1. Introduction
Cutaneous melanoma is the most aggressive form of
skin cancer and its incidence is on the increase worldwide. The
most promising strategy for treating melanoma is represented by the
therapeutic manipulation of the immune system. The key challenge
for the clinical implementation of immunotherapies is controlling
the mechanisms of the immune system, with high responses and low
side effects. This modulation requires administration of the most
appropriate immunomodulator dose during the right time at the most
suitable tissular, cellular and intracellular location (1).
Biomedical nanotechnologies represent a promising
choice through engineering biomaterials, drug-delivery systems,
even immune cells for targeting the immune system in a controlled
manner. Improving the ability of immunomodulatory molecules to
reach disease tissues, immune cells, or their intracellular
compartments, and manipulating immune cells to kill cancer cells
remain two essential objectives for every immuno-nanotechnology
platform. Several categories of NPs or biologic devices that
enhance the efficacy of immunotherapy are cancer vaccines, immune
checkpoint inhibitors and nano-immunostrategies that can be
employed to reprogram the tumor microenvironment.
2. Immune system in cancer
The composition of the immune system includes immune
cells and immune organs, strongly related to other
non-immunological cells and organs, with the purpose of protecting
the host from foreign microorganisms and their bodies. This
protective function is performed simultaneously with the
maintenance and tolerance of self-antigens. Innate and an adaptive
immune responses are described. Innate immunity includes
macrophages, natural killer cells (NKs) and dendritic cells (DCs)
that are responsible for the first barrier against non-selfs. DCs
and macrophages trigger a response manifested by inflammation,
which is followed by innate and adaptive cell alerting.
In cancer, the immune cells from innate and adaptive
immunity constitute the tumor immune microenvironment (TIME). T
cells from TME are represented by tumor-infiltrating lymphocytes
(TILs), which play a major role in tumor initiation and progression
(2) and exert both protumoral and
antitumoral activity. Normally, for the inhibition of tumor growth,
CD4+ T helper 1 (Th1) and T helper 2 (Th2),
CD8+ T cells and natural killer (NK) T cells produce
interferon-gamma (IFN-γ), which activates macrophages for cancer
cell phagocytosis. Macrophages are involved in interleukin 2 (IL-2)
synthesis, which enhances Th1 cell differentiation (3). The balance between Th1 and Th2 cells
is critical in the antitumor immune response. Th1 cells stimulate
IL-2 and IFN-γ production, which trigger the induction of cellular
immunity by eradicating the tumor mass, whereas Th2 cells are
essential in stimulating the humoral immunity by inducing tumor
necrosis (4). IFN-γ is responsible
for the stimulation of the antigen-presenting cells (APC) that
activate cytotoxic CD8+ T cells, which recognize the
peptide antigens presented by MHC class I molecules from the tumor
and promote tumor cell lysis. Most tumors are positive for MHC
class I and negative for MHC class II. Th2 release IL-10, IL-13,
IL-5 and IL-4, enhancing T-regulatory (Treg) cells that inhibit the
CD4+ and CD8+ synthesis (5,6).
Immature myelomonocytic cells are represented by myeloid-derived
suppressive cells (MDSCs) that improve the immunosuppressive
activity on T cells. MDSCs produce arginase-1 (ARG-1) and
indoleamine 2,3 dioxygenase (IDO) that generates inefficient T-cell
receptor complex expression on Ag-activated T cells (7,8) and
are involved in expressing reactive oxygen species, IL-10, TGFβ and
nitric oxide, responsible for the suppression of anti-tumoral
immunity (9). Macrophages are other
major players in cancer progression and various types of activation
of these macrophages are described, related with different signals:
i) The classical activation of macrophages (M1), associated with
the production of proinflammatory cytokines, that produces reactive
oxygen species that causes cytolysis in cancer cells (10), and ii) alternative activation of
macrophages (M2), which generate anti-inflammatory cytokines that
enhance tissue repair and angiogenesis, favoring tumor progression
(11). IFN-γ promotes M1 and
IL-4 enhances M2, leading to the description of a bipolar axis.
Prostaglandin, free fatty acids, IL-10 or high-density lipoprotein
are other factors involved in macrophage activation alongside the
bipolar M1/M2 axis (12,13). The immune checkpoint proteins as
cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and Programmed
Death-1 (PD-1), both expressed by activated T cells, can also
enhance the immunosuppression of TIME. PD-1 blocks the effector
T-cell activation, preventing the interaction with its ligands
PD-L1 or PD-L2. In addition, CTLA-4 binds to the APCs surface with
CD80 and CD86, producing the inhibition of T cells (14).
The response of the immune system to tumor growth is
represented by immunoediting, a dynamic process consisting of three
distinct phases: Elimination, equilibrium and escape. In the
elimination phase, the cells and molecules of innate and adaptive
immunity work together in order to find the presence of the tumor
and eliminate it. In some cases, variants of tumor cells may not be
completely destroyed, but enter the following phase, namely the
equilibrium phase, where the immune system controls the tumor cell
growth. In the equilibrium phase, the innate immune system cannot
totally eliminate cancer cells, but keeps them in a state of
immune-mediated tumor dormancy. The escape phase can be considered
as a failure of the immune system to eliminate or control cancer
cells, enabling the survival of cell variants, in an unrestricted
manner. Related to these dynamic phases, one must take into account
the described immune phenotypes of tumor microenvironment (TME): i)
The immune-desert phenotype is characterized by immunological
tolerance (not the response to antigen presentation), ignorance
(lack of antigen) and lack of T-cell priming; ii) The
immune-excluded phenotype, where the immune cells from the
periphery of the tumor or stroma are impeded by extravascular
stroma and immature vessels; iii) The inflamed-phenotype, where
pro-inflammatory cytokines are expressed by T cells from
parenchyma, representing a failure of antitumor immune response. Of
note, the TME has various compositions in different cancer types,
different patients with the same cancer and also different tumor
sites within the same patient (15).
3. Immunotherapy in melanoma
Immunotherapy aims to manipulate the immune system
and is used for treating diseases. This targeting has two main
goals: The inhibition or enhancement of the immune system,
depending on the intended effect. The general classification
related to therapy includes active and passive immunotherapy
(16). This classification takes
into account how immunotherapy stimulates or inhibits the immune
system. Active immunotherapy is composed of treatments aiming to
enhance the immune response against antigens. The checkpoint
inhibitors and vaccines are included in this category. These are
immune active and act only in close relationship with the host
immune system. Conversely, passive immunotherapy is based on an
intrinsic immune response, mediated by molecules, such as
antibodies and cytokines that stimulates the immune response.
Examples of passive immunotherapy are monoclonal therapeutic
antibodies and adoptive cell transfer therapy (17).
Three main strategies in which immunotherapies for
cancer are classified were described, related to active or passive
therapies. The first strategy is represented by nanoparticles (NPs)
that represent delivery systems for stimulating molecules or
antigens and show promising results in treating melanoma. NPs are
nanocarriers that trigger specific receptors and target the immune
system activation or inhibition. Furthermore, if the molecule
carried by nanoparticles is an antigen, they are known as
nano-vaccines and the purpose is to migrate into the lymph nodes
(LNs) in order to trigger the T lymphocytes and generate a specific
cytotoxic response against the tumor (18). In addition, specific nanoparticles
can target dendritic cells from the tumor microenvironment using
the same mechanism of presenting antigens to stimulate cytotoxic T
cells. Adoptive cell transfer therapy is the second strategy in
which the immune cells are collected from the patient, trained
ex vivo, and reinfused into the patient (19). The third strategy, with promising
results in melanoma treatment, is represented by the delivery of
therapeutics to the immune tumor microenvironment area. These
therapeutics can target cancer cells, but also elements of the
immune system that are present in the tumor immune microenvironment
including DCs, tumor-associated macrophages, cytokines (IFNs,
TGF-β, IL-2) and enzymes involved in the metabolic pathways of
cancer cells. The development of biologic devices as viral
therapies in the last decades opened new routes to modulate the
immune system against tumor cells (Fig.
1). Currently, the main available medical immunotherapies of
melanoma are represented by tumor vaccines, gene therapy,
checkpoint inhibition immunotherapies, T-cell directed therapies
and non-specific approaches including cytotoxic chemotherapy,
photodynamic therapy, photothermal therapy, and radiotherapy,
recommended only in subsets of patients carefully selected for the
clinical benefit (20).
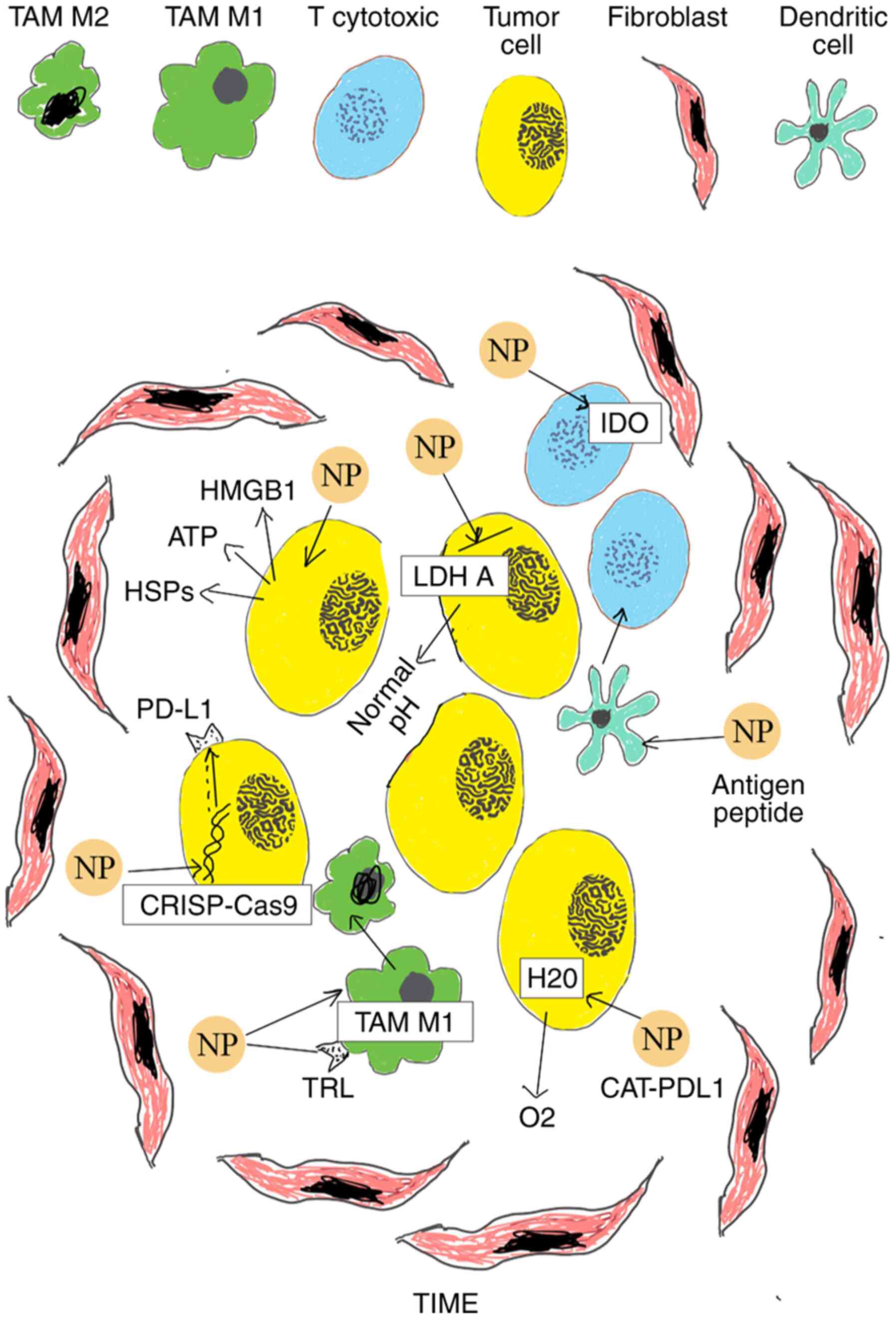 | Figure 1Schematic of various nanomedicines
applied in cancer immunotherapy of cutaneous melanoma. i)
Multifunctional CAT-PDL1 liposomes includes CAT that decreases
tumor hypoxia decomposing H2O2 into
O2 and PDL1 which improves immunotherapeutic effects,
promoting CD4+and CD8+ T cells; ii) PLGA NPs
deliver antigenic peptides that target dendritic cells to promote
cytotoxic T lymphocyte responses; iii) NPs inhibit IDO and block
tryptophan metabolism of cancer cells; iv) NPs block LDH A in tumor
cells leading to normal pH; v) NPs cause the translocation of
calrecutin and lead to the release of ATP, HMGB1 and HSPs in
extracellular environment, inducing ICD of cancer cells; vi)
co-polymer NPs aPBAE knock down Cdk5 and cause PD-L1 downregulation
via CRISPR-Cas9 genome editing; vii) aCD47@CaCO3 NPs increase the
macrophage polarization to M1 phenotype and block the ‘don't eat
me’ signal in cancer cells. NPs, nanoparticles; PLGA,
poly-lactic-co-glycolic acid; TIME, Tumor Immune Microenvironment;
IDO, indoleamine 2,3-dioxygenase; CAT, encapsulated catalase; ICD,
Immunogenic cell death; ATP, adenosine triphosphate; HMGB1, high
mobility group box 1 protein; HSPc, heat shock proteins. |
4. Classification of nanotechnologies for
cancer immunotherapy
Nanomedicine represents the medical application of
nanotechnology and includes medical applications of nanomaterials,
biological devices and applications of molecular technologies. The
main issues of nanomedicine are related to toxicity and
environmental impact of nanoscale materials. Specifically,
nanomaterials and biological devices used for enhancing cancer
immunotherapy are classified into polymeric nanoparticles, lipid
nanocarriers, metal nanoparticles, inorganic non-metallic
nanoparticles, exosome and engineered viruses (21-25).
Polymeric nanoparticles
Polymeric NPs are represented by
poly-lactic-co-glycolic acid (PLGA), dendrimers and micelles and
have been used in many drug delivery systems. Advantages of
polymeric nanoparticles are the versatility in size, morphology,
high loading of therapeutic drugs and surface functionalization.
The disadvantages are represented by the synthesis of
proinflammatory molecules and the inconstant degradation and
inactivation of the therapeutic pay load in the preparation
process.
PLGA
PLGA is FDA approved and represents the most
commonly used polymer which is biocompatible, biodegradable and of
low toxicity. Microspheres of PLGA target the pathways for MHC
class I and II molecules and cause an increase in the maturation of
DCs (26). PLGA delivery systems
were designed for cytokine agonists, siRNAs or CpG-coated tumor
antigen transportation to promote the internalization of antigens
by DC and the generation of immune responses stimulating CTL
(CD8+) and Th (CD4+) (27-29).
In addition, PLGA nanoparticles seem to be more suitable to target
DCs than PLGA microparticles are, with a 10- to 100-fold higher
efficiency in delivery of hD1 for nanoparticles (30).
Dendrimers
Dendrimers are branched macromolecules, composed of
a core and cavities to entrap drugs, suitable for modified drug
delivery due to water solubility, polyvalency and well-defined
chemical structure (31), being
described as a direct interaction between immune cells and
dendrimers. The surface of dendrimers shows many groups with the
possibility to be functionalized.
Lipid nanocarriers
Lipid nanocarriers are represented by liposomes,
solid-lipid NPs and phospholipids micelles. Liposomes are vesicles
with high biocompatibility, including synthetic or natural
phospholipids, with a cell membrane-like structure such as
hydrophobic tails of phospholipids cluster associated with
hydrophilic heads. The existence of hydrophilic and hydrophobic
compartments makes it possible to be encapsulated and released by
different compound mechanisms, without the influence of
intracellular mechanisms (32).
Micelles
Micelles are vesicular particles generated by
spontaneous aggregation of amphiphilic molecules with many
applications in cancer treatment as carriers for imaging,
radiotherapy, chemotherapy and immunotherapy. Compared with other
nanocarriers, the synthesis of micelles is almost easier. Micelles
can deliver intracytoplasmatic, are biodegradable and non-toxic
(33). Ovalbumin (OVA) and
metabolism-related enzymes such as IR780 are involved in IDO
metabolism can be transported also by micelles (34).
Metal NPs Gold nanoparticles
Gold nanoparticles (AuNPs) are carriers for
antigenic proteins and gene oligonucleotides to specific sites.
Covalent and non-covalent interactions with various biomolecules
were described, such as peptides, DNA and antibodies on the surface
of Au NPs (35). AuNPs interact
with selected subcellular organelles in tumor cells, being related
to cancer cell survival, growth, proliferation and death. Combining
AuNPs with photothermal ablation is a promising concept,
investigated in various trials (36). In addition, AuNPs are used in
delivering CgP oligonucleotides that enhance the migration of
macrophages and DCs in the tumor microenvironment (TME) (37). Different sized and shaped gold
nanoparticles, such as nanoshells, nanostars and nanorods were
designed for immunotherapeutic delivery adjuvants as OVA or CpG
(38).
Iron oxide nanoparticles
Iron oxide nanoparticles are promising carriers for
vaccine delivery, polarizing immune cells, such as DCs and
macrophages, and increasing immune response. They can also carry
adjuvants such as OVA to potentiate the immune system (39).
Inorganic non-metallic NPs Mesoporous
silica NPs
Mesoporous silica NPs (MSNs) is a honeycomb-like
porous structure including hundreds of empty mesopores that absorb
large amounts of bioactive molecules (40). Materials from mesoporous silica
interact with biosystems and biodistribution, cellular uptake,
biodegradation, toxicity, and interaction with immune cells are
related with specific physical and chemical properties including
particle shape, size, porosity, and surface functionality of the
materials (41). Mesoporous silica
materials are degradable in physiological conditions via hydrolysis
in the silica matrix, being related to the stability and release
profile of guest molecules, particle size, surface functionality,
concentration, porosity, morphology, degree of condensation, and
the type of degradation medium. Mesoporous silica can be released
to body tissues and excreted via renal clearance, being non-toxic
(42). Larger particles of
mesoporous silica and higher concentrations are more effective on
monocyte-derived dendritic cells (MDDC) than small particles in low
concentrations, suggesting the use of mesoporous silica as a
component of cancer vaccines (43).
An example was designed, a complete vaccine formulation using
mesoporous silica (XLMSNs + OVA + CpG-ODN). This vaccine induces
dendritic cell (DC) maturation with high levels of CD86 expression,
and increases the secretion of pro-inflammatory cytokines,
especially IL-12 and TNF-α (44).
Other MSNs utility was found for transportation of drugs together
with siRNAs which were co-delivered into the body, inducing the
secretion of cytokines (45).
Carbon nanotubes
Carbon nanotubes (CNTs) are cylindrical multi-walled
carbon nanotubes (MWNTs) that have various potential roles as tumor
antigen nanocarriers, represented by ovalbumin (OVA) and
cytosine-phosphate-guanine oligodeoxynucleotide (CpG), which are
delivered to antigen presenting cells (APCs) (24). Single-walled carbon nanotubes
combined with photothermal ablation of primary tumors and with
anti-CTLA-4 antibody therapy aiming to trigger adaptive immune
responses and prevent the metastatic process were also investigated
(46).
Exosomes
Exosomes (EXOs) are extracellular vesicles released
by the majority of cell types, with the size of 30-100 nm, with
functions of intercellular transporters for lipids, proteins and
nucleic acids among cells and organs that play different roles in
various physiological and pathological processes in the immune
system, including mediators, modulators and activators. Exosomes
are generated via plasma membrane invagination initially as
endosomes, which migrate to the center of the cell, resulting in
the generation of multivesicular bodies (MVBs) that carry DNA, mRNA
and non-coding RNA species or protein. The secretion of exosomes is
produced mainly in lymphoid and myeloid lineages but also in many
types of cells involving TME and cancer cell so-called
tumor-derived exosomes containing growth factors and microRNAs
(47,48). Exosomes can also inhibit tumors by
delivering chemical drugs avoiding phagocytosis by macrophages. The
potential benefits of using exosomes as a therapeutic approach to
promote melanoma immunotherapy for inducing strong and lasting
responses is ongoing.
Engineered viruses Virus-like
particles
Virus-like particles (VLPs) are 20-100 nm in size
and are artificial nanostructures containing viruses without the
possibility to replicate. The functions of VLPs are to stimulate
immune responses, being immunogenic, and target immune cells as an
engineered vaccine.
A VLP-based vaccine was designed, using a plant
virus, cowpea mosaic virus, in an empty CPMV (eCPMV) VLP
system, RNA-free and non-infectious. It was reported that eCPMV
nanoparticles have strong immunotherapeutic efficacy and modulate
the tumor immune environment. VLPs are involved in specifically
targeting TME cells and tumor cells and can be used as a
nanocarrier for tumor antigens and drugs (49).
Oncolytic viruses
Oncolytic viruses are engineered viruses that infect
tumor cells selectively, followed by tumor cell death. The aim
during delivery consists in generating systemic and local immune
response against tumor cells with a minimum of collateral effects
on normal cells. Oncolytic viruses are engineered to act
selectively on tumor cells in order to achieve this purpose. Viral
replication is followed by lysis, release of antigens,
damage-associated molecular patterns, and cytokines, promoting the
immunogenic reaction and modelling the antitumor immune system
(50). Consequently, in melanoma
the immune-suppressed microenvironment is transformed into an
immune-inflamed microenvironment. For a successful oncolytic virus,
several conditions are required: It must target and replicate in
tumor cells, it must have in vivo stability and it must not
be integrated into tumor chromosomes (51).
5. Factors that modulate the efficacy of
nanoparticles
Microbiome modulation
Gut microbiota constitute a variety of essential and
opportunistic microorganisms hosted in the gastrointestinal tract
as bacteria, viruses, protozoa, fungi, phages and archaea.
Heterogeneity of the immune treatment effect can be explained by
gut microbiota composition. Gut microbiota has a regulatory effect
on the immune system suggesting that a large number of
microorganisms can influence the functions of immune cells,
especially Tregs, CD4+ and CD8+ T cells. It
has been shown that human commensal Bacteroides fragilis
enhance the transformation of CD4+ naive T cells into
Treg and stimulate the production of anti-inflammatory cytokines
(52). The thymus-derived Tregs
from the colon recognize the antigenic materials from
Clostridiales, Lactobacillus and Bacteroides and
could preserve tolerance to these bacteria. Antibiotics decreasing
mainly the members of Clostridium family in gut microbiota
composition can decrease the number of colonic Tregs (53). It has been demonstrated that some
commensals such as Escherichia coli can improve the
pro-inflammatory gut immunity in a ‘love-hate’ relationship
(54-56).
In addition, increased gut Faecalibacterium is correlated
with elevated CD4+ or CD8+ T cells (57). The efficacy of anti-PD-1 treatment
in metastatic melanoma patients is influenced by gut microbiota.
The presence of some bacteria, such as Bifidobacterium longum,
Bifidobacterium adolescentis, Enterococcus faecium, Klebsiella
pneumoniae, Collinsella aerofaciens, Parabacteroides merdae,
Veillonella parvula and Lactobacillus species is related
with the response to anti-PD-1 treatment through various
mechanisms, such as elevating the secretion of IFN-γ, enhancing DCs
and increasing CD8+ tumor-infiltrating T cells in
contrast to Roseburia intestinalis and Ruminococcus
obeum that were found enriched in nonresponders (58,59).
EPR effect
The enhanced permeability and retention (EPR) effect
was first described in studies of inflammation (60). The enhanced permeability and
retention (EPR) effect represent a unique phenomenon found in solid
tumors, strongly correlated with anatomical and physiological
characteristics. These features can be represented by the
inadequate architecture of the vessels, large branches among
endothelial cells in blood vessels, vascular mediators in excess
and defective lymphatic drainage, followed by the significant
extravasation of components of plasma and nanomedicines. The EPR
effect determined the accelerated development of macromolecular
antitumoral drugs, known as nanomedicines (61,62).
It has been noted that a different EPR effect was observed in
various tumors or different areas of the same tumor, especially in
large tumors. In addition, the EPR effect is a dynamic phenomenon
involving pathophysiological factors, biological events inside the
body, tumoral growth, and inflammatory processes. EPR effect is the
basic concept of tumor targeting with nanomedicines and it is
related with the size, biocompatibility and conformation of
macromolecules. Surface of charge and half-time in circulation are
another critical point for the tumor-targeting nanomedicines
(63-65).
The concept of EPR-based tumor targeting was investigated in recent
studies and it has described the potential possibilities of
investigating transcytosis for tumor targeting by
nanomedicines.
The nanomedicine effectiveness related to the EPR
effect can be enhanced by pharmacological and physical
co-treatments designed to prime the tumor microenvironment.
Improvement of the EPR effect can be obtained by adding
supplementary strategies related to molecular targeting, and
physical or physiological modulation of the tumor microenvironment
(66).
Protein corona
The interactions between nanoparticles and
biological fluids is important to be understood to anticipate the
fate of injected NPs. This interaction is the consequence of
several factors related to nanoparticles, such as shape, size,
charge, or coating agents. These are critical and related to
features of biological fluids including protein concentration,
ionic strength, temperature and pH (67-72).
NPs are in contact with biological fluids and have interactions
with active biological molecules (nucleic acids, lipids, proteins).
Consequently, there is an inappropriate absorption of proteins on
the surface of NPs, with protein corona (PC) formation, a different
biological identity being generated in comparison to normal NPs. PC
can have two roles in biomolecular recognition. Firstly, in a
process defined as ‘immune-blinding’, the PC covers the surface of
NPs and hides the antigen or biomolecule carried by NPs from the
interaction with its specific receptor. Secondly, in some cases,
proteins included in PC can link to the receptors of immune cells
promoting unwanted immune responses. Nanoparticle-based
immunotherapy can fail because of PC formation, inducing two types
of responses: A non-response and an uncontrolled response. The
immune-blinding response (non-response) may be promoted by
partially or totally covering the antigens or stimulating molecules
present on the surface of the nanoparticles, and consequently, the
specific stimulation will be retarded, with the absence of the
immune response. Additionally, in some cases, PC can express an
altered structure during the PC formation on the surface of the NPs
that can bind to scavenger receptors from monocytes and macrophages
and induce phagocytosis. In this situation, recognition of the
stimulating molecules expressed on the surface of NPs is avoided.
In an uncontrolled response, aggregation of NPs triggers toxic
effects by strange-body recognition via the immune system (73-78).
6. Nanomedicine to enhance the immunotherapy
in melanoma
Cancer vaccines
Cancer vaccines administered for enhancing the
treatment of tumors has generated greater interest as an attractive
type of cancer immunotherapy strategy. These vaccines can be
classified into several classes: Neoantigen, dendritic cell,
nucleic acid, and whole tumor cell vaccines (79,80). A
challenge in promoting T-cell responses to eradicate tumor cells
after vaccination is the ability in presenting the antigens to
dendritic cells (DCs). A polyamidoamine dendrimer modified with
guanidinobenzoic acid (DGBA) was found to represent an efficient
cargo for some proteins such as ovalbumin (OVA) representing
antigen, and unmethylated cytosine-guanine dinucleotides (CpG)
representing the adjuvant, followed by an effective antigen
cross-presentation by DCs. This DGBA-OVA-CpG nano-vaccine can
promote powerful antigen-specific cellular immunities and has
demonstrated prophylactic efficacy against B16-OVA melanoma.
Combining anti-PD-1 treatment with DGBA-OVA-CpG nano-vaccine, an
increased percentage of the tumor-infiltrating
CD3+CD8+ and CD3+CD4+
T-cells among CD3+ T-cells in tumors was observed.
Conversely, vaccination with only DGBA-OVACpG or anti-PD-1
treatment is followed by a low infiltration of CD8+
(79). It is well known that tumors
with higher tumor mutational burden (TMB), such as cutaneous
melanoma, produce more neoantigen liable to activate the immune
system for recognizing tumors, in relation with two main elements:
The number and the type of mutations. Neoantigens are specific
non-autologous proteins, produced by non-synonymous mutations in
the tumor cell genome antigens. They are characterized by strong
and specific immunogenicity, higher affinity towards MHC, and lack
of expression in normal tissues; due to these properties, they can
virtually eliminate the risk of off-target side effects, while
reinforcing the immune response to destroy cancer cells (80). Classical tumor-associated antigens
(TAA) are present both in tumor and normal tissues, being highly
enhanced in tumor cells expressing HER2, MART-1, MUC1, and MAGE.
Neoantigens express stronger immunogenicity and higher affinity
towards MHC than TAAs, not being able to be affected by central
immunological tolerance. The first step in neoantigen
identification is the fast comparison of the DNA sequences of tumor
cells and normal cells. The majority of neoantigens are actually
identified using several software applications based on whole-exon
sequencing technology (81-84).
Neoantigens activate T cells and stimulate the production of highly
active T cells with strong affinity towards MHC-neoantigen-peptide
complexes, avoiding recognition by the central immune system
(85). The recently developed
bioinformatics algorithms were combined with sequencing technology;
it is now possible to accurately identify tumor neoantigens and
predict their MHC affinity and immunogenicity. Neoantigen
vaccination can enhance pre-existing neoantigen-specific T-cell
populations and promote an extensive collection of new T-cell
specificities in cancer patients, changing the intratumoral balance
in favor of enhanced tumor control. Some antigens from melanoma
tumors express four peptide sequence epitopes, similar to the
pathogen and more easily recognized by T cells, with sustained
clinical responses to immunosuppressive agents (86).
Anti-tumor therapy is a complex process, where a
neoantigen vaccine initially presents the antigen to be recognized
by T cells, and subsequently attacks the tumor. These processes act
on various targets and are difficult to obtain with a single drug.
In a recent study, a three-in-one immunotherapy nano-platform was
designed, where aPD-L1@HC/PM NPs combining Chlorin e6
(Ce6)-conjugated hyaluronic acid (HC), dextro-1-methyl tryptophan
(1-mt)-conjugated polylysine (PM) with anti-PD-L1 monoclonal
antibodies (aPD-L1) was prepared. A comparison of melanoma mice
model radiotherapy with aPD-L1@HC/PM NPs treatment showed that the
tumor volume in mice receiving radiotherapy was reduced, while the
tumor volume of the mice treated with aPD-L1@HC/PM NPs had
disappeared almost completely (87).
Although neoantigens were considered optimal targets
for an anti-tumor immune response, their discovery and evaluation
became possible only by frequently using parallel sequencing and
machine learning approaches for detecting the mutations within
tumors and to predict mutated peptides with high affinity that bind
autologous human leukocyte antigen (HLA) molecules. In a clinical
study on six patients with advanced melanoma, personalized vaccines
including 20 different fragments of peptide containing neoantigen
and PolyIC:LC as the immune adjuvant were prepared (83). It has been demonstrated that 60% of
the peptides developed a T-cell immune response in the patients,
and out of six treated patients, four patients presented stable
disease 25 months after vaccination and two patients presented
recurrence treated with PD-1 antibody treatment with complete
remission (83).
Aiming to increase the therapeutic effect of PD-1
inhibitors, a vaccine combined with a PD-1 inhibitor was designed,
which also improved the effective response of PD-1 inhibitor. The
antigen-specific vaccine stimulates the immune system to produce
PD-1 positive T-cells that interact and work together with PD-1
inhibitor, sustaining a double attack against the tumor. This
personalized vaccine can accelerate the immune response and remove
the obstacles from the PD-1 inhibitors, reducing the recurrence and
incidence of metastasis (88).
Nucleic acid vaccines were also designed, which include mRNA or DNA
encoding neoantigens delivered to intracellular (mRNA) or
intranuclear (DNA) APCs (89).
Antigens are presented to T-lymphocytes, which
destroy tumor cells expressing antigens with the same epitope. RNA
vaccines have the advantage that they can bypass integration into
host cell genome. Many clinical trials of DNA and RNA vaccines have
failed to actually demonstrate the efficacy due to the delivery
barriers and immunogenicity, but recent promising studies are
ongoing (90). A new strategy for
enhancing immune check-point blockade could be to promote the
antitumor immune response using a liposomal RNA vaccine
intravenously administered, currently under development (FixVac),
which targets four non-mutated, tumor-associated antigens. In an
exploratory analysis of clinical activity from a phase I
dose-escalation trial of FixVac alone or combined with anti-PD-1 in
patients with stage IIIB, IIIC, or IV melanoma (Lipo-MERIT trial,
ClinicalTrials.gov identifier NCT 02410733) in 50
patients, the IFNγ-ELISpot assay showed immune responses in more
than 75% of patients. Regarding the clinical responses in 42
patients with stage IV melanoma, FixVac monotherapy obtained 12%
partial responses and 28% stable disease and a combination of
FixVac with check-point inhibitors revealed partial response in 35%
of the 17 patients (91).
Tumor cell lysate-derived vaccines are also included
in cancer immunotherapies and are classified into autologous cancer
vaccines and allogeneic cancer vaccines. Autologous vaccines are
with tumor cell lysate derived from the patient and allogeneic
cancer vaccination are with another member of the same species.
Tumor cell lysates are presented by MHC (major histocompatibility
complex) molecules to trigger immune responses. It is well known
that the NY-ESO-1 cancer/testis antigen is expressed in 25% of
patients with melanoma. In a study on 11 patients with melanoma,
tumors expressing NY-ESO-1 that received autologous TCR-transduced
T cells plus interleukin-2 reported objective clinical responses in
five patients, representing the first demonstration of the
successful treatment of a non-melanoma tumor using TCR-transduced T
cells (92).
A biomaterial-based vaccination system using an
encapsulated GM-CSF that enhances DCs activity and
cytosine-phosphodiester-guanine oligodeoxynucleotide (CpG ODN), a
specific toll-like receptor (TLR) agonist which activates DCs, into
sponge-like macroporous cryogels was designed. The cryogels were
administered subcutaneously to mice in a melanoma model in order to
deliver immunomodulatory factors (GM-CSF and CpG ODN) in a
controlled manner. This vaccine caused local infiltrates consisting
of DCs that induce a potent, durable, and specific anti-tumor
T-cell response, indicating the potential for cryogels to be used
as a platform for cancer cell vaccinations (93).
Another target in immune therapy of melanoma can be
cancer stem cells (CSC). CSCs have been identified in melanoma,
where their extensive proliferation is responsible for metastasis
and recurrence of the tumor, but how to target and eliminate CSCs
in vivo remains a major issue. Synthetic high-density
lipoprotein nanodiscs represent a novel approach to reduce the
aldehyde dehydrogenase (ALDH), a marker for isolating CSCs. This
vaccine is designed against CSCs that are highly enriched in ALDH,
increasing antigen trafficking to lymph nodes and generating robust
ALDH-specific T-cell responses (94).
A vaccine targeting ALDH highly enriched CSCs
targeting dendritic cells (CSC-DC vaccine) in combination with
anti-PD-L1 and anti-CTLA-4 was designed and it was suggested that
this combination could manipulate T-cell functions and induce the
activation and proliferation of T cells in a B16-F10 murine
melanoma tumor model (95).
Targeting immune checkpoint inhibitors
to improve immunotherapy
The immune check-point blockade treatment in
melanoma is related with adverse events (AEs), with a global
incidence of 26.8% (all grades) in a meta-analysis of 46 studies
including 12,808 cancer patients treated with PD1/PD-L1 inhibitors
(96). Enhancing the efficacy of
checkpoint inhibitors can be obtained by escalation of the doses
for enhancing the efficacy of check-point inhibitors, but it is
hampered by the appearance of AEs and represent an emergent issue
in cancer immunotherapy. Delivery systems containing biomaterials
were experimented such as hydrogels, nanoparticles (NPs) and
microneedle patch-assisted delivery. Celecoxib and an anti-PD-1
monoclonal antibody (PD-1mAb) were locally delivered by way of a
designed alginate hydrogel system for treating a B16-F10 melanoma
model. The alginate hydrogel delivery system was found to
significantly enhance the antitumor activities of celecoxib (CXB),
PD-1mAb, or combination of both. This hydrogel system
synergistically improved the accumulation of CT4+ and
CD8+ T cells within the tumor (97). Another nanocarrier was designed as a
self-degradable microneedle patch containing biocompatible
hyaluronic acid integrated with dextran nanoparticles that
encapsulate aPD1 and glucose oxidase, for the delivery of an
anti-PD1 antibody (aPD1). A single intratumoral injection of
microneedle patch in a B16F10 mouse melanoma was demonstrated to
induce robust immune responses (98). The delivery of anti-PD-1 antibodies
was also examined using encapsulated PLGA nanoparticles (anti-PD-1
NPs) into the spleen in a B16-F10 murine melanoma model,
demonstrating the enhancement of the antitumor effect of this
agent. Administration of a high dose of anti-PD-1 NPs can develop
significantly higher mortality compared with administration of free
anti-PD-1 antibodies, due to the hyperexpression of T cells. By
contrast, administration of anti-PD-1 NPs to splenectomized mice
has produced a decreased mortality and showed the importance of
secondary lymphoid tissues in mediating the toxicity of anti-PD-1
antibodies. It has also been demonstrated that anti-PD-1 NPs
stimulate internalization by DCs in the spleen, followed by the
maturation and activation of T cells (99). (CTLA-4)-siRNA
(NPsiCTLA-4) is another platform biomaterial-based
delivering cytotoxic lymphocyte-associated molecule-4 employed in a
mouse model bearing B16 melanoma, the results of which showed an
increase of cell activation and proliferation of CD4+
and CD8+ T cells, following NPsiCTLA-4 in
vitro treatment (100).
Specific chemotherapeutics (such as oxaliplatin,
doxorubicin) have the competence to induce immunogenic cell death
(ICD) of cancer cells by inducing various signals that release ATP,
CXCL10, calreticulin (CALR) and high mobility group box 1 (HMGB1)
and stimulate the immune system. The combinatory therapy between
chemotherapy agents and check-point inhibitors is another paradigm
in cancer treatment. In a recent study, an anti-CTLA-4 was combined
with chemotherapy (liposomal doxorubicin) encapsulated in a
PEGylated liposome, in order to increase the efficiency of
treatment and decrease the SEs of anti-CTLA-4. In a B16 mouse
melanoma model, the liposomal anti-CTLA-4 produced a reduction in
the size of tumors and increased survival in comparison with
non-liposomal anti-CTLA-4(101).
Nano-immunostrategies to reprogram the
tumor microenvironment Targeting TME conditions
Tumor-infiltrating cytotoxic T lymphocytes have a
major role in controlling tumor development and it has been
observed that they retard their functions in an acidic tumor
microenvironment. Targeting tumor acidity is a promising concept
for the reversal of the anergic state of T cells and the
improvement of T cell-associated immunotherapy. A concept of RNAi
nanoparticles that reversed tumor acidity and rendered T cells for
enhancing the checkpoint blockade therapy functional was developed.
Following this concept, the in vivo use in melanoma tumor
models of an optimized vesicular cationic lipid-assisted
nanoparticle to mediate systematic blocking of lactate
dehydrogenase A (LDH A) in tumor cells has been reported. The
treatment was followed by reduction of lactate production,
neutralization of tumor pH and enhancing of infiltration with
CD8+ T and NK cells with the result in slowing down
tumor growth. The restored tumoral pH stimulated checkpoint
inhibition therapy using the antibody of PD-1(102).
Hypoxia is also a major component of the
tumor-suppressive microenvironment and it has been demonstrated to
have a negative regulatory effect on the activation of T cells. A
multifunctional immunoliposome was developed, known as
CAT@aPDL1-SSL, which contains modified aPDL1s on the surface for
improving the immunotherapeutic effects against the tumor and an
encapsulated catalase (CAT). It was suggested that the
CAT-encapsulated liposomes decreased tumor hypoxia through the
activity of CAT, which decomposes endogenous
H2O2 into O2. Furthermore, these
immunoliposomes promoted the infiltration of CD4+and
CD8+ T cells in tumor tissues and stimulate the blocking
of the PD-1/PD-L1 pathway (103).
Targeting cancer cells
Immunogenic cell death (ICD) is an umbrella term
containing some cell death modalities, including apoptosis,
necroptosis and immunogenic apoptosis. Generally, ICD is
represented by the production of damage-associated molecular
patterns (DAMPs), cytokines, chemokines, leading to the initiation
of enhanced anti-tumor immune responses. ICD can be induced by
radiotherapy, chemotherapy (e.g., oxaliplatin, cyclophosphamide),
magnetic fluid hyperthermia, photodynamic therapy or other stimuli.
New experimental data indicate that the immunogenicity of dying
cancer cells can be enhanced by the use of biomaterials, so-called
‘in situ tumor vaccines’ and constitute a new modality that
makes immunotherapy more efficient by combining with ICD-inducing
modalities (104). In the ICD
process, the translocation of calreticulin (CRT) is produced on the
cell surface and adenosine triphosphate (ATP), the HMGB1 protein
together with heat shock proteins (HSPs) are released into the
extracellular environment. The immune system reacts by activating
APCs and cytotoxic T cells, which eradicate tumors and metastases.
ATP recruit APCs by chemo-attraction, and CRT generates an ‘eat-me’
signal in order to stimulate the APCs to capture the dying tumor
cells and their debris. Concomitantly, HMGB-1 and HSPs enhance
antigen presentation to T cells (105). In addition, ICD induces the
release of pro-inflammatory cytokines such as TNF-α, IL-6, and
IL-1β converting an immunosuppressive TIME to an immunogenic TIME
(106). Antigen-capturing
nanoparticles (AC-NPs) were engineered to sequester TAAs and to
present them to APCs. It has been demonstrated that AC-NPs promoted
the proliferation of CD8+ and CD8+ cytotoxic
T cells, improving the efficacy of anti-PD-1 treatment on the
B16F10 melanoma model with up to a 20% cure rate compared to 0%
without AC-NPs (107). Another
study demonstrated the synergy between low-doses paclitaxel and a
toll-like receptor-7 (TLR-7) agonist-imiquimod administrated in a
co-delivery system for treatment of B16F10 melanoma (108). The researchers observed an
improved proliferation (250%) of DCs and secretion of
pro-inflammatory and Th1 cytokines with an inhibition of tumor
growth, eventually leading to 70% survival as compared to
individual components with 0% survival at day 41(108). Cationic copolymer aPBAE for
delivering CRISPR-Cas9 genome editing system was designed to retard
the PD-L1 expression on tumor cells in vivo. The expression
of PD-L1 on tumor cells was significantly attenuated by knocking
out cyclin-dependent kinase 5 (Cdk5), followed by effective tumor
growth inhibition in murine melanoma. It has been demonstrated that
aPBAE/Cas9-Cdk5 treatment stimulate strong T cell-mediated immune
responses in tumor microenvironment, thereby stimulating the
increasing of CD8 T cells and the decreasing of Tregs (109).
A biological platform for delivery of nanoparticles
comprising biodegradable materials that can genetically reprogram
cancer cells and their microenvironment in situ was also
designed. The reprogrammed cancer cells mimic tumor-associated
antigen-presenting cells (tAPCs) by inducing the expression of an
immunostimulatory cytokine (IL-12) and a costimulatory molecule
(4-1BBL). The nanoparticles combined with checkpoint blockade
significantly retarded tumor growth in B16-F10 melanoma model.
In vitro and in vivo analyses showed that
tAPC-reprogramming nanoparticles locally delivered produce an
enhanced cell-mediated cytotoxic immune response, with systemically
translated effects (110). In
another study, a plasmid DNA expressing small hairpin RNA of PD-L1
(shPD-L1) was loaded in dual-rebound nanoparticles (shPD-L1@NPs)
that were pH-dependent to silence the PD-L1 gene and decrease the
PD-L1/PD-1 interactions between T cells and tumors. Overexpressed
hyaluronic acid (HA) was degraded by Hyaluronidase (HAase) in the
extracellular matrix (ECM) of the tumor tissues to increase the
penetration of the shPD-L1-loaded nanoparticles in tumors. An
enhanced tumor inhibitory effect was reported by the combination
treatment of HAase and shPD-L1@NPs in a malignant melanoma mouse
tumor model (111).
Targeting the tumor immune
microenvironment Targeting antigen-presenting and dendritic
cells
In a study using the vaccination of mice with
melanoma B16 tumors with PLGA nanoparticles (NPs), which contained
encapsulated poorly immunogenic melanoma antigen,
tyrosinase-related protein 2 (TRP2) and Toll-like receptor (TLR)
ligand covered by TLR4 agonist (7-acyl-lipidA) were evaluated. It
has been shown that this vaccine can induce therapeutic anti-tumor
effect by interferon-gamma production in lymph nodes and spleens of
the vaccinated mice and an enhanced level of cytokines was
demonstrated compared to the control group (112). Another
poly(d,l-lactide-co-glycolide) nanoparticle (PLGA-NP) was designed
to deliver antigenic peptides to induce cytotoxic T-lymphocyte
responses against tumor-associated self-antigens in C57BL/6
melanoma mouse models. Vaccination with PLGA-NP carrying both
TRP2180-188 and monophosphoryl lipid A (a toll-like
receptor 4 agonist) slowed down the growth of subcutaneously
inoculated B16 melanoma cells. This anti-tumor potential of the
peptide-loaded DC vaccine was further enhanced when it was
administered in combination with IFN-γ to suppress tumor escape
(113).
Targeting tumor-associated
macrophages
Nanomedicines are first collected in tumors through
passive or active targeting mechanisms and are then involved in
local tumor immunosuppression mediated by MDSC, targeting
tumor-associated macrophages (TAM), and soluble inhibitors,
reducing the immunosuppression in the TIME with the increase of
infiltration, maturation, proliferation, survival, and activity of
effector immune cells. TAM is a major population of immune cells
with an M2-like phenotype in tumors, which have pro-tumoral
functions, reducing the infiltration of effector T cells (114,115). In a recent study, cyclodextrin
nanoparticles were designed that target a small molecule toll-like
receptor 7/8 agonist to macrophages from the TIME, stimulating M2
to M1 polarization and increasing the efficacy of
checkpoint-inhibiting immunotherapy in anti-PD-1 unresponsive
tumors (116). CaCO3
nanoparticles combined with anti-CD47 antibodies also increase the
macrophages polarization towards an M1 phenotype followed by
improving the outcome of checkpoint blockade therapy.
CaCO3 nanoparticles were locally administrated as
hydrogel during tumor surgery and an interaction between
CaCO3 and the protons in the TIME was demonstrated. The
embedded anti-CD47 antibodies have the function to block the ‘don't
eat me’ signal on tumor cells, increasing phagocytosis of cancer
cells by macrophages (117).
Tumor-targeted delivery systems can also increase
the antitumor efficacy of statins. A long-circulating liposome that
encapsulates simvastatin (LCL-SIM) was compared with free SIM in
B16.F10 murine melanoma-bearing mice as antitumor activity. It has
been previously demonstrated that B16.F10 melanoma growth was
strongly inhibited by LCL-SIM (by 85%), whereas free SIM has no
antitumor activity. The efficacy of LC-SIM was related with the
reduction of the TAM-mediated oxidative stress as well as of the
production of the hypoxia-inducible factor 1 α (HIF-1 α) in tumors,
concluding that the tumor-targeting property of the liposome
formulation is correlated with the presence of TAM in tumor tissue
(118).
Other designed carriers are M2-like TAM
dual-targeting nanoparticles (M2NPs). By loading anti-colony
stimulating factor-1 receptor (anti-CSF-1R) small-interfering RNA
(siRNA) on the M2NPs, a molecular-targeted immunotherapeutic
approach was designed that blocks the survival signal of M2-like
TAMs, reducing them from melanoma tumors. After administration to
tumor-bearing mice, a notable elimination of M2-like TAMs (52%) was
reported, with a tumor size decrease (87%) and prolonged survival.
In addition, M2NP-based siRNA delivery system inhibited the IL-10
and TGF-β production and increased the cytokine (IL-12 and IFN-γ)
expression and CD8+ T-cell infiltration in the TME and
retardation of the expression of PD-1 and Tim-3 on infiltrating
CD8+ T cells, restoring the T-cell immune function
(119).
Targeting indoleamine 2,3-dioxygenase
(IDO1)
Indolamine-2,3-dioxygenase 1 (IDO1) is a cytosolic
enzyme with a heme prosthetic group secreted by DCs that converts
tryptophan (Trp) from the tumor microenvironment to kynurenine
(Kyn). IDO1 is overexpressed in more than 50% of tumors that use
the mechanisms of IDO1 to enhance their spread and survival
(120). In the ‘elimination’
phase, IDO1 is produced at low levels within the TME and inhibits
tumor proliferation. During the degradation of IDO1, tolerogenic
dendritic cells (DCs) are converted into immunogenic cells
(121). In the ‘equilibrium’
phase, surviving tumor cells become ‘edited’ by the permanent
attack of the immune system and accumulate mutations. In the
‘escape’ phase, high IDO1 level is described, produced by tumor
cells and tolerogenic immune cells (DCs, MDSCs, TAMs). Trp
depletion and Kyn accumulation inhibit the effector T cell and NK
cell functions, switching DCs to and stimulating regulatory T cells
(122). Small molecules of IDO
inhibitors incorporated in nanomedicine formulations were tested in
preclinical and clinical trials (123).
A three-in-one immunotherapy nanoplatform involved
in the three phases of cancer immunity cycle (elimination,
equilibrium and escape) was reported. An aPD-L1@HC/PM NPs platform
(Ce6-conjugated hyaluronic acid, dextro-1-methyl
tryptophan-conjugated polylysine and aPD-L1) was designed against
tumor metastasis relapses and postsurgical regrowth. A bilateral
mouse tumor model of B16F10 melanoma was also developed to verify
the abscopal effect of aPD-L1@HC/PM NPs. Through the simultaneous
collaboration of the enhancing tumor antigen for DC maturation
followed by lymphocyte activation (elimination), the suppression of
the IDO pathway (equilibrium), and the blocking of the PD-1/PD-L1
pathway for supporting tumor elimination (escape), all three phases
of cancer immunity cycle were efficiently manipulated to enhance
the immune response and immune memory (88). Peptide-based nanoparticles were also
designed to promote a dual function: IDO inhibitor by blocking
tryptophan metabolism and antagonist of programmed cell
death-ligand 1 (PD-L1). This NP creates an environment, which
enhances the survival and activation of cytotoxic T lymphocytes and
effectively inhibits melanoma growth in mice by stimulating
anticancer immunity (124). A
synergistic immunotherapy strategy was also developed; it targets
the immunoinhibitory receptor programmed cell death protein 1 (PD1)
and immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) into
the TIME for the treatment of melanoma through an embedded
immunotherapeutic nanocapsule microneedle-based transcutaneous
delivery approach (125).
Targeting TGF-β
TGF-β, a pleiotropic cytokine, is a key signal
produced in the tumor microenvironment promoting tumor evasion from
the immune response. Transforming the signaling of growth factor-β
(TGF-β) is an important mechanism of immune suppression in the
tumor microenvironment, but systemic blockade of TGF-β signaling
pathway may induce multifocal inflammation, autoimmune disease and
significant cardiac toxicities in animal models (126). Current nanoparticle designs have
an inefficient accumulation in tumors after systemic administration
due to slow passage through vascular barriers in tumors and rapid
clearance of particles by the reticuloendothelial system. A
PEGylated liposomal form antibody-targeted of TGF-βI that inhibits
TGF-β signaling in primary T-cells was synthesized, maintaining
T-cell proliferation and cytotoxicity in B16F10 melanoma tumors.
The liposomal delivery of TGF-βI that targets an internalizing
receptor (CD90, or Thy1) was also compared with a TGF-βI that
targets non-internalizing receptor (CD45) and it was demonstrated
that T-cells pre-loaded ex vivo with liposomes that target
CD45-infiltrated tumors are more efficient (127). NPs coated with a T-cell membrane
(TCMNPs) that represents T-cell-mimicking nanoparticles were
developed. TCMNPs can eliminate tumors due to T-cell
membrane-derived proteins on TCMNPs. In addition, TCMNPs can
release anticancer drugs and stimulate the suppressed CTLs by
inhibition of TGF-β1 and PD-L1. In combination with dacarbazine,
TCMNP produced a higher reduction of tumor growth in a B16F10
melanoma model, increasing the percentages of CD8+
Granzyme B+ and CD8+ IFN-γ + T
cells in tumors. In current cancer immunotherapies, TCMNPs have
potential advantages of being cost-effective and less
time-consuming than adoptive T-cell transfer therapy, as they are
prepared from T-cell lines and synthetic polymers within 2 days
(128).
Targeting the peripheral immune
system
Immune compartments situated outside of tumors
represented by the peripheral immune system have aroused increased
interest in nanomedicine in recent years. The secondary lymphoid
organs, such as lymph nodes and the spleen are parts of the
peripheral immune system where antigen presentation and cytotoxic
T-cell generation occurs. These compartments are often affected in
terms of cancer occurrence and progression. Restoration of the
functions of the peripheral immune system can lead to potentiation
of antigen presentation by engineering T-cells (129). Findings of a previous study
(130) showed that the
administration of tumor-draining lymph nodes (TDLN)-targeting NPs,
which contain tumor-associated antigen TRP-2 or CpG oligonucleotide
to B16-F10 melanoma cancer model is followed by the induction of
strong cytotoxic lymphocyte (CTL) responses. It has been
demonstrated that this strategy could significantly slow down
immunosuppressive cells and enhance antitumor immune cells in TDLN.
The antigen-adjuvant combination in NPs could promote the delivery
to DCs from TLDN and induce anti-tumor T-cell responses. Generally,
oncolytic viruses mediate anti-tumor activity expressing a dual
mechanism of selective replication and lysis within infected cancer
cells and inducing host anti-tumor immunity. Talimogene
Laherparepvec (T-VEC) is a type I herpes simplex virus (HSV-1)
genetically modified which is preferentially replicated in tumor
cells and induces a systemic antitumor immunity capable of
eradicating tumor at a distance. T-VEC was engineered by deleting
the neurovirulence genes responsible for fever development and
deleting a viral gene that blocks antigen presentation. T-VEC was
further modified to enhance antigen presentation and T-cell priming
by deleting the ICP47 viral gene, human GM-CSF being incorporated
into the virus design. It was demonstrated that T-VEC selectively
replicates in tumor cells through oncogenic disruption of the PKR
pathway. Locally, T-VEC acts on an immunosuppressive tumor
microenvironment, producing the local release of interferons,
chemokines, pathogen-associated molecular pattern (PAMP) and
danger-associated molecular pattern (DAMP) factors. It has been
observed that Toll-like receptor agonists help reverse the
suppressed tumor milieu into a more pro-immunogenic environment
capable of enhancing anti-tumor immune responses. Local GM-CSF
expression promoted by T-VEC enhances migration and maturation of
dendritic cells, which form phagocyte soluble tumor antigens and
apoptotic tumor cells. The dendritic cells then migrate to regional
lymph nodes where they present antigens to specific CD4 C helper
and CD8 C cytotoxic T-cells, initiating a systemic immune response.
The T-VEC generates a higher immune response in injected tumor
compared to the response rate of distant metastases as compared to
distant metastasis, due to an inadequate effector T-cell expansion
and/or inability of circulating effectors to defeat the
immunosuppressive tumor microenvironment at distant sites. This is
the reason of combinatory therapy with TVEC and immune checkpoint
blockade, suggesting its efficacy in retarding the progression of
melanoma (131-133).
T-VEC is the first viral oncolytic immunotherapy,
FDA approved in 2015 for the local treatment of unresectable,
cutaneous, subcutaneous and nodal lesions in patients with melanoma
recurrent after initial surgery based on data from OPTiM, a
randomized phase III open-label trial. OPTiM trial comparing T-VEC
vs. GM-CSF have demonstrated a 4.4-month longer median overall
survival (OS) in patients receiving T-VEC than GM-CSF, with
estimated 5-year survival for the T-VEC arm of 33.4%, reaching
48.9% in patients with early metastatic melanoma (stage
IIIB-IVM1a), with an acceptable safety profile (134). T-VEC combined with check-point
inhibitors in melanoma has shown improved efficacy vs. CPIs alone.
A phase III trial of T-VEC/placebo plus pembrolizumab is underway
in unresectable stage IIIB-IVM1c melanoma (MASTERKEY 265;
NCT02263508) (135). Another phase
IB trial of ipilimumab C TVEC (NCT01740297) in 19 patients with
advanced melanoma showed tolerability of standard dose TVEC
combined with ipilimumab (136).
TVEC plus ipilimumab vs. ipilimumab were compared in a randomized,
open-label phase II trial in 198 patients with advanced melanoma
and an improved overall response rate (ORR) was demonstrated with
the combination (39 vs. 18%, P=0.002) (135). A phase II randomized trial on
resectable stage III B/C or IV melanoma in 150 patients treated
with immediate surgical resection versus 12 weeks of neoadjuvant
intratumoral TVEC followed by surgery (NCT02211131) was also
carried out. Most recently, results of the interim 1-year analysis
of recurrence-free survival results demonstrated that in the T-VEC
treatment group, a great percentage of patients remained
recurrence-free (33.5 vs. 21.9%, P=0.05) and overall survival after
1 year was higher in patients treated with T-VEC prior to surgery
(95.9 vs. 85.8%) (137-139).
7. Conclusions and future directions
Current immunotherapy for melanoma has reached a
limit of clinical responses. New methods are needed to increase the
effectiveness of the treatments. One of the major ways to improve
clinical responses is represented by nanomedicine modalities to
manipulate the immune responses. The major challenge for
nanomedicine-based immunotherapy remains the optimization of tumor
targeting, drug delivery vs. clearance and control of toxicity
(140-143).
In order to achieve a sustained and efficient
anti-tumor immune response, a controlled release of
immunostimulating substances, together with antineoplastic drugs
combined with specific targeting are needed. In addition to
classical well-known check-point inhibitors PD-1, PD-L1 and CRLA-4,
other potential molecular targets in immunomodulatory therapy of
melanoma can be represented by newly identified small-molecule
immune checkpoint co-stimulators (GITR, OX40 with their ligands),
inhibitors (VISTA, LAG-3, TIM-3, TIGIT) (143-145).
The immune system can also be modulated by small molecules that
enhance cellular immunity, such as IDO/TDO, STING agonists, TLR
agonists, GSK-3 inhibitors. The tumor microenvironment modulators
including CSF-1R inhibitors, TGF-β or CXCR antagonists and
epigenetic regulators of immune response as HDAC inhibitors, BET,
EZH2 inhibitors are also promising for the enhancement of
immunotherapy.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: Series E no. 0048).
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
CV supervised the study. CV, SV, SN, CRS, CCV
contributed equally to the conception and design of the study and
wrote the original draft. CL, DS, BMC, CS, CG, IA, CLU edited and
critically revised the manuscript, read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Munro N: Immunology and immunotherapy in
critical care: An overview. AACN Adv Crit Care. 30:113–125.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nishimura T, Iwakabe K, Sekimoto M, Ohmi
Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M and Ohta A:
Distinct role of antigen-specific T helper type 1 (Th1) and Th2
cells in tumor eradication in vivo. J Exp Med. 190:617–627.
1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: Crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9(212)2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mailliard RB, Egawa S, Cai Q, Kalinska A,
Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ and Kalinski P:
Complementary dendritic cell-activating function of CD8+
and CD4+ T cells: Helper role of CD8+ T cells
in the development of T helper type 1 responses. J Exp Med.
195:473–483. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Srivastava MK, Sinha P, Clements VK,
Rodriguez P and Ostrand-Rosenberg S: Myeloid derived suppressor
cells inhibit T-cell activation by depleting cystine and cysteine.
Cancer Res. 70:68–77. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumorassociated macrophages (TAM) as major players of
the cancerrelated inflammation. J Leukoc Biol. 86:1065–1073.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Franklin RA, Liao W, Sarkar A, Kim MV,
Bivona MR, Liu K, Pamer EG and Li MO: The cellular and molecular
origin of tumor-associated macrophages. Science. 344:921–925.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nielsen SR and Schmid MC: Macrophages as
key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017(9624760)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lesterhuis WJ, Haanen JB and Punt CJ:
Cancer immunotherapy-revisited. Nat Rev Drug Discov. 10:591–600.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Galluzzi L, Vacchelli E, Bravo-San Pedro
JM, Buqué A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado
JP, Agostinis P, et al: Classification of current anticancer
immunotherapies. Oncotarget. 5:12472–12508. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cobaleda-Siles M, Henriksen-Lacey M, Ruiz
de Angulo A, Bernecker A, Gómez Vallejo V, Szczupak B, Llop J,
Pastor G, Plaza-Garcia S, Jauregui-Osoro M, et al: An iron oxide
nanocarrier for dsRNA to target lymph nodes and strongly activate
cells of the immune system. Small. 10:5054–5067. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khalil D, Smith E, Brentjens R and Wolchok
JD: The future of cancer treatment: Immunomodulation, CARs and
combination immunotherapy. Nat Rev Clin Oncol. 13:273–290.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Michielin O, van Akkooi ACJ, Ascierto PA,
Dummer R and Keilholz U: ESMO Guidelines Committee: Electronic
address: simpleclinicalguidelines@esmo.org.
Cutaneous melanoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up†. Ann Oncol. 30:1884–1901.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rosalia RA, Cruz LJ, van Duikeren S, Tromp
AT, Silva AL, Jiskoot W, de Gruijl T, Löwik C, Oostendorp J, van
der Burg SH and Ossendorp F: CD40-targeted dendritic cell delivery
of PLGA-nanoparticle vaccines induce potent anti-tumor responses.
Biomaterials. 40:88–97. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yuba E, Yamaguchi A, Yoshizaki Y, Harada A
and Kono K: Bioactive polysaccharide-based pH-sensitive polymers
for cytoplasmic delivery of antigen and activation of
antigen-specific immunity. Biomaterials. 120:32–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Buonaguro L, Tagliamonte M, Tornesello ML
and Buonaguro FM: Developments in virus-like particle-based
vaccines for infectious diseases and cancer. Expert Rev Vaccines.
10:1569–1583. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hassan HA, Smyth L, Wang JT, Costa PM,
Ratnasothy K, Diebold SS, Lombardi G and Al-Jamal KT: Dual
stimulation of antigen presenting cells using carbon nanotube-based
vaccine delivery system for cancer immunotherapy. Biomaterials.
104:310–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wong HL, Rauth AM, Bendayan R, Manias JL,
Ramaswamy M, Liu ZS, Erhan SZ and Wu XY: A new polymer-lipid hybrid
nanoparticle system increases cytotoxicity of doxorubicin against
multidrug-resistant human breast cancer cells. Pharm Res.
23:1574–1585. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Waeckerle-Men Y and Groettrup M: PLGA
microspheres for improved antigen delivery to dendritic cells as
cellular vaccines. Adv Drug Deliv Rev. 57:475–482. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim H, Niu L, Larson P, Kucaba TA, Murphy
KA, James BR, Ferguson DM, Griffith TS and Panyam J: Polymeric
nanoparticles encapsulating novel TLR7/8 agonists as
immunostimulatory adjuvants for enhanced cancer immunotherapy.
Biomaterials. 164:38–53. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Heo MB and Lim YT: Programmed
nanoparticles for combined immunomodulation, antigen presentation
and tracking of immunotherapeutic cells. Biomaterials. 35:590–600.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kokate RA, Chaudhary P, Sun XL, Thamake
SI, Maji S, Chib R, Vishwanatha JK and Jones HP: Rationalizing the
use of functionalized poly-lactic-co-glycolic acid nanoparticles
for dendritic cell-based targeted anticancer therapy. Nanomedicine
(Lond). 11:479–494. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cruz LJ, Tacken PJ, Fokkink R, Joosten B,
Stuart MC, Albericio F, Torensma R and Figdor CG: Targeted PLGA
nano-but not microparticles specifically deliver antigen to human
dendritic cells via DC-SIGN in vitro. J Control Release.
144:118–126. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nanjwade BK, Bechra HM, Derkar GK, Manvi
FV and Nanjwade VK: Dendrimers: Emerging polymers for drug-delivery
systems. Eur J Pharm Sci. 38:185–196. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Torchilin VP: Recent advances with
liposomes as pharmaceutical carriers. Nat Rev Drug Discov.
4:145–160. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peng J, Xiao Y, Li W, Yang Q, Tan L, Jia
Y, Qu Y and Qian Z: Photosensitizer micelles together with IDO
inhibitor enhance cancer photothermal therapy and immunotherapy.
Adv Sci (Weinh). 5(1700891)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li H, Li Y, Wang X, Hou Y, Hong X, Gong T,
Zhang Z and Sun X: Rational design of polymeric hybrid micelles to
overcome lymphatic and intracellular delivery barriers in cancer
immunotherapy. Theranostics. 7:4383–4398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kong FY, Zhang JW, Li RF, Wang ZX, Wang WJ
and Wang W: Unique roles of gold nanoparticles in drug delivery,
targeting and imaging applications. Molecules.
22(1445)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kodiha M, Wang YM, Hutter E, Maysinger D
and Stochaj U: Off to the organelles-killing cancer cells with
targeted gold nanoparticles. Theranostics. 5:357–370.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lin AY, Almeida JP, Bear A, Liu N, Luo L,
Foster AE and Drezek RA: Gold nanoparticle delivery of modified CpG
stimulates macrophages and inhibits tumor growth for enhanced
immunotherapy. PLoS One. 8(e63550)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dykman LA, Staroverov SA, Fomin AS,
Khanadeev VA, Khlebtsov BN and Bogatyrev VA: Gold nanoparticles as
an adjuvant: Influence of size, shape, and technique of combination
with CpG on antibody production. Int Immunopharmacol. 54:163–168.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao Y, Zhao X, Cheng Y, Guo X and Yuan W:
Iron oxide nanoparticles-based vaccine delivery for cancer
treatment. Mol Pharm. 15:1791–1799. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Slowing II, Vivero-Escoto JL, Wu CW and
Lin VS: Mesoporous silica nanoparticles as controlled release drug
delivery and gene transfection carriers. Adv Drug Deliv Rev.
60:1278–1288. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nguyen TL, Choi Y and Kim J: Mesoporous
silica as a versatile platform for cancer immunotherapy. Adv Mater.
31(e1803953)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Croissant JG, Fatieiev Y and Khashab NM:
Degradability and clearance of silicon, organosilica,
silsesquioxane, silica mixed oxide, and mesoporous silica
nanoparticles. Adv Mater 29, 2017.
|
|
43
|
Vallhov H, Gabrielsson S, Strømme M,
Scheynius A and Garcia-Bennett AE: Mesoporous silica particles
induce size dependent effects on human dendritic cells. Nano Lett.
7:3576–3582. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kwon D, Cha BG, Cho Y, Min J, Park EB,
Kang SJ and Kim J: Extra-large pore mesoporous silica nanoparticles
for directing in vivo M2 Macrophage polarization by delivering
IL-4. Nano Lett. 17:2747–2756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guo HC, Feng XM, Sun SQ, Wei YQ, Sun DH,
Liu XT, Liu ZX, Luo JX and Yin H: Immunization of mice by hollow
mesoporous silica nanoparticles as carriers of porcine circovirus
type 2 ORF2 protein. Virol J. 9(108)2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang C, Xu L, Liang C, Xiang J, Peng R and
Liu Z: Immunological responses triggered by photothermal therapy
with carbon nanotubes in combination with anti-CTLA-4 therapy to
inhibit cancer metastasis. Adv Mater. 26:8154–8162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang H, Fu H, Wang B, Zhang X, Mao J, Li
X, Wang M, Sun Z, Qian H and Xu W: Exosomal miR-423-5p targets SUFU
to promote cancer growth and metastasis and serves as a novel
marker for gastric cancer. Mol Carcinog. 57:1223–1236.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lizotte PH, Wen AM, Sheen MR, Fields J,
Rojanasopondist P, Steinmetz NF and Fiering S: In situ vaccination
with cowpea mosaic virus nanoparticles suppresses metastatic
cancer. Nat Nanotechnol. 11:295–303. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Guo ZS, Liu Z and Bartlett DL: Oncolytic
immunotherapy: Dying the right way is a key to eliciting potent
antitumor immunity. Front Oncol. 4(74)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fu LQ, Wang SB, Cai MH, Wang XJ, Chen JY,
Tong XM, Chen XY and Mou XZ: Recent advances in oncolytic
virus-based cancer therapy. Virus Res. 270(197675)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Round JL and Mazmanian SK: Inducible
Foxp3+ regulatory T-cell development by a commensal
bacterium of the intestinal microbiota. Proc Natl Acad Sci USA.
107:12204–12209. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cebula A, Seweryn M, Rempala GA, Pabla SS,
McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P and Ignatowicz L:
Thymus-derived regulatory T cells contribute to tolerance to
commensal microbiota. Nature. 497:258–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ivanov II, Atarashi K, Manel N, Brodie EL,
Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al:
Induction of intestinal Th17 cells by segmented filamentous
bacteria. Cell. 139:485–498. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gaboriau-Routhiau V, Rakotobe S, Lécuyer
E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M,
Brandi G, et al: The key role of segmented filamentous bacteria in
the coordinated maturation of gut helper T cell responses.
Immunity. 31:677–689. 2009.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tomkovich S and Jobin C: Microbiota and
host immune responses: A love-hate relationship. Immunology.
147:1–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103.
2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes
antitumor immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Matsumoto K, Yamamoto T, Kamata R and
Maeda H: Pathogenesis of serratial infection: Activation of the
Hageman factor-prekallikrein cascade by serratial protease. J
Biochem. 96:739–749. 1984.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Maeda H: Tumor-selective delivery of
macromolecular drugs via the EPR effect: Background and future
prospects. Bioconjug Chem. 21:797–802. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhao M, Yang M, Li XM, Jiang P, Baranov E,
Li S, Xu M, Penman S and Hoffman RM: Tumor-targeting bacterial
therapy with amino acid auxotrophs of GFP-expressing Salmonella
typhimurium. Proc Natl Acad Sci USA. 102:755–760. 2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Marshall JS, Green AM, Pensky J, Williams
S, Zinn A and Carlson DM: Measurement of circulating desialylated
glycoproteins and correlation with hepatocellular damage. J Clin
Invest. 54:555–562. 1974.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Campbell RB, Fukumura D, Brown EB, Mazzola
LM, Izumi Y, Jain RK, Torchilin VP and Munn LL: Cationic charge
determines the distribution of liposomes between the vascular and
extravascular compartments of tumors. Cancer Res. 62:6831–6836.
2002.PubMed/NCBI
|
|
65
|
Matsumura Y, Hamaguchi T, Ura T, Muro K,
Yamada Y, Shimada Y, Shirao K, Okusaka T, Ueno H, Ikeda M and
Watanabe N: Phase I clinical trial and pharmacokinetic evaluation
of NK911, a micelle-encapsulated doxorubicin. Br J Cancer.
91:1775–1781. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Park J, Choi Y, Chang H, Um W, Ryu JH and
Kwon IC: Alliance with EPR effect: Combined strategies to improve
the EPR effect in the tumor microenvironment. Theranostics.
9:8073–8090. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Franqui LS, De Farias MA, Portugal RV,
Costa CAR, Domingues RR, Souza Filho AG, Coluci VR, Leme AFP and
Martinez DST: Interaction of graphene oxide with cell culture
medium: Evaluating the fetal bovine serum protein corona formation
towards in vitro nanotoxicity assessment and nanobiointeractions.
Mater Sci Eng C Mater Biol Appl. 100:363–377. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Glancy D, Zhang Y, Wu JLY, Ouyang B, Ohta
S and Chan WCW: Characterizing the protein corona of sub-10 nm
nanoparticles. J Control Release. 304:102–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lai W, Wang Q, Li L, Hu Z, Chen J and Fang
Q: Interaction of gold and silver nanoparticles with human plasma:
Analysis of protein corona reveals specific binding patterns.
Colloids Surf B Biointerfaces. 152:317–325. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Giulimondi F, Digiacomo L, Pozzi D,
Palchetti S, Vulpis E, Capriotti AL, Chiozzi RZ, Laganà A,
Amenitsch H, Masuelli L, et al: Interplay of protein corona and
immune cells controls blood residency of liposomes. Nat Commun.
11(1697)2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Partikel K, Korte R, Stein NC, Mulac D,
Herrmann FC, Humpf HU and Langer K: Effect of nanoparticle size and
PEGylation on the protein corona of PLGA nanoparticles. Eur J Pharm
Biopharm. 141:70–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ekdahl KN, Fromell K, Mohlin C, Teramura Y
and Nilsson B: A human whole-blood model to study the activation of
innate immunity system triggered by nanoparticles as a demonstrator
for toxicity. Sci Technol Adv Mater. 20:688–698. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Gossmann R, Fahrländer E, Hummel M, Mulac
D, Brockmeyer J and Langer K: Comparative examination of adsorption
of serum proteins on HSA- and PLGA-based nanoparticles using
SDS-PAGE and LC-MS. Eur J Pharm Biopharm. 93:80–87. 2015.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kah JC, Wong KY, Neoh KG, Song JH, Fu JW,
Mhaisalkar S, Olivo M and Sheppard CJ: Critical parameters in the
pegylation of gold nanoshells for biomedical applications: An in
vitro macrophage study. J Drug Target. 17:181–193. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fleischer CC and Payne CK: Secondary
structure of corona proteins determines the cell surface receptors
used by nanoparticles. J Phys Chem B. 118:14017–14026.
2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Mortimer GM, Butcher NJ, Musumeci AW, Deng
ZJ, Martin DJ and Minchin RF: Cryptic epitopes of albumin determine
mononuclear phagocyte system clearance of nanomaterials. ACS Nano.
8:3357–3366. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lartigue L, Wilhelm C, Servais J, Factor
C, Dencausse A, Bacri JC, Luciani N and Gazeau F: Nanomagnetic
sensing of blood plasma protein interactions with iron oxide
nanoparticles: Impact on macrophage uptake. ACS Nano. 6:2665–2678.
2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Barbero F, Russo L, Vitali M, Piella J,
Salvo I, Borrajo ML, Busquets-Fité M, Grandori M, Bastús NG, Casals
E and Puntes V: Formation of the protein corona: The interface
between nanoparticles and the immune system. Semin Immunol.
34:52–60. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Xu J, Wang H, Xu L, Chao Y, Wang C, Han X,
Dong Z, Chang H, Peng R, Cheng Y and Liu Z: Nanovaccine based on a
protein-delivering dendrimer for effective antigen
cross-presentation and cancer immunotherapy. Biomaterials. 207:1–9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li L, Goedegebuure SP and Gillanders WE:
Preclinical and clinical development of neoantigen vaccines. Ann
Oncol. 28 (Suppl 12):xii11–xii17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Gubin MM, Zhang X, Schuster H, Caron E,
Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et
al: Checkpoint blockade cancer immunotherapy targets
tumour-specificmutant antigens. Nature. 515:577–581.
2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sahin U, Derhovanessian E, Miller M, Kloke
BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B,
et al: Personalized RNA mutanome vaccines mobilize poly-specific
therapeutic immunity against cancer. Nature. 547:222–226.
2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Yadav M, Jhunjhunwala S, Phung QT,
Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J,
Weinschenk T, et al: Predicting immunogenic tumour mutations by
combining mass spectrometry and exome sequencing. Nature.
515:572–576. 2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Stone JD, Harris DT and Kranz DM: TCR
affinity for p/MHC formed by tumor antigens that are self-proteins:
Impact on efficacy and toxicity. Curr Opin Immunol. 33:16–22.
2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Atif SM, Gibbings SL, Redente EF, Camp FA,
Torres RM, Kedl RM, Henson PM and Claudia V: Immune surveillance by
natural IgM is required for early neoantigen recognition and
initiation of adaptive immunity. Am J Respir Cell Mol Biol.
59:580–591. 2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Li Q, Zhang D, Zhang J, Jiang Y, Song A,
Li Z and Luan Y: A three-in-one immunotherapy nanoweapon via
cascade-amplifying cancer-immunity cycle against tumor metastasis,
relapse, and postsurgical regrowth. Nano Lett. 19:6647–6657.
2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Nonomura C, Otsuka M, Kondou R, Iizuka A,
Miyata H, Ashizawa T, Sakura N, Yoshikawa S, Kiyohara Y, Ohshima K,
et al: Identification of a neoantigen epitope in a melanoma patient
with good response to anti-PD-1 antibody therapy. Immunol Lett.
208:52–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kreiter S, Selmi A, Diken M, Koslowski M,
Britten CM, Huber C, Türeci O and Sahin U: Intranodal vaccination
with naked antigen-encoding RNA elicits potent prophylactic and
therapeutic antitumoral immunity. Cancer Res. 70:9031–9040.
2010.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur
V, Stevanović S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V,
van der Burg SH, et al: Actively personalized vaccination trial for
newly diagnosed glioblastoma. Nature. 565:240–245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Sahin U, Oehm P, Derhovanessian E,
Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D,
Kuhn AN, Omokoko T, et al: An RNA vaccine drives immunity in
checkpoint-inhibitor-treated melanoma. Nature. 585:107–112.
2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Robbins PF, Morgan RA, Feldman SA, Yang
JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ,
Mackall CL, et al: Tumor regression in patients with metastatic
synovial cell sarcoma and melanoma using genetically engineered
lymphocytes reactive with NY-ESO-1. J Clin Oncol. 29:917–924.
2011.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Bencherif SA, Warren Sands R, Ali OA, Li
WA, Lewin SA, Braschler TM, Shih TY, Verbeke CS, Bhatta D, Dranoff
G and Mooney DJ: Injectable cryogel-based whole-cell cancer
vaccines. Nat Commun. 6(7556)2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Hassani Najafabadi A, Zhang J, Aikins ME,
Najaf Abadi ZI, Liao F, Qin Y, Okeke EB, Scheetz LM, Nam J, Xu Y,
et al: Cancer immunotherapy via targeting cancer stem cells using
vaccine nanodiscs. Nano Lett. 20:7783–7792. 2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zheng F, Dang J, Zhang H, Xu F, Ba F,
Zhang B, Cheng F, Chang AE, Wicha MS and Li Q: Cancer stem cell
vaccination with PD-L1 and CTLA-4 blockades enhances the
eradication of melanoma stem cells in a mouse tumor model. J
Immunother. 41:361–368. 2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Aggarwal S: Adverse effects of
immuno-oncology drugs-Awareness, diagnosis, and management: A
literature review of immune-mediated adverse events. Indian J
Cancer. 56 (Suppl):S10–S22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Li Y, Fang M, Zhang J, Wang J, Song Y, Shi
J, Li W, Wu G, Ren J, Wang Z, et al: Hydrogel dual delivered
celecoxib and anti-PD-1 synergistically improve antitumor immunity.
Oncoimmunology. 5(e1074374)2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang C, Ye Y, Hochu GM, Sadeghifar H and
Gu Z: Enhanced cancer immunotherapy by microneedle patch-assisted
delivery of anti-PD1 antibody. Nano Lett. 16:2334–2340.
2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Ordikhani F, Uehara M, Kasinath V, Dai L,
Eskandari SK, Bahmani B, Yonar M, Azzi JR, Haik Y, Sage PT, et al:
Targeting antigen-presenting cells by anti-PD-1 nanoparticles
augments antitumor immunity. JCI Insight. 3(e122700)2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Li SY, Liu Y, Xu CF, Shen S, Sun R, Du XJ,
Xia JX, Zhu YH and Wang J: Restoring anti-tumor functions of T
cells via nanoparticle-mediated immune checkpoint modulation. J
Control Release. 231:17–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Alimohammadi R, Alibeigi R, Nikpoor AR,
Chalbatani GM, Webster TJ, Jaafari MR and Jalali SA: Encapsulated
checkpoint blocker before chemotherapy: The optimal sequence of
anti-CTLA-4 and doxil combination therapy. Int J Nanomedicine.
15:5279–5288. 2020.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zhang YX, Zhao YY, Shen J, Sun X, Liu Y,
Liu H and Wang Y and Wang Y: Nanoenabled modulation of acidic tumor
microenvironment reverses anergy of infiltrating T cells and
potentiates anti-PD-1 therapy. Nano Lett. 19:2774–2783.
2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Hei Y, Teng B, Zeng Z, Zhang S, Li Q, Pan
J, Luo Z, Xiong C and Wei S: Multifunctional immunoliposomes
combining catalase and PD-L1 antibodies overcome tumor hypoxia and
enhance immunotherapeutic effects against melanoma. Int J
Nanomedicine. 15:1677–1691. 2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Mishchenko T, Mitroshina E, Balalaeva I,
Krysko O, Vedunova M and Krysko DV: An emerging role for
nanomaterials in increasing immunogenicity of cancer cell death.
Biochim Biophys Acta Rev Cancer. 1871:99–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Obeid M, Panaretakis T, Joza N, Tufi R,
Tesniere A, van Endert P, Zitvogel L and Kroemer G: Calreticulin
exposure is required for the immunogenicity of gamma-irradiation
and UVC light-induced apoptosis. Cell Death Differ. 14:1848–1850.
2007.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Garg AD, Martin S, Golab J and Agostinis
P: Danger signalling during cancer cell death: Origins, plasticity
and regulation. Cell Death Differ. 21:26–38. 2014.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Min Y, Roche KC, Tian S, Eblan MJ,
McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, et
al: Antigen-capturing nanoparticles improve the abscopal effect and
cancer immunotherapy. Nat Nanotechnol. 12:877–882. 2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Seth A, Heo MB and Lim YT: Poly
(γ-glutamic acid) based combination of water-insoluble paclitaxel
and TLR7 agonist for chemo-immunotherapy. Biomaterials.
35:7992–8001. 2014.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Deng H, Tan S, Gao X, Zou C, Xu C, Tu K,
Song Q, Fan F, Huang W and Zhang Z: Cdk5 knocking out mediated by
CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced
antitumor immunity. Acta Pharm Sin B. 10:358–373. 2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Tzeng SY, Patel KK, Wilson DR, Meyer RA,
Rhodes KR and Green JJ: In situ genetic engineering of tumors for
long-lasting and systemic immunotherapy. Proc Natl Acad Sci USA.
117:4043–4052. 2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Guan X, Lin L, Chen J, Hu Y, Sun P, Tian
H, Maruyama A and Chen X: Efficient PD-L1 gene silence promoted by
hyaluronidase for cancer immunotherapy. J Control Release.
293:104–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Hamdy S, Molavi O, Ma Z, Haddadi A,
Alshamsan A, Gobti Z, Elhasi S, Samuel J and Lavasanifar A:
Co-delivery of cancer-associated antigen and Toll-like receptor 4
ligand in PLGA nanoparticles induces potent CD8+ T
cell-mediated anti-tumor immunity. Vaccine. 26:5046–5057.
2008.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Zhang Z, Tongchusak S, Mizukami Y, Kang
YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL and Sasada
T: Induction of anti-tumor cytotoxic T cell responses through
PLGA-nanoparticle mediated antigen delivery. Biomaterials.
32:3666–3678. 2011.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Peranzoni E, Lemoine J, Vimeux L, Feuillet
V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J,
Ouakrim H, et al: Macrophages impede CD8 T cells from reaching
tumor cells and limit the efficacy of anti-PD-1 treatment. Proc
Natl Acad Sci USA. 115:E4041–E4050. 2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Prendergast GC, Malachowski WP, DuHadaway
JB and Muller AJ: Discovery of IDO1 inhibitors: From bench to
bedside. Cancer Res. 77:6795–6811. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Rodell CB, Arlauckas SP, Cuccarese MF,
Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ and Weissleder R:
TLR7/8-agonist-loaded nanoparticles promote the polarization of
tumour-associated macrophages to enhance cancer immunotherapy. Nat
Biomed Eng. 2:578–588. 2018.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li
H, Wang J, Wen D, Zhang Y, Lu Y, et al: In situ sprayed
bioresponsive immunotherapeutic gel for post-surgical cancer
treatment. Nat Nanotechnol. 14:89–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Alupei MC, Licarete E, Patras L and Banciu
M: Liposomal simvastatin inhibits tumor growth via targeting
tumor-associated macrophages-mediated oxidative stress. Cancer
Lett. 356:946–952. 2015.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L,
Yu X, Luo Q and Zhang Z: Molecular-targeted immunotherapeutic
strategy for melanoma via dual-targeting nanoparticles delivering
small interfering RNA to tumor-associated macrophages. ACS Nano.
11:9536–9549. 2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Hornyák L, Dobos N, Koncz G, Karányi Z,
Páll D, Szabó Z, Halmos G and Székvölgyi L: The role of
indoleamine-2,3-dioxygenase in cancer development, diagnostics, and
therapy. Front Immunol. 9(151)2018.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Orabona C, Pallotta MT, Volpi C, Fallarino
F, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Grohmann U and
Puccetti P: SOCS3 drives proteasomal degradation of indoleamine
2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis.
Proc Natl Acad Sci USA. 105:20828–20833. 2008.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Shou D, Liang W, Song Z, Yin J, Sun Q and
Gong W: Suppressive role of myeloid-derived suppressor cells
(MDSCs) in the microenvironment of breast cancer and targeted
immunotherapies. Oncotarget. 7:64505–64511. 2016.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li
TJ and Zheng L: Activated CD69+ T cells foster immune
privilege by regulating IDO expression in tumor-associated
macrophages. J Immunol. 188:1117–1124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Cheng K, Ding Y, Zhao Y, Ye S, Zhao X,
Zhang Y, Ji T, Wu H, Wang B, Anderson GJ, et al: Sequentially
responsive therapeutic peptide assembling nanoparticles for
dual-targeted cancer immunotherapy. Nano Lett. 18:3250–3258.
2018.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang
C and Gu Z: Synergistic transcutaneous immunotherapy enhances
antitumor immune responses through delivery of checkpoint
inhibitors. ACS Nano. 10:8956–8963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Aoki CA, Borchers AT, Li M, Flavell RA,
Bowlus CL, Ansari AA and Gershwin ME: Transforming growth factor
beta (TGF-beta) and autoimmunity. Autoimmun Rev. 4:450–459.
2005.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Zheng Y, Tang L, Mabardi L, Kumari S and
Irvine DJ: Enhancing adoptive cell therapy of cancer through
targeted delivery of small-molecule immunomodulators to
internalizing or noninternalizing receptors. ACS Nano.
11:3089–3100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Kang M, Hong J, Jung M, Kwon SP, Song SY,
Kim HY, Lee JR, Kang S, Han J, Koo JH, et al: T-cell-mimicking
nanoparticles for cancer immunotherapy. Adv Mater.
32(e2003368)2020.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Shi Y and Lammers T: Combining
nanomedicine and immunotherapy. Acc Chem Res. 52:1543–1554.
2019.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Xu Z, Wang Y, Zhang L and Huang L:
Nanoparticle-delivered transforming growth factor-β siRNA enhances
vaccination against advanced melanoma by modifying tumor
microenvironment. ACS Nano. 8:3636–3645. 2014.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Kohlhapp FJ and Kaufman HL: Molecular
pathways: Mechanism of action for talimogene laherparepvec, a new
oncolytic virus immunotherapy. Clin Cancer Res. 22:1048–1054.
2016.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Ott PA and Hodi FS: Talimogene
laherparepvec for the treatment of advanced melanoma. Clin Cancer
Res. 22:3127–3131. 2016.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Conry RM, Westbrook B, McKee S and Norwood
TG: Talimogene laherparepvec: First in class oncolytic virotherapy.
Hum Vaccin Immunother. 14:839–846. 2018.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Andtbacka RHI, Collichio F, Harrington KJ,
Middleton MR, Downey G, Ӧhrling K and Kaufman HL: Final analyses of
OPTiM: A randomized phase III trial of talimogene laherparepvec
versus granulocyte-macrophage colony-stimulating factor in
unresectable stage III-IV melanoma. J Immunother Cancer.
7(145)2019.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Long GV, Dummer R, Ribas A, Puzanov I,
Michielin O, Vanderwalde AM, Andtbacka RHI, Cebon J, Fernandez E,
Malvehy J, et al: A phase I/III, multicenter, open-label trial of
talimogene laherparepvec (T-VEC) in combination with pembrolizumab
for the treatment of unresected, stage IIIb-IV melanoma
(MASTERKEY-265). J Immunother Cancer. 3 (Suppl 2)(P181)2015.
|
|
136
|
Puzanov I, Milhem MM, Minor D, Hamid O, Li
A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, et al:
Talimogene laherparepvec in combination with ipilimumab in
previously untreated, unresectable stage IIIB-IV melanoma. J Clin
Oncol. 34:2619–2626. 2016.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Dummer R, Gyorki DE, Hyngstrom JR, Berger
AC, Conry RM, Demidov LV, Sharma A, Treichel S, Faries MB and Ross
MI: One-year (yr) recurrence-free survival (RFS) from a randomized,
open label phase II study of neoadjuvant (neo) talimogene
laherparepvec (T-VEC) plus surgery (surgx) versus surgx for
resectable stage IIIB-IVM1a melanoma (MEL). J Clin Oncol. 37 (Suppl
15)(S9520)2019.
|
|
139
|
Trager MH, Geskin LJ and Saenger YM:
Oncolytic viruses for the treatment of metastatic melanoma. Curr
Treat Options Oncol. 21(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Ancuceanu R, Dinu M, Neaga I, Laszlo FG
and Boda D: Development of QSAR machine learning-based models to
forecast the effect of substances on malignant melanoma cells.
Oncol Lett. 17:4188–4196. 2019.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Ion A, Popa IM, Papagheorghe LML,
Lisievici C, Lupu M, Voiculescu V, Caruntu C and Boda D: Proteomic
approaches to biomarker discovery in cutaneous T-cell lymphoma. Dis
Markers. 2016(9602472)2016.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Boda D, Negrei C, Arsene AL, Caruntu C,
Lupuleasa D and Ion RM: Spectral and photochemical properties of
hyperbranched nanostructures based on gardiquimod and TPPS4.
Farmacia. 63:218–223. 2015.
|
|
143
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013.
|
|
144
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016.PubMed/NCBI View Article : Google Scholar
|















