Non-traumatic osteonecrosis of the femoral head
(NONFH) is the result of impaired blood supply to the femoral head
and structural and functional disruption of local articular
cartilage, subchondral bone and blood vessels (Fig. 1), which culminate in subchondral
osteonecrosis, femoral head collapse and hip joint pain (1). Mont et al (2) estimate that the total number of
patients with NONFH worldwide could reach >20 million in the
next decade. The pain and limited hip joint mobility resulting from
femoral head necrosis can seriously affect the quality of life of
the patient.
NONFH is a chronic disease with risk factors
including long-term steroid use, excessive alcohol consumption and
other underlying disease (3,4).
Early diagnosis is difficult and there is currently no specific
diagnostic standard or treatment that can reverse early-stage
femoral head necrosis. Therefore, most patients with NONFH already
show substantial necrosis at the time of diagnosis. The early-stage
treatments are typically palliative and experimental, rather than
preventive and curative. Given the personal and social burden of
NONFH, the optimal treatment method is based on the individual
needs of the patients. The most suitable course of action is to
perform appropriate physical and minimally invasive surgery when
first diagnosed. In case the symptoms are not sufficiently relieved
within a reasonable period, hip replacement surgery needs to be
performed. The present review examined recent developments
regarding the treatment methods of NONFH and the underlying
mechanisms.
NONFH has a complex etiology and the most common
causes are hormonal disturbances and excessive alcohol consumption.
The risk of osteonecrosis is significantly higher in adolescents
and adult compared with children (3,4).
Every year in the United States alone ~10,000-20,000 new cases of
ONFH are diagnosed; it is common in individuals aged between 20-50
years and 5-12% of the patients eventually undergo total hip
replacement (5). In India, the
mean age of onset of femoral head necrosis is 34.71 years and the
male to female ratio is 5:1(3).
More than one-third (37.3%) of patients with ONFH have a history of
long-term steroid usage, whereas 21.3% of the cases are idiopathic
and chronic alcoholism is the underlying cause in 20.1% of the
patients (3). In Japan, the
incidence of femoral head necrosis is relatively low in men aged
40-49 years and significantly higher in individuals aged >50
years. Among women, the incidence rate is lower in the 16-29 years
age bracket and increases markedly in the 30-39 and 60-69 age
groups (4). In addition, 48 and
35% of ONFH cases among males are respectively alcohol- and
hormone-induced. By contrast, 70% of the cases among females have a
hormonal basis (6). Studies have
shown that high-dose corticosteroids (>40 mg/day) significantly
increase the risk of NONFH (7,8) and
every 10 mg increase in the dose increases the incidence rate by
3.6% (9).
Studies have shown that femoral head necrosis is
associated with several underlying diseases (Table I), including trauma or surgery of
the hip joint, excessive corticosteroid production, hyperlipidemia,
abnormal blood pressure, autoimmune diseases, endotoxin poisoning,
smoking, excessive alcohol consumption and blood hypercoagulability
(10). All of these pathological
changes can eventually lead to vascular damage, bone marrow
infarction and avascular necrosis.
Alcohol abuse is one of the main risk factors of
bone deterioration. Ethanol impairs the proliferation of human bone
mesenchymal stem cells (hBMSCs) and induces their differentiation
into adipocytes, which eventually leads to bone loss and structural
damage (16,17). Recent studies have shown that
abnormal bone metabolism in patients with alcoholic ONFH is
associated with the inhibition of the Akt/GSK-3β/β-catenin pathway
in bone mesenchymal stem cells (BMSCs), which is negatively
affected by ethanol-induced increase in tensin homology phosphatase
(18).
Sickle cell leukemia can lead to microthrombosis,
resulting in osteonecrosis and occasionally osteomyelitis (19). Further aggravation can also lead to
hemolysis, increased red blood cell activity and expansion of the
bone marrow cavity (19). Femoral
head necrosis in patients with HIV is associated with
hyperlipidemia and drug treatment (20). A meta-analysis conducted by Matos
et al (20) shows that
protease inhibitors can cause hyperlipidemia and ultimately lead to
osteonecrosis. Mazzotta et al (21) conducted a multi-center case-control
study wherein the highly active antiretroviral therapy
significantly increased the triglyceride and cholesterol levels of
patients with HIV and altered the total IgE levels in the serum. In
a study on 539 patients with severe acute respiratory syndrome who
received different types of steroid therapy, 39.5% of the males and
19.3% of the females were diagnosed with ONFH. In addition, ONFH
was more common in patients aged 20-49 years (25.9%) compared with
that in the 50-59 years group (6.3%). The incidence of ONFH in
patients receiving one steroid was 12.5% as opposed to 28.6 and
37.1% in patients treated respectively with two and three types of
steroids (37.1%) (22). The
COVID-19 patients are also at a higher risk of developing ONFH. The
COVID-19 virus is more sensitive to ONFH and the cumulative dose of
steroids is smaller (23).
The current treatments for NONFH can be classified
into non-surgical and surgical methods (Fig. S1). The non-surgical approaches
include protective weight bearing, physical therapy and drug
therapy and examples of surgical intervention are non-vascular
transplantation, osteotomy, core decompression, vascularized
transplantation and joint replacement. Some therapeutic strategies
developed in recent years for treating avascular necrosis of the
femoral head are discussed in the following sections.
The patients are advised to lose weight, reduce the
force exerted on the femoral head of the affected side, avoid
mutual and reverse movement, use crutches for walking and avoid
sitting or lying in bed for a long period of time (24). Studies show (21,24)
that local non-weight bearing can reduce the occurrence of femoral
head deformity following ischemic osteonecrosis by increasing
revascularization and reducing bone resorption in the infarcted
epiphysis, although it does not stimulate new bone formation.
Nevertheless, it can optimize osteogenic therapies and the healing
of necrotic femoral heads by controlling the rate of bone
resorption (24). Therefore,
weight bearing can prevent the occurrence and development of
osteonecrosis, especially femoral head collapse, in subjects at the
early stage of the disease [association research circulation
osseous (ARCO Ia)] and with small lesions. However, this
non-surgical intervention is ineffective for 80-90% of the patients
with ONFH (25,26).
Lipid-lowering agents, anticoagulants, vasoactive
substances, statins and bisphosphonates have been used to prevent
and treat femoral head necrosis at the early-stages.
Bisphosphonates such as alendronate (trade name Fosamax) were
originally prescribed to treat osteoporosis, They improve bone
density by reducing osteoclast-mediated bone resorption (27-33).
Animal studies and clinical trials have shown that bisphosphonates
can accelerate the recovery of joint function, delay disease
progression, reduce pain and lower the risk of femoral head
collapse associated with ONFH without significant side effects
(27-33).
Nevertheless, the therapeutic effect of bisphosphonates against
corticosteroid-induced ONFH is controversial (27) and there is no clear recommendation
for the dose and duration of treatment. Overall, bisphosphonate
drugs can be considered for treating femoral head necrosis caused
due to metabolic disturbances.
Prostacyclin is a vasodilatory agent and an
antagonist of thromboxane that improves blood flow by preventing
platelet aggregation. While short-term use of prostacyclin is
associated with significant improvements in the clinical and
radiological indices of early-stage ONFH, the long-term efficacy is
still being evaluated (34). One
study showed that a combination of intravenous prostacyclin and
core decompression can alleviate the symptoms of osteonecrosis
(35). Iloprost is a synthetic
analog of prostacyclin that is routinely used to treat pulmonary
arterial hypertension and has proved to effective against bone
marrow edema and ONFH (36,37).
A prospective study on 30 cases of ONFH with coagulopathy showed
that 53% of the patients did not progress to Ficat and Arlet stages
I and II following treatment with the anticoagulant enoxaparin. In
addition, the combination of enoxaparin, ginkgo biloba extract
(vasodilators) and sildenafil improved femoral head perfusion in an
animal model of steroid-induced ONFH (36,37).
Studies show that the development of ONFH in the hip
joint is associated with an increase in the number and size of
circulating fat cells (38,39).
Therefore, the lipid-lowering statins that are used to treat
steroid-related inflammatory disorders (38,39)
can potentially be therapeutic against ONFH. Indeed, lovastatin
decreases adipogenesis and bone death in a chicken model of
steroid-induced ONFH and increases the expression of osteogenic
genes in bone marrow cells (40).
One study on patients receiving statins and high-dose steroids
showed that after an mean follow-up period of 7.5 years, the
incidence of ONFH was 1% at annual follow-up, as opposed to 3%
usually reported by patients receiving high-dose steroids.
Therefore, statins may prevent osteonecrosis in subjects with
long-term steroid use (38).
Traditional Chinese medicine formulations have been
used to treat osteonecrosis for decades. Ye et al (41) showed that the ginsenoside Rb1
inhibits steroid-induced avascular necrosis, osteonecrosis and the
elevation of serum osteocalcin in a rat model of Steroid-induced
avascular necrosis of the femoral head (SANFH) by blocking the
vascular endothelial growth factor (VEGF)/runt-related
transcription factor 2/bone morphogenetic protein 2 signaling
pathway. In addition, Rb1 also reduces inflammation, oxidative
stress, total cholesterol and low/high lipoprotein levels, alkaline
phosphatase activity and bone calcium loss in the SANFH rats.
High-energy ESWT is a non-invasive approach that has
been used for treating ONFH since the end of the last century
(42,43). Its potential mechanisms of action
include restoration of tissue oxygenation, reduction of bone marrow
edema and increased blood supply to focal lesions (42,43).
ESWT is a promising alternative to the more invasive surgical
methods currently used to treat ONFH at different stages. Ludwig
et al (44) subjected 22
patients with ONFH to the shock wave treatment and assessed the
visual analog scale (VAS) and HHS scores after one year follow-up.
The VAS scores of the patients decreased from 8.5 points before
surgery to 1.2 points and the HHS scores increased from 43.3 points
to 92 points, indicating high efficacy of ESWT. Other studies have
shown that ESWT is only effective against early-stage ONFH
(45). Algarni and Al Moallem
(46) treated 21 patients (33
hips) with early-stage ONFH using this approach and found that the
VAS scores and HHS scores of 21 hip joints improved significantly
after 8 months. After 5 years, 4 cases received THA treatment.
Among them, 26 cases showed no significant progression from the
first stage. Magnetic resonance imaging (MRI) results showed that
in 23 cases, bone marrow edema was significantly reduced. Thus,
ESWT significantly improved the quality of life of patients with
ONFH, delayed the progression of disease and avoided the need for
total hip arthroplasty (THA). Xie et al (43) retrospectively analyzed the data of
31 patients with ONFH (44 hips) who underwent ESWT and were
followed up over an mean duration of 130.6 months. The imaging
findings showed lack of disease progression in all stage I, 64.3%
of stage II and 12.5% of stage III hips. Thus, ESWT is a suitable
option for ARCO I and II patients.
HBO has proved to be highly effective against early
stages of femoral head necrosis, particularly in Asian populations
(52-55).
In a recent study (56) on 19
patients with stage II ONFH, 12 (52.2%) underwent core
decompression (CD) surgery and 11 (47.8%) received HBO treatment.
Over the mean follow-up period of 34.2±18.4 months, 66.7% of
patients in the CD group and 81.8% of patients in the HBO group
showed satisfactory hip joint function. However, 8 patients (34.7%)
progressed to a higher radiological stage during the first year of
follow-up and the progression rate was similar in both groups.
Studies have also shown that HBO therapy is as effective as CD for
treating non-traumatic pre-collapsed femoral head AVN and therefore
can be used a non-invasive alternative (52-56).
The clinical efficacy of HBO depends on the synthesis of growth
factors, which promote wound healing and reduce post-ischemia and
post-inflammatory damage (57,58).
In addition, the increase in hydrostatic pressure compresses all
gas-filled spaces in the human body (Ball's law), which can reverse
decompression-related diseases (59,60).
HBO therapy reduces edema, increases tissue oxygenation and
restores venous drainage in the affected bone area by inducing
proliferation of endothelial progenitor cells, promoting
neo-angiogenesis and improving local microcirculation (57,61-65).
A 2017 systematic review found that HBO therapy is effective
against early-stage ONFH and may reduce cellular ischemia by
increasing the concentration of extracellular oxygen (66). In summary, HBO therapy can be
considered for the prevention and treatment of early-stage femoral
head necrosis.
The literature related to the non-surgical treatment
methods for NONFH are summarized in Table II.
CD is the commonest surgical procedure for
early-stage osteonecrosis and is used to relieve pain and promote
bone regeneration and repair (67,68).
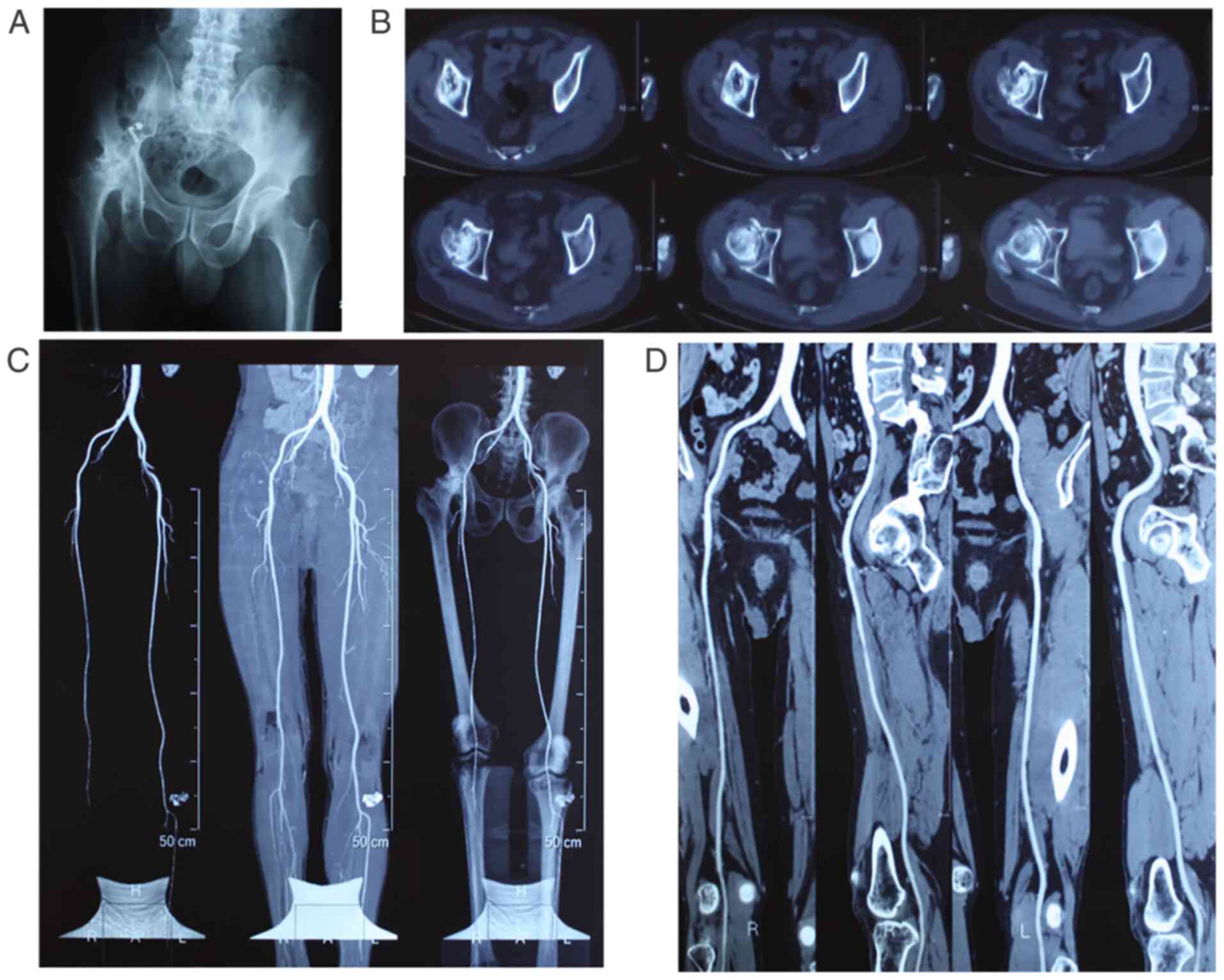
As shown in Fig. 2, pure CD can
repair and delay the progression of NONFH (67) by reducing intramedullary pressure
on the inside of the femoral head, accelerating bone regeneration,
reversing femoral head necrosis and improving blood flow. However,
the clinical results so far are inconclusive and indicate that CD
may have an improved effect on early-stage ONFH (68).
In one study, CD surgery prevented further
deterioration of the hip joints of 87-90% of Ficat I patients with
NONFH, whereas only 59-70% of stage IIa patients derived a clinical
benefit from this procedure (69,70).
CD surgery can also significantly reduce hip pain in patients with
stage I and II a avascular necrosis of the femoral head and
increase the range of motion of the hip joint (69,71).
Simank et al (72) found
that the therapeutic effect of CD can be weakened in case of
corticosteroid usage, smoking, drinking and other risk factors. In
addition, some studies report that CD can only exert a short-term
therapeutic effect and the long-term curative effect is poor and
does not preclude the need for total hip replacement (73-76).
Multiple drilling cannot effectively reduce the rate of THA
conversion in early-stage NONFH. In fact, the risk of conversion to
THA is increased after multiple drilling in case of larger necrotic
lesions, presence of bone marrow edema and higher postoperative
workload (74,75). Furthermore, Sadile et al
(76) report lower efficacy of CD
against NONFH compared with that of other palliative surgical
techniques in terms of clinical status, imaging characteristics and
the need for total hip replacement.
Despite the inconsistencies regarding the
therapeutic effects of CD alone, the combination of CD with other
treatment methods has shown encouraging results. For instance, the
HHS score, hip function recovery and imaging performance of
patients with NONFH that received bone marrow mesenchymal stem cell
therapy are superior compared with patients who underwent CD,
although the combination treatment had the optimal effect.
Tabatabaee et al (77)
subjected 28 cases of early NONFH to CD or a combination of CD with
concentrated bone marrow implantation and found that the VAS pain
index and MRI results improved to a significant greater extent in
the combination treatment group. In a recent study conducted on 52
hips (65%) with Ficat IIa and 28 hips (35%) with Ficat IIb ONFH, 46
hips (30 Ficat IIa and 16 Ficat IIb) received HBO therapy and 34
hips (22 Ficat IIa and 12 Ficat IIb) were treated with a
combination of CD and HBO. Although the VAS and HHS scores improved
in each group compared with the pre-treatment scores (P<0.001),
the recovery was markedly higher in the CD + HBO group
(P<0.001). Furthermore, the functional and pain scores as per
the SF-36 scale (78) were also
significantly different between the two groups (P<0.005). HBO
treatment can reduce the degree of pain in Ficat II patients and
increase their functional scores. The combination of CD and HBO
treatment achieves greater pain reduction in stage IIa patients
compared with stage IIb patients (78). The BMSCs-bone morphogenetic protein
2 (BMP-2) complex on the magnesium alloy rod was implanted into the
metaphysis of the left femur of the rabbit to the femoral head,
BMP-2 coated magnesium alloy can promote the expression of bone
growth factor in rabbit bone marrow implants, thereby delaying
femoral head necrosis and promoting repair (79).
PRP has 8-fold higher load of platelets compared
with whole blood, along with high levels of regenerative cytokines
such as platelet-derived growth factor, transforming growth factor
β, basic fibroblast growth factor, endothelial growth factor,
insulin-like growth factor and VEGF (80). Karakaplan et al (81) found that PRP alleviates the
symptoms of early-stage hormonal ONFH in a rabbit model. Zhang
et al (82) further showed
that the fusion of PRP with tricalcium phosphate (TCP) promotes the
formation of new bone and inhibited inflammation in the rabbit
model of ONFH. In addition, the triple combination of CD surgery,
PRP fusion and autologous granular bone transplantation relieves
traumatic ONFH with a success rate of 80% (83). Houdek et al (84) treated 22 steroid-induced patients
with ONFH (35 hips) with BMSCs and PRP and found that the mean HSS
increased from 57 points pre-transplantation to 85 points over an
mean follow-up duration of 3 years and 93% of the patients were
stable without any disease progression or complications. Although
PRP cannot reverse the pathophysiological process of ONFH, it can
induce osteogenic activity and stimulate the differentiation of
stem cells in ARCO stage I and II patients when used in conjunction
with CD and stem cell transplantation or bone grafting (85).
Studies increasingly show that femoral head necrosis
is associated with a weakened regenerative ability of the affected
tissue, which can be attributed to the decrease in the number of
BMSCs and lower osteogenic differentiation (90-92).
Since BMSCs support angiogenesis, transplantation of bone marrow
MSCs can potentially achieve clinical resolution of ONFH. However,
there are several challenges in the practical application of stem
cell therapy, such as patient selection, standardization of
procedures, safety assessment and the fate of transplanted cells
in vivo. Further studies are needed to identify ideal
sources of cells, the appropriate transplantation methods, as well
as the optimal number of cells (90-92).
Bone transplantation has been the mainstay of early
NONFH treatment for more than 70 years (104). The common methods included simple
bone transplantation, osteochondral transplantation and
vascularized bone flap transplantation, all of which have exhibited
good clinical effects.
Bone impaction grafting prevents the development of
osteoarthritis by restoring the collapsed spherical head and
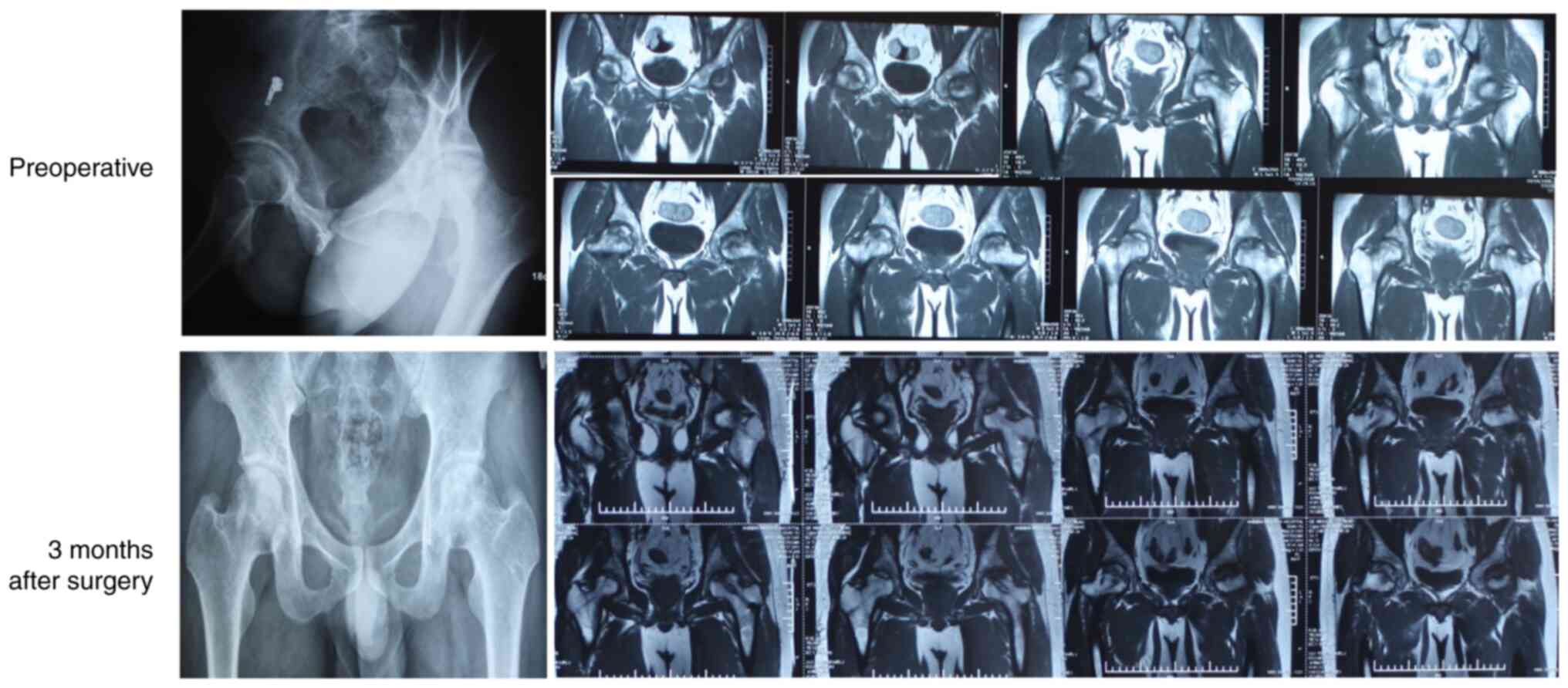
remodeling the necrotic area using autologous bone tissue (105). Guo et al (106) performed allogeneic fibula
transplantation in patients with early-stage femoral head necrosis
and recorded rapid recovery of joint function, low level of trauma
and significant improvement in clinical symptoms, indicating that
this method is ideal for short-term management (107,108).
Vascularized bone grafts have been developed to
restore the blood supply to the necrotic femoral head and improve
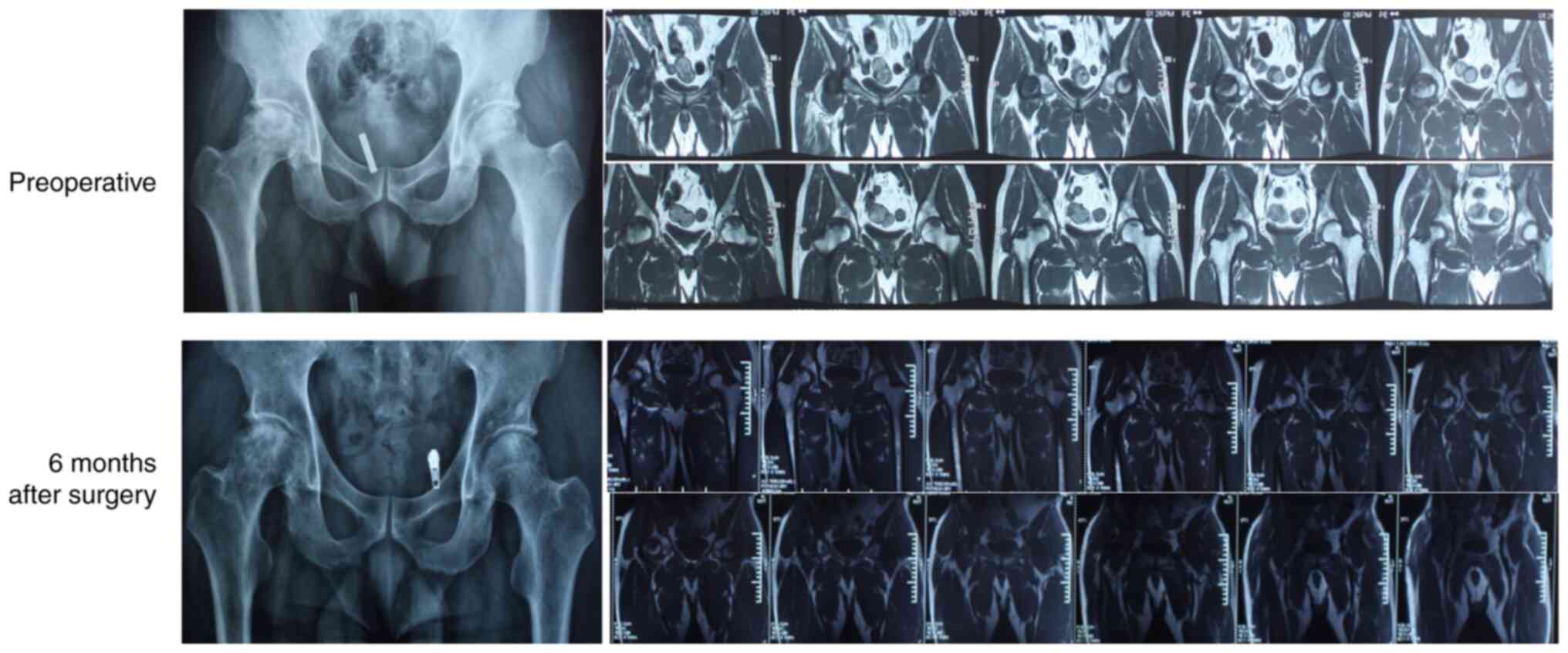
the ischemic state. Zhu and Zhou (112) subjected 6 patients with NONFH to
vascularized iliac periosteum transplantation and detected
significant recovery during the follow-up period of 3 to 7.5 years.
The patients reported complete cessation of pain in the hip joint
and were able to move freely. X-ray images showed that the shape of
the femoral head was normal with a clear outline and the bone
density was also restored. Thus, vascularized bone transplantation
improves the blood supply of the femoral head, which is conducive
to its revascularization and may reverse early-stage avascular
necrosis of the femoral head (113).
For vascularized iliac bone transplantation with
femoral head necrosis, medium and short-term therapeutic effects
that can effectively delay the need for total hip replacement have
been observed (114,115). Scully et al (116) compared the therapeutic effects of
vascularized fibula transplantation and cord decompression in
patients with ONFH at different stages. The combination of both
treatments had a significant curative effect in the Ficat I
patients. In patients at Ficat stages II and III, the success rate
of vascularized fibula transplantation was 89 and 81% respectively
compared with only 65 and 21% with CD, indicating that vascularized
bone transplantation is superior to CD against both early and late
stage ONFH. Heinrich and McBeath (117) used the gluteal muscle pedicle
bone grafting technique to treat 16 patients (20 hips) with Ficat
II-III NONFH. After an mean follow-up duration of 47 months, X-ray
imaging showed recovery in 12 cases whereas no obvious progress was
seen in 8 cases.
Cartilage transplantation, including autologous
chondrocyte implantation, autologous osteochondral transplantation
and allogeneic osteochondral transplantation (118), is widely used for treating talus
osteochondral disease and knee cartilage injury (119,120). Gagala et al (121) treated 7 early stage patients with
NONFH via osteochondral transplantation and 13 advanced-stage
patients with a combination of autologous osteochondral and
allogeneic bone transplantation. The mean follow-up duration was
46.14 months. At the final follow-up, the HHS scores of both groups
had improved significantly, especially in patients with early-stage
NONFH, indicating that osteochondral transplantation can
significantly slow the progression of NONFH and delay the need for
THA. However, due to lack of relevant research, the curative effect
of cartilage transplantation is not completely clear.
A porous tantalum rod is a biocompatible material
with an elastic modulus similar to the human fibula and can
therefore provide structural support for the femoral head. Tantalum
implants have been widely used for treating orthopedic diseases.
Studies show that porous tantalum implants can effectively slow the
progression of femoral head necrosis (122) and that its therapeutic effect on
early-stage NONFH is superior to that of traditional bone
transplantation (123). Nadeau
et al (124) used porous
tantalum implants for treating 15 patients with NONFH (18 hips), of
which 3 cases were at Steinberg stage III and 15 cases were at
stage IV. Most patients did not need further surgical treatment
after 12 months and the success rate was 77.8%. The postoperative
HHS score of the patients had improved significantly over an mean
follow-up period of 23 months, which was indicative of good
short-term effects. At the last follow-up however, the total
success rate dropped to 44.5%, indicating that the long-term
treatment effect requires further resolution. Liu et al
(125) compared the postoperative
hospital stay, number of days of PCA, HHS score and survival rate
of the hip joint in patients who underwent traditional bone
transplantation (control group) and patients who had received
porous tantalum implants. The HHS score of the tantalum rod
implantation group was significantly higher compared with the
control group and the hip joint survival rate was 74.1% compared
with only 49% in the bone-transplanted controls. Thus, implantation
of tantalum rod can have a significant therapeutic effect in
patients with NONFH without bone marrow edema and can delay or even
avoid the need for THA. Likewise, Liu et al (126) found that whole tantalum rod
implantation can effectively treat early-stage to mid-term NONFH.
However, they observed high-density metal particles remnants in the
femoral bone marrow cavity on X-ray images, which may be the cause
of post-treatment pain and treatment failure.
The composite scaffold is expected to be a promising
device for regulating the microenvironment of osteonecrosis and
overcoming the challenges related to bone regeneration. A number of
organic [for example, poly(lactide-co-glycolide) (PLGA),
poly(ε-caprolactone), polylactide and chitosan], inorganic (for
example, nano-hydroxyapatite, β-tricalcium phosphate and ceramics)
and composite materials have attracted increasing attention as the
matrix of bone tissue engineering scaffolds. This is due to their
excellent biocompatibility, controllable degradation, easy
processing, excellent mechanical properties, osteoconductivity and
the ability to promote bone regeneration (127-130).
The combined application of polymers and various other substances
utilizes the advantages of various substances and the advantages of
polymers to meet the needs of a wider range of osteonecrosis
research. The addition of various substances improves the
biological activity and mechanical support performance of the pure
polymer. Various biologically active substances are added to
polymers to produce functionalized polymers. The addition of stem
cells, growth factors, small molecule drugs and metal ions in
polymer bone substitute materials give the polymer bone-forming and
vascular properties, which is conducive to repairing osteonecrosis
(129-131).
The research on functionalized polymer bone substitute materials
has become a development trend and the production of various
functionalized polymer biomaterials may improve the treatment of
osteonecrosis (131).
Magnesium powder, PLGA and β-TCP are elements used
to formulate novel porous PLGA/TCP/Mg (PTM) scaffolds using low
temperature-rapid prototyping technology. The biological safety
assessment from 0-12 weeks after implantation did not cause an
increase in the serum magnesium ion concentration and the immune
response and liver and kidney function parameters were at normal
levels. These findings indicate that PTM scaffolds have osteogenic
and angiogenic capabilities and they have a synergistic effect in
enhancing the formation of new bone and enhancing the quality of
newly formed bone in ONFH (132).
Osteotomy can effectively delay the need for THA in
patients with NONFH and the most common type is intertrochanteric
valgus flexion osteotomy. The postoperative situation is related to
the degree of the femoral head necrosis (133,134). Portigliatti Barbos et al
(135) performed flexion
osteotomy on 19 patients with avascular necrosis of the femoral
head and followed them up for 8 years. Almost 85% of patients
showed significant recovery, indicating that osteotomy is an ideal
choice for treating NONFH (136).
Mont et al (137) followed
up 37 patients with Ficat II-III NONFH who underwent
intertrochanteric osteotomy for an mean of 11.5 years and found
that 28 showed good recovery with improved HHS score, while 9 had
to undergo THA. Among the 17 patients with hormonal NONFH, the
treatment failed in 6 cases, of which 5 had a necrosis angle
exceeding 200 degrees. Thus, intertrochanteric osteotomy can be
effective against Ficat II-III NONFH, but is not recommended for
patients with long-term corticosteroid usage or excessive necrosis.
Inao et al (138) and
Masui and Hasegawa (139)
performed transtrochanteric osteotomy on patients with NONFH and
confirmed good recovery of the femoral heads during the mean
follow-up of 13.2 years, with <2 mm collapse over 15 years after
surgery.
The adaptation period and therapeutic effects of
non-traumatic NONFH with non-replacement surgery are summarized in
Table III.
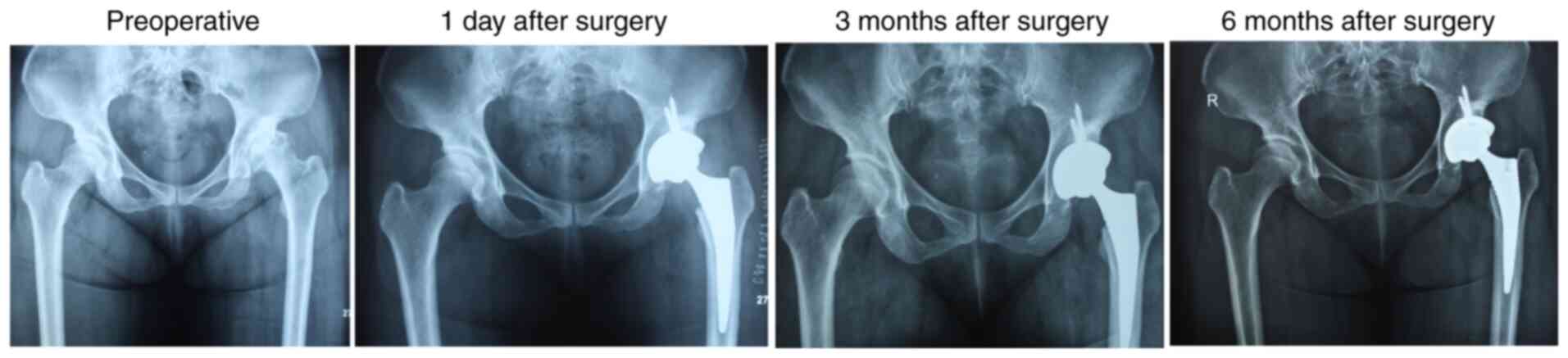
For the Ficat stage III or IV patients with NONFH
with femoral head depression measuring >2 mm and large lesions,
it is frequently difficult to achieve the desired therapeutic
effect using palliative surgery and the patients often require
total hip replacement or hip resurfacing. The number of cases
opting for THA is increasing, which can be attributed to the
improvements in long-term efficacy. Furthermore, younger patients
with ONFH are unwilling to undergo joint-sparing surgery that
requires long-term hospitalization (143). Thus, joint replacement surgery is
currently the most preferred treatment method for advanced
NONFH.
The aforementioned studies underscore the influence
of age on femoral head necrosis and that of various underlying
diseases on joint function. The surgical approach of joint
replacement has no significant impact on the lifespan of joint
replacement and rather depends on individual differences (149-153).
Compared to THA, hip resurfacing (HRA) can preserve
more femoral bone mass and maintain normal biomechanics with
similar outcomes (154,155). Therefore, HRA can be used as an
alternative to total hip replacement. Beaulé et al (156) performed half-face replacement
therapy on 37 patients with Ficat stage II, III or IV NONFH. The
clinical, imaging and functional indices showed considerable
improvement during the mean follow-up duration of 6.5 years and the
5-, 10- and 15-year survival rates were 79, 59 and 45%
respectively. However, some studies have reported suboptimal
outcomes of HRA. Calder et al (157) performed HRA on 12 patients with
NONFH (15 hips; 1 stage Ⅱ, 9 stage III and 5 stage IV), of which 9
had to undergo revision surgery within two years during the mean
follow-up period of 22.8 months. In addition, the overall results
were not satisfactory. In addition, Hsieh et al (158) found that temperatures >50˚C
prolonged bone cement polymerization during HRA, resulting in
higher heat loss compared with normal bone. Therefore, more
research is needed to determine the therapeutic efficacy of HRA. As
summarized in Table IV, THA has a
significantly lower revision rate compared with HRA.
Femoral head necrosis is a slow progressive disease
that causes irreversible structural changes in the hip joint,
leading to chronic pain and disability. However, there are no
standard criteria for early screening and diagnosis, thus making
early treatment less likely. Based on the current literature, the
present review recommended CD combined with bone transplantation,
PRP, HBO therapy, stem cell therapy, high-energy ESWT and PEMF for
patients at ARCO stage Ⅰ or Ⅱ following the failure of non-surgical
treatment. For patients at ARCO stage III, these methods may be
effective, although there is currently lack of evidence. THA must
be performed for the ARCO stage IV patients. However, this surgical
plan does not consider patient age, comorbidities and other
influencing factors. Therefore, an individualized plan based on the
patient's condition and the mechanisms of the therapeutic strategy
should be developed.
Not applicable.
Funding: No funding was received.
Not applicable.
NL and QW collected images from clinical work. NL
performed literature review and wrote the manuscript. CZ performed
literature review of pathophysiology and diagnosis. QW edited the
manuscript and figures. ZH designed the study and wrote the
manuscript. NL and ZH confirm the authenticity of all raw data. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Jin H, Li L, Yu W and Fu Y: The efficacy
of acupuncture and moxibustion for early and middle-stage
osteonecrosis of the femeral head: A systematic review and
meta-analysis of randomized controlled trials. Medicine
(Baltimore). 100(e26210)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mont MA, Zywiel MG, Marker DR, McGrath MS
and Delanois RE: The natural history of untreated asymptomatic
osteonecrosis of the femoral head: A systematic literature review.
J Bone Joint Surg Am. 92:2165–2170. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vardhan H, Tripathy SK, Sen RK, Aggarwal S
and Goyal T: Epidemiological profile of femoral head osteonecrosis
in the North Indian population. Indian J Orthop. 52:140–146.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tomaru Y, Yoshioka T, Sugaya H, Shimizu Y,
Aoto K, Wada H, Akaogi H, Yamazaki M and Mishima H: Mid-term
results of concentrated autologous bone marrow aspirate
transplantation for corticosteroid-associated osteonecrosis of the
femoral head in systemic lupus erythematosus. Int Orthop.
42:1623–1630. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gun BK, Frank RM, Gratton RW, Bader JO,
Kusnezov N, Orr JD and Waterman BR: Non-modifiable risk factors
associated with avascular necrosis in the US military. Mil Med.
185:e178–e182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takahashi S, Fukushima W, Yamamoto T,
Iwamoto Y, Kubo T, Sugano N and Hirota Y: Japanese Sentinel
Monitoring Study Group for Idiopathic Osteonecrosis of the Femoral
Head. Temporal trends in characteristics of newly diagnosed
nontraumatic osteonecrosis of the femoral head from 1997 to 2011: A
hospital-based sentinel monitoring system in Japan. J Epidemiol.
25:437–444. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shigemura T, Nakamura J, Kishida S, Harada
Y, Ohtori S, Kamikawa K, Ochiai N and Takahashi K: Incidence of
osteonecrosis associated with corticosteroid therapy among
different underlying diseases: Prospective MRI study. Rheumatology
(Oxford). 50:2023–2028. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mont MA, Pivec R, Banerjee S, Issa K,
Elmallah RK and Jones LC: High-dose corticosteroid use and risk of
hip osteonecrosis: Meta-analysis and systematic literature review.
J Arthroplasty. 30:1506–1512.e5. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gladman DD, Dhillon N, Su J and Urowitz
MB: Osteonecrosis in SLE: Prevalence, patterns, outcomes and
predictors. Lupus. 27:76–81. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Semin Arthritis Rheum. 32:94–124. 2002.PubMed/NCBI
|
|
11
|
Zhu Z, Li S, Yu H, Huang J and Tong P:
Correlation between continuation of glucocorticoid treatment and
risk of femoral head collapse: A retrospective cohort study of
patients with glucocorticoid-induced osteonecrosis of femoral head
after hip-preserving interventions. Orthopade. 50:143–149.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Q, L VJ and Jin L: Role of
coagulopathy in glucocorticoid-induced osteonecrosis of the femoral
head. J Int Med Res. 46:2141–2148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kuroda T, Tanabe N, Wakamatsu A, Takai C,
Sato H, Nakatsue T, Wada Y, Nakano M and Narita I: High
triglyceride is a risk factor for silent osteonecrosis of the
femoral head in systemic lupus erythematosus. Clin Rheumatol.
34:2071–2077. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu X, Sun W and Tan M: Noncoding RNAs in
steroid-induced osteonecrosis of the femoral head. Biomed Res Int.
2019(8140595)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yue J, Wan F, Zhang Q, Wen P, Cheng L, Li
P and Guo W: Effect of glucocorticoids on miRNA expression spectrum
of rat femoral head microcirculation endothelial cells. Gene.
651:126–133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tan B, Li W, Zeng P, Guo H, Huang Z, Fu F,
Gao H, Wang R and Chen W: Epidemiological study based on China
osteonecrosis of the femoral head database. Orthop Surg.
13:153–160. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoon BH, Jones LC, Chen CH, Cheng EY, Cui
Q, Drescher W, Fukushima W, Gangji V, Goodman SB, Ha YC, et al:
Etiologic classification criteria of ARCO on femoral head
osteonecrosis part 2: Alcohol-associated osteonecrosis. J
Arthroplasty. 34:169–174.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen YX, Zhu DY, Gao J, Xu ZL, Tao SC, Yin
WJ, Zhang YL, Gao YS and Zhang CQ: Diminished membrane recruitment
of Akt is instrumental in alcohol-associated osteopenia via the
PTEN/Akt/GSK-3β/β-catenin axis. FEBS J. 286:1101–1119.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aguilar C, Vichinsky E and Neumayr L: Bone
and joint disease in sickle cell disease. Hematol Oncol Clin North
Am. 19:929–941, viii. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Matos MA, Alencar RW and Matos SS:
Avascular necrosis of the femoral head in HIV infected patients.
Braz J Infect Dis. 11:31–34. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mazzotta E, Agostinone A, Rosso R, Di
Biagio A, De Socio GV, Cappelletti A, Zicolella R, Polilli E,
Bonfanti P, Di Matteo L, et al: Osteonecrosis in human
immunodeficiency virus (HIV)-infected patients: A multicentric
case-control study. J Bone Miner Metab. 29:383–388. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo KJ, Zhao FC, Guo Y, Li FL, Zhu L and
Zheng W: The influence of age, gender and treatment with steroids
on the incidence of osteonecrosis of the femoral head during the
management of severe acute respiratory syndrome: A retrospective
study. Bone Joint J. 96-B:259–262. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Agarwala SR, Vijayvargiya M and Pandey P:
Avascular necrosis as a part of ‘long COVID-19’. BMJ Case Rep.
14(e242101)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim HK, Aruwajoye O, Stetler J and Stall
A: Effects of non-weight-bearing on the immature femoral head
following ischemic osteonecrosis: An experimental investigation in
immature pigs. J Bone Joint Surg Am. 94:2228–2237. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hungerford DS and Mont MA: Current options
and approaches for blood management in orthopaedic surgery. J Bone
Joint Surg Am. 80A(765)1998.
|
|
26
|
Arai R, Takahashi D, Inoue M, Irie T,
Asano T, Konno T, Terkawi MA, Onodera T, Kondo E and Iwasaki N:
Efficacy of teriparatide in the treatment of nontraumatic
osteonecrosis of the femoral head: A retrospective comparative
study with alendronate. BMC Musculoskelet Disord.
18(24)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee YK, Ha YC, Cho YJ, Suh KT, Kim SY, Won
YY, Min BW, Yoon TR, Kim HJ and Koo KH: Does zoledronate prevent
femoral head collapse from osteonecrosis? A prospective,
randomized, open-label, multicenter study. J Bone Joint Surg Am.
97:1142–1148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yuan HF, Guo CA and Yan ZQ: The use of
bisphosphonate in the treatment of osteonecrosis of the femoral
head: A meta-analysis of randomized control trials. Osteoporos Int.
27:295–299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Agarwala S, Banavali SD and Vijayvargiya
M: Bisphosphonate combination therapy in the management of
postchemotherapy avascular necrosis of the femoral head in
adolescents and young adults: A retrospective study from India. J
Glob Oncol. 4:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li D, Yang Z, Wei Z and Kang P: Efficacy
of bisphosphonates in the treatment of femoral head osteonecrosis:
A PRISMA-compliant meta-analysis of animal studies and clinical
trials. Sci Rep. 8(1450)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sheng H, Lao Y, Zhang S, Ding W, Lu D and
Xu B: Combined pharmacotherapy with alendronate and desferoxamine
regulate the bone resorption and bone regeneration for preventing
glucocorticoids-induced osteonecrosis of the femoral head. Biomed
Res Int. 2020(3120458)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Roth A, Beckmann J, Bohndorf K, Heiß C,
Jäger M, Landgraeber S, Maus U, Nöth U, Peters KM, Rader C, et al:
Update of the German S3 guideline on atraumatic femoral head
necrosis in adults. Orthopade. 47:757–769. 2018.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
33
|
Agarwala S and Vijayvargiya M:
Bisphosphonate combination therapy for non-femoral avascular
necrosis. J Orthop Surg Res. 14(112)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mardones R, Camacho D, Monsalvo F, Zulch
N, Jofre C and Minguell JJ: Treatment of osteonecrosis of the
femoral head by core decompression and implantation of fully
functional ex vivo-expanded bone marrow-derived mesenchymal stem
cells: A proof-of-concept study. Stem Cells Cloning. 12:11–16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Beckmann J, Schmidt T, Schaumburger J,
Rath B, Lüring C, Tingart M and Grifka J: Infusion, core
decompression, or infusion following core decompression in the
treatment of bone edema syndrome and early avascular osteonecrosis
of the femoral head. Rheumatol Int. 33:1561–1565. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Osmani F, Thakkar S and Vigdorchik J: The
utility of conservative treatment modalities in the management of
osteonecrosis. Bull Hosp Jt Dis (2013). 75:186–192. 2017.PubMed/NCBI
|
|
37
|
Song Q, Ni J, Jiang H and Shi Z:
Sildenafil improves blood perfusion in steroid-induced avascular
necrosis of femoral head in rabbits via a protein kinase
G-dependent mechanism. Acta Orthop Traumatol Turc. 51:398–403.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pritchett JW: Statin therapy decreases the
risk of osteonecrosis in patients receiving steroids. Clin Orthop
Relat Res. 173–178. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jiang Y, Zhang Y, Zhang H, Zhu B, Li P, Lu
C, Xu Y, Chen W and Lin N: Pravastatin prevents steroid-induced
osteonecrosis in rats by suppressing PPARγ expression and
activating Wnt signaling pathway. Exp Biol Med (Maywood).
239:347–355. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pengde K, Fuxing P, Bin S, Jing Y and
Jingqiu C: Lovastatin inhibits adipogenesis and prevents
osteonecrosis in steroid-treated rabbits. Joint Bone Spine.
75:696–701. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ye J, Wei D, Peng L and Chang T:
Ginsenoside Rb1 prevents steroid-induced avascular necrosis of the
femoral head through the bone morphogenetic protein-2 and vascular
endothelial growth factor pathway. Mol Med Rep. 20:3175–3181.
2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hao Y, Guo H, Xu Z, Qi H, Wang Y, Lu C,
Liu J and Yuan P: Meta-analysis of the potential role of
extracorporeal shockwave therapy in osteonecrosis of the femoral
head. J Orthop Surg Res. 13(166)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xie K, Mao Y, Qu X, Dai K, Jia Q, Zhu Z
and Yan M: High-energy extracorporeal shock wave therapy for
nontraumatic osteonecrosis of the femoral head. J Orthop Surg Res.
13(25)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ludwig J, Lauber S, Lauber HJ, Dreisilker
U, Raedel R and Hotzinger H: High-energy shock wave treatment of
femoral head necrosis in adults. Clin Orthop Relat Res.
137:119–126. 2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Russo S, Sadile F, Esposito R, Mosillo G,
Aitanti E, Busco G and Wang CJ: Italian experience on use of E.S.W.
therapy for avascular necrosis of femoral head. Int J Surg.
24:188–190. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Algarni AD and Al Moallem HM: Clinical and
radiological outcomes of extracorporeal shock wave therapy in
early-stage femoral head osteonecrosis. Adv Orthop.
2018(7410246)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bassett CA, Pawluk RJ and Pilla AA:
Augmentation of bone repair by inductively coupled electromagnetic
fields. Science. 184:575–577. 1974.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Massari L, Benazzo F, Falez F, Perugia D,
Pietrogrande L, Setti S, Osti R, Vaienti E, Ruosi C and Cadossi R:
Biophysical stimulation of bone and cartilage: State of the art and
future perspectives. Int Orthop. 43:539–551. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Al-Jabri T, Tan JYQ, Tong GY, Shenoy R,
Kayani B, Parratt T and Khan T: The role of electrical stimulation
in the management of avascular necrosis of the femoral head in
adults: a systematic review. BMC Musculoskelet Disord.
18(319)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Seber S, Omeroğlu H, Cetinkanat H and Köse
N: The efficacy of pulsed electromagnetic fields used alone in the
treatment of femoral head osteonecrosis: A report of two cases.
Acta Orthop Traumatol Turc. 37:410–413. 2003.PubMed/NCBI(In Turkish).
|
|
51
|
Massari L, Fini M, Cadossi R, Setti S and
Traina GC: Biophysical stimulation with pulsed electromagnetic
fields in osteonecrosis of the femoral head. J Bone Joint Surg Am.
88 (Suppl 3):S56–S60. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Paderno E, Zanon V, Vezzani G, Giacon TA,
Bernasek TL, Camporesi EM and Bosco G: Evidence-supported HBO
therapy in femoral head necrosis: A systematic review and
meta-analysis. Int J Environ Res Public Health.
18(2888)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li W, Ye Z, Wang W, Wang K, Li L and Zhao
D: Clinical effect of hyperbaric oxygen therapy in the treatment of
femoral head necrosis: A systematic review and meta-analysis.
Orthopade. 46:440–446. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chandrinou A, Korompeli A, Grammatopoulou
E, Gaitanou K, Tsoumakas K and Fildissis G: Avascular necrosis of
the femoral head: Evaluation of hyperbaric oxygen therapy and
quality of life. Undersea Hyperb Med. 47:561–569. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li H, Bai X and Pan S: Repetitive 1.6 ATA
hyperbaric oxygen therapy for bilateral ARCO stage II
steroid-associated osteonecrosis of the femoral head. Undersea
Hyperb Med. 47:625–633. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Moghamis I, Alhammoud AA, Kokash O and
Alhaneedi GA: The outcome of hyperbaric oxygen therapy versus core
decompression in the non-traumatic avascular necrosis of the
femoral head: Retrospective cohort study. Ann Med Surg (Lond).
62:450–454. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Camporesi EM and Bosco G: Mechanisms of
action of hyperbaric oxygen therapy. Undersea Hyperb Med.
41:247–252. 2014.PubMed/NCBI
|
|
58
|
Bennett M: Hyperbaric oxygen therapy
improved both pain scores and range of motion in patients with
early idiopathic femoral head necrosis (Ficat stage II). Diving
Hyperb Med. 41(105)2011.PubMed/NCBI
|
|
59
|
Camporesi E, Vezzani G, Zanon V, Manelli
D, Enten G, Quartesan S and Bosco G: Review on hyperbaric oxygen
treatment in femoral head necrosis. Undersea Hyperb Med.
44:497–508. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Vann RD, Butler FK, Mitchell SJ and Moon
RE: Decompression illness. Lancet. 377:153–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Thom SR: Hyperbaric oxygen: Its mechanisms
and efficacy. Plast Reconstr Surg. 127 (Suppl 1):131S–141S.
2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Al Hadi H, Smerdon GR and Fox SW:
Hyperbaric oxygen therapy accelerates osteoblast differentiation
and promotes bone formation. J Dent. 43:382–388. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Al Hadi H, Smerdon GR and Fox SW:
Hyperbaric oxygen therapy suppresses osteoclast formation and bone
resorption. J Orthop Res. 31:1839–1844. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Vezzani G, Iezzi M, Rizzato A, Quartesan
S, Mangar D, Camporesi E, Paganini M and Bosco G: Effects of
hyperbaric oxygen exposure on mobilization of endothelial
progenitor cells in healthy volunteers. Acta Med Mediterr.
33:801–805. 2017.
|
|
65
|
Bosco G, Vezzani G, Mrakic Sposta S,
Rizzato A, Enten G, Abou-Samra A, Malacrida S, Quartesan S, Vezzoli
A and Camporesi E: Hyperbaric oxygen therapy ameliorates
osteonecrosis in patients by modulating inflammation and oxidative
stress. J Enzyme Inhib Med Chem. 33:1501–1505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Banerjee S, Issa K, Pivec R, Kapadia BH,
Khanuja HS and Mont MA: Osteonecrosis of the hip: Treatment options
and outcomes. Orthop Clin North Am. 44:463–476. 2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Pakos EE, Megas P, Paschos NK, Syggelos
SA, Kouzelis A, Georgiadis G and Xenakis TA: Modified porous
tantalum rod technique for the treatment of femoral head
osteonecrosis. World J Orthop. 6:829–837. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rajagopal M, Balch Samora J and Ellis TJ:
Efficacy of core decompression as treatment for osteonecrosis of
the hip: A systematic review. Hip Int. 22:489–493. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Leder K and Knahr K: Results of medullary
space decompression in the early stage of so-called idiopathic
femur head necrosis. Z Orthop Ihre Grenzgeb. 131:113–119.
1993.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
70
|
Specchiulli F: Core decompression in the
treatment of necrosis of the femoral head. Long-term results. Chir
Organi Mov. 85:395–402. 2000.PubMed/NCBI(In English, Italian).
|
|
71
|
Etemadifar M, Kooskzari M, Khalilollah N,
Ali MK and Mahsa B: The results of core decompression treatment in
patients with avascular necrosis of femoral head in patients at
Isfahan City educational hospitals in 2010-2011. Adv Biomed Res.
3(93)2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Simank HG, Brocai DR, Strauch K and
Lukoschek M: Core decompression in osteonecrosis of the femoral
head: Risk-factor-dependent outcome evaluation using survivorship
analysis. Int Orthop. 23:154–159. 1999.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Miyahara HS, Rosa BB, Hirata FY, Gurgel
HMC, Ejnisman L and Vicente JRN: What is the role of core
decompression in the early stages of osteonecrosis of the femoral
head? Evaluation of the surgical result by functional score and
radiological follow-up. Rev Bras Ortop. 53:537–542. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu Z, Yang X, Li Y, Zeng WN, Zhao E and
Zhou Z: Multiple drilling is not effective in reducing the rate of
conversion to total hip Arthroplasty in early-stage nontraumatic
osteonecrosis of the femoral head: A case-control comparative study
with a natural course. BMC Musculoskelet Disord.
22(535)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Moon JK, Yoon JY, Kim CH, Lee SH,
Kekatpure AL, Lee JS and Yoon PW: Multiple drilling and multiple
matchstick-like bone allografts for large osteonecrotic lesions in
the femoral head: An average 3-year follow-up study. Arch Orthop
Trauma Surg. 140:1655–1663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Sadile F, Bernasconi A, Russo S and
Maffulli N: Core decompression versus other joint preserving
treatments for osteonecrosis of the femoral head: A meta-analysis.
Br Med Bull. 118:33–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Tabatabaee RM, Saberi S, Parvizi J,
Mortazavi SM and Farzan M: Combining concentrated autologous bone
marrow stem cells injection with core decompression improves
outcome for patients with early-stage osteonecrosis of the femoral
head: A comparative study. J Arthroplasty. 30 (9 Suppl):S11–S15.
2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bozkurt I, Yalcin N, Uluyardimci E and
Akgul EA: Combination of hyperbaric oxygen and core decompression
therapies improve outcomes in the treatment of hip osteonecrosis.
Hip Int: April 12, 2021 (Epub ahead of print).
|
|
79
|
Katiella KA, Yanru Z and Hui Z: Magnesium
alloy transfected BMSCs-BMP-2 composite in repair of femoral head
necrosis with assessment of visceral organs. Springerplus.
5(1857)2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Eppley BL, Woodell JE and Higgins J:
Platelet quantification and growth factor analysis from
platelet-rich plasma: Implications for wound healing. Plast
Reconstr Surg. 114:1502–1508. 2004.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Karakaplan M, Gülabi D, Topgül H and
Elmalı N: Does platelet-rich plasma have a favorable effect in the
early stages of steroid-associated femoral head osteonecrosis in a
rabbit model? Eklem Hastalik Cerrahisi. 28:107–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang XL, Wang YM, Chu K, Wang ZH, Liu YH,
Jiang LH, Chen X, Zhou ZY and Yin G: The application of PRP
combined with TCP in repairing avascular necrosis of the femoral
head after femoral neck fracture in rabbit. Eur Rev Med Pharmacol
Sci. 22:903–909. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Xian H, Luo D, Wang L, Cheng W, Zhai W,
Lian K and Lin D: Platelet-rich plasma-incorporated autologous
granular bone grafts improve outcomes of post-traumatic
osteonecrosis of the femoral head. J Arthroplasty. 35:325–330.
2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Houdek MT, Wyles CC, Collins MS, Howe BM,
Terzic A, Behfar A and Sierra RJ: Stem cells combined with
platelet-rich plasma effectively treat corticosteroid-induced
osteonecrosis of the hip: A prospective study. Clin Orthop Relat
Res. 476:388–397. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
D'Ambrosi R, Biancardi E, Massari G,
Ragone V and Facchini RM: Survival analysis after core
decompression in association with platelet-rich plasma, mesenchymal
stem cells, and synthetic bone graft in patients with osteonecrosis
of the femoral head. Joints. 6:16–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Han J, Gao F, Li Y, Ma J, Sun W, Shi L, Wu
X and Li T: The use of platelet-rich plasma for the treatment of
osteonecrosis of the femoral head: A systematic review. Biomed Res
Int. 2020(2642439)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Huang Z, Wang Q, Zhang T, Fu Y and Wang W:
Hyper-activated platelet lysates prevent glucocorticoid-associated
femoral head necrosis by regulating autophagy. Biomed Pharmacother.
139(111711)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Aggarwal AK, Poornalingam K, Jain A and
Prakash M: Combining platelet-rich plasma instillation with core
decompression improves functional outcome and delays progression in
early-stage avascular necrosis of femoral head: A 4.5- to 6-year
prospective randomized comparative study. J Arthroplasty. 36:54–61.
2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Huang Z, Zhao Z, Lang J and Wang W, Fu Y
and Wang W: Therapeutic study of thermosensitive hydrogel loaded
with super-activated platelet lysate combined with core
decompression technology for the treatment of femoral head
necrosis. Stem Cells Int. 2021(7951616)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Xu Y, Jiang Y, Xia C, Wang Y, Zhao Z and
Li T: Stem cell therapy for osteonecrosis of femoral head:
Opportunities and challenges. Regen Ther. 15:295–304.
2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Maruyama M, Nabeshima A, Pan CC, Behn AW,
Thio T, Lin T, Pajarinen J, Kawai T, Takagi M, Goodman SB and Yang
YP: The effects of a functionally-graded scaffold and bone
marrow-derived mononuclear cells on steroid-induced femoral head
osteonecrosis. Biomaterials. 187:39–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Tomaru Y, Yoshioka T, Sugaya H, Kumagai H,
Hyodo K, Aoto K, Wada H, Akaogi H, Yamazaki M and Mishima H:
Ten-year results of concentrated autologous bone marrow aspirate
transplantation for osteonecrosis of the femoral head: A
retrospective study. BMC Musculoskelet Disord.
20(410)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hernigou P and Beaujean F: Treatment of
osteonecrosis with autologous bone marrow grafting. Clin Orthop
Relat Res. 14–23. 2002.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB,
Guo WS, Xu SQ, Lou JN and Li ZR: Treatment of nontraumatic
osteonecrosis of the femoral head with the implantation of core
decompression and concentrated autologous bone marrow containing
mononuclear cells. Arch Orthop Trauma Surg. 130:859–865.
2010.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Rastogi S, Sankineani SR, Nag HL, Mohanty
S, Shivanand G, Marimuthu K, Kumar R and Rijal L: Intralesional
autologous mesenchymal stem cells in management of osteonecrosis of
femur: A preliminary study. Musculoskelet Surg. 97:223–228.
2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Khan M, Abbas K, Ayling EA, Waqas Ilyas M
and Dunlop DG: Autologous stem cell implantation with core
decompression for avascular necrosis of the femoral head using a
new device. Ann R Coll Surg Engl. 103:508–513. 2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Mao Q, Jin H, Liao F, Xiao L, Chen D and
Tong P: The efficacy of targeted intraarterial delivery of
concentrated autologous bone marrow containing mononuclear cells in
the treatment of osteonecrosis of the femoral head: A five year
follow-up study. Bone. 57:509–516. 2013.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Yoshioka T, Mishima H, Akaogi H, Sakai S,
Li M and Ochiai N: Concentrated autologous bone marrow aspirate
transplantation treatment for corticosteroid-induced osteonecrosis
of the femoral head in systemic lupus erythematosus. Int Orthop.
35:823–829. 2011.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Daltro GC, Fortuna V, de Souza ES, Salles
MM, Carreira AC, Meyer R, Freire SM and Borojevic R: Efficacy of
autologous stem cell-based therapy for osteonecrosis of the femoral
head in sickle cell disease: A five-year follow-up study. Stem Cell
Res Ther. 6(110)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Tomaru Y, Yoshioka T, Sugaya H, Aoto K,
Wada H, Akaogi H, Yamazaki M and Mishima H: Hip preserving surgery
with concentrated autologous bone marrow aspirate transplantation
for the treatment of asymptomatic osteonecrosis of the femoral
head: Retrospective review of clinical and radiological outcomes at
6 years postoperatively. BMC Musculoskelet Disord.
18(292)2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Nandeesh NH, Janardhan K, Subramanian V,
Ashtekar AB, Srikruthi N, Koka PS and Deb K: Treatment of AVN using
autologous BM stem cells and activated platelet-derived growth
factor concentrates. J Stem Cells. 11:135–148. 2016.PubMed/NCBI
|
|
102
|
Emadedin M, Karimi S, Karimi A, Labibzadeh
N, Niknejadi M, Baharvand H and Aghdami N: Autologous bone
marrow-derived CD133 cells with core decompression as a novel
treatment method for femoral head osteonecrosis: A pilot study.
Cytotherapy. 21:107–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Pak J: Autologous adipose tissue-derived
stem cells induce persistent bone-like tissue in osteonecrotic
femoral heads. Pain Physician. 15:75–85. 2012.PubMed/NCBI
|
|
104
|
Wu CT, Yen SH, Lin PC and Wang JW:
Long-term outcomes of phemister bone grafting for patients with
non-traumatic osteonecrosis of the femoral head. Int Orthop.
43:579–587. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Osawa Y, Seki T, Okura T, Takegami Y,
Ishiguro N and Hasegawa Y: Long-term outcomes of curved
intertrochanteric varus osteotomy combined with bone impaction
grafting for non-traumatic osteonecrosis of the femoral head. Bone
Joint J. 103-B:665–671. 2021.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Guo X, Dou B, Zhou Y and Li Y: Surgical
treatment of necrosis of the femoral head in early stages with core
depression and allo-fibular grafting. Zhongguo Xiu Fu Chong Jian
Wai Ke Za Zhi. 19:697–699. 2005.PubMed/NCBI(In Chinese).
|
|
107
|
Yue J, Gao H, Guo X, Wang R, Li B, Sun Q,
Liu W, Chen J and Li Y: Fibula allograft propping as an effective
treatment for early-stage osteonecrosis of the femoral head: A
systematic review. J Orthop Surg Res. 15(206)2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Alam MT, Alam QS, Alam MK, Islam MA, Saha
MK, Rahman MM, Hossain MZ, Roy MK, Islam MK, Hossain M, et al: Core
decompression with non vascularized fibular graft as modern
surgical treatment of early hip avascular necrosis. Mymensingh Med
J. 30:323–328. 2021.PubMed/NCBI
|
|
109
|
Bednarek A, Atras A, Gągała J and Kozak Ł:
Operative technique and results of core decompression and filling
with bone grafts in the treatment of osteonecrosis of femoral head.
Ortop Traumatol Rehabil. 12:511–518. 2010.PubMed/NCBI
|
|
110
|
Vahid Farahmandi M, Abbasian M, Safdari F
and Emami Moghaddam Tehrani M: Midterm results of treating femoral
head osteonecrosis with autogenous corticocancellous bone grafting.
Trauma Mon. 19(e17092)2014.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Bakx PA, van Biezen FC and van Linge B:
Failure of tibial bone grafting for femoral head necrosis. Acta
Orthop Scand. 62:230–231. 1991.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhu SX and Zhou MW: Iliac periosteal graft
with vascular pedicle in the treatment of avascular necrosis of the
femoral head. Experimental study and clinical application. Chin Med
J (Engl). 105:849–855. 1992.PubMed/NCBI
|
|
113
|
Ligh CA, Nelson JA, Fischer JP, Kovach SJ
and Levin LS: The effectiveness of free vascularized fibular flaps
in osteonecrosis of the femoral head and neck: A systematic review.
J Reconstr Microsurg. 33:163–172. 2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Zhang NF, Li ZR, Zhang XZ and Wang W:
Vascularized iliac bone grafting for avascular necrosis of the
femoral head. Zhonghua Wai Ke Za Zhi. 41:125–129. 2003.PubMed/NCBI(In Chinese).
|
|
115
|
Lau HW, Wong KC, Ho K, Chung KY, Chiu WK
and Kumta SM: Long-term outcome of vascularized iliac bone grafting
for osteonecrosis of femoral head: A retrospective study with
17-year follow-up. J Orthop Surg (Hong Kong).
29(2309499021996842)2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Scully SP, Aaron RK and Urbaniak JR:
Survival analysis of hips treated with core decompression or
vascularized fibular grafting because of avascular necrosis. J Bone
Joint Surg Am. 80:1270–1275. 1998.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Heinrich JT and McBeath AA: The gluteus
minimus muscle pedicle graft in the treatment of femoral head
avascular necrosis. Am J Orthop (Belle Mead NJ). 24:615–623.
1995.PubMed/NCBI
|
|
118
|
Chahla J, LaPrade RF, Mardones R, Huard J,
Philippon MJ, Nho S, Mei-Dan O and Pascual-Garrido C: Biological
therapies for cartilage lesions in the hip: A new horizon.
Orthopedics. 39:e715–e723. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Seow D, Shimozono Y, Gianakos AL,
Chiarello E, Mercer N, Hurley ET and Kennedy JG: Autologous
osteochondral transplantation for osteochondral lesions of the
talus: High rate of return to play in the athletic population. Knee
Surg Sports Traumatol Arthrosc. 29:1554–1561. 2021.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Carey JL, Shea KG, Lindahl A, Vasiliadis
HS, Lindahl C and Peterson L: Autologous chondrocyte implantation
as treatment for unsalvageable osteochondritis dissecans: 10- to
25-year follow-up. Am J Sports Med. 48:1134–1140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Gagala J, Tarczyńska M and Gawęda K:
Clinical and radiological outcomes of treatment of avascular
necrosis of the femoral head using autologous osteochondral
transfer (mosaicplasty): Preliminary report. Int Orthop.
37:1239–1244. 2013.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Tsao AK, Roberson JR, Christie MJ, Dore
DD, Heck DA, Robertson DD and Poggie RA: Biomechanical and clinical
evaluations of a porous tantalum implant for the treatment of
early-stage osteonecrosis. J Bone Joint Surg Am. 87 (Suppl
2):S22–S27. 2005.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Shuler MS, Rooks MD and Roberson JR:
Porous tantalum implant in early osteonecrosis of the hip:
Preliminary report on operative, survival, and outcomes results. J
Arthroplasty. 22:26–31. 2007.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Nadeau M, Séguin C, Theodoropoulos JS and
Harvey EJ: Short term clinical outcome of a porous tantalum implant
for the treatment of advanced osteonecrosis of the femoral head.
Mcgill J Med. 10:4–10. 2007.PubMed/NCBI
|
|
125
|
Liu Y, Yan L, Zhou S, Su X, Cao Y, Wang C
and Liu S: Tantalum rod implantation for femoral head
osteonecrosis: Survivorship analysis and determination of
prognostic factors for total hip arthroplasty. Int Orthop.
40:1397–1407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Liu G, Wang J, Yang S, Xu W, Ye S and Xia
T: Effect of a porous tantalum rod on early and intermediate stages
of necrosis of the femoral head. Biomed Mater.
5(065003)2010.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Zhu T, Jiang M, Zhang M, Cui L, Yang X,
Wang X, Liu G, Ding J and Chen X: Biofunctionalized composite
scaffold to potentiate osteoconduction, angiogenesis, and favorable
metabolic microenvironment for osteonecrosis therapy. Bioact Mater.
9:446–460. 2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Benedini L, Laiuppa J, Santillán G,
Baldini M and Messina P: Antibacterial alginate/nano-hydroxyapatite
composites for bone tissue engineering: Assessment of their
bioactivity, biocompatibility, and antibacterial activity. Mater
Sci Eng C Mater Biol Appl. 115(111101)2020.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Zhou H, Boys AJ, Harrod JB, Bonassar LJ
and Estroff LA: Mineral distribution spatially patterns bone marrow
stromal cell behavior on monolithic bone scaffolds. Acta Biomater.
112:274–285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Zhao D, Zhu T, Li J, Cui L, Zhang Z,
Zhuang X and Ding J: Poly(lactic-co-glycolic acid)-based composite
bone-substitute materials. Bioact Mater. 6:346–360. 2020.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Dong H, Zhu T and Zhang M, Wang D, Wang X,
Huang G, Wang S and Zhang M: Polymer scaffolds-enhanced bone
regeneration in osteonecrosis therapy. Front Bioeng Biotechnol.
9(761302)2021.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Lai Y, Li Y, Cao H, Long J, Wang X, Li L,
Li C, Jia Q, Teng B, Tang T, et al: Osteogenic magnesium
incorporated into PLGA/TCP porous scaffold by 3D printing for
repairing challenging bone defect. Biomaterials. 197:207–219.
2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Wagner H, Baur W and Wagner M:
Joint-preserving osteotomy in segmental femur head necrosis.
Orthopade. 19:208–218. 1990.PubMed/NCBI(In German).
|
|
134
|
Lee YK, Park CH, Ha YC, Kim DY, Lyu SH and
Koo KH: Comparison of surgical parameters and results between
curved varus osteotomy and rotational osteotomy for osteonecrosis
of the femoral head. Clin Orthop Surg. 9:160–168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Portigliatti Barbos M, Balbo C and Rossi
P: Middle and long-term results of flexion osteotomy for avascular
necrosis of the femoral head. Ital J Orthop Traumatol. 18:53–61.
1992.PubMed/NCBI
|
|
136
|
Xu YX, Ren YZ, Zhao ZP, Wang YZ, Wang T
and Li T: Hip survival rate in the patients with avascular necrosis
of femoral head after transtrochanteric rotational osteotomy: A
systematic review and meta-analysis. Chin Med J (Engl).
132:2960–2971. 2019.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Mont MA, Fairbank AC, Krackow KA and
Hungerford DS: Corrective osteotomy for osteonecrosis of the
femoral head. J Bone Joint Surg Am. 78:1032–1038. 1996.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Inao S, Ando M, Gotoh E and Matsuno T:
Minimum 10-year results of Sugioka's osteotomy for femoral head
osteonecrosis. Clin Orthop Relat Res. 141–148. 1999.PubMed/NCBI
|
|
139
|
Masui T and Hasegawa Y: Curved
intertrochanteric varus osteotomy. Clin Calcium. 17:931–937.
2007.PubMed/NCBI(In Japanese).
|
|
140
|
Motomura G, Yamamoto T, Suenaga K,
Nakashima Y, Mawatari T, Ikemura S and Iwamoto Y: Long-term outcome
of transtrochanteric anterior rotational osteotomy for
osteonecrosis of the femoral head in patients with systemic lupus
erythematosus. Lupus. 19:860–865. 2010.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Schneider W, Aigner N and Knahr K:
Intertrochanteric rotational osteotomies in idiopathic femur head
necrosis-comparison of different procedures. Z Orthop Ihre
Grenzgeb. 136:147–153. 1998.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
142
|
Morita D, Hasegawa Y, Okura T, Osawa Y and
Ishiguro N: Long-term outcomes of transtrochanteric rotational
osteotomy for non-traumatic osteonecrosis of the femoral head. Bone
Joint J. 99-B:175–183. 2017.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Kuroda Y, Okuzu Y, Kawai T, Goto K and
Matsuda S: Difference in therapeutic strategies for
joint-preserving surgery for non-traumatic osteonecrosis of the
femoral head between the United States and Japan: A review of the
literature. Orthop Surg. 13:742–748. 2021.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Kirschenbaum IH, Vernace JV, Booth RE Jr,
Balderston RA and Rothman RH: Total hip arthroplasty for
osteonecrosis. Semin Arthroplasty. 2:234–240. 1991.PubMed/NCBI
|
|
145
|
Ritter MA, Helphinstine J, Keating EM,
Faris PM and Meding JB: Total hip arthroplasty in patients with
osteonecrosis. The effect of cement techniques. Clin Orthop Relat
Res. 94–99. 1997.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Dong W, Yu N and Bai B: Total hip
replacement in patients with steroid-induced femoral head necrosis.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 11:161–163.
1997.PubMed/NCBI(In Chinese).
|
|
147
|
Yuan B, Taunton MJ and Trousdale RT: Total
hip arthroplasty for alcoholic osteonecrosis of the femoral head.
Orthopedics. 32(400)2009.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Al-Mousawi F, Malki A, Al-Aradi A,
Al-Bagali M, Al-Sadadi A and Booz MM: Total hip replacement in
sickle cell disease. Int Orthop. 26:157–161. 2002.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Holzapfel BM, Rak D, Kreuzer S, Arnholdt
J, Thaler M and Rudert M: Short stem hip arthroplasty via the
minimally invasive direct anterior approach. Oper Orthop Traumatol.
33:288–303. 2021.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Anthony CA, Wasko MK, Pashos GE, Barrack
RL, Nunley RM and Clohisy JC: Total hip arthroplasty in patients
with osteoarthritis associated with legg-calve-perthes disease:
Perioperative complications and patient-reported outcomes. J
Arthroplasty. 36:2518–2522. 2021.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Moschetti WE, Kunkel S, Keeney BJ and
Jevsevar D: Do patients with higher preoperative functional outcome
scores preferentially seek direct anterior approach total hip
arthroplasty? Arthroplast Today. 10:6–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Bose VC, Kalaivanan K, Manohar M, Kumar A,
Patil S and Suryanarayan P: Is the revision rate higher after hip
arthroplasty in teenage patients? A prospective study with
long-term follow-up of more than 10 years. Indian J Orthop.
55:993–1002. 2021.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Agrawal Y, Kerry RM, Stockley I and Hamer
AJ: Review of total hip arthroplasty in patients younger than 30
years: Mid- to long-term results. Hip Int. 31:533–541.
2021.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Mont MA, Rajadhyaksha AD and Hungerford
DS: Outcomes of limited femoral resurfacing arthroplasty compared
with total hip arthroplasty for osteonecrosis of the femoral head.
J Arthroplasty. 16 (8 Suppl 1):S134–S139. 2001.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Dong T, Sun J, Qu Y and Wang Y:
Resurfacing arthroplasty for treatment of avascular necrosis of
femoral head in young and middle-aged patients. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 19:707–709. 2005.PubMed/NCBI(In Chinese).
|
|
156
|
Beaulé PE, Schmalzried TP, Campbell P,
Dorey F and Amstutz HC: Duration of symptoms and outcome of
hemiresurfacing for hip osteonecrosis. Clin Orthop Relat Res.
104–117. 2001.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Calder PR, Hynes MC and Scott G:
Short-term results of hemi-resurfacing for osteonecrosis of the
femoral head. Hip Int. 14:174–181. 2004.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Hsieh PH, Tai CL, Liaw JW and Chang YH:
Thermal damage potential during hip resurfacing in osteonecrosis of
the femoral head: An experimental study. J Orthop Res.
26:1206–1209. 2008.PubMed/NCBI View Article : Google Scholar
|