1. Introduction
The kidney is responsible for multiple vital
functions in the body. It regulates blood volume and pressure,
reabsorbs nutrients, excretes wastes, and secretes hormones
(1,2). Thyroid hormones are involved in renal
development, kidney hemodynamics, glomerular filtration rate (GFR),
sodium, and water homeostasis. Hypothyroidism and hyperthyroidism
affect renal function (3). Acute
kidney injury (AKI), chronic kidney disease (CKD), end stage renal
disease (ESRD), kidney stones and kidney cancer-renal cell
carcinoma, represent major kidney pathologies (3-6).
AKI and CKD are associated with an increased risk of morbidity and
mortality. AKI is a rapid and reversible decline in renal function
and evolves rapidly to CKD (7).
This acute renal injury is diagnosed based on an increase in the
serum creatinine level of above ≥0.3 mg/dl (≥26.5 µmol/l) within 48
h; an increase in serum creatinine to ≥1.5 times baseline; or urine
volume <0.5 ml/kg/h for 6 h (8).
Drugs (including non-steroidal anti-inflammatory compounds),
toxins, diuretics, sepsis, age, genetic factors, race, diabetes
mellitus (DM), and hypertension are risk factors for AKI, which may
lead to reduced GFR and further to acute tubular cell destruction
(9). Worldwide, CKD is an urgent
medical issue, with a rapidly increasing incidence particularly in
diabetic and hypertensive patients, predisposing these patients to
diabetic nephropathy, hypertensive nephrosclerosis and focal and
segmental glomerulosclerosis (10-14).
Anemia, dyslipidemia, malnutrition, mineral and bone disorders, are
the most common complications in CKD patients (15).
The presence of albuminuria or estimated GFR (eGFR)
from serum creatinine <60 ml/min/1.73 m2, is the main
diagnostic criterion for CKD (16).
According to the Kidney Disease Quality Outcome Initiative
(K/DOQI), CKD is defined as kidney damage or GFR less than 60
ml/min/1.73 m2 for 3 months or more, irrespective of the
cause (17). GFR is measured from
calibrated serum creatinine and estimating equations, such as the
Modification of Diet in Renal Disease (MDRD) study equation or the
Cockcroft-Gault formula (17).
Albuminuria is the most commonly used marker to
reflect kidney damage, and elevated levels are associated with an
increased risk of CKD and ESRD, independent of eGFR. Based on
severity, albuminuria is classified as A1 (albuminuria <30
mg/g-optimal or normal), A2 (albuminuria 30-300 mg/g-high) and A3
(albuminuria >300 mg/g-very high) (18).
Previous studies have previously described CKD
stages (18-21).
CKD is an independent risk factor for many systemic disorders such
as, angina, acute myocardial infarction, heart failure, stroke,
peripheral vascular disease, and arrhythmias (13). According to the World Health
Organization (WHO), obesity is considered a disease (22). In the Western world, obesity rates
are increasing rapidly, which mirrors the increase in comorbidities
such as cancer, cardiovascular diseases, diabetes, and CKD
(22). Obesity is involved in the
progression of CKD in two ways: Indirectly through DM and
hypertension or directly through adipose tissue (13,22-26).
According to the WHO, the definition of obesity is based on body
mass index (BMI) as follows: Underweight (<18.5
kg/m2), normal weight (18.5-25 kg/m2),
overweight (25-30 kg/m2) and obese (>30
kg/m2), obese class I (30.0-34.9 kg/m2),
obese class II (35-39.9 kg/m2) and obese class III
(>40 kg/m2) (27).
Between 1975 and 2016, the worldwide incidence of obesity was found
to triple, with a high prevalence of overweight individuals (over
30%) and obesity (over 10%) (27).
Moreover, childhood obesity is currently increasing worldwide (27,
28). In Europe, one child out of 3 is overweight or obese, and over
60% of them will be overweight before puberty or overweight in
early adulthood (27,28).
Initially, adipose tissue was considered a passive
reservoir for energy storage, involved in mechanical and heat
insulation, involved in thermogenesis regulation. However, adipose
tissue secretes various bioactive peptides, called ‘adipokines’,
which are involved in both autocrine/paracrine and endocrine
activity (29). Thus, adipose
tissue may directly affect the kidney through its endocrine
activity via the production of adiponectin, leptin, and other
adipokines (22).
2. Obesity and CKD
Increased body weight is associated with lower urine
pH, increased urinary oxalate and the excretion of sodium,
phosphate, and uric acid (30-32).
Obesity is also involved in nephrolithiasis pathogenesis (33,34).
Studies have revealed that individuals presenting
with no kidney disease but with higher BMI, develop proteinuria
(22,35). Patients who do not present with DM
and hypertension, but who present with juvenile obesity, may
present a 3-fold increased risk of CKD (36). Fox et al published the
results of a study over a 19-year period involving 2,585
individuals. The authors observed that BMI predicted new onset
kidney diseases (37). In patients
with pre-existing CKD, it was also observed that increased levels
of BMI led to a rapid progression of CKD (22). In obesity, the kidneys are
compressed by increased visceral and retroperitoneal fat, which may
increase blood pressure. Moreover, excess fat accumulation in and
around the kidneys conduces to increased intra-renal pressure,
impaired pressure natriuresis, and hypertension (38). In patients with visceral obesity,
intra-abdominal pressure rises to 35-40 mmHg, in proportion to
sagittal abdominal diameter, which leads to compression of the
renal veins, lymph vessels, ureters and renal parenchyma. Increased
sagittal abdominal diameter is associated with increased
intra-abdominal pressure which leads to obesity-related
comorbidity, such as type II diabetes, hypertension, and CKD
(39).
Previous studies performed on obese dogs, rabbits
and even humans have demonstrated that retroperitoneal fat
encapsulates the kidneys, adheres tightly to the renal capsule, and
invades the renal sinuses, causing additional compression and
increasing intra-renal pressure (40,41).
Animals fed a hypercaloric diet, such as rabbits, accumulate fat in
the renal sinuses, followed by the distortion and prolapse of the
renal medullary ducts of Bellini, and urinary outflow restriction
(42,43). In obese adults, a higher amount of
retroperitoneal and renal sinus fat is associated with
hypertension. Moreover, higher BMI seems to be correlated with a
decrease in GFR, which will conduce over time to a rapid loss of
GFR, and an increased incidence of ESRD (44). In obese adults, central obesity is
associated with a 70% increased risk of microalbuminuria when
compared with lean adults (45).
Obesity can alter renal hemodynamics through two
pathogenic mechanisms: Glomerular hyperperfusion and
hyperfiltration (46,47). To describe glomerular hyperperfusion
and hyperfiltration, Henegar et al conducted a study on
non-obese dogs fed a high-fat diet for 7-9 or 24 weeks. The study
reported increased blood pressure, pulse rate, GFR and renal plasma
flow in the obese vs. the lean dogs. Increased plasma levels of
insulin and renin were detected in dogs treated with the fat diet,
compared with the control group. The histological analysis revealed
enlarged Bowman's space, increased glomerular cell proliferation,
increased mesangial expression, thickening of the basement
membranes and even higher expression for renal transforming growth
factor (TFG)-β. Interestingly, the study did not find any
association between the glomerulosclerosis score of the obese dogs
and the lean dogs. Acute obesity did not conduce to renal scarring,
instead chronic obesity was involved in renal scarring (46). Chronic obesity contributes to CKD
progression by glomerular hyperfiltration,
microalbuminuria/proteinuria development, hypofiltration and
decreased GFR (48-50).
Ectopic lipid accumulation, together with renal
sinus fat accumulation, may lead to glomerular hypertension
development and increased glomerular permeability, caused by the
hyperfiltration associated with glomerular filtration barrier
damage, leading to glomerulomegaly and focal or segmental
glomerulosclerosis (51-54).
Glomerular hyperfiltration, glomerular hypertrophy
and increased filtration fraction promote proteinuria and
glomerulosclerosis, processes that further stimulate the renin
angiotensin-aldosterone system (RAAS), release of TGF-β, a cascade
of events that cause kidney damage (55).
Adipose tissue expresses all RAAS components;
angiotensin receptors 1 and 2 are present in human and animal
adipocytes (56,57). Sodium retention and aldosterone
secretion are normal RAAS functions, but in adipose tissue it may
be involved in obesity-related hypertension. Moreover, adipocytes
from inflamed visceral and perivascular tissues are involved in
RAAS activation (58). In obese
individuals, increased levels of angiotensin II, directly
contribute to the increase in oxidant stress (OS) at the vascular
level (59). Boustany et al
conducted a study on rats fed a high-fat diet for 11 weeks to
induce obesity. The results of the study revealed higher systemic
blood pressure, increased levels of the plasmatic angiotensin and
angiotensin gene expression in the retroperitoneal adipose tissue
(60).
In patients with metabolic syndrome, which is
characterized by the presence of hypertension, dyslipidemia and
insulin resistance, angiotensin II presents higher levels (61). Adipocytes may contribute up to 30%
to the production of circulating angiotensinogen II (58). Increased angiotensin II levels
affect renal hemodynamic, contributing to hyperfiltration,
glomerulomegaly, and further focal glomerulosclerosis via afferent
arteriolar dilation. Moreover, afferent renal arteriolar
vasoconstriction, angiotensin II with endocrine and paracrine
properties, links the intrarenal and the systemic RAAS. Adipose
tissue dysfunction, together with insulin resistance and
hypertension contributes to CKD development and eventually to ESRD
(61). In vascular tissue,
aldosterone may induce mineralocorticoid receptor activation,
promoting vascular stiffness caused by oxidative stress (OS)
(62), inflammation (63), maladaptive immune modulation, and
fibrosis (58).
3. Adipose tissues as an endocrine
organ
White adipose tissue exhibits endocrine, paracrine,
and autocrine activities. The adipocytes secreted are involved in
body weight regulation (leptin and adiponectin), in local
inflammation (TNF-α, IL-6 and IL-1β), in vascular function and even
in breeding (64). Cytokines,
hormones, leptin, and adiponectin are secreted also by brown
adipose tissue, which acts as an endocrine organ (64). The last form of adipose tissue,
perivascular adipose tissue, is located around the coronary artery,
the aorta (periaortic adipose tissue), and the microcirculatory bed
of the kidney and adipose tissue, which releases adiponectin,
leptin, interleukin (IL)-6, and tumor necrosis factor (TNF)-α. In
men, the most common form of obesity is central or abdominal
obesity, and consists of an accumulation of visceral adipose tissue
(64). According to various
studies, this type of obesity has been associated, with a higher
risk of diseases such as insulin resistance, type 2 diabetes, and
cardiovascular risk (65-67).
Adipose tissue presents a variety of cell populations such as
macrophages, endothelial cells, fibroblasts, and leukocytes
(64). Hypercaloric diet
consumption induces lipid accumulation in adipocytes, triggering
cellular stress and activation of c-Jun N-terminal kinase (JNK) and
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)
signaling pathways (64). These
inflammatory signaling pathways are involved in the phosphorylation
of different proteins and transcriptional factors, causing an
increased secretion of proinflammatory molecules (TNF-α, IL-6),
leptin, chemokines [monocyte chemoattractant protein 1 (MCP-1)],
and proatherogenic mediators [plasminogen activator inhibitor-1
(PAI-1)] (64). Obesity can be
defined as a proinflammatory state of low grade, where the
adipokine secretion increases with visceral fat mass. IL-6 and
TNF-α activate the production of other inflammatory cytokines, such
as C-reactive protein (CRP) (59,64).
Inflammation is associated with leukocyte infiltration, via NADPH
oxidase and reactive oxygen species (ROS) generation. Ηydroxyl
radical (HO•), anion superoxide radical
(O2•-) and hydrogen peroxide
(H2O2) are ROS generated by inflammatory
pathways, adipokine increased levels (leptin) and leukocyte
infiltration in white adipose tissue (59). In isolated adipocytes, it was
reported that TNF-α suppresses insulin signal transduction and
expression of the insulin receptor, leading to hyperglycemia, and
even to pancreatic β-cell destruction. Hyperglycemia induces OS
(59).
Obesity stimulates the production of leptin, which
further increases OS, activity of the sympathetic nervous system
(SNS) similar to sleep deprivation (68) leading to glomerulosclerosis, renal
fibrosis and finally proteinuria (69). In obese patients, renal compression
conduces to increased sodium reabsorption, promoting renal
vasodilatation, glomerular hyperfiltration, and increased renin
secretion (38). Fat accumulation
in and around the kidney, leads to OS, mitochondrial dysfunction,
and endoplasmic reticulum stress (70).
4. Adiponectin and CKD
Adipocytes secrete adiponectin, a 26.4-kDa protein,
which presents in healthy individuals and has anti-inflammatory,
anti-atherogenic and insulin-sensitizing properties, being involved
in lipid and glucose metabolism, partially through activation of
AMPK (69,71). Decreased levels of adiponectin are
associated with several systemic disorders such as insulin
resistance, obesity, type II diabetes (early stages) and
dyslipidemia (72). Adiponectin
levels are significantly decreased in obesity, being negatively
correlated with the percent of fat mass (72). Based on distributions and molecular
affinities of adiponectin complexes, adiponectin presents two
receptors. Adiponectin receptor 1 (AdipoR1) is mainly expressed in
skeletal muscle and is moderately expressed in other tissues, such
as heart and brain (73). At the
renal level, AdipoR1 is found in the glomerulus and proximal
tubule. AdipoR2 is predominantly expressed in the liver.
Adiponectin binds to AdipoR1 and AdipoR2 and further activates the
AMPK signaling pathway which is involved in energy homeostasis
(73). Moreover, adiponectin
activates in vitro the MAPK signaling pathway, increasing
glucose uptake (73).
Kuo and co-researchers conducted a study which
included 196 non-diabetic CKD patients with eGFR ranging between 10
and 60 ml/min/1.73 m2, divided into two groups based on
the presence of metabolic syndrome. The study reported, over a
period of 5 years, 48 (24.5%) incident cases of end-stage renal
disease (ESRD) and 33 (16.8%) deaths. Adiponectin levels were
inversely related to BMI (r=-0.29; P<0.001) and waist
circumference (r=-0.35; P<0.001). A decreased adiponectin level
was associated with a higher risk of ESRD independent of
conventional risk factors, BMI, and even metabolic syndrome
(74).
Coimbra et al studied 194 ESRD patients on
dialysis and 22 controls and evaluated the lipid profile
[lipoprotein subpopulations and oxidized LDL (oxLDL)], CRP,
adiponectin, leptin, and paraoxonase 1 activity. In diabetic and
obese patients (n=45) they observed the lowest values for
adiponectin vs. the normoponderal patients (n=81) (75). Yaturu et al studied 43
subjects with CKD and 34 control subjects and evaluated plasma and
urinary levels of adiponectin. In patients with CKD, the plasma
levels of adiponectin were not decreased compared with the
controls. The study revealed a negative correlation between urinary
adiponectin levels and GFR (r=-0.4; P<0.05) and a positive
correlation with plasma adiponectin levels (r=0.9; P<0.0001)
(76). Moreover, increased
consumption of sugars was found to lead to obesity (77) and further to a decreased level of
adiponectin (72).
Regarding anti-inflammatory effects, adiponectin was
found to suppress IL-6 and TNF-α expression, which are activated by
NF-κB. Adiponectin was found to bind to its two receptors and to
activate adaptor protein containing a pleckstrin homology domain 1
(APPL1) (78). Once activated,
APPL1 was found to further activate peroxisome
proliferator-activated receptor-α (PPAR-α) and phosphorylation of
5'activated protein kinase (AMPK), and mitogen-activated protein
kinase (p38-MAPK) was found to occur (78). Phospho-AMPK downstream was found to
phosphorylate acetyl-CoA carboxylase (ACC), promote fatty acid
oxidation, and inhibit lipogenesis. Phosphorylation of endothelial
nitric oxide synthase (eNOS) by AMP was found to stimulate NO
production, which results in vasodilation (78). In inflammation, adiponectin presents
a cytoprotective effect, activates AMP, suppresses mammalian target
for rapamycin (mTOR) and the inhibitor of nuclear factor κB kinase
subunit γ-phosphatase and tensin homology (IKK-NF-κB-PTEN)
signaling pathways (78). The
phosphatidylinositol 3-kinase-protein kinase B (PI3K-AKT) signaling
pathway controls the metabolic effects of insulin, which is
involved in glycogen synthesis, increases glucose uptake, inhibits
lipolysis also being involved in other physiological processes such
as motility (78). Adiponectin may
activate insulin receptor substrate ½ (IRS1/2) increasing insulin
sensitivity (78).
Hypoadiponectinemia is associated with CKD. A
potential mechanism for this was identified in mice presenting with
hypoadiponectinemia. These mice exhibit podocyte fusion with
adiponectin treatment improving the glomerular podocyte foot
processes via activation of AMPK, which downregulates podocyte
NADPH oxidase (Nox)4. Importantly, these mice exhibit albuminuria,
clearly demonstrating a link between hypoadiponectinemia and kidney
dysfunction (79). Moreover,
podocytes present AdipoR1, an alteration of the receptor that may
lead to obesity-related dysfunction. Increased urinary levels of
both the low- and high-molecular-weight isoforms of adiponectin
were detected in patients with established kidney disease and
patients with type 2 diabetes (79). In vivo, it has been observed
that only a week of consuming a high-fat diet, contributes to
kidney inflammation, which is associated with albuminuria, and
further triggers urinary excretion of monocyte chemotactic protein
(MCP-1) and H2O2 (79).
5. Leptin and CKD
Leptin is a small peptide hormone, secreted mainly
by visceral, subcutaneous, and pericardial adipose compartments,
but can be produced even by normal human osteoblasts, subchondral
osteoblasts, placental syncytiotrophoblasts, and the gastric
epithelium. This hormone regulates bone metabolism and food intake
after it binds to its receptors in the hypothalamus (80). Moreover, leptin exhibits additional
important metabolic effects on peripheral tissues of liver,
skeletal muscle, and bone marrow. In CKD patients, serum levels of
leptin are increased with a decline in GFR. Leptin may be
implicated in patients with CKD in hematopoiesis, nutrition, and
bone metabolism. Increased leptin levels seem to be a risk factor
for CKD development (80).
Shankar et al conducted a large
cross-sectional study which included 5,000 patients, and revealed
that the risk of CKD development significantly increases as blood
levels of leptin rise (81). Leptin
may be involved in CKD pathogenesis and progression through two
mechanism. First, by stimulating the sympathetic nervous system, it
promotes renal sodium reabsorption and increases blood pressure.
Second, leptin may induce renal injury by stimulating renal
endothelial cell proliferation, increased mesangial cell
production, which leads to renal scarring by collagen type I and IV
production, renal fibrosis, and proteinuria (81-84).
Canpolat et al studied CKD patients divided
into four groups (patients with non-dialysis, dialysis, kidney
transplant and control group) and evaluated levels of leptin.
Plasma levels of leptin did not differ at all in the four mentioned
groups (85). Noor and
co-researchers conducted a cross-sectional study at the Nephrology
Department of Jinnah Post Graduate Medical Center from January 2014
to September 2014, which included CKD patients divided by GFR
values in II, III, and IV stages. The study excluded CKD patients
with DM, steroid therapy and any inflammatory disease. Serum
leptin, CRP, and lipid profile (HDL, LDL) were measured. The serum
levels of leptin and CRP were increased with CKD progression. The
control group presented increased HDL/LDL ratio vs. the CKD group
(P<0.001). Leptin presented a positive correlation with CRP
(r=0.994; P<0.001), suggesting that inflammation contributes to
hyperleptinemia, and a negative correlation with HDL/LDL ratio
(r=-0.403; P<0.001) was also observed (86).
Studies performed in-vitro and in animals
have demonstrated that leptin and adiponectin may mediate
pathological and functional changes in renal parenchyma (87,88).
Elevated leptin levels have also been detected in diabetic and
obese non-diabetic CKD patients (89,90).
Korczyńska et al reported that leptin gene expression from
subcutaneous adipose tissue of patients with CKD contributes to
elevated serum leptin levels. In patients with CKD, serum levels of
leptin were three times higher both in men and women, compared with
healthy controls. In CKD women, serum leptin levels were two times
higher than in men. The study also revealed that the mRNA level for
leptin gene expression from subcutaneous adipose tissue of CKD
patients was three times higher compared with that noted in the
controls. In addition, total saturated fatty acids (SFA) and
monounsaturated FA (MUFA) presented higher serum levels in CKD
patients vs. the control group. Serum levels of total n-3
polyunsaturated FAs (n-3PUFAs) and n-6 polyunsaturated (n-6 PUFAs)
were decreased in patients with CKD vs. healthy controls. To test
whether the serum FA has an impact on adipose leptin gene
expression, 3T3-L1 adipocyte cells were treated with various FAs;
such as SFA (palmitic acid 16:0; PA), MUFA (oleic acid 18:1, OA),
n-3 PUFA (docosahexaenoic acid 22:6 n-3 DHA) and n-6 PUFA
(arachidonic acid 20:4 n-6 AA) at different concentrations. After
48 h of incubation with PA and OA, it was observed that FAs, which
were increased in serum CKD patients, contributed to elevated
leptin gene expression. Instead, DHA and AA FAs, which were
decreased in the serum of patients with CKD, had decreased
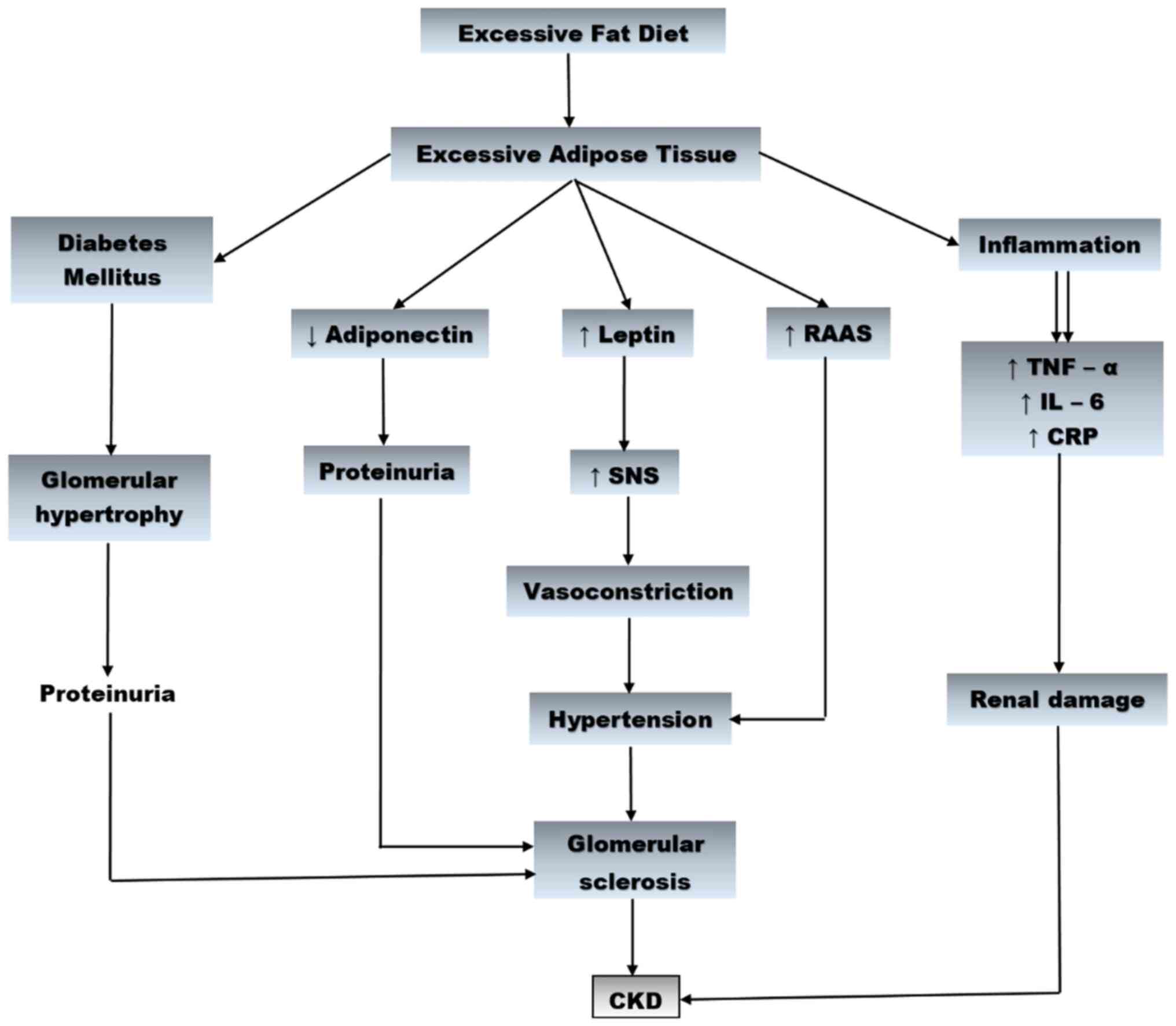
expression of the leptin gene (91). Collectively, inflammation, OS, RAAS
activation, changes in leptin and adiponectin levels, increased
secretion of insulin and insulin resistance, contribute to CKD
development (Fig. 1).
6. Conclusions
Diabetic and hypertensive overweight and obese
patients present an increased risk to develop CKD. Higher amounts
of fat in diet leads to renal fat accumulation, which is associated
with hypertension. Obesity affects renal hemodynamic by increasing
blood pressure, pulse rate, and RAAS activity, by reducing GFR and
by inducing histological perturbations. Decreased adiponectin
level, increased leptin level, increased secretions of
proinflammatory cytokines and activation of RAAS, result in the
development of glomerulopathy. Chronic obesity is involved in
glomerular hyperfiltration, glomerular hypertrophy, promotes
proteinuria, glomerulomegaly and focal and segmental
glomerulosclerosis. Weight loss has beneficial effects on the
entire body, including renal functions, which are associated even
with the improvement in glomerular hemodynamics.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All information in this review is documented by
relevant references.
Authors' contributions
DM, DGB, AT, OS, IAV, DAM, CCP, ME, ASN, AEN and CS
designed the study, performed the literature search and selected
the included studies and wrote the manuscript. DM, DGB, AT, OS,
IAV, DAM, CCP, ME, ASN, AEN and CS critically revised the
manuscript. All authors read and approved the final manuscript. The
contributions of all the authors on this review are greatly valued
and appreciated.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abed AB, Kavvadas P and Chadjichristos CE:
Functional roles of connexins and pannexins in the kidney. Cell Mol
Life Sci. 72:2869–2877. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dantzler WH: Challenges and intriguing
problems in comparative renal physiology. J Exp Biol. 208:587–594.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iglesias P, Bajo MA, Selgas R and Díez JJ:
Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab
Disord. 18:131–144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mandita A, Timofte D, Balcangiu-Stroescu
AE, Balan D, Raducu L, Tanasescu MD, Diaconescu A, Dragos D,
Cosconel CI, Stoicescu SM and Ionescu D: Treatment of high blood
pressure in patients with chronic renal disease. Rev Chim Buchar.
70:993–995. 2019.
|
|
5
|
Young RH and Eble JN: The history of
urologic pathology: An overview. Histopathology. 74:184–212.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dan Spinu A, Gabriel Bratu O, Cristina
Diaconu C, Maria Alexandra Stanescu A, Bungau S, Fratila O,
Bohiltea R and Liviu Dorel Mischianu D: Botulinum toxin in low
urinary tract disorders-over 30 years of practice (Review). Exp
Ther Med. 20:117–120. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Siew ED and Davenport A: The growth of
acute kidney injury: A rising tide or just closer attention to
detail? Kidney Int. 87:46–61. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khwaja A: KDIGO clinical practice
guidelines for acute kidney injury. Nephron Clin Pract.
120:C179–C184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sanz AB, Sanchez-Niño MD, Martin-Cleary C,
Ortiz A and Ramos AM: Progress in the development of animal models
of acute kidney injury and its impact on drug discovery. Expert
Opin Drug Discov. 8:879–895. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Timofte D, Dragoș D, Balcangiu-Stroescu
AE, Tănăsescu MD, Gabriela Bălan D, Răducu L, Tulin A, Stiru O and
Ionescu D: Abdominal aortic calcification in predialysis patients:
Contribution of traditional and uremia-related risk factors. Exp
Ther Med. 20:97–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Totan A, Balcangiu-Stroescu AE, Melescanu
Imre M, Miricescu D, Balan DG, Stanescu II, Ionescu D, Timofte D,
Tanasescu MD and Greabu M: XOR-possible correlations with oxidative
stress and inflammation markers in the context of diabetic kidney
disease. Rev Chim Buchar. 70:1396–1398. 2019.
|
|
12
|
Balcangiu-Stroescu AE, Tanasescu MD,
Diaconescu AC, Raducu L, Balan DG, Mihai A, Tanase M, Stanescu II
and Ionescu D: Diabetic nephropathy: A concise assessment of the
causes, risk factors and implications in diabetic patients. Rev
Chim Buchar. 69:3118–3121. 2018.
|
|
13
|
Silva Junior GB, Bentes AC, Daher EF and
Matos SM: Obesity and kidney disease. J Bras Nefrol. 39:65–69.
2017.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
14
|
Balan DG, Tanasescu MD, Diaconescu A,
Raducu L, Mihai A, Tanase M, Stanescu II, Ionescu D and
Balcangiu-Stroescu AE: Nutritional intervention in patients with
diabetic renal disease a brief presentation. Rev Chim Buchar.
69:4078–4082. 2018.
|
|
15
|
Zhang J and Ningning W: Leptin in chronic
kidney disease: A link between hematopoiesis, bone metabolism, and
nutrition. Int Urol Nephrol. 46:1169–1174. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Andrassy KM: Comments on ‘KDIGO 2012
clinical practice guideline for the evaluation and management of
chronic kidney disease’. Kidney Int. 84:622–623. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Levey AS, Eckardt KU, Tsukamoto Y, Levin
A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N and
Eknoyan G: Definition and classification of chronic kidney disease:
A position statement from kidney disease: Improving global outcomes
(KDIGO). Kidney Int. 67:2089–2100. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
KDIGO CKD Work Group. KDIGO 2012 clinical
practice guideline for the evaluation and management of chronic
kidney disease. Kidney Int Suppl. 3:1–150. 2013.
|
|
19
|
Balcangiu-Stroescu AE, Tanasescu MD,
Diaconescu AC, Raducu L, Constantin AM, Balan DG, Ţarmure V and
Ionescu D: Cardiovascular comorbidities, inflammation and serum
albumin levels in a group of hemodialysis patients. Rev Chim
Buchar. 69:926–929. 2018.
|
|
20
|
Timofte D, Ionescu D, Medrihan L, Mandita
A, Rasina A and Damian L: Vascular calcification and bone disease
in hemodialysis patients assessment, association and risk factors.
Nephrol Dial Transplant. 22:325–326. 2007.
|
|
21
|
Craver L, Marco MP, Martínez I, Rue M,
Borràs M, Martín ML, Sarró F, Valdivielso JM and Fernández E:
Mineral metabolism parameters throughout chronic kidney disease
stages 1-5-achievement of K/DOQI target ranges. Nephrol Dial
Transplant. 22:1171–1176. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kovesdy CP, Furth S and Zoccali C: World
Kidney Day Steering Committee. Obesity and kidney disease: Hidden
consequences of the epidemic. Indian J Nephrol. 27:85–92.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Timofte D, Mandita A, Balcangiu-Stroescu
AE, Balan D, Raducu L, Tanasescu MD, Diaconescu A, Dorin D,
Cosconel CI and Ionescu D: Hyperuricemia and cardiovascular
diseases-clinical and paraclinical correlations. Rev Chim Buchar.
70:1045–1046. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Popa AR, Vesa CM, Uivarosan D, Jurca CM,
Isvoranu G, Socea B, Stanescu AM, Iancu MA, Scarneciu I and Zaha
DC: Cross sectional study regarding the association between
sweetened beverages intake, fast-food products, body mass index,
fasting blood glucose and blood pressure in the young adults from
north-western Romania. Rev Chim. 70:156–160. 2019.
|
|
25
|
Stanescu AM, Grajdeanu IV, Iancu MA,
Stoian AP, Bratu OG, Socea B, Socea LI and Diaconu CC: Correlation
of oral vitamin D administration with the severity of psoriasis and
the presence of metabolic syndrome. Rev Chim Buchar. 69:1668–1672.
2018.
|
|
26
|
Dan SA, Bratu OG, Marcu DR, Stanciu AE,
Gherghiceanu F, Ionita-Radu F, Bungau S, Stanescu AMA and Mischianu
D: Underactive bladder-an underestimated entity. J Mind Med Sci.
7:23–28. 2020.
|
|
27
|
Nittari G, Scuri S, Petrelli F, Pirillo I,
di Luca NM and Grappasonni I: Fighting obesity in children from
European world health organization member states. Epidemiological
data, medicalsocial aspects, and prevention programs. Clin Ter.
170:e223–e230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chomba H, Martin HD and Kimywe J:
Prevalence and predictors of obesity among 7- to 17-year-old
schoolchildren in urban arusha, Tanzania. J Nutr Metab.
2019(3106597)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Smitka K and Marešová D: Adipose tissue as
an endocrine organ: An update on pro-inflammatory and
anti-inflammatory microenvironment. Prague Med Rep. 116:87–111.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maalouf NM, Sakhaee K, Parks JH, Coe FL,
Adams-Huet B and Pak CY: Association of urinary pH with body weight
in nephrolithiasis. Kidney Int. 65:1422–1425. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Siener R, Glatz S, Nicolay C and Hesse A:
The role of overweight and obesity in calcium oxalate stone
formation. Obes Res. 12:106–113. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tessaro CZ, Ramos CI and Heilberg IP:
Influence of nutritional status, laboratory parameters and dietary
patterns upon urinary acid excretion in calcium stone formers. J
Bras Nefrol. 40:35–43. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
33
|
Taylor EN, Stampfer MJ and Curhan GC:
Diabetes mellitus and the risk of nephrolithiasis. Kidney Int.
68:1230–1235. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Poore W, Boyd CJ, Singh NP, Wood K, Gower
B and Dean GA: Obesity and its impact on kidney stone formation.
Rev Urol. 22:17–23. 2020.PubMed/NCBI
|
|
35
|
Toto RD, Greene T, Hebert LA, Hiremath L,
Lea JP, Lewis JB, Pogue V, Sika M and Wang X: AASK Collaborative
Research Group. Relationship between body mass index and
proteinuria in hypertensive nephrosclerosis: Results from the
African American study of kidney disease and hypertension (AASK)
cohort. J Kidney Dis. 56:896–906. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ejerblad E, Fored CM, Lindblad P, Fryzek
J, McLaughlin JK and Nyrén O: Obesity and risk for chronic renal
failure. J Am Soc Nephrol. 17:1695–1702. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fox CS, Larson MG, Leip EP, Culleton B,
Wilson PW and Levy D: Predictors of new-onset kidney disease in a
community-based population. JAMA. 291:844–850. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hall ME, do Carmo JM, da Silva AA, Juncos
LA, Wang Z and Hall JE: Obesity, hypertension, and chronic kidney
disease. Int J Nephrol Renovasc Dis. 7:75–88. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sugerman H, Windsor A, Bessos M and Wolfe
L: Intra-abdominal pressure, sagittal abdominal diameter and
obesity comorbidity. J Intern Med. 241:71–79. 1997.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hall JE, Crook ED, Jones DW, Wofford MR
and Dubbert PM: Mechanisms of obesity-associated cardiovascular and
renal disease. Am J Med Sci. 324:127–137. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eckel RH, Barouch WW and Ershow AG: Report
of the national heart, lung, and blood institute-national institute
of diabetes and digestive and kidney diseases working group on the
pathophysiology of obesity-associated cardiovascular disease.
Circulation. 105:2923–2928. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dwyer TM, Bigler SA, Moore NA, Carroll JF
and Hall JE: The altered structure of renal papillary outflow
tracts in obesity. Ultrastruct Pathol. 24:251–257. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dwyer TM, Banks SA, Alonso-Galicia M,
Cockrell K, Carroll JF, Bigler SA and Hall JE: Distribution of
renal medullary hyaluronan in lean and obese rabbits. Kidney Int.
58:721–729. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chandra A, Neeland IJ, Berry JD, Ayers CR,
Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA and Turer AT:
The relationship of body mass and fat distribution with incident
hypertension: Observations from the Dallas heart study. J Am Coll
Cardiol. 64:997–1002. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pinto-Sietsma SJ, Navis G, Janssen WM, de
Zeeuw D, Gans RO and de Jong PE: PREVEND Study Group. A central
body fat distribution is related to renal function impairment, even
in lean subjects. Am J Kidney Dis. 41:733–741. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Henegar JR, Bigler SA, Henegar LK, Tyagi
SC and Hall JE: Functional and structural changes in the kidney in
the early stages of obesity. J Am Soc Nephrol. 12:1211–1217.
2001.PubMed/NCBI
|
|
47
|
Rutkowski P, Klassen A, Sebekova K, Bahner
U and Heidland A: Renal disease in obesity: The need for greater
attention. J Ren Nutr. 16:216–223. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
De Jong PE, Verhave JC, Pinto-Sietsma SJ
and Hillege HL: PREVEND study group. Obesity and target organ
damage: The kidney. Int J Obes Relat Metab Disord. 26 (Suppl
4):S21–S24. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mascali A, Franzese O, Nisticò S, Campia
U, Lauro D, Cardillo C, Di Daniele N and Tesauro M: Obesity and
kidney disease: Beyond the hyperfiltration. Int J Immunopathol
Pharmacol. 29:354–363. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Amann K and Benz K: Structural renal
changes in obesity and diabetes. Semin Nephrol. 33:23–33.
2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kambham N, Markowitz GS, Valeri AM, Lin J
and D'Agati VD: Obesity-related glomerulopathy: An emerging
epidemic. Kidney Int. 59:1498–1509. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tsuboi N, Utsunomiya Y, Kanzaki G, Koike
K, Ikegami M, Kawamura T and Hosoya T: Low glomerular density with
glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc
Nephrol. 7:735–741. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Okabayashi Y, Tsuboi N, Sasaki T, Haruhara
K, Kanzaki G, Koike K, Miyazaki Y, Kawamura T, Ogura M and Yokoo T:
Glomerulopathy associated with moderate obesity. Kidney Int Rep.
1:250–255. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tsuboi N, Utsunomiya Y and Hosoya T:
Obesity-related glomerulopathy and the nephron complement. Nephrol
Dial Transplant. 28 (Suppl 4):iv108–iv113. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wickman C and Kramer H: Obesity and kidney
disease: Potential mechanisms. Semin Nephrol. 33:14–22.
2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cassis LA, Police SB, Yiannikouris F and
Thatcher SE: Local adipose tissue renin-angiotensin system. Curr
Hypertens Rep. 10:93–98. 2008.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Marcus Y, Shefer G and Stern N: Adipose
tissue renin-angiotensin-aldosterone system (RAAS) and progression
of insulin resistance. Mol Cell Endocrinol. 378:1–14.
2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cabandugama PK, Gardner MJ and Sowers JR:
The renin angiotensin aldosterone system in obesity and
hypertension: Roles in the cardiorenal metabolic syndrome. Med Clin
North Am. 101:129–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vincent HK and Taylor AG: Biomarkers and
potential mechanisms of obesity-induced oxidant stress in humans.
Int J Obes (Lond). 30:400–418. 2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Boustany CM, Bharadwaj K, Daugherty A,
Brown DR, Randall DC and Cassis LA: Activation of the systemic and
adipose renin-angiotensin system in rats with diet-induced obesity
and hypertension. Am J Physiol Regul Integr Comp Physiol.
287:R943–R949. 2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rüster C and Wolf G: The role of the
renin-angiotensin-aldosterone system in obesity-related renal
diseases. Semin Nephrol. 33:44–53. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Papacocea T, Buraga I, Papacocea R,
Badarau AI, Buraga M, Ciornei C, Mihai G, Stoian I and Adam D:
Antioxidant enzymes-potential targets in intracerebral haemorrhage.
Farmacia. 62(1118)2014.
|
|
63
|
Olariu L, Dumitriu B, Craciun L, Buse E,
Rosoiu N, Bojinca M and Papacocea T: The in vitro influence of a
pharmaceutically active small sea fish extract on apoptosis and
proliferation mechanisms amplified by inflammatory conditions.
Farmacia. 66:524–529. 2018.
|
|
64
|
Gómez-Hernández A, Beneit N,
Díaz-Castroverde S and Escribano Ó: Differential role of adipose
tissues in obesity and related metabolic and vascular
complications. Int J Endocrinol. 2016(1216783)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Le Jemtel TH, Samson R, Milligan G,
Jaiswal A and Oparil S: Visceral adipose tissue accumulation and
residual cardiovascular risk. Curr Hypertens Rep.
20(77)2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Shinar S, Berger H, De Souza LR and Ray
JG: Difference in visceral adipose tissue in pregnancy and
postpartum and related changes in maternal insulin resistance. J
Ultrasound Med. 38:667–673. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Reijrink M, de Boer SA, Spoor DS, Lefrandt
JD, Lambers Heerspink HJ, Boellaard R, Greuter MJ, Borra RJ,
Hillebrands JL, Slart RH and Mulder DJ: Visceral adipose tissue
volume is associated with premature atherosclerosis in early type 2
diabetes mellitus independent of traditional risk factors.
Atherosclerosis. 290:87–93. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Papacocea IR, Badarau IA, Ciornei MC,
Burciulescu SL and Papacocea MT: The effects of caffeine intake on
cardiovascular parameters in sleep deprived medical residents. Rev
Chim Buchar. 70:1445–1448. 2019.
|
|
69
|
Rhee CM, Ahmadi SF and Kalantar-Zadeh K:
The dual roles of obesity in chronic kidney disease: A review of
the current literature. Curr Opin Nephrol Hypertens. 25:208–216.
2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Unger RH, Scherer PE and Holland WL:
Dichotomous roles of leptin and adiponectin as enforcers against
lipotoxicity during feast and famine. Mol Biol Cell. 24:3011–3015.
2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kadowaki T, Yamauchi T, Kubota N, Hara K,
Ueki K and Tobe K: Adiponectin and adiponectin receptors in insulin
resistance, diabetes, and the metabolic syndrome. J Clin Invest.
116:1784–1792. 2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Slee AD: Exploring metabolic dysfunction
in chronic kidney disease. Nutr Metab (Lond). 9(36)2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Run H and Dong LQ: Adiponectin signaling
and function in insulin target tissues. J Mol Cell Bio. 8:101–109.
2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kuo IC, Wu PH, Lin HY, Niu SW, Huang JC,
Hung CC, Chiu YW and Chen HC: The association of adiponectin with
metabolic syndrome and clinical outcome in patients with
non-diabetic chronic kidney disease. PLoS One.
14(e0220158)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Coimbra S, Reis F, Nunes S, Viana S,
Valente MJ, Rocha S, Catarino C, Rocha-Pereira P, Bronze-da-Rocha
E, Sameiro-Faria M, et al: The protective role of adiponectin for
lipoproteins in end-stage renal disease patients: Relationship with
diabetes and body mass index. Oxid Med Cell Longev.
2019(3021785)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Yaturu S, Reddy RD, Rains J and Jain SK:
Plasma and urine levels of resistin and adiponectin in chronic
kidney disease. Cytokine. 37:1–5. 2007.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Papacocea T, Papacocea R, Rădoi M, Pițuru
S and Balan DG: Stomach ‘tastes’ the food and adjusts its emptying:
A neurophysiological hypothesis (Review). Exp Ther Med.
20:2392–2395. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Choi HM, Doss HM and Kim KS: Multifaceted
physiological roles of adiponectin in inflammation and diseases.
Int J Mol Sci. 21(1219)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Briffa JF, McAinch AJ, Poronnik P and
Hryciw DH: Adipokines as a link between obesity and chronic kidney
disease. Am J Physiol Renal Physiol. 305:F1629–F1636.
2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tesauro M, Mascali A, Franzese O, Cipriani
S, Cardillo C and Di Daniele N: Chronic kidney disease, obesity,
and hypertension: The role of leptin and adiponectin. Int J
Hyperten. 2012(943605)2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Shankar A, Syamala S, Xiao J and Muntner
P: Relationship between plasma leptin level and chronic kidney
disease. Int J Nephrol. 2012(269532)2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Nasrallah MP and Ziyadeh FN: Overview of
the physiology and pathophysiology of leptin with special emphasis
on its role in the kidney. Semin Nephrol. 33:54–65. 2013.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Han DC, Isono M, Chen S, Casaretto C, Hong
SW, Wolf G and Ziyadeh FN: Leptin stimulates type I collagen
production in db/db mesangial cells: Glucose uptakeand TGF-beta
type II receptor expression. Kidney Int. 59:1315–1323.
2001.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Mao S, Fang L, Liu F, Jiang S, Wu L and
Zhang J: Leptin and chronic kidney diseases. J Recept Signal
Transduct Res. 38:89–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Canpolat N, Sever L, Agbas A, Tasdemir M,
Oruc C, Ekmekci OB and Caliskan S: Leptin and ghrelin in chronic
kidney disease: Their associations with protein-energy wasting.
Pediatr Nephrol. 33:2113–2122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Noor S, Alam F, Fatima SS, Khan M and
Rehman R: Role of leptin and dyslipidemia in chronic kidney
disease. Pak J Pharm Sci. 31:893–897. 2018.PubMed/NCBI
|
|
87
|
Sharma K, Ramachandrarao S, Qiu G, Usui
HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, et al:
Adiponectin regulates albuminuria and podocyte function in mice. J
Clin Invest. 118:1645–1656. 2008.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Lee MP, Orlov D and Sweeney G: Leptin
induces rat glomerular mesangial cell hypertrophy, but does not
regulate hyperplasia or apoptosis. Int J Obes (Lond). 29:1395–1401.
2005.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Galletti F, D'Elia L, Barba G, Siani A,
Cappuccio FP, Farinaro E, Iacone R, Russo O, De Palma D, Ippolito R
and Strazzullo P: High-circulating leptin levels are associated
with greater risk of hypertension in men independently of body mass
and insulin resistance: Results of an eight-year follow-up study. J
Clin Endocrinol Metab. 93:3922–3926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lu JW, Chi PJ, Lin YL, Wang CH and Hsu BG:
Serum leptin levels are positively associated with aortic stiffness
in patients with chronic kidney disease stage 3-5. Adipocyte.
9:206–211. 2010.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Korczyńska J, Czumaj A, Chmielewski M,
Śledziński M, Mika A and Śledziński T: Increased expression of the
leptin gene in adipose tissue of patients with chronic kidney
disease-the possible role of an abnormal serum fatty acid profile.
Metabolites. 10(98)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Silva Junior and Matos SMA: Padrões
alimentares e doença renal crônica. In Cruz J, Cruz HMM, Kirsztajn
GM, Oliveira RB, Barros RT, eds. Atualidades em Nefrologia 14. São
Paulo: Sarvier, 2016.
|