCOCO2 suppressed cyclin-E1
protein levels in both DU145 (Fig.
5A and B) and PC-3 (Fig. 5C and D) cells. However, silencing of CALCOCO2
increased p53 protein levels in DU145 cells (Fig. 5E and F).
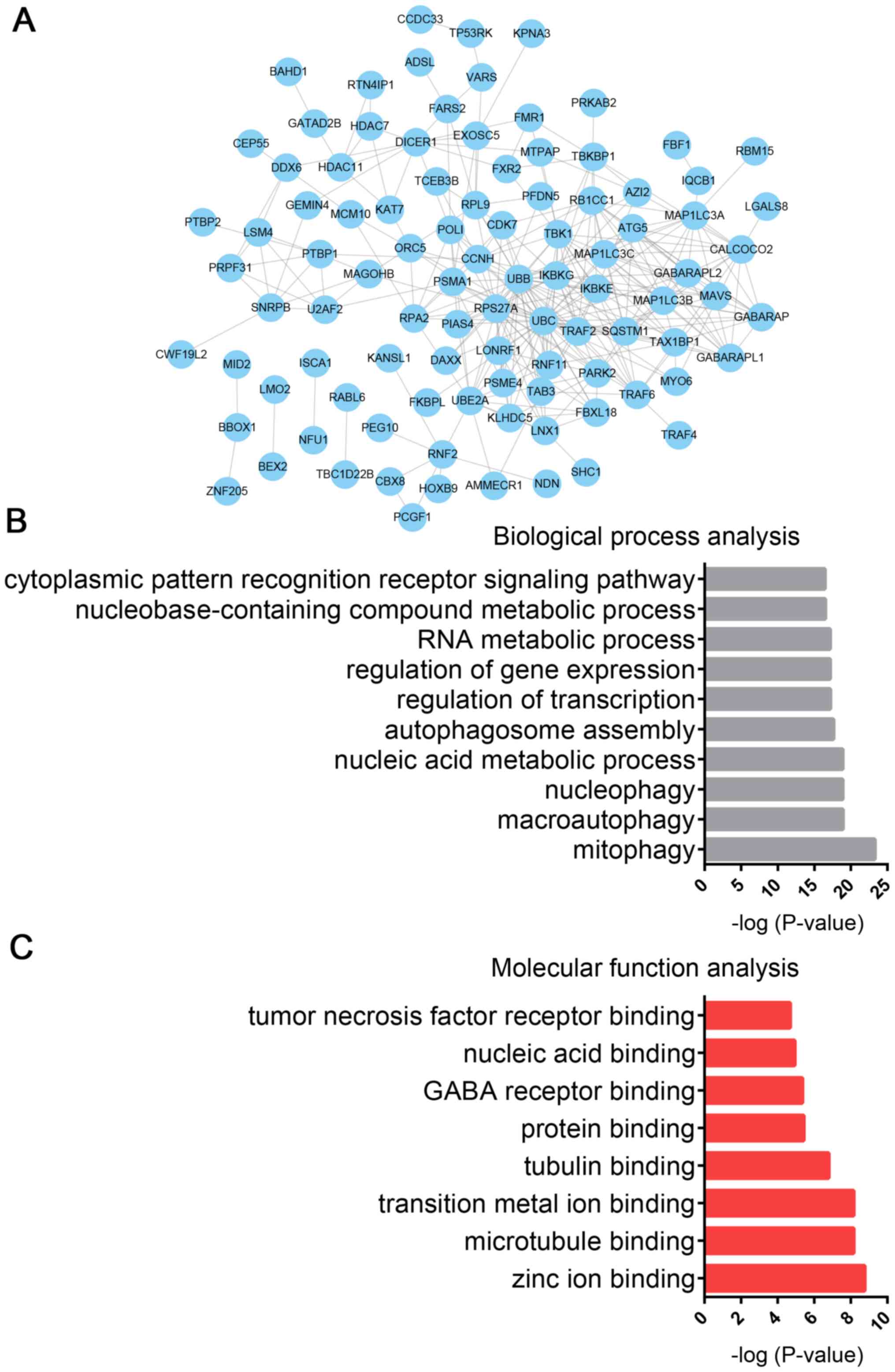
Bioinformatics analysis of CALCOCO2 in
PCa
In the present study, CALCOCO2-mediated PPI networks
were constructed to predict its potential roles in PCa progression.
A total of 190 proteins, including GABARAPL1, MAP1LC3B, GABARAP,
IκB kinase subunit γ, MAP1LC3C, SQSTM1 and IκB kinase epsilon, were
identified as CALCOCO2 cofactors using a CALCOCO2 interacting
proteins dataset, which was downloaded from NCBI (https://www.ncbi.nlm.nih.gov/gene/10241).
Subsequently, CALCOCO2-mediated PPI networks were constructed using
the STRING database. A total of 97 nodes and 310 edges were
included in the CALCOCO2-associated PPI network (Fig. 6A).
GO analysis was also performed to predict the
potential roles of CALCOCO2 using STRING database. GO analysis
revealed that CALCOCO2 was primarily associated with ‘mitophagy’,
‘macroautophagy’, ‘nucleophagy’, ‘nucleic acid metabolic process’,
‘autophagosome assembly’, ‘regulation of transcription’,
‘regulation of gene expression’, ‘RNA metabolic process’,
‘nucleobase-containing compound metabolic process’ and ‘cytoplasmic
pattern recognition receptor signaling pathway’ (Fig. 6B). Molecular function analysis
revealed that CALCOCO2 was associated with ‘zinc ion binding’,
‘microtubule binding’, ‘transition metal ion binding’, ‘tubulin
binding’, ‘protein binding’, ‘GABA receptor binding’, ‘nucleic acid
binding’ and ‘tumor necrosis factor receptor binding’ (Fig. 6C).
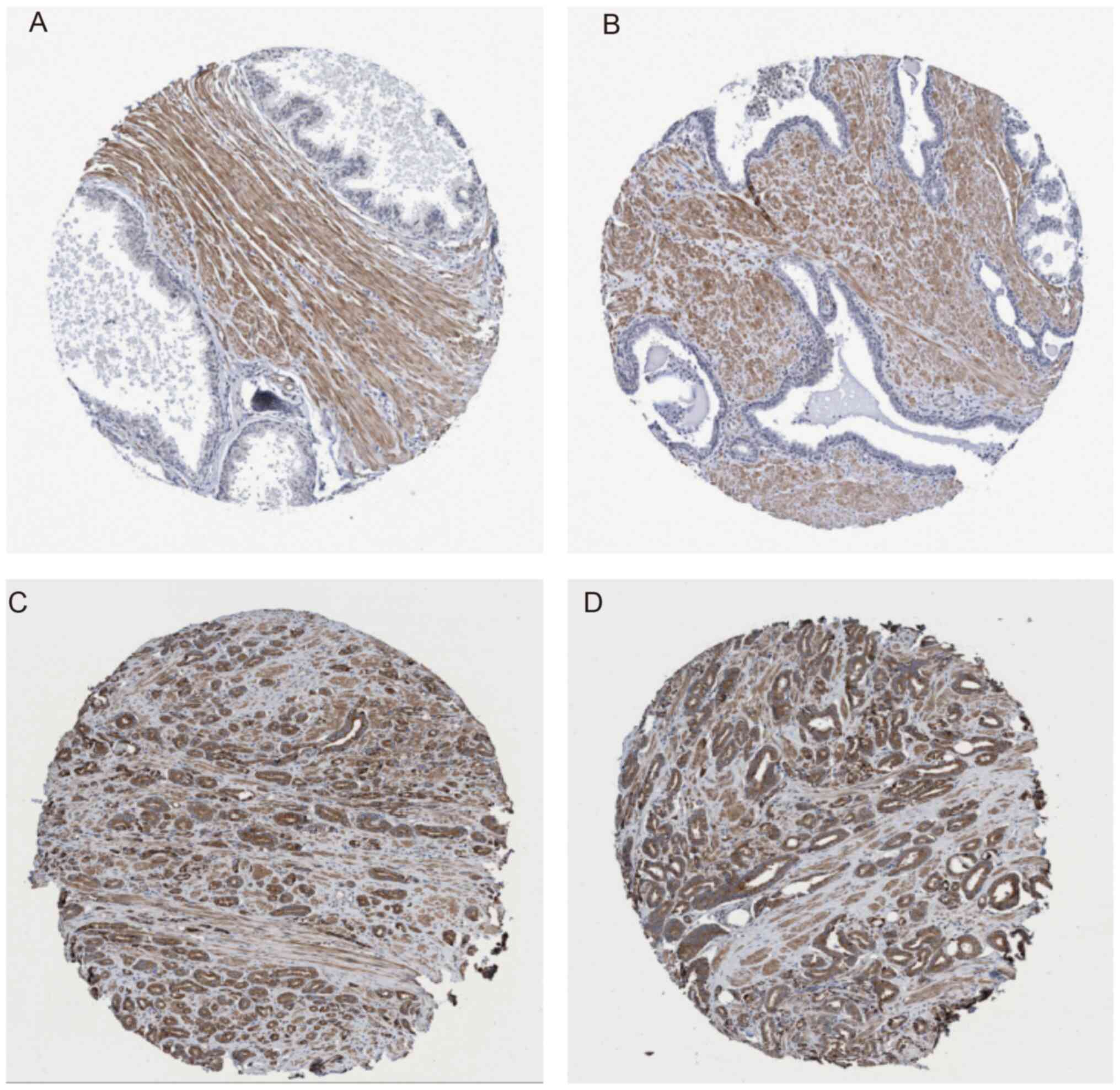
Protein levels of CALCOCO2 are
increased in PCa samples
In order to additionally validate the oncogenic role
of CALCOCO2 in PCa, CALCOCO2 protein levels were analyzed in PCa
tissues using a public dataset from The Human Protein Atlas. The
results demonstrated that the protein levels of CALCOCO2 were not
detectable in normal prostate tissues (Fig. 7A and B; https://www.proteinatlas.org/ENSG00000136436-CALCOCO2/tissue/prostate#img).
However, ~9% (1/11) of PCa samples exhibited high CALCOCO2 protein
levels, 18% (2/11) of PCa samples exhibited intermediate CALCOCO2
protein levels, 36% (4/11) of PCa samples exhibited low CALCOCO2
protein levels (Fig. 7C and
D, https://www.proteinatlas.org/ENSG00000136436-CALCOCO2/pathology/tissue/prostate+cancer)
and 36% (4/11) of PCa samples exhibited no CALCOCO2 expression
(data not shown). These results suggested that CALCOCO2 is
overexpressed in PCa samples compared with normal tissues.
Discussion
PCa is considered to be one of the most common types
of cancer affecting men worldwide (32). Autophagy has been indicated to serve
an important role in cancer (33).
A number of autophagy regulators have been associated with PCa
progression. For example, beclin-1 has been revealed to be
downregulated in PCa samples (34).
Autophagy-associated protein p62 has been demonstrated to inhibit
the autophagic flux and promote epithelial-to-mesenchymal
transition (EMT) in metastatic PCa by sustaining histone
deacetylase 6 expression (35).
Moreover, ATG7 deficiency has been indicated to delay
PTEN-deficient PCa progression (9).
CALCOCO2 is considered to be the most important known xenophagy
receptor; however, only few studies have focused on its potential
role in human cancer (15). In the
present study, CALCOCO2 expression was knocked down in PCa cell
lines and the effect of this knockdown on the proliferation, colony
formation and apoptosis of PCa cells was evaluated. The present
study demonstrated that CALCOCO2 knockdown significantly inhibited
cell proliferation and colony formation, whereas it promoted
apoptosis of PCa cells. Apoptosis, which is a type of programmed
cell death, has been indicated to serve an important role in cancer
progression and evasion of apoptosis is considered to be a hallmark
of cancer (36). Knockdown of
CALCOCO2 in PCa cells reduced cyclin-E1 and increased p53 protein
levels. Moreover, CALCOCO2 protein levels were indicated to be
higher in PCa samples compared with normal prostate tissues in the
analysis performed using The Human Protein Atlas. To the best of
our knowledge, these results are the first to demonstrate that
CALCOCO2 functions as an oncogene in PCa.
Previous studies have reported that CALCOCO2 can
interact with a number of proteins, such as galectin-8(19) and PB1-F2(20), to mediate xenophagy. Several studies
indicated that CALCOCO2 may be associated with cancer progression
(20,37,38).
For example, Leymarie et al (20) observed that CALCOCO2 interacted with
type I interferon production and IκB kinase/NF-κB signaling using
bioinformatics tools. Type I interferon production has been
reported to be associated with antitumor immunity (37), whereas NF-κB signaling may promote
tumor cell proliferation and EMT, and suppress apoptosis of cancer
cells (38). In the present study,
bioinformatics analysis was performed to determine the potential
mechanisms via which CALCOCO2 regulates PCa progression. A total of
190 proteins were indicated to be cofactors of CALCOCO2. A PPI
network mediated by CALCOCO2 was also constructed, which may
provide useful information to understand the potential roles of
CALCOCO2 in regulating PCa progression. Bioinformatics analysis
revealed that CALCOCO2 was primarily associated with ‘mitophagy’,
‘macroautophagy’, ‘nucleophagy’, ‘nucleic acid metabolic process’,
‘autophagosome assembly’, ‘regulation of transcription’,
‘regulation of gene expression’, ‘RNA metabolic process’, ‘zinc ion
binding’, ‘microtubule binding’ and ‘transition metal ion
binding’.
In conclusion, the present study demonstrated that
CALCOCO2 functions as an oncogene in PCa. CALCOCO2 knockdown
decreased cell proliferation and colony formation and promoted
apoptosis in PCa cell lines. Bioinformatics analysis revealed that
CALCOCO2 was involved in regulating ‘autophagy’, ‘nucleophagy’ and
‘nucleic acid metabolic process’ via interacting with SQSTM1. The
current study also demonstrated that the CALCOCO2 protein levels
were upregulated in PCa tissue samples. These results suggested
that CALCOCO2 may be a useful diagnostic and therapeutic target in
PCa.
Supplementary Material
CALCOCO2 knockdown suppresses the
proliferation of androgen receptor-dependent LNCaP cells. DepMap
database analysis indicated that CALCOCO2 knockdown suppressed the
proliferation of androgen receptor-dependent LNCaP cells.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Social Development
Plan of Jiangsu Province-Standardization of Key Disease Diagnosis
and Treatment Projects (grant no. BE2016715) and Jiangsu Province
Youth Medical Key Talent Program (grant no. QNRC2016457).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC and JH were responsible for the study conception
and design. FC, SW, JT and HT performed the experiments. FC, HT and
YF analyzed and interpreted the data. All authors wrote, reviewed
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dou Z, Xu C, Donahue G, Shimi T, Pan JA,
Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, et al: Autophagy
mediates degradation of nuclear lamina. Nature. 527:105–109.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Singh R, Kaushik S, Wang Y, Xiang Y, Novak
I, Komatsu M, Tanaka K, Cuervo AM and Czaja MJ: Autophagy regulates
lipid metabolism. Nature. 458:1131–1135. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wan X, Huang W, Yang S, Zhang Y, Pu H, Fu
F, Huang Y, Wu H, Li T and Li Y: Identification of
androgen-responsive lncRNAs as diagnostic and prognostic markers
for prostate cancer. Oncotarget. 7:60503–60518. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhu X, Zhou M, Liu G, Huang X, He W, Gou X
and Jiang T: Autophagy activated by the c-Jun N-terminal
kinase-mediated pathway protects human prostate cancer PC3 cells
from celecoxib-induced apoptosis. Exp Ther Med. 13:2348–2354.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18(1088)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nie C, Zhou J, Qin X, Shi X, Zeng Q, Liu
J, Yan S and Zhang L: Diosgenininduced autophagy and apoptosis in a
human prostate cancer cell line. Mol Med Rep. 14:4349–4359.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang L, Kim D, Wise J, Shi X, Zhang Z and
DiPaola RS: p62 as a therapeutic target for inhibition of autophagy
in prostate cancer. Prostate. 78:390–400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chang MA, Morgado M, Warren CR, Hinton CV,
Farach-Carson MC and Delk NA: p62/SQSTM1 is required for cell
survival of apoptosis-resistant bone metastatic prostate cancer
cell lines. Prostate. 74:149–163. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Santanam U, Banach-Petrosky W, Abate-Shen
C, Shen MM, White E and DiPaola RS: Atg7 cooperates with Pten loss
to drive prostate cancer tumor growth. Genes Dev. 30:399–407.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chandra P and Kumar D: Selective autophagy
gets more selective: Uncoupling of autophagy flux and xenophagy
flux in Mycobacterium tuberculosis-infected macrophages. Autophagy.
12:608–609. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bauckman KA, Owusu-Boaitey N and Mysorekar
IU: Selective autophagy: Xenophagy. Methods. 75:120–127.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mao K and Klionsky DJ: Xenophagy: A
battlefield between host and microbe, and a possible avenue for
cancer treatment. Autophagy. 13:223–224. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sui X, Liang X, Chen L, Guo C, Han W, Pan
H and Li X: Bacterial xenophagy and its possible role in cancer: A
potential antimicrobial strategy for cancer prevention and
treatment. Autophagy. 13:237–247. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang L, Sung JJ, Yu J, Ng SC, Wong SH,
Cho CH, Ng SS, Chan FK and Wu WK: Xenophagy in Helicobacter
pylori- and Epstein-Barr virus-induced gastric cancer. J
Pathol. 233:103–112. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Verlhac P, Viret C and Faure M: Dual
function of CALCOCO2/NDP52 during xenophagy. Autophagy. 11:965–966.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Verlhac P, Viret C and Faure M: Handcuffs
for bacteria-NDP52 orchestrates xenophagy of intracellular
Salmonella. Microb Cell. 2:214–215. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
von Muhlinen N, Akutsu M, Ravenhill BJ,
Foeglein A, Bloor S, Rutherford TJ, Freund SM, Komander D and
Randow F: LC3C, bound selectively by a noncanonical LIR motif in
NDP52, is required for antibacterial autophagy. Mol Cell.
48:329–342. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Verlhac P, Gregoire IP, Azocar O, Petkova
DS, Baguet J, Viret C and Faure M: Autophagy receptor NDP52
regulates pathogen-containing autophagosome maturation. Cell Host
Microbe. 17:515–525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thurston TL, Wandel MP, von Muhlinen N,
Foeglein A and Randow F: Galectin 8 targets damaged vesicles for
autophagy to defend cells against bacterial invasion. Nature.
482:414–418. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leymarie O, Meyer L, Tafforeau L, Lotteau
V, Costa BD, Delmas B, Chevalier C and Le Goffic R: Influenza virus
protein PB1-F2 interacts with CALCOCO2 (NDP52) to modulate innate
immune response. J Gen Virol. 98:1196–1208. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cui F, Hu J, Fan Y, Tan J and Tang H:
Knockdown of spindle pole body component 25 homolog inhibits cell
proliferation and cycle progression in prostate cancer. Oncol Lett.
15:5712–5720. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nabzdyk CS, Chun M, Pradhan NL, Yoshida S
and LoGerfo FW: Differential susceptibility of human primary aortic
and coronary artery vascular cells to RNA interference. Biochem
Biophys Res Commun. 425:261–265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dennis GJ, Sherman BT, Hosack DA, Yang J,
Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol.
4(P3)2003.PubMed/NCBI
|
|
26
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347(1260419)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
McFarland JM, Ho ZV, Kugener G, Dempster
JM, Montgomery PG, Bryan JG, Krill-Burger JM, Green TM, Vazquez F,
Boehm JS, et al: Improved estimation of cancer dependencies from
large-scale RNAi screens using model-based normalization and data
integration. Nat Commun. 9(4610)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nakayama N, Nakayama K, Shamima Y,
Ishikawa M, Katagiri A, Iida K and Miyazaki K: Gene amplification
CCNE1 is related to poor survival and potential therapeutic target
in ovarian cancer. Cancer. 116:2621–2634. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu Y, Zhang X, Han C, Wan G, Huang X,
Ivan C, Jiang D, Rodriguez-Aguayo C, Lopez-Berestein G, Rao PH, et
al: TP53 loss creates therapeutic vulnerability in colorectal
cancer. Nature. 520:697–701. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cancer Genome Atlas Research Network. The
molecular taxonomy of primary prostate cancer. Cell. 163:1011–1025.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhong Z, Sanchez-Lopez E and Karin M:
Autophagy, inflammation, and immunity: A Troika governing cancer
and its treatment. Cell. 166:288–298. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu C, Xu P, Chen D, Fan X, Xu Y, Li M,
Yang X and Wang C: Roles of autophagy-related genes Beclin-1 and
LC3 in the development and progression of prostate cancer and
benign prostatic hyperplasia. Biomed Rep. 1:855–860.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiang X, Huang Y, Liang X, Jiang F, He Y,
Li T, Xu G, Zhao H, Yang W, Jiang G, et al: Metastatic prostate
cancer-associated P62 inhibits autophagy flux and promotes
epithelial to mesenchymal transition by sustaining the level of
HDAC6. Prostate. 78:426–434. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348.
2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fuertes MB, Woo SR, Burnett B, Fu YX and
Gajewski TF: Type I interferon response and innate immune sensing
of cancer. Trends Immunol. 34:67–73. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014.PubMed/NCBI View Article : Google Scholar
|