Introduction
Lung cancer (LC) remains the leading cause of
cancer-related mortality and the most common diagnosed cancer
worldwide, in both sexes and for all age groups; in 2018 an
estimated 1.7 million deaths, with an incidence of approximately 2
million cases were reported (1).
The histology of LC is represented by small cell lung cancer at a
proportion of 15%, while non-small cell lung cancer (NSCLC)
represents about 85% of all LC cases (2).
In recent years, the role of immunotherapy for the
treatment of cancer has been highlighted and the use of immune
checkpoint inhibitors (ICIs) has led to improved patient survival
(3). Nivolumab, a fully human
anti-programmed cell death-1 (PD-1) immunoglobulin G4 monoclonal
antibody (mAb) is the first ICI to be approved by the U.S. Food and
Drug Administration (FDA) for the treatment of previously treated
advanced or metastatic NSCLC after prior chemotherapy in adults
(4). However, the treatment
response with nivolumab varies and a reliable biomarker to assess
the prediction of clinical outcome using this anti-PD-1 mAb has not
yet been established (4).
It is widely known that inflammation plays an
important role in the development and propagation of many diseases,
including cancer. Moreover, many retrospective studies regarding
various cancer sites have concluded that systemic inflammation,
indexed commonly by the neutrophil-to-lymphocyte ratio (NLR),
calculated as the ratio between the absolute neutrophil count and
the absolute lymphocyte count in the peripheral blood, is
associated with a poorer prognosis in patients with cancer
(5,6).
Another key factor in the development and
therapeutic response in cancer is represented by the body mass
index (BMI), where obesity appears to influence the immune system
and to induce a state of low-grade inflammation (7). Furthermore, Cortellini et al
found that overweight/obese patients who suffer from different
types of diseases, including lung cancer, have a better response to
anti-PD-1/PD-L1 antibodies (8), a
fact also observed in other retrospective studies regarding
advanced/metastatic melanoma (9,10).
The association between inflammatory and nutritional
status may help predict treatment efficacy and allow patient
selection for different types of treatment. Therefore, our study
aimed to investigate the influence of baseline NLR and BMI on
progression-free survival (PFS) in NSCLC patients treated with
nivolumab.
Patients and methods
Study population
A retrospective study was carried out on 80 patients
with NSCLC who were treated with nivolumab after failed response to
platinum-based chemotherapy, from January 2018 to April 2020, at
the OncoHelp Oncology Center, Timisoara, Romania.
Inclusion criteria were: Patients older than 18
years of age, diagnosed with NSCLC as confirmed by
histopathological analysis, who failed first-line treatment.
Exclusion criteria were patients who did not have the available
biochemical tests and evaluation of nutritional status. Nivolumab
(3 mg/m2 or 240 mg total dose) was administered every 2
weeks until the occurrence of disease progression, unacceptable
toxicity, treatment withdrawal or patient death.
PFS was the time from the start of nivolumab
treatment to disease progression or death.
All patients gave their informed consent for data
collection. The study protocol was conducted according to the
Helsinki Declaration after the approval by OncoHelp Oncology
Center's Ethics Committee (no. 1b/27.04.2020).
Clinical assessment
Clinical assessment, anthropometric and demographic
data were collected from the medical records including: Age, sex,
hemogram parameters (leukocyte count, neutrophil count, lymphocyte
count, platelet count, hemoglobin value, NLR) at initial diagnosis,
after 2 and after 4 weeks, pathological diagnosis, tumor stage,
treatment, progression, and death. TNM staging was recorded for all
patients. The NLR ratio was obtained from the absolute neutrophil
count and the absolute lymphocyte count and for the first analysis,
it was dichotomized according to previous literature (11), NLR ≥3 and NLR <3. BMI was
calculated as weight in kilograms divided by square of the height
in meters. Underweight, normal weight, overweight and obesity were
defined as BMI <18.5 kg/m², BMI ≥18.5 and <25 kg/m², BMI ≥25
and <30 kg/m² and BMI ≥30 kg/m², respectively.
Statistical analysis
MedCalc software for Windows (v. 19.2.0) (https://www.medcalc.org/) and the R software packages
(v.3.3) (R Foundation for Statistical Computing, Vienna, Austria,
https://cran.r-project.org/) were used
for statistical computing. The Kolmogorov-Smirnov test was used for
testing the distribution of numerical variables. Qualitative
variables are presented as numbers and percentages. Parametric
tests (t-test, ANOVA) were used for the assessment of differences
between numerical variables with normal distribution and
nonparametric tests (Mann-Whitney or Kruskal-Wallis tests) for
variables with non-normal distribution. The Chi-square (χ²) test
was used for comparing proportions expressed as percentages (‘n’
designates the total number of patients included in a particular
subgroup). Univariate and multivariate logistic regression analysis
was performed to assess the association between variables. Survival
curves were calculated with the Kaplan-Meier method and differences
between groups were assessed with the log-rank test. Multivariate
survival analysis was carried out using the Cox proportional
hazards model. For the best threshold, the area under the receiver
operating characteristic (AUROC) curve analysis was used, by
identifying the optimal cut-off values using the Youden index. We
considered a P-value of 0.05 as the threshold for statistical
significance and a confidence level of 95% for estimating
intervals.
Results
Baseline characteristics
A total of 80 patients were included in the study
(mean age 60.91±8.42, 70% male). Patient characteristics are
documented in Table I. A total of
54/80 (67.5%) patients were diagnosed with adenocarcinomas, 20/80
(25.0%) patients with squamous cell carcinoma and 6/80 (7.5%)
patients with uncategorized NSCLC. A total of 4/80 (5%) patients
had epidermal growth factor receptor (EGFR) mutation and
1/80 (1.2%) patients had echinoderm microtubule-associated
protein-like 4-anaplastic lymphoma kinase (ALK) fusion gene.
The most prevalent nutritional status was normal weight (51.2%); 50
subjects (62.5%) had NLR ≥3. Additional characteristics are
presented in Table I.
 | Table IBaseline characteristics of the NSCLC
patients (N=80). |
Table I
Baseline characteristics of the NSCLC
patients (N=80).
| Parameter | Data values |
|---|
| Age (years), mean ±
SD | 60.91±8.42 |
| Sex (male), n
(%) | 56 (70.0) |
| BMI (kg/m²), mean ±
SD | 25.03±5.36 |
| NLR ≥3, n (%) | |
|
Yes | 50 (62.5) |
|
No | 30 (37.5) |
| Nutritional status,
n (%) | |
|
Underweight | 3 (3.7) |
|
Normal
weight | 41 (51.2) |
|
Overweight/obese | 36 (45.0) |
| Histological type,
n (%) | |
|
Adenocarcinoma | 54 (67.5) |
|
Squamous
cell carcinoma | 20 (25.0) |
|
Uncategorized
NSCLC | 6 (7.5) |
| Targetable driver
mutation, n (%) | |
|
EGFR | 4 (5.0) |
|
ALK | 1 (1.2) |
| Stage, n (%) | |
|
1 | 2 (2.5) |
|
2 | 5 (6.2) |
|
3 | 25 (31.2) |
|
4 | 48 (60.0) |
| Progressive
disease, n (%) | |
|
Yes | 21 (26.2) |
|
No | 59 (67.5) |
| Status, n (%) | |
|
Alive | 52 (65.0) |
|
Deceased | 28 (35.0) |
The frequency of nutritional status, evaluated by
BMI, showed some differences according to age (P=0.01). Subjects
under 65 years had a higher prevalence of normal weight (54%),
while subjects over 65 years had a higher prevalence of
overweight/obese (50%). There were no differences between the NLR
distribution in subjects under 65 years and subjects over 65 years
(70 vs. 50%, P=0.12).
Treatment response and survival
analysis
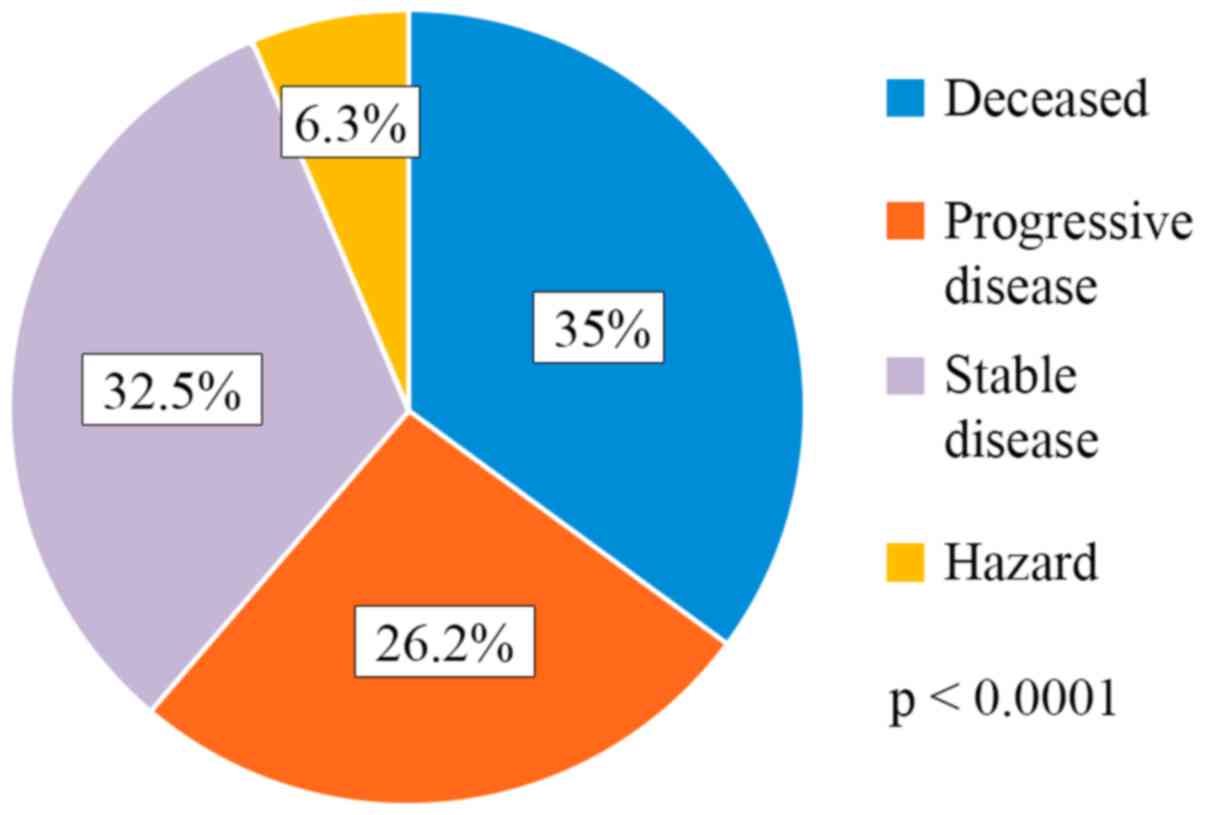
A total of 35% of the patients succumbed to the
disease (28/80), 26.2% of the patients had progressive disease
(21/80), 32.5% (26/80) had stable disease and 6.3% (5/80) were
hazard (lost from evidence due to non-oncological causes or low
compliance for treatment) (Fig. 1).
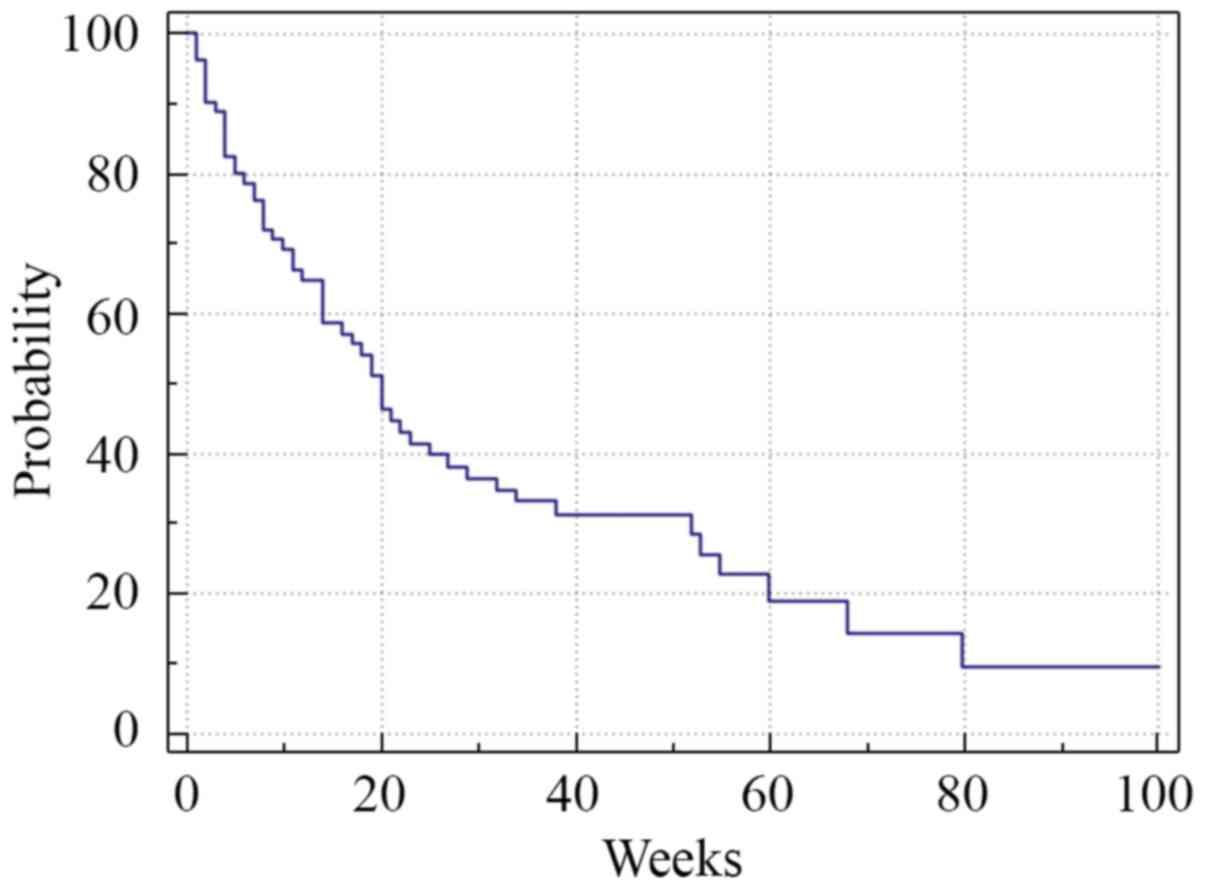
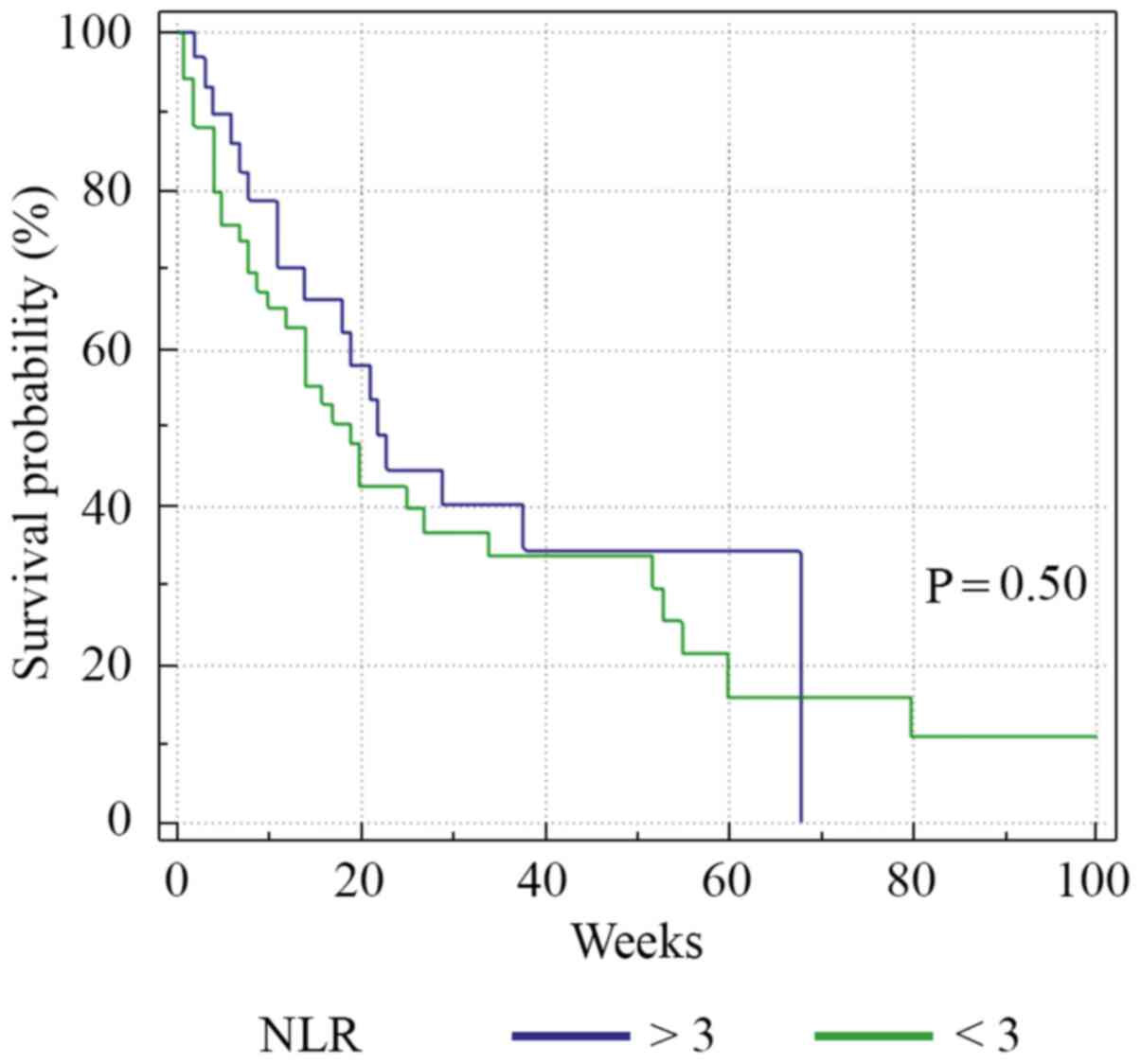
Median PFS was 13 weeks (range 1-80) (Fig. 2). Analysis of the survival curve
showed that the NLR above the proposed cut-off point was
significantly associated with underweight patients (P=0.04) and
lower survival (P=0.01). The differences between survival time of
the patients with NLR >3 and those with NLR <3 are showed in
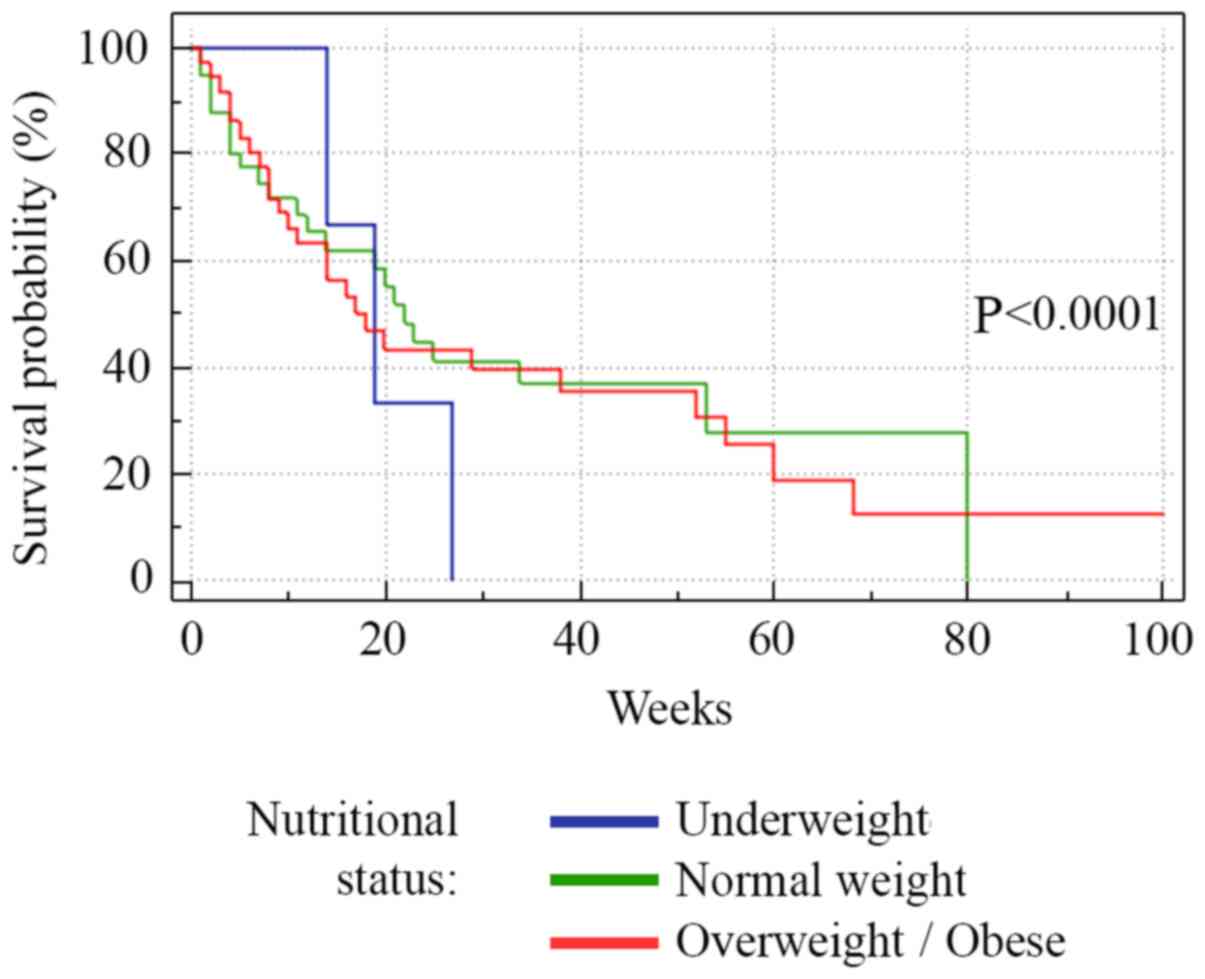
Fig. 3. Regarding nutritional
status, overweight/obese subjects had a higher survival rate
(P=0.001), while underweight subjects had a lower survival rate
(P=0.0001) (Fig. 4).
Relationship between NLR and PFS
We analyzed initial NLR, NLR at 2 weeks (NLR2) and
NLR at 4 weeks (NLR4). The median PFS for subjects with NLR <3
before treatment was 18.5 weeks, while in subjects with NLR ≥3 the
median PFS was 14 weeks (P=0.50). The median PFS for subjects with
NLR2 <3 at 2 weeks after treatment was 21 weeks, while in
subjects with NLR2 ≥3 the PFS was 14 weeks (P=0.17). The median PFS
for subjects with NLR4 <3 at 4 weeks after treatment was 23
weeks, while in subjects with NLR4 ≥3, the PFS was 19 weeks
(P=0.33).
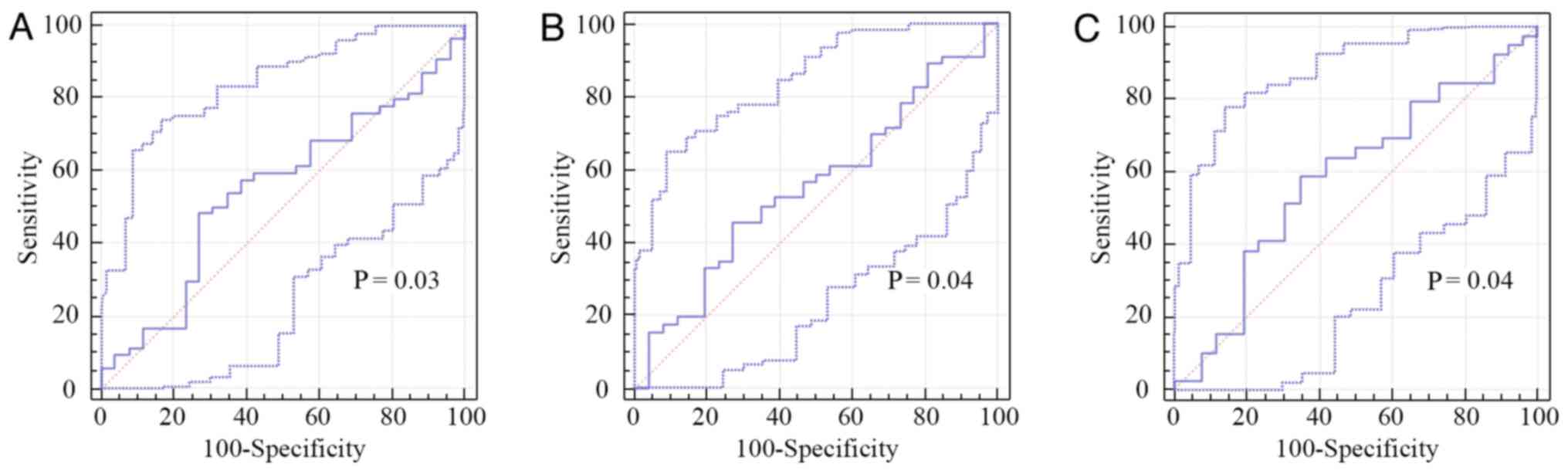
As initial NLR, NLR2 and NLR4 are good predictors
for PFS (P=0.03, P=0.04 and P=0.04, respectively), we obtained new
cut-off values for predicting PFS (Table II; Fig.
5).
 | Table IIPerformance of baseline NLR, NLR2 and
NLR4 for predicting PFS. |
Table II
Performance of baseline NLR, NLR2 and
NLR4 for predicting PFS.
| Variable | Cut-off | AUROC | P-value | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|
| NLR | 3.28 | 0.55 | <0.0001 | 71.4 | 49.1 | 33.3 | 82.9 |
| NLR2 | 3.26 | 0.56 | <0.0001 | 60.8 | 46.1 | 66.7 | 40 |
| NLR4 | 3.49 | 0.59 | <0.0001 | 58.9 | 65.3 | 71.9 | 51.5 |
Univariate and multivariate
analysis
We also investigated factors that are associated
with NLR (Table III) and other
factors that may be associated with the outcome of nivolumab
treatment, such as age of >65 years, sex, BMI and nutritional
status. The factors associated with NLR in univariate analysis were
male sex, age over 65 years, BMI value and underweight patients. In
multivariate analysis, only BMI value and underweight patients were
independently associated. Underweight status was able to increase
the NLR value by 27 times (OR=27), normal weight patients by 2
times (OR=2.07), while overweight/obese patients appeared to have a
protective role over NLR value (OR=0.60). For PFS, in univariate
logistic regression analysis (Table
IV), NLR, male sex and BMI value were associated (P=0.001,
P=0.02, P=0.01, respectively). Multivariate analysis for the
association with PFS showed that the same variables, NLR, male sex
and BMI, were associated independently, thus we were able to
develop a significant statistical model [AUROC=0.76, 95% CI
(0.45-0.89), P=0.03], a new predictive score for PFS:
 | Table IIIUnivariate and multivariate logistic
regression model for NLR by clinical characteristics of the NSCLC
patients. |
Table III
Univariate and multivariate logistic
regression model for NLR by clinical characteristics of the NSCLC
patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age over 65
years | 0.42
(0.16-1.09) | 0.04 | 0.89
(0.65-1.2) | 0.48 |
| Male sex | 1.05
(0.38-2.91) | 0.03 | 1.28
(0.54-1.8) | 0.91 |
| BMI value | 0.96
(0.58-1.56) | 0.01 | 1.01
(0.23-1.9) | 0.001 |
| Nutritional
status | | | | |
|
Underweight | 27 (7.1-30) | 0.04 | 15 (5.7-21.3) | 0.01 |
|
Normal
weight | 2.07
(0.82-5.20) | 0.11 | - | - |
|
Overweight/obese | 0.60
(0.24-1.49) | 0.27 | - | - |
 | Table IVUnivariate and multivariate logistic
regression model for PFS by clinical characteristics of the NSCLC
patients. |
Table IV
Univariate and multivariate logistic
regression model for PFS by clinical characteristics of the NSCLC
patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age over 65
years | 0.86
(0.20-1.10) | 0.56 | - | - |
| NLR | 1.03
(0.37-2.88) | 0.001 | 1.10
(0.38-3.12) | 0.01 |
| Male sex | 0.80
(0.27-2.35) | 0.02 | 0.95
(0.86-1.05) | 0.04 |
| BMI value | 0.95
(0.87-1.01) | 0.01 | 0.96
(0.96-1.91) | 0.001 |
| Nutritional
status | | | | |
|
Underweight | 1.42
(0.12-16.5) | 0.77 | - | - |
|
Normal
weight | 1.37
(0.50-3.76) | 0.55 | - | - |
|
Overweight/obese | 0.84
(0.28-2.51) | 0.75 | - | - |
PFS-NSCLC Score=0.43-0.08 x NLR value + 0.02 x BMI
value + 1 (if male)
The best cut-off value for a poor PFS for this score
in our cohort was 1.32, with a sensitivity of 90.4% and a negative
predictive value of 95.7%.
Multivariate Cox regression demonstrated that NLR ≥3
(HR=2.21, P=0.03) and male sex (HR=1.10, P=0.01) were identified as
independent poor prognostic factors for PFS. Conversely, normal
weight subjects (HR=0.52, P<0.0001) and overweight/obese
subjects (HR=0.45, P<0.0001) presented a better prognosis
(Table V).
 | Table VUnivariate and multivariate Cox
regression models of factors that may influence patient
survival. |
Table V
Univariate and multivariate Cox
regression models of factors that may influence patient
survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| | 95% CI | 95% CI |
|---|
| Variables | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value |
|---|
| Age (years) | | | | | | | | |
|
<65 | 1.20 | 1.01 | 1.68 | 0.85 | 1.10 | 0.35 | 1.94 | 0.76 |
|
≥65 | 0.97 | 0.55 | 1.72 | 0.93 | 0.99 | 0.55 | 1.76 | 0.97 |
| Nutritional
status | | | | | | | | |
|
Underweight | 1.27 | 0.31 | 5.20 | 0.73 | 1.08 | 0.24 | 4.79 | 0.91 |
|
Normal
weight | 0.90 | 0.52 | 1.56 | 0.72 | 0.52 | 0.15 | 1.73 | <0.0001 |
|
Overweight/obese | 1.10 | 0.64 | 1.89 | 0.72 | 0.45 | 0.40 | 1.25 | <0.0001 |
| Sex | | | | | | | | |
|
F | 1.01 | 0.56 | 1.81 | 0.96 | - | - | - | - |
|
M | 1.06 | 1.02 | 1.10 | 0.01 | 1.10 | 0.95 | 1.90 | 0.01 |
| NLR ≥3 | | | | | | | | |
|
Yes | 1.05 | 1.01 | 1.09 | 0.02 | 2.21 | 0.65 | 2.24 | 0.03 |
|
No | 1.05 | 0.96 | 1.14 | 0.25 | 1.10 | 0.85 | 1.25 | 0.48 |
Discussion
The identification of prognostic indicators and
predictive markers related to the clinical evolution of lung cancer
(LC) is extremely relevant, since the disease stands as number one
in regards to patient mortality and incidence worldwide (1). Our study revealed that LC patients
treated with nivolumab who showed high baseline
neutrophil-to-lymphocyte ratio (NLR) and were underweight had a
significantly lower progression-free survival (PFS) rate and that
overweight/obese patients had a prolonged PFS rate. Furthermore,
independently, patients who presented NLR ≥3 and those of male sex
had a poor prognosis, while normal weight and overweight/obese
patients had a better prognosis. Ultimately, we managed to develop
a significant statistical model, a new predictive score for PFS,
based on the association between NLR, body mass index (BMI) and
male sex.
It is known that cancer-associated inflammation
plays an important role in disease progression and survival in a
variety of solid tumors (12),
while the absence of inflammation could favor outcomes, even
though, in this situation, tumors could develop unnoticed (13,14).
Accordingly, NLR, as an indicator of systemic inflammation
associated with alterations in peripheral blood leukocytes, could
play a significant role in various cancers and it has been
extensively studied in this matter (6,15). An
integral part of the innate immune system is represented by
neutrophils, with both immune-suppressive and tumor-promoting roles
being described (16-20).
Beside the fact that neutrophils produce chemokines and cytokines
that influence tumor progression, they can also suppress the immune
activity of lymphocytes to further promote metastasis (21-23).
Lymphocytes have been proven to exhibit a vital role in host
cell-mediated immune regulation and their increased infiltration
into tumors has been linked with a better response to cytotoxic
treatment and progression in cancer patients (24,25).
Furthermore, tumor-infiltrating lymphocytes (TILs) have been shown
to have prognostic significance in cancer clinical outcomes
(26,27). Considering that immune checkpoint
inhibitors (ICIs) block negative regulators of T-cell function,
thus enhancing antitumor immunity (28), an alteration in peripheral blood
leukocytes in favor of neutrophils, with a commonly associated
lymphopenia, could influence the efficacy of ICIs. Bagley et
al demonstrated that higher pretreatment NLR in patients with
advanced NSCLC treated with nivolumab was independently associated
with lower PFS and overall survival (OS) (29). However, the pre-specified cut-off
value for NLR used in this study was 5, based on the validation of
a previous study that assessed patients with metastatic melanoma
treated with ipilimumab (30).
Conversely, in an Asian cohort, Nakaya et al did not report
a significant association between baseline NLR and median PFS, but
revealed that a NLR <3 at 2 and 4 weeks after nivolumab
initiation may be an independent predictive indicator in patients
with advanced NSCLC (31). In the
present study, high pretreatment NLR was associated with lower PFS
and a poor outcome.
BMI has also become a subject of interest in the
context of clinical outcomes of cancer patients. It is considered
to be the second highest risk factor after tobacco smoking, causing
approximately 20% of the number of cancer cases (32). Moreover, studies have found that
obesity is associated with lower survival and poorer cancer
treatment response (32-35),
until in the recent years, when the ‘obesity paradox’ was
described, a phenomenon which suggests a protective effect
increased BMI has in chronic diseases, including cancer (36-39).
Furthermore, since the development of ICIs, there is growing
evidence that highlights a connection between high BMI and a better
response to immunotherapy and improved cancer survival, with the
evidence by Naik et al that improved OS was associated in
male patients who had high serum creatinine levels (a marker for
high muscle mass) (8,9). A separate study that analyzed
individual participant data from 4 different clinical trials found
a linear connection between increased BMI and improved survival in
patients with NSCLC treated with atezolizumab (an anti-PD-L1). In
comparison, the same connection was not found in the groups treated
with the chemotherapy agent docetaxel (40). The present study adds to the
evidence that high BMI may improve PFS and response to
immunotherapy. In addition, despite the small number of underweight
patients, the results of our study showed that this category
presented a significantly lower PFS, intersecting with what
Cortellini et al revealed in their retrospective
observational study, which included NSCLC patients receiving ICIs
(41).
The biological basis that stands between the
association of BMI and cancer survival following immunotherapy is
just at the beginning of understanding. The obese state induces a
low-grade systemic inflammation and an impaired immune response,
including T-cell dysfunction and a growing number of exhausted
PD-1-presenting T-cells in adipose tissue and tumor
microenvironment via a leptin-dependent mechanism, which are known
to have a strong affinity for PD-L1, a ligand located on tumor
cells, meant to further suppress T-cell function (7). Based on this hypothesis, nivolumab,
which acts as an anti-PD-1 antibody, blocking the bonding between
PD-L1/PD-1 molecules, might induce a better response in patients
with increased BMI and an established PD-1 T-cell exhausted
state.
Despite not being the main aim of the present study,
our statistical analysis revealed that the male sex may represent a
poor predictive factor for PFS, a fact that proves to be
inconsistent with other studies that showed variation in ICI
outcomes related to sex (10,42,43).
At the same time, according to univariate and multivariate logistic
regression analysis, male sex, NLR and BMI were found to be
associated with PFS. Consequently, we proposed a new predictive
score for PFS. PFS-NSCLC Score has the ability to rule-out the poor
outcome at a cut-off value of more than 1.32 with a specificity of
90.4% and a negative predictive value of 95.7%.
Nevertheless, our study has its limitations. The
number of patients in our cohort was relatively moderate with a few
disproportions regarding baseline characteristics, such as sex, NLR
and nutritional status distributions. In addition, being a
retrospective study, we did not use any of the Response Evaluation
Criteria in Solid Tumors (RECIST) methodologies and the expression
of PD-L1 was examined only in a few patients, because of insurance
policy constraints and succession of treatments. Despite these
limitations, we managed to set a new NLR cut-off value (3.26) for
predicting PFS, which resembles the one used in previous literature
(11). In addition, new cut-off
values were calculated for NLR after 2 and 4 weeks after the
initiation of nivolumab (NLR2=3.26, NLR4=3.49, respectively).
Regarding the proposed predictive score for PFS, the performance in
the present study appears to be good and, to our knowledge, this
type of tool is the first one to be proposed. We consider our study
significant, as the power of the test was 75%. However, more
research is required, with larger populations and specific
characteristics.
In conclusion, NLR and BMI may represent simple and
useful biomarkers and, by combining them and taking into
consideration the male sex, they may predict PFS in patients with
advanced NSCLC treated with nivolumab.
Acknowledgements
Professional editing, linguistic and technical
assistance were performed by Irina Radu, Individual Service
Provider.
Funding
No funding was received.
Availability of data and materials
The data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
RD and RL organized the study, analyzed and
interpreted the study data and wrote the manuscript. ASD, AN, SS,
DP and MS analyzed the data and helped to draft the findings and
critically reviewed the manuscript; SN interpreted the data and
critically reviewed the manuscript for intellectual content. All
the authors have read and approved the final version of the
manuscript for publication.
Ethics approval and consent to
participate
All patients provided written informed consent for
the study participation and data collection. The study protocol was
conducted according to the principles of the Declaration of
Helsinki after the approval by OncoHelp Oncology Center's Ethics
Committee (no. 1b/27.04.2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization. Lung cancer.
Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Accessed June 4, 2020.
|
|
2
|
Inamura K: Lung cancer: Understanding its
molecular pathology and the 2015 WHO classification. Front Oncol.
7(193)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Onoi K, Chihara Y, Uchino J, Shimamoto T,
Morimoto Y, Iwasaku M, Kaneko Y, Yamada T and Takayama K: Immune
checkpoint inhibitors for lung cancer treatment: A review. J Clin
Med. 9(1362)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Malhotra J, Jabbour SK and Aisner J:
Current state of immunotherapy for non-small cell lung cancer.
Transl Lung Cancer Res. 6:196–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han F, Liu Y, Cheng S, Sun Z, Sheng C, Sun
X, Shang X, Tian W, Wang X, Li J, et al: Diagnosis and survival
values of neutrophil-lymphocyte ratio (NLR) and red blood cell
distribution width (RDW) in esophageal cancer. Clin Chim Acta.
488:150–158. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Woodall MJ, Neumann S, Campbell K,
Pattison ST and Young SL: The effects of obesity on anti-cancer
immunity and cancer immunotherapy. Cancers (Basel).
12(1230)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cortellini A, Bersanelli M, Buti S,
Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di
Marino P, et al: A multicenter study of body mass index in cancer
patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors:
When overweight becomes favorable. J Immunother Cancer.
7(57)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Naik GS, Waikar SS, Johnson AE, Buchbinder
EI, Haq R, Hodi RS, Schoenfeld JD and Ott PA: Complex
inter-relationship of body mass index, sex and serum creatinine on
survival: Exploring the obesity paradox in melanoma patients
treated with checkpoint inhibition. J Immunother Cancer.
7(89)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McQuade JL, Daniel CR, Hess KR, Mak C,
Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al:
Association of body-mass index and outcomes in patients with
metastatic melanoma treated with targeted therapy, immunotherapy,
or chemotherapy: A retrospective, multicohort analysis. Lancet
Oncol. 19:310–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ozyurek BA, Ozdemirel TS, Ozden SB,
Erdogan Y, Kaplan B and Kaplan T: Prognostic value of the
neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian
Pac J Cancer Prev. 18:1417–1421. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fouad TM, Kogawa T, Liu DD, Shen Y, Masuda
H, El-Zein R, Woodward WA, Chavez-MacGregor M, Alvarez RH, Arun B,
et al: Erratum: Overall survival differences between patients with
inflammatory and noninflammatory breast cancer presenting with
distant metastasis at diagnosis. Breast Cancer Res Treat.
152:407–416. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Faur CI, Pop DL, Motoc AGM, Folescu R,
Grigoraş ML, Gurguş D, Zamfir CL, Iacob M, Vermeşan D, Deleanu BN,
et al: Large giant cell tumor of the posterior iliac bone-an
atypical location. A case report and literature review. Rom J
Morphol Embryol. 61:247–252. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hao S, Andersen M and Yu H: Detection of
immune suppressive neutrophils in peripheral blood samples of
cancer patients. Am J Blood Res. 3:239–245. 2013.PubMed/NCBI
|
|
17
|
Pillay J, Kamp VM, van Hoffen E, Visser T,
Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P and Koenderman
L: A subset of neutrophils in human systemic inflammation inhibits
T cell responses through mac-1. J Clin Invest. 122:327–336.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Pillay J, Tak T, Kamp VM and Koenderman L:
Immune suppression by neutrophils and granulocytic myeloid-derived
suppressor cells: Similarities and differences. Cell Mol Life Sci.
70:3813–3827. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Uribe-Querol E and Rosales C: Neutrophils
in cancer: Two sides of the same coin. J Immunol Res.
2015(983698)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cools-Lartigue J, Spicer J, Najmeh S and
Ferri L: Neutrophil extracellular traps in cancer progression. Cell
Mol Life Sci. 71:4179–4194. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Galdiero MR, Garlanda C, Jaillon S, Marone
G and Mantovani A: Tumor associated macrophages and neutrophils in
tumor progression. J Cell Physiol. 228:1404–1412. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Coffelt SB, Kersten K, Doornebal CW,
Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M,
Hawinkels LJ, Jonkers J and de Visser KE: IL-17-producing γδ T
cells and neutrophils conspire to promote breast cancer metastasis.
Nature. 522:345–348. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fridlender ZG, Albelda SM and Granot Z:
Promoting metastasis: Neutrophils and T cells join forces. Cell
Res. 25:765–766. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: A systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J,
Wu C and Jiang J: Prognostic role of tumor-infiltrating lymphocytes
in lung cancer: A meta-analysis. Cell Physiol Biochem.
37:1560–1571. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ,
Xie CM and Hu QG: Prognostic and predictive value of
tumor-infiltrating lymphocytes for clinical therapeutic research in
patients with non-small cell lung cancer. Oncotarget.
7:13765–13781. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Bagley SJ, Kothari S, Aggarwal C, Bauml
JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson
JC, et al: Pretreatment neutrophil-to-lymphocyte ratio as a marker
of outcomes in nivolumab-treated patients with advanced
non-small-cell lung cancer. Lung Cancer. 106:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ferrucci PF, Gandini S, Battaglia A,
Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis
F, Marchetti P, Amato G, et al: Baseline neutrophil-to-lymphocyte
ratio is associated with outcome of ipilimumab-treated metastatic
melanoma patients. Br J Cancer. 112:1904–1910. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakaya A, Kurata T, Yoshioka H, Takeyasu
Y, Niki M, Kibata K, Satsutani N, Ogata M, Miyara T and Nomura S:
Neutrophil-To-Lymphocyte ratio as an early marker of outcomes in
patients with advanced non-small-cell lung cancer treated with
nivolumab. Int J Clin Oncol. 23:634–640. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wolin KY, Carson K and Colditz GA: Obesity
and cancer. Oncologist. 15:556–565. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
De Pergola G and Silvestris F: Obesity as
a major risk factor for cancer. J Obes. 2013(291546)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reeves GK, Pirie K, Beral V, Green J,
Spencer E and Bull D: Million Women Study Collaboration. Cancer
incidence and mortality in relation to body mass index in the
million women study: Cohort study. BMJ. 335(1134)2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vucenik I and Stains JP: Obesity and
cancer risk: Evidence, mechanisms, and recommendations. Ann NY Acad
Sci. 1271:37–43. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tobias DK, Pan A, Jackson CL, O'Reilly EJ,
Ding EL, Willett WC, Manson JE and Hu FB: Body-Mass index and
mortality among adults with incident type 2 diabetes. N Engl J Med.
370:233–244. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Andersen KK and Olsen TS: The obesity
paradox in stroke: Lower mortality and lower risk of readmission
for recurrent stroke in obese stroke patients. Int J Stroke.
10:99–104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Curtis JP, Selter JG, Wang Y, Rathore SS,
Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F,
et al: The obesity paradox: Body mass index and outcomes in
patients with heart failure. Arch Intern Med. 168:55–61. 2005.2008.
PubMed/NCBI View Article : Google Scholar
|
|
39
|
Silva TH, Schilithz AO, Peres WAF and
Murad LB: Neutrophil-lymphocyte ratio and nutritional status are
clinically useful in predicting prognosis in colorectal cancer
patients. Nutr Cancer. 72:1345–1354. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kichenadasse G, Miners JO, Mangoni AA,
Rowland A, Hopkins AM and Sorich MJ: Association between body mass
index and overall survival with immune checkpoint inhibitor therapy
for advanced non-small cell lung cancer. JAMA Oncol. 6:512–518.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cortellini A, Bersanelli M, Santini D,
Buti S, Tiseo M, Cannita K, Perrone F, Giusti R, De Tursi M,
Zoratto F, et al: Another side of the association between body mass
index (BMI) and clinical outcomes of cancer patients receiving
programmed cell death protein-1 (PD-1)/Programmed cell death-ligand
1 (PD-L1) checkpoint inhibitors: A multicentre analysis of
immune-related adverse events. Eur J Cancer. 128:17–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wallis CJD, Butaney M, Satkunasivam R,
Freedland SJ, Patel SP, Hamid O, Pal SK and Klaassen Z: Association
of patient sex with efficacy of immune checkpoint inhibitors and
overall survival in advanced cancers: A systematic review and
meta-analysis. JAMA Oncol. 5:529–536. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sagerup CMT, Småstuen M, Johannesen TB,
Helland A and Brustugun OT: Sex-Specific trends in lung cancer
incidence and survival: A population study of 40 118 cases. Thorax.
66:301–307. 2011.PubMed/NCBI View Article : Google Scholar
|