Introduction
Spontaneous intracerebral hemorrhage (sICH) has a
disproportionate socioeconomic impact considering its low incidence
rate (26% of all incident strokes in 2017, with higher prevalence
rates in East European countries) (1). An aging population and repeated
unsuccessful research endeavors for curative treatment contribute
to its high mortality and morbidity, resulting in a 1-month case
fatality of 40% with only 12% of patients regaining long-term
functional independence (2). At
present, the focus is on improving early outcome prediction to
individualize patient management, and to also identify individuals
at risk before sICH occurs, as to date, no reliable premonitory
onset markers have been determined. Therefore, biomarker testing is
an area of interest, as it is minimally invasive, low cost and
could potentially enable accurate risk stratification and outcome
estimation. Routinely, the standard hematologic evaluation consists
of complete blood count (CBC), including platelet (PLT) count,
coagulation profile and serum glucose (3,4). As
sICH is a time-sensitive condition, readily available point-of-care
(POC) devices reduce delays and facilitate prompt management.
Over the past decade, the role of inflammation in
sICH progression and neurological impairment has been further
clarified (5,6), and inflammatory biomarkers are
currently regarded as potential prognostication tools. CBC upon
admission, which includes hemoglobin (Hb), red blood cells and
their distribution width (RDW), and derived inflammatory indexes,
such as neutrophils-to-lymphocytes ratio (NLR),
lymphocytes-to-monocytes ratio (LMR), platelets-to-lymphocytes
ratio (PLR) and C-reactive protein (CRP), have been associated with
mortality (5,7-11).
Moreover, early neurological worsening (ENW) (12), hematoma volume expansion and the
expansion of the surrounding edema (5,13,14),
and day 90 functional outcome (FO) (6,13,15-19)
have also been reported to be associated with mortality.
Furthermore, systemic immune-inflammation index (SII) has been
recently reported as a relevant predictor of poor hospital
discharge outcome (20).
Leukocyte count has been consistently associated
with larger ICH volumes (5), but no
consensus has been reached as to ICH progression, infection risk
and mortality (11). Higher
admission neutrophils have been associated with larger baseline
volumes (21), mortality (10,22,23)
and morbidity (10,23). With regard to monocyte (MON) count,
increased admission levels are associated with poor outcome and
mortality (5), but not with ICH
volume (21), as MON are thought to
contribute to secondary injury (13). Admission lymphopenia has been
correlated with higher stroke severity, larger baseline hematoma
volume and intraventricular extension, along with infection risk
and 3-month mortality rate (5). NLR
and LMR mirror post-ICH proinflammation and immunosuppression
(12), as higher NLR has reflected
larger baseline volumes, stroke severity, severe perihematomal
edema (PHE) growth and poor 3-month outcome (5), and lower LMR has indicated neurologic
deterioration and day 90 mortality (12).
CRP has been significantly linked with hematoma
growth (HG), ENW, mortality and 3-month outcome (5,7,9,24).
Its early presence at the hemorrhagic site could be due to local
synthesis or transformation of the circulating liver-synthetized
pentameric form (24).
Regarding Hb, anemia is associated with larger ICH
volumes (25), increased HG
(26) and worse outcomes (26-28).
RDW is another inexpensive, automatically generated hematology
parameter that is impacted by inflammation and is currently
associated with day 30 FO (29).
Moreover, stroke is considered a systemic condition
that induces cardiac, lung and immune dysfunctions (5,30);
therefore, cardiac biomarkers, such as troponin I (cTnI), have been
linked to stroke severity (31),
in-hospital mortality (30,32) and unfavorable outcomes (33,34).
On the other hand, D-dimer levels have been associated with an
increased risk (35,36) and severity (37) of hemorrhagic stroke, and an
increased hematoma volume (37),
although it has not been proved sufficiently accurate for molecular
stroke diagnosis (38).
Furthermore, admission hyperglycemia has also been related to
mortality (6,8) and day 90 FO (6).
The emergency department (ED) provides a unique
opportunity for POC testing, both for standard and additional
biomarkers (e.g., cTnI, D-dimer and CRP). When addressing
time-sensitive conditions such as sICH, targeted escalation of the
standard protocol could benefit these hyperacute patients. The
contribution of additional POC testing could enable early risk
stratification strategies to be identified and facilitate
improvements in outcomes for patients with sICH. Nevertheless,
information about the applicability of POC testing on cerebral
hemorrhage is scarce.
The present study aimed to assess the predictive
role of ED-based POC biomarkers (standard and additional) and
derived inflammatory indexes on day 90 FO in patients with acute
sICH.
Materials and methods
Patient recruitment
The design and enrolment processes of this
prospective, single-center, ED-based pilot study have been
previously published (39). To
summarize, adult patients presenting with acute sICH (<8 h from
onset) to the ED of the County Emergency Hospital (Cluj-Napoca,
Romania) were recruited over 18 months (December 2017 to June 2018)
provided that Glasgow Coma Scale (GCS) was ≥8 and no exclusion
criteria were met. The exclusion criteria were as follows:
Identifiable secondary ICH causes, thromboembolic/ischemic disease,
seizures, severe pre-ICH disability [modified Rankin Scale
(mRS)≥4], coagulopathy, treatment with heparin,
low-molecular-weight heparin, glycoprotein IIb/IIIa antagonists or
oral anticoagulants, pregnancy/breastfeeding, scheduled
neurosurgical/hemostatic treatment, enrolment in other studies
within the last 30 days or terminal disease. The study protocol was
approved by the Institutional Review Board of the ‘Iuliu Hațieganu’
University of Medicine and Pharmacy Cluj-Napoca (approval no.
441/24.11.2016). The procedures and interventions in the present
study were in accordance with the principles stated by the
Declaration of Helsinki. All participants or legal representatives
provided written informed consent.
Data sources/measurements
Demographic, clinical and laboratory data were
documented upon ED admission. The routine management of patients
with acute sICH in our department and the study of specific
interventions are present in Fig.
1. ED-based POC whole-blood analyzers included the Fujifilm
Dry-Chem NX500 biochemistry analyzer and the Swelab Alfa Plus
hematology analyzer. CBC included granulocytes [GRA; composed of
neutrophiles (NEU) and the largest proportion of eosinophils (EOS)]
and mid-size (MID) population of cells (composed of mid-size
population of MON, basophils, EOS, blasts and other immature
cells). Calculated hematology indexes included the following
ratios: NLR (incorporating GRA values), LMR (incorporating MID
values) and PLR, alongside SII [calculated as NEU x
PLT/(lymphocytes (LYM) x1,000)] and incorporating GRA results. An
ED-based PathFast™ fully automatic chemiluminescence
enzyme immunoassay was used to study additional biomarkers,
including cTnI, D-dimer and high sensitive CRP (hs-CRP).
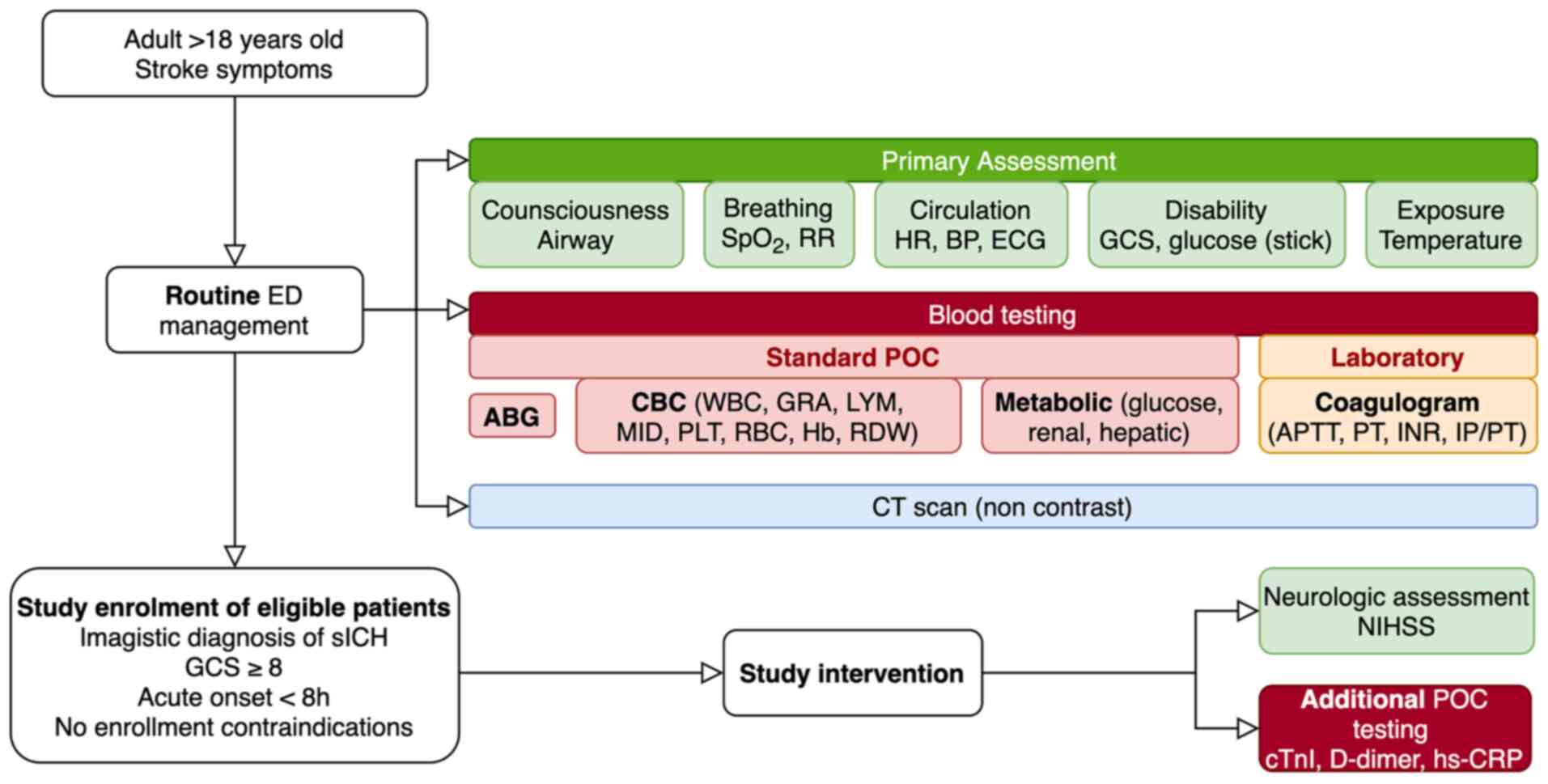 | Figure 1Routine ED baseline assessment of
patients with sICH and study specific interventions. ED, emergency
department; SpO2, peripheral oxygen saturation; RR,
respiratory rate; HR, heart rate; BP, blood pressure; ECG,
electrocardiogram; GCS, Glasgow Coma Scale score; POC,
point-of-care; ABG, arterial blood gases; CBC, complete blood
count; WBC, white blood cells; GRA, granulocytes; LYM, lymphocytes;
MID, mid-cell population; PLT, platelets; RBC, red blood cells; Hb,
hemoglobin; RDW, red cell distribution width; APTT, activated
partial prothrombin time; PT, prothrombin time; INR, international
normalized ratio; IP/PT, prothrombin index/prothrombin time; CT,
computer tomography; sICH, spontaneous intracerebral hemorrhage;
NIHSS, National Institute of Health Stroke Scale; cTnI, cardiac
troponin I; hs-CRP, high-sensitive C reactive protein. |
Patients were clinically assessed on days 2 and 7
(or on discharge). Follow-up telephone interviews on day 90
included FO (mRS) and independence on daily living activities
[Barthel Index (BI)].
Diagnosis and imagistic controls were performed on a
General Electric Optima 64 scanner (Cytiva). The hemorrhage volume
was measured using manual segmentation with the inclusion of the
entire visible lesion area. The post-processing analysis was
performed on a General Electric AW Server 2.0 workstation by two
independent radiologists, blinded for patient outcome.
Statistical analysis
In this analysis, the primary endpoint was day 90
FO. A favorable outcome was considered as an mRS of 0-3, whereas an
mRS of 4-6 was considered as an unfavorable outcome. Secondary
clinical endpoints included ENW [defined as a GCS decrease of ≤2
points or a National Institute of Health Stroke Scale (NIHSS)
increase ≥4], day 7/discharge neurological impairment (NIHSS ≤15),
and day 90 assessment of quality of life and independence.
Radiological endpoints included HG (change in baseline hematoma
volume of >33% or >6 ml by day 2) and PHE growth [difference
in the largest PHE linear dimension between the diagnostic and
control computed tomography (CT) scans].
Statistical analyses was performed using
MedCalc® Statistical software (version 19.6; MedCalc
Software byba). Quantitative data was assessed for normality of
distribution using the Shapiro-Wilk test, and presented as the
median and 25-75th percentiles. Qualitative data are presented as
the frequency and percentage. Comparisons between groups were
analyzed using the Mann-Whitney U test or χ2 test.
Spearman's rho was used to assess correlations between variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
A cohort of 39 patients was recruited, with 35
completing the in-hospital follow-up and 23 (66%) alive on day 90.
All deaths were registered within the first month and in-hospital
mortality was 23%.
Baseline characteristics of the cohort according to
day 90 FO are presented in Table I.
Older age, previous history of ischemic stroke, and the presence of
intraventricular hemorrhage (IVH), enlarged contralateral ventricle
(ECV) and cerebral atrophy on the initial CT scan significantly
predicted an unfortunate FO scoring on day 90 follow-up. Median
baseline hematoma volume and PHE did not differ significantly
between surviving [hematoma volume, 9.15 cm3
(6.69-22.18); PHE, 8.35 mm (6.81-11.58)] and deceased [hematoma
volume, 16.83 cm3 (9.2-31.89); PHE, 9.35 mm
(6.50-13.25)] day 90 outcome groups (P=0.234 and P=0.470,
respectively).
 | Table IBaseline clinical and imagistic
characteristics. |
Table I
Baseline clinical and imagistic
characteristics.
| | Day 90 FO | |
|---|
| Characteristic | Favorable
(n=16) | Unfavorable
(n=19) | P-value |
|---|
| Median age (range),
years | 62 (57-68.5) | 75 (73-81) | <0.001 |
| >70 | 3 (18.8) | 17 (89.5) | <0.001 |
| Male, n (%) | 11 (68.8) | 8 (42.1) | 0.217 |
| Hypertension, n
(%) | 12 (75.0) | 15 (78.9) | 1.000 |
| >2
antihypertensive drugs | 7 (43.8) | 10 (52.6) | 0.854 |
| Diabetes mellitus,
n (%) | 6 (37.5) | 4 (21.1) | 0.454 |
| Dyslipidemia, n
(%) | 7 (43.8) | 7 (36.8) | 0.945 |
| Statin use prior to
admission, n (%) | 4(25) | 6 (31.6) | 0.723 |
| Antiplatelet agent,
n (%) | 4 (25.0) | 5 (26.3) | 1.000 |
| Smoking
(former/active), n (%) | 11 (68.8) | 8 (42.1) | 0.217 |
| GCS, median
(range) | 15 (14; 15) | 13 (12; 15) | 0.080 |
| NIHSS score, median
(range) | 8.5 (6.2;
14.5) | 15 (6; 21) | 0.288 |
| Median SBP (range),
mmHg | 164.5
(154.5-192.5) | 163
(147.2-174.2) | 0.174 |
| >170 mmHg, n
(%) | 7 (43.8) | 8 (42.1) | 1.000 |
| Median HR (range),
beats/min | 81.5
(71.5-97.5) | 75 (65-86) | 0.267 |
| Atrial
fibrillation, n (%) | 1 (6.2) | 0 (0) | 0.457 |
| Hematoma location,
n (%) | | | 0.581 |
|
Supra-tentorial
lobar | 2 (12.5) | 4 (21.1) | |
|
Supra-tentorial
deep | 14 (87.5) | 13 (68.4) | |
|
Supra-tentorial
mixte | 0 (0) | 1 (5.3) | |
|
Infratentorial | 0 (0) | 1 (5.3) | |
| Hematoma volume,
cm3, n (%) | | | 0.316 |
|
<30 | 14 (87.5) | 14 (73.7) | |
|
30-60 | 2 (12.5) | 2 (10.5) | |
|
>60 | 0 (0) | 3 (15.8) | |
| Median
perihematomal edema (range), mm | 9.07
(7.16-28.21) | 21.47
(6.97-32.86) | 0.371 |
| IVH, n (%) | 1 (6.2) | 8 (42.1) | 0.022 |
| MLS >10 mm, n
(%) | 3 (18.8) | 10 (52.6) | 0.086 |
| Mass effect, n
(%) | 12 (75.0) | 17 (89.5) | 0.379 |
| CVC, n (%) | 12 (75.0) | 16 (84.2) | 0.677 |
| ECV, n (%) | 1 (6.2) | 8 (42.1) | 0.022 |
| Periventricular
leucoaraiosis, n (%) | 5 (31.2) | 13 (68.4) | 0.064 |
| Lacunarism, n
(%) | 8 (50.0) | 16 (84.2) | 0.071 |
| Cerebral atrophy, n
(%) | 4 (25.0) | 16 (84.2) | 0.001 |
| Median length of
hospital stay (range), days | 15
(11.5-16.75) | 14 (8-19) | 0.715 |
| Discharge
disposition | | | 0.741 |
|
Family
care | 9 (56.2) | 12 (63.2) | |
|
Rehabilitation/lower
rank hospital | 1 (6.2) | 2 (10.5) | |
Baseline POC biomarker values and calculated indexes
according to day 90 mRS are presented in Table II. Higher values in the unfavorable
outcome group were documented for RDWa, GRA, NLR, PLR, SII, hs-CRP
and D-dimer, but the differences were only significant for D-dimer
(P<0.001). When further considering day 90 independence on daily
living activities, D-dimer values were significantly higher
(P=0.032) in the dependent patients [BI <60; 3.610 µg/ml FEU
(0.900-5.010) vs. 0.758 µg/ml FEU (0.383-0.890)] compared with
those in the day 90 independent group. Negative correlations were
documented between admission D-dimer and admission GCS (rho=-0.342;
P=0.044) and day 90 independence status (rho=-0.670; P=0.001).
 | Table IIComparison of admission POC
biomarkers according to day 90 outcome. |
Table II
Comparison of admission POC
biomarkers according to day 90 outcome.
| | Day 90 FO | |
|---|
| POC biomarker | Favorable
(n=16) | Unfavorable
(n=19) | P-value |
|---|
| Hb, g/dl | 13.85
(12.75-15.07) | 13.40
(12.70-14.80) | 0.417 |
| RBC,
x1012/l | 4.55
(4.33-4.91) | 4.55
(4.08-4.94) | 0.729 |
| RDWa, fl | 60.30
(58.13-65.38) | 63.20
(58.20-66.40) | 0.943 |
| WBC,
x109/l | 9.35
(6.45-11.58) | 9.30
(6.70-11.30) | 0.895 |
| GRA,
x109/l | 5.40
(3.80-8.60) | 6.20
(4.70-8.30) | 0.667 |
| LYM,
x109/l | 2.15
(1.33-2.68) | 1.80
(1.40-2.20) | 0.127 |
| MID,
x109/l | 0.85
(0.60-1.10) | 0.80
(0.60-1.30) | 0.934 |
| PLT,
x109/l | 168.50
(140.00-221.50) | 162.00
(150.00-192.00) | 0.740 |
| NLR | 2.26
(1.82-3.70) | 3.14
(2.79-5.14) | 0.145 |
| LMR | 2.11
(1.71-4.33) | 2.11
(1.20-3.25) | 0.486 |
| PLR | 92.94
(55.2-125.00) | 95.21
(72.5-116.48) | 0.655 |
| SII | 0.39
(0.25-1.15) | 0.53
(0.36-0.88) | 0.868 |
| hs-CRP, mg/l | 2.49
(0.89-4.01) | 3.27
(0.68-5.20) | 0.446 |
| cTnI, ng/ml | 0.003
(0.002-0.070) | 0.003
(0.001-0.006) | 0.181 |
| D-dimer, µg/ml
FEU | 0.75
(0.38-0.89) | 2.31
(0.92-5.01) | <0.001 |
| Glucose,
mmol/l | 146 (143-168) | 140 (119-183) | 0.585 |
ENW was documented in 9/35 patients, with only two
alive by day 90, equally divided between outcome groups. Baseline
median hematoma volume and PHE did not differ significantly
(P=0.051 and P=0.094, respectively) between those with [hematoma
volume, 24.60 cm3 (14.16-70.00); PHE, 10.50 mm
(9.23-20.63)] and without ENW [hematoma volume, 9.20 cm3
(5.99-21.25); PHE, 7.5 mm (6.00-10.40)]. All ENW patients received
antibiotic treatment by day 7 (ATB 7) and a modest correlation
between ENW and ATB 7 was observed (rho=0.367, P=0.033). In
patients who developed ENW, white blood cell [WBC;
10.60x109/l (8.00-15.25)], GRA [7.30x109/l
(5.95-11.60)] and D-dimer [3.73 µg/ml FEU (1.22-5.01)] values were
significantly higher compared with those in patients without ENW
[WBC, 8.10x109/l (6.60-10.60); GRA,
5.30x109/l (3.90-7.55); D-dimer, 0.86 µg/ml FEU
(0.63-1.63)] (P=0.042, P=0.025 and P=0.024, respectively). D-dimer
was also significantly higher in the 8/35 patients with a worse day
7 neurological status, defined as NIHSS score ≥16 [2.01 (1.20-5.01)
vs. 0.84 (0.59-1.93); P=0.017). By contrast, SII values were not
significantly higher in either ENW group [0.45 (0.33-0.86) vs. 0.83
(0.37-1.36); P=0.265], nor in those patients with a worse day 7
neurological status [0.45 (0.32-1.01) vs. 0.49 (0.37-0.81);
P=0.315].
The median length of hospital stay was 15 days. Two
participants required neurosurgery and another one required
advanced intensive care unit (ICU) airway management. By day 7,
medical complications occurred in 20 (57%) participants, all of
whom were undergoing antibiotic treatments. A day 90 favorable
outcome was reached in only 25% patients. No significant POC
biomarker differences were documented between patients with and
without day 7 antibiotic treatment.
Control CT scans were performed for 29/35
participants (83%). Only 7 controls were performed within the first
48 h (median time of 6 days and 13 h since the onset of symptoms).
Any hematoma expansion occurred in 15/29 participants [median 2.46
cm3 (1.78-11.18 cm3)], yet the criteria for
HG was fulfilled in only 6 (40%) patients, with 4 dying before the
follow-up. A median PHE growth of 3.65 mm (1.38-8.38 mm) was
documented in 25 participants (12/19 unfavorable outcome group).
Moderate or weak negative correlations were detected between POC
inflammatory markers and indexes and PHE growth (rho=-0.511,
P=0.005 for WBC; rho=-0.548, P=0.002 for GRA; rho=-0.373, P=0.047
for SII; rho=-0.378, P=0.043 for glucose). D-dimer was correlated
with admission PHE (rho=0.398, P=0.018).
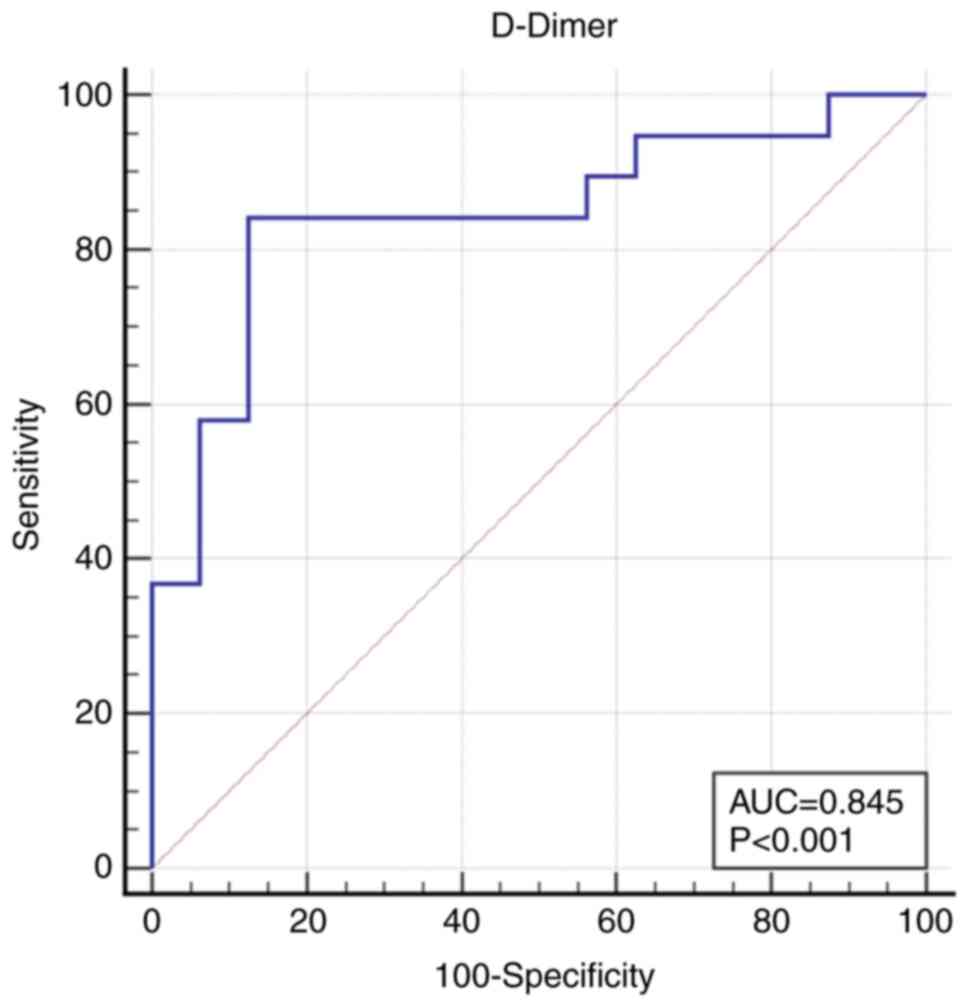
Cut-off values of the variables mostly associated
with the primary outcome were calculated, namely age, admission GCS
and D-dimer. As such, an unfavorable day 90 FO was indicated by age
≥72 years [area under the curve (AUC) 0.908 (95% confidence
interval (CI), 0.761-0.979), Se 84.2 (60.4-96.6), Sp 93.7
(69.8-99.8), P<0.001)], GCS ≤13 [AUC 0.661 (95% CI 0.482-0.812),
Se 52.63 (28.9-75.6), Sp 81.25 (54.4-96.0), P=0.0598)] and D-dimer
>0.905 µg/ml fibrinogen equivalent unit [FEU; AUC 0.845 (95% CI
0.683-0.945), Se 84.21 (60.4-96.6), Sp 87.50 (61.7-98.4),
P<0.001]. The receiver operating characteristic curve of
admission D-dimer predicting day 90 FO is presented in Fig. 2.
Discussion
In this observational cohort of patients with
spontaneous ICH, lower admission D-dimer indicated an improved day
90 FO and independence status. Increased age, previous stroke and
certain initial imagistic parameters, including IVH, ECV and
cerebral atrophy, implied an unfavorable outcome. The results
indicated that D-dimer may also anticipate the development of ENW,
and modestly reflect admission PHE, whereas certain inflammatory
markers may correlate with PHE growth.
Our POC results of baseline D-dimer supported
previously published data on the use of D-dimer in prognosing day
90 unfavorable FO (40,41), with an admission level of 0.905
µg/ml FEU predicting a poor 3-month outcome with a sensitivity and
specificity of ~85%. Previous reports identified values ~0.5 mg/l
FEU as estimating a poor outcome (41,42).
Nevertheless, the correlation between D-dimer and 3-month
dependence status should be investigated further to verify the
results of the present study. The mechanism through which D-dimer
impacts the sICH prognostic value is yet to be established, as it
is traditionally a hypercoagulability marker and more recently has
been considered for potential application in ischemic stroke
diagnosis (43). Evidence of
elevated D-dimer as expression of increased fibrinolysis, hence
contributing to a hypocoagulable status and extensive hemorrhages,
is rather scarce (35,36,44).
The present study consistently associated D-dimer with clinical
endpoints, including admission neurological status expressed as
GCS, throughout the entire follow-up period, alongside baseline
PHE. Nevertheless, a correlation with ICH volume was not identified
in the present study, despite being frequently reported in previous
studies (37,40,41).
With ICH volume as a known determinant of admission neurological
status and ENW (40,44,45), a
larger cohort might associate D-dimer with baseline volume and the
evolution of neurological status, thus supporting the
hypocoagulability theory.
ICH induces a state of systemic and peripheral
inflammation, thus increasing circulating WBC and recruiting
certain molecules within the affected area, which amplifies local
damage (13,22). Previous studies have reported
results for several inflammatory biomarkers and calculated indexes
(Hb, absolute RDW, GRA, LYM, PLT, NLR, PLR) that contribute to the
existing data on FO prognostication (5,6,17,19,28,29,46,47),
but the cohort assessed in the present study did not display
statistically significant results, only in-line tendencies with
previously published evidence. Furthermore, moderate negative
correlations were determined between WBC, GRA, SII and PHE growth.
However, these were not consistent with existing theories on the
contribution of acute inflammation to PHE enlargement (13), and subsequently to an unfavorable FO
(6,13,15,16).
This contradiction might reside in the modest sample size of the
current analysis and of the fact that the GRA population was
reported as a substitute for NEU, the former also including EOS
alongside NEU. Recent evidence has shown a significant increase in
peripheral WBC, GRA and MON population in patients with acute ICH,
whereas the LYM population has decreased (48). The contribution of EOS to sICH
prognostication is not completely understood; however, Chen et
al (14) correlated EOS count
with increased risk of HE. Moreover, the short interval from
symptom onset to CBC sampling in the present cohort (39) might have prevented the documentation
of the activation of local and systemic inflammation (13,22,48).
In the present study, MID was documented as a surrogate for MON,
but its values incorporate multiple cell populations. SII is a
parameter that is documented in regard to hospital discharge
outcome of patients with sICH (20); NEU and LYM reflect inflammation,
whilst PLT reflect vascular permeability. As such, SII components
could depict local PHE metabolism. The results of the present study
were in line with previous data on larger SII values in the
unfavorable FO subgroup (20),
without reaching statistical significance. Furthermore, there was
no association with day 7 neurological status (as a proxy for the
reported discharge FO) (20) or day
90 FO, which indicated that the timing of the most effective
inflammatory panel requires further investigation.
As all reported individual inflammatory markers
(WBC, GRA, LYM, MID and CRP) failed to predict day 90 FO, the
analysis on calculated indexes (NLR, LMR, PLR and SII) produced
similar results. Subsequently, further extensive research is
required to validate whether such POC derived indexes can impact
outcome prognostication in a similar manner as previous evidence
has indicated (5,12,17,46).
At present, the results regarding CRP are
inconclusive, despite existing evidence of its association with HG,
ENW, mortality and 3-month outcome (5,7,9,24),
including in-hospital mortality data reported on a consistent
ED-based cohort (9). Currently,
hs-CRP assays can only measure plasma pentameric CRP, thus failing
to incorporate the extent of the neuroinflammatory response to ICH
(24). Furthermore, a pre-existing
subliminal inflammatory status, though not detected as an infection
or chronic inflammation, might affect the coagulation function and
the vessel wall pathophysiology, contributing to persistent vessel
leakage.
PLT and PLR are well-known indicators of mortality
(8,49) and increase the chances of a negative
day 90 FO (6,17-19).
Although a similar 25% of each study group was under antiplatelet
medication when sICH occurred, the present analysis only documented
a moderate negative correlation of PLT with admission ICH volume
and day 7 neurological status, in spite of previous discussions on
its implications within the existing inflammatory process
accompanying sICH (19).
Nevertheless, Zhang and Shen (19)
demonstrated that ICU rather than ED admission PLR values are
relevant for outcome estimation.
In regard to Hb levels, lower mean admission values
were recorded in the unfavorable outcome group, without statistical
significance or without meeting the criteria of the definition of
anemia (39). RDW is another
inexpensive automatically generated hematology parameter that is
impacted by inflammation (29), and
our results indicated a modest correlation with admission PHE, but
this was not associated with day 90 FO.
If sICH is considered as having a systemic impact,
then metabolic and cardiac biomarkers could indicate its amplitude.
However, random admission hyperglycemia did not reflect the
severity of sICH (50), nor was it
associated with day 90 FO estimation (6), in spite of the modest negative
correlation with PHE growth. Mean admission cTnI levels did not
differ among outcome groups and the analysis conducted in the
present study did not correlate this parameter with any of the
study endpoints. Previous studies on troponin levels have concluded
that peak serum cTnI values rather than admission values reflect a
poor outcome, including mortality (33,34).
Therefore, we speculate that the results of the present study
suggest further investigating POC testing.
To the best of our knowledge, the present study was
one of the few studies on POC testing in acute sICH within an ED,
yet its value is limited due to its one-center location and modest
sample size, meaning that the current results require further
investigation in order to be validated in sICH management. As the
sICH population is of an increasing age, D-dimer interpretation
should be considered cautiously. The heterogeneity of control CT
scan acquisition is another restraint, as the timing of the second
scan varied greatly and, as such, ICH and PHE progression were not
reflected uniformly. Therefore, serial sampling of inflammatory
biomarkers and a more restrictive protocol for control scans might
identify the most relevant time point for a predictive inflammatory
panel.
In conclusion, several biomarkers showed modest
correlations with the progression of sICH and the day 90 FO,
advocating for extensive research on the contribution of ED POC
routine biomarkers as outcome assessment tools in hemorrhagic
stroke. D-dimer could be a promising maker, as lower admission
values could indicate an improved day 90 FO and anticipate the
development of PHE growth and ENW. The predictive utility of
D-dimer on independence status is a novelty at the present moment
and further research is needed to validate the current observations
in the acute care setting.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the ‘Iuliu
Hațieganu’ University of Medicine and Pharmacy Cluj-Napoca via an
internal PhD research program (grant no. 7690/74/15.04.2016 and PCD
no. 5200/64/01.03.2017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EMM, AG and LPD conceptualized the study. EMM, AG,
ML, CC and LPD performed the experiments. Formal analysis was
conducted by EMM and SCV. Investigations were performed by EMM, AG,
ML, CC and LPD. Resources were accrued by EMM, AG and LPD. The
original draft preparation was carried out by EMM. Reviewing and
editing of the manuscript were performed by EMM, AG, SCV, ML, CC
and LPD. AG and LPD provided supervision. EMM and AG confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
Declaration of Helsinki and was approved by the Ethics Committee of
the ‘Iuliu Hațieganu’ University of Medicine and Pharmacy (approval
no. 441/24.11.2016). All patients or legal representatives provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krishnamurthi RV, Ikeda T and Feigin VL:
Global, regional and country-specific burden of ischaemic stroke,
intracerebral haemorrhage and subarachnoid haemorrhage: A
systematic analysis of the global burden of disease study 2017.
Neuroepidemiology. 54:171–179. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
An SJ, Kim TJ and Yoon BW: Epidemiology,
risk factors, and clinical features of intracerebral hemorrhage: An
update. J Stroke. 19:3–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McGurgan IJ, Ziai WC, Werring DJ, Al-Shahi
Salman R and Parry-Jones AR: Acute intracerebral haemorrhage:
Diagnosis and management. Pract Neurol. 21:128–136. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Romanian Ministry of Health: Priority
action regarding the treatment of acute stroke. Standard
operational procedure regarding patient referral and therapeutic
protocol. Romanian Ministry of Health, Bucharest, 2015. https://www.neurology.ro/protocoale-si-ghiduri-meniu-main/protocol-avc.
Accessed June 28, 2021.
|
|
5
|
Saand AR, Yu F, Chen J and Chou SH:
Systemic inflammation in hemorrhagic strokes-a novel neurological
sign and therapeutic target? J Cereb Blood Flow Metab. 39:959–988.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fonseca S, Costa F, Seabra M, Dias R,
Soares A, DIas C, Azevedo E and Castro P: Systemic inflammation
status at admission affects the outcome of intracerebral hemorrhage
by increasing perihematomal edema but not the hematoma growth. Acta
Neurol Belg. 121:649–659. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Di Napoli M, Parry-Jones AR, Smith CJ,
Hopkins SJ, Slevin M, Masotti L, Campi V, Singh P, Papa F,
Popa-Wagner A, et al: C-reactive protein predicts hematoma growth
in intracerebral hemorrhage. Stroke. 45:59–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim Y, Han MH, Kim CH, Kim JM, Cheong JH
and Ryu JI: Increased short-term mortality in patients with
spontaneous intracerebral hemorrhage and its association with
admission glucose levels and leukocytosis. World Neurosurg.
98:503–511. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoshinaga R, Doi Y, Ayukawa K and Ishikawa
S: High-sensitivity C reactive protein as a predictor of inhospital
mortality in patients with cardiovascular disease at an emergency
department: A retrospective cohort study. BMJ Open.
7(e015112)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tao C, Hu X, Wang J, Ma J, Li H and You C:
Admission neutrophil count and neutrophil to lymphocyte ratio
predict 90-day outcome in intracerebral hemorrhage. Biomark Med.
11:33–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu S, Arima H, Heeley E, Delcourt C,
Krause M, Peng B, Yang J, Wu G, Chen X, Chalmer J, et al: White
blood cell count and clinical outcomes after intracerebral
hemorrhage: The INTERACT2 trial. J Neurol Sci. 361:112–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qi H, Wang D, Deng X and Pang X:
Lymphocyte-to-monocyte ratio is an independent predictor for
neurological deterioration and 90-day mortality in spontaneous
intracerebral hemorrhage. Med Sci Monit. 24:9282–9291.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Urday S, Kimberly WT, Beslow LA, Vortmeyer
AO, Selim MH, Rosand J, Simard JM and Sheth KN: Targeting secondary
injury in intracerebral haemorrhage-perihaematomal oedema. Nat Rev
Neurol. 11:111–122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Q, Liu J, Xu H, He W, Li Y, Jiao L,
Xiang Y, Zhan C, Chen J, Yang X, et al: Association between
eosinophilic leukocyte count and hematoma expansion in acute
spontaneous intracerebral hemorrhage. Front Neurol.
10(1164)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Urday S, Beslow LA, Dai F, Zhang F, Battey
TW, Vashkevich A, Ayres AM, Leasure AC, Selim MH, Simard M, et al:
Rate of perihematomal edema expansion predicts outcome after
intracerebral hemorrhage. Crit Care Med. 44:790–797.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Grunwald Z, Beslow LA, Urday S, Vashkevich
A, Ayres AM, Greenberg SM, Goldstein JN, Leasure A, Shi FD, Kahle
KT, et al: Perihematomal edema expansion rates and patient outcomes
in deep and lobar intracerebral hemorrhage. Neurocrit Care.
26:205–212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang W and Shen Y: Platelet-to-lymphocyte
ratio as a new predictive index of neurological outcomes in
patients with acute intracranial hemorrhage: A retrospective study.
Med Sci Monit. 24:4413–4420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mayda-Domaç F, Mısırlı H and Yılmaz M:
Prognostic role of mean platelet volume and platelet count in
ischemic and hemorrhagic stroke. J Stroke Cerebrovasc Dis.
19:66–72. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin CY, Chang CY, Sun CH, Li TY, Chen LC,
Chang ST and Wu YT: Platelet count and early outcome in patients
with spontaneous cerebellar hemorrhage: A retrospective study. PLoS
One. 10(e0119109)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Trifan G and Testai FD: Systemic
immune-inflammation (SII) index predicts poor outcome after
spontaneous supratentorial intracerebral hemorrhage. J Stroke
Cerebrovasc Dis. 29(105057)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Adeoye O, Walsh K, Woo JG, Haverbusch M,
Moomaw CJ, Broderick JP, Kissela BM, Kleindorfer D, Flaherty ML and
Woo D: Peripheral monocyte count is associated with case fatality
after intracerebral hemorrhage. J Stroke Cerebrovasc Dis.
23:e107–e111. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tapia-Pérez JH, Karagianis D, Zilke R,
Koufuglou V, Bondar I and Schneider T: Assessment of systemic
cellular inflammatory response after spontaneous intracerebral
hemorrhage. Clin Neurol Neurosurg. 150:72–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang F, Ren Y, Fu W, Yang Z, Wen D, Hu X,
Tao C, Li X, You C, Xin T and Yang M: Predictive accuracy of
neutrophil-to-lymphocyte ratio on long-term outcome in patients
with spontaneous intracerebral hemorrhage. World Neurosurg.
125:e651–e657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Di Napoli M, Slevin M, Popa-Wagner A,
Singh P, Lattanzi S and Divani AA: Monomeric C-reactive protein and
cerebral hemorrhage: From bench to bedside. Front Immunol.
9(1921)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kumar MA, Rost NS, Snider RW, Chanderraj
R, Greenberg SM, Smith EE and Rosand J: Anemia and hematoma volume
in acute intracerebral hemorrhage. Crit Care Med. 37:1442–1447.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roh DJ, Albers DJ, Magid-Bernstein J,
Doyle K, Hod E, Eisenberger A, Murthy S, Witsch J, Park S, Agarwal
S, et al: Low hemoglobin and hematoma expansion after intracerebral
hemorrhage. Neurology. 93:e372–e380. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Diedler J, Sykora M, Hahn P, Heerlein K,
Schölzke MN, Kellert L, Bösel J, Poli S and Steiner T: Low
hemoglobin is associated with poor functional outcome after
non-traumatic, supratentorial intracerebral hemorrhage. Crit Care.
14(R63)2010.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Zhang S, Pan X, Wei C, Wang L, Cheng Y, Hu
Z, Dong W, Liu M and Wu B: Associations of anemia with outcomes in
patients with spontaneous intracerebral hemorrhage: A
meta-analysis. Front Neurol. 10(406)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cui Z, Liu C, Sun G, Huang L and Zhou W: A
prognostic nomogram incorporating red cell distribution width for
patients with intracerebral hemorrhage. Medicine (Baltimore).
99(e23557)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hays A and Diringer MN: Elevated troponin
levels are associated with higher mortality following intracerebral
hemorrhage. Neurology. 66:1330–1334. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu M, Lin J, Wang D, Liu M, Hao Z and Lei
C: Cardiac troponin and cerebral herniation in acute intracerebral
hemorrhage. Brain Behav. 7(e00697)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sandhu R, Aronow WS, Rajdev A, Sukhija R,
Amin H, D'aquila K and Sangha A: Relation of cardiac troponin I
levels with in-hospital mortality in patients with ischemic stroke,
intracerebral hemorrhage, and subarachnoid hemorrhage. Am J
Cardiol. 102:632–634. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gerner ST, Auerbeck K, Sprügel MI, Sembill
JA, Madžar D, Gölitz P, Hoelter P, Kuramatsu JB, Schwab S and
Huttner HB: Peak troponin I levels are associated with functional
outcome in intracerebral hemorrhage. Cerebrovasc Dis. 46:72–81.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
He Y, Liu Q, Wang J, Wang DW, Ding H and
Wang W: Prognostic value of elevated cardiac troponin I in patients
with intracerebral hemorrhage. Clin Cardiol. 43:338–345.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhou Z, Liang Y, Zhang X, Xu J, Kang K, Qu
H, Zhao C and Zhao M: Plasma D-dimer concentrations and risk of
intracerebral hemorrhage: A systematic review and meta-analysis.
Front Neurol. 9(1114)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Di Castelnuovo A, Agnoli C, de Curtis A,
Giurdanella MC, Sieri S, Mattiello A, Matullo G, Panico S,
Sacerdote C, Tumino R, et al: Elevated levels of D-dimers increase
the risk of ischaemic and haemorrhagic stroke. Findings from the
EPICOR Study. Thromb Haemost. 112:941–946. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng X, Zhang L, Xie NC, Ma YQ and Lian
YJ: High plasma levels of D-dimer are independently associated with
a heightened risk of deep vein thrombosis in patients with
intracerebral hemorrhage. Mol Neurobiol. 53:5671–5678.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bustamante A, López-Cancio E, Pich S,
Penalba A, Giralt D, García-Berrocoso T, Ferrer-Costa C, Gasull T,
Hernández-Pérez M, Millan M, et al: Blood biomarkers for the early
diagnosis of stroke: The stroke-chip study. Stroke. 48:2419–2425.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mureșan EM, Golea A, Bolboacă S and
Perju-Dumbravă L: Feasibility of a pilot study on point-of-care
biomarkers in spontaneous intracerebral hemorrhage in an emergency
setting. Med Pharm Rep. 94:307–317. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Delgado P, Alvarez-Sabín J, Abilleira S,
Santamarina E, Purroy F, Arenillas JF, Molina CA, Fernández-Cadenas
I, Rosell A and Montaner J: Plasma d-dimer predicts poor outcome
after acute intracerebral hemorrhage. Neurology. 67:94–98.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hu X, Fang Y, Ye F, Lin S, Li H, You C and
Liu M: Effects of plasma D-dimer levels on early mortality and
long-term functional outcome after spontaneous intracerebral
hemorrhage. J Clin Neurosci. 21:1364–1367. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhou Q, Zhang D, Chen X, Yang Z, Liu Z,
Wei B, Jin M, Feng K, Guo C, Sun J, et al: Plasma D-dimer predicts
poor outcome and mortality after spontaneous intracerebral
hemorrhage. Brain Behav. 11:462–468. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Misra S, Montaner J, Ramiro L, Arora R,
Talwar P, Nath M, Kumar A, Kumar P, Pandit AK, Mohania D, et al:
Blood biomarkers for the diagnosis and differentiation of stroke: A
systematic review and meta-analysis. Int J Stroke. 15:704–721.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cho TG, Lee JC, Park SW, Chung C, Nam TK
and Hwang SN: Relationship between systemic thrombogenic or
thrombolytic indices and acute increase of spontaneous
intracerebral hemorrhage. J Cerebrovasc Endovasc Neurosurg.
16:159–165. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Specogna AV, Turin TC, Patten SB and Hill
MD: Factors associated with early deterioration after spontaneous
intracerebral hemorrhage: A systematic review and meta-analysis.
PLoS One. 9(e96743)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lattanzi S, Brigo F, Trinka E, Cagnetti C,
Di Napoli M and Silvestrini M: Neutrophil-to-lymphocyte ratio in
acute cerebral hemorrhage: A system review. Transl Stroke Res.
10:137–145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu S, Liu X, Chen S, Xiao Y and Zhuang W:
Neutrophil-lymphocyte ratio predicts the outcome of intracerebral
hemorrhage: A meta-analysis. Medicine (Baltimore).
98(e16211)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang C, Wang Y, Hu Q, Shou J, Zhu L, Tian
N, Sun L, Luo H, Zuo F, Li F, et al: Immune changes in peripheral
blood and hematoma of patients with intracerebral hemorrhage. FASEB
J. 34:2774–2791. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zou Y, Zhang W, Huang C and Zhu Y:
Clinical significance of neutrophil to lymphocyte ratio and
platelet to lymphocyte ratio in acute cerebral hemorrhage with
gastrointestinal hemorrhage, and logistic regression analysis of
risk factors. Exp Ther Med. 18:1522–1538. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao Y, Yang J, Zhao H, Ding Y, Zhou J and
Zhang Y: The association between hyperglycemia and the prognosis of
acute spontaneous intracerebral hemorrhage. Neurol Res. 39:152–157.
2017.PubMed/NCBI View Article : Google Scholar
|