Introduction
Excessive alcohol intake, as result of binge
drinking as well as chronic alcohol consumption of >40 g per
day, is becoming a global healthcare concern (1,2).
Globally, ~2 billion individuals consume alcohol regularly, where
>75 million are diagnosed with disorders associated with alcohol
abuse and are at risk of developing alcohol-associated liver
diseases (3). The liver typically
sustains the earliest and the greatest degree of tissue injury
caused by excessive drinking, since it is the primary site of
ethanol metabolism (1,4). Excessive alcohol consumption causes
alcoholic liver disease (ALD), which is characterized by a wide
spectrum of hepatic pathologies, from reversible fatty liver
(simple steatosis) to acute alcoholic hepatitis, chronic fibrosis
and cirrhosis and superimposed hepatocellular carcinoma (HCC)
(2,5,6).
Mechanistically, pathogenesis of ALD includes genetic
susceptibility, oxidative stress, hepatic metabolism alteration and
steatosis, acetaldehyde-mediated toxicity, cytokine- and
chemokine-induced inflammation, alterations in immunity and
dysbiosis, epigenetic changes and modifications in the regenerative
process (2,6-8).
Alcohol abstinence achieved by psychosomatic intervention is at
present the best treatment for all stages of ALD (2). However, drinking discontinuation may
be difficult, for example, the cheap price of hard liquor, easy
accessibility to alcohol, and alcohol advertisement make it very
difficult to prevent the increase in ALD (9). Therefore, new strategies for
alleviating alcohol-induced liver injury remain to be in
demand.
Hydrogen gas (H2) is the lightest and
diffusible gas molecule that has been shown to confer potent
antioxidant and anti-inflammatory properties (10,11).
It can be absorbed into the blood circulation, such that it reaches
the target organ either by blood circulation or free diffusion
(11). Therefore, treatments
involving exogenous H2, including breathing
H2 gas, injection with H2-rich saline and
drinking H2-rich water, may protect against excessive
oxidative stress- and inflammation-related liver damage, including
liver injury induced by drugs, sepsis, bile duct ligation,
ischemia/reperfusion (I/R), CO2 pneumoperitoneum and
chronic intermittent hypoxia, in addition to non-alcoholic fatty
liver disease (NAFLD) (12-17).
Furthermore, drinking H2-rich water has been indicated
to protect against chronic ethanol-induced hepatotoxicity (18). These previous observations suggest
that H2 serves an important role in modulating hepatic
redox, immune and inflammatory homeostasis (11).
Previous studies have demonstrated that
supplementation with exogenous H2 by intraperitoneal
injection may improve lipopolysaccharide (LPS)-induced cardiac
dysfunction, isoproterenol-induced cardiac hypertrophy, pressure
overload-induced vascular hypertrophy, and cardiopulmonary cerebral
resuscitation in a cardiac arrest rabbit model (19-23).
These were mediated by the suppression of excessive oxidative
stress and inflammatory responses (19-23).
However, the effects of intraperitoneal injection of H2
on ALD remain unclear.
Therefore, the aim of the present study was to
investigate the effect of H2 intraperitoneal injection
on acute alcohol-induced liver injury in a mouse model and to
elucidate the potential underlying mechanisms.
Materials and methods
Drugs
In total, 99,999% H2 (Dalian Special
Gases Co., Ltd.) was injected into a vacuumed aseptic soft plastic
infusion bag (100 ml; Hebei Tiancheng Pharmaceutical Co., Ltd.)
from a seamless steel gas cylinder under sterile conditions, as
previously described (Fig. 1)
(20-23).
Anhydrous ethanol (Guangzhou Chemical Reagent Factory) was
dissolved in double-distilled water to obtain 33% (v/v) ethanol
(0.26 g/ml) (24).
Animal model of acute alcohol-induced
liver injury and treatment protocol
Male C57BL/6J mice (Animal license no.
44007200061823) were purchased from the Guangdong Medical
Laboratory Animal Center (Foshan, China). A total of 28 mice aged
8-10 weeks were used in this study. All animals were housed in a
temperature-controlled animal facility (21-24˚C) with a 12-hour
light-dark cycle, and the animals had access to rodent chow and
water ad libitum (20). All
mice were provided with humane care according to the Principles of
Laboratory Animal Care formulated by the National Society of
Medical Research and the Guide for the Care and Use of Laboratory
Animals published by the NIH (8th Edition, Revised 2011)
(20,25). All animal procedures were approved
by the Institutional Animal Care and Use Committee of Zhongshan
School of Medicine, Sun Yat-sen University (Guangzhou, China).
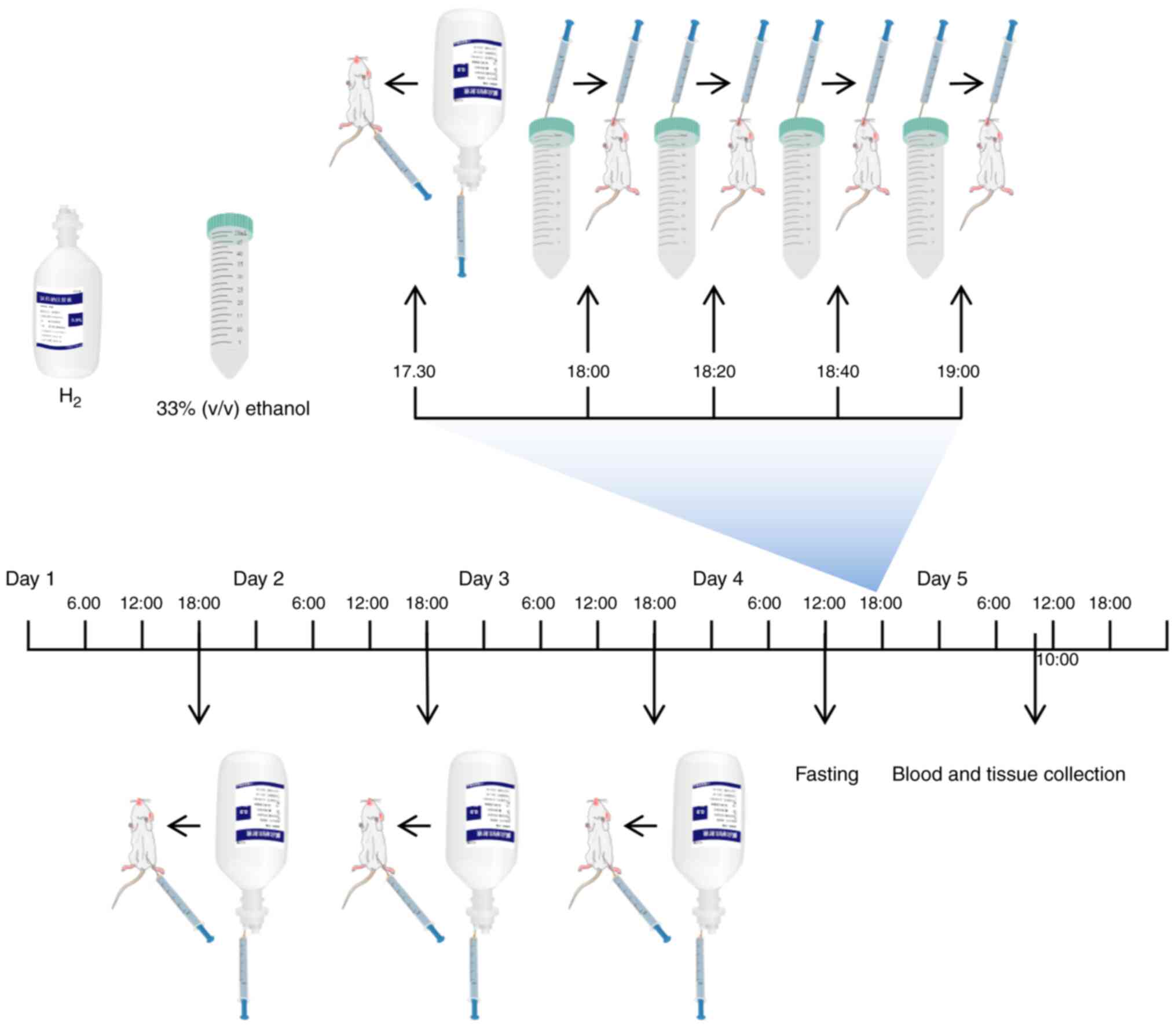
Mice were randomly assigned into the following four
groups (n=7 mice per group): i) Control; ii) Alcohol; iii)
Alcohol+H2; and iv) H2. In the
Alcohol+H2 and H2 groups, H2 was
administered daily at the dose of 1.0 ml/100 g by intraperitoneal
injection for 4 days. On day 4, mice in each group were fasted for
6 h before the mice in the Alcohol and Alcohol+H2 groups
were orally administered with 33% (v/v) ethanol at a cumulative
dose of 4.5 g/kg body weight (17.3 ml/kg body weight) by four
equally divided gavages at 20-min intervals (24). Mice in the Alcohol+H2 and
H2 groups were administered with an intraperitoneal
injection (1.0 ml/100 g) of H2 30 min before the first
ethanol administration (20). In
addition, mice in the Control and H2 groups received the
same volume of double-distilled water (17.3 ml/kg body weight) by
four equally divided gavages at 20 min intervals. The animals were
anesthetized by intraperitoneal injection of ketamine (100 mg/kg)
and xylazine (10 mg/kg) after 16 h of the first ethanol gavage,
cardiac puncture was performed when the animals reached the
surgical plane of anesthesia and ~0.4-0.5 ml blood was collected
from each mouse (26-28).
Euthanasia was then performed by cervical dislocation in a state of
deep anesthesia before liver tissues were extracted (Fig. 2) (24). The body weight of animals in each
group was recorded at the baseline (Control, 25.19±1.58 g; Alcohol,
24.63±1.42 g; Alcohol+H2, 25.17±1.15 g; H2,
23.9±0.70 g) and after intervention (Control, 24.90±1.36 g;
Alcohol, 25.16±1.53 g; Alcohol+H2, 25.00±0.93 g;
H2, 23.71±0.63 g).
Biochemical analysis
Blood biochemical analysis was performed as
previously described (29).
Briefly, the blood samples were centrifuged at the speed of 986 x g
for 10 min at 4˚C to separate the serum. Levels of liver function
markers alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) in addition to the levels of lipid markers
triglyceride (TG) and total cholesterol (TC) in the serum were
analyzed using an Automatic Clinical Analyzer (Hitachi 7600;
Hitachi High-Technologies Corporation) at the Department of
Clinical Laboratory, The Third Affiliated Hospital of Sun Yat-sen
University.
Western blotting
JNK antibody (cat. no. 9252S), phosphorylated (p-)
JNK antibody (cat. no. 9255S), anti-rabbit IgG horseradish
peroxidase (HRP)-conjugated secondary antibody (cat. no. 7074S) and
anti-mouse IgG HRP-conjugated secondary antibody (cat. no. 7076S)
were purchased from Cell Signaling Technology, Inc. GAPDH antibody
(cat. no. MB001) was obtained from Bioworld Technology, Inc. BSA
(cat. no. ST023-200g) was purchased from Beyotime Institute of
Biotechnology.
Western blotting was performed as previously
described (30). The proteins were
transferred onto polyvinylidene fluoride membranes (EMD Millipore).
Membranes were incubated in blocking buffer (1X TBST with 5% BSA)
for 30 min at room temperature. For antibody incubations, membranes
were incubated with primary antibodies in antibody dilution buffer
(1X TBST with 5% BSA) with gentle agitation overnight at 4˚C (p-JNK
and JNK: 1:2,000; GAPDH: 1:10,000), and with secondary antibodies
in antibody dilution buffer (1X TBST with 5% BSA) with gentle
agitation for 1 h at room temperature (anti-rabbit IgG
HRP-conjugated secondary antibody and anti-mouse IgG HRP-conjugated
secondary antibody: 1:2,000). Immobilon™ Western Chemiluminescent
HRP Substrate (ECL; cat. no. WBKLS0100, EMD Millipore) was used to
reveal the bands using the ChemiDoc™ Touch Imaging System (Bio-Rad
Laboratories, Inc.). Image J software (Version 1.52; National
Institutes of Health) was used for estimating the ‘IntDen’ value of
the western blot bands for quantification.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc.). Data were expressed
as the mean ± SD. For biochemical analysis, n=7 mice in each group.
For western blotting, n=5 mice in each group. Statistical analysis
was performed by one-way ANOVA followed by Bonferroni's post hoc
test. P<0.05 was considered to indicate statistically
significant differences.
Results
H2 treatment prevents acute
alcohol-induced liver damage
To investigate the effects of H2 on
alcohol-induced liver injury, an acute alcohol-induced
hepatotoxicity mouse model was established as previously described
(24). This model was shown to
closely mimic excessive ethanol consumption in humans in terms of
blood alcohol levels, behavioral and physiological effects
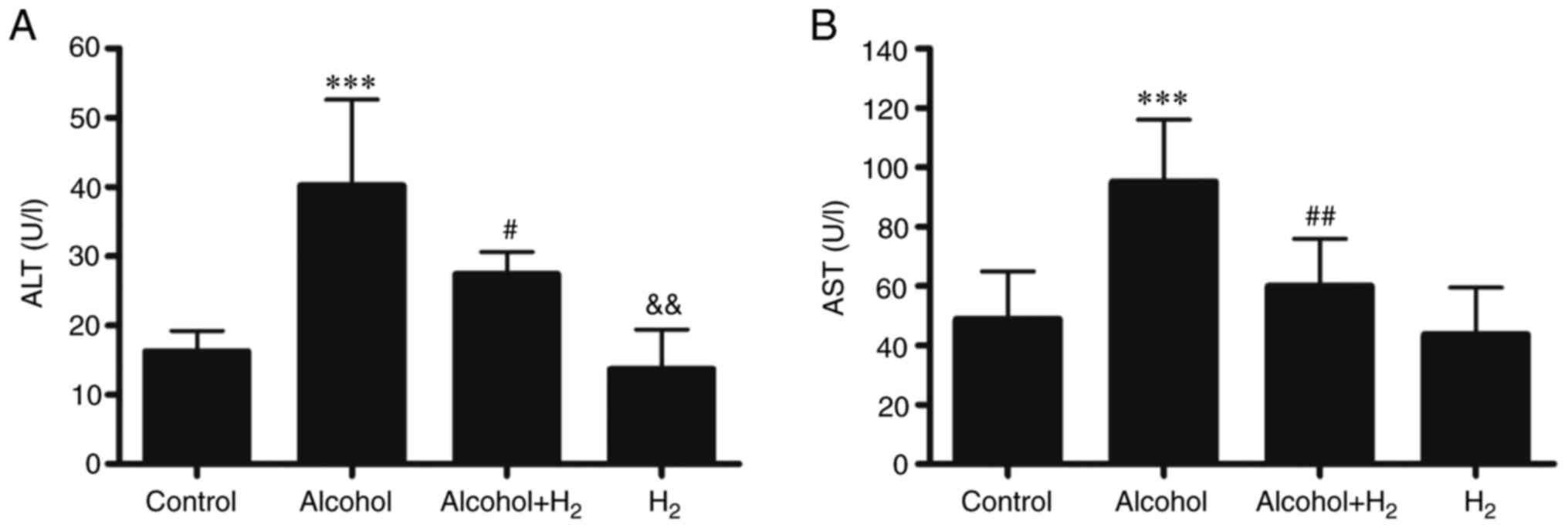
(24). Compared with those in the
Control group, serum ALT and AST levels in the Alcohol group were
significantly higher following acute alcohol gavage (Fig. 3), whilst intraperitoneal injection
of H2 significantly prevented these elevations in serum
ALT and AST levels in mice following alcohol gavage (Fig. 3). However, H2 alone
exerted no effects on serum ALT and AST levels compared with those
in the Control group (Fig. 3).
Therefore, these observations suggest that intraperitoneal
injection of H2 effectively alleviated acute
alcohol-induced liver injury in mice.
Effects of H2 on serum
lipid levels in acute alcohol-induced liver injury
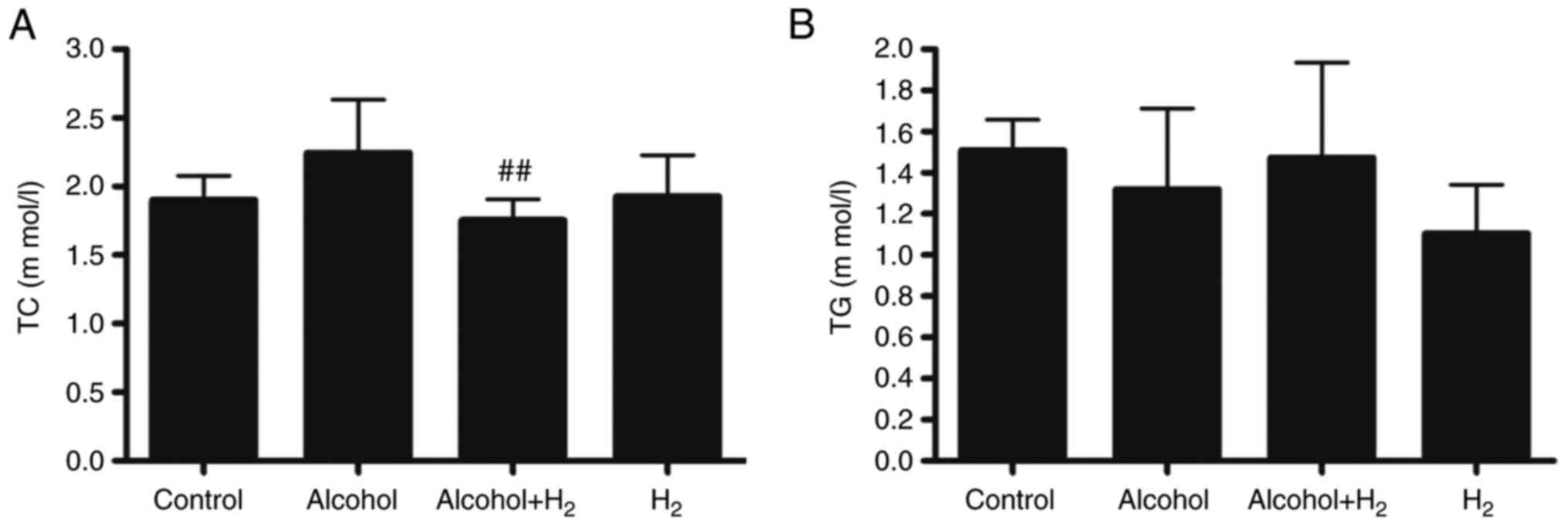
Long-term chronic alcohol consumption may lead to
disruptions in lipid metabolism (6). Therefore, the serum levels of TC and
TG were next examined. Although acute alcohol treatment slightly
increased serum TC levels, there was no significant difference
between those in the Control and Alcohol groups (Fig. 4A). Serum TC levels in the
Alcohol+H2 group were significantly lower compared with
those in the Alcohol group (Fig.
4A). However, there were no significant differences in TG
levels among the four groups (Fig.
4B). These data suggest that intraperitoneal injection of
H2 may modulate blood TC levels in an acute
alcohol-induced liver injury mice model.
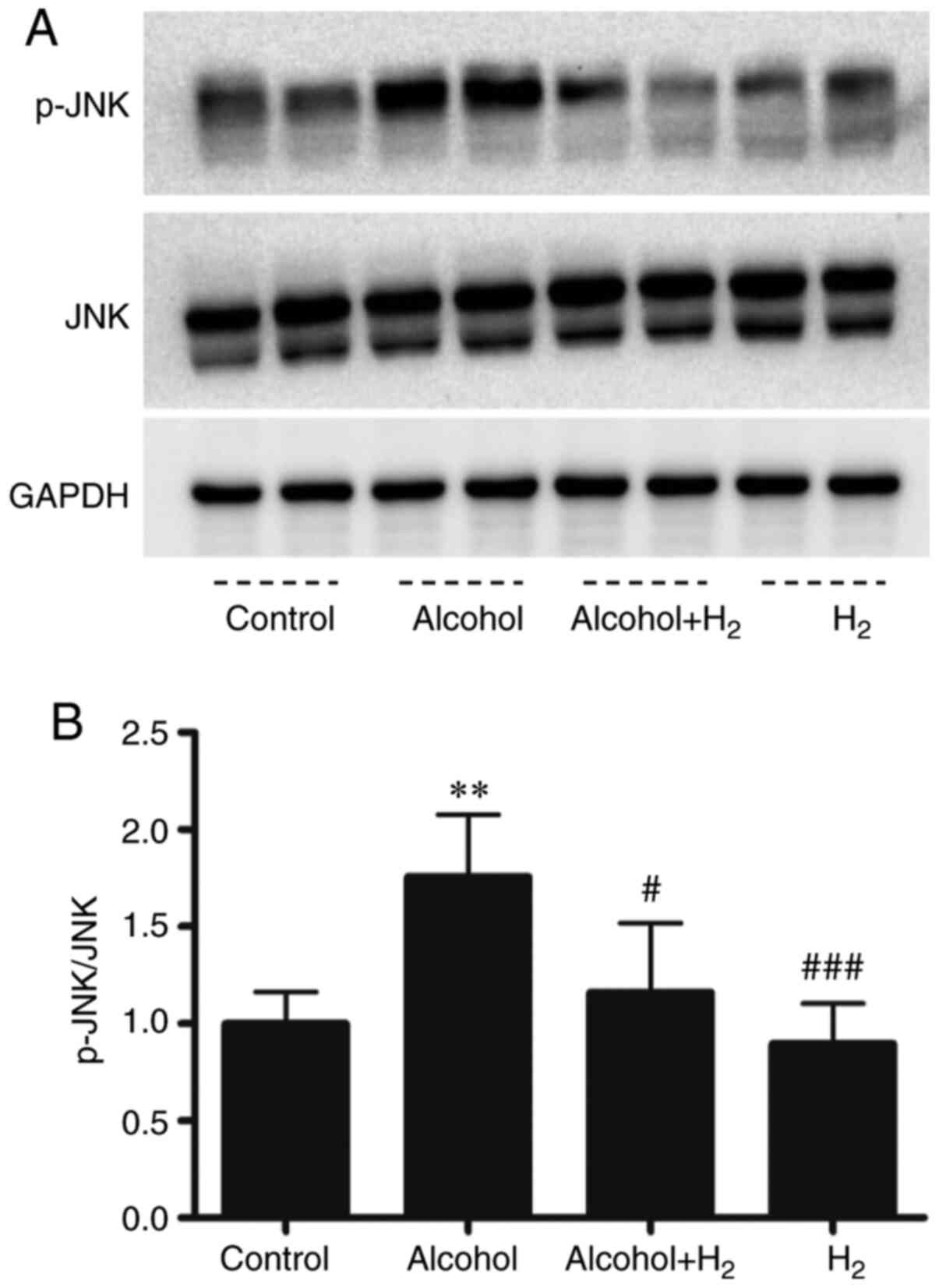
H2 alleviates acute
alcohol-induced hepatic JNK activation
Acute ethanol loading causes oxidative stress and
activates cell-death signaling through the JNK pathway in the liver
(31). In addition, JNK is a key
mediator in hepatic steatosis, where it regulates transcription
factor activity associated with lipid metabolism (32,33).
In the present study, the upregulation of serum ALT and AST levels
after ethanol treatment indicated hepatocytes damage. Therefore,
the role of hepatic JNK activation in the potentially protective
effects of H2 against acute alcohol-induced liver injury
was examined. Western blotting data demonstrated that acute ethanol
treatment significantly increased hepatic JNK phosphorylation,
which was significantly prevented by intraperitoneal injection with
H2 (Fig. 5). Therefore,
these data suggest that intraperitoneal injection with
H2 conferred protective effects against acute
alcohol-induced liver injury by preventing hepatic JNK
activation.
Discussion
Inflammatory and cytokine signaling, oxidative
stress and mitochondrial dysfunction, alterations in hepatic
metabolism and hepatocyte cell death, and abnormalities in immunity
and dysbiosis are all key to the pathogenesis of liver diseases,
including ALD and NAFLD (2,34-36).
Therefore, strategies preventing fatty liver disease progression
were previously developed using combinations of naturally occurring
compounds products in animal models. For example, the combination
of docosahexaenoic acid and the antioxidant hydroxytyrosol was
demonstrated to prevent high-fat diet-induced liver steatosis, by
inhibiting oxidative stress, mitochondrial dysfunction and
inflammation associated with steatosis (34,35).
H2 was first reported to alleviate skin tumors by
neutralizing toxic free radicals in 1975(37). In 2007, H2 was
demonstrated to act as a therapeutic antioxidant by selectively
reducing cytotoxic hydroxyl radical levels to improve focal
I/R-induced brain injury in rats (38). Since then, H2 has been
extensively investigated, where it has been shown to be an able
antioxidant, anti-inflammatory and anti-apoptotic agent (10). The present study was undertaken to
determine the effect of supplementation with exogenous
H2 on acute alcohol-induced liver injury in mice.
Exogenous H2 can be supplied by 2%
H2 gas inhalation (38),
drinking H2-rich water (39), intraperitoneal injection of
H2-rich saline (12) and
intraperitoneal injection of H2 gas (19). Previous studies found that
intraperitoneal injection of H2 gas can alleviate
vascular remodeling (23), cardiac
hypertrophy and dysfunction (20,21),
and display neuroprotective effects in rabbits experiencing cardiac
arrest (19). In the present study,
it was observed that ethanol consumption induced hepatocyte injury
as indicated by the upregulation of serum ALT and AST levels.
Intraperitoneal injection with H2 gas was found to
effectively protect against acute alcohol-induced liver injury and
reduced serum TC levels. However, body weight was not influenced by
the acute intraperitoneal injection of H2 gas or acute
alcohol feeding. The lack of food intake records, liver weight,
liver histological analysis and steatosis score and hepatic TG
analysis are limitations of the present study. It has been
documented that chronic alcohol over consumption may lead to
hepatic steatosis, fibrosis and cirrhosis and eventually HCC
(2,5,6). The
long-term effects of intraperitoneal H2 injection on
pathological features, such as hepatic fibrosis, and the effects of
other H2 delivery methods, such as drinking
H2-rich alcohol, on ALD, require further study.
Acute alcohol feeding activates cytochrome P450 2E1
and causes oxidative stress to activate JNK in hepatocytes, and
JNK, in turn, reciprocally increases oxidative stress (33). JNK activation can cause programmed
cell death, and increases the expression of lipogenic transcription
factor sterol regulatory element binding protein (SREBP)-activated
lipid synthesis enzymes, resulting in hepatic steatosis (31,33,40,41).
Additionally, JNK may suppress hepatic peroxisome
proliferator-activated receptor-α (PPAR-α) activation, which is a
transcription factor and a positive regulator of intracellular free
fatty acid and TG metabolism by regulating gene transcription
involved in fatty acid transport and degradation in mitochondria
and peroxisomes (32). Therefore,
hepatic JNK activation increases oxidative stress, leads to
hepatocyte apoptosis and injury, and contributes to hepatic
steatosis via modulating lipid metabolic transcription factor
activation. H2 has been shown to inhibit JNK activation
in numerous liver disease and cardiovascular disease animal models
(11,20,22,23).
JNK inhibitor can improve acute alcohol-induced fatty liver and
oxidative stress in mice (33). In
the present study, phosphorylation of JNK in the liver induced by
acute alcohol consumption was inhibited by the intraperitoneal
injection of H2. Therefore, the protective effect of
H2 against acute alcohol-induced liver injury is
hypothesized to be partially mediated by reducing JNK
phosphorylation. In addition to the inhibition of JNK activation,
H2 has been shown to attenuate the activation of NF-κB
in an LPS-induced cardiac dysfunction mice model (20). H2 has also been shown to
increase the hepatic expression of nuclear factor erythroid
2-related factor 2 (Nrf2) (42) and
PPAR-α (43) and reduced the
hepatic expression of SREBP-1c (44) in NAFLD animal models. These
transcription factors are essential mediators of inflammation
(NF-κB), oxidative stress (Nrf2) and lipid metabolism (SREBP-1c and
PPAR-α), where they have been reported to serve key roles in the
pathogenesis of both ALD and NAFLD (35). Therefore, strategies modulating the
expression or activation of these transcription factors may
alleviate ALD and NAFLD (35,45-51).
However, whether the protective effects of intraperitoneal
injection of H2 against acute alcohol-induced liver
injury is mediated by regulating the expression and/or activation
of these transcription factors aforementioned requires further
investigation.
The gut microbiome serves an important role in liver
homeostasis, intestinal dysbiosis, including quantitative (such as
intestinal bacterial overgrowth) and qualitative (such as colonic
Firmicutes and Bacteroidetes levels) changes in the gut microbiota,
and pathological bacterial translocation are fundamental for the
pathogenesis of ALD (8,52). A number of these intestinal
microbiota are affected by alcohol, which express hydrogenases and
act as the main producers of endogenous H2 in humans and
animals (10,20,52,53).
Endogenous H2 is crucial for hepatic redox homeostasis,
glucose and lipid homeostasis, in addition to immune and
inflammatory homeostasis (11,54-57).
Therefore, it remains of importance to investigate the effects of
endogenous H2 in the pathogenesis of ALD.
In summary, findings of the present study indicated
that intraperitoneal injection with exogenous H2
attenuated acute alcohol-induced liver injury in mice by inhibiting
hepatic JNK activation. Therefore, it would be possible to treat
alcohol-induced liver injury with H2, such as drinking
H2-rich alcohol or H2-rich water, where
H2 can be a potentially useful natural agent for the
treatment of ALD.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 81900376), Natural Science
Foundation of Guangdong Province (grant no. 2018A030313657), the
Project funded by China Postdoctoral Science Foundation (grant no.
2019M653238) and Guangdong famous Traditional Chinese Medicine
inheritance studio construction project (grant no. 20180137).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, WG, MD and HY conceived and designed the
experiments. YZ, GZ, ZC, MB and YH performed the experiments and
analyzed the data. YZ drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Sun Yat-sen
University (IACUC code no: 2018-057).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Osna NA, Donohue TM Jr and Kharbanda KK:
Alcoholic liver disease: Pathogenesis and current management.
Alcohol Res. 38:147–161. 2017.PubMed/NCBI
|
|
2
|
Seitz HK, Bataller R, Cortez-Pinto H, Gao
B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G and Tsukamoto
H: Alcoholic liver disease. Nat Rev Dis Primers.
4(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lieber CS: Alcoholic liver disease: New
insights in pathogenesis lead to new treatments. J Hepatol. 32
(Suppl):113–128. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ding WX and Yang L: Alcohol and
drug-induced liver injury: Metabolism, mechanisms, pathogenesis and
potential therapies. Liver Res. 3:129–131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao B and Bataller R: Alcoholic liver
disease: Pathogenesis and new therapeutic targets.
Gastroenterology. 141:1572–1585. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Louvet A and Mathurin P: Alcoholic liver
disease: Mechanisms of injury and targeted treatment. Nat Rev
Gastroenterol Hepatol. 12:231–242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bajaj JS: Alcohol, liver disease and the
gut microbiota. Nat Rev Gastroenterol Hepatol. 16:235–246.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Y, Tan S, Xu J and Wang T: Hydrogen
therapy in cardiovascular and metabolic diseases: From bench to
bedside. Cell Physiol Biochem. 47:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang Y, Xu J and Yang H: Hydrogen: An
endogenous regulator of liver homeostasis. Front Pharmacol.
11(877)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q,
Chang Y, Liu Q, Sun X, Wu M, et al: The protective role of
hydrogen-rich saline in experimental liver injury in mice. J
Hepatol. 54:471–480. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Q, Li BS, Song YJ, Hu MG, Lu JY, Gao
A, Sun XJ, Guo XM and Liu R: Hydrogen-rich saline protects against
mitochondrial dysfunction and apoptosis in mice with obstructive
jaundice. Mol Med Rep. 13:3588–3596. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang SC, Chen LL, Fu T, Li WY and Ji ES:
Improvement of hydrogen on liver oxidative stress injury in chronic
intermittent hypoxia rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
34:61–64. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
16
|
Chen M, Jiang L, Li Y, Bai G, Zhao J,
Zhang M and Zhang J: Hydrogen protects against liver injury during
CO2 pneumoperitoneum in rats. Oncotarget. 9:2631–2645.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kawai D, Takaki A, Nakatsuka A, Wada J,
Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y, et
al: Hydrogen-rich water prevents progression of nonalcoholic
steatohepatitis and accompanying hepatocarcinogenesis in mice.
Hepatology. 56:912–921. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin CP, Chuang WC, Lu FJ and Chen CY:
Anti-oxidant and anti-inflammatory effects of hydrogen-rich water
alleviate ethanol-induced fatty liver in mice. World J
Gastroenterol. 23:4920–4934. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang G, Zhou J, Zhan W, Xiong Y, Hu C, Li
X, Li X, Li Y and Liao X: The neuroprotective effects of
intraperitoneal injection of hydrogen in rabbits with cardiac
arrest. Resuscitation. 84:690–695. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan S, Long Z, Hou X, Lin Y, Xu J, You X,
Wang T and Zhang Y: H2 protects against
lipopolysaccharide-induced cardiac dysfunction via blocking
TLR4-mediated cytokines expression. Front Pharmacol.
10(865)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Long Z, Xu J, Tan S, Zhang N, Li
A, Wang L and Wang T: Hydrogen inhibits isoproterenol induced
autophagy in cardiomyocytes in vitro and in vivo. Mol
Med Rep. 16:8253–8258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Xu J, Long Z, Wang C, Wang L, Sun
P, Li P and Wang T: Hydrogen (H2) inhibits
isoproterenol-induced cardiac hypertrophy via antioxidative
pathways. Front Pharmacol. 7(392)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang YX, Xu JT, You XC, Wang C, Zhou KW,
Li P, Sun P, Wang L and Wang TH: Inhibitory effects of hydrogen on
proliferation and migration of vascular smooth muscle cells via
down-regulation of mitogen/activated protein kinase and
ezrin-radixin-moesin signaling pathways. Chin J Physiol. 59:46–55.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao
W, Lu B, Stolz DB, Clemens DL and Yin XM: Autophagy reduces acute
ethanol-induced hepatotoxicity and steatosis in mice.
Gastroenterology. 139:1740–1752. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Polhemus DJ, Trivedi RK, Gao J, Li Z,
Scarborough AL, Goodchild TT, Varner KJ, Xia H, Smart FW, Kapusta
DR, et al: Renal sympathetic denervation protects the failing heart
via inhibition of neprilysin activity in the kidney. J Am Coll
Cardiol. 70:2139–2153. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hill JA, Karimi M, Kutschke W, Davisson
RL, Zimmerman K, Wang Z, Kerber RE and Weiss RM: Cardiac
hypertrophy is not a required compensatory response to short-term
pressure overload. Circulation. 101:2863–2869. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Frankenberg L: Cardiac puncture in the
mouse through the anterior thoracic aperture. Lab Anim. 13:311–312.
1979.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kaur H, Fisher K and Othman M:
Thromboelastography testing in mice following blood collection from
facial vein and cardiac puncture. Blood Coagul Fibrinolysis.
30:366–369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Y, Zhou G, Chen Z, Guan W, Zhang J,
Bi M, Wang F, You X, Liao Y, Zheng S, et al: Si-Wu-Tang alleviates
nonalcoholic fatty liver disease via blocking TLR4-JNK and
Caspase-8-GSDMD signaling pathways. Evid Based Complement Alternat
Med. 2020(8786424)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y, Zhang J, Xu K, Chen Z, Xu X, Xu
J, Zheng S, Dai M and Yang H: Helium protects against
lipopolysaccharide-induced cardiac dysfunction in mice via
suppressing Toll-like receptor 4-nuclear factor κB-tumor necrosis
factor-alpha/interleukin-18 signaling. Chin J Physiol. 63:276–285.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nishitani Y and Matsumoto H: Ethanol
rapidly causes activation of JNK associated with ER stress under
inhibition of ADH. FEBS Lett. 580:9–14. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Manieri E, Folgueira C, Rodríguez ME,
Leiva-Vega L, Esteban-Lafuente L, Chen C, Cubero FJ, Barrett T,
Cavanagh-Kyros J, Seruggia D, et al: JNK-mediated disruption of
bile acid homeostasis promotes intrahepatic cholangiocarcinoma.
Proc Natl Acad Sci USA. 117:16492–16499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang L, Wu D, Wang X and Cederbaum AI:
Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute
alcohol-induced fatty liver. Free Radic Biol Med. 53:1170–1180.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ortiz M, Soto-Alarcón SA, Orellana P,
Espinosa A, Campos C, López-Arana S, Rincón MA, Illesca P,
Valenzuela R and Videla LA: Suppression of high-fat diet-induced
obesity-associated liver mitochondrial dysfunction by
docosahexaenoic acid and hydroxytyrosol co-administration. Dig
Liver Dis. 52:895–904. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Valenzuela R and Videla LA: Impact of the
co-administration of N-3 fatty acids and olive oil components in
preclinical nonalcoholic fatty liver disease models: A mechanistic
view. Nutrients. 12(499)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nevzorova YA, Boyer-Diaz Z, Cubero FJ and
Gracia-Sancho J: Animal models for liver disease-A practical
approach for translational research. J Hepatol. 73:423–440.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dole M, Wilson FR and Fife WP: Hyperbaric
hydrogen therapy: A possible treatment for cancer. Science.
190:152–154. 1975.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Sun Q, Kawamura T, Masutani K, Peng X, Sun
Q, Stolz DB, Pribis JP, Billiar TR, Sun X, Bermudez CA, et al: Oral
intake of hydrogen-rich water inhibits intimal hyperplasia in
arterialized vein grafts in rats. Cardiovasc Res. 94:144–153.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chang L, Kamata H, Solinas G, Luo JL,
Maeda S, Venuprasad K, Liu YC and Karin M: The E3 ubiquitin ligase
itch couples JNK activation to TNFalpha-induced cell death by
inducing c-FLIP(L) turnover. Cell. 124:601–613. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kamata H, Honda S, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote
TNFalpha-induced death and sustained JNK activation by inhibiting
MAP kinase phosphatases. Cell. 120:649–661. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang X and Wang J: High-content hydrogen
water-induced downregulation of miR-136 alleviates non-alcoholic
fatty liver disease by regulating Nrf2 via targeting MEG3. Biol
Chem. 399:397–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhai X, Chen X, Lu J, Zhang Y, Sun X,
Huang Q and Wang Q: Hydrogen-rich saline improves non alcoholic
fatty liver disease by alleviating oxidative stress and activating
hepatic PPARα and PPARγ. Mol Med Rep. 15:1305–1312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu B, Xue J, Zhang M, Wang M, Ma T, Zhao
M, Gu Q and Qin S: Hydrogen inhalation alleviates nonalcoholic
fatty liver disease in metabolic syndrome rats. Mol Med Rep.
22:2860–2868. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hernández-Rodas MC, Valenzuela R,
Echeverría F, Rincón-Cervera MÁ, Espinosa A, Illesca P, Muñoz P,
Corbari A, Romero N, Gonzalez-Mañan D, et al: Supplementation with
docosahexaenoic acid and extra virgin olive oil prevents liver
steatosis induced by a high-fat diet in mice through PPAR-α and
Nrf2 upregulation with concomitant SREBP-1c and NF-κB
downregulation. Mol Nutr Food Res. 61(61)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li L, Fu J, Liu D, Sun J, Hou Y, Chen C,
Shao J, Wang L, Wang X, Zhao R, et al: Hepatocyte-specific Nrf2
deficiency mitigates high-fat diet-induced hepatic steatosis:
Involvement of reduced PPARγ expression. Redox Biol.
30(101412)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ambade A, Catalano D, Lim A, Kopoyan A,
Shaffer SA and Mandrekar P: Inhibition of heat shock protein 90
alleviates steatosis and macrophage activation in murine alcoholic
liver injury. J Hepatol. 61:903–911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lamlé J, Marhenke S, Borlak J, von
Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M and
Vogel A: Nuclear factor-eythroid 2-related factor 2 prevents
alcohol-induced fulminant liver injury. Gastroenterology.
134:1159–1168. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ji C, Chan C and Kaplowitz N: Predominant
role of sterol response element binding proteins (SREBP) lipogenic
pathways in hepatic steatosis in the murine intragastric ethanol
feeding model. J Hepatol. 45:717–724. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nakajima T, Kamijo Y, Tanaka N, Sugiyama
E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ and
Aoyama T: Peroxisome proliferator-activated receptor alpha protects
against alcohol-induced liver damage. Hepatology. 40:972–980.
2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Valenzuela R and Videla LA: Crosstalk
mechanisms in hepatoprotection: Thyroid hormone-docosahexaenoic
acid (DHA) and DHA-extra virgin olive oil combined protocols.
Pharmacol Res. 132:168–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hartmann P, Seebauer CT and Schnabl B:
Alcoholic liver disease: The gut microbiome and liver cross talk.
Alcohol Clin Exp Res. 39:763–775. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wolf PG, Biswas A, Morales SE, Greening C
and Gaskins HR: H2 metabolism is widespread and diverse
among human colonic microbes. Gut Microbes. 7:235–245.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tanabe H, Sasaki Y, Yamamoto T, Kiriyama S
and Nishimura N: Suppressive effect of high hydrogen generating
high amylose cornstarch on subacute hepatic ischemia-reperfusion
injury in rats. Biosci Microbiota Food Health. 31:103–108.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kajiya M, Sato K, Silva MJ, Ouhara K, Do
PM, Shanmugam KT and Kawai T: Hydrogen from intestinal bacteria is
protective for concanavalin A-induced hepatitis. Biochem Biophys
Res Commun. 386:316–321. 2009.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yu J, Zhang W, Zhang R, Ruan X, Ren P and
Lu B: Lactulose accelerates liver regeneration in rats by inducing
hydrogen. J Surg Res. 195:128–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhao L, Wang Y, Zhang G, Zhang T, Lou J
and Liu J: L-arabinose elicits gut-derived hydrogen production and
ameliorates metabolic syndrome in C57BL/6J mice on high-fat-diet.
Nutrients. 11(3054)2019.PubMed/NCBI View Article : Google Scholar
|