Introduction
Relapsing polychondritis (RP) is a clinical disease,
which is characterized by the inflammation of systemic cartilage
tissues such as the external auricle, nose, respiratory tract and
joints, and a pattern of repeated remission and recurrence
(1). However, identifying
effective treatment strategies for RP remains a challenge. It is
well known that inflammatory response in chondrocytes is one of the
most important risk factors in the pathogenesis of RP, which is
influenced by oxidative stress (2). H2O2 is a common
agent and may lead to DNA damage by inducing oxidative stress. It
is necessary to deeply explore the molecular mechanisms underlying
the suppression of inflammation in chondrocytes under oxidative
stress, which is responsible for cartilage destruction in the
progression of RP. Therefore, the suppression of inflammatory
responses in chondrocytes may be an effective strategy to delay RP
progression.
Curcumin is a promising pharmacologically active
natural product, which is extracted from turmeric (Curcuma
longa) and has significant anti-inflammatory, anti-oxidant and
anti-cancer properties (3).
Previous studies have reported on the anti-inflammatory effects of
curcumin in various common diseases, including chronic
inflammation, cancer, cardiovascular disease and osteoarthritis
(4-7).
Furthermore, it was suggested that curcumin may exert
anti-inflammatory effects in several chronic diseases by activating
the nuclear factor E2-related factor 2 (Nrf2) signaling pathway
(8). However, the roles of
curcumin in chondrocytes and the underlying mechanisms remain
elusive.
Previous studies have indicated that the
reactivation of autophagy is a promising therapeutic strategy for
the suppression of inflammation (9,10).
Autophagy, a cellular conservation and self-digestion system, is
accurately regulated by a family of autophagy regulators and
autophagy-related proteins and homologues. It is controlled by a
series of different signaling pathways such as MAPK,
phosphoinositide-3 kinase (PI3K) and mTOR (11-13),
which coordinate autophagy by regulating autophagosome formation
and autophagosome-lysosome fusion. Autophagy mediates the
degradation of dysfunctional proteins and damaged organelles for
energy recycling to maintain the metabolic regulation and nutrition
maintenance of the cell under oxidative stress (14). Recently, pharmacological
suppression studies to reduce cell inflammation have repeatedly
demonstrated the protective effect of autophagy on cells under
abnormal physiological conditions, including external pressure,
hypoalimentation, hypoxia and endoplasmic reticulum stress
(15,16). In rats with osteoarthritis,
β-ecdysterone promoted the autophagic flux of chondrocytes by
regulating the PI3K/AKT/mTOR signaling pathway and attenuated the
inflammatory response (17). In
addition, curcumin was reported to exert a neuroprotective effect
by inducing autophagic activities via the PI3K/Akt/mTOR pathway and
suppresses inflammatory reactions through the Toll-like receptor
4/p38/MAPK pathway (18). However,
whether curcumin mediates the suppression of the inflammatory
response by inducing autophagic activities in chondrocytes has
remained elusive.
Therefore, the present study aimed to explore the
role of curcumin on the inflammatory response of chondrocytes and
its correlation with autophagy in a hydrogen peroxide
(H2O2)-induced inflammation model in
vitro.
Materials and methods
Materials
Dulbecco's Modified Eagle's Medium F-12 (DMEM-F12)
and FBS were purchased from Corning Life Sciences. Curcumin (cat.
no. HY-N0005, >96.0%) purchased from MedChemExpress was
dissolved in DMSO and then diluted with culture medium for cell
experiments. H2O2 solution (cat. no. 106097;
34.5-36.5%) was purchased from Merck KGaA. 3-Methyladenine (3-MA;
cat. no. HY-19312) was obtained from MedChemExpress and prepared as
a 100 mM stock solution in PBS. Protease inhibitors were purchased
from MilliporeSigma. An ECL chemiluminescence detection kit
(SuperSignal HRP; cat. no. 46640) was from Pierce (Thermo Fisher
Scientific, Inc.).
Cell isolation and culture
A total of 20 male Sprague-Dawley rats (weight,
200-220 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. All Sprague Dawley rats were reared
under specific pathogen-free conditions. Rats were housed under
laminar flow in an isolated room with controlled temperature and at
a 12-h light/dark cycle. Food and water were available ad
libitum. The rats were sacrificed by injecting 100-200 mg/kg
pentobarbital sodium at the end of the experiments. Death of rats
was confirmed by observation of respiration and heartbeat. Primary
chondrocytes were isolated from the bilateral hip joints of
4-week-old male rats. The cartilage of the rat hip joint was cut
into 1 mm3 pieces in a sterile manner and then treated
with 0.25% (V/V) trypsin/EDTA (cat. no. C0201; Beyotime Institute
of Biotechnology) for 1 h and digested with 0.2% (V/V) collagenase
II (cat. no. C2-28; Sigma-Aldrich; Merck KGaA) in DMEM-F12 at 37˚C
in an atmosphere with 5% CO2 for 6 h. Next, the
suspensions were centrifuged at 1,609 x g at room temperature for 5
min and cultured at 37˚C under 5% CO2 in DMEM-F12 with
10% (V/V) FBS, 1% (V/V) penicillin and streptomycin. Primary
chondrocytes from the first passage were used for in vitro
experiments.
Cell proliferation assay
The effect of curcumin and
H2O2 on chondrocytes was assessed with a
CCK-8 (cat. no. CK04; Dojindo Laboratories, Inc.) according to the
manufacturer's protocol. In brief, after treatment, cells were
cultured in 96-well plates with a density of 5x103
cells/well for 24 h. Subsequently, CCK-8 solution was added to each
well and the cells were further incubated at 37˚C in the dark for 1
h. The optical density values were detected at wavelengths of 450
nm.
Monodansylcadaverine (MDC) assay
MDC was used to fluorescently label autophagic
lysosomes in the cytoplasm. Cells were seeded on sterile glass
slides in cell culture media. In the
curcumin+H2O2+3-MA group, chondrocytes were
pretreated with 20 µM curcumin for ~2 h, followed by incubation
with 20 µM H2O2 and 10 mM 3-MA at 37˚C for 24
h. In the rapamycin group, chondrocytes were pretreated with 7.5 µM
rapamycin. In the other groups, chondrocytes were pretreated with
or without 20 µM curcumin for ~2 h, followed by treatment with or
without 20 µM H2O2 at 37˚C for 24 h.
Subsequently, chondrocytes were treated with MDC (0.05 mM) at 37˚C
for 30 min and were then washed with PBS three times. The samples
were immediately analyzed by confocal microscopy (Olympus
Corporation). Excitation wavelengths were 360-380 nm and images
were captured under a microscope at x200 magnification from 20
separate randomly selected microscopic fields. MDC-specific
activity was calculated as the number of cells with morphological
features of autophagy as determined by scoring 100 cells from 20
different microscopic fields.
Western blot analysis
To examine the function of curcumin on apoptosis
induced by H2O2 on chondrocytes, the total
cellular protein was extracted by using a radioimmunoprecipitation
assay lysis buffer (main components, pH 7.4; 50 mM Tris, 150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium
orthovanadate, sodium fluoride, EDTA and leupeptin; cat. no.
P0013B; Beyotime Institute of Biotechnology) supplemented with
protease inhibitor cocktail (cat. no. 1081; Beyotime Institute of
Biotechnology). The concentration of the protein in different
groups was measured by a BCA protein assay kit (cat. no. P0009;
Beyotime Institute of Biotechnology). Subsequently, 30 µg of
protein in each group was separated by 15% SDS-PAGE and
electrotransferred to PVDF membranes (cat. no. 162-0177; Bio-Rad
Laboratories, Inc.). Following blocking with 5% skimmed milk powder
(cat. no. P0216; Beyotime Institute of Biotechnology) in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 90 min at
room temperature, membranes were incubated overnight at 4˚C with
the following primary antibodies: IL-1β (dilution, 1:1,000; cat.
no. ab283818; Abcam), IL-6 (dilution, 1:1,000; cat. no. ab259341;
Abcam), inducible nitric oxide synthase (iNOS; dilution, 1:1,000;
cat. no. ab178945; Abcam), beclin-1 (dilution, 1:1,000; cat. no.
ab207612; Abcam), P62 (dilution, 1:1,000; cat. no. ab109012;
Abcam), light chain (LC)3 (dilution, 1:1,000; cat. no. ab192890;
Abcam) and β-actin (dilution, 1:1,000; cat. no. ab6276, Abcam). The
next day, the blots were washed with TBST five times for 30 min and
incubated with secondary antibodies (dilution, 1:20,000; cat. no.
ab288151; Abcam) at room temperature for 90 min. Then membranes
were washed with TBST five times. An imaging system (Li-Cor
Biosciences, Inc.) was used to detect and analyze the density of
each band.
Reverse transcription-quantitative
(RT-q)PCR analysis
Following the manufacturer's protocol, the total RNA
was isolated by an RNA extraction kit (cat. no. 28306; Qiagen
GmbH). The quality [criterion, optical density at 260 nm
(OD260)/OD280=1.8-2.0] and concentration of
RNA in each group were assessed using a NanoDrop 2000 (Thermo
Fisher Scientific, Inc.), while any RNA contamination and
degradation were detected on 1% agarose gels. Eventually, 1,000 ng
of total RNA was reverse transcribed to synthesize cDNA with the
PrimeScript™ RT Master Mix (cat. no. RR036A; Takara Biotechnology,
Co., Ltd.). Real-time PCR was performed in triplicate by using SYBR
green PCR Master Mix (cat. no. 640210; Takara Biotechnology, Co.,
Ltd.). The amplification was conducted using the following cycling
conditions: 5 sec at 95˚C, 20 sec at 63.5˚C and 10 sec at 72˚C for
40 cycles. The amplification efficiency of the qPCR was 95.6% and
the relative mRNA expression level of the target gene was
determined using the 2-ΔΔCq method (19). The sequences of the forward and
reverse primers of target genes are presented in Table I.
 | Table IPrimer sequences used for real-time
PCR. |
Table I
Primer sequences used for real-time
PCR.
| Primer/gene
name | Sequences (5' to
3') |
|---|
| IL-1β F |
CAACCAACAAGTGATATTCTCCATG |
| IL-1β R |
GATCCACACTCTCCAGCTGCA |
| IL-6 F |
ACTTCCATCCAGTTGCCTTCTTGG |
| IL-6 R |
TTAAGCCTCCGACTTGTGAAGTGG |
| iNOS F |
AGTCAACTACAAGCCCCACG |
| iNOS R |
GCAGCTTGTCCAGGGATTCT |
| GAPDH F |
GCATCTTCTTGTGCAGTGCC |
| GAPDH R |
GATGGTGATGGGTTTCCCGT |
ELISA
The concentrations of IL-1β and IL-6 in the culture
supernatants from chondrocytes treated with the different stimuli
were determined using commercial ELISA kits (cat. nos. KE1002 and
KE1003; Proteintech Group, Inc.) following the manufacturer's
instructions. The absorbance at 450 nm was detected with a
Multiskan Ascant (SPECTRAFluor Plus; Tecan Group, Ltd.).
Statistical analysis
The experiments were performed as at least three
independent experiments. The results are presented as the mean ±
standard deviation. SPSS 13.0 software (SPSS Inc.) was used to
analyze the data. Comparisons among multiple groups were performed
using a one-way or two-way ANOVA followed by a Tukey's post-hoc
test. P<0.05 was considered to indicate statistical
significance.
Results
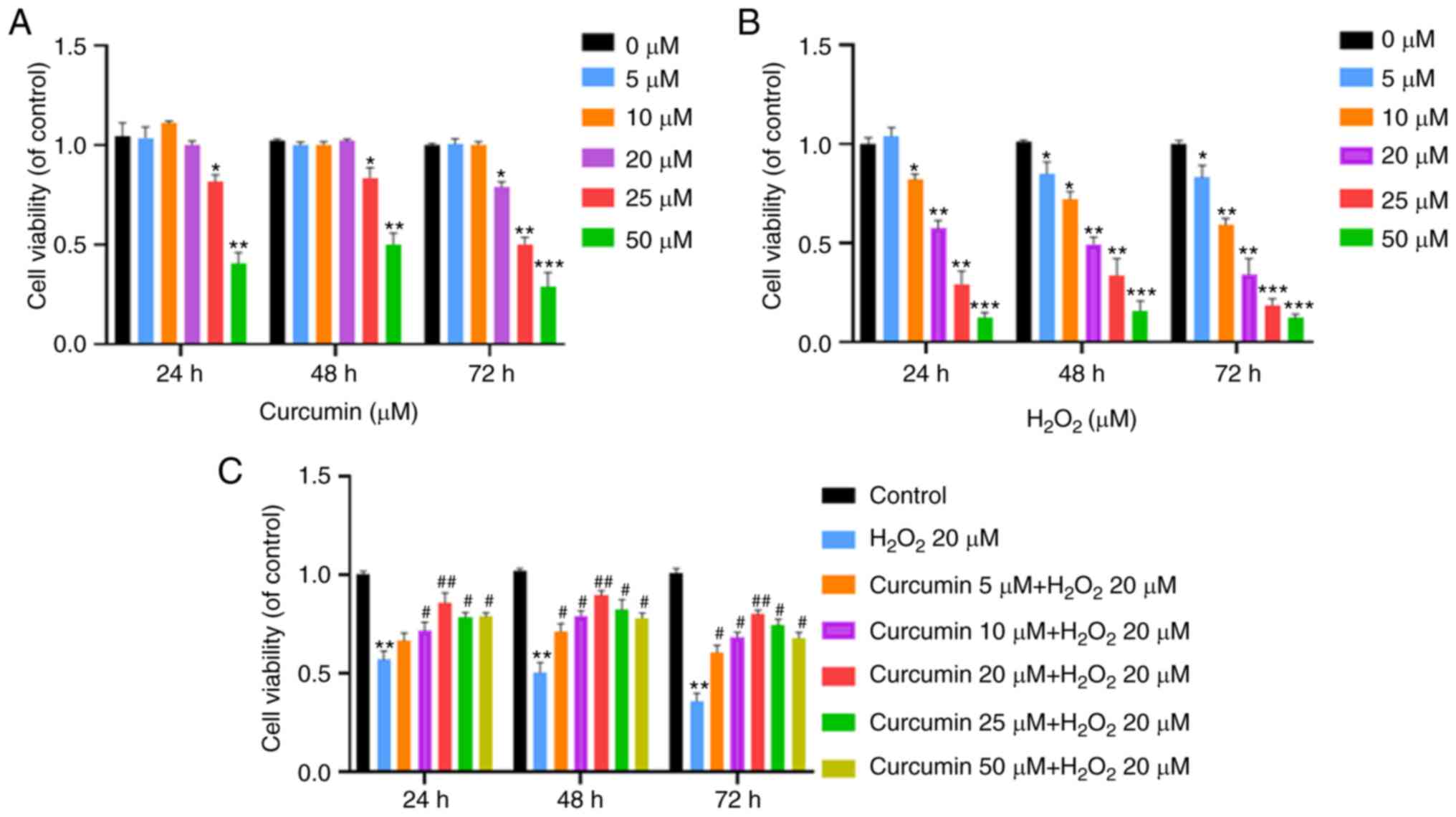
Effect of various concentrations of
curcumin on chondrocyte viability in the presence or absence of
H2O2
The effect of curcumin on chondrocyte viability with
or without H2O2 was studied at different
concentrations for 24, 48 and 72 h by the CCK-8 assay. Curcumin
exerted no significant cytotoxic effect at concentrations of up to
20 µM at different time-points (Fig.
1A). The results also indicated that 20 µM
H2O2 significantly inhibited the viability of
chondrocytes (P<0.05; Fig. 1B),
whereas curcumin (<20 µM) markedly increased the viability of
chondrocytes in a dose-dependent manner (P<0.01; Fig. 1C). To mimic the oxidative stress of
RP in vitro, 20 µM H2O2 was used to
treat rat chondrocytes for 24 h. Thus, 20 µM of curcumin and 20 µM
of H2O2 were used for the next
experiments.
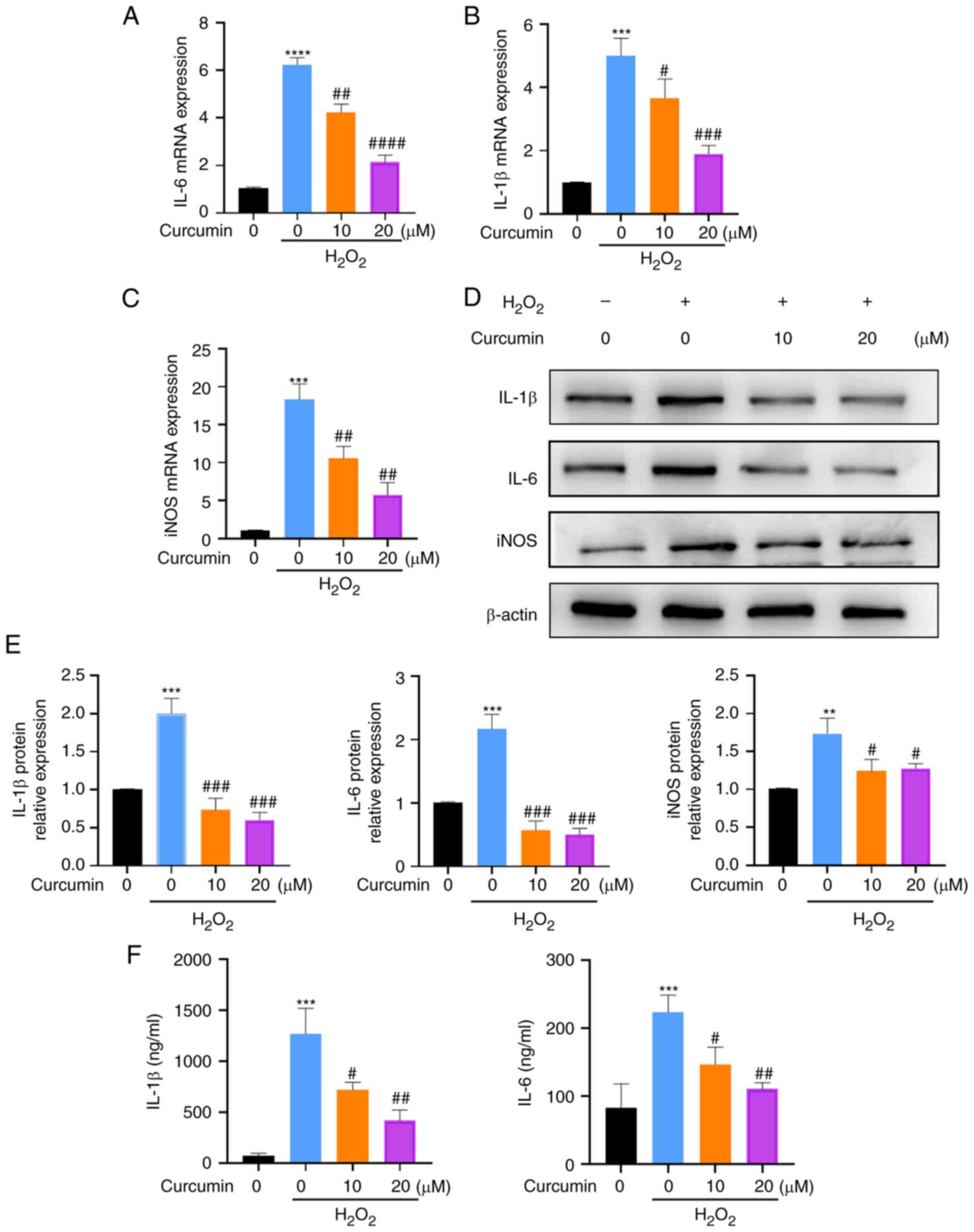
Curcumin inhibits
H2O2-induced chondrocyte inflammation
To explore the effects of curcumin on
H2O2-induced chondrocyte inflammation,
chondrocytes were pretreated with curcumin for 2 h at 10 and 20 µM
and were exposed to H2O2 for another 24 h.
Subsequently, it was examined whether curcumin affects
H2O2-induced IL-1β, IL-6 and iNOS mRNA
levels. RT-qPCR analyses indicated that curcumin treatment
inhibited IL-1β, IL-6 and iNOS mRNA expression levels as compared
to the levels found in cells treated only with
H2O2 (P<0.05; Fig. 2A-C). In addition, western blot
analysis also indicated that curcumin treatment inhibited the
H2O2-induced increases in the protein levels
of inflammatory indicators, including IL-1β, IL-6 and iNOS
(P<0.05; Fig. 2D and E). Furthermore, the production of IL-1β
and IL-6 in the culture supernatant was detected by an ELISA kit
and the results suggested that curcumin treatment markedly
inhibited the H2O2-induced secretion of IL-1β
and IL-6 (P<0.05; Fig. 2F). In
conclusion, curcumin inhibited H2O2-induced
chondrocyte inflammation at the RNA and protein levels.
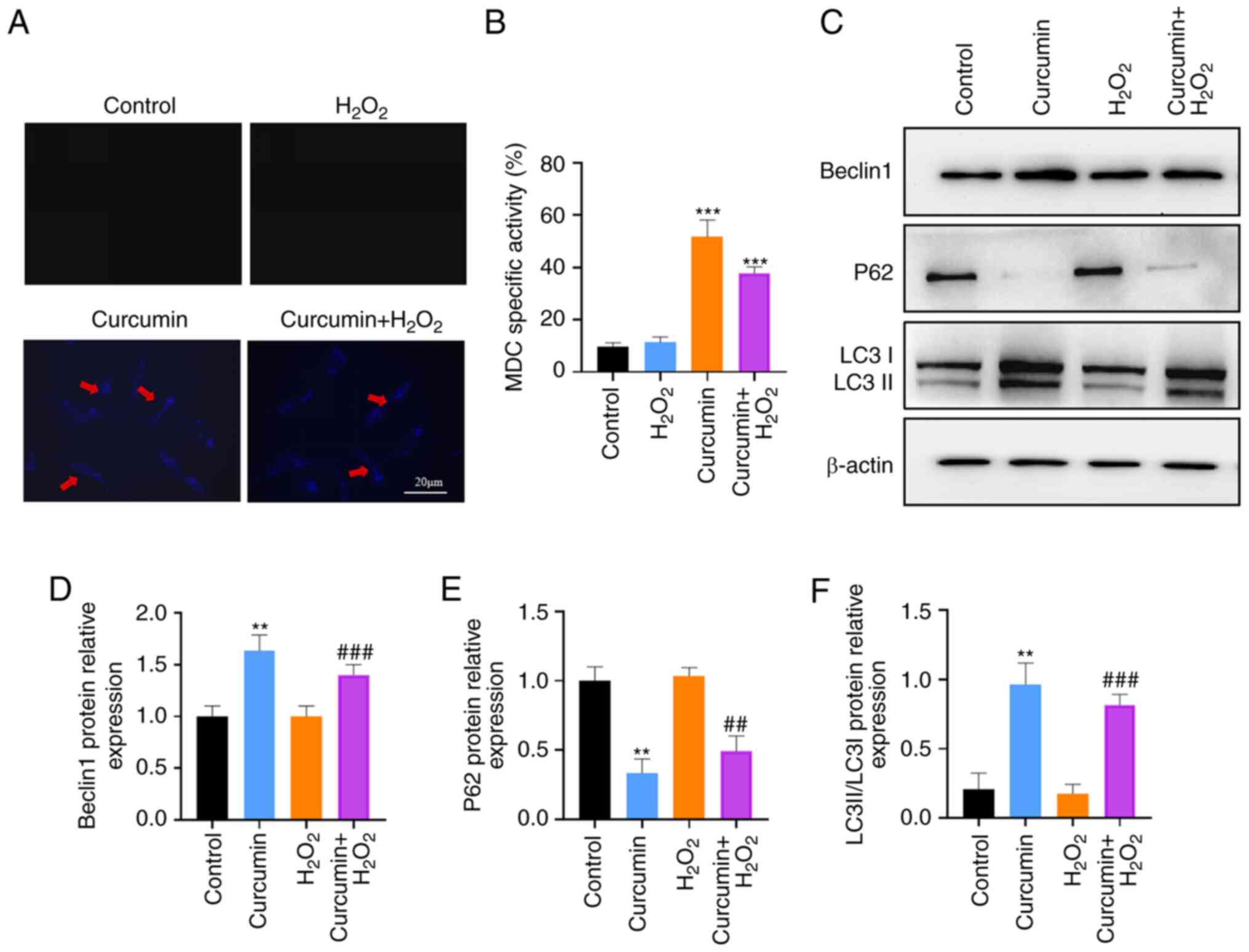
Curcumin treatment promotes autophagy
of chondrocytes
MDC staining and western blot analysis were used to
examine whether curcumin is able to induce autophagy in
chondrocytes. In early endosome compartments, no MDC accumulates
may be observed, but certain accumulates are present in mature
autophagic vacuoles (AVs), such as autophagolysosomes (20). AVs stained by MDC appear as
distinct dot-like structures distributed within the cytoplasm or
localized in the perinuclear regions and were detected under a
fluorescence microscope by scanning the cells. As presented in
Fig. 3A, there was an increase in
the number of MDC-labeled positive vesicles at 24 h after curcumin
treatment. The effects of curcumin were inhibited by
H2O2. This observation was further confirmed
by examining the expression levels of the autophagy-related markers
LC3-I/II using western blot analysis. The expression of LC3II was
significantly increased, while the expression of P62 was
significantly reduced after curcumin treatment compared to the
group without treatment, but there was no significant effect in
inducing LC3II expression in the H2O2 group
(P<0.01; Fig. 3C-F). Therefore,
curcumin promoted autophagy of chondrocytes.
Autophagy inhibition abrogates the
anti-inflammatory and protective effects of curcumin in
inflammatory chondrocytes
To study the role of autophagy in the
anti-inflammatory and chondroprotective effects of curcumin,
chondrocytes were treated with the inhibitor of autophagy 3-MA.
Under stimulation with H2O2, inhibition of
autophagy abolished the curcumin-mediated downregulation of the
mRNA and protein levels of IL-1β, IL-6 and iNOS (P<0.05;
Fig. 4). These findings indicated
that autophagy is a pivotal factor in the curcumin-mediated
suppression of inflammation in chondrocytes.
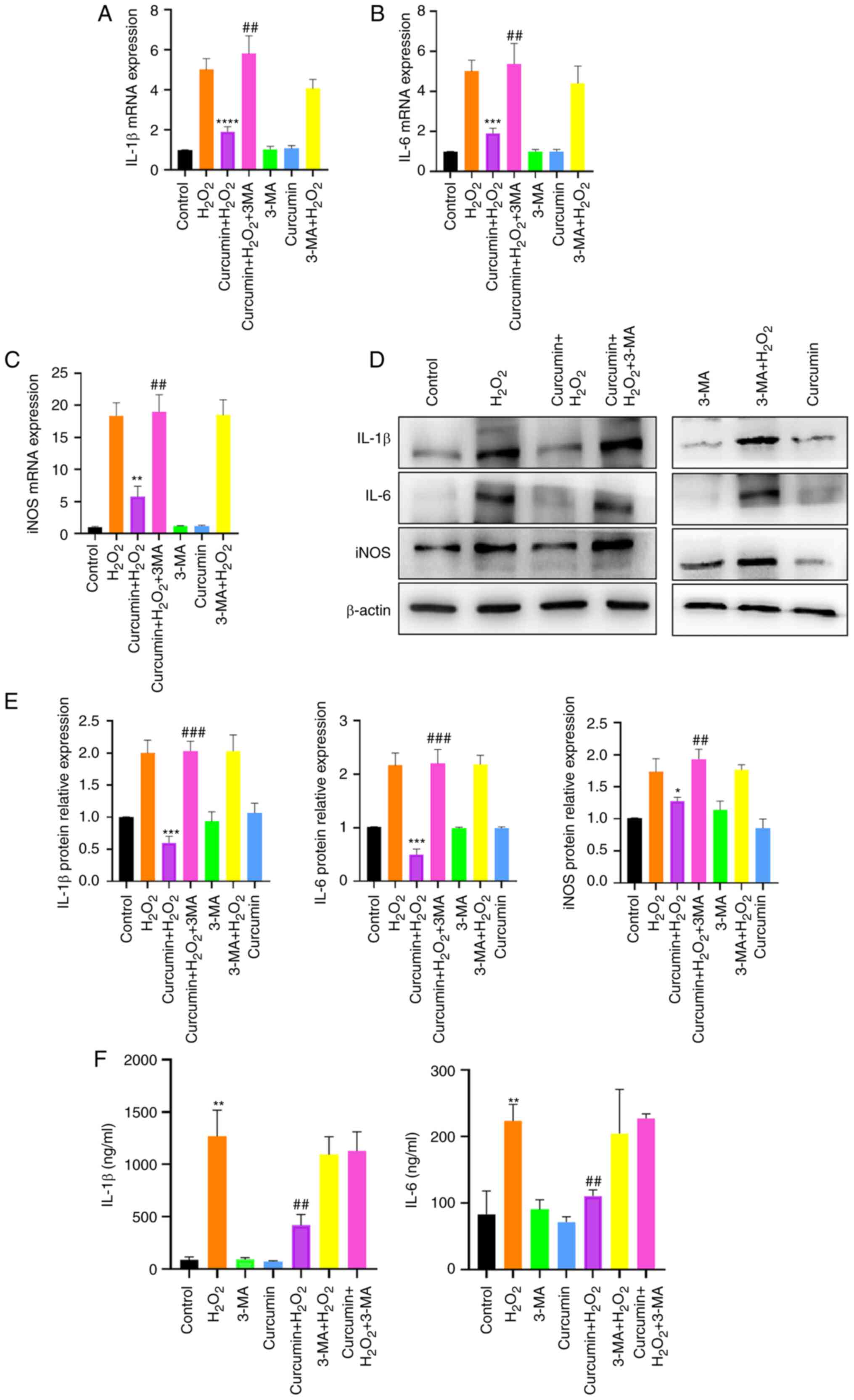 | Figure 4Autophagy inhibition abrogates the
anti-inflammatory and chondroprotective effects of curcumin in
inflammatory chondrocytes. Chondrocytes were pretreated with or
without curcumin (20 µM) for 2 h, followed by treatment with or
without H2O2 (20 µM) for 24 h. Chondrocytes
were then treated with 3-MA (50 nM), curcumin (20 µM) or
3-MA+curcumin. (A) IL-1β, (B) IL-6 and (C) iNOS mRNA expression was
measured by reverse transcription-quantitative PCR. GAPDH was used
as an internal control. (D and E) IL-1β, IL-6 and iNOS protein
expression was determined using western blot analysis. (D)
Representative western blot and (E) quantified protein levels. (F)
The production of IL-1β and IL-6 was detected by ELISA. Values are
expressed as the mean ± standard deviation (n=3). One-way ANOVA
followed by Tukey's post-hoc test was used for statistical
analysis. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs.
H2O2 treatment group; ##P<0.01,
###P<0.001 vs. curcumin + H2O2
treatment group. iNOS, inducible nitric oxide synthase; 3-MA,
3-methyladenine. |
Discussion
The present study reported the following: i)
Treatment of chondrocytes with 20 µM H2O2
results in viability inhibition and inflammation; ii)
H2O2-induced chondrocyte inflammation was
decreased by pretreatment with curcumin in a time-dependent manner;
iii) curcumin's anti-inflammatory effects were mediated by the
induction of chondrocyte autophagy; and iv) autophagy inhibitor
3-MA abolished the curcumin-mediated downregulation of inflammation
factors.
The present results implied that autophagy is
necessary to suppress chondrocyte inflammation. Curcumin is known
for its underlying anti-inflammatory and antioxidant activity.
Curcumin suppresses IL-1β secretion and prevents inflammation
through inhibition of the NLR family pyrin domain containing 3
inflammasome (21). More
importantly, curcumin is able to inhibit oxidative stress by
regulating the Nrf2/heme oxygenase-1 signaling pathway to prevent
aflatoxin B1-induced hepatotoxicity (4). In Saos-2 cells, curcumin ameliorated
apoptosis by inhibiting oxidative stress and it attenuated palmitic
acid-induced cell apoptosis by inhibiting endoplasmic reticulum
stress (22-24).
Further, related mechanistic studies suggested that curcumin is
able to modulate autophagy (25).
These data are consistent with the present results.
Various previous studies have reported that
autophagy is constitutively active in chondrocytes (26,27).
Appropriate autophagy has a housekeeping role in preventing
diseases such as cancer, cardiomyopathy, diabetes, liver diseases
and autoimmune diseases, as well as neurodegeneration and
infections (28-34).
It is a well-conserved mechanism and has been confirmed to be
important in various physical events. Beclin-1 and LC3 are major
regulators and markers of the autophagy pathway among the human
autophagy genes (35). The
nucleation of autophagic vesicles relies on beclin-1, which may
consequently lead to the formation of a complex with type III
phosphatidylinositol. LC3-I is converted to LC3-II, which is then
attached to the membrane of the autophagosome during autophagy
activation. The BH3 domain of beclin-1 interacts with Bcl-2 and
lead to inhibition of beclin-1-induced autophagy activation.
Sequestosome 1 (SQSTM1/p62) is an important autophagy receptor
protein. It is able to bind and deliver polyubiquitinated proteins
to the autophagy pathway for degradation. It is important that
SQSTM1/p62 is able to induce NF-κB signaling pathway activation by
recruiting TNF receptor-associated factors (TRAFs) to TRAF binding
sites in CD40(36), but its effect
may be inhibited after the silencing of p62(37). Of note, p62 and certain other
proteins that activate the NF-κB signaling pathway may also be
degraded in the selective autophagy pathway (38). There is a complex regulatory
relationship between p62-mediated autophagy and NF-κB signaling
pathway activation (39). A study
reported that p62 is able to improve the expression of antioxidant
genes by binding to kelch-like ECH-associated protein 1, leading to
activation of the Nrf2 signaling pathway (40). In short, p62-mediated induction of
Nrf2 reduces inflammatory responses by inhibiting the activation of
the NF-κB signaling pathway during autophagy (41). In the present study, it was
observed that curcumin raised the LC3-II/LC3-I ratio and decreased
p62 expression in chondrocytes. This effect was reconciled with
accelerated autophagy and mitigated inflammatory responses.
RP is a clinical disease characterized by a pattern
of repeated remission and recurrence of systemic inflammation, in
some cases followed by destruction, affecting the cartilage of the
ears, nose, larynx, joints and tracheobronchial tree (42,43).
Previous studies have revealed that inflammation, oxidative stress
and matrix degradation are important factors associated with RP
(2). It has been indicated that
cell inflammation may be effectively inhibited by moderate
autophagy activity (44). In the
present study, an increase in autophagic activity induced by
curcumin treatment was observed. In chondrocytes, autophagy is
likely to be a self-protective process induced by curcumin in
response to H2O2 stimulation. To confirm
this, it was demonstrated that when chondrocytes were pretreated
with curcumin and then cotreated with H2O2
and 3-MA, chondrocyte inflammation clearly increased and autophagy
was decreased.
In conclusion, the present study suggested that
autophagy is vital for chondrocyte inflammation, whereas the
self-activation of autophagy is a protective mechanism against
inflammation under curcumin treatment. This suggested that the
anti-inflammatory effects of curcumin are mediated, at least in
part, by the autophagy signaling pathway. The present study
provided a theoretical basis for the treatment of RP in the
clinic.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 82103181) and the Scientific
Research Foundation of Hebei Administration of Traditional Chinese
Medicine (grant no. 2022383).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZL was responsible for the conception and design of
the study. HLQ and ZYC were involved in data acquisition. YHQ was
involved in the development of the study methodology, analysis and
interpretation of the data. HLQ, ZYC, YHQ and YZL were involved in
the writing, reviewing and revision of the article, and analyzed
the relevant literature. HLQ and YZL confirmed the authenticity of
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Laboratory
Animal Care and Use Committee of Hebei Medical University (approval
ID: HebMU 20200026; Shijiazhuang, China) and were conducted in
accordance with National Institutes of Health guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Norooznezhad F, Rodriguez-Merchan EC,
Asadi S and Norooznezhad AH: Curcumin: Hopeful treatment of
hemophilic arthropathy via inhibition of inflammation and
angiogenesis. Expert Rev Hematol. 13:5–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Montmollin N, Dusser D, Lorut C, Dion
J, Costedoat-Chalumeau N, Mouthon L, Chassagnon G, Revel MP and
Puéchal X: Tracheobronchial involvement of relapsing
polychondritis. Autoimmun Rev. 8(102353)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
D'Cruz DP and Ferrada MA: Relapsing
polychondritis and large-vessel vasculitis. J Rheumatol.
47:1732–1733. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yin H, Guo Q, Li X, Tang T, Li C, Wang H,
Sun Y, Feng Q, Ma C, Gao C, et al: Curcumin suppresses IL-1β
secretion and prevents inflammation through inhibition of the NLRP3
inflammasome. J Immunol. 200:2835–2846. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Burge K, Gunasekaran A, Eckert J and
Chaaban H: Curcumin and intestinal inflammatory diseases: Molecular
mechanisms of protection. Int J Mol Sci. 20(1912)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Deguchi A: Curcumin targets in
inflammation and cancer. Endocr Metab Immune Disord Drug Targets.
15:88–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yavarpour-Bali H, Ghasemi-Kasman M and
Pirzadeh M: Curcumin-loaded nanoparticles: A novel therapeutic
strategy in treatment of central nervous system disorders. Int J
Nanomedicine. 14:4449–4460. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kunnumakkara AB, Bordoloi D, Padmavathi G,
Monisha J, Roy NK, Prasad S and Aggarwal BB: Curcumin, the golden
nutraceutical: Multitargeting for multiple chronic diseases. Br J
Pharmacol. 174:1325–1348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao Y, Chen J, Ren G, Zhang Y, Tan X and
Yang L: Punicalagin prevents inflammation in LPS-induced RAW264.7
macrophages by inhibiting FoxO3a/Autophagy signaling pathway.
Nutrients. 11(2794)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Matsuzawa-Ishimoto Y, Hwang S and Cadwell
K: Autophagy and inflammation. Annu Rev Immunol. 36:73–101.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong
WT, Leung CC, Choi G, Wang MH, Gin T, et al: Autophagy in sepsis:
Degradation into exhaustion? Autophagy. 12:1073–1082.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pozuelo-Rubio M: 14-3-3 proteins are
regulators of autophagy. Cells. 1:754–773. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Martinet W, Agostinis P, Vanhoecke B,
Dewaele M and De Meyer GR: Autophagy in disease: A double-edged
sword with therapeutic potential. Clin Sci (Lond). 116:697–712.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuma A, Komatsu M and Mizushima N:
Autophagy-monitoring and autophagy-deficient mice. Autophagy.
13:1619–1628. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Renga G, Oikonomou V, Stincardini C,
Pariano M, Borghi M, Costantini C, Bartoli A, Garaci E, Goldstein
AL and Romani L: Thymosin β4 limits inflammation through autophagy.
Expert Opin Biol Ther. 18 (Suppl 1):S171–S175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cui SN, Chen ZY, Yang XB, Chen L, Yang YY,
Pan SW, Wang YX, Xu JQ, Zhou T, Xiao HR, et al: Trichostatin A
modulates the macrophage phenotype by enhancing autophagy to reduce
inflammation during polymicrobial sepsis. Int Immunopharmacol.
77(105973)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang Y, Mo Y, Xin D, Zeng L, Yue Z and Xu
C: β-ecdysterone alleviates osteoarthritis by activating autophagy
in chondrocytes through regulating PI3K/AKT/mTOR signal pathway. Am
J Transl Res. 12:7174–7186. 2020.PubMed/NCBI
|
|
18
|
Huang L, Chen C, Zhang X, Li X, Chen Z,
Yang C, Liang X, Zhu G and Xu Z: Neuroprotective effect of curcumin
against cerebral ischemia-reperfusion via mediating autophagy and
inflammation. J Mol Neurosci. 64:129–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Munafó DB and Colombo MI: A novel assay to
study autophagy: Regulation of autophagosome vacuole size by amino
acid deprivation. J Cell Sci. 114:3619–3629. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Muhammad I, Wang X, Li S, Li R and Zhang
X: Curcumin confers hepatoprotection against
AFB1-induced toxicity via activating autophagy and
ameliorating inflammation involving Nrf2/HO-1 signaling pathway.
Mol Biol Rep. 45:1775–1785. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shakibaei M, Mobasheri A and Buhrmann C:
Curcumin synergizes with resveratrol to stimulate the MAPK
signaling pathway in human articular chondrocytes in vitro. Genes
Nutr. 6:171–179. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mobasheri A, Henrotin Y, Biesalski HK and
Shakibaei M: Scientific evidence and rationale for the development
of curcumin and resveratrol as nutraceutricals for joint health.
Int J Mol Sci. 13:4202–4232. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: Inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11(R165)2009.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Chen T, Zhou R, Chen Y, Fu W, Wei X, Ma G,
Hu W and Lu C: Curcumin ameliorates IL-1β-induced apoptosis by
activating autophagy and inhibiting the NF-κB signaling pathway in
rat primary articular chondrocytes. Cell Biol Int. 45:976–988.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Caramés B, Taniguchi N, Otsuki S, Blanco
FJ and Lotz M: Autophagy is a protective mechanism in normal
cartilage, and its aging-related loss is linked with cell death and
osteoarthritis. Arthritis Rheum. 62:791–801. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Settembre C, Arteaga-Solis E, McKee MD, de
Pablo R, Al Awqati Q, Ballabio A and Karsenty G: Proteoglycan
desulfation determines the efficiency of chondrocyte autophagy and
the extent of FGF signaling during endochondral ossification. Genes
Dev. 22:2645–2650. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Patergnani S, Missiroli S, Morciano G,
Perrone M, Mantovani CM, Anania G, Fiorica F, Pinton P and Giorgi
C: Understanding the role of autophagy in cancer formation and
progression is a real opportunity to treat and cure human cancers.
Cancers (Basel). 13(5622)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ikeda S, Zablocki D and Sadoshima J: The
role of autophagy in death of cardiomyocytes. J Mol Cell Cardiol.
165:1–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Farber E, Hanut A, Tadmor H, Ruth A,
Nakhoul F and Nakhoul N: Autophagy and diabetic nephropathy.
Harefuah. 160:740–745. 2021.PubMed/NCBI(In Hebrew).
|
|
31
|
Zhou JC, Wang JL, Ren HZ and Shi XL:
Autophagy plays a double-edged sword role in liver diseases. J
Physiol Biochem. Oct 18, 2021. (Epub ahead of print). doi:
10.1007/s13105-021-00844-7.
|
|
32
|
Wu MY, Wang EJ, Feng D, Li M, Ye RD and Lu
JH: Pharmacological insights into autophagy modulation in
autoimmune diseases. Acta Pharm Sin B. 11:3364–3378.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sriram N and Sah MK: Regulatory insights
into progression of cancer and Alzheimer's disorder from autophagy
perspective. Mol Biol Rep. 48:8227–8232. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Leonardi L, Sibéril S, Alifano M, Cremer I
and Joubert PE: Autophagy modulation by viral infections influences
tumor development. Front Oncol. 11(743780)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ravanan P, Srikumar IF and Talwar P:
Autophagy: The spotlight for cellular stress responses. Life Sci.
188:53–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chang CP, Su YC, Hu CW and Lei HY:
TLR2-dependent selective autophagy regulates NF-κB lysosomal
degradation in hepatoma-derived M2 macrophage differentiation. Cell
Death Differ. 20:515–523. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Qing G, Yan P, Qu Z, Liu H and Xiao G:
Hsp90 regulates processing of NF-kappa B2 p100 involving protection
of NF-kappa B2 p100 involving protection of NF-kappa B-inducing
kinase (NIK) from autophagy-mediated degradation. Cell Res.
17:520–530. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Moscat J and Diaz-Meco MT: p62 at the
crossroads of autophagy, apoptosis, and cancer. Cell. 137:1001–004.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Komatsu M, Kurokawa H, Waguri S, Taguchi
K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et
al: The selective autophagy substrate p62 activates the stress
responsive transcription factor Nrf2 through inactivation of Keap1.
Nat Cell Biol. 12:213–223. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Song C, Mitter SK, Qi X, Beli E, Rao HV,
Ding J, Ip CS, Gu H, Akin D, Dunn WA Jr, et al: Oxidative
stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and
promotes retinal pigmented epithelial cell survival through
increased autophagy. PLoS One. 12(e0171940)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Puéchal X, Terrier B, Mouthon L,
Costedoat-Chalumeau N, Guillevin L and Le Jeunne C: Relapsing
polychondritis. Joint Bone Spine. 81:118–124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vitale A, Sota J, Rigante D, Lopalco G,
Molinaro F, Messina M, Iannone F and Cantarini L: Relapsing
polychondritis: An update on pathogenesis, clinical features,
diagnostic tools, and therapeutic perspectives. Curr Rheumatol Rep.
18(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Qin Y, Zheng B, Yang GS, Yang HJ, Zhou J,
Yang Z, Zhang XH, Zhao HY, Shi JH and Wen JK: Salvia
miltiorrhiza-derived Sal-miR-58 induces autophagy and attenuates
inflammation in vascular smooth muscle cells. Mol Ther Nucleic
Acids. 21:492–511. 2020.PubMed/NCBI View Article : Google Scholar
|