Introduction
Allergic rhinitis (AR) is one of the more common
chronic inflammatory diseases. Its main feature is nasal mucositis,
which is a non-infectious rhinitis caused by an immune response
mediated by immunoglobulin E (IgE) (1). AR induces upper respiratory tract
inflammation, which is associated with the mediators released by
several types of hypersensitive immune cells (2). At present, patients with AR present
with various complications, such as chronic rhinosinusitis and
asthma (3). Two types of AR have
been identified, including seasonal and perennial. Seasonal AR is
caused by exposure to seasonal allergens. Common seasonal allergens
are mainly pollen, grass and weeds, while perennial allergens are
mainly dust mites, mold, animal dander and other allergens
(4). AR affects the social life of
sufferers to a large extent, and can cause significant financial
burden to patients (1). The
advancement of medical technology has enabled the development of
several treatments for AR, including intranasal steroids,
antihistamines, leukotriene receptor antagonists and immunotherapy
(5,6). However, 20% of patients with AR do
not exhibit improved symptoms (7).
Therefore, the development of novel treatment strategies for AR is
imperative.
Lidocaine is a local anesthetic with
anti-inflammatory effects. It is the only local anesthetic compound
that can be injected intravenously (8,9). At
present, lidocaine is widely used for invasive anesthesia, central
axonal anesthesia and peripheral nerve block (10). In addition, this compound can
prevent myocardial infarction and heart disease (11). Detailed studies on lidocaine have
revealed that it exhibits optimal effects in the treatment of
specific diseases, including epilepsy, asthma, acute
gastric-induced abdominal pain and cancer (12-16).
Liu et al (15) indicated
that lidocaine inhibited cell viability by regulating the transient
receptor potential cation channel, subfamily M, member 7 in breast
cancer. Zhao et al (16)
indicated that lidocaine inhibited cancer development via the
circular RNA_itchy E3 ubiquitin protein ligase/microRNA
(miR)-421/cytoplasmic polyadenylation element binding protein 3
axis in hepatocellular carcinoma. Previous studies have revealed
that injection of lidocaine can reduce inflammation and relieve
pain following double maxillary surgery (17). Guang et al (18) indicated that lidocaine exerted its
anti-inflammatory effects by inhibiting the NF-κB signaling
pathway. In recent years, an increasing number of studies have
revealed the anti-inflammatory effects of lidocaine (19,20).
In addition, a recent study indicated that nebulized lidocaine
could ameliorate allergic airway inflammation via downregulation of
toll-like receptor (TLR)2(21). As
AR is a chronic inflammatory disease, it was hypothesized that
lidocaine may play a protective role in AR by inhibiting
inflammation. However, the effects of lidocaine on AR have not been
previously reported. Therefore, an AR mouse model was established
to explore the role of lidocaine in AR and further analyze its
potential molecular mechanisms.
Materials and methods
Establishment of an ovalbumin
(OVA)-induced AR model
A total of 80 male BALB/c mice (age, 6 weeks;
weight, ~20 g) were purchased from the Experimental Animal Center
of Nanjing University. All animal experiments were performed
according to the protocol approved by the Wuhan Jinyintan Hospital
Committee on Care and Use of Laboratory Animals (Wuhan, China).
The mice were kept in a sterile environment at a
temperature of 20±1˚C and a 12-h dark/light cycle. All mice were
allowed full access to water and food. The animal experiments were
conducted according to strict procedures. The mice were sensitized
for the first time on days 0, 7 and 14 by intraperitoneal injection
of 200 µl saline containing 25 µg OVA (Sigma-Aldrich; Merck KGaA)
and 2 mg aluminum hydroxide. Then, 1 week after the last
intraperitoneal injection, the mice were challenged intranasally
daily and 3% OVA was diluted in 20 µl saline for a second
immunization. The mice of the control group were injected with
saline without OVA and aluminum hydroxide (22).
Intranasal administration of
lidocaine
The total amount for intranasal administration is
usually 20 µl (23). During the
experiment, 1, 3 and 5 mg/kg/day lidocaine was intranasally
administered to mice (n=10) 3 h prior to OVA challenge on days
28-34. The mice were intranasally administered 20 µl saline 3 h
prior to every daily OVA challenge (once a day) on days 28-34 in
the AR group. Mice were divided into the following groups: Control,
mice (n=10) without any treatment; AR, mice (n=10) intranasally
administered 20 µl saline 3 h prior to every daily OVA challenge
(once a day) on days 28-34; AR + Lidocaine (1 mg/kg): 1 mg/kg/day
lidocaine was intranasally administered to mice (n=10) 3 h prior to
OVA challenge on days 28-34; AR + Lidocaine (3 mg/kg), 3 mg/kg/day
lidocaine was intranasally administered to mice (n=10) 3 h prior to
OVA challenge on days 28-34; and AR + Lidocaine (5 mg/kg), 5
mg/kg/day lidocaine was intranasally administered to mice (n=10) 3
h prior to OVA challenge on days 28-34.
For NF-κB inhibitor IMD-0354 (Sigma-Aldrich; Merck
KGaA) and p38 MAPK inhibitor SB 203580 (Sigma-Aldrich; Merck KGaA)
treatment, mice were divided into the following groups: AR, mice
(n=10) were intranasally administered 20 µl saline 3 h prior to
every daily OVA challenge (once a day) on days 28-34; AR + IMD, 5
mg/kg/day IMD-0354 was intranasally administered to mice (n=10) 3 h
prior to OVA challenge on days 28-34; AR + SB 203580, 2 mg/kg/day
SB 203580 was intranasally administered to mice (n=10) 3 h prior to
OVA challenge on days 28-34.
The mice were anesthetized with an intraperitoneal
injection of pentobarbital sodium (Sigma-Aldrich; Merck KGaA; 50
mg/kg) and sacrificed by cervical dislocation on day 35. Animal
sacrifice was defined as the lack of heartbeat and breathing. The
peripheral blood and nasal lavage fluid (NLF) were subsequently
harvested following euthanasia. Blood and NLF samples were
collected from mice, followed by centrifugation at 4˚C for 10 min
at 1,600 x g. The serum and NLF supernatant were stored at -80˚C
for subsequent measurements.
Evaluation of nasal symptoms
Following the last OVA challenge, the animals were
kept in their cages for 30 min and the parameters including
sneezing frequency and nose rubbing were recorded. The following
scores were calculated: i) Slight rubbing of the nose several times
or sneezing <3 times; ii) repeated nose rubbing or sneezing
>3 times and <10 times; and iii) rubbing from nose to face or
sneezing ≥11 times.
Inflammatory cell counting
The inflammatory cells (leucocytes, eosinophils,
neutrophils and lymphocytes) in NLF were resuspended in 1 ml PBS
(100 mM) with 1% BSA (Beyotime Institute of Biotechnology). The
number of leukocytes was counted using a hemocytometer.
Wright's-Giemsa staining (cat. no. E607315; Sangon Biotech) was
performed at 37˚C for 20 min according to the manufacturer's
protocol, and the numbers of eosinophils, neutrophils and
lymphocytes were evaluated with a light microscope at a
magnification of x200.
ELISA
The assay was performed to examine the expression
levels of IL-4 (cat. no. ab100710; Abcam), IL-5 (cat. no. ab204523;
Abcam), IL-13 (cat. no. ab219634; Abcam), IL-17 (cat. no. ab100702;
Abcam), TNF-α (cat. no. ab208348; Abcam), IFN-γ (cat. no. PI508;
Beyotime Institute of Biotechnology), OVA-specific IgE (cat. no.
439807-1; BioLegend, Inc.), leukotriene C4 (LTC4; cat. no.
EK-M28299; EK-Bioscience) and IL-2 (cat. no. PI575; Beyotime
Institute of Biotechnology) in inflammation. Each specific ELISA
kit was used to detect the expression levels of specific markers
according to manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was acquired using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
procedure provided by the manufacturer. RNA concentration was
detected using NanoDrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). Total RNA was transformed into cDNA by HiScript
1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd.) according
to the manufacturer's instructions. Subsequently, cDNA was used for
amplification. RT-qPCR was performed with a SYBR-Green PCR kit
(Vazyme Biotech Co., Ltd.) as determined by the manufacturer's
instructions. Thermocycling conditions were used as follows:
Initial denaturation at 94˚C for 15 min; followed by 38 cycles at
94˚C for 15 sec (denaturation), 60˚C for 15 sec (annealing) and
72˚C for 15 sec (extension). GAPDH was used as an endogenous
control. The 2-ΔΔCq method (24) was used to quantify relative gene
expression. The primer sequences for qPCR were as follows: GAPDH
forward, 5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; p38 forward,
5'-GACGAATGGAAGAGCCTGAC-3' and reverse,
5'-AGATACATGGACAAACGGACA-3'; p65 forward,
5'-ACCAACACAGACCCAGGGAGT-3' and reverse,
5'-CAGTCACCAGGCGAGTTATAG-3'.
Western blot analysis
The total protein from the mouse nasal mucosa was
obtained using RIPA buffer (Beijing Solarbio Science &
Technology Co., Ltd.). A bicinchoninic acid assay kit (Pierce;
Thermo Fisher Scientific, Inc.) was used to quantify the total
protein. Equal amounts of proteins (20 µg protein per lane) were
separated by 12% SDS-PAGE for 40 min and subsequently transferred
to polyvinylidene fluoride membranes. The membranes were blocked at
room temperature for 1.5 h with 5% non-fat milk to prevent
non-specific binding and subsequently incubated with primary
antibodies including anti-phosphorylated (p)-p65 (1:1,000; product
code ab86299), anti-p65 (1:1,000; product code ab16502), anti-p38
(1:1,000; product code ab170099) and anti-p-p38 (1:1,000; product
code ab4822; all from Abcam) at 4˚C overnight. The following
morning, the membranes were incubated with HRP-linked secondary
antibody (1:2,000; cat no. 7074; Cell Signaling Technology, Inc.)
at room temperature for 2 h. The protein bands were visualized by
enhanced chemiluminescence method (Cytiva). GAPDH (1:1,000; product
code ab181602; Abcam) was used as the loading control for
normalization. ImageJ v.2.0 software (National Institutes of
Health) was used to quantify the band intensity.
Statistical analysis
Experiments were repeated three times. Data are
presented as the mean ± SD from at least three independent
experiments. The results were analyzed using GraphPad Prism 6.0
(GraphPad Software, Inc.) software. Statistical significance of the
differences between groups was determined by one-way ANOVA followed
by Tukey's post hoc test. The data were estimated from three
independent experiments. P<0.05 was considered to indicate
statistically significant differences.
Results
Effects of lidocaine on AR mice
To explore the role of lidocaine in AR, an AR mouse
model was initially established. Different concentrations (1, 3 and
5 mg/kg/day) of lidocaine were injected into mice by intranasal
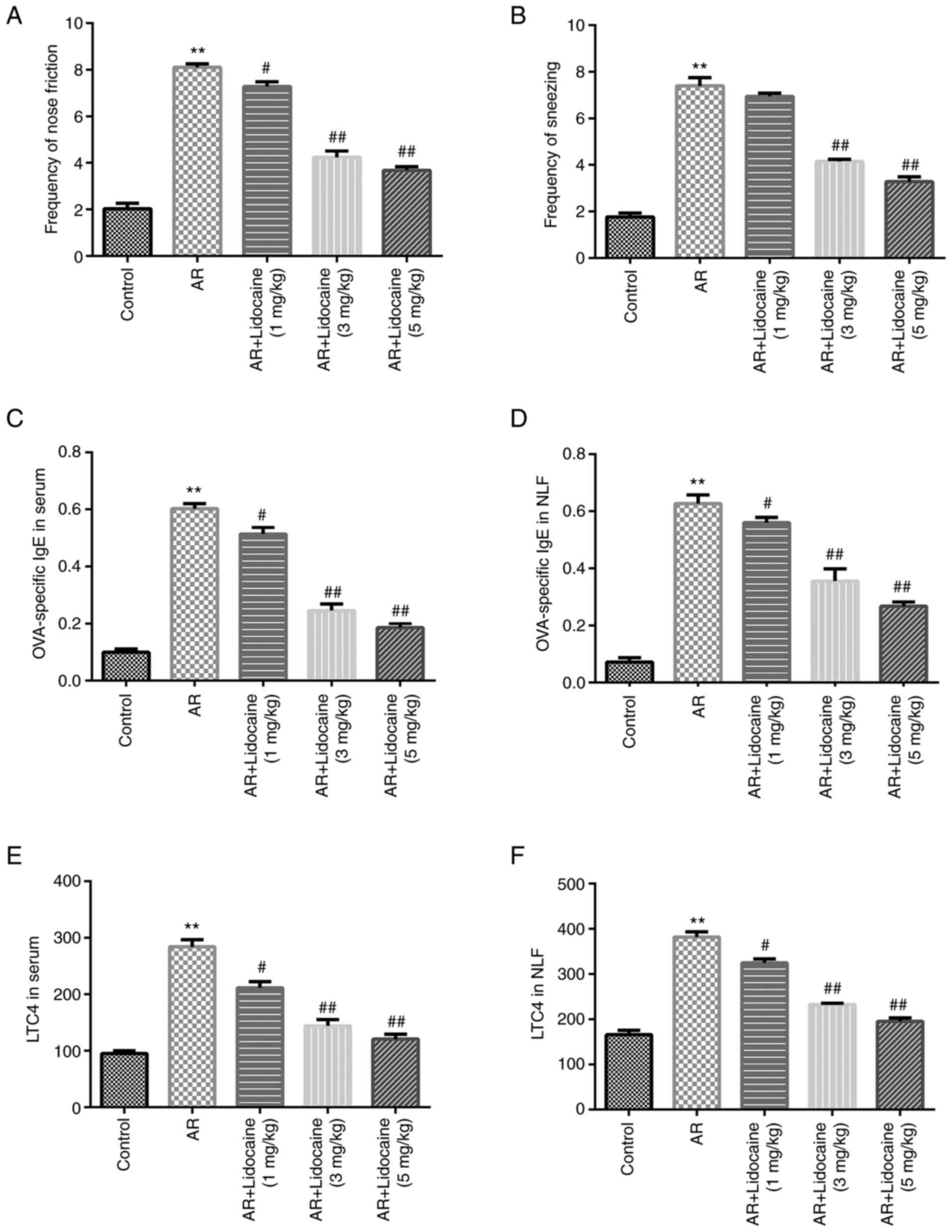
administration. The results indicated that the frequency of
sneezing and nose rubbing of the mice in the AR group was
significantly higher compared with that of the control group. In
addition, lidocaine reduced the frequency of nose rubbing (Fig. 1A) and sneezing (Fig. 1B) in AR mice in a dose-dependent
manner. Furthermore, the expression levels of OVA-specific IgE and
LTC4 were increased in serum and NLF in the AR group. However,
lidocaine significantly inhibited OVA-specific IgE expression
(Fig. 1C and D) and LTC4 expression (Fig. 1E and F).
Effects of lidocaine on inflammatory
cells in AR mice
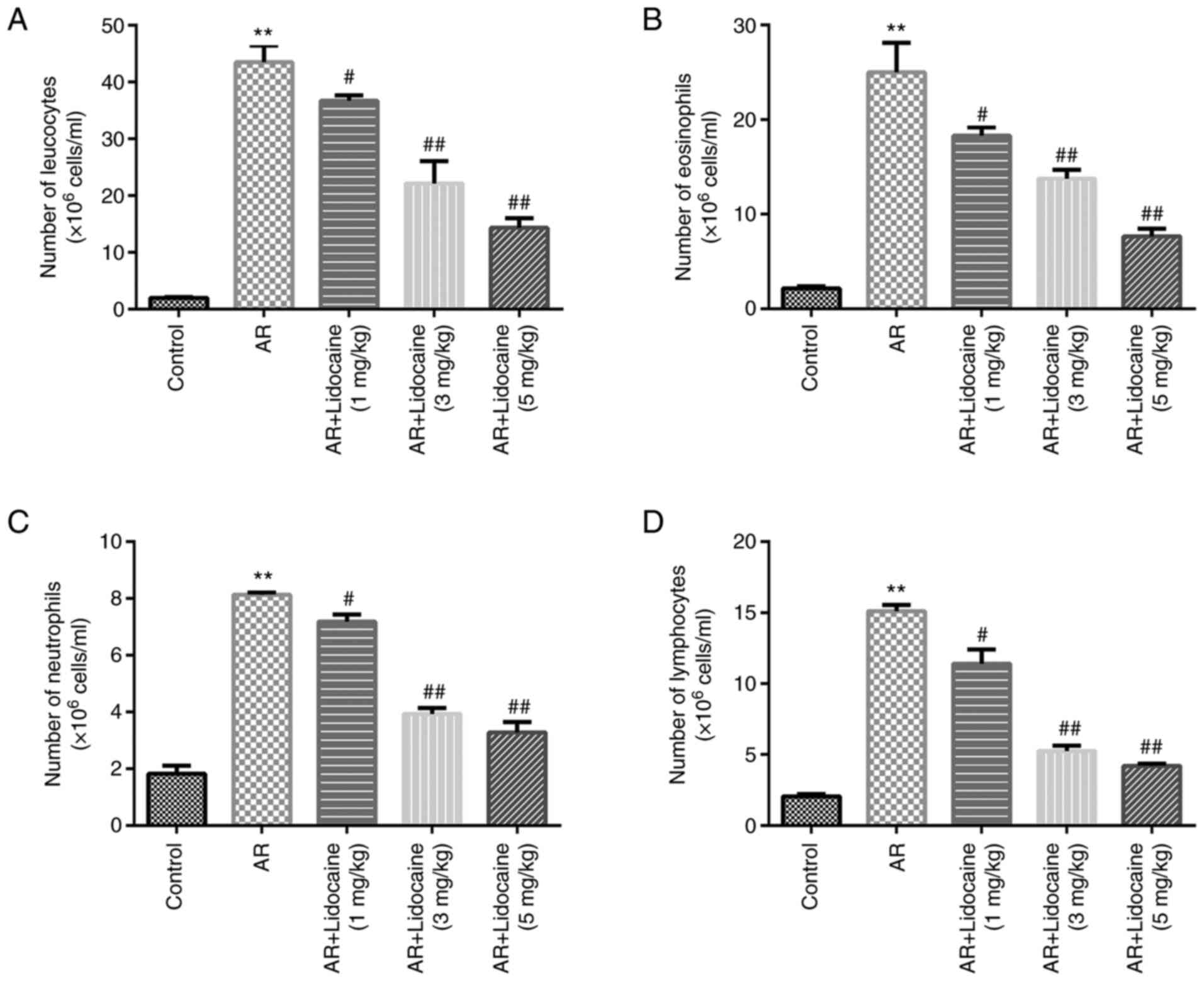
Subsequently, the number of inflammatory cells was
examined. The numbers of leukocytes, eosinophils, neutrophils and
lymphocytes were significantly increased in the AR group, whereas
lidocaine decreased the numbers of leukocytes, eosinophils,
neutrophils and lymphocytes (Fig.
2A-D) in AR mice.
Effects of lidocaine on the
inflammatory response of AR mice
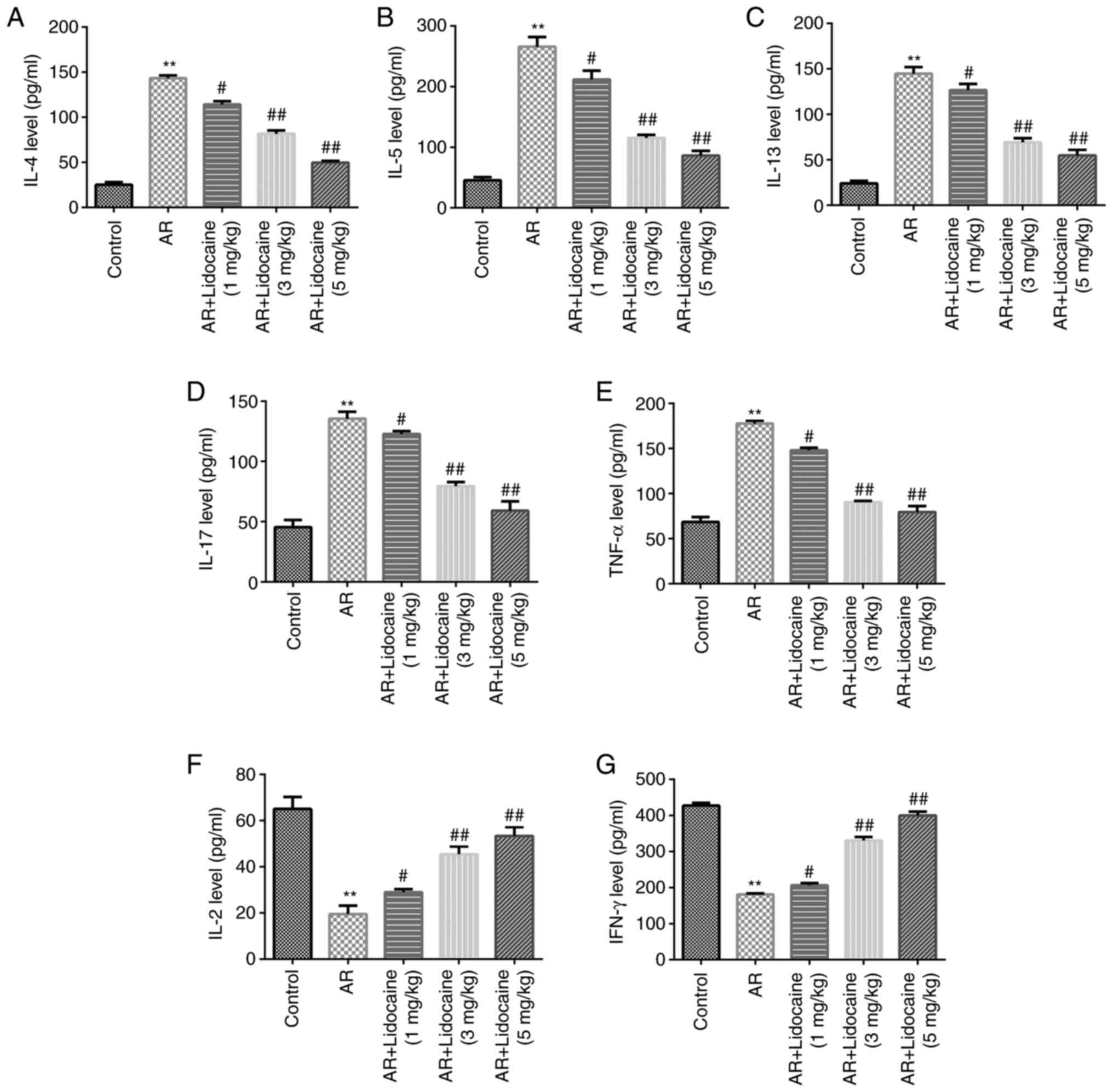
The ability of lidocaine to affect the T helper type
(Th)1/Th2/Th17 imbalance was assessed in AR mice. The expression
levels of the inflammatory factors IL-4, IL-5, IL-13, IL-17, TNF-α
and IFN-γ were determined by ELISA. The results indicated that
IL-4, IL-5, IL-13, IL-17 and TNF-α were positively expressed in the
serum of AR mice, whereas the release of IFN-γ and IL-2 was
significantly reduced. However, lidocaine caused a downregulation
in the expression levels of IL-4, IL-5, IL-13, IL-17 and TNF-α,
whereas it increased IFN-γ and IL-2 release in AR mice (Fig. 3A-G).
NF-κB and p38 MAPK signaling pathways
are involved in the lidocaine-mediated reduction of AR in mice
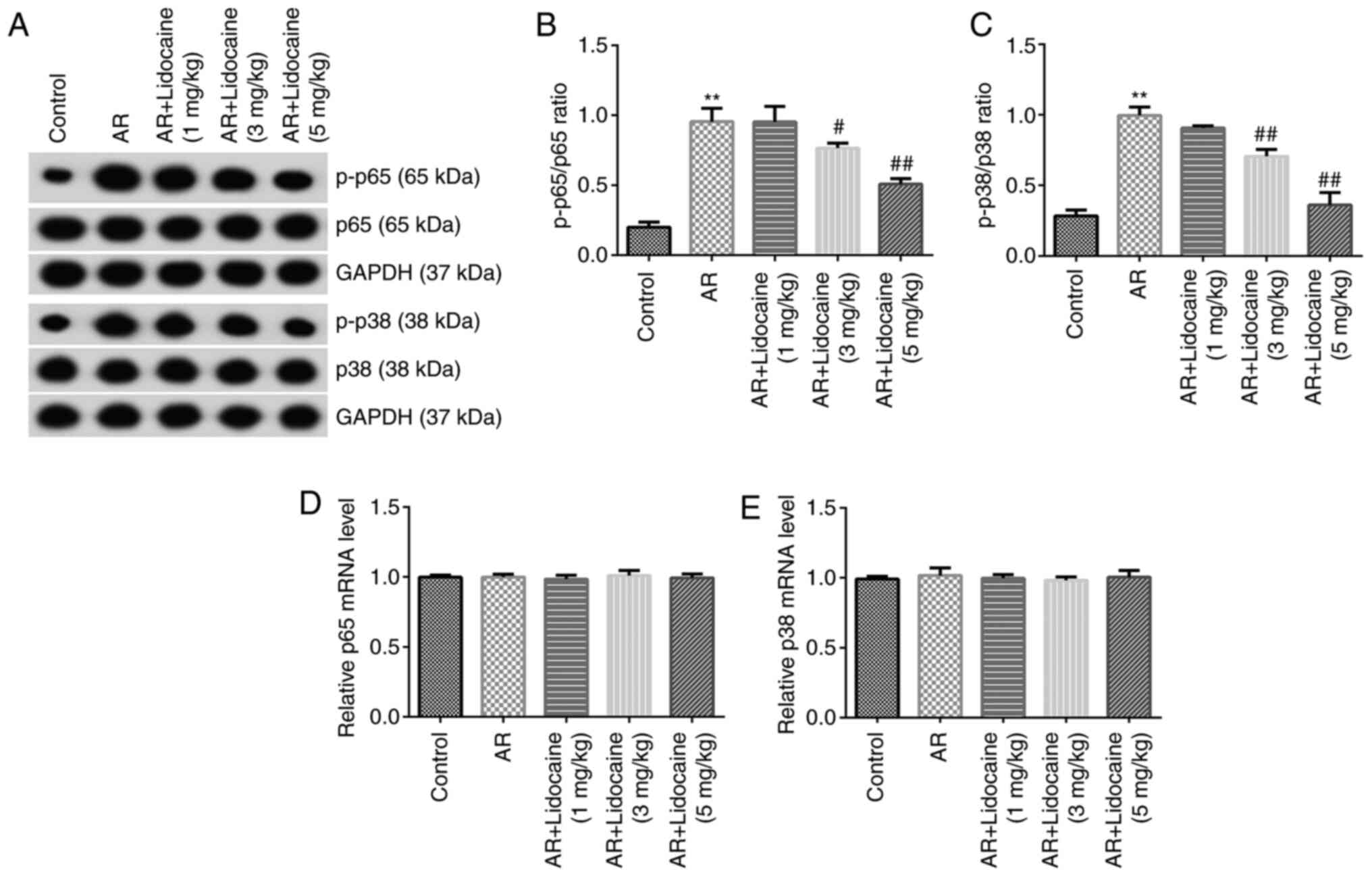
Subsequently, the expression levels of the proteins
associated with the NF-κB and p38 MAPK signaling pathways were
examined by western blot analysis. The results indicated that the
expression levels of p-p65 and p-p38 were upregulated in the AR
group and the corresponding ratios of p-p65/p65 and p-p38/p38 were
increased. Lidocaine decreased p-p38 and p-p65 expression and
reduced the ratio of p-p65/p65 and p-p38/p38 (Fig. 4A-C). The mRNA expression levels of
p65 and p38 were not significantly different among all groups
(Fig. 4D and E).
NF-κB inhibitor (IMD-0354) and p38
MAPK inhibitor (SB 203580) suppress the expression levels of the
proteins associated with the NF-κB and p38 MAPK signaling
pathways
To further investigate the roles of the NF-κB and
p38 MAPK signaling pathways in the development of AR in mice, AR
mice were treated with IMD-0354 (5 mg/kg) or SB 203580 (2 mg/kg) by
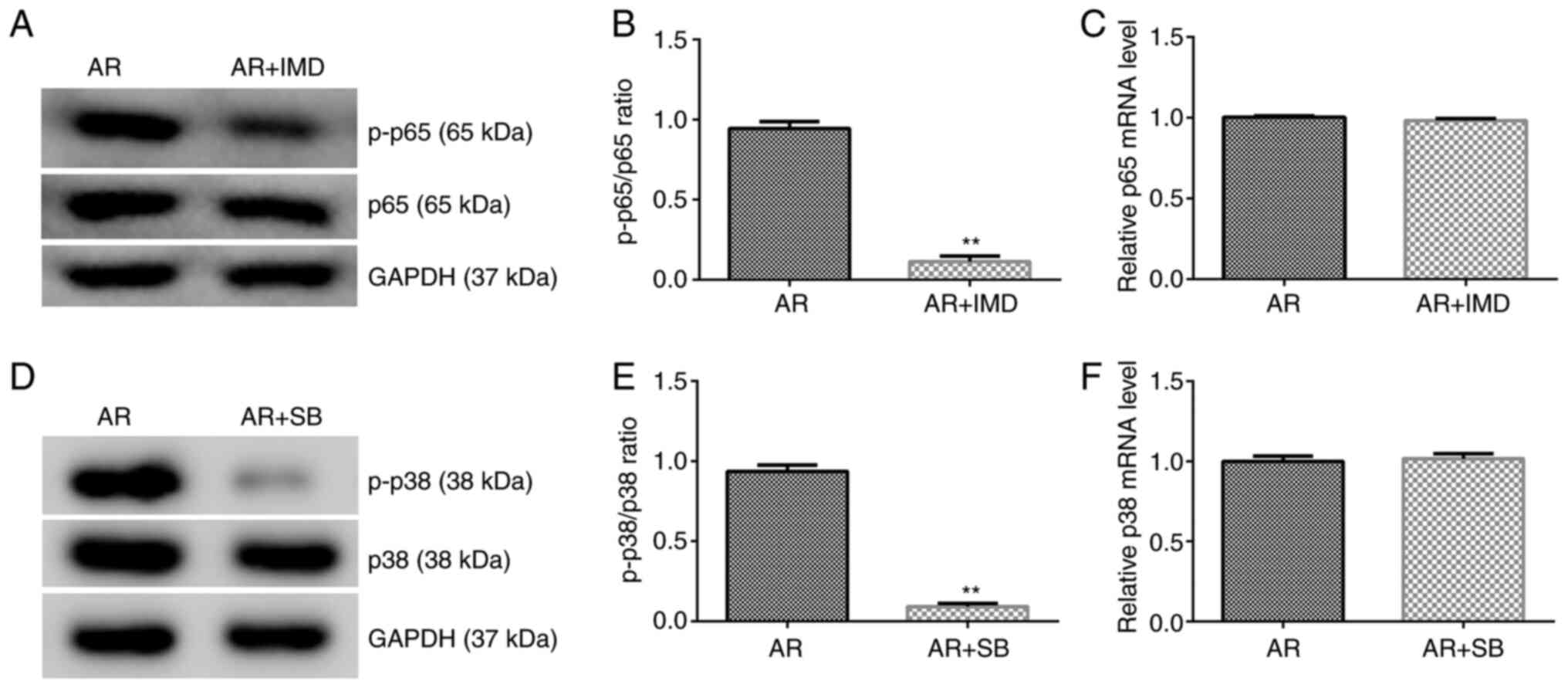
intranasal administration. The NF-κB inhibitor IMD-0354 suppressed
p-p65 protein expression and reduced the ratio of p-p65/p65
compared with that of the AR group (Fig. 5A and B). However, the mRNA expression levels of
p65 did not change significantly between the groups (Fig. 5C). The p38 MAPK inhibitor SB 203580
suppressed p-p38 protein expression (Fig. 5D) and reduced the ratio of
p-p38/p38 (Fig. 5D and E). However, the mRNA expression levels of
p38 did not change significantly between the different groups
examined (Fig. 5F).
Effects of the NF-κB and p38 MAPK
inhibitors on AR mice
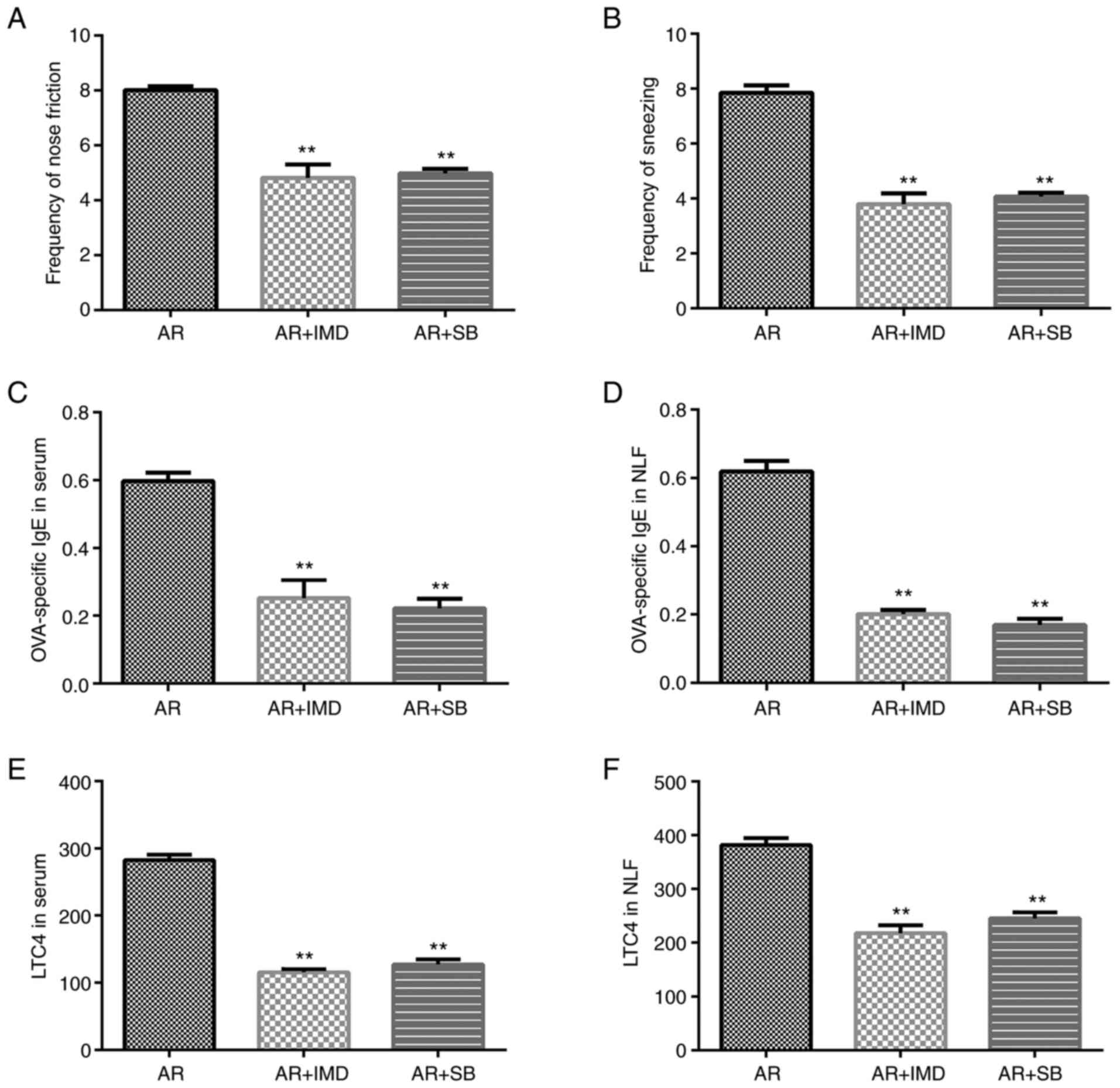
Subsequently, the effects of IMD-0354 and SB 203580
were investigated on the nasal symptoms of AR mice. Both IMD-0354
and SB 203580 reduced the frequency of sneezing and nose rubbing of
the mice compared with that of the AR group (Fig. 6A and B). In addition, the expression levels of
OVA-specific IgE and LTC4 were increased in the serum and NLF
derived from the AR group. However, IMD-0354 and SB 203580
significantly inhibited OVA-specific IgE (Fig. 6C and D) and LTC4 (Fig. 6E and F) expression levels.
Effects of the NF-κB and the p38 MAPK
inhibitors on inflammatory cells in AR mice
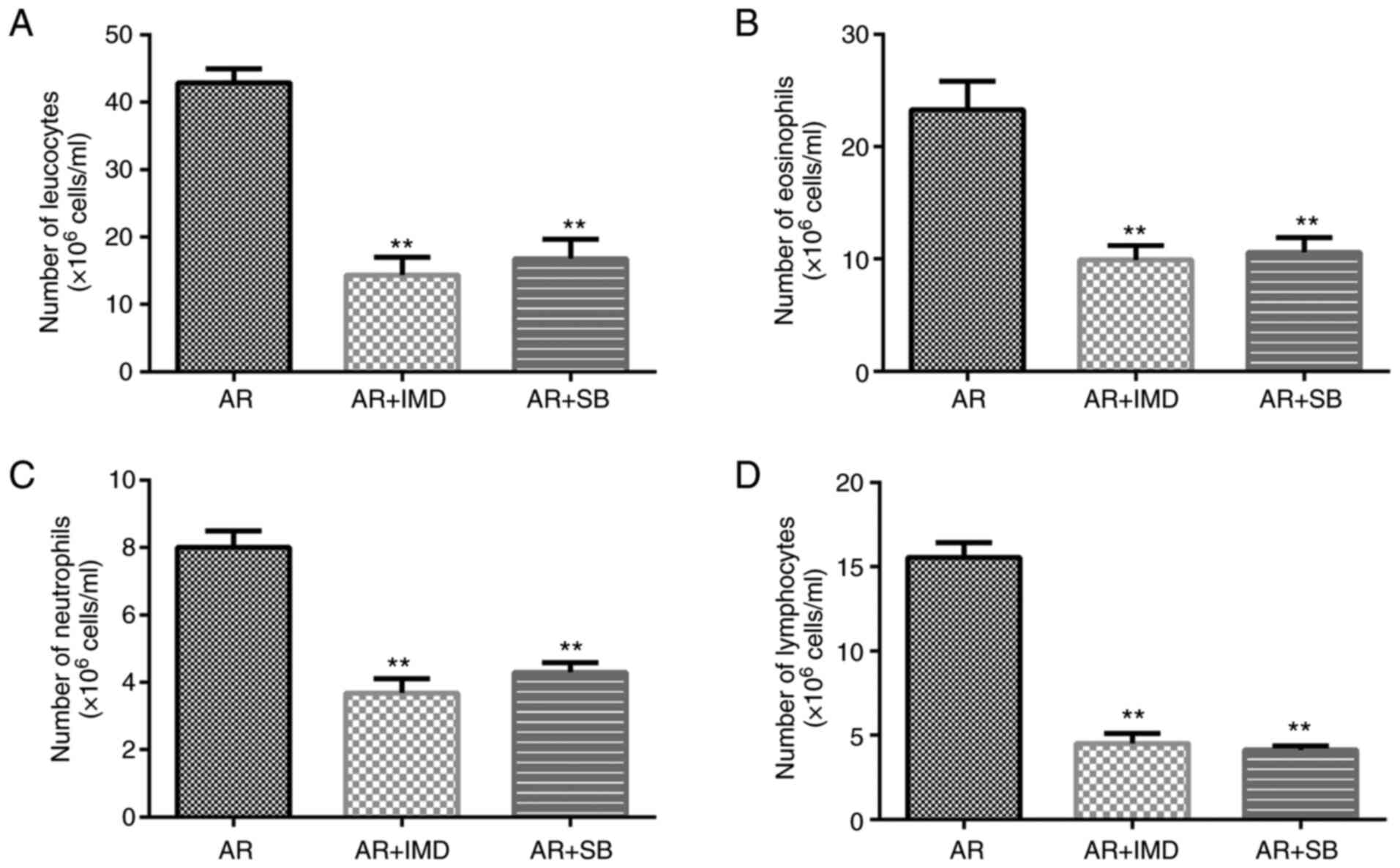
The number of inflammatory cells was also counted.
The data indicated that IMD-0354 and SB 203580 decreased the
numbers of leukocytes, eosinophils, neutrophils and lymphocytes
compared with those of the AR group (Fig. 7).
Effects of the NF-κB and MAPK
inhibitors on the inflammatory response of AR mice
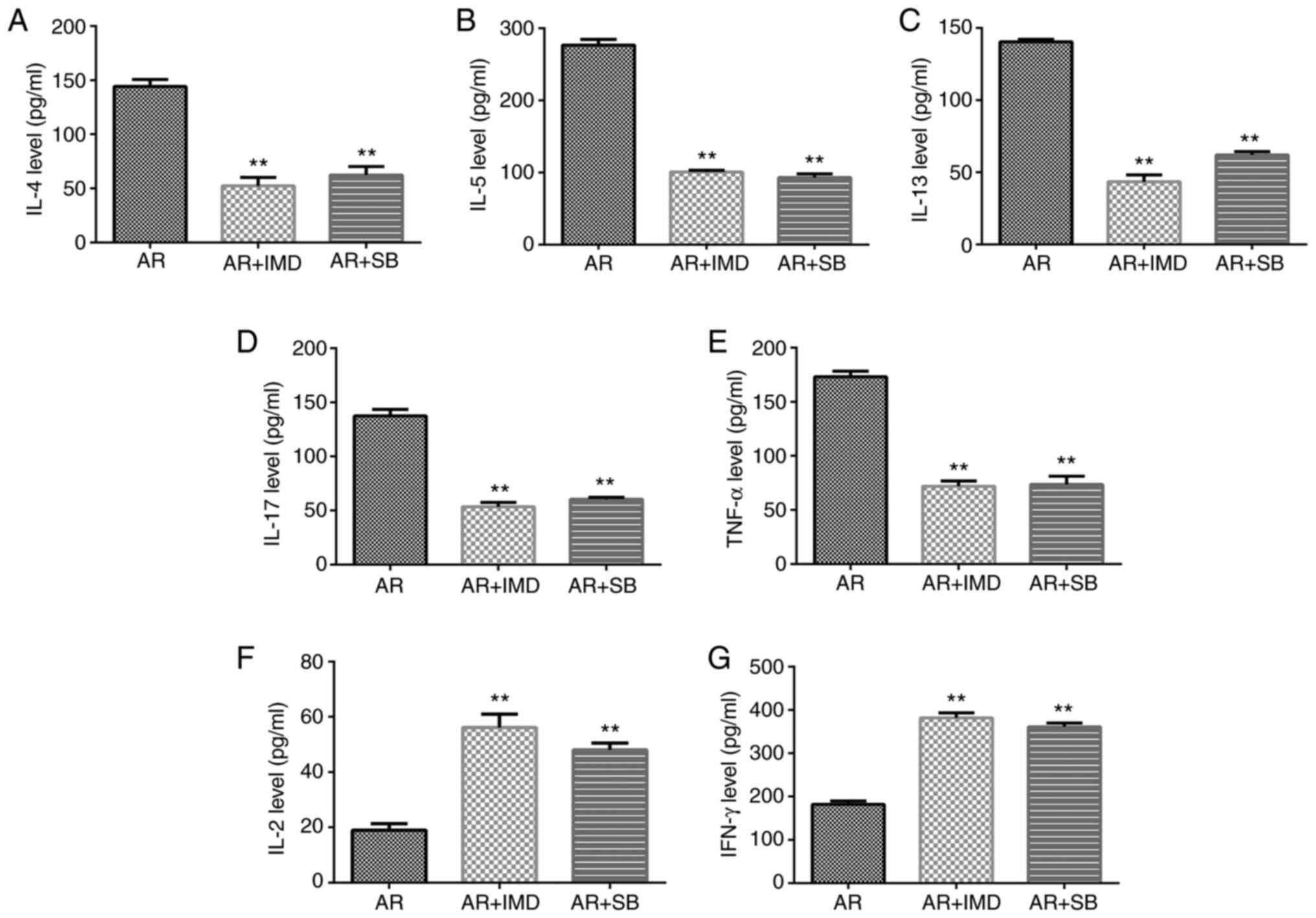
ELISA was used to detect the expression levels of
the inflammatory markers IL-4, IL-5, IL-13, IL-17 and TNF-α. The
data indicated that their expression levels were downregulated in
the AR + IMD and AR + SB groups, whereas the release of IFN-γ and
IL-2 was significantly increased (Fig.
8).
Discussion
Lidocaine is a local anesthetic used clinically,
which can usually prevent arrhythmia and it can also block nerve
conduction. Its mechanism of action involves the inhibition of the
voltage-gated sodium channels, which reduces neuropathic pain and
chronic pain tolerance (25).
Furthermore, the anti-inflammatory effects of lidocaine have also
been confirmed (19-21).
However, to the best of our knowledge, the role of lidocaine in AR
(a chronic inflammatory disease) has not been previously reported.
In the present study, an AR mouse model was established as
previously described (22).
AR is an inflammatory disease, which mainly occurs
during spring and autumn. AR manifestations mainly involve repeated
sneezing, nasal congestion, itchy nose, rhinorrhea and tearing
(26,27). Previous studies demonstrated that
the average incidence of AR was 10-20% in 2013 and that the number
was constantly increasing every year (28,29).
Therefore, it is urgent to identify new targets for AR. The present
study investigated the effects of lidocaine on AR through
intranasal administration. However, whether nasal drip will affect
the sense of smell and whether it affects the ciliary swing of the
nose were not evaluated in this study, and this was a limitation of
the present study. The results indicated that lidocaine relieved
allergic inflammation, which significantly reduced the frequency of
sneezing and nose rubbing in mice in a dose-dependent manner.
Lidocaine further decreased OVA-specific IgE and LTC4 expression.
It should be noted that the representative images of nasal mucosa
morphology and eosinophils stained by Giemsa will render our
results more convincing, and the absence of these images was a
further limitation of the present study.
IgE-mediated release of inflammatory mediators such
as histamine, with the participation of a variety of
pro-inflammatory cells, immunocompetent cells and cytokines, a
variety of inflammatory factors, promote the development of AR
(1). Suppression of the release of
inflammatory factors in the serum of the patient can significantly
reduce the inflammatory response in the body of the patient and
relieve the symptoms of AR (30,31).
It has been previously reported that the development of AR is
mediated via an imbalance between Th1 and Th2 cells (32). Li et al (33) indicated Th17/Treg cell imbalance in
patients with AR. In addition, allergic inflammation is mainly
controlled by the Th2-mediated immune response (34,35).
It has also been revealed that Th2 cell depletion can inhibit
allergic inflammation in AR. IL-4 is involved in allergic reactions
(36). IL-5 is the main
differentiation and maturation factor of eosinophils and
contributes to eosinophil activation, development and survival
(37). TNF-α is released by mast
cells and eosinophils and is essential for the development of AR
(38). The data of the present
study indicated that lidocaine decreased the expression levels of
IL-4, IL-5, IL-13, IL-17 and TNF-α. However, a previous study
indicated that IFN-γ expression was downregulated in AR mice
(39). The present study
demonstrated that IFN-γ was negatively expressed in AR and that its
expression was upregulated following lidocaine treatment, which is
consistent with previously reported findings. IL-2 exhibited
similar expression levels as IFN-γ.
NF-κB has long been considered a prototypical
proinflammatory signaling pathway, largely based on NF-κB
activation induced by proinflammatory cytokines such as IL-1 and
TNF-α, and the role of NF-κB in the expression of other
proinflammatory genes including adhesion molecules, chemokines, and
cytokines (40). Activation of the
mitogen-activated protein kinase (MAPK) pathways, including p38, is
also required for NF-κB subunit p65 transactivation (41). The p38 MAPK belongs to the MAPK
family, the members of which are important signal transducers. In
the immune system, the p38 MAPK signaling cascade plays a key role
in the regulation of innate and adaptive immunity (42). p38 MAPK has been revealed to
regulate the activity of the regulator of the IL-4 and IL-5
promoter in T cells, and regulate Th2-specific transcription factor
GATA-3(43). p38 MAPK is also
known to upregulate specific cytokines such as IL-6, IL-8, and
TNF-α in some biological contexts (44). Previous studies have indicated that
the NF-κB signaling pathway mediated AR inflammation (39,45).
Studies have also revealed the important role of p38 MAPK in AR
(46,47). The present study further indicated
that lidocaine relieved AR in mice via the NF-κB and p38 MAPK
signaling pathways. Lidocaine inhibited both NF-κB and p38 MAPK
signaling pathway activation in AR mice. However, which was the
target of lidocaine remains to be explored. Thus, lack of
identification of the target of lidocaine was a limitation of the
present study. Since multiple studies (18,48-51)
have revealed that lidocaine inhibits the activation of NF-κB and
p38 MAPK, it was hypothesized that both NF-κB and p38 MAPK may be
the targets of lidocaine. Moreover, as it is well known, the
activation of MAPK/NF-κB signaling pathway requires the increase in
Ca2+ levels in cytosol (52). On the other hand, lidocaine exerts
analgesic effects through the blocking of sodium channels (53). The reason why the blocker of sodium
channels, lidocaine, is able to inhibit the MAPK/NF-κB signaling
pathway through blocking remains to be explored. These issues will
be studied in the future. The association between the NF-κB and the
p38 MAPK signaling pathways with AR was assessed by treatment of
the mice with NF-κB and p38 MAPK signaling pathway inhibitors. The
results indicated that these two inhibitors exhibited similar
inhibitory effects on AR. Unfortunately, only one dose of NF-κB and
p38 MAPK signaling pathway inhibitors was studied, and this was a
limitation of the present study. Moreover, which pathway is more
prominent/significant was not investigated in the present
study.
In conclusion, the results of the present study
confirmed that lidocaine relieved AR in mice by regulating the
NF-κB and p38 MAPK pathways. However, this study is only a
preliminary study of the role of lidocaine in AR. In order to
render the effect of lidocaine on AR more convincing, extensive
in-depth research is required. For example, examination of the
levels of neuropeptides, which are well known to be responsible for
the development of clinical symptoms of allergic rhinitis, in NLFs
should be performed. In addition, which cells, within the mucosa
tissue (epithelial, immune cells), were modulating p38 MAPK and
NF-κB after treatment with lidocaine need to be identified.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and ZY contributed to the study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. QZ contributed to data collection and
statistical analysis. JX and ZY confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the protocol approved by the Wuhan Jinyintan Hospital Committee on
Care and Use of Laboratory Animals (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Incorvaia C, Cavaliere C, Frati F and
Masieri S: Allergic rhinitis. J Biol Regul Homeost Agents. 32 (1
Suppl 1):S61–S66. 2018.PubMed/NCBI
|
|
2
|
Paiva Ferreira LKD, Paiva Ferreira LAM,
Monteiro TM, Bezerra GC, Bernardo LR and Piuvezam MR: Combined
allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol.
74(105718)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rosati MG and Peters AT: Relationships
among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J
Rhinol Allergy. 30:44–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Prenner BM and Schenkel E: Allergic
rhinitis: Treatment based on patient profiles. Am J Med.
119:230–237. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sur DK and Plesa ML: Treatment of allergic
rhinitis. Am Fam Physician. 92:985–992. 2015.PubMed/NCBI
|
|
6
|
Bernstein DI, Schwartz G and Bernstein JA:
Allergic rhinitis: Mechanisms and treatment. Immunol Allergy Clin
North Am. 36:261–278. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Steelant B, Farré R, Wawrzyniak P, Belmans
J, Dekimpe E, Vanheel H, Van Gerven L, Kortekaas Krohn I, Bullens
DM, Ceuppens JL, et al: Impaired barrier function in patients with
house dust mite-induced allergic rhinitis is accompanied by
decreased occludin and zonula occludens-1 expression. J Allergy
Clin Immunol. 137:1043–1053.e5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin S, Jin P, Shao C, Lu W, Xiang Q, Jiang
Z, Zhang Y and Bian J: Lidocaine attenuates
lipopolysaccharide-induced inflammatory responses and protects
against endotoxemia in mice by suppressing HIF1α-induced
glycolysis. Int Immunopharmacol. 80(106150)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leng T, Lin S, Xiong Z and Lin J:
Lidocaine suppresses glioma cell proliferation by inhibiting TRPM7
channels. Int J Physiol Pathophysiol Pharmacol. 9:8–15.
2017.PubMed/NCBI
|
|
10
|
Křikava I, Nováková M and Ševčík P: The
effects of trimecaine on bupivacaine induced cardiotoxicity in the
isolated rat heart: A pilot study. Physiol Res. 57(18)2008.
|
|
11
|
Martí-Carvajal AJ, Simancas-Racines D,
Anand V and Bangdiwala S: Prophylactic lidocaine for myocardial
infarction. Cochrane Database Syst Rev.
2015(CD008553)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakazawa M, Okumura A, Niijima S,
Yamashita S, Shimono K, Hirose S and Shimizu T: Oral mexiletine for
lidocaine-responsive neonatal epilepsy. Brain Dev. 35:667–669.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Slaton RM, Thomas RH and Mbathi JW:
Evidence for therapeutic uses of nebulized lidocaine in the
treatment of intractable cough and asthma. Ann Pharmacother.
47:578–585. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chinn E, Friedman BW, Naeem F, Irizarry E,
Afrifa F, Zias E, Jones MP, Pearlman S, Chertoff A, Wollowitz A and
Gallagher EJ: Randomized trial of intravenous lidocaine versus
hydromorphone for acute abdominal pain in the emergency department.
Ann Emerg Med. 74:233–240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu H, Dilger JP and Lin J: Lidocaine
suppresses viability and migration of human breast cancer Cells:
TRPM7 as a target for some breast cancer cell lines. Cancers
(Basel). 13(234)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao L, Ma N, Liu G, Mao N, Chen F and Li
J: Lidocaine inhibits hepatocellular carcinoma development by
modulating circ_ITCH/miR-421/CPEB3 axis. Dig Dis Sci. 66:4384–4397.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee U, Choi YJ, Choi GJ and Kang H:
Intravenous lidocaine for effective pain relief after bimaxillary
surgery. Clin Oral Investig. 21:2645–2652. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guang F, Liu S, Wang GL and Liu GJ:
Lidocaine attenuates lipopolysaccharide-induced acute lung injury
through inhibiting NF-kappaB activation. Pharmacology. 81:32–40.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin S, Jin P, Shao C, Lu W, Xiang Q, Jiang
Z, Zhang Y and Bian J: Lidocaine attenuates
lipopolysaccharide-induced inflammatory responses and protects
against endotoxemia in mice by suppressing HIF1α-induced
glycolysis. Int Immunopharmacol. 80(106150)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leon-Constantin MM, Alexa-Stratulat T,
Luca A, Tamba BI, Trandafir LM, Harabagiu V and Cojocaru E: The
morphofunctional impact of topical lidocaine formulation in
inflammatory pain-experimental study. Rom J Morphol Embryol.
60:869–874. 2019.PubMed/NCBI
|
|
21
|
Wang L, Wang M, Li S, Wu H, Shen Q, Zhang
S, Fang L and Liu R: Nebulized lidocaine ameliorates allergic
airway inflammation via downregulation of TLR2. Mol Immunol.
97:94–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiao L, Jiang L, Hu Q and Li Y:
MicroRNA-133b ameliorates allergic inflammation and symptom in
murine model of allergic rhinitis by targeting Nlrp3. Cell Physiol
Biochem. 42:901–912. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu H, Shu H, Zhu J and Song J: Inhibition
of TLR4 inhibits allergic responses in murine allergic rhinitis by
regulating the NF-κB pathway. Exp Ther Med. 18:761–768.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng Y, Hou X and Yang S: Lidocaine
potentiates SOCS3 to attenuate inflammation in microglia and
suppress neuropathic pain. Cell Mol Neurobiol. 39:1081–1092.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khan DA: Allergic rhinitis and asthma:
Epidemiology and common pathophysiology. Allergy Asthma Proc.
35:357–361. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mandhane SN, Shah JH and Thennati R:
Allergic rhinitis: An update on disease, present treatments and
future prospects. Int Immunopharmacol. 11:1646–1662.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Casale TB and Dykewicz MS: Clinical
implications of the allergic rhinitis-asthma link. Am J Med Sci.
327:127–138. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brożek JL, Bousquet J, Agache I, Agarwal
A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R,
Canonica GW, Casale T, Chavannes NH, et al: Allergic rhinitis and
its impact on asthma (ARIA) guidelines-2016 revision. J Allergy
Clin Immunol. 140:950–958. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Drazdauskaitė G, Layhadi JA and Shamji MH:
Mechanisms of allergen immunotherapy in allergic rhinitis. Curr
Allergy Asthma Rep. 21(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu S and Xiao D: Effect of curcumin on
nasal symptoms and airflow in patients with perennial allergic
rhinitis. Ann Allergy Asthma Immunol. 117:697–702.e1.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu S, Han B, Liu S, Wang H, Zhuang W,
Huang Y and Zhang R: Derp1-modified dendritic cells attenuate
allergic inflammation by regulating the development of T helper
type1(Th1)/Th2 cells and regulatory T cells in a murine model of
allergic rhinitis. Mol Immunol. 90:172–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li J, Lin XY, Liu X, Ma ZQ and Li Y:
Baicalin regulates Treg/Th17 cell imbalance by inhibiting autophagy
in allergic rhinitis. Mol Immunol. 125:162–171. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bae JS, Kim JH, Kim EH and Mo JH: The role
of IL-17 in a lipopolysaccharide-induced rhinitis model. Allergy
Asthma Immunol Res. 9:169–176. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bachert C, Zhang L and Gevaert P: Current
and future treatment options for adult chronic rhinosinusitis:
Focus on nasal polyposis. J Allergy Clin Immunol. 136:1431–1440.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Deo SS, Mistry KJ, Kakade AM and Niphadkar
PV: Role played by Th2 type cytokines in IgE mediated allergy and
asthma. Lung India. 27:66–71. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Coffman RL, Seymour BW, Hudak S, Jackson J
and Rennick D: Antibody to interleukin-5 inhibits helminth-induced
eosinophilia in mice. Science. 245:308–310. 1989.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jung HW, Jung JK and Park YK: Comparison
of the efficacy of KOB03, ketotifen, and montelukast in an
experimental mouse model of allergic rhinitis. Int Immunopharmacol.
16:254–260. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Piao CH, Fan YJ, Nguyen TV, Song CH and
Chai OH: Mangiferin alleviates ovalbumin-induced allergic rhinitis
via Nrf2/HO-1/NF-κB signaling pathways. Int J Mol Sci.
21(3415)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Vanden Berghe W, Plaisance S, Boone E, De
Bosscher K, Schmitz ML, Fiers W and Haegeman G: p38 and
extracellular signal-regulated kinase mitogen-activated protein
kinase pathways are required for nuclear factor-kappaB p65
transactivation mediated by tumor necrosis factor. J Biol Chem.
273:3285–3290. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dodeller F, Skapenko A, Kalden JR, Lipsky
PE and Schulze-Koops H: The p38 mitogen-activated protein kinase
regulates effector functions of primary human CD4 T cells. Eur J
Immunol. 35:3631–3642. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dodeller F and Schulze-Koops H: The p38
mitogen-activated protein kinase signaling cascade in CD4 T cells.
Arthritis Res Ther. 8(205)2006.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Ono K and Han J: The p38 signal
transduction pathway: Activation and function. Cell Signal.
12:1–13. 2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kang OH, Jang HJ, Chae HS, Oh YC, Choi JG,
Lee YS, Kim JH, Kim YC, Sohn DH, Park H and Kwon DY:
Anti-inflammatory mechanisms of resveratrol in activated HMC-1
cells: Pivotal roles of NF-kappaB and MAPK. Pharmacol Res.
59:330–337. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu J, Liu L, Cui Y, Zhang J and Jiang H:
p38 MAPK regulates Th2 cytokines release in PBMCs in allergic
rhinitis rats. J Huazhong Univ Sci Technolog Med Sci. 30:222–225.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gao X, Li N and Zhang J: SB203580, a
p38MAPK inhibitor, attenuates olfactory dysfunction by inhibiting
OSN apoptosis in AR mice (activation and involvement of the p38
mitogen-activated protein kinase in olfactory sensory neuronal
apoptosis of OVA-induced allergic rhinitis). Brain Behav.
9(e01295)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang R, Liao J, Yang MC, Deng J, Hu YX,
Li P and Li MT: Lidocaine mediates the progression of cerebral
ischemia/reperfusion injury in rats via inhibiting the activation
of NF-κB p65 and p38 MAPK. Ann Transl Med. 8(548)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen LJ, Ding YB, Ma PL, Jiang SH, Li KZ,
Li AZ, Li MC, Shi CX, Du J and Zhou HD: The protective effect of
lidocaine on lipopolysaccharide-induced acute lung injury in rats
through NF-κB and p38 MAPK signaling pathway and excessive
inflammatory responses. Eur Rev Med Pharmacol Sci. 22:2099–2108.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Haller I, Hausott B, Tomaselli B, Keller
C, Klimaschewski L, Gerner P and Lirk P: Neurotoxicity of lidocaine
involves specific activation of the p38 mitogen-activated protein
kinase, but not extracellular signal-regulated or c-jun N-terminal
kinases, and is mediated by arachidonic acid metabolites.
Anesthesiology. 105:1024–1033. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jin H and Yu J: Lidocaine protects H9c2
cells from hypoxia-induced injury through regulation of the
MAPK/ERK/NF-κB signaling pathway. Exp Ther Med. 18:4125–4131.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Niu L, Wei J, Li X, Jin Y and Shi X:
Inhibitory activity of narirutin on RBL-2H3 cells degranulation.
Immunopharmacol Immunotoxicol. 43:68–76. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
van der Wal SE, van den Heuvel SA, Radema
SA, van Berkum BF, Vaneker M, Steegers MA, Scheffer GJ and Vissers
KC: The in vitro mechanisms and in vivo efficacy of intravenous
lidocaine on the neuroinflammatory response in acute and chronic
pain. Eur J Pain. 20:655–674. 2016.PubMed/NCBI View Article : Google Scholar
|