Introduction
Epidemiological studies have indicated that the
incidence of diabetes mellitus (DM) is increasing annually
worldwide, while diabetic cardiomyopathy (DCM) is the main factor
contributing to heart failure in diabetic patients without coronary
heart disease or hypertension (1,2).
However, the pathogenesis of DCM is very complex and not yet fully
understood. Long-term hyperglycemia can act on the respiratory
chain, increase production of reactive oxygen species (ROS) and
oxidative stress, and further induce myocardial apoptosis (3,4). Due
to the non-renewable characteristics of myocardial cells, cardiac
function gradually declines with cardiomyocyte apoptosis.
MicroRNAs (miRNAs or miRs) are non-coding small
molecule RNAs regulating post-transcriptional gene expression. They
can inhibit mRNA translation or target mRNA degradation by binding
to specific mRNAs, thus regulating gene expression (5). MiRNAs play important roles in
cardiovascular disease. For example, miR-17-5p and miR-1594 are
important regulators of cellular responses to heart injury
(6,7). miR-21-5p is involved in numerous
diseases, including lung and endometrial fibrosis (8,9), but
its exact function in heart disease remains controversial. Qiao
et al reported that miR-21-5p can enhance angiogenesis and
myocardial cell survival by regulating the phosphatase and tensin
homolog (PTEN)-Akt signaling pathway, thus contributing to cardiac
repair (10). Expression of
miR-21-5p is affected by isoflurane preconditioning in a rat model
of myocardial infarction (11).
However, the mechanism by which miR-21-5p regulates cardiomyocyte
injury under high glucose and high fat (HG-HF) conditions is
unclear.
In the present study, a miR-21-5p mimic was
constructed to study its effect on apoptosis in cardiomyocytes
under HG-HF conditions and to provide improved understanding of its
mechanism of action. The results of the present study have
important implications for the pathogenesis of DCM and for research
on possible treatments.
Materials and methods
Cell culture and treatments
H9c2 cells were purchased from American Type Culture
Collection (ATCC) and cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences). The cells were passaged when
their density reached 80-90%. The supernatant was then discarded
and the cells were washed twice with 1X PBS. The cells were then
treated with 0.25% trypsin (containing 0.02% EDTA; 3 min at 37˚C)
to detach them from the culture vessel. Subsequently, the cells
became round, and complete medium was added to terminate the
digestion. The cells were centrifuged at 875 x g for 3 min at room
temperature. The cell suspension was divided into new culture
dishes at a ratio of 1:3, marked and placed in a 5% CO2
incubator at 37˚C. H9c2 cells were cultured in DMEM containing 33
mM glucose (HG) and 250 µM sodium palmitate (HF) for 24, 48 and 72
h to induce HG-HF injury as previously described (12). DMEM supplemented with 5.5 mM
glucose was used as a control.
Transfection
A total of two sterilized Eppendorf tubes were
prepared for each group of cells. Each tube was filled with 62.5 µl
Opti-MEM™ (Thermo Fisher Scientific, Inc.). In addition, one tube
was filled with 2.5 µl Lipofectamine® 3000 (Thermo
Fisher Scientific, Inc.) while the other with 6.25 µl of either a
negative control (NC) mimic (5'-UCACAA
CCUCCUAGAAAGAGUAGAUCUACUCUUUCUAGGA GGUUGUGA-3') or a miR-21-5p
mimic (5'-UAGCUUAUC AGACUGAUGUUGAUCAACAUCAGUCUGAUAAG CUA-3') from
General Biosystems (Anhui) Co., Ltd., and both were then incubated
at room temperature for 5 min. The two tubes were evenly mixed and
incubated at room temperature for 15 min, after which the mixed
solution was dropped into wells in a 6-well plate before the cells
were returned to the incubator. Following 48 h of transfection of
the H9c2 cells, transfection efficiency was detected by reverse
transcription-quantitative PCR (RT-qPCR) and the treatment groups
were cultured in HG-HF medium for an additional 48 h at 37˚C.
TUNEL assay
Cells (3x105 cells/ml) were fixed with 4%
paraformaldehyde for 15 min at room temperature, washed 3 times
with PBS, and then permeated for 20 min at room temperature in PBS
containing 0.5% Triton X-100. PBS was used to wash the culture
dishes 3 times (3 min each). TUNEL solution was added to each well
and incubated for 1.5 h at 45˚C. DAPI (5 µg/ml) was added to stain
the nuclei for 5 min at room temperature. The culture dish was
sealed with 50% glycerol, and images from at least five fields in
each section were taken under a fluorescence microscope
(magnification, x200).
Measurement of ROS
To assess the levels of ROS, the cells
(3x105 cells/ml) were incubated with DCFH-DA (10 µM)
(Beyotime Institute of Biotechnology) at 37˚C for 20 min.
Subsequently, they were washed three times with serum-free medium
to remove excess DCFH-DA. ROS levels were then analyzed by flow
cytometry (FACSCalibur; BD Biosciences). The data were analyzed by
FlowJo 7.6 (FlowJo LLC).
Measurement of nitric oxide (NO)
level
The levels of NO were measured in the cells
(3x105 cells/ml) using the double antibody sandwich
method according to the manufacturer's instructions (cat. no.
MM-20607R1; Jiangsu Enzyme Industry Co., Ltd.).
RT-qPCR
Total RNA was extracted from cells using an
Ultrapure RNA extraction kit according to the manufacturer's
instructions (CoWin Biosciences). The concentration and purity of
RNA (OD260/OD280) were determined by a UV-Vis
spectrophotometer. RNA was reversely transcribed into cDNA using a
miRNA cDNA Synthesis Kit according to the manufacturer's
instructions (cat. no. CW2141S; CoWin Biosciences). The reaction
system for qPCR was as follows: RNase free dH2O, 9.5 µl;
cDNA, 1 µl; upstream primer, 1 µl; downstream primer, 1 µl; and 2X
SYBR Green PCR Master Mix (miRNA qPCR Assay Kit; cat. no. CW2142S;
CoWin Biosciences), 12.5 µl. The reaction steps were as follows:
Pre-denaturation at 95˚C for 10 min; denaturation at 95˚C for 10
sec; annealing at 58˚C for 30 sec; and extension at 72˚C for 30
sec, carried out over 40 cycles. The primers were designed based on
poly(A) tailing reaction method (13) and synthesized by General Biosystems
(Anhui) Co., Ltd., using the following sequences: miR-21-5p
forward, 5'-TAGCTTATCAGACTGATGTTGA-poly(A)-3' and the reverse
primer (5'-AGTGCAGGGTCCGAGGTATT-3') was a general primer in the kit
(2X SYBR Green PCR Master Mix; CoWin Biosciences); U6 forward,
5'-GCTTCGGCAGCACATT ACTACTATAAAAT-3' and reverse, 5'-CGCTTCACGGAATT
TGCGTGTCAT-3'. The target gene expression was calculated using the
2-ΔΔCq method (14).
Western blotting
Cells were collected and total protein was extracted
using TriplePrep kit according to the manufacturer's instructions
(cat. no. 28-9425-44; ReadyPrep; Cytiva). After 30 min on ice, the
lysate was centrifuged at 8798 x g for 10 min at 4˚C. Protein
concentration was determined using a BCA kit (Beyotime Institute of
Biotechnology). A total of (20 µg) per protein sample was
denatured, and the samples were separated by 12% SDS-PAGE for 2 h,
followed by transfer to a PVDF membrane. Following blocking in 5%
skim milk for 2 h at room temperature, the membranes were incubated
with primary antibodies at 4˚C overnight, and then the membranes
were incubated with a secondary antibody at room temperature for 2
h. The primary antibodies included mouse monoclonal anti-GAPDH
(1:2,000; cat. no. TA-08; ZSGB-BIO), rabbit anti-phosphorylated
(p)-PTEN (1:1,000; cat. no. AF3351; Affinity Biosciences), rabbit
anti-p-AKT (1:1,000; cat. no. AF0016; Affinity Biosciences) rabbit
anti-p-FOXO3a (1:1,000; cat. no. AF3020; Affinity Biosciences),
rabbit anti-Bax (1:1,000; cat. no. A0207; ABclonal Biotech Co.,
Ltd.), and mouse anti-Bcl-2 (1:500; product code ab692; Abcam). The
secondary antibodies (1:10,000) were HRP-labeled goat anti-rabbit
IgG (cat. no. 65-6120; Thermo Fisher Scientific, Inc.) and
HRP-labeled goat anti-mouse IgG (cat. no. 31430; Thermo Fisher
Scientific, Inc.). Enhanced chemiluminescence exposure solution
(cat. no. CW0049; CoWin Biosciences) was added to the membrane and
exposed in a gel imaging system. The gray value was analyzed by
Quantity One software (version 4.62; Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD; n=6 in each group) and analyzed by Graphpad Prism
version 7 (GraphPad Software, Inc.). Results from the two groups
were compared using unpaired Student's t-test and one-way ANOVA
followed by Bonferroni's correction were applied to compare three
or more groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
HG-HF downregulates the expression of
miR-21-5p in H9c2 cells
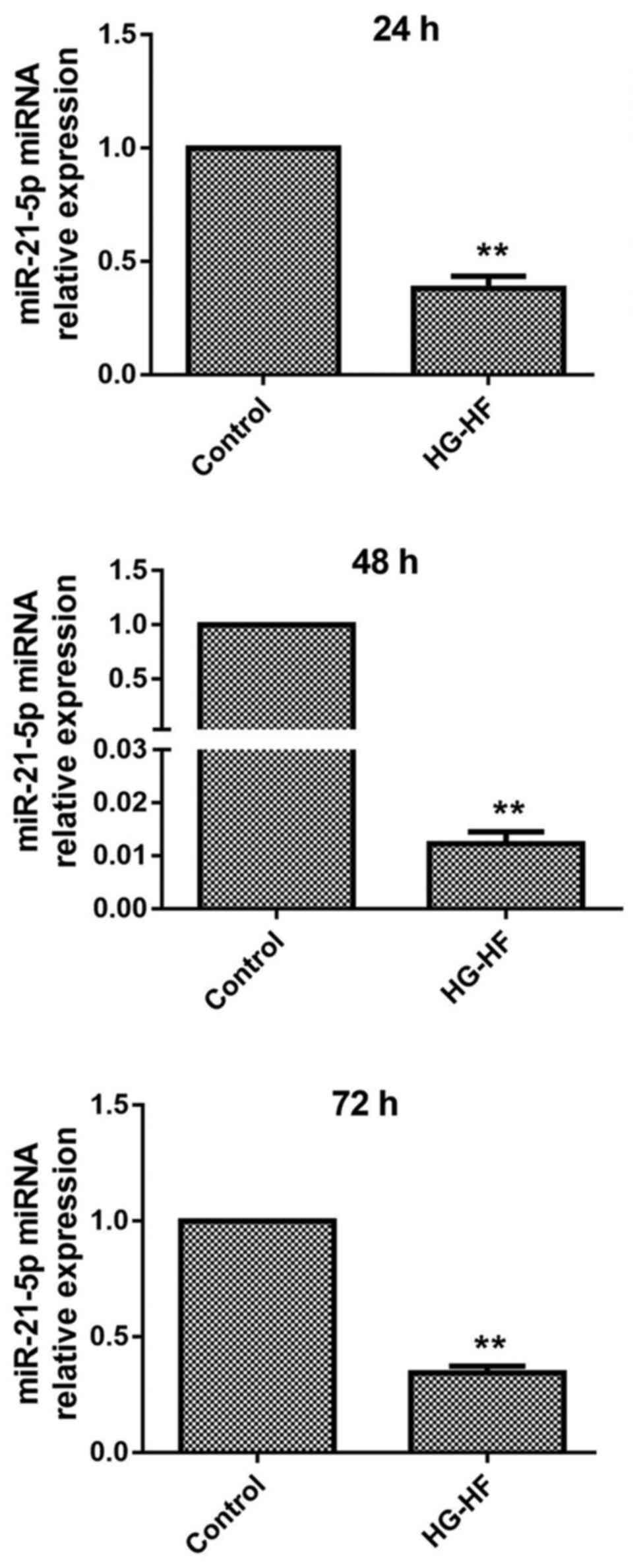
To explore the effect of HG-HF on the expression of
miR-21-5p in H9c2 cells, RT-qPCR was used to detect the expression
of miR-21-5p mRNA. The results are revealed in Fig. 1. Compared with the control group,
the expression of miR-21-5p in H9c2 cells was significantly reduced
by treatment with HG-HF for 24, 48, and 72 h.
HG-HF triggers apoptosis-related
protein expression and modulates PTEN/Akt/FOXO3a signaling
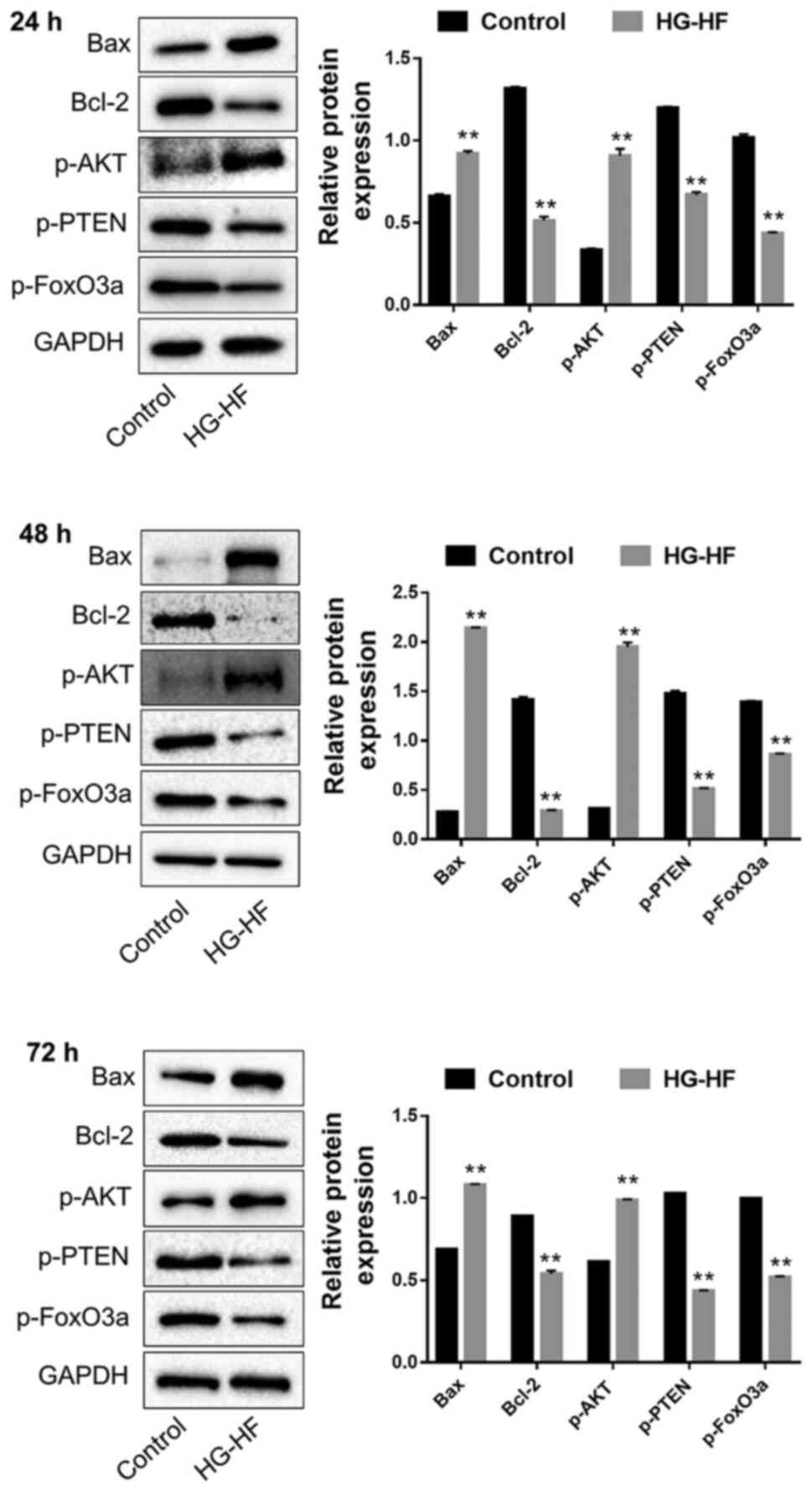
The expression levels of the apoptosis-related
proteins Bax/Bcl-2 and PTEN/Akt/FOXO3a were detected by western
blotting. Compared with the control group, HG-HF treatment for 24,
48, and 72 h significantly increased the expression of the
pro-apoptotic proteins Bax and p-Akt, while it significantly
decreased the expression of the anti-apoptotic proteins Bcl-2,
p-PTEN and p-FOXO3a (Fig. 2).
miR-21-5p mimic promotes miR-21-5p
expression in H9c2 cells
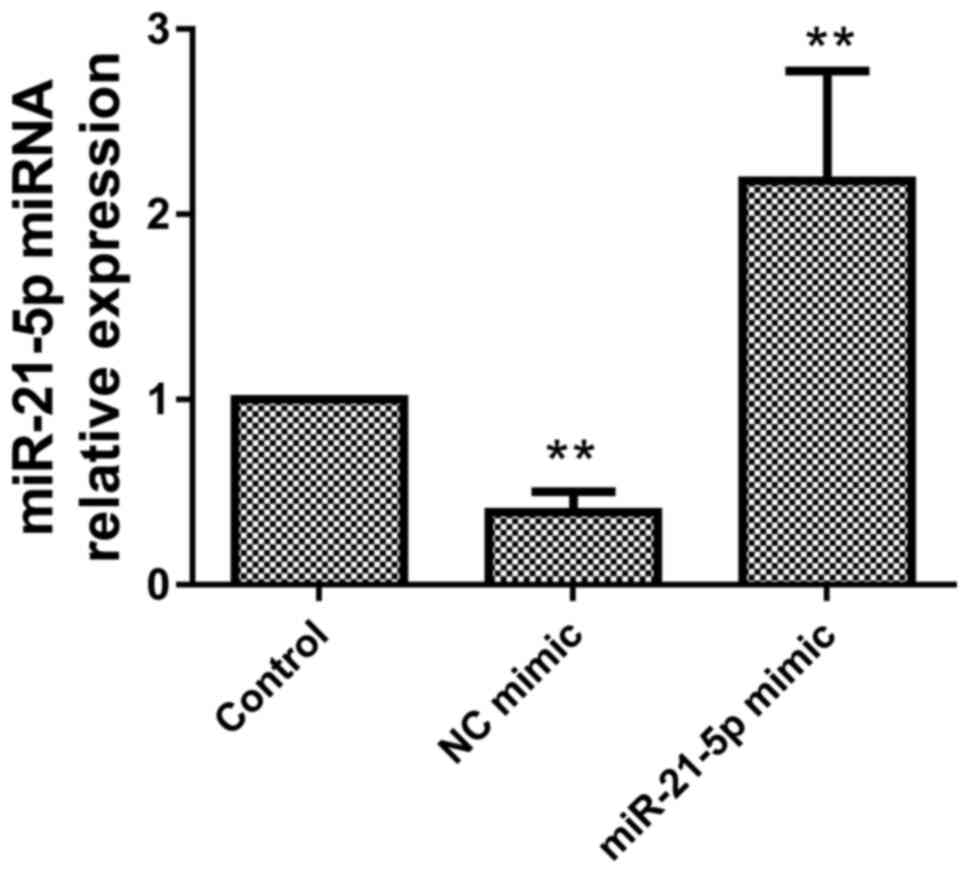
To verify transfection of miR-21-5p in H9c2 cells,
the expression of miR-21-5p was assessed using RT-qPCR. Compared
with the control group, the miR-21-5p mimic significantly increased
the expression of miR-21-5p (Fig.
3), indicating that miR-21-5p was overexpressed in the H9c2
cells.
miR-21-5p mimic reduces apoptosis of
H9c2 cells caused by HG-HF
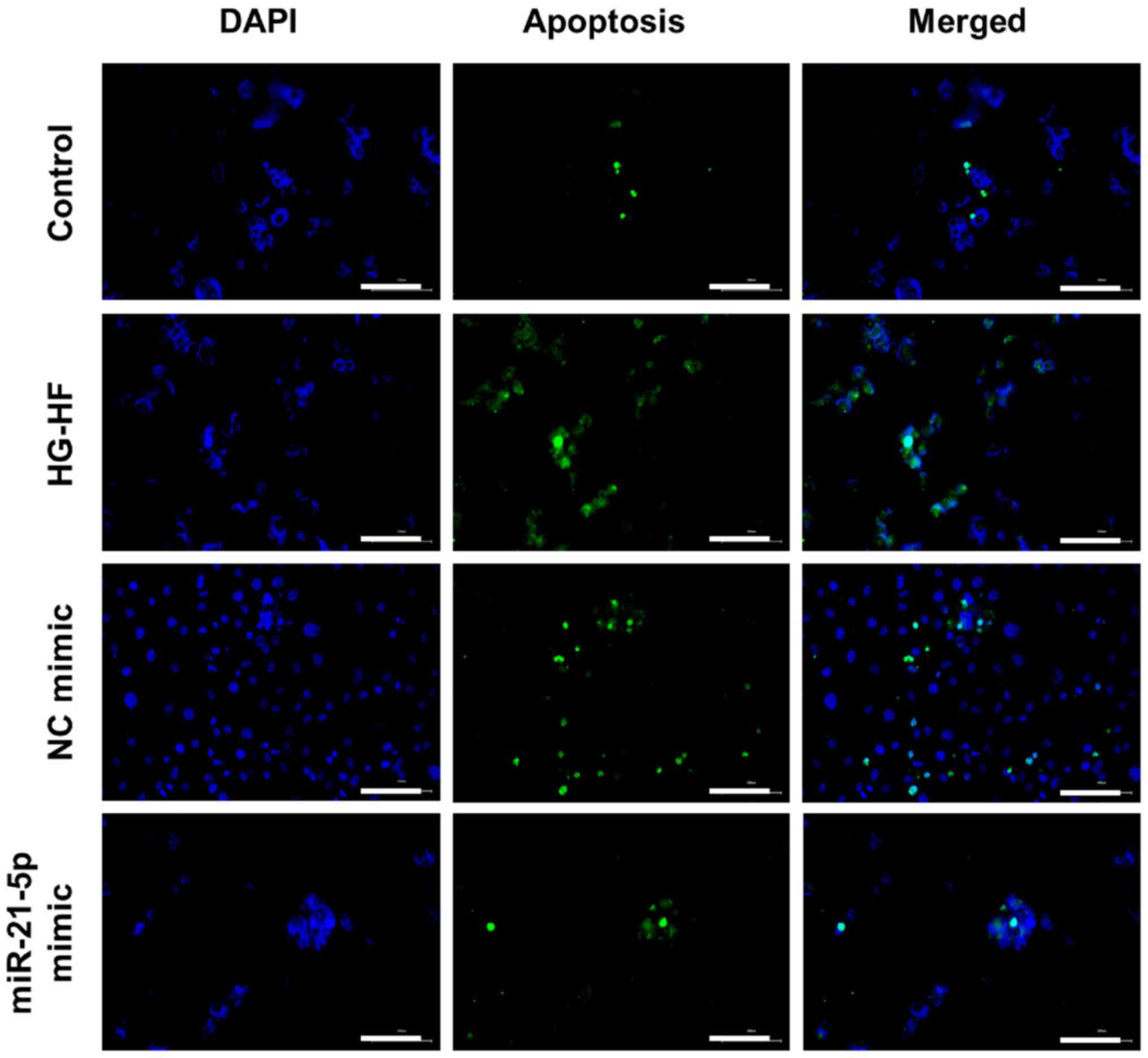
TUNEL staining was used to detect apoptosis, and the
results were revealed in Fig. 4.
Compared with the control H9c2 cells, HG-HF treatment significantly
promoted apoptosis while the miR-21-5p mimic inhibited apoptosis
induced by HG-HF (Fig. 4).
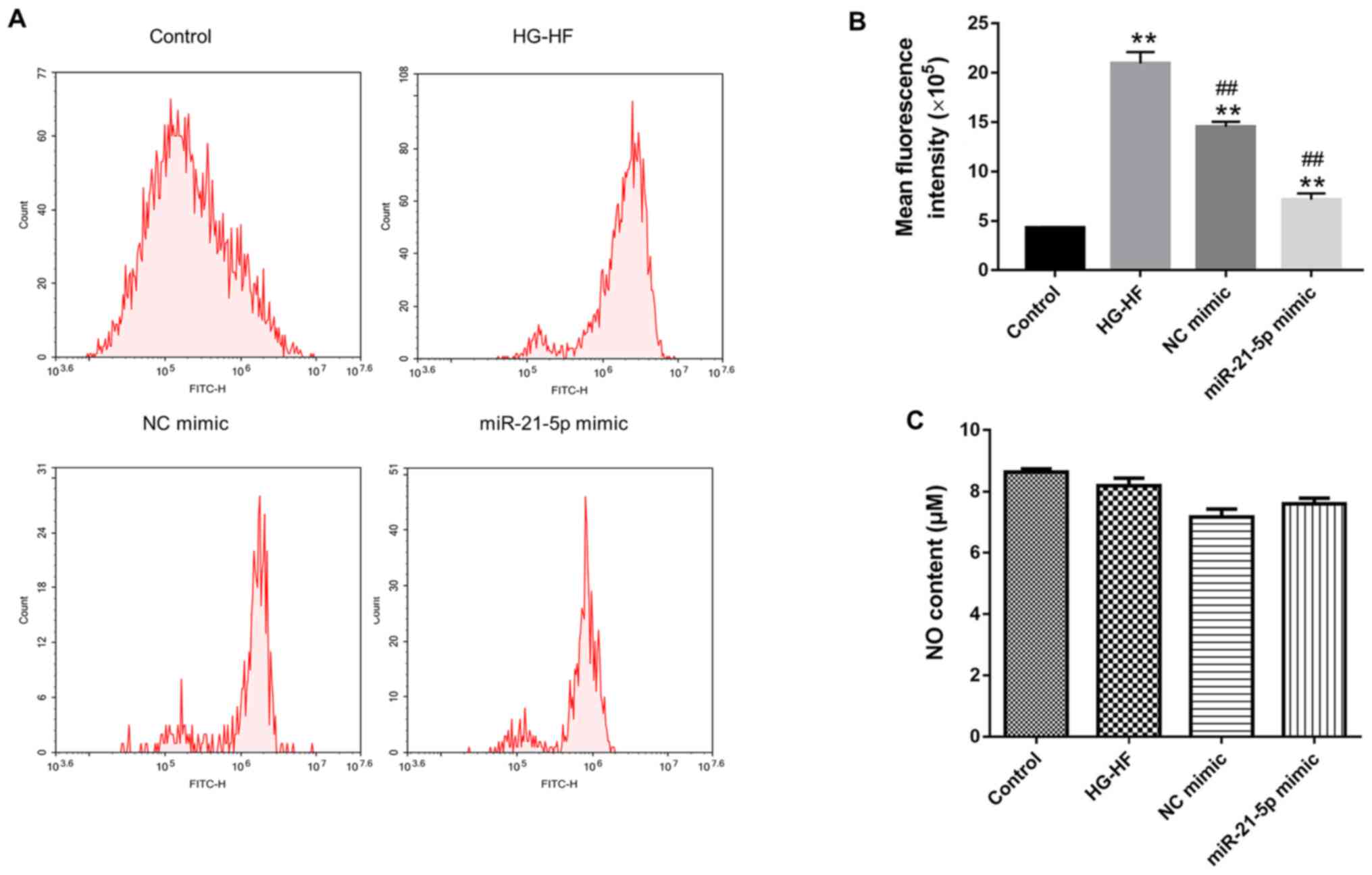
Effects of the miR-21-5p mimic on NO
levels and oxidative stress in H9c2 cells
In order to explore the effect of miR-21-5p mimic on
oxidative stress in H9c2 cells, flow cytometry was used to detect
ROS. Compared with the control group, HG-HF treatment significantly
increased the level of ROS, while the miR-21-5p mimic significantly
decreased ROS levels in the HG-HF group (Fig. 5A and B). Conversely, the miR-21-5p mimic had no
significant effect on NO levels in H9c2 cells (Fig. 5C).
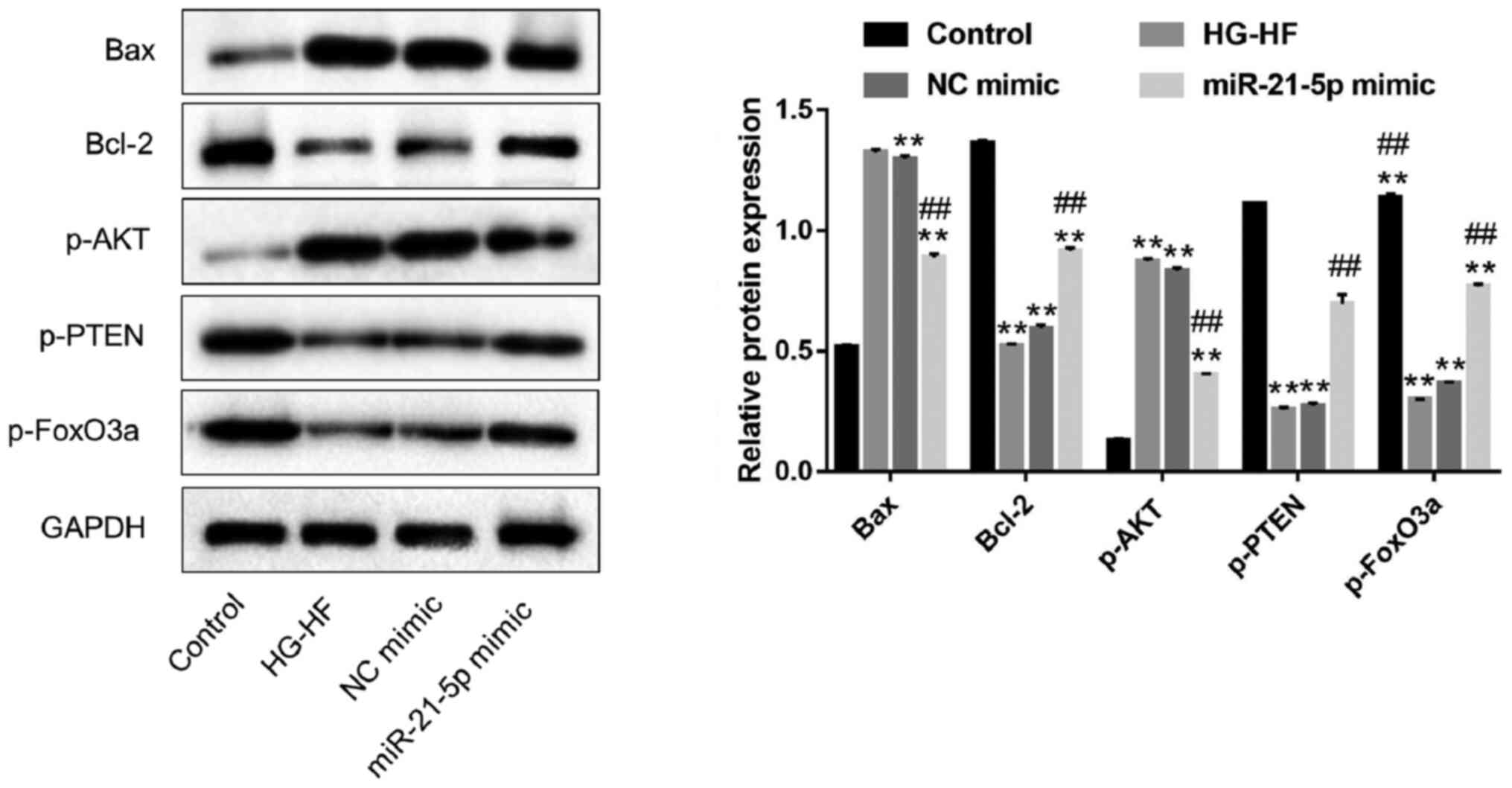
miR-21-5p mimic inhibits apoptosis
induced by HG-HF in H9c2 cells, likely via the PTEN/Akt/FOXO3a
signaling pathway
To further explore the effect of miR-21-5p on
apoptosis of H9c2 cells induced by HG-HF and to identify its
molecular mechanism, the expression levels of the apoptosis-related
proteins Bax/Bcl-2 and the signaling pathway proteins
PTEN/Akt/FOXO3a were detected by western blotting. Compared with
the control group, HG-HF treatment significantly increased the
expression of the pro-apoptotic proteins Bax and p-Akt and
decreased the expression of the anti-apoptotic proteins Bcl-2,
p-PTEN and p-FOXO3a, but these effects were greatly reduced in the
miR-21-5p mimic group (Fig. 6).
These data indicated that the miR-21-5p mimic inhibited H9c2 cell
apoptosis induced by HG-HF, likely via the PTEN/Akt/FOXO3a
signaling pathway.
Discussion
The steady increase in the number and mortality of
diabetic patients is partly due to DM-related heart disease
(15), including abnormal cardiac
structure and function such as left ventricular dysfunction,
myocardial apoptosis, and myocardial fibrosis (16). Cardiomyocyte apoptosis has been
considered a potential mechanism for the development of
cardiomyopathy and heart failure (17,18).
It has been observed that the PTEN/Akt signaling
pathway is important for regulation of cell apoptosis,
inflammation, and synaptic plasticity (19,20).
PTEN was initially recognized as a tumor suppressor that can
antagonize the effect of PI3K and downregulate PIP3 (21-23).
PIP3 can increase the level of p-Akt and participate in the growth
and survival of cells. PTEN is a negative regulator of the PI3K/Akt
pathway and plays an important role in regulation of cell growth,
differentiation, apoptosis, migration, and neuronal plasticity
(19,20). The FOXO protein is a member of the
forkhead transcription factor family. Its common characteristic is
the forkhead protein (Fox) domain, which plays an important role in
apoptosis via regulation of the PI3K/Akt pathway. Li et al
(24) found that the
PTEN/Akt/FOXO3a pathway plays an important role in hypoxia and
ischemia-induced neuronal apoptosis in rats. In the present study,
it was revealed that p-Akt was significantly upregulated and p-PTEN
and p FOXO3a were significantly downregulated. Additionally,
upregulation of the pro-apoptotic protein Bax and downregulation of
the anti-apoptotic protein Bcl-2 were observed after 24, 48, and 72
h of HG-HF treatment, which indicated that HG-HF promoted H9c2 cell
apoptosis through the PTEN/Akt/ FOXO3a signaling pathway.
miR-21 (miRBase Accession number: MI0000077) is a
stem-loop precursor sequence and is processed into two mature miRNA
sequences, miR-21-5p (miRBase Accession number: MIMAT0000076) and
miR-21-3p (miRBase Accession number: MIMAT0004494). miR-21-5p and
miR-21-3p are derived from 5' and 3' ends of miR-21, respectively
(25). MiR-21 is differentially
expressed in numerous cardiovascular diseases, including neointimal
injury, myocardial infarction, heart failure, as well as other
pathological states, and in cardiomyocyte apoptosis associated with
a variety of conditions (10).
Sayed et al (26) revealed
that miR-21 was sensitive to sustained hypoxia, which could
downregulate the expression of miR-21 in cardiomyocytes. Cheng
et al (27) identified that
miR-21 was sensitive to hydrogen peroxide, which could upregulate
the expression of miR-21 in cardiomyocytes. Regulation of PTEN by
miR-21 has been reported in cancer and cardiovascular injury
(28,29). Interestingly, miR-21-5p has also
been revealed to perform key roles in heart diseases. Knockdown of
miR-21-5p decreases myocardial infarction injury, indicating that
miR-21-5p may play an active role in post-myocardial infarction
repair (30,31). In the present study, it was
revealed that the expression of miR-21-5p in HG-HF-induced
cardiomyocytes was significantly lower than in a normal control
group. miR-21-5p mimic inhibited Bax expression and increased Bcl-2
expression to inhibit cell apoptosis, and also reduced the effect
of HG-HF on the PTEN/Akt/FOXO3a signaling pathway, indicating that
miR-21-5p regulates the PTEN/Akt/FOXO3a signaling pathway to
inhibit HG-HF-induced apoptosis in cardiomyocytes. Although the
specific function of miR-21 and miR-21-5p in heart diseases was not
distinguished, the mimic of miR-21 may also exert a similar
function, since miR-21-5p is a mature product of miR-21. In
addition, the function of miR-21-3p should also be investigated in
future.
Oxidative stress is widely reported in numerous
pathophysiological processes, such as aging, inflammation, and
psychiatric disorders (32).
Studies have revealed that autophagy and apoptosis are
ROS-dependent (33), and ROS are
involved in regulation of apoptosis (34). In the present study, ROS levels in
the experimental groups were also detected using flow cytometry.
HG-HF treatment increased ROS in H9c2 cells, while the miR-21-5p
mimic decreased ROS in the HG-HF group. These data may suggest that
the miR-21-5p mimic inhibits HG-HF-induced apoptosis of
cardiomyocytes by regulating ROS. NO generated within the heart has
long been known to influence vascular homoeostasis (35), but it was revealed that the
miR-21-5p mimic had no significant effect on NO levels in the H9c2
cells. These data indicated that regulation of apoptosis by
miR-21-5p is independent of NO.
There are a few limitations in the present study.
Firstly, whether miR-21-5p has other anti-apoptotic mechanisms and
whether the PTEN/Akt/FOXO3a pathway is the downstream target of
miR-21-5p in cardiomyocyte apoptosis induced by HG-HF remains
unclear. Therefore, the relationship between miR-21-5p and
PTEN/Akt/FOXO3a signaling in inhibiting apoptosis in cardiomyocytes
needs further study. Secondly, our research was limited to in vitro
cell experiments; animal experiments and human clinical trials have
not yet been carried out. The present study provided a preliminary
conceptual and experimental basis for the use of miR-21-5p in the
treatment of diabetic cardiomyopathy.
In conclusion, our results indicated that miR-21-5p
inhibits apoptosis in cardiomyocytes induced by HG-HF, and may act
via the PTEN/Akt/FOXO3a signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Fujian (grant no. 2018J01166), the Medical Innovation
Project of Fujian Health Commission (grant no. 2019-cx-29) and the
Promotion Project of Fujian Health and Family Planning Commission
for Rural Urban Communities (grant no. 2018017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, XC, MP, JG, WC, DL and CX performed the
experiments and analyzed the data. YH designed the study and wrote
the manuscript. All authors have read and approved the final
manuscript. YH, XC, MP, JG, WC, DL and CX confirmed the
authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Picano E: Diabetic cardiomyopathy the
importance of being earliest. J Am Coll Cardi-ol. 42:454–457.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Avogaro A, Vigili de Kreutzenberg S, Negut
C, Tiengo A and Scognamiglio R: Diabetic cardiomyopathy: A
metabolic perspective. Am J Cardiol. 93:13A–16A. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang BB, Zhou G and Li C: AMPK: An
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li B, Zheng Z, Wei Y, Wang M, Peng J, Kang
T, Huang X, Xiao J, Li Y and Li Z: Therapeutic effects of
neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol.
10(69)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y, Zou L, Wu T, Xiong L, Zhang T,
Kong L, Xue Y and Tang M: Identification of mRNA-miRNA crosstalk in
human endothelial cells after exposure of PM2.5 through integrative
transcriptome analysis. Ecotoxicol Environ Saf. 169:863–873.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang J, Shi G, Gong Y, Cai J, Zheng Y and
Zhang Z: LncRNA 0003250 accelerates heart autophagy and binds to
miR-17-5p as a competitive endogenous RNA in chicken induced by
selenium deficiency. J Cell Physiol. 236:157–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang J, Gong Y, Cai J, Liu Q and Zhang Z:
lnc-3215 Suppression Leads to Calcium Overload in Selenium
Deficiency-Induced Chicken Heart Lesion via the
lnc-3215-miR-1594-TNN2 Pathway. Mol Ther Nucleic Acids. 18:1–15.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Song M, Zhao G, Sun H, Yao S, Zhou Z,
Jiang P, Wu Q, Zhu H, Wang H, Dai C, et al: circPTPN12/miR-21-5
p/Np63alpha pathway contributes to human endometrial fibrosis.
eLife 10: e65735.
|
|
9
|
Liu E, Lv L, Zhan Y, Ma Y, Feng J, He Y,
Wen Y, Zhang Y, Pu Q, Ji F, et al: METTL3/N6-methyladenosine/
miR-21-5p promotes obstructive renal fibrosis by regulating
inflammation through SPRY1/ERK/NF-κB pathway activation. J Cell Mol
Med. 25:7660–7674. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qiao L, Hu S, Liu S, Zhang H, Ma H, Huang
K, Li Z, Su T, Vandergriff A, Tang J, et al: microRNA-21-5p
dysregulation in exosomes derived from heart failure patients
impairs regenerative potential. J Clin Invest. 129:2237–2250.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raupach A, Torregroza C, Niestegge J,
Feige K, Klemm-Meyer S, Bauer I, Brandenburger T, Grievink H,
Heinen A and Huhn R: MiR-21-5p but not miR-1-3p expression is
modulated by preconditioning in a rat model of myocardial
infarction. Mol Biol Rep. 47:6669–6677. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dai B, Li H, Fan J, Zhao Y, Yin Z, Nie X,
Wang DW and Chen C: MiR-21 protected against diabetic
cardiomyopathy induced diastolic dysfunction by targeting gelsolin.
Cardiovasc Diabetol. 17(123)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Balcells I, Cirera S and Busk PK: Specific
and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC
Biotechnol. 11(70)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xia Y, Gong L, Liu H, Luo B, Li B, Li R,
Li B, Lv M, Pan J and An F: Inhibition of prolyl hydroxylase 3
ameliorates cardiac dysfunction in diabetic cardiomyopathy. Mol
Cell Endocrinol. 403:21–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang CC and Reusch JE: Diabetes and
cardiovascular disease: Changing the focus from glycemic control to
improving long-term survival. Am J Cardiol. 110 (Suppl 9):58B–68B.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee Y and Gustafsson AB: Role of apoptosis
in cardiovascular disease. Apoptosis. 14:536–548. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shekhar A, Heeger P, Reutelingsperger C,
Arbustini E, Narula N, Hofstra L, Bax JJ and Narula J: Targeted
Imaging for Cell Death in Cardiovascular Disorders. JACC Cardiovasc
Imaging. 11:476–493. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song Z, Shen F, Zhang Z, Wu S and Zhu G:
Calpain inhibition ameliorates depression-like behaviors by
reducing inflammation and promoting synaptic protein expression in
the hippocampus. Neuropharmacology. 174(108175)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song Z, Chen H, Xu W, Wu S and Zhu G:
Basolateral amygdala calpain is required for extinction of
contextual fear-memory. Neurobiol Learn Mem. 155:180–188.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Myers MP, Stolarov JP, Eng C, Li J, Wang
SI, Wigler MH, Parsons R and Tonks NK: P-TEN, the tumor suppressor
from human chromosome 10q23, is a dual-specificity phosphatase.
Proc Natl Acad Sci USA. 94:9052–9057. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shi YH, Wang YX, You JF, Heng WJ, Zhong HH
and Fang WG: Activation of HIF-1 by bFGF in breast cancer: Role of
PI-3K and MEK1/ERK pathways. Zhonghua Yi Xue Za Zhi. 84:1899–1903.
2004.PubMed/NCBI(In Chinese).
|
|
23
|
Song ZJ, Yang SJ, Han L, Wang B and Zhu G:
Postnatal calpeptin treatment causes hippocampal neurodevelopmental
defects in neonatal rats. Neural Regen Res. 14:834–840.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero
D and Mu D: Involvement of the PTEN-AKT-FOXO3a pathway in neuronal
apoptosis in developing rat brain after hypoxia-ischemia. J Cereb
Blood Flow Metab. 29:1903–1913. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sayed D, He M, Hong C, Gao S, Rane S, Yang
Z and Abdellatif M: MicroRNA-21 is a downstream effector of AKT
that mediates its antiapoptotic effects via suppression of Fas
ligand. J Biol Chem. 285:20281–20290. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng Y, Liu X, Zhang S, Lin Y, Yang J and
Zhang C: MicroRNA-21 protects against the H(2)O(2)-induced injury
on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol.
47:5–14. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He Z, Long J, Yang C, Gong B, Cheng M,
Wang Q and Tang J: LncRNA DGCR5 plays a tumor-suppressive role in
glioma via the miR-21/Smad7 and miR-23a/PTEN axes. Aging (Albany
NY). 12:20285–20307. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang J, Yue J, Xia Q, Jiao X and Zhi J:
Angiotensin II type i receptor agonistic autoantibodies induces
apoptosis of cardiomyocytes by downregulating miR21 in
preeclampsia: A mechanism study. Am J Transl Res. 11:2339–2349.
2019.PubMed/NCBI
|
|
30
|
Roy S, Khanna S, Hussain SR, Biswas S,
Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ and Sen CK: MicroRNA
expression in response to murine myocardial infarction: miR-21
regulates fibroblast metalloprotease-2 via phosphatase and tensin
homologue. Cardiovasc Res. 82:21–29. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shen F, Song Z, Xie P, Li L, Wang B, Peng
D and Zhu G: Polygonatum sibiricum polysaccharide prevents
depression-like behaviors by reducing oxidative stress,
inflammation, and cellular and synaptic damage. J Ethnopharmacol.
275(114164)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qian M, Tan HM, Yu N, Wang T and Zhang Q:
Inactivated Sendai Virus Induces ROS-dependent Apoptosis and
Autophagy in Human Prostate Cancer Cells. Biomed Environ Sci.
31:280–289. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang H, Xie Y, Yang D and Ren D: Oxidative
stress-induced apoptosis in granulosa cells involves JNK, p53 and
Puma. Oncotarget. 8:25310–25322. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sears CE, Ashley EA and Casadei B: Nitric
oxide control of cardiac function: Is neuronal nitric oxide
synthase a key component? Philos Trans R Soc Lond B Biol Sci.
359:1021–1044. 2004.PubMed/NCBI View Article : Google Scholar
|