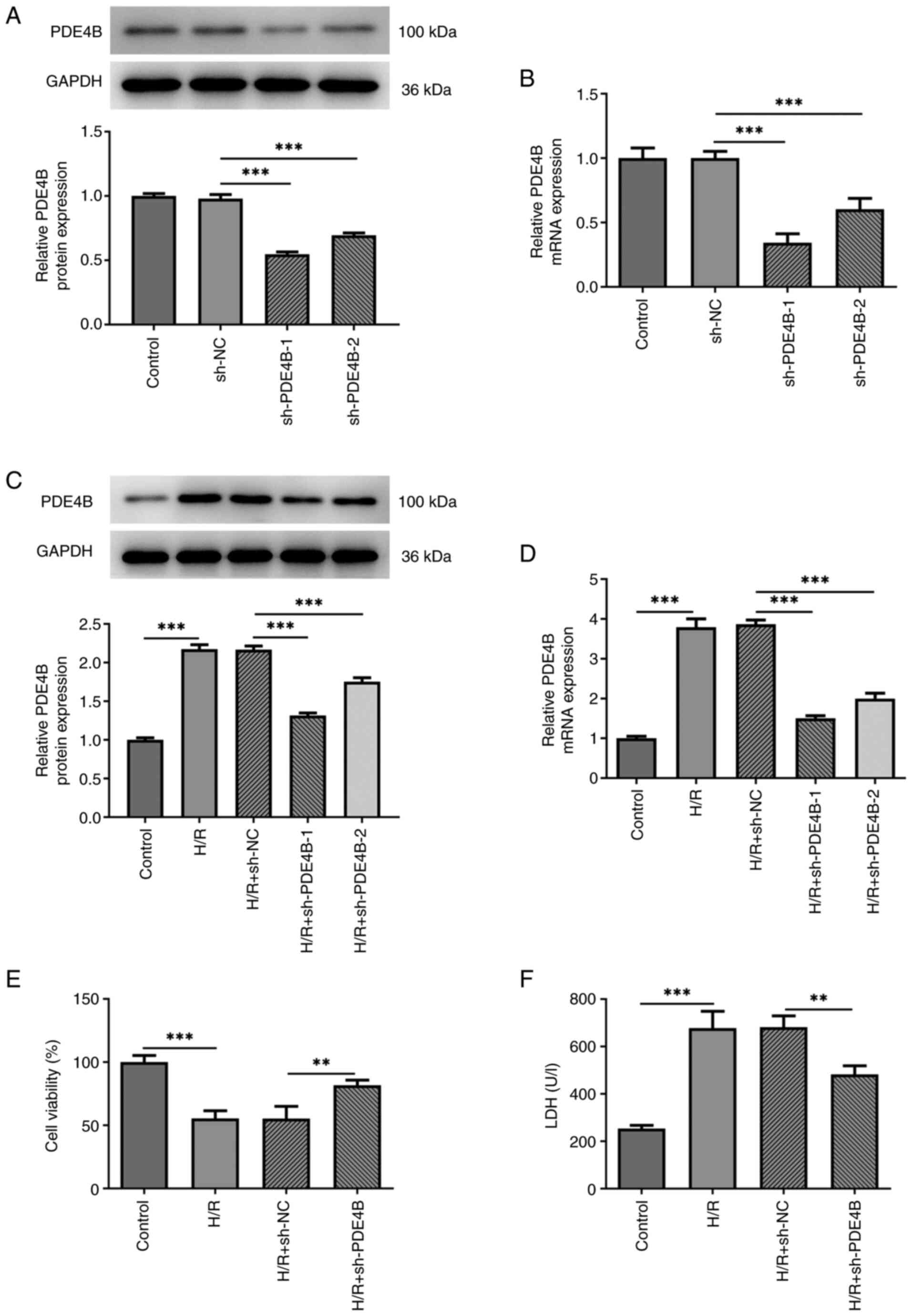
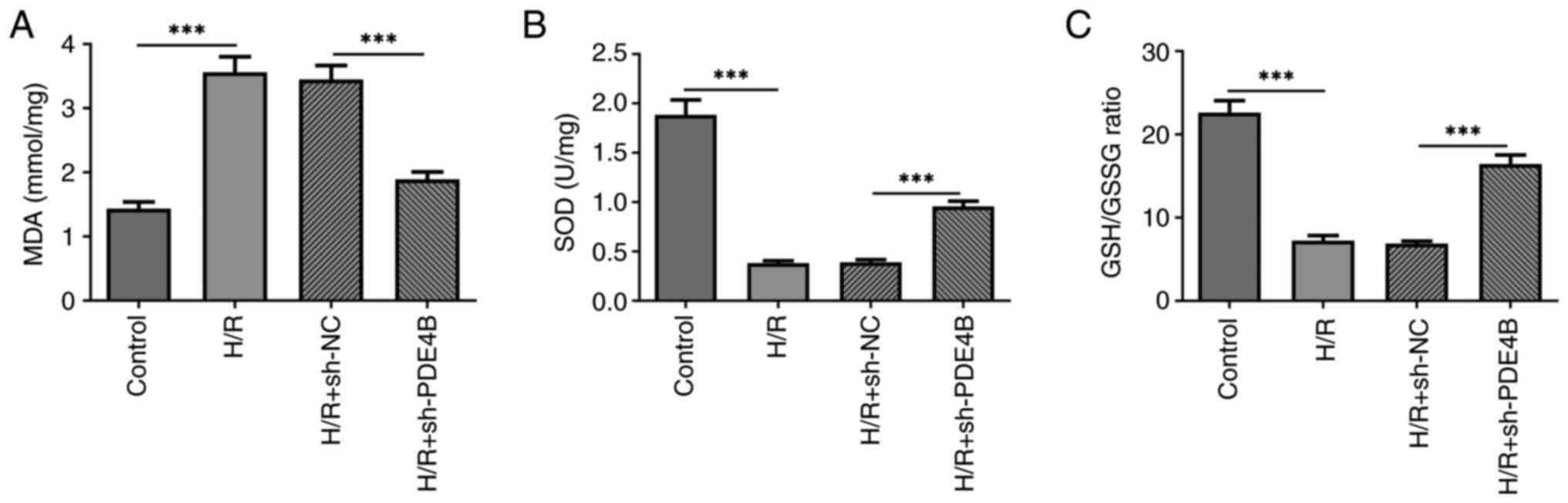
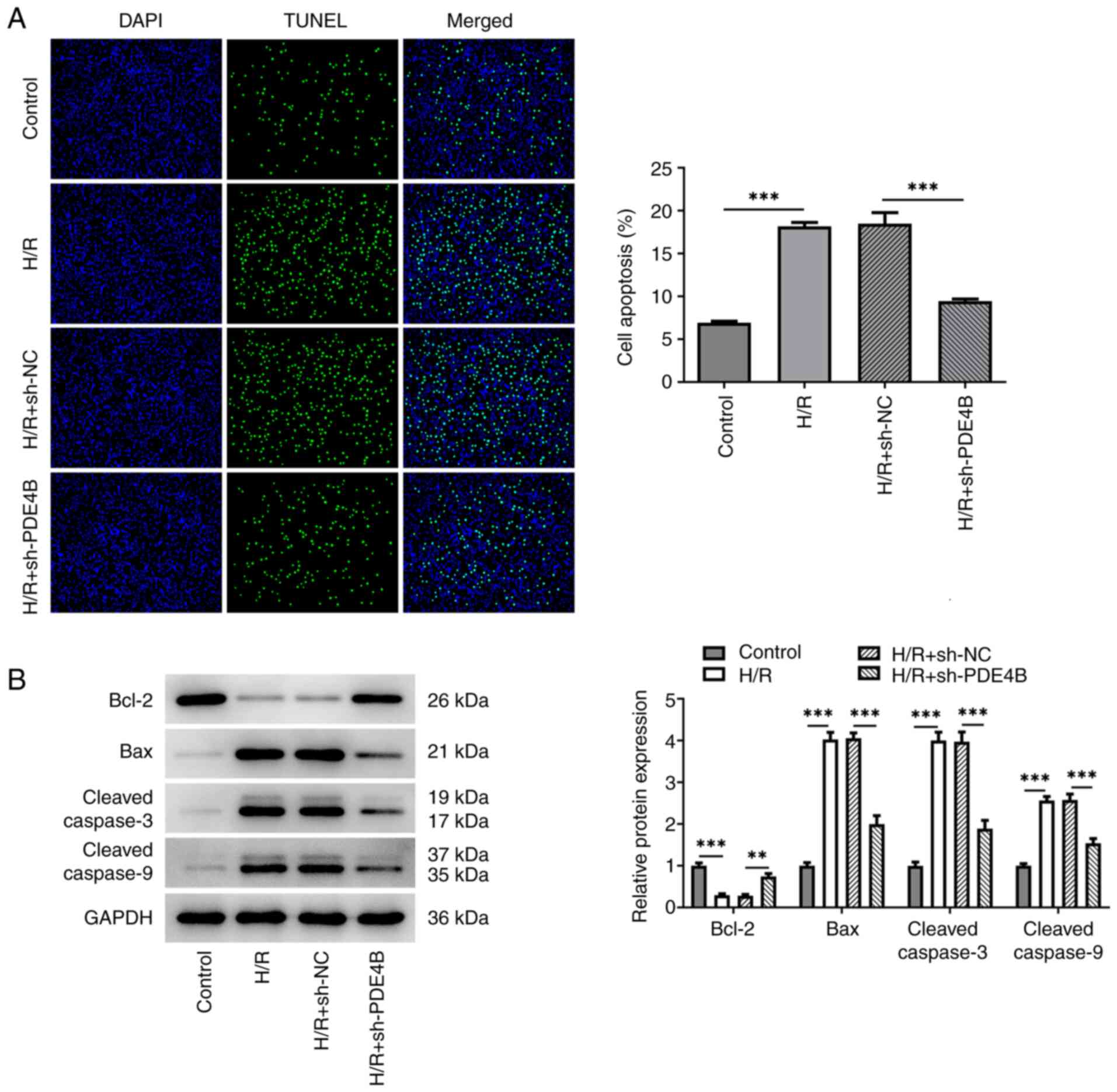
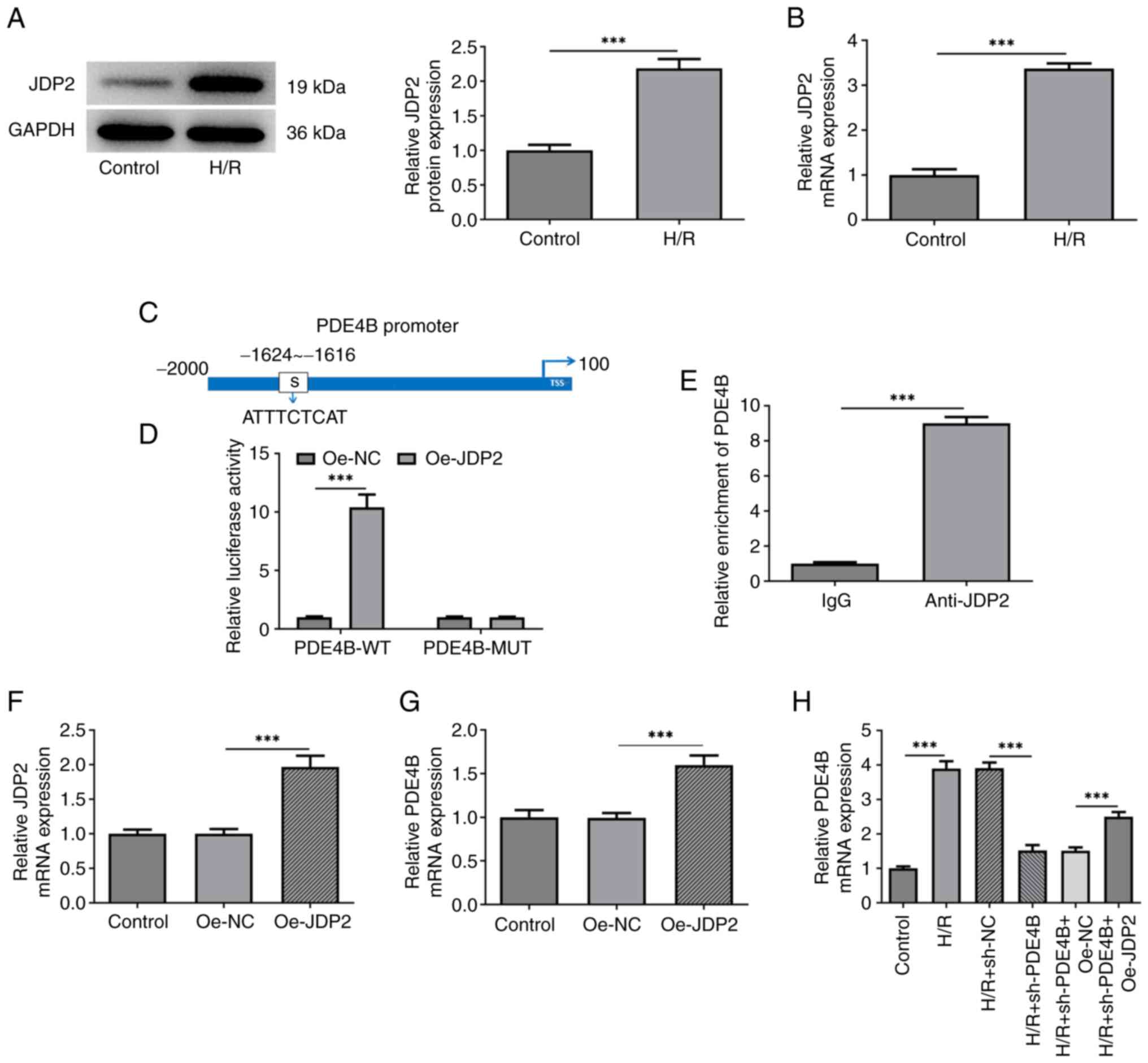
|
1
|
Pollard TJ: The acute myocardial
infarction. Prim Care. 27:631–649;vi. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Beltrame JF: Nitrate therapy in acute
myocardial infarction: Potion or poison? Cardiovasc Drugs Ther.
22:165–168. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jensen AE, Reikvam A and Asberg A:
Diagnostic efficiency of lactate dehydrogenase isoenzymes in serum
after acute myocardial infarction. Scand J Clin Lab Invest.
50:285–289. 1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hurst JW: Thoughts about the abnormalities
in the electrocardiogram of patients with acute myocardial
infarction with emphasis on a more accurate method of interpreting
ST-segment displacement: Part I. Clin Cardiol. 30:381–390.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sattar Y and Alraies MC: Ventricular
aneurysm. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2021.
|
|
6
|
Lindower P, Embrey R and Vandenberg B:
Echocardiographic diagnosis of mechanical complications in acute
myocardial infarction. Clin Intensive Care. 4:276–283.
1993.PubMed/NCBI
|
|
7
|
Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi
FA, Spertus JA, Krumholz HM and Jiang L: China PEACE Collaborative
Group. ST-segment elevation myocardial infarction in China from
2001 to 2011 (the China PEACE-retrospective acute myocardial
infarction study): A retrospective analysis of hospital data.
Lancet. 385:441–451. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017(7018393)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bagai A, Dangas GD, Stone GW and Granger
CB: Reperfusion strategies in acute coronary syndromes. Circ Res.
114:1918–1928. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Borer JS and Lewis BS: Therapeutic
coronary reperfusion and reperfusion injury: An introduction.
Cardiology. 135(13)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou H and Toan S: Pathological roles of
mitochondrial oxidative stress and mitochondrial dynamics in
cardiac microvascular ischemia/reperfusion injury. Biomolecules.
10(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tibbo AJ and Baillie GS: Phosphodiesterase
4B: Master regulator of brain signaling. Cells.
9(1254)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xie H, Zha E and Zhang Y: Identification
of featured metabolism-related genes in patients with acute
myocardial infarction. Dis Markers. 2020(8880004)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo H, Cheng Y, Wang C, Wu J, Zou Z, Niu
B, Yu H, Wang H and Xu J: FFPM, a PDE4 inhibitor, reverses learning
and memory deficits in APP/PS1 transgenic mice via cAMP/PKA/CREB
signaling and anti-inflammatory effects. Neuropharmacology.
116:260–269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhong J, Yu H, Huang C, Zhong Q, Chen Y,
Xie J, Zhou Z, Xu J and Wang H: Inhibition of phosphodiesterase 4
by FCPR16 protects SH-SY5Y cells against MPP+-induced
decline of mitochondrial membrane potential and oxidative stress.
Redox Biol. 16:47–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsai MH, Wuputra K, Lin YC, Lin CS and
Yokoyama KK: Multiple functions of the histone chaperone Jun
dimerization protein 2. Gene. 590:193–200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Heger J, Bornbaum J, Würfel A, Hill C,
Brockmann N, Gáspár R, Pálóczi J, Varga ZV, Sárközy M, Bencsik P,
et al: JDP2 overexpression provokes cardiac dysfunction in mice.
Sci Rep. 8(7647)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duan Y, Cheng S, Jia L, Zhang Z and Chen
L: PDRPS7 protects cardiac cells from hypoxia/reoxygenation injury
through inactivation of JNKs. FEBS Open Bio. 10:593–606.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rocha EAV: Fifty years of coronary artery
bypass graft surgery. Braz J Cardiovasc Surg. 32:2–3.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang MX, Liu X, Li JM, Liu L, Lu W and
Chen GC: Inhibition of CACNA1H can alleviate endoplasmic reticulum
stress and reduce myocardial cell apoptosis caused by myocardial
infarction. Eur Rev Med Pharmacol Sci. 24:12887–12895.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen YQ, Yang X, Xu W, Yan Y, Chen XM and
Huang ZQ: Knockdown of lncRNA TTTY15 alleviates myocardial
ischemia-reperfusion injury through the miR-374a-5p/FOXO1 axis.
IUBMB Life. 73:273–285. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Tibaut M, Mekis D and Petrovic D:
Pathophysiology of myocardial infarction and acute management
strategies. Cardiovasc Hematol Agents Med Chem. 14:150–159.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Molecular machinery and interplay of apoptosis and
autophagy in coronary heart disease. J Mol Cell Cardiol. 136:27–41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shen Y, Liu X, Shi J and Wu X: Involvement
of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol
Macromol. 125:496–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee SJ, Lee CK, Kang S, Park I, Kim YH,
Kim SK, Hong SP, Bae H, He Y, Kubota Y and Koh GY: Angiopoietin-2
exacerbates cardiac hypoxia and inflammation after myocardial
infarction. J Clin Invest. 128:5018–5033. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Liu Z, Ma C, Gu J and Yu M: Potential
biomarkers of acute myocardial infarction based on weighted gene
co-expression network analysis. Biomed Eng Online.
18(9)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sheng X, Fan T and Jin X: Identification
of key genes involved in acute myocardial infarction by comparative
transcriptome analysis. Biomed Res Int.
2020(1470867)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pearse DD and Hughes ZA: PDE4B as a
microglia target to reduce neuroinflammation. Glia. 64:1698–1709.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng XY, Chen JC, Xie QM, Chen JQ and
Tang HF: Anti-inflammatory effect of ciclamilast in an allergic
model involving the expression of PDE4B. Mol Med Rep. 19:1728–1738.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pleiman JK, Irving AA, Wang Z, Toraason E,
Clipson L, Dove WF, Deming DA and Newton MA: The conserved
protective cyclic AMP-phosphodiesterase function PDE4B is expressed
in the adenoma and adjacent normal colonic epithelium of mammals
and silenced in colorectal cancer. PLoS Genet.
14(e1007611)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karam S, Margaria JP, Bourcier A, Mika D,
Varin A, Bedioune I, Lindner M, Bouadjel K, Dessillons M, Gaudin F,
et al: Cardiac overexpression of PDE4B blunts β-adrenergic response
and maladaptive remodeling in heart failure. Circulation.
142:161–174. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu B, Qin Y, Li D, Cai N, Wu J, Jiang L,
Jie L, Zhou Z, Xu J and Wang H: Inhibition of PDE4 protects neurons
against oxygen-glucose deprivation-induced endoplasmic reticulum
stress through activation of the Nrf-2/HO-1 pathway. Redox Biol.
28(101342)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
You T, Cheng Y, Zhong J, Bi B, Zeng B,
Zheng W, Wang H and Xu J: Roflupram, a phosphodiesterase 4
inhibitior, suppresses inflammasome activation through autophagy in
microglial cells. ACS Chem Neurosci. 8:2381–2392. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ma H, Shi J, Wang C, Guo L, Gong Y, Li J,
Gong Y, Yun F, Zhao H and Li E: Blockade of PDE4B limits lung
vascular permeability and lung inflammation in LPS-induced acute
lung injury. Biochem Biophys Res Commun. 450:1560–1567.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu XQ, Jin J, Li Z, Jiang L, Dong YH, Cai
YT, Wu MF, Wang JN, Ma TT, Wen JG, et al: Rutaecarpine derivative
Cpd-6c alleviates acute kidney injury by targeting PDE4B, a key
enzyme mediating inflammation in cisplatin nephropathy. Biochem
Pharmacol. 180(114132)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang S and Cao N: Uncovering potential
differentially expressed miRNAs and targeted mRNAs in myocardial
infarction based on integrating analysis. Mol Med Rep.
22:4383–4395. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Huang S, Tao W, Guo Z, Cao J and Huang X:
Suppression of long noncoding RNA TTTY15 attenuates hypoxia-induced
cardiomyocytes injury by targeting miR-455-5p. Gene. 701:1–8.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang XH, Liu Q and Shao ZT: Deletion of
JDP2 improves neurological outcomes of traumatic brain injury (TBI)
in mice: Inactivation of caspase-3. Biochem Biophys Res Commun.
504:805–811. 2018.PubMed/NCBI View Article : Google Scholar
|