Introduction
Atherosclerosis is a chronic inflammatory arterial
angiopathy that is a common cause of cardiovascular disease
(1). There is considerable
evidence demonstrating that diabetes significantly exacerbates the
progression of atherosclerosis (2). Blood glucose and lipid metabolism
disorders are among the commonest pathophysiological mechanisms of
diabetic atherosclerosis (3). It
has been found that macrophage dysfunction contributes to the
development of atherosclerosis. This dysfunction is characterized
by an increased inflammatory response and problems with phagocytes,
which ultimately increase the likelihood of plaque rupture
(4). A large number of
inflammatory factors, including IL-1β and IL-18, are released from
pyroptotic macrophages and contribute to the instability of
atherosclerotic plaques in diabetes (5). It is also reported that sinapic acid
attenuates diabetic atherosclerosis by suppressing macrophage
pyroptosis (6). Therefore,
preventing macrophage death may be an effective method for
preventing diabetic atherosclerosis. Pyroptosis is a form of
proinflammatory programmed cell death that requires NLR family
pyrin domain containing 3 (NLRP3) inflammasome activation and
mutation of gasdermin D (GSDMD) (7). Interaction of NLRP3 with
apoptosis-associated speck-like protein (ASC) containing a caspase
recruitment domain (CARD) causes recruitment of caspase-1. This
triggers the cleavage and release of IL-1β through pores formed by
N-GSDMD (8). Macrophage pyroptosis
serves an important role in the worsening of atherosclerotic
plaques (9). NLRP3 and caspase-1
gene deletions significantly slow the progression of
arteriosclerosis (10). Thus, it
is of great significance to identify drugs that are protective
against pyroptosis in order to prevent and treat diabetic
atherosclerosis.
Oxidative stress is considered a potential agonist
of inflammasome activation. Previous studies have shown that
excessive production of reactive oxygen species (ROS) can activate
NLRP3 inflammasome and lead to pyroptosis (7,11).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a common
transcription factor that exerts antioxidant, anti-apoptotic and
anti-inflammatory effects by interacting with multiple promoters
(12). When increased oxidative
stress is sensed, Nrf2 translocates into the nucleus and promotes
the transcription of antioxidant proteins (13). Heme oxygenase-1 (HO-1) and NADPH
quinone oxidoreductase-1 (NQO-1) are important antioxidant proteins
downstream of Nrf2, which inhibit oxidative stress-induced
pyroptosis (14). In a diabetic
atherosclerosis model, exogenous hydrogen sulfide stimulates the
Nrf2/HO-1 pathway, thus improving macrophage function and
inhibiting atherosclerosis progression (15). In addition, pharmacological
activation of Nrf2 suppresses endothelial pyroptosis by reducing
the overproduction of ROS (16).
It is reported that Nrf2 may be an ideal therapeutic target for
prevention of diabetic atherosclerosis (17).
Spermine is a small polyamine that is a natural
product of cellular metabolism (18). It is reported that spermine is
involved in regulating cell proliferation, apoptosis and
inflammation (19). Notably,
metabolomic analysis shows that problems with spermine metabolism
can lead to a decrease in spermine levels, which is often observed
in atherosclerotic plaques (20).
Furthermore, spermine can directly activate the Nrf2 pathway to
attenuate cardiomyopathy in diabetic rats (21). However, it is unclear whether
spermine activation of the Nrf2 pathway can inhibit macrophage
pyroptosis.
The present study investigated whether spermine
could attenuate macrophage pyroptosis under high glucose (HG) and
oxidized low-density lipoprotein (ox-LDL) conditions. Furthermore,
it explored the underlying mechanism by which spermine prevented
macrophage pyroptosis and investigated its ability to suppress ROS
production via Nrf2 activation.
Materials and methods
Reagents
Human monocytic THP-1 cells were obtained from
American Type Culture Collection. Spermine was purchased from
MilliporeSigma. Ox-LDL was purchased from Guangzhou Yiyuan
Biological Technology Co. Ltd. Phorbol 12-myristate 13-acetate
(PMA), DCFDA Assay kit, lactate dehydrogenase (LDH) Assay kit,
Lipid Peroxidation MDA Assay kit, GSH and GSSG Assay kit and
Hoechst/PI Staining kit were obtained from Beyotime Institute of
Biotechnology. The CCK-8 kit was obtained from Dojindo
Laboratories, Inc. SYBR Green and reverse transcription kits were
obtained from Roche Diagnostics. Primary antibodies against NLRP3
(cat. no. ab263899), N-GSDMD (cat. no. ab210070), cleaved caspase-1
(cat. no. ab179515), Nrf2 (cat. no. ab137550), HO-1 (cat. no.
ab52947), NQO-1 (cat. no. ab28947) and IL-1β (cat. no. ab254360)
were purchased from Abcam. The secondary antibody was HRP-labeled
Goat Anti-Rabbit IgG (H+L) (cat. no. A0208), purchased from
Beyotime Institute of Biotechnology.
Cell culture and induction
THP-1 cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Cell culture
medium was replaced every 2 days and the cultures were maintained
at 37˚C, 5% CO2 in a fully humidified incubator. THP-1
cells were induced into macrophages using PMA pretreatment (50 nM
PMA for 48 h before the experiment at 37˚C). The cells were then
divided into the following groups: The control group, which was
treated with phosphate-balanced saline (PBS) or dimethyl sulfoxide;
the ox-LDL/HG group, which was treated with D-glucose (D-Glu, 25
mmol/l) and ox-LDL (100 µg/ml) (15,22);
the spermine group, which was treated with spermine (5 µmol/l),
D-Glu (25 mmol/l) and ox-LDL (100 ng/ml); and the ML385 group,
which was treated with NRF2 inhibitor ML385 (5 µmol/l), spermine (5
µmol/l), D-Glu (25 mmol/l) and ox-LDL (100 ng/ml).
Enzyme-linked immunosorbent assay
(ELISA)
The cell supernatant of the macrophages was
collected and the levels of IL-1β were determined using their
corresponding ELISA kit (cat. no. EK0392; Wuhan Boster Biological
Technology, Ltd.) according to the manufacturer's protocols.
Scanning electron microscopy
(SEM)
Macrophages were planted in glass sliders, and the
cells were divided into experimental groups. Cells were immobilized
at room temperature with glutaraldehyde (2.5%) for 48 h. The sample
was rinsed with acetone and immersed in iso-amyl acetate and then
exposed to air at room temperature for dehydration for ~3 h.
Finally, gold palladium film was added to the sample using a vacuum
plating method and observed under scanning electron microscope at
x3,000 magnification.
Cell viability assays
Macrophages were cultured in 96-well plates with
10,000 cells per well. The CCK-8 assays were performed according to
the manufacturer's instructions. After preparing the experimental
groups and performing the cell treatments, a 1:10 dilution of the
CCK-8 working solution was prepared. Then, 100 µl working solution
was removed and added to 96-well plates. The plates were placed in
an incubator at 37˚C for 60 min to allow the reaction to occur.
After the incubation, absorbance was measured at 450 nm using a
microplate spectrophotometer (Tecan Group, Ltd.).
LDH assay
Macrophages were cultured in 96-well plates with
10,000 cells per well. According to the LDH Assay kit
manufacturer's instructions, the cell culture plates were
centrifuged at 400 x g for 5 min using a multi-well plate
centrifuge following drug stimulation. The LDH-releasing reagent
provided in the kit was diluted 10-fold with PBS and mixed well.
The cell supernatant was aspirated and 150 µl of the diluted
LDH-releasing reagent was added to each cell sample. The plates
were shaken to assure that the LDH-releasing reagent in each well
was mixed well. The plates were then incubated at 37˚C for 1 h. The
cell culture plates were subsequently centrifuged for 5 min at 400
x g in a multi-well plate centrifuge. Thereafter, 120 µl
supernatant was collected from each well and separately added to
the corresponding wells of a new 96-well plate. subsequently, the
absorbance was measured at 490 nm using a microplate
spectrophotometer.
Reverse transcription-quantitative
(RT-q) PCR
A total of 1x106 cells/ml were seeded in
6-well plates for RNA extraction (23). Total RNA was isolated from the
macrophages using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. RNA concentration was determined using a
spectrophotometer (BioSpec-nano; Shimadzu Corporation) to measure
absorbance at 260 nm. Complementary DNA (cDNA) was synthesized
using the RNA as template using a Reverse Transcription kit (Roche
Diagnostics). qPCR was then performed using SYBR Green (Roche
Diagnostics). Each reaction was performed in triplicate. The
cycling profile was as follows: Initial denaturation at 95˚C for 5
min, followed by 40 cycles of denaturation at 94˚C for 10 sec,
annealing at temperatures specific for each pair of primers
(ranging from 53 to 62˚C) for 10 sec, extension at 72˚C for 28 sec
and a final elongation at 72˚C for 5 min. A melt-curve analysis was
performed to quantify the PCR products. The primer sequences for
the target genes used in this study was shown in Table SI. All data were normalized to
β-actin mRNA levels.
Hoechst/propidium iodide (PI)
staining
Cells were seeded into 24-well plates with 50,000
cells per well. After the cells were treated according to the
experimental design, the cells were washed three times with PBS.
Then, 5 µl Hoechst staining solution was added, followed by 5 µl PI
stain. The cells were mixed and incubated in an ice bath at 4˚C for
20-30 min. The cells were carefully washed three times with PBS and
an anti-fluorescence quenching agent (Beyotime Institute of
Biotechnology) was added. Cells were observed under a fluorescence
microscope at x200 magnification and images captured. To calculate
the percentage of PI-positive cells, the number of PI-positive
cells was divided by the number of Hoechst-positive cells. Three
random fields were chosen for this calculation.
Western blot analysis
Following treatment, the cells were washed once with
pre-cooled PBS. Radioimmunoprecipitation assay buffer containing
phenylmethylsulfonyl fluoride was added to fully lyse the cells.
The lysate was collected and placed on ice for 30 min. The lysate
was then centrifuged at 14,000 x g at 4˚C for 15 min to collect the
supernatant. The concentration of the protein in the macrophage
lysates was detected using a BCA kit (Beijing Solarbio Science
& Technology Co., Ltd.). Aliquots of each sample containing 30
µg of protein were prepared and loading buffer was added to these.
The proteins were then separated using 10% SDS-polyacrylamide gel
electrophoresis. The separated proteins were transferred onto
0.22-µm polyvinylidene fluoride membranes (MilliporeSigma). The
PVDF membranes were blocked with 5% skimmed milk at room
temperature for 1 h to prevent non-specific antibody binding and
then washed three times with PBS. The PVDF membranes were then
incubated with the indicated primary antibody for 12 h at 4˚C. The
membranes were then washed three times with PBS. The appropriate
secondary antibody (HRP-labeled Goat Anti-Rabbit IgG; 1:10,000) was
added to the membrane, followed by a 1-h incubation at room
temperature. The membranes were washed three times at room
temperature for 1 h each. The protein bands were visualized using
an enhanced chemiluminescence reagent (Beijing Solarbio Science
& Technology Co., Ltd.). ImageJ software v. 1.4 (National
Institutes of Health) was used to analyze the gray level intensity
of the blot strips.
Total ROS measurements
Macrophages were cultured in 96-well plates with
10,000 cells per well. Macrophages were treated according to the
experimental design. 2',7'-Dichlorodihydrofluorescein diacetate
(DCFH-DA) was diluted 1:1,000 with serum-free medium to obtain a
final concentration of 10 mM. The cell culture medium was removed,
100 µl diluted DCFH-DA was added and the cells were incubated for
20 min at 37˚C. The cells were then washed three times with
serum-free cell culture medium to fully remove any DCFH-DA that had
not entered the cells. The cells were then subjected to
fluorescence spectrophotometric analysis, as well as fluorescence
microscopy at x200 magnification using an excitation wavelength of
488 nm and emission wavelength of 525 nm.
Immunofluorescent staining
Macrophages were seeded into 24-well plates, treated
with 4% paraformaldehyde for 10 min at room temperature, washed
three times with PBS and then treated with 0.3% Triton for 5 min to
disrupt the cell membrane at room temperature. The cells were then
washed three times with PBS and then 5% bovine serum albumin was
added followed by incubation for 60 min to reduce non-specific
staining at room temperature. After washing the samples three times
with PBS, anti-Nrf2 primary antibody (1:100) was added and the
mixture was left to incubate overnight at 4˚C. The secondary
antibody (1:400) was added and the samples were incubated at room
temperature for 1 h at room temperature. To stain the nuclei, the
cells were incubated with 4',6-diamidino-2-phenylindole (DAPI) for
5 min at room temperature. The cells were washed three times with
PBS and then observed using a fluorescence microscope at x400
magnification.
Statistical analysis
The data were collected from three independent
experiments and all results are presented as the mean ± SD.
Differences between two or more groups were estimated using
unpaired Student's t-test or one-way ANOVA followed by Tukey's post
hoc test, respectively. The statistical significance of the data
was determined using analysis of variance with the SPSS v22.0
software (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
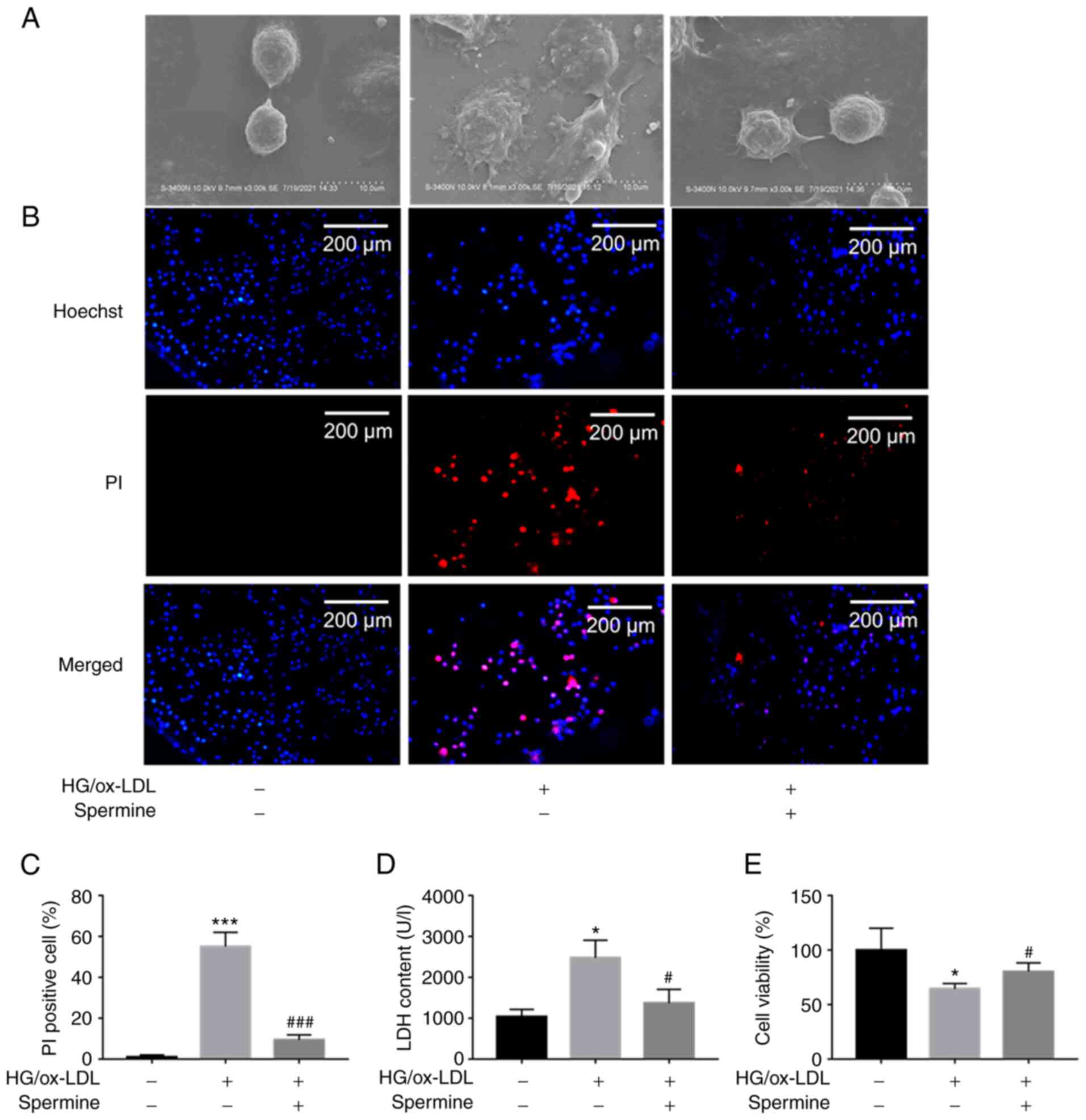
Spermine prevented macrophage
pyroptosis induced by HG/ox-LDL
The results of CCK-8 assay showed that <5 µM
spermine had no significant cytotoxicity on THP-1 macrophages
(Fig. S1A). Cell swelling and
cell membrane rupture are common characteristics of pyroptosis. To
determine the effect of spermine on HG/ox-LDL-induced pyroptosis in
macrophages, THP-1 derived macrophages were treated with D-glucose
(25 mmol/l) and ox-LDL (100 ng/ml) for 24 h in the absence or
presence of spermine (5 µmol/l) at 37˚C. SEM was used to detect
changes in macrophage morphology, PI staining and LDH assays were
used to evaluate cell membrane integrity and CCK-8 assays were
performed to determine cell viability. Macrophages swelled and
enlarged in response to HG/ox-LDL stimulation and spermine
pretreatment reversed these effects (Fig. 1A). PI staining revealed that
spermine decreased the percentage of PI-positive macrophages in the
HG/ox-LDL group (Fig. 1B and
C). In addition, it was found that
HG/ox-LDL treatment led to increased LDH levels in the supernatant.
However, spermine administration markedly decreased the LDH levels
in HG/ox-LDL-treated macrophages (Fig.
1D), indicating a decrease in the rates of cell swelling and
membrane rupture. The CCK-8 assays showed that spermine
pretreatment significantly reversed the decrease in cell viability
caused by HG/ox-LDL conditions (Fig.
1E). These data suggested that spermine inhibited
HG/ox-LDL-induced macrophage pyroptosis.
Spermine suppressed macrophage
pyroptosis by inhibiting NLRP3 inflammasome activation
Activation of the NLRP3 inflammasome leads to IL-1β
expression and the mutation of GSDMD (N-GSDMD), which is cleaved by
cleaved caspase-1. It was hypothesized that spermine pretreatment
would suppress NLRP3 inflammasome activation. To this end, PCR and
western blot analyses were performed to evaluate NLRP3, caspase-1,
cleaved caspase-1, N-GSDMD and IL-1β expression levels. As
expected, the levels of NLRP3 and IL-1β mRNAs were downregulated in
the macrophages of the spermine group compared with that of the
macrophages in the HG/ox-LDL group, which did not undergo spermine
pretreatment (Fig. 2A and B). There are no significantly differences
of caspase-1 and GSDMD mRNA level in these three groups (Fig. S2A and B). Furthermore, it was found that the
increase in NLRP3, cleaved caspase-1, IL-1β and GSDMD expression in
the HG/ox-LDL group was suppressed by spermine pretreatment
(Fig. 2D and E). However, spermine treatment alone did
not affect the expression level of cleaved caspase-1 and GSDMD in
macrophages (Fig. S1B and
C). IL-1β levels in the cell
supernatant were also evaluated using ELISA and the results were
consistent with those of the western blot analysis. IL-1β levels
were measured in the HG/ox-LDL group and compared with those of the
control group and it was found that spermine blocked the
upregulated expression of IL-1β (Fig.
2C).
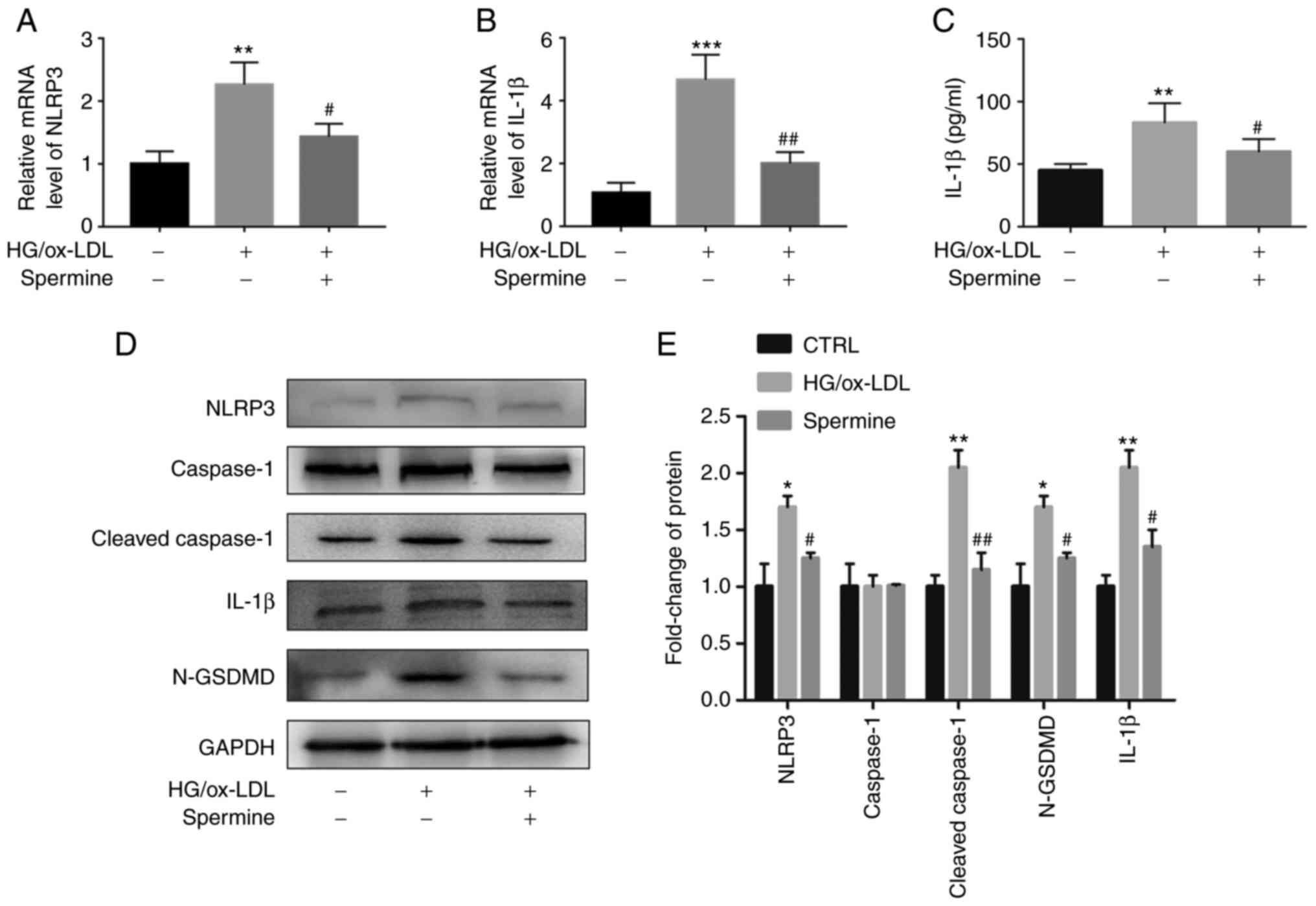 | Figure 2Spermine suppresses NLRP3
inflammasome activation under HG/ox-LDL condition. PCR was used to
detect NLRP3 and IL-1β mRNA levels in macrophages. ELISA was used
to detect IL-1β levels in cell supernatant. Western blotting was
used to detect the levels of NLRP3, caspase-1, cleaved-caspase-1,
IL-1β and N-GSDMD in lysate. GAPDH was the internal reference. (A)
NLRP3 mRNA level. (B) IL-1β mRNA level. (C) IL-1β level in
supernatant. (D) Protein level of NLRP3, caspase-1,
cleaved-caspase-1, IL-1β and N-GSDMD in control, HG/ox-LDL and
spermine group. (E) Quantification of NLRP3, caspase-1,
cleaved-caspase-1, IL-1β and N-GSDMD. *P<0.05,
**P<0.01, ***P<0.001 compared with the
control group; #P<0.05, ##P<0.01
compared with the HG/ox-LDL group. NLRP3, NLR family pyrin domain
containing 3; HG, high glucose; ox-LDL, oxidized low-density
lipoprotein; GSDMD, gasdermin D. |
Spermine enhanced Nrf2 nuclear
translocation and promoted transcription of antioxidant
proteins
It is well established that Nrf2 serves an important
role in pyroptosis. Therefore, it was explored whether spermine
could activate Nrf2 and its downstream antioxidant proteins. First,
nuclear lysates were extracted from the different experimental
groups and Nrf2 levels in the lysates were evaluated using western
blotting. The results showed that the level of Nrf2 in the nucleus
was significantly lower in the HG/ox-LDL group than in the control
group, but spermine pretreatment caused an increase in nuclear Nrf2
levels (Fig. 3A). Furthermore,
spermine treatment reduced NRF2 expression in cytoplasm, suggesting
that spermine promoted NRF2 translocation from the cytoplasm to the
nucleus (Fig. 3A). Second, levels
of HO-1 and NQO-1, which are common antioxidant proteins downstream
of Nrf2, were measured in the experimental groups of macrophages.
It was found that there were higher levels of HO-1 and NQO-1 in the
total lysates of the HG/ox-LDL treatment group than in the lysates
of the control group. Notably, this effect was significantly
amplified in the spermine group (Fig.
3B and C). Third,
immunofluorescence was used to visualize the cellular localization
of Nrf2. The results demonstrated that spermine increased
localization of Nrf2 to the nucleus (Fig. 3D and E). These results indicate that spermine
promotes Nrf2 activation by enhancing Nrf2 nuclear
translocation.
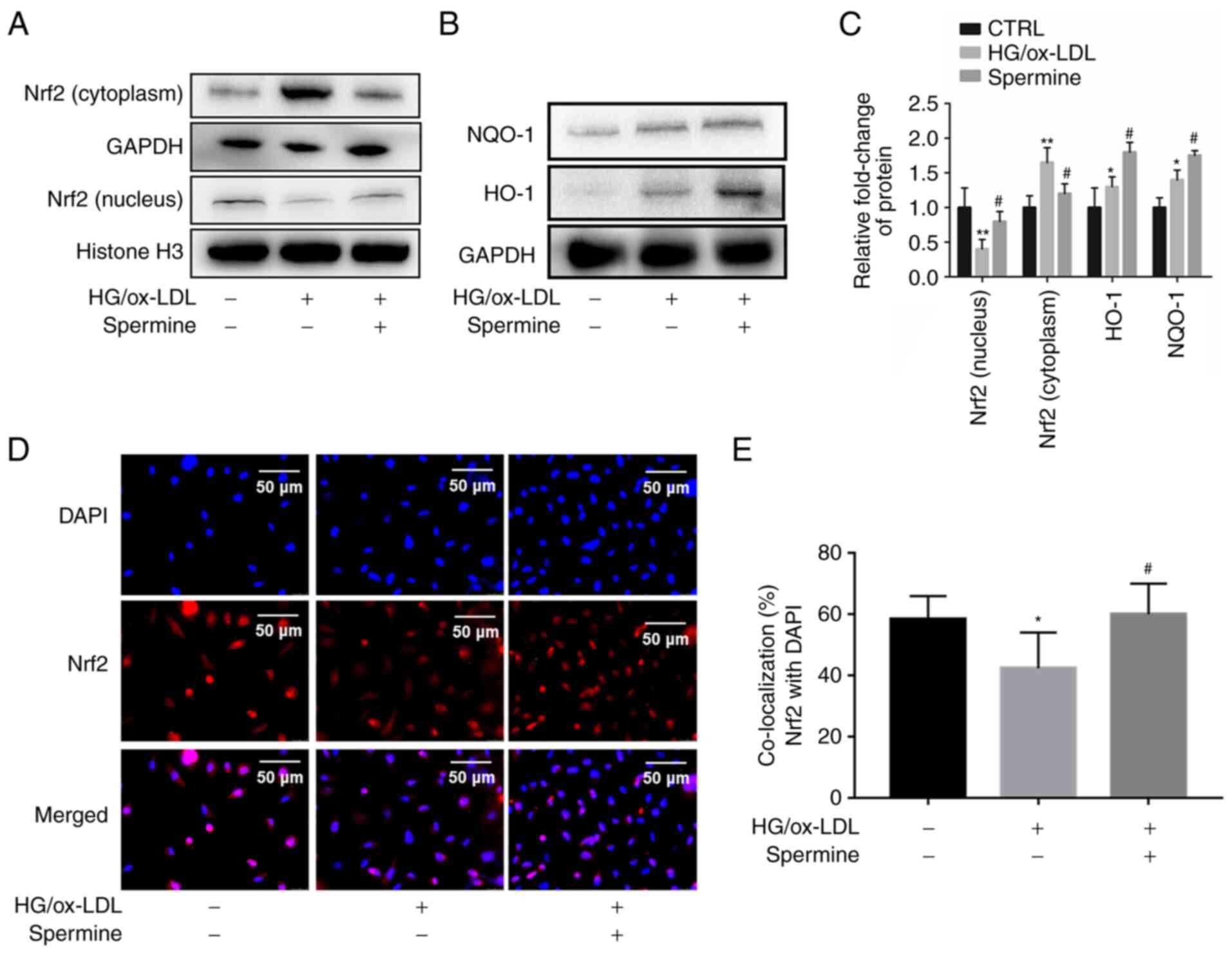 | Figure 3Spermine promotes Nrf2 to enter the
nucleus and promotes antioxidant protein expression. Western
blotting was used to detect the levels of Nrf2, HO-1 and NQO-1,
Histone H3 and GAPDH were used as internal references for nuclear
and cell total protein, respectively. Immunofluorescence was used
to detect Nrf2 nuclear translocation. (A) Nrf2 level in nuclear and
cytoplasm lysates. (B) HO-1 and NQO-1 level in total lysates. (C)
The levels of Nrf2, HO-1 and NQO-1 were quantified. (D and E)
Immunofluorescence was used to detect Nrf2 cell localization.
*P<0.05, **P<0.01 compared with the
control group; #P<0.05 compared with the HG/ox-LDL
group. Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase-1; NQO-1, NADPH quinone oxidoreductase-1; HG, high
glucose; ox-LDL, oxidized low-density lipoprotein. |
Spermine reduced oxidative stress in
HG/ox-LDL-treated macrophages via the Nrf2 pathway
Previous studies have provided robust evidence to
support the hypothesis that excessive ROS contribute to NLRP3
inflammasome activation and pyroptosis (11,24).
The present study aimed to confirm that spermine could reduce ROS
production and investigate whether this reduction occurs via the
Nrf2 pathway. The macrophages in the different experimental groups
were pretreated with ML385, a specific inhibitor of Nrf2. The
levels of ROS were detected in the different groups using DCFH-DA.
As shown in Fig. 4A-C, ROS
production and malondialdehyde (MDA) levels, a major marker of
lipid peroxidation, were decreased in the spermine pretreatment
group compared with those in the HG/ox-LDL group. In addition,
ML385 completely abolished the anti-ROS effect of spermine. ML385
increased oxidative levels after HG/ OX-LDL treatment, however,
ML385 alone had no significant effect. Furthermore, the expression
of Nrf2 downstream proteins were evaluated. Analysis of glutathione
(GSH) and superoxide dismutase (SOD) levels in the cell lysates
revealed that spermine pretreatment resulted in increased levels of
both GSH and SOD expression in macrophages subjected to HG/ox-LDL
treatment and this was reversed by ML385. ML385 combined with HG/
ox-LDL treatment further reduced SOD and GSH levels, but ML385
alone did not affect SOD and GSH levels (Fig. 4D and E). In addition, western blotting was used
to evaluate the protein expression levels of HO-1 and NQO-1. The
findings revealed that ML385 attenuated the spermine-induced
increases in HO-1 and NQO-1 expression in HG/ox-LDL-treated
macrophages. ML385 attenuated the upregulation of HO-1 and NQO-1
induced by ox-LDL, but treatment with ML385 alone did not affect
HO-1 and NQO-1 levels (Fig. 4F and
G).
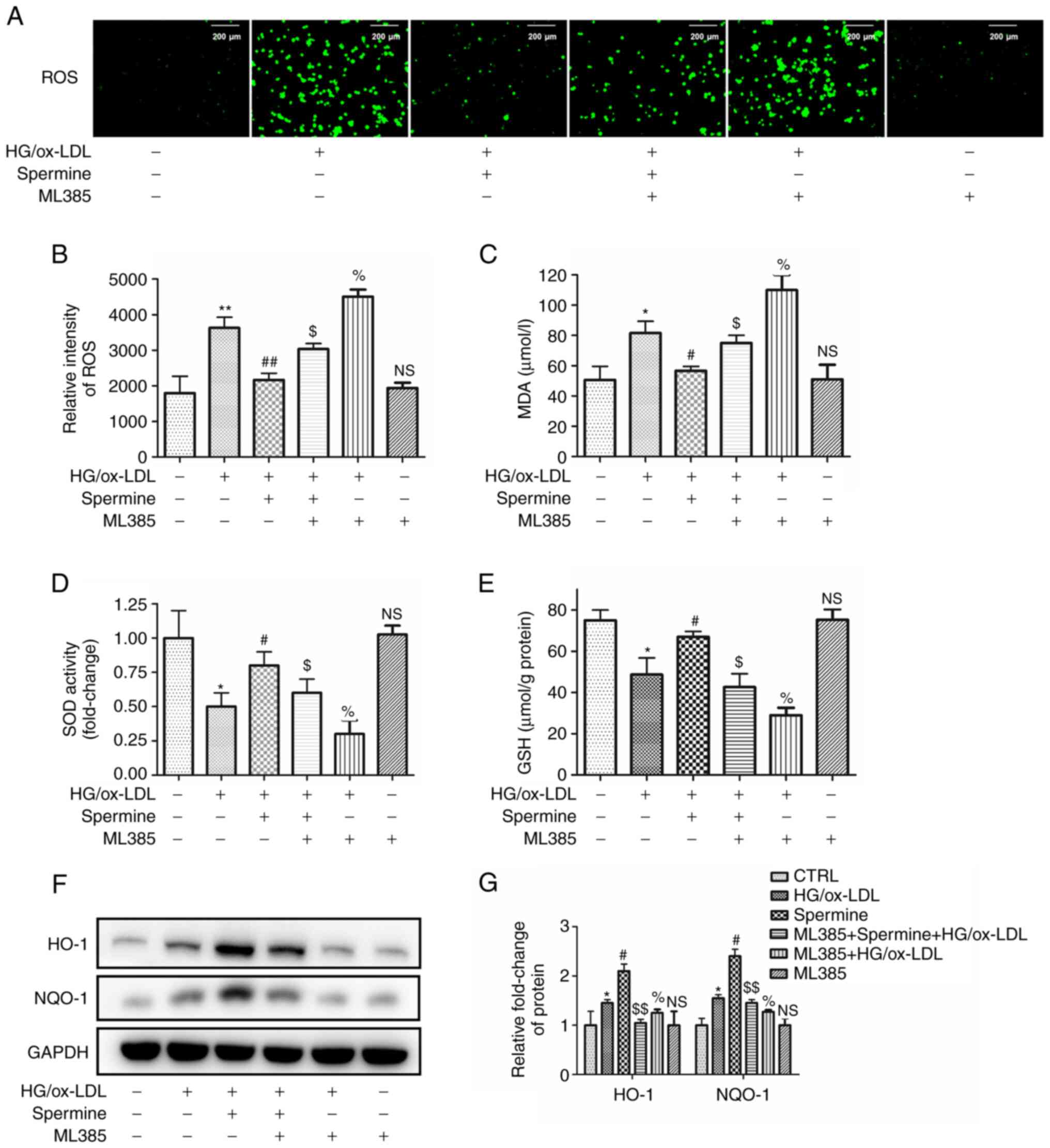 | Figure 4Spermine improves oxidative stress in
macrophages exposed to HG/ox-LDL independent of Nrf2. Macrophages
were divided into six groups, control group (PBS), HG/ox-LDL group
(25 mmol/l D-Glu and 100 µg/ml ox-LDL treatment for 24 h) and
Spermine group (5 µM spermine pretreatment for 30 min followed by
the addition of 25 mmol/l D-Glu and 100 µg/ml ox-LDL treatment for
24 h), Spermien+ML385+HG/ox-LDL group (5 µM spermine and ML385
pretreatment for 30 min followed by the addition of 25 mmol/l D-Glu
and 100 µg/ml ox-LDL treatment for 24 h), ML385 group (5 µM ML385)
and ML385+HG/ox-LDL group (5 µM ML385 and 5 mmol/l D-Glu and 100
µg/ml ox-LDL treatment for 24 h). DCFH-DA was used to evaluated ROS
level. GSH, SOD and MDA levels in cell lysates were detected. HO-1
and NQO-1 level in lysates was analysis by western blotting. (A and
B) Fluorescence microscopy showed the total ROS level. (C) MDA
level. (D) SOD activity. (E) GSH level. (F and G) Protein level of
HO-1 and NQO-1. *P<0.05, **P<0.01
compared with the control group; #P<0.05,
##P<0.01 compared with the HG/ox-LDL group.
$P<0.05, $$P<0.01 compared with the
spermine group. %P<0.05 compared with the HG/ox-LDL
group. HG, high glucose; ox-LDL, oxidized low-density lipoprotein;
Nrf2, nuclear factor erythroid 2-related factor 2; D-Glu,
D-glucose; DCFH-DA. 2',7'-Dichlorodihydrofluorescein diacetate;
ROS, reactive oxygen species; GSH, glutathione; SOD, superoxide
dismutase; MDA, malondialdehyde; NQO-1, NADPH quinone
oxidoreductase-1; NS, no statistical significance. |
The anti-pyroptosis effect of spermine
on macrophages required Nrf2 activation
The experimental groups of cells were pretreated
with ML385 to further explore whether spermine inhibited macrophage
pyroptosis by stimulating the Nrf2 pathway and to investigate the
mechanism underlying this process. Results from the CCK-8 assays
demonstrated that the increase in cell viability caused by spermine
pretreatment was completely abolished by ML385 treatment (Fig. 5A). The LDH assays showed a
significant increase in LDH levels following ML385 and HG/ox-LDL
treatment without spermine (Fig.
5B). Spermine reduced the HG/ox-LDL-induced increase in the
percentage of PI-positive cells, which was also reversed by ML385
treatment (Fig. 5C and D). These findings suggest that ML385
abolished the anti-pyroptosis effect of spermine. In addition,
inhibition of NLRP3 inflammasome activation by spermine was
alleviated in the ML385 group compared with the groups with
HG/ox-LDL treatment alone (Fig. 5E
and F). Collectively, these
results indicate that the Nrf2 signaling pathway contributes to the
protective effect of spermine against HG/ox-LDL injury in
macrophages.
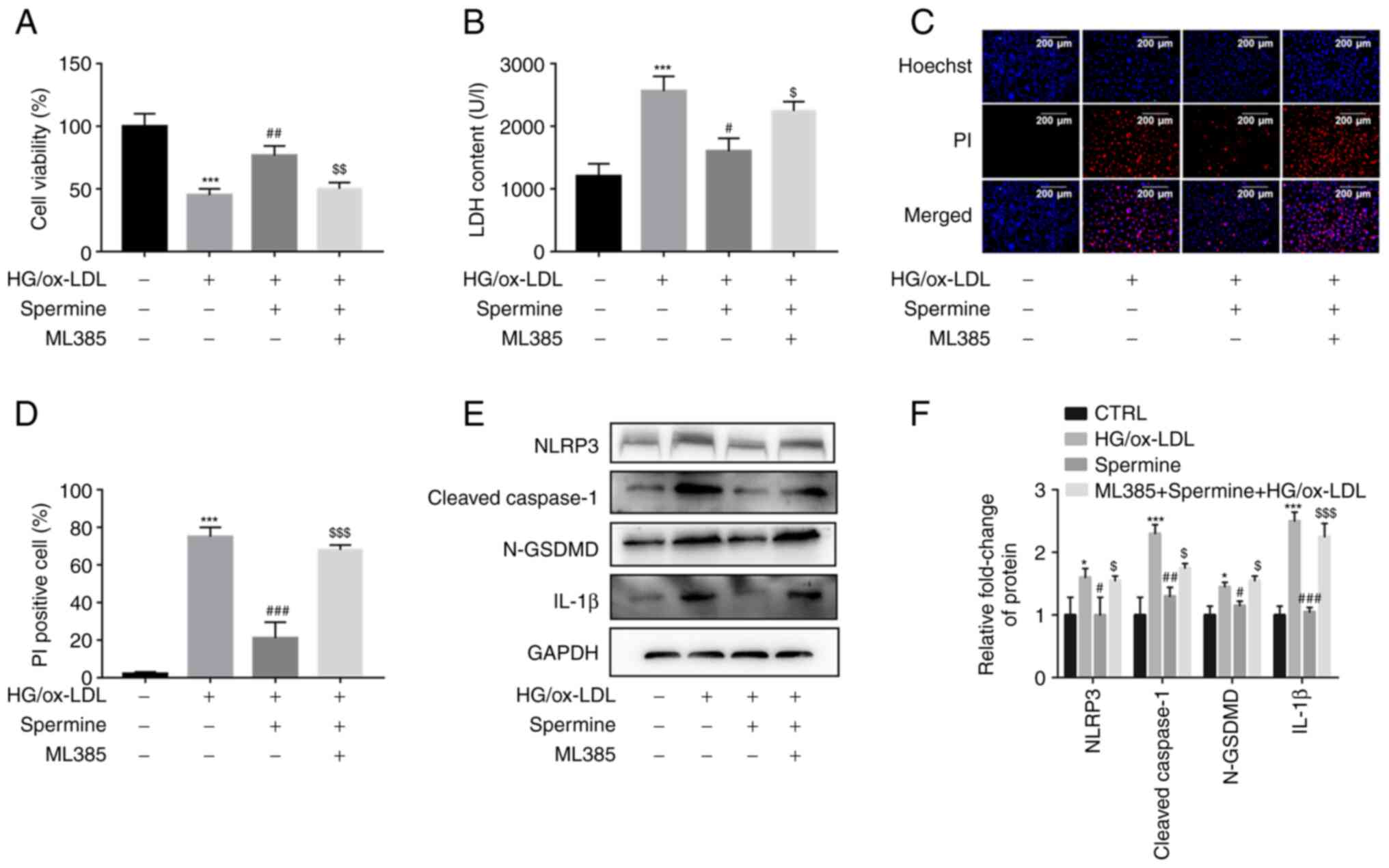 | Figure 5Spermine inhibits macrophage
pyroptosis by activating the Nrf2 pathway. Macrophages were divided
into three groups, control group (PBS), HG/ox-LDL group (25 mmol/l
D-Glu and 100 µg/ml ox-LDL treatment for 24 h) and Spermine group
(5 µM spermine pretreatment for 30 min followed by the addition of
25 mmol/l D-Glu and 100 µg/ml ox-LDL treatment for 24 h), ML385
group (5 µM spermine and ML385 pretreatment for 30 min followed by
the addition of 25 mmol/l D-Glu and 100 µg/ml ox-LDL treatment for
24 h). CCK-8 assay was used to detect cell viability and LDH kit
was used to detect LDH level in cell supernatant. Hoechst/PI
staining kit was used to detect cell membrane continuity. Western
blotting was used to detected NLRP3, cle-caspase-1, N-GSDMD and
IL-1β levels. (A) CCK-8 assay. (B) LDH level in cell supernatant.
(C and D) Hoechst/PI was used to detect cell membrane permeability.
(E) Protein level of NLRP3, cleaved-caspase-1, IL-1β and N-GSDMD in
control, HG/ox-LDL, spermine and ML385 group. (F) Quantification of
NLRP3, caspase-1, cleaved-caspase-1, IL-1β and N-GSDMD.
*P<0.05, ***P<0.001 compared with the
control group; #P<0.05, ##P<0.01,
###P<0.001 compared with the HG/ox-LDL group.
$P<0.05, $$P<0.01,
$$$P<0.001 compared with the spermine group. Nrf2,
nuclear factor erythroid 2-related factor 2; HG, high glucose;
ox-LDL, oxidized low-density lipoprotein; D-Glu, D-glucose; LDH,
lactate dehydrogenase; NLRP3, NLR family pyrin domain containing 3;
GSDMD, gasdermin D. |
Discussion
In diabetic atherosclerosis, hyperglycemia and
lipids contribute to macrophage dysfunction, which exacerbates
inflammatory responses and increases plaque instability (25,26).
The present study recreated an environment similar to that in
patients with diabetic atherosclerosis in vitro using
HG/ox-LDL conditions. The current study investigated the protective
effects of spermine against HG/ox-LDL-induced oxidative stress and
pyroptosis in THP-1-derived macrophages. It also investigated the
mechanisms underlying this protection. The findings revealed that
spermine suppressed HG/ox-LDL-induced macrophage pyroptosis by
inhibiting NLRP3 inflammasome activation. In addition, the Nrf2
signaling pathway mediated the protective effects of spermine
against HG/ox-LDL injury and oxidative stress and contributed to
the inhibitory effect of spermine on HG/ox-LDL-induced NLRP3
inflammasome-mediated pyroptosis in macrophages. These results
indicated that spermine attenuated HG/ox-LDL injury in macrophages
by inhibiting NLRP3 inflammasome-mediated pyroptosis via activation
of the Nrf2 signaling pathway. This provided new insight into the
functional role of spermine in diabetic atherosclerosis.
Pyroptosis is a newly discovered proinflammatory
mode of programmed cell death and there is abundant evidence that
pyroptosis promotes the progression of atherosclerosis (26). In addition, various natural
compounds, such as melatonin, colchicine and oxymatrine, have been
reported to exert anti-atherogenic effects (27). In addition, Han et al
(6) found that sinapic acid
attenuated diabetic atherosclerosis by inhibiting bone
marrow-derived macrophage pyroptosis in a rat model. Spermine, a
natural product of cellular metabolism, has been shown to possess
anti-inflammatory and antioxidant properties (28). However, the specific effects of
spermine on macrophages remain to be elucidated. The present study
found that spermine prevented loss of cell viability usually
induced by HG and ox-LDL stimulation. Furthermore, the LDH levels
in the supernatant and percentage of PI-positive cells were both
significantly decreased in the spermine group compared with those
in the HG/ox-LDL group, suggesting spermine increased cell
viability and reduced cell membrane disruptions caused by
HG/ox-LDL. Western blot analysis indicated that the expression of
NLRP3, cleaved caspase-1 and N-GSDMD were also decreased by
spermine pretreatment. Furthermore, the levels of IL-1β in the
culture supernatants and cell lysates were decreased by spermine
treatment. Accordingly, the results suggested that spermine could
inhibit macrophage pyroptosis induced by exposure to HG/ox-LDL. It
should be noted that in the present study, only PCR and western
blotting were used to detect the changes of pyroptosis related
genes and proteins in macrophages and flow cytometry was not used
to detect the levels of these proteins. This is a limitation of the
current study.
Oxidative stress has been shown to contribute to the
activation of NLRP3 inflammation and pyroptosis (29). It was previously demonstrated that
ROS enhance the assembly of NLRP3, ASC and caspase-1, which lead to
the mutation of cleaved caspase-1(30). Furthermore, it is observed that
ox-LDL induced endothelial pyroptosis by causing ROS overproduction
(31). In the present study, it
was also found that HG/ox-LDL increased ROS and MDA levels in
macrophages and inhibited expression of antioxidant proteins. These
effects were reversed by spermine treatment. These data suggested
that spermine attenuated macrophage pyroptosis by inhibiting ROS
overproduction.
Nrf2 is a common transcription factor that regulates
the transcription of anti-inflammatory and antioxidant proteins,
including HO-1, NQO-1, SOD and GSH. The activation of Nrf2,
enhanced by luteolin and oxymatrine, suppresses endothelial cell
and macrophage pyroptosis (32).
Notably, several reports demonstrate that spermine effectively
activates Nrf2 in a range of cell types (21,33).
Spermine is also regarded as a ROS scavenger and is capable of
protecting DNA from free radical attack (34). However, it is unclear whether
spermine has antioxidant effects and whether it activates Nrf2 in
HG/ox-LDL-induced pyroptotic macrophages. The current study found
that spermine pretreatment decreased ROS and MDA levels, increased
SOD and GSH levels and enhanced Nrf2 nuclear translocation. All
these effects were reversed by ML385 pretreatment. To test the
hypothesis that spermine inhibits macrophage pyroptosis by
activating Nrf2 and decreasing oxidative stress, the experimental
groups of cells were pretreated with ML385 and then pyroptosis
levels and NLRP3 inflammasome protein expression were measured.
ML385 attenuated the anti-pyroptosis activity of spermine, causing
decreased cell viability and increased levels of NLRP3, cleaved
caspase-1 and N-GSDMD. These findings support the hypothesis that
spermine attenuates macrophage pyroptosis by activating the Nrf2
pathway.
The biological activity of spermine, which is a
common polyamine, has been extensively studied. Spermine has been
shown to protect against cardiovascular diseases, including
diabetic cardiomyopathy and pulmonary hypertension (21,35).
However, to the best of the authors' knowledge, there has been no
direct evidence that spermine protects against diabetic
atherosclerosis. In the current study, HG/ox-LDL exposure led to
macrophage pyroptosis and oxidative stress, which are crucial
mechanisms involved in the progression of advanced diabetic
atherosclerosis. It was observed that spermine pretreatment
significantly increased macrophage viability and suppressed ROS
production under HG/ox-LDL conditions. In conclusion, the present
findings demonstrated that spermine was protective against
macrophage pyroptosis. Notably, the current study provided new
evidence that spermine may be a potential drug for the treatment of
diabetic atherosclerosis.
Supplementary Material
Toxic effects of spermine treatment
alone on macrophages. (A) CCK-8, (B and C) the protein level of
GSDMD and cleaved-caspase-1. GSDMD, gasdermin D; NS, no statistical
significance. *P<0.05, ***P<0.001 vs.
24 h; #P<0.05, ###P<0.001 vs. 48
h.
The mRNA level of caspase-1 and GSDMD.
(A) Caspase-1, (B) GSDMD. GSDMD, gasdermin D; HG, high glucose;
ox-LDL, oxidized low-density lipoprotein; NS, no statistical
significance.
Primers for quantitative PCR.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81770820).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL was responsible for the conception and design of
the study. YQ, JL and XG aquired and analyzed the data. YQ, LX and
LL were involved in drafting the manuscript, the interpretation of
the data and revising the manuscript. All authors have read and
approved the final manuscript. YL and YQ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soehnlein O and Libby P: Targeting
inflammation in atherosclerosis-from experimental insights to the
clinic. Nat Rev Drug Discov. 20:589–610. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen X, Zhang D, Li Y, Wang W, Bei W and
Guo J: NLRP3 inflammasome and IL-1β pathway in type 2 diabetes and
atherosclerosis: Friend or foe? Pharmacol Re.
173(105885)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Senior PA: Glucose as a modifiable cause
of atherosclerotic cardiovascular disease: Insights from type 1
diabetes and transplantation. Atherosclerosis. 335:16–22.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poznyak AV, Nikiforov NG, Starodubova AV,
Popkova TV and Orekhov AN: Macrophages and foam cells: Brief
overview of their role, linkage, and targeting potential in
atherosclerosis. Biomedicines. 9(1221)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tall AR and Westerterp M: Inflammasomes,
neutrophil extracellular traps, and cholesterol. J Lipid Res.
60:721–727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han Y, Qiu H, Pei X, Fan Y, Tian H and
Geng J: Low-dose sinapic acid abates the pyroptosis of macrophages
by downregulation of lncRNA-MALAT1 in rats with diabetic
atherosclerosis. J Cardiovasc Pharmacol. 71:104–112.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang C, Song JW, Huang HH, Fan X, Huang
L, Deng JN, Tu B, Wang K, Li J, Zhou MJ, et al: NLRP3 inflammasome
induces CD4+ T cell loss in chronically HIV-1-infected
patients. J Clin Invest. 131(e138861)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y,
Wang Y, Li D, Liu W, Zhang Y, et al: Granzyme a from cytotoxic
lymphocytes cleaves GSDMB to trigger pyroptosis in target cells.
Science (New York, N.Y.). 368(eaaz7548)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Fang C, Xu L, Yang B, Song E and
Song Y: Polybrominated diphenyl ether quinone exposure induces
atherosclerosis progression via CD36-mediated lipid accumulation,
NLRP3 inflammasome activation, and pyroptosis. Chem Res Toxicol.
34:2125–2134. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen S, Markman JL, Shimada K, Crother TR,
Lane M, Abolhesn A, Shah PK and Arditi M: Sex-specific effects of
the Nlrp3 inflammasome on atherogenesis in LDL receptor-deficient
mice. JACC Basic Transl Sci. 5:582–598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang H, Lv H, Li H, Ci X and Peng L:
Oridonin protects LPS-induced acute lung injury by modulating
Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB
pathways. Cell Commun Signal. 17(62)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jayasuriya R, Dhamodharan U, Ali D,
Ganesan K, Xu B and Ramkumar KM: Targeting Nrf2/Keap1 signaling
pathway by bioactive natural agents: Possible therapeutic strategy
to combat liver disease. Phytomedicine. 92(153755)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiegman CH, Li F, Ryffel B, Togbe D and
Chung KF: Oxidative stress in ozone-induced chronic lung
inflammation and emphysema: A facet of chronic obstructive
pulmonary disease. Front Immunol. 11(1957)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang X, Ding M, Zhu P, Huang H, Zhuang Q,
Shen J, Cai Y, Zhao M and He Q: New insights into the Nrf-2/HO-1
signaling axis and its application in pediatric respiratory
diseases. Oxid Med Cell Longev. 2019(3214196)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie L, Gu Y, Wen M, Zhao S, Wang W, Ma Y,
Meng G, Han Y, Wang Y, Liu G, et al: Hydrogen sulfide induces keap1
S-sulfhydration and suppresses diabetes-accelerated atherosclerosis
via Nrf2 activation. Diabetes. 65:3171–3184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu Y, Yang X, Liu Y, Jiang T, Ren S, Chen
J, Xiong H, Yuan M, Li W, Machens HG and Chen Z: NRF2 signalling
pathway: New insights and progress in the field of wound healing. J
Cell Mol Med. 25:5857–5868. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alonso-Piñeiro JA, Gonzalez-Rovira A,
Sánchez-Gomar I, Moreno JA and Durán-Ruiz MC: Nrf2 and heme
oxygenase-1 involvement in atherosclerosis related oxidative
stress. Antioxidants (Basel). 10(1463)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Grancara S, Martinis P, Manente S,
García-Argáez AN, Tempera G, Bragadin M, Via LD, Agostinelli E and
Toninello A: Bidirectional fluxes of spermine across the
mitochondrial membrane. Amino Acids. 46:671–679. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li QZ, Zuo ZW, Zhou ZR and Ji Y: Polyamine
homeostasis-based strategies for cancer: The role of combination
regimens. Eur J Pharmacol. 910(174456)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Matsumoto M: Prevention of atherosclerosis
by the induction of microbial polyamine production in the
intestinal lumen. Biol Pharm Bull. 43:221–229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Y, Chen J, Li S, Zhang X, Guo Z, Hu
J, Shao X, Song N, Zhao Y, Li H, et al: Exogenous spermine
attenuates rat diabetic cardiomyopathy via suppressing ROS-p53
mediated downregulation of calcium-sensitive receptor. Redox Biol.
32(101514)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao S, Song T, Gu Y, Zhang Y, Cao S, Miao
Q, Zhang X, Chen H, Gao Y, Zhang L, et al: Hydrogen sulfide
alleviates liver injury through the S-sulfhydrated-kelch-like
ECH-associated protein 1/nuclear erythroid 2-related factor
2/low-density lipoprotein receptor-related protein 1 pathway.
Hepatology. 73:282–302. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Verreth W, De Keyzer D, Davey PC, Geeraert
B, Mertens A, Herregods MC, Smith G, Desjardins F, Balligand JL and
Holvoet P: Rosuvastatin restores superoxide dismutase expression
and inhibits accumulation of oxidized LDL in the aortic arch of
obese dyslipidemic mice. Br J Pharmacol. 151:347–355.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019(8151836)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Josefs T, Barrett TJ, Brown EJ, Quezada A,
Wu X, Voisin M, Amengual J and Fisher EA: Neutrophil extracellular
traps promote macrophage inflammation and impair atherosclerosis
resolution in diabetic mice. JCI insight. 5(e134796)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li P, Wang Y, Liu X, Liu B, Wang ZY, Xie
F, Qiao W, Liang ES, Lu QH and Zhang MX: Loss of PARP-1 attenuates
diabetic arteriosclerotic calcification via Stat1/Runx2 axis. Cell
Death Dis. 11(22)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moss JW and Ramji DP: Nutraceutical
therapies for atherosclerosis. Nat Rev Cardiol. 13:513–532.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Igarashi K and Kashiwagi K: Functional
roles of polyamines and their metabolite acrolein in eukaryotic
cells. Amino Acids. 53:1473–1492. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu J, Xu L, Zeng Z, Xue C, Li J, Chen X,
Zhou P, Lin S, Liao Y, Du X, et al: Normothermic ex vivo heart
perfusion combined with melatonin enhances myocardial protection in
rat donation after circulatory death hearts via inhibiting NLRP3
inflammasome-mediated pyroptosis. Front Cell Dev Biol.
9(733183)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen JJ, Tao J, Zhang XL, Xia LZ, Zeng JF,
Zhang H, Wei DH, Lv YC, Li GH and Wang Z: Inhibition of the
ox-LDL-induced pyroptosis by FGF21 of human umbilical vein
endothelial cells through the TET2-UQCRC1-ROS pathway. DNA Cell
Biol. 39:661–670. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zou Y, Luo X, Feng Y, Fang S, Tian J, Yu B
and Li J: Luteolin prevents THP-1 macrophage pyroptosis by
suppressing ROS production via Nrf2 activation. Chem Biol Interact.
345(109573)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang J, Li S, Wang J, Wu F, Chen Y, Zhang
H, Guo Y, Lin Y, Li L, Yu X, et al: Spermidine alleviates cardiac
aging by improving mitochondrial biogenesis and function. Aging
(Albany NY). 12:650–671. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mohammadi M, Aelaei M and Saidi M:
Pre-harvest spray of GABA and spermine delays postharvest
senescence and alleviates chilling injury of gerbera cut flowers
during cold storage. Sci Rep. 11(14166)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He YY, Yan Y, Jiang X, Zhao JH, Wang Z, Wu
T, Wang Y, Guo SS, Ye J, Lian TY, et al: Spermine promotes
pulmonary vascular remodelling and its synthase is a therapeutic
target for pulmonary arterial hypertension. Eur Respir J.
56(2000522)2020.PubMed/NCBI View Article : Google Scholar
|