Introduction
Ischemic heart disease (IHD) has become a major
public human issue, with a decreasing age of onset. Coronary heart
disease (CHD) is a leading cause of death all over the world
according to the World Health Organization (1). With the advent of cardiac surgery,
including percutaneous coronary intervention (PCI) and coronary
artery bypass graft (CABG), some clinical symptoms have been
alleviated, but ischemia/reperfusion (I/R) injury induces
arrhythmia, heart failure and cardiomyocyte death (2). Therefore, I/R injury is an important
concern of doctors following cardiac surgery. According to previous
studies, I/R injury is associated with calcium overload (3), oxidative stress (4) and myocardial apoptosis (5). Therefore, the search for a drug that
is able to prevent or treat myocardial I/R injury is a popular
focus of research.
Oxymatrine (OMT), an alkaloid that originates from
the traditional Chinese herb Sophora flavescens Aiton,
possesses numerous pharmacological properties, including
anti-hepatic fibrosis (6),
anti-inflammatory (7) and antitumor
activities (8). The use of OMT in
patients with cardiovascular diseases has attracted increasing
attention, because studies have identified that OMT has a wide
range of pharmacological properties, including activities against
arrhythmia (9), shock (10) and hypertension (11). The nuclear factor
erythroid-2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway
and the phosphatidylinositol 3-kinase (PI3K)/Akt/glycogen synthase
kinase-3β (GSK3β) pathway are important pathophysiological
mechanisms that are relevant to I/R injury, and previous studies
have shown that OMT attenuates I/R injury in the brain through the
p-Akt/GSK3β/HO-1/Nrf2 signaling pathway (12-14).
However, the effects of OMT on I/R injury in cardiomyocytes, and
the specific signaling pathways by which OMT exerts these effects
have not yet been explored. Therefore, a hypoxia/reoxygenation
(H/R) model of H9c2 cardiomyocytes was established in the present
study to detect the potential effects and signaling pathways of
OMT.
Materials and methods
Cell culture
The H9c2 cardiomyocyte cell line was provided by the
Tianjin Key Laboratory of Hepatopancreatic Fibrosis and Molecular
Diagnosis and Treatment, and were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with
4,500 mg/l glucose containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin. The
H9c2 cardiomyocytes were grown in an incubator with 100% humidity
containing 95% air and 5% CO2 at 37˚C.
H/R treatment
The H/R model was established according to
previously published methods (15,16).
The H9c2 cardiomyocytes were cultured with Hank's balanced salt
solution in an incubator containing 5% CO2 and 95%
N2 at 37˚C for 2 h to establish hypoxia. Then, Hank's
balanced salt solution was replaced with complete DMEM containing
10% FBS, and reoxygenation was conducted at 37˚C with 5%
CO2 for 4 h.
Cell grouping
Cell groups were established and the concentrations
and durations of treatment chosen with reference to previously
published methods (14,16,17).
Also, the H9c2 cardiomyocytes were treated with different
concentrations of OMT (0, 10, 30, 50, 70 and 90 µM) for 12 h under
normoxic conditions to assess their cytotoxicity and select the
concentrations for further analysis. OMT (cat. no. YM-0074) was
purchased from Shanghai Yuanmu Biotech Co., Ltd. The H9c2
cardiomyocytes grown on plates were randomly divided into seven
groups: i) Normally cultured H9c2 cardiomyocytes (control) group,
in which the cells were cultured under standard conditions, without
H/R or any additional treatments; ii) hypoxia/reoxygenation (model)
group, in which the cells were exposed to hypoxia for 2 h followed
by reoxygenation for 4 h; iii) model + 10 µmol/l OMT (10 µmol/l
OMT) group, in which the cells were pretreated with 10 µmol/l OMT
for 12 h and then exposed to H/R; iv) model + 30 µmol/l OMT (30
µmol/l OMT) group; v) model + 50 µmol/l OMT (50 µmol/l OMT) group;
vi) model + LY294002 group, in which the cells were pretreated with
20 µmol/l LY294002(L9908, Sigma) for 1 h and then exposed to H/R;
and vii) model + OMT + LY294002 (OMT + LY294002) group, in which
the cells were maintained under the same conditions as those in the
50 µmol/l OMT group, but were also pretreated with 20 µmol/l
LY294002 for 1 h beforethey were treated with OMT.
Cell viability assay
Cell viability was analyzed using the
3-(4,5-dimethylthiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)
assay. The H9c2 cardiomyocytes in the various groups were grown to
a density of 1x104 cells/well on 96-well plates, 20 µl
MTT (5 mg/ml) was then added to each well, and the cells were
incubated at 37˚C with 5% CO2 for 4 h. The medium was
removed, and 100 µl dimethylsulfoxide was added to the H9c2 cells
in each well to dissolve the formazan crystals. Finally, the
absorbance was read at 490 nm using a microplate reader (5200Multi;
Tanon Science and Technology Co., Ltd.).
Observation of cell morphology
Cell morphology was observed with hematoxylin and
eosin (H&E) staining. The cell supernatant of each group was
discarded after 5 min of centrifugation at 132 x g at 4˚C. The H9c2
cardiomyocytes were sequentially washed with phosphate-buffered
saline (PBS) and deionized water two or three times, incubated with
hematoxylin for 5 min at room temperature, and then placed in eosin
solution for 2 min at room temperature. Finally, the H9c2
cardiomyocytes were dried under ventilated conditions, and the cell
morphology was observed using an optical microscope.
Detection of lactate dehydrogenase
(LDH) levels
The severity of H9c2 cardiomyocyte injuries was
evaluated by detecting the release of LDH into the cell
supernatant. This was performed using an LDH kit (A020-2-2; Nanjing
Jiancheng Bioengineering Institute), with measurement of the
absorbance at 450 nm according to the manufacturer's
instructions.
Detection of cellular malondialdehyde
(MDA) levels, superoxide dismutase (SOD) activity and catalase
(CAT) activity
The MDA levels, SOD activity and CAT activity of the
cells were determined after the various treatments. H9c2
cardiomyocytes from the different groups were collected, washed
three times with cold PBS, and then cell lysis buffer (RABLYSIS1;
Sigma-Aldrich; Merck KGaA)was added for cell lysis. Following
centrifugation at 206 x g for 5 min at 4˚C the supernatant was
collected for detection. MDA levels, SOD activity and CAT activity
were measured with corresponding kits (cat. nos. A003-3-1, A001-3-2
and A007-1-1; Nanjing Jiancheng Bioengineering Institute) at
absorbances of 530, 450 and 405 nm, respectively, according to the
manufacturer's instructions.
Cell apoptosis analysis with flow
cytometric and TUNEL assays
The percentage of apoptotic cells in each group was
analyzed using an Annexin V-FITC/PI apoptosis kit (Beijing 4A
Biotech Co., Ltd.) for flow cytometry according to the
manufacturer's instructions. Following treatment, the H9c2
cardiomyocytes from the different groups were collected and washed
twice with cold PBS. Then, 5 µl Annexin V/FITC was added to the
cells, which were then incubated for 5 min in the dark at room
temperature for the labeling of early apoptotic cells. This was
followed by incubation with 10 µl PI (20 µg/ml) for 10 min in the
dark at room temperature to label late apoptotic cells. The
analysis was performed using a flow cytometer (BeamCyte-1026;
Changzhou Beamdiag Biotech Co., Ltd.), and quantitative processing
was performed using FlowJo 10.6.2 software (FlowJo LLC).
The apoptosis of the H9c2 cardiomyocytes was also
assessed using a TUNEL kit (KA4159; Abnova) according to the
manufacturer's instructions. The H9c2 cardiomyocytes were fixed
with xylene for 10 min at room temperature and washed with PBS
three times. The cells were then blocked with FBS in a humid
atmosphere at 37˚C for 60 min and incubated with the antibody from
the kit at 4˚C overnight. Afterwards, the slides were rinsed with
PBS three times, the TUNEL reaction mixture was added and the
slides were incubated for 1 h at 37˚C in the dark. The apoptotic
cells were incubated in the mounting medium containing 0.05% DAPI
for 10 min in the dark and then analyzed under a fluorescence
microscope; green fluorescence was observed at 520 nm with a
standard fluorescence filter and blue DAPI was observed at 460 nm.
Image-Pro Plus 6.0 software (Media Cybernetics) was used for
quantification.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RT-qPCR was used to detect the expression of B cell
lymphoma/leukemia-2 (Bcl-2), Bax, caspase-3, PI3K, Akt, GSK3β, Nrf2
and HO-1 in each group. TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA, and UV
spectrophotometry was used to measure the purity. Then, RNA was
reverse transcribed into cDNAs using a HiFiScript cDNA Synthesis
Kit (CoWin Biosciences) according to the manufacturer's
instructions. The cDNA templates were analyzed by qPCR using the
UltraSYBR Mixture (Low ROX) kit (CoWin Biosciences) under the
following conditions: 40 cycles of 10 sec at 95˚C, 30 sec at 60˚C
and 32 sec at 72˚C. The nucleotide sequences of the forward and
reverse primers are shown in Table
I. The relative expression level of each mRNA was calculated by
using the 2-ΔΔCq method (18).
 | Table ISequences of the primer pairs used
for quantitative PCR. |
Table I
Sequences of the primer pairs used
for quantitative PCR.
| Primer | Sequence |
|---|
| Bcl-2 | F:
5'-ATAACCGGGAGATCGTGATGA-3' |
| | R:
5'-CTCTCAGGCTGGAAGGAGAAG-3' |
| Bax | F:
5'-CCACCAGCTCTGAACAGATCA-3' |
| | R:
5'-GCTCCATGTTGTTGTCCAGT-3' |
| Caspase-3 | F:
5'-GAGCAGAGTCAAAGGCTGGT-3' |
| | R:
5'-TGTCGTCATGTCCACCACT-3' |
| Nrf2 | F:
5'-TCCTCTGCTGCCATTAGTCA-3' |
| | R:
5'-GTGCCTTCAGTGTGCTTCT-3' |
| HO-1 | F:
5'-TCTGGAATGGAAGGAGATGC-3' |
| | R:
5'-AGTTCTGGGGCTCTGTTGC-3' |
| PI3K | F:
5'-GACTCCAAGATGAAGAAGATGTG-3' |
| | R:
5'-GAGCATTCGCAGGTCCAAGCC-3' |
| Akt | F:
5'-CGAGGCCCAACACCTTCATC-3' |
| | R:
5'-CCGGAAGTCCATCGTCTCCT-3' |
| GSK3β | F:
5'-CCAGGTGGAGGACCATTTGC-3' |
| | R:
5'-ACTCTACACCAGCAGCAGCC-3' |
| β-actin | F:
5'-TCAGGTCATCACTATCGGCAAT-3' |
| | R:
5'-AAAGAAAGGGTGTAAAACGCA-3' |
Protein preparation and western blot
analysis
H9c2 cardiomyocytes from the various groups were
washed three times with PBS, and then lysed in complete RIPA buffer
(R0020; Beijing Solarbio Science & Technology Co., Ltd.) at 4˚C
for 20 min. The total protein concentrations were determined using
a BCA kit (A045-4-2; Nanjing Jiancheng Bioengineering Institute).
Equal amounts of protein from each group (30 µg) were loaded onto
10% polyacrylamide gels for electrophoresis and transferred to
nitrocellulose membranes (EMD Millipore). Then, the membranes were
blocked with Tris-buffered saline and 0.05%Tween 20 buffer
containing 5% skimmed milk for 3 h at room temperature, followed by
incubation with the following primary antibodies overnight at 4˚C:
Bax (cat. no. 50599-2-Ig; 1:2,000;), Bcl-2 (cat. no. 60178-1-Ig;
1:2,000), pro caspase-3 (cat. no. 66470-2-Ig; 1:1,000), cleaved
caspase-3 (cat. no. 66470-2-Ig; 1:1,000), PI3K (cat. no.
20584-1-AP; 1:1,000), Akt (cat. no. 10176-2-AP, 1:1000), GSK3β
(cat. no. 22104-1-AP; 1:1,000), Nrf2 (cat. no. 16396-1-AP,
1:1,000), HO-1 (cat. no. 16396-1-AP, 1:1,000) and β-actin (cat. no.
4970S; 1:1,000), all from ProteinTech Group, Inc.; phosphorylated
(p-)PI3K (cat. no. bs-3332R, 1:1,000; BIOSS); p-Akt (cat. no.
4060s; 1:2,000; Cell Signaling Technology, Inc.) and p-GSK3β (cat.
no. 9327s; 1:1,000; Cell Signaling Technology, Inc.). The membranes
were then incubated with horseradish peroxidase-conjugated
secondary antibodies (cat. no. 7074V; 1:5,000; Cell Signaling
Technology, Inc.) for 1 h at room temperature. Signals were
observed using ECL reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Band densities were
detected using ImageJ 1.52a software (National Institutes of
Health).
Statistical analysis
Results are presented as the mean ± SD (n=10).
Multigroup comparisons of the means were performed using one-way
ANOVA followed by Tukey's post hoc test for multiple comparisons.
SPSS version 25.0 (IBM Corp.) statistical software was used to
perform the analysis. P<0.05 was considered to indicate a
statistically significant result. All experiments were repeated
three times.
Results
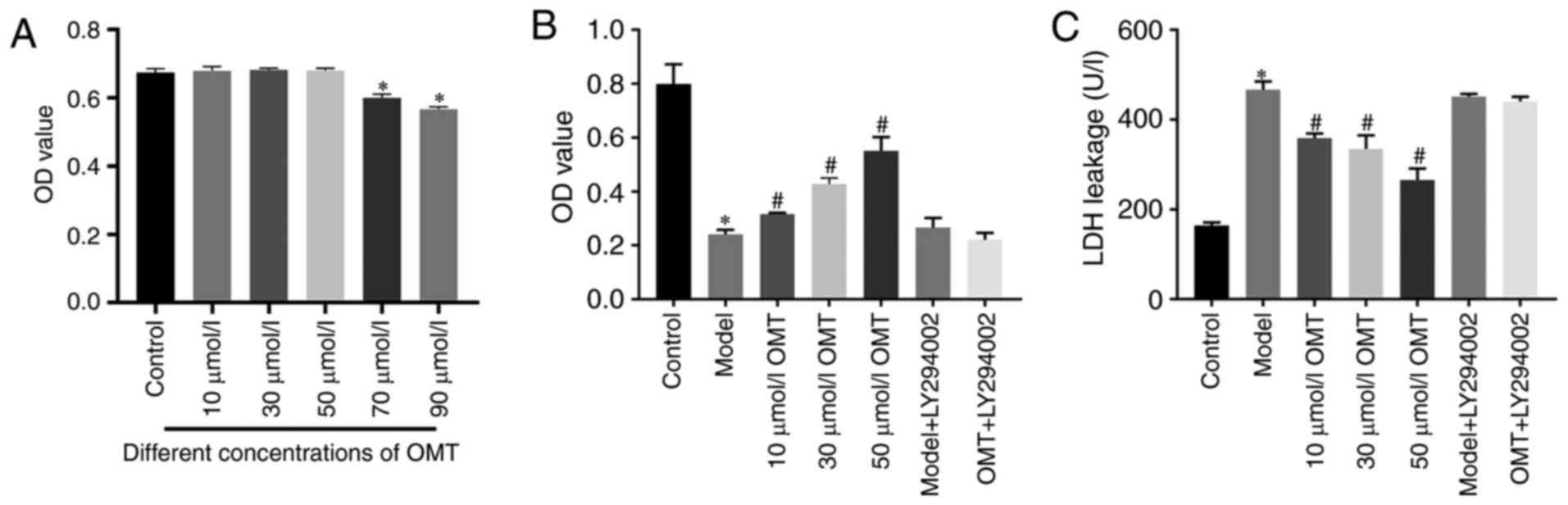
OMT increases the viability of H9c2
cardiomyocytes exposed to H/R
H9c2 cardiomyocytes were treated with different
concentrations of OMT for 12 h under normoxic conditions to explore
the effects of OMT on these cells. As evidenced by the MTT assay,
OMT did not exert marked cytotoxic effects or reduce the viability
of H9c2 cardiomyocytes pretreated with 10, 30 or 50 µM OMT under
normoxic conditions (Fig. 1A).
Therefore, 10, 30 and 50 µM OMT were chosen as the low, medium and
high concentrations for subsequent experiments. The viability of
H9c2 cardiomyocytes was significantly decreased compared with that
of the control group after H/R injury, and
concentration-dependently increased in the cells treated with OMT
for 12 h prior to H/R injury compared with that of the model group
(P<0.05; Fig. 1B). The LDH
release assay revealed that the H/R injury-induced increase in LDH
release was significantly reduced when the cells were pretreated
with OMT (P<0.05; Fig. 1C). The
results of the cell viability and LDH release assays indicate a
protective effect of OMT against H/R injury. Furthermore, no
difference in the viability and LDH release of the H9c2
cardiomyocytes was observed between the model group and the model
group treated with the PI3K inhibitor LY294002, indicating that
LY294002 was not toxic to cells. When LY294002 was added before the
OMT pretreatment, the cell viability was significantly decreased
and LDH release was significantly increased, indicating that the
protection provided by OMT may be mediated by the PI3K/Akt
signaling pathway.
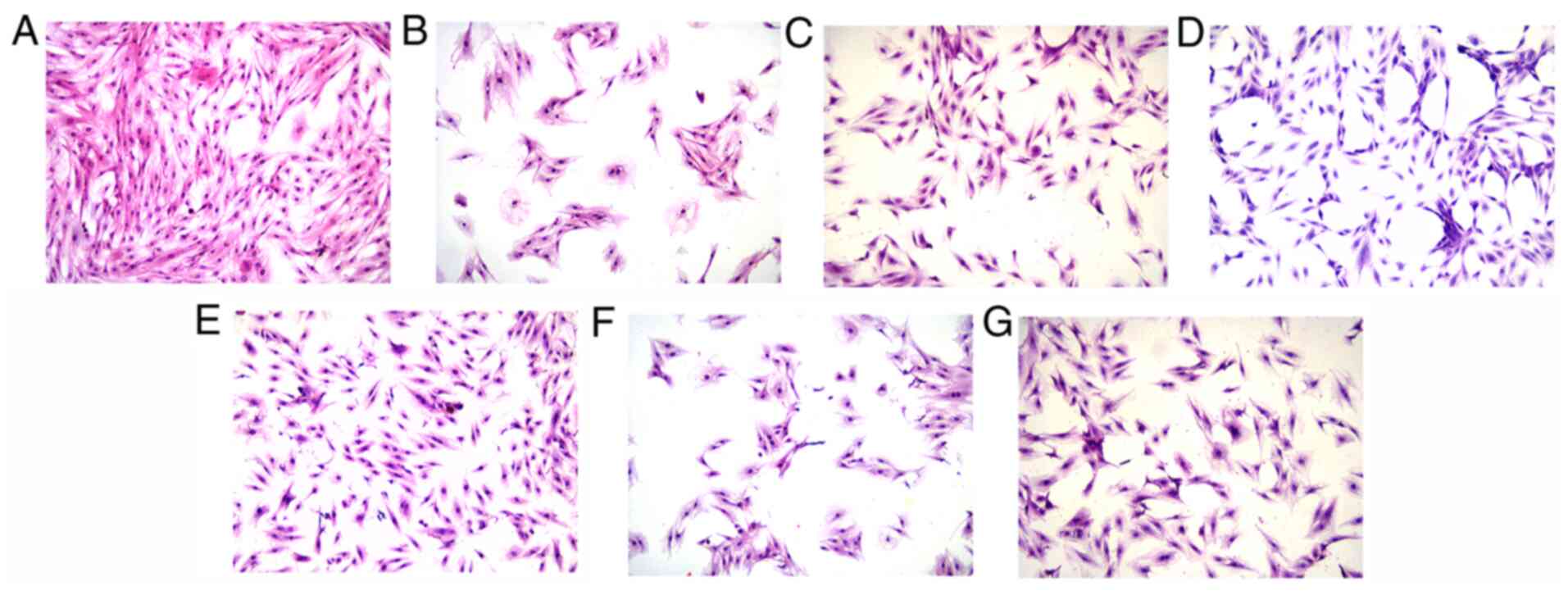
OMT improves the morphology of H9c2
cardiomyocytes exposed to H/R
As shown in the images of H&E staining, the H9c2
cardiomyocytes in the control group (Fig. 2A) showed good growth and good
adhesion to the well. The cells had an elongated spindle morphology
with a full cytoplasm and intact structure. A large number of
suspended cells were present in the model group (Fig. 2B), which exhibited a marked loss of
basic structure and evident shrinkage. In addition, the cytoplasm
appeared cloudy and the intracellular structures were unclear. The
cell morphology was clearly ameliorated by the OMT pretreatment at
different concentrations (Fig.
2C-E). Compared with the model group, the cells gradually
recovered their spindle-like morphology, the cytoplasm became
fuller, the intracellular structures became clearer and the
intercellular space was significantly reduced, and thus the number
of cells observed under the microscope increased. No differences
were observed between the cells in the model group and model +
LY294002 group (Fig. 2F),
confirming the previous finding that LY294002 was not toxic to
cells. However, the group treated with LY294002 prior to the OMT
pretreatment exhibited cell morphology similar to that in the model
group, suggesting that the protective effect of OMT on H9c2
cardiomyocytes subjected to H/R injury may be mediated by the
PI3K/Akt signaling pathway (Fig.
2G).
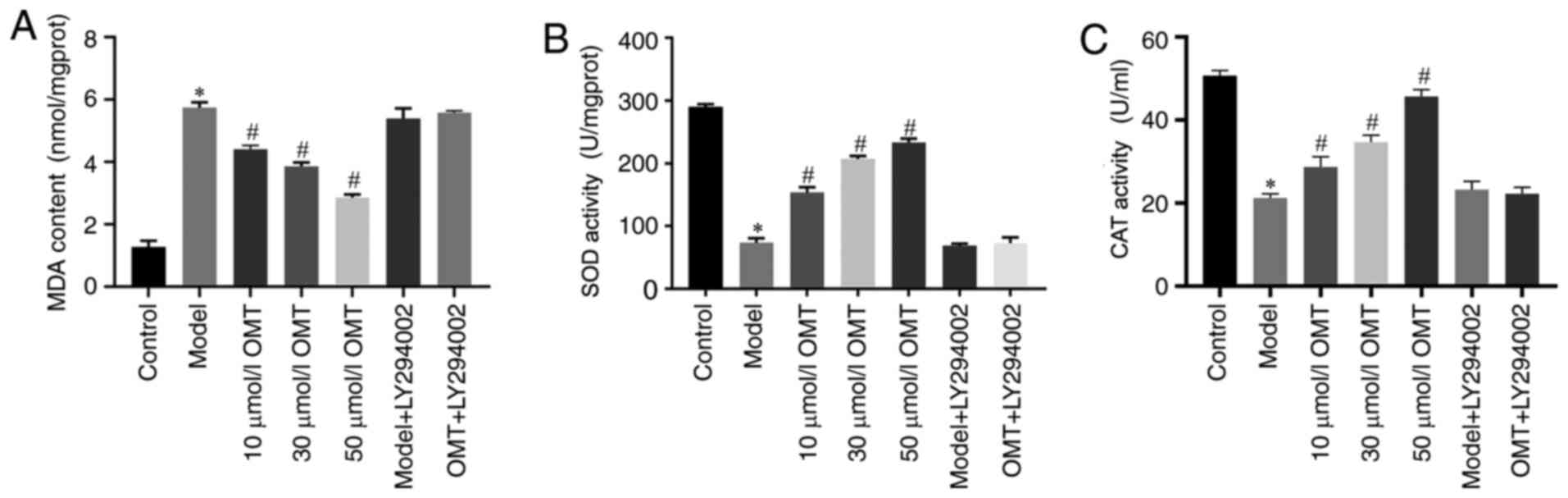
OMT suppresses oxidative stress in
H9c2 cardiomyocytes exposed to H/R
The activities of SOD and CAT and the quantity of
MDA in each group were detected using the corresponding kits, to
investigate whether the protective effect of OMT on H9c2
cardiomyocytes exposed to H/R was associated with the suppression
of oxidative stress. Compared with the control group, the
activities of the antioxidants SOD and CAT were decreased and the
content of the lipid peroxide marker MDA was increased in H9c2
cardiomyocytes in the model group, indicating that H/R injury
increased the oxidative stress response. No differences in results
were detected between the model group and the model + LY294002
group, indicating that LY294002 had no effect on the cells.
Compared with the model group, the H9c2 cardiomyocytes pretreated
with 10, 30 and 50 µM OMT exhibited significantly increased SOD and
CAT activities and significantly decreased MDA content, suggesting
that the protective effect of OMT was associated with the
suppression of oxidative stress. The SOD and CAT activities and MDA
content in the cells treated with LY294002 prior to OMT
pretreatment were comparable with those in the model group. These
results indicate that the OMT pretreatment protected H9c2
cardiomyocytes from H/R injury by preserving their antioxidant
capacity, which may be associated with the PI3K/Akt signaling
pathway (P<0.05; Fig. 3).
OMT inhibits apoptosis in H9c2
cardiomyocytes exposed to H/R
TUNEL staining (Fig.
4A) and flow cytometry (Fig.
4B) were performed to evaluate the effect of OMT on the
H/R-induced apoptosis of H9c2 cardiomyocytes, and the rates of
apoptosis were determined (Fig.
4C). A significantly increased number of TUNEL-positive cells
and apoptotic cells were detected in the model group compared with
the control group, indicating that H/R injury promoted the
apoptosis of H9c2 cardiomyocytes. Furthermore, the levels of Bcl-2
and Bax and caspase-3, biomarkers of mitochondrial apoptosis, were
detected using western blotting and RT-qPCR (Fig. 4D and E). Regardless of whether mRNA or protein
levels were analyzed, the results indicated that H/R injury
accelerated the apoptosis of H9c2 cardiomyocytes by increasing the
levels of the pro-apoptotic factors Bax and cleaved caspase-3, and
reducing the level of the anti-apoptotic factor Bcl-2, which are
mainly associated with the mitochondrial apoptotic pathway. No
differences were observed between the model group and the model +
LY294002 group, indicating that LY294002 did not alter apoptosis.
However, compared with the model group, the apoptosis of H9c2
cardiomyocytes was significantly attenuated by the OMT
pretreatment. Following treatment with increasing concentrations of
OMT, the number of TUNEL-positive cells gradually decreased and the
proportion of apoptotic cells also decreased, indicating that OMT
exerts an anti-apoptotic effect on cells with H/R injury. However,
the protective effects of OMT were markedly reduced by the addition
of LY294002 prior to the OMT pretreatment, indicating that the
anti-apoptotic effects of OMT were potentially mediated by the
PI3K/Akt signaling pathway.
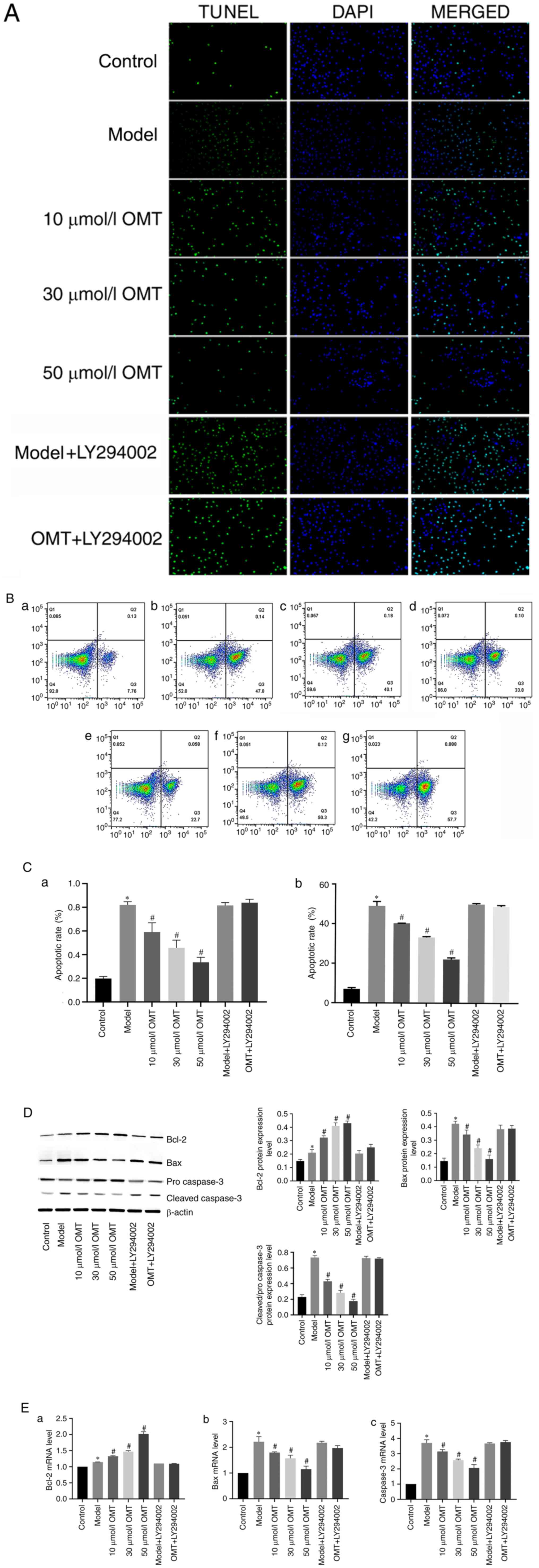 | Figure 4OMT inhibits apoptosis in H9c2
cardiomyocytes exposed to hypoxia/reoxygenation. (A) The apoptosis
of H9c2 cardiomyocytes in different groups was determined using the
TUNEL assay. TUNEL-positive cells are green, and nuclei are stained
blue with DAPI (magnification, x100). (B) Flow cytometric analysis
of the apoptosis of H9c2 cardiomyocytes in the (a) control, (b)
model, (c) 10 µM OMT, (d) 30 µM OMT and (e) 50 µM OMT pretreatment,
(f) model with LY294002 and (g) OMT + LY294002 pretreatment groups.
(C) Apoptosis rates determined using (a) TUNEL assay and (b) flow
cytometry. (D) The levels of apoptosis-associated proteins, namely
Bax, Bcl-2, pro caspase-3 and cleaved caspase-3, were detected
using western blot analysis. (E) The mRNA expression levels of the
apoptosis-associated proteins (a) Bcl-2, (b) Bax and (c) caspase-3
were measured using RT-qPCR. *P<0.05 compared with
the control group; #P<0.05 compared with the model
group. OMT, oxymatrine; Bcl-2, B cell lymphoma/leukemia-2. |
OMT protects H/R-exposed H9c2
cardiomyocytes by activating the PI3K/Akt signaling pathway
The PI3K/Akt/GSK3β and Nrf2/HO-1 signaling pathways
were analyzed using western blotting and RT-qPCR to examine the
molecular mechanism of OMT in H9c2 cardiomyocytes with H/R injury
(Fig. 5). Western blots (Fig. 5A) revealed that the levels of
p-PI3K, p-Akt and p-GSK3β in the model group were increased
compared with those in the the control group, indicating that H/R
injury activated the PI3K/Akt/GSK3β pathway. No difference was
observed between the phosphorylated protein levels in the model
group and the model + LY294002 group, suggesting that LY294002 did
not modulate the activity of the PI3K/Akt/GSK3β pathway.
Pretreatment with OMT concentration-dependently increased the
levels of these phosphorylated proteins in H9c2 cardiomyocytes
exposed to H/R injury, indicating that the protective effect of OMT
was associated with the PI3K/Akt/GSK3β pathway. Furthermore,
LY294002 attenuated the OMT-induced increases in the levels of
p-Akt and p-GSK3β, confirming that the protective effect of OMT was
mediated by the PI3K/Akt pathway. The expression of the PI3K and
Akt mRNAs in different groups detected using RT-qPCR (Fig. 5B) were consistent with the protein
levels. However, a difference was observed between the levels of
the GSK3β mRNA and protein. Compared with the control group, the
expression of the GSK3β mRNA was increased in the model group,
indicating that H/R injury increased the expression of the GSK3β
mRNA. However, expression of the GSK3β mRNA was markedly decreased
in the 10, 30 and 50 µM OMT pretreatment groups compared with the
model group, suggesting that the protective effect of OMT was
related to a reduction in the expression of GSK3β mRNA.
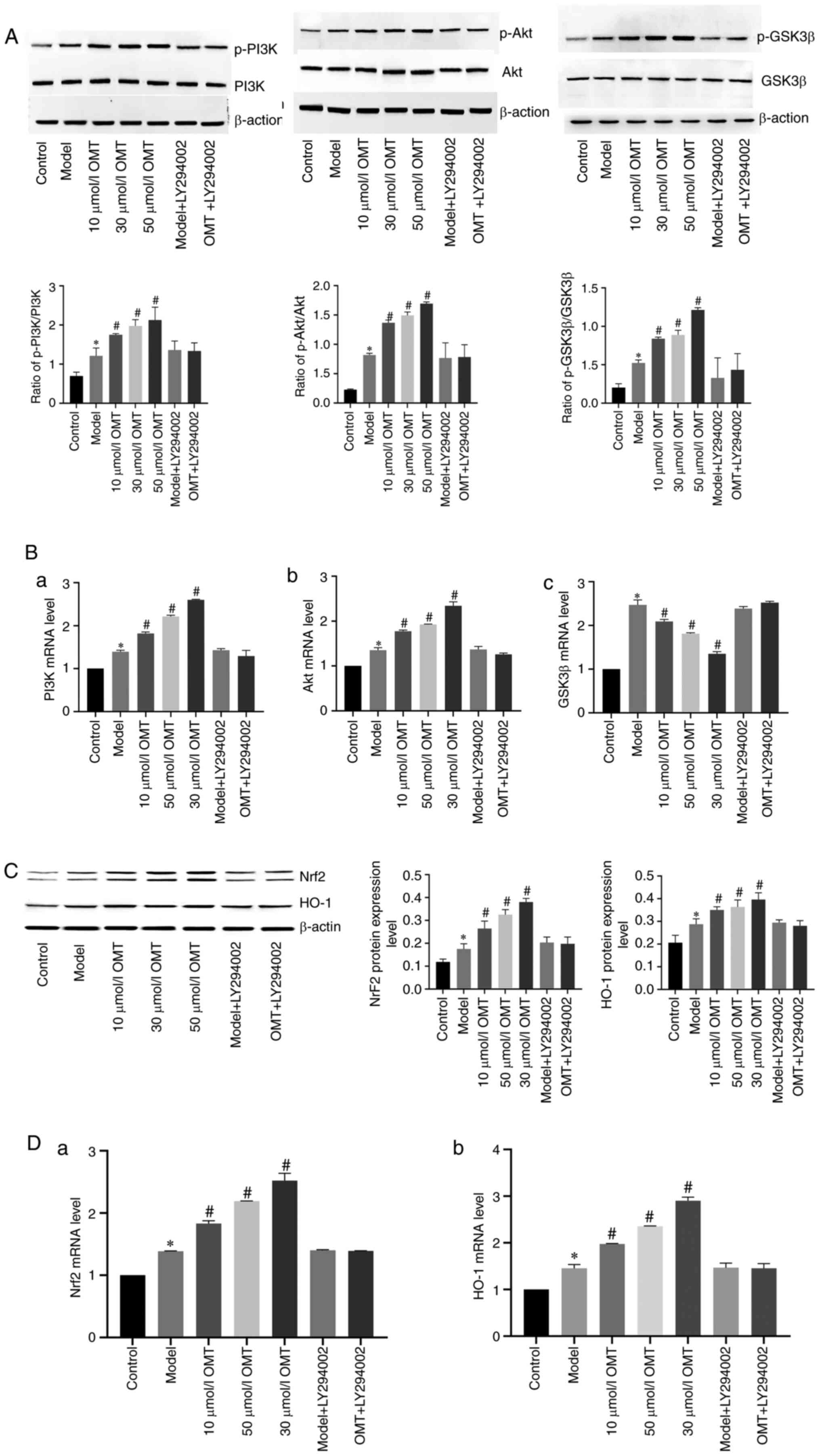 | Figure 5OMT protects H9c2 cardiomyocytes
exposed to H/R by activating the Akt/GSK3β/Nrf2/HO-1 pathway. (A)
After H/R injury and treatment with different concentrations of
OMT, the levels of proteins involved in the PI3K/Akt/GSK3β pathway
were detected using western blotting. (B) The expression of (a)
PI3K, (b) Akt and (c) GSK3β mRNAs measured using RT-qPCR. (C) In
addition, the levels of Nrf2 and HO-1 proteins, which are
downstream targets of the PI3K/Akt/GSK3β pathway, were detected
using western blotting and (D) the expression of (a) Nrf2 and (b)
HO-1 mRNAs were measured using RT-qPCR. *P<0.05
compared with the control group; #P<0.05 compared
with the model group. OMT, oxymatrine; H/R, hypoxia/reoxygenation;
GSK3β, glycogen synthase kinase-3β; Nrf2, nuclear factor
erythroid-2-related factor 2; HO-1, heme oxygenase-1; PI3K,
phosphatidylinositol 3-kinase; RT-qPCR, reverse
transcription-quantitative PCR. |
The Nrf2/HO-1 pathway is a crucial component of the
antioxidant defenses against H/R injury. Western blots (Fig. 5C) revealed increased levels of Nrf2
and HO-1 proteins in the model group compared with the control
group. Thus, H/R injury activated the Nrf2/HO-1 pathway.
Furthermore, no differences were observed between the cells in the
model group and model + LY294002 group, indicating that LY294002
does not alter the Nrf2/HO-1 pathway. The levels of Nrf2 and HO-1
proteins were significantly increased in the 10, 30 and 50 µM OMT
pretreatment groups compared with those in the model group,
suggesting that the protective effect of OMT was associated with
the Nrf2/HO-1 pathway. However, LY294002 reduced the levels of
these factors, indicating that the protective effect of OMT was
mediated by the activation of the Nrf2/HO-1 pathway via the
PI3K/Akt pathway. The expression levels of the Nrf2 and HO-1 mRNAs
in different groups were measured using RT-qPCR (Fig. 5D) and were consistent with the
protein levels, confirming that OMT increased the expression of the
Nrf2 and HO-1 mRNAs and proteins to function as an antioxidant.
Discussion
IHD is a serious threat to human health worldwide.
Acute myocardial infarction (AMI) is one of the main diseases that
constitute IHD. Patients with AMI often have a history of coronary
atherosclerotic heart disease (CAD), and the basic pathological
change in patients with CAD is atherosclerosis (19). In some patients with CAD, the
rupture of atherosclerotic plaques in the coronary arteries due to
fatigue, stress and other factors leads to the rapid accumulation
of platelets, neutrophils and macrophages, which form emboli and
block the vascular cavity, leading to the necrosis of
cardiomyocytes. Myocardial cells are non-renewable, and thus
myocardial blood perfusion must be restored as soon as possible
(20). However, although the
continuous development of PCI, CABG and other technologies has
effectively improved myocardial blood perfusion, I/R injury is a
major problem that remains to be addressed. The apoptosis of
cardiomyocytes mostly occurs during reperfusion and is mainly
mediated by the mitochondrial apoptosis pathway (21). Therefore, according to the
pathogenesis of I/R injury, the identification of a drug that
protects cardiomyocytes exposed to I/R injury is the focus of the
present study.
OMT is an alkaloid that has been widely used
clinically and possesses various biological activities. OMT
pretreatment has been shown to have a protective effect on
cardiomyocytes exposed to I/R injury, but the protective mechanism
has not been fully elucidated (22). Therefore, the present study
simulated human myocardial I/R injury using an in vitro H9c2
cardiomyocyte H/R injury model, and an OMT pretreatment was
administered to explore the protective effect of OMT on
cardiomyocyte H/R injury. OMT effectively protected H9c2
cardiomyocytes with H/R injury. The protective mechanism may be
associated with activation of PI3K/Akt signaling pathway and an
increase in the expression of the downstream proteins GSK3β and
Nrf2.
Under normal physiological conditions, the serum
concentration of LDH is low, and LDH in cells is released only
after cell membrane damage (23).
Therefore, the degree of cell damage can be evaluated by measuring
the LDH level. When cells were exposed to H/R in the present study,
a large amount of LDH was released due to damage of the myocardial
cell membrane, which increased the LDH content in the cell
supernatant. When OMT was added to the cells before H/R injury, the
LDH content of the cell supernatant decreased as the OMT
concentration increased, suggesting that OMT exerted a protective
effect on cell membranes and reduced cell damage. When LY294002 was
added to the cells prior to OMT, the protective effect of OMT on
the cell membrane was weakened, suggesting that the protective
effect of OMT was mediated by the PI3K/Akt signaling pathway. This
result also laid the foundation for the follow-up experiments.
Oxidative stress is an imbalance between oxidant
levels and antioxidant activity in the body. It is often
accompanied by the infiltration of a large number of inflammatory
cells and increased lipid oxidation and decomposition. Oxidative
stress is considered one of the pathological processes that
promotes apoptosis in I/R injury (23,24).
I/R injury causes the production of a large amount of hydrogen
peroxide (H2O2) in cells.
H2O2 interacts with iron in the nucleus to
generate a large quantity of reactive oxygen species (ROS) and
thereby accelerates cell damage. In addition,
H2O2 also interacts with lipids to generate
the lipid oxidation product MDA, which promotes protein
polymerization and accelerates cell apoptosis (25). SOD and CAT are important endogenous
antioxidants in vivo, which effectively remove excess oxygen
free radicals, reduce mitochondrial damage and maintain cell
homeostasis (26). As shown in the
present study, cardiomyocytes exposed to H/R were extensively
damaged, as evidenced by a significant reduction in intracellular
SOD and CAT activities, and a significant increase in the MDA
content, which prevents cells from removing excess ROS and results
in excessive ROS deposition and the exacerbation of cell damage.
When the cardiomyocytes were pretreated with OMT, their SOD and CAT
activities were significantly increased and MDA content was
significantly decreased, indicating that OMT increased the
antioxidant capacity of the cells by increasing the activities of
these antioxidant enzymes in cardiomyocytes and reducing lipid
peroxide levels. However, this biological effect was weakened by
LY294002, suggesting that OMT increased the antioxidant capacity of
H9c2 cardiomyocytes exposed to H/R through the PI3K/Akt signaling
pathway.
In-depth study of myocardial I/R injury has
demonstrated that the Nrf2/HO-1 pathway, a downstream signaling
pathway of the PI3K/Akt pathway (27), plays an important role in oxidative
stress. Under normal circumstances, Nrf2 exists in the cytoplasm in
the form of an inactive complex with its inhibitor, Kelch-like ECH
associated protein 1 (Keap1), and Nrf2 is degraded by the ubiquitin
proteasome pathway. When myocardial tissue undergoes I/R injury and
myocardial cells are exposed to ROS, the Nrf2-Keap 1 complex
quickly separates and Nrf2 translocates to the nucleus, where it
binds the antioxidant response element and initiates the
transcription of downstream antioxidant genes and the phase II
antioxidant enzyme HO-1 to activate antioxidant defenses (28). In addition, activation of the
Nrf2/HO-1 pathway has been shown to upregulate the expression of
the Bcl-2 gene and exert an anti-apoptotic effect (29). In the present study, RT-qPCR
revealed that OMT significantly increased the expression of Nrf2
and the downstream gene HO-1 in H/R-injured cardiomyocytes. Western
blot analyses of these proteins were consistent with the RT-qPCR
analyses of mRNA expression, indicating that OMT activated the
Nrf2/HO-1 signaling pathway to provide an antioxidant effect and
concurrently increased the activity of antioxidant enzymes. When
LY294002 was applied prior to IMP, the ability of OMT to upregulate
Nrf2 and HO-1 was significantly attenuated, suggesting that OMT
activated the Nrf2/HO-1 pathway via the PI3K/Akt pathway while
simultaneously upregulating the expression of HO-1 to exert its
antioxidant effect. In summary, the results indicate that OMT
exerted antioxidant effects through multiple pathways to protect
cardiomyocytes.
The PI3K/Akt/GSK3β signaling pathway is an important
pathway involved in the intracellular transduction of signals from
transmembrane receptors that serve key roles in cell survival
(30), proliferation (31) and apoptosis (32). It is one of the more extensively
investigated signaling pathways in clinical research. According to
numerous studies, this pathway is activated following I/R injury,
and effectively reduces the area of myocardial infarction, which is
also the target of a number of biological molecules and drugs
(14,33,34).
Components of this signaling pathway were analyzed at the protein
and mRNA levels to determine whether the protective effect of OMT
on cardiomyocytes subjected to H/R injury was associated with this
signaling pathway and to verify the pathway upstream of the
Nrf2/HO-1 pathway. Western blotting revealed significantly
increased levels of p-PI3K, p-Akt and p-GSK3β in the OMT group
compared with the model group, suggesting that OMT protected
H/R-injured cardiomyocytes via the activation of PI3K/Akt/GSK3β
signaling. However, RT-qPCR revealed significant increases in the
expression of PI3K and Akt mRNAs in the OMT pretreatment groups
compared with the model group, whereas the expression of the GSK3β
mRNA was significantly decreased. This finding differs from the
western blotting results. According to previous studies, in this
pathway, Akt phosphorylates GSK3β at Ser9, inactivating GSK3β and
phosphorylating β-catenin, thereby promoting cell survival
(35,36). Therefore, OMT may inhibit myocardial
injury by activating the PI3K/Akt/GSK3β and Nrf2/HO-1 pathways.
Apoptosis is a type of programed death characterized
by morphological changes, such as cell shrinkage, nucleolysis and
DNA fragmentation (37). I/R injury
activates the mitochondrial apoptosis pathway, and cardiomyocyte
apoptosis is mainly mediated by the mitochondrial apoptosis pathway
(21). A previous study by Sun
et al (22) demonstrated
that OMT is able to inhibit the mitochondrial apoptosis of
cardiomyocytes injured by H/R in vivo. The mitochondrial
apoptosis pathway mainly involves the Bcl-2 protein family, which
is composed of the proapoptotic protein Bax and the antiapoptotic
protein Bcl-2. The extent of cell necrosis and apoptosis is
determined by regulation of the permeability of the mitochondrial
membrane (38). The caspase protein
family also plays an important role in cell apoptosis. When Bax
binds to the mitochondrial membrane, the gradient in the ion
concentration between the inner and outer membrane of the
mitochondria changes, and cytochrome c flows into the
cytoplasm, forming apoptotic bodies with the apoptotic protein
caspase-9 and activating caspase-3 to induce cell apoptosiss
(39). In the present study, the
expression of apoptosis-associated markers was detected at the mRNA
and protein levels. OMT significantly increased the levels of the
anti-apoptotic protein Bcl-2 and reduced those of the pro-apoptotic
proteins Bax and cleaved caspase-3, suggesting that OMT inhibited
the mitochondrial apoptosis pathway to protect cardiomyocytes
injured by H/R. When the PI3K inhibitor LY294002 was added to
cardiomyocytes prior to OMT, the ability of OMT to regulate the
expression of the anti-apoptotic protein Bcl-2 was significantly
reduced, indicating that the PI3K/Akt signaling pathway was
involved in the anti-apoptotic effect of OMT. In addition TUNEL
staining and flow cytometry assays were also performed in the
present study to supplement and verify these conclusions. The
results of these assays more comprehensively showed that OMT
exerted its anti-apoptotic effects through the PI3K/Akt signaling
pathway and protected H/R-injured cardiomyocytes. By analyzing
different pathological mechanisms, the present study demonstrated
that OMT protected cells from H/R injury and inhibited the
mitochondrial apoptosis pathway in cardiomyocytes.
In summary, the present study provides new insights
into the protective effects of OMT against myocardial I/R injury.
The reperfusion injury salvage kinase signaling pathway, Nrf2/HO-1
signaling pathway and mitochondrial apoptosis pathway were used as
entry points to clarify that the PI3K/Akt signaling pathway is
involved in the protective effect of OMT on H9c2 cardiomyocytes
subjected to H/R injury. The mechanism is hypothesized to be as
follows: When H/R injury occurs in cardiomyocytes, upstream
signaling activates PI3K via the stimulation of membrane receptors
to transduce a signal in the cell. PI3K then transmits the
extracellular signal to the downstream kinase Akt and activates it.
Akt phosphorylates the downstream protein GSK3β to inactivate it,
and finally apoptosis is inhibited via regulation of mitochondrial
permeability. Concurrently, Nrf2, an important transcription factor
downstream of the PI3K/Akt/GSK3β signaling pathway, is also
activated by Akt, functions as an antioxidant and inhibits cell
apoptosis by increasing the expression of the anti-apoptotic
protein Bcl-2. When the H9c2 cardiomyocytes were exposed to H/R
injury after OMT pretreatment, OMT significantly increased the
expression of proteins involved in the Akt/GSK3β/Nrf2/HO-1
signaling pathway, while the PI3K inhibitor LY294002 blocked this
biological effect. The occurrence of this phenomenon strongly
suggests that the PI3K/Akt signaling pathway is involved in the
protective effects of OMT. The OMT pretreatment protected H9c2
cardiomyocytes from H/R-induced cell damage, oxidative stress and
cell apoptosis via a common upstream PI3K/Akt pathway. Based on
these findings, OMT might be a potential candidate treatment for
myocardial I/R injury.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the Key
Project of Tianjin Natural Science Foundation (grant no.
16JCZDJC31900).
Availability of data and materials
All data generated or used during the study are
included in this published article.
Authors' contributions
ZZ, YL, WZ and MZ designed the experiments,
conducted the experiments and wrote the manuscript. ZZ, FC and ZW
performed RT-qPCR, MTT and H&E staining assays. XQ, RC and CL
performed flow cytometry and TUNEL assays. ZZ, CL, ZW, RC and WZ
analyzed the datasets and supervised the project. All authors
reviewed the data and provided feedback on the manuscript. ZZ and
MZ confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hausenloy DJ, Boston-Griffiths E and
Yellon DM: Cardioprotection during cardiac surgery. Cardiovasc Res.
94:253–265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. New Engl J Med. 357:1121–1135. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ma HJ, Li Q, Ma HJ, Guan Y, Shi M, Yang J,
Li DP and Zhang Y: Chronic intermittent hypobaric hypoxia
ameliorates ischemia/reperfusion-induced calcium overload in heart
via Na/Ca2+ exchanger in developing rats. Cell Physiol
Biochem. 34:313–324. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Konstantinidis K, Whelan RS and Kitsis RN:
Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc
Biol. 32:1552–1562. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee S, Kim K, Kim YH, Chung MH, Kang I, Ha
J and Choe W: Preventive role of propofol in
hypoxia/reoxygenation-induced apoptotic H9c2 rat cardiac myoblast
cell death. Mol Med Rep. 4:351–356. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP
and Wu BY: Oxymatrine liposome attenuates hepatic fibrosis via
targeting hepatic stellate cells. World J Gastroenterol.
18:4199–4206. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dong XQ, Du Q, Yu WH, Zhang ZY, Zhu Q, Che
ZH, Chen F, Wang H and Chen J: Anti-inflammatory effects of
oxymatrine through inhibition of nuclear factor-kappa B and
mitogen-activated protein kinase activation in
lipopolysaccharide-induced BV2 microglia cells. Iran J Pharm Res.
12:165–174. 2013.PubMed/NCBI
|
|
8
|
Ye J, Zou µM, Li P, Lin XJ, Jiang QW, Yang
Y, Huang JR, Yuan ML, Xing ZH and Wei MN: Oxymatrine and cisplatin
synergistically enhance anti-tumor immunity of CD8(+) T cells in
non-small cell lung cancer. Front Oncol. 8(631)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao YG, Jing S, Li L, Gao JQ, Shen ZY, Liu
Y, Xing Y, Wu ML, Wang Y and Xu CQ: Antiarrhythmic effects and
ionic mechanisms of oxymatrine from Sophora flavescens.
Phytother Res. 24:1844–1849. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zhang M, Wang X, Wang X, Hou X, Teng P,
Jiang Y, Zhang L, Yang X, Tian J and Li G: Oxymatrine protects
against myocardial injury via inhibition of JAK2/STAT3 signaling in
rat septic shock. Mol Med Rep. 7:1293–1299. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dai G, Li B, Xu Y, Zeng Z and Yang H:
Oxymatrine prevents the development of monocrotaline-induced
pulmonary hypertension via regulation of the N(G),
N(G)-dimethyl-L-arginine metabolism pathways in rats. Eur J
Pharmacol. 842:338–344. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ge XH, Shao L and Zhu GJ: Oxymatrine
attenuates brain hypoxic-ischemic injury from apoptosis and
oxidative stress: Role of p-Akt/ GSK3β//HO-1/Nrf-2 signaling
pathway. Metab Brain Dis. 33:1869–1875. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li M, Zhang X, Cui L, Yang R, Wang L, Liu
L and Du W: The neuroprotection of oxymatrine in cerebral
ischemia/reperfusion is related to nuclear factor erythroid
2-related factor 2 (nrf2)-mediated antioxidant response: Role of
nrf2 and hemeoxygenase-1 expression. Biol Pharm Bull. 34:595–601.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Wang H, Liu N, Du J, Lan XB, Qi X,
Zhuang CL, Sun T, Li YX and Yu JQ: Oxymatrine protects neonatal rat
against hypoxic-ischemic brain damage via PI3K/Akt/GSK3β pathway.
Life Sci. 254(116444)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao TT, Yang TL, Gong L and Wu P:
Isorhamnetin protects against hypoxia/reoxygenation-induced injure
by attenuating apoptosis and oxidative stress in H9c2
cardiomyocytes. Gene. 666:92–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Min J and Wei C: Hydroxysafflor yellow A
cardioprotection in ischemia-reperfusion (I/R) injury mainly via
Akt/hexokinase II independent of ERK/GSK-3β pathway. Biomed
Pharmacother. 87:419–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meng Y, Li WZ, Shi YW, Zhou BF, Ma R and
Li WP: Danshensu protects against ischemia/reperfusion injury and
inhibits the apoptosis of H9c2 cells by reducing the calcium
overload through the p-JNK-NF-κB-TRPC6 pathway. Int J Mol Med.
37:258–266. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(-Delta Delta C(T) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kingstone L, Currie GM and Torres C: The
pathogenesis, analysis, and imaging methods of atherosclerotic
disease of the carotid artery: Review of the literature. J Med
Imaging Radiat Sci. 43:84–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tong G, von Garlen NNA, Wowro SJ, Lam PD,
Krech J, Berger F and Schmitt KRL: Post-TTM rebound pyrexia after
ischemia-reperfusion injury results in sterile inflammation and
apoptosis in cardiomyocytes. Mediators Inflamm.
2019(6431957)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ilmarinen P, Moilanen E and Kankaanranta
H: Mitochondria in the center of human eosinophil apoptosis and
survival. Int J Mol Sci. 15:3952–3969. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun HL, Lei L, Lei S, Dan Z, De-Li D,
Guo-Fen Q, Yan L, Wen-Feng C and Bao-Feng Y: Cardioprotective
effects and underlying mechanisms of oxymatrine against Ischemic
myocardial injuries of rats. Phytother Res. 22:985–989.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Li T, Chen L, Yu Y, Yang B, Li P and Tan
XQ: Resveratrol alleviates hypoxia/reoxygenation injuryinduced
mitochondrial oxidative stress in cardiomyocytes. Mol Med Rep.
19:2774–2780. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sung HK, Song E, Jahng JWS, Pantopoulos K
and Sweeney G: Iron induces insulin resistance in cardiomyocytes
via regulation of oxidative stress. Sci Rep. 9(4668)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Heusch G, Libby P, Gersh B, Yellon D, Bohm
M, Lopaschuk G and Opie L: Cardiovascular remodelling in coronary
artery disease and heart failure. Lancet. 383:1933–1943.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dai DF, Chiao YA, Marcinek DJ, Szeto HH
and Rabinovitch PS: Mitochondrial oxidative stress in aging and
healthspan. Longev Healthspan. 6(3)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang Y, Che J, Zhao H, Tang J and Shi G:
Platycodin D inhibits oxidative stress and apoptosis in H9c2
cardiomyocytes following hypoxia/reoxygenation injury. Biochem
Biophys Res Commun. 503:3219–3224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kansanen E, Kuosmanen SM, Leinonen H and
Levonen AL: The Keap1-Nrf2 pathway: Mechanisms of activation and
dysregulation in cancer. Redox Biol. 1:45–49. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fan J, Xu G, Jiang T and Qin Y:
Pharmacologic induction of heme oxygenase-1 plays a protective role
in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci.
53:6541–6556. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang D, Zhang X, Li D, Hao W, Meng F, Wang
B, Han J and Zheng Q: Kaempferide protects against myocardial
ischemia/reperfusion injury through activation of the
PI3K/Akt/GSK-3beta pathway. Mediators Inflamm.
2017(5278218)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu L, Jiang X, Wei F and Zhu H: Leonurine
protects cardiac function following acute myocardial infarction
through antiapoptosis by the PI3K/AKT/GSK3β signaling pathway. Mol
Med Rep. 18:1582–1590. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang Q, Zheng X and Zhang J: Long
non-coding RNA CRNDE promotes heptaocellular carcinoma cell
proliferation by regulating PI3K/Akt/β-catenin signaling. Biomed
Pharmacother. 103:1187–1193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao Q, Li H, Chang L, Wei C, Yin Y, Bei
H, Wang Z, Liang J and Wu Y: Qiliqiangxin attenuates oxidative
stress-induced mitochondrion-dependent apoptosis in cardiomyocytes
via PI3K/AKT/GSK3β signaling pathway. Biol Pharm Bull.
42:1310–1321. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bhat RV, Shanley J, Correll MP, Fieles WE,
Keith RA, Scott CW and Lee CM: Regulation and localization of
tyrosine216 phosphorylation of glycogen synthase kinase-3beta in
cellular and animal models of neuronal degeneration. Proc Natl Acad
Sci U S A. 97:11074–11079. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li X, Zhang J, Zhu X, Wang P, Wang X and
Li D: Progesterone reduces inflammation and apoptosis in neonatal
rats with hypoxic ischemic brain damage through the PI3K/Akt
pathway. Int J Clin Exp Med. 8:8197–8203. 2015.PubMed/NCBI
|
|
37
|
Xiao TT, Wang YY, Zhang Y, Bai CH and Shen
XC: Similar to spironolactone, oxymatrine is protective in
aldosterone-induced cardiomyocyte injury via inhibition of calpain
and apoptosis-inducing factor signaling. PLoS One.
9(e88856)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Javadov S, Hunter JC, Barreto-Torres G and
Parodi-Rullan R: Targeting the mitochondrial permeability
transition: Cardiac ischemia-reperfusion versus carcinogenesis.
Cell Physiol Biochem. 27:179–190. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX,
Lv BJ, Nie SF, Wang J, Iwakura Y and Xiao H: Interleukin-17A
contributes to myocardial ischemia/reperfusion injury by regulating
cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll
Cardiol. 59:420–429. 2012.PubMed/NCBI View Article : Google Scholar
|