1. Introduction
Over 2 years have passed since the World Health
Organization (WHO) declared coronavirus disease 2019 (COVID-19) as
a global public health emergency (1). During this period, significant
efforts have been made to describe, study, and understand the
clinical manifestations of the disease and its repercussions on
physical and mental health (2-7).
During the acute phase of COVID-19, apart from the
typical systemic and pulmonary manifestations, such as fever,
cough, sore throat and dyspnea, neuropsychiatric symptoms related
to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
infection may occur (8-10).
Neuropsychiatric manifestations of acute COVID-19 include
hyposmia/anosmia, consciousness disorders, delirium, agitation,
encephalopathy, encephalitis, acute ischemic stroke,
hypoxic/ischemic brain injury, seizures, vertigo,
numbness/paresthesia, anxiety, depression and insomnia, while
new-onset psychosis has also been reported in the setting of acute
COVID-19 infection (11-20).
Crucially, while the acute phase of COVID-19 has
been well-characterized, the data concerning the long-term outcomes
of the disease are comparatively rather limited (21). There is increasing evidence to
indicate that a number of patients may experience new, recurring or
ongoing symptoms, as well as clinical signs, that persist beyond
the acute illness; a condition that is colloquially referred to as
‘long COVID’ (22-25).
Over the course of the pandemic, several definitions have been
proposed and various terms have been used to describe the long-term
symptoms and consequences following SARS-CoV-2 infection, including
‘post-COVID conditions’, ‘chronic COVID’, ‘long-haul COVID’,
‘long-term COVID-19’, ‘post-acute COVID-19’, ‘post-acute COVID
syndrome’, ‘post COVID syndrome’ and ‘post-acute sequelae of
SARS-COV-2 infection (PASC)’ (21,22,26,27).
However, it is important to note that i) the lack of a standardized
definition for ‘long COVID’; ii) the use of different temporal
criteria for ‘long COVID’ diagnosis (i.e., 3 weeks up to several
months after SARS-CoV-2 infection); and iii) the inclusion and
attribution of diverse symptoms, conditions or signs to ‘long
COVID’, may hinder the accurate classification of patients and may
thus limit the elucidation of ‘long COVID’ syndrome (22,23,26-28).
‘Long COVID’ can become debilitating for some
patients, while it has also been associated with a higher risk of
mortality (23,29). In particular, individuals with
‘long COVID’ may present a wide spectrum of clinical
manifestations, both pulmonary and extrapulmonary ones (including
nervous system and neurocognitive disorders, musculoskeletal pain,
mental health disorders, metabolic disorders, cardiovascular
disorders, gastrointestinal disorders, and anemia), as well as
signs and symptoms related to poor physical wellbeing (including
malaise and fatigue) (30).
Patients affected by ‘long COVID’ include also those
who initially had mild or asymptomatic disease, pointing to
residual effects that involve multiple organ systems, including the
peripheral and central nervous system (CNS) (23,31,32).
Research evidence suggests that the neuroinvasive and neurotrophic
properties of SARS-CoV-2, as well as the rapid overproduction of
cytokines and immune cell hyperactivation (i.e., cytokine storm)
may influence the manifestation of neuropsychiatric symptoms during
and after COVID-19 (33-35).
Furthermore, the indirect effects of COVID-19, such as social
isolation and feelings of loneliness, uncertainty of the prognosis
or incomplete physical health recovery, changes in sleep and
lifestyle behaviors and the economic burden may also affect the
manifestation of neuropsychiatric symptoms (36,37).
The systematic research of neuropsychiatric
manifestations in patients beyond the resolution of acute COVID-19
is thus crucial in order to broaden the current understanding
regarding the sequelae experienced by COVID-19 survivors and may
facilitate the development of targeted evidence-based approaches
towards an integrated patient care.
2. Neuropsychiatric manifestations in
COVID-19 survivors with ‘long COVID’
There is increasing scientific evidence to indicate
that a significant proportion of COVID-19 survivors experience a
range of neuropsychiatric symptoms persisting or even presenting
months after the initial infection (29,32,38).
Of note, Mazza et al (39), in 2020, assessed 402 COVID-19
survivors at 1 month following treatment at the hospital emergency
department, using self-rated psychometric instruments, and found
that overall, 56% scored in the pathological range in at least one
clinical dimension [i.e., including depression, anxiety,
post-traumatic stress disorder (PTSD) and obsessive-compulsive
symptomatology] (39). Moreover,
females, patients with a pre-existing psychiatric diagnosis and
patients who were managed at home exhibited increased levels in the
majority of psychopathological measurements (39). In a later cohort study, reassessing
a subsample of 226 COVID-19 survivors of the aforementioned study
at 3 months, Mazza et al (40), reported that 35.8% of the patients
still scored in the pathological range in at least one
psychopathological dimension (40).
Furthermore, Huang et al (41) evaluated a sample of 1,733 patients
who were hospitalized due to COVID-19, 6 months following symptom
onset and demonstrated that a significant proportion (76%) still
reported at least one neuropsychiatric symptom, with the most
common being fatigue/muscle weakness (63%) and sleep disturbances
(26%). In a later study, 1-year follow-up data were available from
a subsample of 1,276 COVID-19 survivors (36). Of note, within 1 year after acute
infection, the majority of individuals exhibited a good physical
and functional recovery over time, and had returned to their
original work and life, albeit their health status remained lower
compared to non-COVID-19 participants (controls) matched for age,
sex and comorbidities (36).
Notably, Taquet et al (42) reported that among 236,379 patients
diagnosed with COVID-19, the estimated incidence of a neurological
or psychiatric diagnosis in the succeeding 6 months was 33.62%,
with a proportion of 12.84% receiving such a diagnosis for the
first time, whereas the estimated incidence was even higher for
patients who had been admitted to an intensive care unit (ICU).
Depression, anxiety, PTSD, sleep disturbances,
fatigue and cognitive deficits are among the most commonly reported
neuropsychiatric symptoms in published studies investigating ‘long
COVID’ (20,43), and thus should be explicitly
assessed when treating patients with symptoms beyond the phase of
acute SARS-COV-2 infection.
Anxiety, depression and ‘long
COVID’
According to previous systematic reviews, the most
frequently reported psychiatric symptoms in the context of ‘long
COVID’ are depression and anxiety (20,38).
Considering that, particularly persisting, depression and anxiety
symptoms are associated with substantial individual morbidity with
severe repercussions on the quality of life of patients, the
psychiatric manifestations of ‘long COVID’ may also pose a
significant healthcare challenge with major societal implications
(44,45).
Of note, a previous retrospective cohort study found
that among 236,379 patients, 17.39% were diagnosed with anxiety
disorder (7.11% received first such diagnosis) and 13.66% with mood
disorder (4.22% received first such diagnosis) in the 6 months
following COVID-19 diagnosis. Notably, as regards patients who were
admitted in the ICU, estimated incidences were 22.43% for anxiety
disorder (9.24% for first diagnosis) and 22.52% for mood disorder
(8.07% for first diagnosis) (42).
Furthermore, another prospective cohort study,
including 251 patients with COVID-19 reported that 29.6% of the
survivors presented state anxiety one month after hospital
discharge, that was persistent at the 3-month follow-up assessment
(i.e., in 25.5% of patients), while no changes in
anxiety/depression symptoms were noted at 3-month follow-up
(evaluated with EuroQoL-5 Dimensions, EQ-5D) (46).
Notably, a previous cohort study including 134
patients with COVID-19 assessed at a median of ~3.8 months (46-167
days) post-discharge, reported that at follow-up, 47.8% of the
survivors experienced anxiety and 39.6% a low mood, with female
patients being at a higher risk in comparison to males (47).
Accordingly, another prospective uncontrolled cohort
study that evaluated, via telephone interview, 478 patients who
were hospitalized due to COVID-19, 4 months after discharge,
indicated that 31.4% of these patients reported anxiety and 20.6%
depression (48), while another
4-month follow-up study found that among 94 COVID-19 patients who
presented COVID-19 related pneumonia with respiratory failure, 21%
presented anxiety post-hospital discharge (49).
Of note, a retrospective, case series of 200
hospitalized patients with severe-to-critical COVID-19 infection
reported that at 4-7 months from disease-onset, 20% of patients
presented anxiety or a low mood, sometimes associated with
intrusive thoughts or flashbacks, while patients with pre-existing
mental health issues presented a deterioration of their symptoms
both during hospitalization and after discharge (50).
Furthermore, another cohort study, including 402
COVID-19 survivors who were hospitalized, demonstrated that at a
1-month assessment, 31% presented with depression and 42% with
anxiety. Crucially, a significant decrease was recorded from the 1-
to the 3-month follow-up assessment with regards to anxiety,
whereas depression rates were not altered. It is important to note
that the severity of baseline systemic inflammation predicted the
severity of depressive psychopathology at the 3-month follow-up
(40). Accordingly, a recent
multimodal magnetic resonance imaging study assessing 42 COVID-19
survivors without brain lesions, at ~3 months (90.59±54.66 days)
after COVID-19 infection, revealed that the systemic
immune-inflammation index measured in the emergency department
predicted increased depression several weeks after the clearance of
the virus, while the severity of depression symptoms was also
associated with decreasing grey matter (GM) volumes in the anterior
cingulate cortex (ACC) (51).
Of note, a multicenter observational study including
1,142 COVID-19 survivors reported that at ~7 months after hospital
discharge 16.2% of the patients presented self-rated anxiety
symptoms, while 19.7% depressive symptoms. The female sex, the
number of days spent at the hospital, the number of pre-existing
medical comorbidities, and the number of lingering symptoms at
hospital admission were significantly associated with depressive
symptoms, whereas only the number of symptoms at hospital admission
was associated with anxiety (52).
Moreover, a longitudinal study that assessed 165
consecutive non-neurological patients 6 months after
hospitalization due to COVID-19, reported that 26.7% of the
patients presented depressive symptoms or anxiety (53). Similarly, a larger cohort study
that assessed 1,733 hospitalized patients 6 months following
symptom onset demonstrated that 23% of these patents experienced
anxiety or depression (41). It is
important to note that the 1-year follow-up findings suggested an
exacerbation of psychiatric symptoms, as the proportion of
individuals with anxiety or depression increased significantly from
23% at the 6-month assessment to 26% at the 12-month assessment
(36). Another 1-year follow-up
study including 171 discharged patients with COVID-19 without a
mental health history revealed that 35.1% of these patients
reported anxiety and 32.2% depression (54). Accordingly, a cohort study
including 2,433 COVID-19 survivors, also identified anxiety
symptoms (10.4%) 1 year after hospital discharge, albeit to a
lesser extent (55).
Findings concerning the trajectory of depression and
anxiety symptoms are contradictory, as there are reports of an
increase over time [e.g., higher anxiety and depression from the 6-
to the 12-month assessment (36)],
or results, suggesting symptom amelioration [e.g., decrease in
anxiety at 1 month after admission (56), and also from the 1- to the 3-month
assessment (40), as well as a
decrease in depression at 1-month after admission (56)]. However, there are also reports of
non-significant changes noted at the follow-up assessment [e.g.,
persistent anxiety 1 month after hospital discharge (46), as well as depression 1 month after
admission (57) that remained
unaltered between the 1- and 3-month assessment (40)].
As regards factors that may be associated with
depression and anxiety in patients with ‘long COVID’, a number of
studies have identified an elevated risk among COVID-19 survivors
with an increased disease severity, with differences arising among
patients who were treated at home or in outpatient settings, those
who were hospitalized or patients requiring treatment in the ICU
(30,41,42,58),
although this connection has been suggested to be rather weak for
psychiatric compared to neurological outcomes (42). Nevertheless, de Graaf et al
(59) indicated that depression
and anxiety symptoms did not differentiate between hospitalized
patients treated in the ICU and those in a general ward. Similar
findings were obtained in another study on a sample of COVID-19
survivors, with no pre-existing neurological, psychiatric, or
severe medical condition, referred to an outpatient rehabilitation
program due to persistent symptoms and/or sequelae of COVID-19
>3 months following acute COVID-19 infection (60). Accordingly, other studies did not
find either differences in anxiety or depression between COVID-19
survivors with varying degrees of severity (e.g., mild, moderate,
severe) (61,62), while Yuan et al (63) reported that depression was not
associated with the severity of initial infection or a history of
hospital admittance. As regards the impact of comorbidities, while
associations with either depression (28) or both anxiety and depression
symptoms (64), have been
suggested, other studies have not found any association between
comorbidities and ‘long COVID’ symptoms (63,65,66).
There is evidence to indicate that patients with a
history of mental health issues may be at a higher risk of
presenting persisting depression symptoms (39,40);
however, there are also findings revealing that among COVID-19
survivors with self-rated anxiety and depression, a high proportion
does not have pre-existing mental health conditions (58). Furthermore, it should be noted that
often, studies investigating mental health sequalae among COVID-19
survivors, either define psychiatric history as an exclusion
criterion or do not report findings using standardized assessment
with respect to this (43).
Crucially, a number of studies suggest that females are, in
general, more likely to display anxiety or depression at follow-up
(40,41,47,67);
however, the absence of such an association has also been reported
(63).
Post-traumatic stress disorder and
‘long COVID’
Taking into consideration the findings of previous
studies regarding previous coronavirus outbreaks, such as SARS and
Middle East respiratory syndrome (MERS), that reported
significantly elevated PTSD rates in survivors even after several
months, it is evident that the investigation of PTSD with respect
to ‘long COVID’ is of utmost importance (68,69).
Of note, in a previous study, among 402 patients
assessed at 1 month after hospital treatment, 28% presented PTSD
(39), while when a subsample of
226 survivors was reassessed at 3 months, a significant decrease
from the 1- to the 3-month follow-up was identified with respect to
PTSD symptoms (40). Nevertheless,
another prospective cohort study, assessing 251 hospitalized
patients both at 1 and 3 months post-discharge, reported that at 1
month, 24.5% of patients experienced PTSD, a proportion that did
not change significantly at the 3-month post-discharge evaluation
(46).
Furthermore, in a large cohort study including 760
hospitalized patients, assessed at a median of 65 days (i.e., ~9
weeks) post-discharge, 10.5% screened positive for PTSD, with
patients having more physical symptoms at admission and at
follow-up being more likely to present PTSD symptoms. Patients with
PTSD were more likely to experience persistent symptoms of
breathlessness, myalgia, anorexia and confusion, whereas they were
less likely to have returned to work (70). Notably, in another large cohort
study, among 767 COVID-19 survivors assessed at a median time of 81
days after discharge, 30.5% reported PTSD (71).
Crucially, in a cohort study, at 4 months after
discharge, PTSD was identified in 14.2% of 478 patients
hospitalized due to COVID-19(48).
Similarly, in a prospective, longitudinal cohort study, among 1,077
patients assessed at a median of 5.9 months after hospital
discharge, 12.2% reported symptoms compatible with PTSD (72). Nevertheless, another 4-month
follow-up study including 238 hospitalized patients due to severe
COVID-19 found that at 4 months after discharge, 42.9% of the
patients presented PTSD symptoms, while 17.2% of the patients
reported moderate-to-severe symptom severity (73).
Notably, a 12-month longitudinal study revealed that
24.6% of the 171 discharged patients with COVID-19, without a
mental health history, reported PTSD at the follow-up assessment
(54). Of note, another study
using a multimodal brain imaging approach demonstrated that
systemic immune-inflammation during the acute phase predicted
increased the risk of PTSD symptoms at ~3 months (90.59±54.66 days)
post-COVID, while such symptoms were also associated with
decreasing GM volumes both, in the ACC and in the bilateral insular
cortex (51).
As regards the risk factors, there is evidence to
indicate that psychological distress at the onset of illness may be
predictive of PTSD development (74), while higher levels of anxiety and
depression symptoms during the first week of hospitalization have
also been suggested to independently predict a higher risk of
developing PTSD symptoms at 1 month after hospitalization (56).
The setting of care (e.g., ICU, general hospital
ward, outpatient setting) has emerged as a risk factor for PTSD in
some studies (58,74); however, this is not the case in
others (40,59,73,75).
Of note, in previous studies, no differences were found among
COVID-19 survivors with different levels of disease severity (e.g.,
mild, moderate, severe, or critical disease) (61,62).
In another study, hospitalization was identified as a protective
factor against developing PTSD, when comparing to patients
discharged from the emergency department (76).
With respect to physical comorbidities, the findings
suggest that there is no association with PTSD development,
particularly following adjustment for other confounding factors
(73,76). Crucially, patients with
pre-existing mental health problems are likely to be at a greater
risk of presenting PTSD (39,40),
even when controlling for other demographic or clinically relevant
factors (75); nevertheless,
non-significant associations have also been reported (77).
Furthermore, studies using either univariate or
multivariate analyses, have demonstrated that PTSD symptoms more
commonly present in females following COVID-19 (39,40,75,77),
particularly when treated in an ICU (58). However, it is important to note
that there is also evidence to indicate that when adjusting for
other variables, the sex effect may lose statistical significance
(46,76). On the other hand, Bellan et
al (73) indicated that the
male sex was independently associated with the presence of
moderate-to-severe PTSD symptoms.
The findings are not consistent regarding the role
of body mass index (BMI) with regards to PTSD. There is evidence to
suggest that a higher BMI (i.e., obesity) is associated with PTSD,
independently of other factors (75), while it has also been indicated
that this association is more pronounced in patients treated in the
ICU (58); nevertheless, other
studies have not found any such associations (73,76).
Further factors suggested to predict PTSD severity include previous
traumatic events, prolonged COVID-19 symptoms, stigmatization and a
negative view of the COVID-19 pandemic (77).
Sleep disturbances, fatigue, other
neuropsychiatric symptoms and ‘long COVID’
Sleep difficulties of varying degrees, as well as
fatigue, that can potentially be debilitating and negatively affect
the quality of life of patients, are among the long-term effects
that COVID-19 survivors may continue to experience or even present
for the first time weeks to months following the initial infection
(20,38). Furthermore, post-acute
neuropsychiatric manifestations of ‘long COVID’ include memory
impairment, concentration difficulties, headaches, disorientation
or confusion and obsessive-compulsive symptoms, among others
(31,78).
In a previous prospective cohort study that assessed
251 survivors, at 1 month after discharge, 41.8% of the patients
experienced insomnia, 35.3% pain/discomfort, 26.8% problems in
usual daily activities and 10.3% problems in self-care. Among the
aforementioned symptoms, none was significantly altered at the
3-month post-discharge evaluation, apart from insomnia, which
improved significantly (i.e., affecting 25.5% of patients) albeit a
number of patients still experienced sleep disturbances (46).
Accordingly, in another study, 402 patients with
COVID-19 who were assessed at 1 month after hospital treatment, 40%
reported insomnia and 20% obsessive-compulsive symptoms (39). Crucially, a significant decrease
from the 1- to the 3-month follow-up assessment (in a subsample of
226 survivors) was recorded for insomnia, whereas notably,
obsessive-compulsive symptomatology worsened (40). Furthermore, a significant
proportion of patients (78%) displayed poor performances in at
least one cognitive domain, with executive functions (50%) and
psychomotor coordination (57%) emerging as the most impaired,
followed by attention and information processing (33%), verbal
fluency (32%), working memory (24%) and verbal memory (10%).
Notably, baseline systemic inflammation predicted neurocognitive
performance at the 3-month follow-up (40).
Another study demonstrated that among 134 patients
with COVID-19 assessed after a median of ~3.8 months
post-discharge, 51.5% reported myalgia, 39.6% extreme fatigue,
37.3% memory impairment, 35.1% sleep disturbances, 25.4% attention
deficits and 9.7% cognitive impairment. Females were more likely to
suffer from sleep disturbances, fatigue, myalgia and memory
impairment compared to males, while higher a BMI was significantly
associated with myalgia and fatigue (47). Crucially, in a larger retrospective
cohort study, including 932 hospitalized patients with COVID-19, a
relatively low proportion with respect to fatigue was noted at 3
months after discharge (i.e., 1.8%) (79).
Of note, in another study with a 4-month follow-up,
including 94 survivors who had COVID-19-related pneumonia with
respiratory failure, at 4 months after their discharge, 52% of
patients reported experiencing fatigue, 10% anorexia and 31%
insomnia (49). Similarly, in a
4-month post-discharge assessment of 478 patients who were
hospitalized due to COVID-19, the neuropsychiatric symptoms
reported included insomnia (53.6%), fatigue (31.1%), memory
difficulties (17.5%), persistent paresthesia (12.1%), mental
slowness (10.1%), memory difficulties (10.0%) and headaches (5.5%),
while cognitive impairment was found in 38.4% of patients. Notably,
51% of the COVID-19 survivors reported at least one symptom that
did not exist prior to the disease (48). Accordingly, in another study among
200 hospitalized patients with severe-to-critical COVID-19
infection, it was found that at 4-7 months from disease-onset a
significant proportion of patients experienced significant fatigue
(53.5%), decreased mobility (37.5%) and pain (36.8%). Further
neuropsychiatric symptoms included cognitive difficulties, mainly
in concentration and short-term recall (12.5%), sleeping
disturbances (14.6%), while 12.2% of patients became frail
(50).
A prospective, longitudinal cohort study that
assessed 1,077 patients at a median of 5.9 months following
hospital discharge found that the most commonly reported persistent
symptoms at follow-up were aching muscles/pain (57.2%), fatigue
(worsening of symptoms reported by 56.2%), physical slowing down
(49.9%), slowing down in thinking (42.4%), impaired sleep quality
(worsening of symptoms reported by 41.8%), joint pain or swelling
(47.8%), limb weakness (46.3%), difficulty with concentration
(40.2%), short-term memory loss (42.0%) and headaches (33.4%).
Moreover, less frequently reported symptoms included, among others,
confusion/fuzzy head, dizziness or lightheadedness, altered
personality/behavior and difficulty with communication. Of note,
29% of the patients reported that they felt fully recovered at
follow-up, while non-recovery was associated with female sex,
middle age (40-59 years), two or more comorbidities and more severe
acute illness (72).
Of note, a previous cohort study, following a total
of 952 patients with absent-to-mild COVID-19 symptoms for a median
time period of 6.8 months (i.e., 442 and 353 patients observed over
a period of 4 and 7 months after symptom onset, respectively),
demonstrated that while the most common symptoms at disease onset
included cough (64.4%), ageusia (59.1%), anosmia (54.3%), body
aches (53.2%), headaches (53.1%) and fever (44.6%), the most
frequently reported symptoms at 4-month follow-up were anosmia
(12.4%), ageusia (11.1%), fatigue (9.7%) and shortness of breath
(8.6%). Intriguingly, symptoms such as anosmia (14.7%), shortness
of breath (13.6%), fatigue (14.7%) and ageusia (11.0%) were also
present at the 7-month assessment, while headaches (3.7%) were also
recorded (80).
Furthermore, a retrospective observational follow-up
study including 797 COVID-19 survivors who were hospitalized,
demonstrated that at 6 months post-discharge, 22.1% presented
fatigue, 3.8% muscle weakness, 15.3% musculoskeletal pain, 5.3%
headache, 3.4% paresthesia, 2.6% disorientation or confusion, and
4.9% sleep disturbances (67).
Another 6-month follow-up cohort study that evaluated 796 patients
with severe COVID-19 rehabilitation, found that the most common
neuropsychiatric symptoms at follow-up comprised fatigue (25.3%)
and sleep disorder (23.2%), while hypomnesia (8.7%), dizziness and
headache (1.9%) were also recorded (81). Crucially, among 165 patients, 6
months after hospitalization due to COVID-19, fatigue (33.9%),
memory/concentration complaints (31.5%), sleep disorders (31.5%),
myalgias (30.3%) and the loss of dependency in instrumental
activities of daily living (20.7%) emerged as the most common
neuropsychiatric symptoms (53).
In another cohort study, poor sleep quality was recorded 7 months
after discharge in 34.5% of 1,142 patients who were hospitalized
due to COVID-19, while females, patients with a higher number of
days of hospital stay, a higher number of comorbidities and a
higher number of symptoms at hospital admission were more likely to
report poor sleep quality (52).
Notably, a retrospective cohort study reported that
among 236,379 patients diagnosed with COVID-19, the estimated
incidences at 6 months post-COVID were 1.40% for psychotic
disorders (0.42% for first such diagnosis), 6.58% for substance use
disorder (1.92% for first diagnosis), 5.42% for insomnia (2.53% for
first diagnosis), 2.10, and 0.67% for dementia (42).
Furthermore, in another study, among 1,733 patients
hospitalized due to COVID-19, within 6 months from symptom onset,
63% experienced symptoms of fatigue or muscle weakness, 27% pain or
discomfort, 26% sleep difficulties, 6% dizziness, 2% myalgia and 2%
headache (41). In a subsample of
1,276 COVID-19 survivors who were assessed both at 6 and 12 months
following symptom onset, the majority of the aforementioned
neuropsychiatric symptoms appeared to have improved. In particular,
symptoms of fatigue or muscle weakness decreased from 52 to 20%,
while sleep difficulties from 27 to 17%. However, a statistically
significant increase was observed with respect to myalgia (from 3
to 4%) and headache (from 2 to 5%), whereas no statistically
significant changes were noted for pain or discomfort (from 27 to
29%) and dizziness (from 6 to 5%) (36).
Notably, the neuropsychiatric symptoms reported in a
retrospective cohort study that assessed 2,433 COVID-19 survivors
at 1 year after their hospital discharge included fatigue (27.7%)
and myalgia (7.9%), while patients with severe disease during
hospitalization presented more frequently with post-COVID symptoms
(55). Importantly, in the
aforementioned study, an older age, female sex and severe disease
during hospital stay were associated with a higher risk of fatigue,
while an advanced age and severe disease were also associated with
an increased risk of having more post-infection symptoms.
Furthermore, another 1-year follow-up study on 171 discharged
patients, reported neuropsychiatric symptoms including fatigue
(48.5%), memory complaints (32.2%), headaches (15.8%) and
paresthesia (7%). Notably, the most affected cognitive domain was
semantic verbal fluency (32.7%) followed by immediate verbal
memory/learning (20.5%), working memory/executive function (12.3%)
and delayed verbal memory (7.6%) (54).
Sleep disturbances persisting in patients even
months after acute COVID-19 infection have been highlighted in
several studies, with some of these indicating that almost half of
the survivors experience difficulties related to sleep (39,48),
although there is evidence for improvement over time (40,46).
The pivotal essential role of sleep in physical and mental health
is well-established (82). Vice
versa, there is evidence to indicate that insomnia and other mental
health conditions not only share common pathophysiological causes,
but also exhibit a bidirectional association, with disrupted sleep
most likely being a contributory causal factor for the occurrence
of major types of mental health disorders (83). Among patients with ‘long COVID’,
the female sex (39,40,47,52,81),
a history of mental health issues (39,40)
and the number of comorbidities (52) are likely associated with an
increased risk of experiencing sleep disturbances. Of note, there
are studies that indicate no difference between patients admitted
to hospital as compared to those discharged from the emergency
department (76), or an
association of sleep disorders with disease severity (41,62,81);
however there are also studies that associate insomnia with the
care setting/disease severity (e.g., patients without
hospitalization, patients with hospitalization, patients with ICU
admission or patients with encephalopathy) (30,42),
or highlight the number of symptoms at hospital admission and the
number of days spent at hospital, as risk factors for a poor sleep
quality (52). Nevertheless, a
previous study also found that patients that were not treated in
the ICU presented a lower sleep quality and higher daytime
dysfunction compared to those treated in the ICU, while no other
differences were noted in any of the other sleep-related dimensions
(i.e., latency, duration, efficacy, disturbance or medication use)
(60).
Fatigue is among the most commonly reported symptoms
that patients experience following the resolution of acute
COVID-19, while according to a recent meta-analysis, ~32% of
individuals experienced fatigue at ≥12 weeks following COVID-19
diagnosis (84). Females are, in
general, at a greater risk of experiencing persisting fatigue
post-COVID (41,47,67,81,85,86),
either when treated in a hospital ward or in the ICU (58), with differences being observed even
at 1 year of follow-up (36,55).
Nevertheless, other studies have reported no such associations
(87,88). An older age has been independently
associated with fatigue or muscle weakness (41) and higher rates of fatigue (87) at 6 months post-discharge, as well
as at the 1-year follow-up (55),
whereas other findings suggesting an absence of significant
association have also been reported at the 10-week (85) and 1-year follow-up following
discharge (36). Furthermore,
patients with a higher BMI are likely to experience increased
fatigue (47,89), although findings not supporting
this association have also been reported (58,85,87).
Crucially, as regards the impact of COVID-19 severity on post-viral
fatigue, the findings are not consistent. There are reports of
severe disease linked to a higher risk of fatigue at 3 months
(62) and 6 months after COVID-19
(30,53,90),
as well as of fatigue or muscle weakness (41) at 6 months post-discharge and
fatigue up to 1 year post-discharge (55); however, in the study sample of
Huang et al this association has emerged only for the
6-month assessment (41), but not
the 12 month assessment (36). Of
note, another study assessing patients, with no neurological or
psychiatric history, at >3 months after acute COVID-19
infection, indicated that there was no significant association
between previous admission to ICU and fatigue (60), while another study assessing
fatigue at a median of 10 weeks after the initial COVID-19 symptoms
demonstrated that there was no association between COVID-19
severity (need for inpatient admission, supplemental oxygen or
critical care) and fatigue following COVID-19(85). Accordingly, Shang et al
(81) did not find any differences
between severe and critical illness at 6-month follow-up assessment
among patients with severe COVID-19. Other risk factors for fatigue
may include high symptom load during COVID-19(89), the length of hospital stay
(87), dyspnea during
COVID-19(89), confusion during
COVID-19(89), previous depression
(89), a pre-existing diagnosis of
depression/anxiety (85), sleep
disturbances (62), as well as new
neurological diagnoses (62). Of
note, in the study by Huang et al (36), therapy using corticosteroids during
the acute phase was associated with an increased risk of fatigue or
muscle weakness at 1 year post-discharge, whereas intravenous
immunoglobulin therapy in the acute phase decreased the risk of
persistent fatigue or muscle weakness. Townsend et al
(85) reported no association
between routine laboratory markers of inflammation and cell
turnover (leukocyte, neutrophil or lymphocyte counts,
neutrophil-to-lymphocyte ratio, lactate dehydrogenase, C-reactive
protein) or pro-inflammatory molecules [interleukin (IL)-6 or
sCD25] and fatigue post COVID-19, while Liang et al
(91) found that serum troponin-I
levels during acute illness correlated positively with fatigue
after hospital discharge.
Cognitive impairment persisting in patients with
COVID-19, is particularly concerning, as apart from the individual
physical/mental health and social repercussions, this may also
translate into a major global healthcare and economic burden
(84). Cognitive
impairment/deficits reported following the resolution of acute
COVID-19 symptoms, may include difficulties in concentration,
memory, attention, language, executive function, encoding and
verbal fluency, and visuospatial function, among others (20,78,92).
A previous systematic review and meta-analysis regarding the
psychiatric and neuropsychiatric presentations associated with
severe coronavirus infections, such as SARS or MERS, demonstrated
that during the acute illness, common cognitive problems among
hospitalized patients included confusion [prevalence, 27.9%; 95%
confidence interval (CI), 20.5-36.0], an impaired concentration or
attention (prevalence, 38.2%; 95% CI, 29.0-47.9) and impaired
memory (prevalence, 34.1%; 95% CI, 26.2-42.5), while an impaired
concentration or attention (prevalence, 19.9%; 95% CI, 14.2-26.2)
and memory impairment (prevalence, 18.9%; 95% CI, 14.1-24.2) were
frequently reported during the post-illness stage as well (93). Notably, a recent meta-analysis
revealed that ~22% of individuals experienced fatigue ≥12 weeks
following the diagnosis of COVID-19(84). Disease severity has been associated
with cognitive deficits, such as deficits related to memory [i.e.,
memory complaints (53), memory
deficits (30), hypomnesia
(81)], confusion (53) and visual disturbances (53). Nevertheless, there are studies that
fail to support such a connection. In particular, de Graaf et
al (2021) did not find any significant differences with regards
to cognitive function between patients treated in a general
hospital ward and those admitted to the ICU (59). Accordingly, in the study by De
Lorenzo et al (76),
patients admitted to hospital as compared to those discharged from
the emergency department did not differ as regards self-reported
cognitive impairment measures. Furthermore, a study assessing
patients in an outpatient rehabilitation program due to persistent
symptoms and/or sequelae of COVID-19, with no prior neurological or
psychiatric history, indicated that there was no significant
association between previous admission to the ICU and orientation,
attention, verbal learning, long-term verbal memory, verbal
recognition, working memory or executive control (60). Importantly, Méndez et al
(94) did not find an association
between sex and neurocognitive impairment at the 2-month
post-discharge assessment, while Shang et al (81) did not find any differences in
hypomnesia between male and female patients at the 6-month
follow-up among patients with severe COVID-19. Nevertheless,
according to other studies, females were more likely than males to
report memory impairment (47), a
poor performance in working memory (40), disorientation or confusion
(67). Of note, delirium during
hospitalization and stress-related symptoms have been found to be
associated with an increased likelihood of neurocognitive
impairment at 2-month assessment (94), while according to Mazza et
al (40) neurocognitive
impairments were associated with severity of depressive
psychopathology, while processing speed, verbal memory and fluency,
and psychomotor coordination were predicted by baseline systemic
inflammation.
3. Pathophysiological mechanisms underlying
neuropsychiatric symptoms of ‘long COVID’
Significant strides have been made recently in the
understanding of the underlying pathophysiology of neuropsychiatric
complications following acute COVID-19 infection (Fig. 1). The neuroinvasive potential of
coronaviruses has long been recognized; however, the precise route
of entry of SARS-COV-2 in the nervous system remains only partially
elucidated (95). The retrograde
transport of viral antigens along the axons of both the olfactory
neurons (96-99)
and vagus nerve (100) has been
suggested by research using animal models, while the Spike (S)
viral protein has been shown to have the capacity of crossing the
blood-brain barrier in mice (101). Additionally, SARS-COV-2 has the
ability to enter host cells directly by binding its Spike protein
to the angiotensin-I converting enzyme 2 (ACE2) (102), which is abundantly expressed in
neural tissue-namely endothelial cells, neurons, glial cells, brain
nuclei, etc. (103). The strong
binding affinity of the Spike protein to ACE2(104) and the potential use of CD147 as a
route of entry into cells (105),
leads to a robust immune response, with infected macrophages
releasing significant amounts of Th-1 (IL-1β, IL-6, interferon-γ,
tumor necrosis factor-α, CXCL10 and CCL2) and Th-2 cytokines (IL-4,
IL-10 and IL-1 receptor antagonist) (106,107). Notably, IL-6 is the main
contributor to the dysregulated inflammatory response induced by
the virus (108).
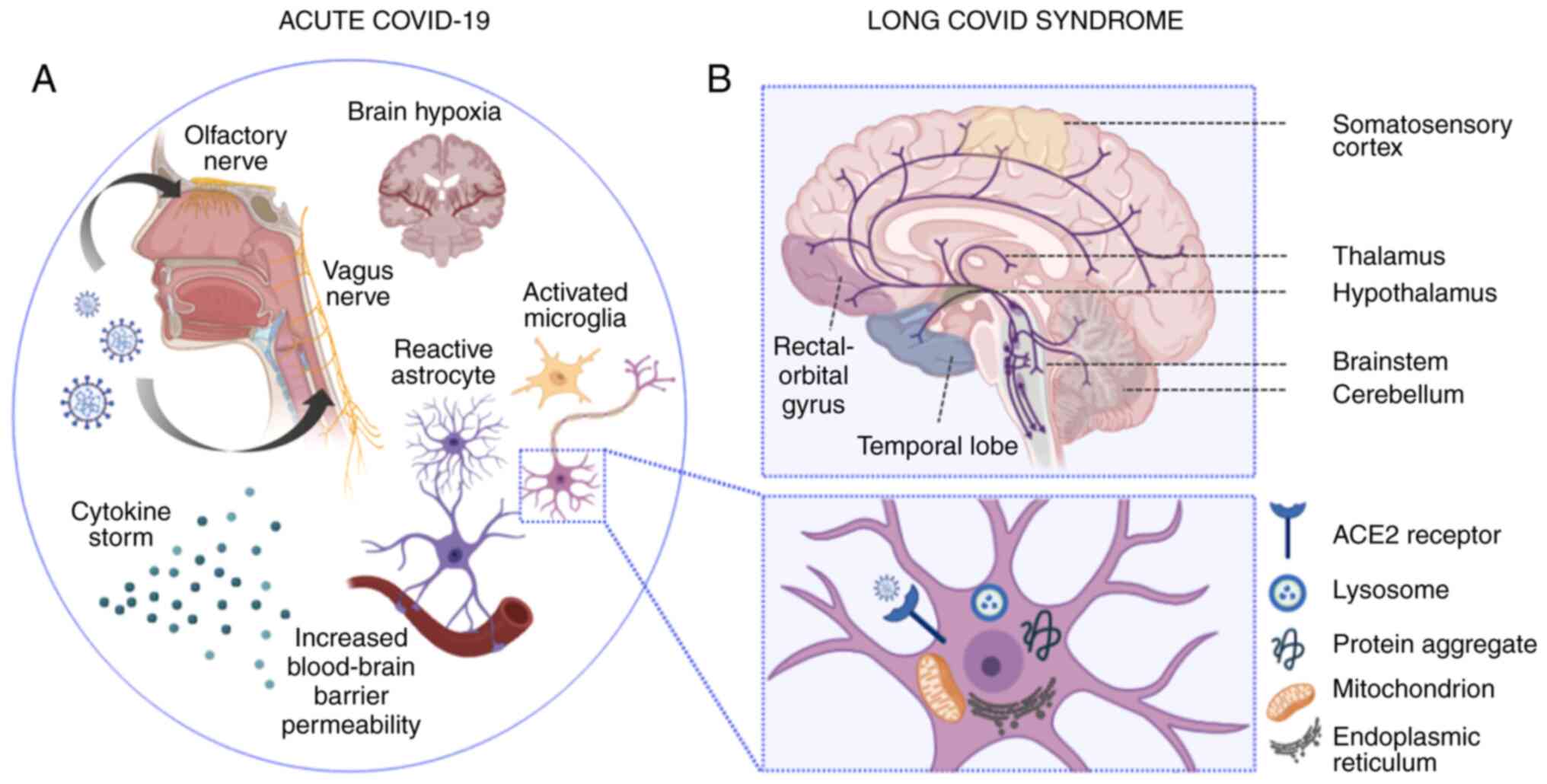 | Figure 1Pathophysiological mechanisms
implicated in acute and ‘long-COVID’ neuropsychiatric sequelae. (A)
In acute COVID-19 infection, SARS-CoV-2 enters the CNS via
hematogenous transmission or retrograde transport along the axons
of the olfactory (94-97)
and vagus nerve (100). Vagus
nerve affection may lead to autonomic dysregulation, impaired
cerebral autoregulation and subsequent brain hypoxia (100). Moreover, the SARS-CoV-2-induced
cytokine storm results in impaired blood-brain barrier function and
increased blood-brain barrier permeability. The latter induces the
transmigration of virus-infected leukocytes into the CNS, the
activation of microglial cells and astrocytes, which in turn
trigger apoptotic cascades and demyelination, respectively. At the
neuronal level (illustrated in the magnified inset), SARS-CoV-2
cell entry is mainly facilitated by the ACE2 receptor, located on
the surface of neuronal cells. Intracellular inflammatory responses
in the setting of acute SARS-CoV-2 infection induce lysosomal,
mitochondrial and endoplasmic reticulum dysfunction, while protein
misfolding and intracellular accumulation of protein aggregates may
induce long-lasting neurodegenerative cascades (132). (B) In ‘long-COVID’ syndrome,
neuropsychiatric deficits have been mainly associated with ongoing
inflammatory, metabolic and degenerative processes, which have been
linked to ‘ACE2-rich’ brain areas, extending from the somatosensory
cortex to the rectal/orbital gyrus, the temporal lobe, the thalamus
and hypothalamus, and further, to brainstem and cerebellar regions
(133). Serotoninergic pathways
are depicted in purple. Apart from serotoninergic pathways, further
neurotransmitter imbalances (not illustrated), including
acetylcholine, dopamine and histamine have been found to be linked
to lingering neuropsychiatric symptoms noted in patients with
‘long-COVID’ (134). The image
was created using BioRender (https://biorender.com). CNS, central nervous system;
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2,
angiotensin-converting enzyme 2. |
Those direct effects of acute COVID-19 infection
may subsequently contribute to the symptomatology noted in ‘long
COVID’. It should, nevertheless, be noted that the exact mechanisms
of ‘long COVID’ remain poorly delineated to date, while new
evidence continues to emerge. Anxiety syndromes, depression and
cognitive impairment are considered to have a multifactorial
origin, with somatic, functional and psychosocial factors all
contributing to the clinical picture. The psychological stressors
of social isolation and infection with a novel potentially fatal
virus, as well as the fear of infecting others or being
stigmatized, all play a key role (109-111).
The activation of the hypothalamus-pituitary-adrenal glands axis
observed in patients with COVID-19 mediates glucocorticoid
secretion (i.e., the main hormonal response to physical and mental
stress stimuli) and is also one of the main neurobiological
mechanisms in depression as it inhibits neurogenesis and decreases
the proliferation and survival of nerve cells in the dentate gyrus
of the hippocampus (112-114).
The cytokine storm as part of the systemic
hyperinflammatory state observed in acute COVID-19 infection, also
plays a key role in persistent maladies, precipitated by changes in
cerebral perfusion, an increased permeability of the blood-brain
barrier, changes in astrocytes involved in synaptogenesis and the
imbalance of neurotransmitters (115). These molecules dysregulate
neurogenesis, causing neurons, oligodendrocytes and glial cells to
lose their physiological function (116). This occurring disruption of
neuronal plasticity, synaptic function, myelination and blood-brain
barrier maintenance can subsequently impair cognitive function and
may lead to a number of the long-term neuropsychiatric symptoms of
COVID-19(117). Of note, previous
studies have demonstrated that the systemic immune-inflammation
index (SII) was elevated at the 3-month follow-up in patients
reporting depressive and cognitive impairment symptomatology
(39,118). The SII is an objective marker of
host systemic inflammation and immune response, implicating
neutrophils, platelets and lymphocytes, cells involved in various
inflammatory pathways. Individuals who presented with a marked
decrease in the SII exhibited a decrease in the severity of
depression, while by contrast, increased levels of SII had a
negative effect on neurocognitive performance (memory, verbal
fluency, speed of information processing, psychomotor
coordination), since this elevation reflects prolonged systemic
inflammation (40).
Another factor that also may account for the
delayed sequelae of COVID-19 is the failure of reactive neuroglia
to return to the physiological state. Neuroglia undergo complex
remodeling in response to systemic pathology, a process known as
gliosis, in order to remove pathogens, strengthen brain-organism
barriers and contribute to the regenerative potential of the CNS
(116). This reactive response
results in the formation of a glial scar isolating the damaged
area, protecting the adjacent healthy nervous tissue (119). The resolution of systemic
pathology is what enables the restoration of homeostatic status of
neuroglial cells. Post-mortem examinations of the brains of
patients with COVID-19 have highlighted a perturbed glial
homeostasis, with significant changes in both astrocytes and
microglia (120). These
alterations are consistent with morphological and functional
astrocyte remodeling in chronic stress and major psychiatric
diseases (121,122).
Autonomic dysfunction, either due to dysfunction
related to the initial infection (123-126)
or mediated by autoantibodies (127) (possibly to adrenoceptors and
muscarinic receptors), has been hypothesized to cause orthostatic
intolerance syndrome in patients with ‘long COVID’. The concomitant
involvement of other organs, such as the heart or lungs, is
prominent and associated with neurological sequelae, while
perturbed viral infection-induced colon inflammation, gut microbial
imbalance and α-synuclein upregulation play a role in the
disruption of interplay between the gastrointestinal tract and the
CNS (128).
The formation of thrombi due to endothelial
dysfunction, hypercoagulability and the lingering cytokine storm in
patients with ‘long COVID’ may be associated with a high incidence
of thrombotic cerebral complications (129). Additionally, direct damage to the
blood-brain barrier by the virus and hypertension due to elevation
in ACE2 may cause hemorrhagic complications with persistent
sequelae following the resolution of acute SARS-CoV-2 infection
(130). The high susceptibility
of white matter to ischemia renders it particularly vulnerable to
ischemic changes. Furthermore, long-term alterations have recently
been confirmed by the neuroradiological evidence of structural
damage and impaired functional integrity of the brain, at a 3-month
follow-up of COVID-19 survivors (131).
4. Conclusion
The present review article has provided insight
into the long-term neuropsychiatric effects of COVID-19, while
presenting a comprehensive overview of published epidemiological
data to date, as well as research evidence on the
pathophysiological mechanisms underlying the neuropsychiatric
manifestations of ‘long COVID’. Considering the disconcerting
effects of COVID-19 and the global dimensions of the pandemic,
interdisciplinary cooperation is warranted for the early
identification of patients who are at a high risk of developing
persistent neuropsychiatric deficits following recovery from acute
disease. To this end, it is of paramount importance to ensure that
appropriate integrated physical and mental health support is
provided, with the aim of mitigating the risks of long-term
disability at an individual and societal level.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VE, MIS, MD, NSi and MM wrote the original draft,
and edited and critically revised the manuscript. GT, VZ, SPK, JNT,
DAS, NSm and ER critically revised and edited the manuscript. All
authors substantially contributed to the conception, writing and
revision of the work, and have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but has
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Sohrabi C, Alsafi Z, O'Neill N, Khan M,
Kerwan A, Al-Jabir A, Iosifidis C and Agha R: World Health
Organization declares global emergency: A review of the 2019 novel
coronavirus (COVID-19). Int J Surg. 76:71–76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Leaune E, Samuel M, Oh H, Poulet E and
Brunelin J: Suicidal behaviors and ideation during emerging viral
disease outbreaks before the COVID-19 pandemic: A systematic rapid
review. Prev Med. 141(106264)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kahil K, Cheaito MA, El Hayek R, Nofal M,
El Halabi S, Kudva KG, Pereira-Sanchez V and El Hayek S: Suicide
during COVID-19 and other major international respiratory
outbreaks: A systematic review. Asian J Psychiatr.
56(102509)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zoumpourlis V, Goulielmaki M, Rizos E,
Baliou S and Spandidos DA: [Comment] The COVID-19 pandemic as a
scientific and social challenge in the 21st century. Mol Med Rep.
22:3035–3048. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Efstathiou V, Stefanou MI, Siafakas N,
Makris M, Tsivgoulis G, Zoumpourlis V, Spandidos DA, Smyrnis N and
Rizos E: Suicidality and COVID-19: Suicidal ideation, suicidal
behaviors and completed suicides amidst the COVID-19 pandemic
(Review). Exp Ther Med. 23(107)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsamakis K, Tsiptsios D, Ouranidis A,
Mueller C, Schizas D, Terniotis C, Nikolakakis N, Tyros G,
Kympouropoulos S, Lazaris A, et al: COVID-19 and its consequences
on mental health (Review). Exp Ther Med. 21(244)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsamakis K, Triantafyllis AS, Tsiptsios D,
Spartalis E, Mueller C, Tsamakis C, Chaidou S, Spandidos DA, Fotis
L, Economou M and Rizos E: COVID-19 related stress exacerbates
common physical and mental pathologies and affects treatment
(Review). Exp Ther Med. 20:159–162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Han Y, Yuan K, Wang Z, Liu WJ, Lu ZA, Liu
L, Shi L, Yan W, Yuan JL, Li JL, et al: Neuropsychiatric
manifestations of COVID-19, potential neurotropic mechanisms, and
therapeutic interventions. Transl Psychiatry.
11(499)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lechien JR, Chiesa-Estomba CM, Place S,
Van Laethem Y, Cabaraux P, Mat Q, Huet K, Plzak J, Horoi M, Hans S,
et al: Clinical and epidemiological characteristics of 1420
European patients with mild-to-moderate coronavirus disease 2019. J
Intern Med. 288:335–344. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ellul MA, Benjamin L, Singh B, Lant S,
Michael BD, Easton A, Kneen R, Defres S, Sejvar J and Solomon T:
Neurological associations of COVID-19. Lancet Neurol. 19:767–783.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Varatharaj A, Thomas N, Ellul MA, Davies
NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M,
et al: Neurological and neuropsychiatric complications of COVID-19
in 153 patients: A UK-wide surveillance study. Lancet Psychiatry.
7:875–882. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Helms J, Kremer S, Merdji H, Clere-Jehl R,
Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S and
Ohana M: Neurologic features in severe SARS-CoV-2 infection. N Engl
J Med. 382:2268–2270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iltaf S Sr, Fatima M, Salman S Sr, Salam
JU and Abbas S: Frequency of neurological presentations of
coronavirus disease in patients presenting to a tertiary care
hospital during the 2019 coronavirus disease pandemic. Cureus.
12(e9846)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q,
Chang J, Hong C, Zhou Y, Wang D, et al: Neurologic manifestations
of hospitalized patients with coronavirus disease 2019 in Wuhan,
China. JAMA Neurol. 77:683–690. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fan S, Xiao M, Han F, Xia P, Bai X, Chen
H, Zhang H, Ding X, Zhao H, Zhao J, et al: Neurological
manifestations in critically ill patients with COVID-19: A
retrospective study. Front Neurol. 11(806)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiong W, Mu J, Guo J, Lu L, Liu D, Luo J,
Li N, Liu J, Yang D and Gao H: New onset neurologic events in
people with COVID-19 in 3 regions in China. Neurology.
95:e1479–e1487. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Frontera JA, Sabadia S, Lalchan R, Fang T,
Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A,
et al: A prospective study of neurologic disorders in hospitalized
patients with COVID-19 in New York City. Neurology. 96:e575–e586.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bo HX, Li W, Yang Y, Wang Y, Zhang Q,
Cheung T, Wu X and Xiang YT: Posttraumatic stress symptoms and
attitude toward crisis mental health services among clinically
stable patients with COVID-19 in China. Psychol Med. 51:1052–1053.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu Y, Chen Y, Zheng Y, You C, Tan J, Hu L,
Zhang Z and Ding L: Factors related to mental health of inpatients
with COVID-19 in Wuhan, China. Brain Behav Immun. 89:587–593.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schou TM, Joca S, Wegener G and
Bay-Richter C: Psychiatric and neuropsychiatric sequelae of
COVID-19-A systematic review. Brain Behav Immun. 97:328–348.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Michelen M, Manoharan L, Elkheir N, Cheng
V, Dagens A, Hastie C, Hara M, Suett J, Dahmash D, Bugaeva P, et
al: Characterising long COVID: A living systematic review. BMJ
Global Health. 6(e005427)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Centers for Disease Control and Prevention
(CDC): Post-COVID Conditions: Information for Healthcare Providers.
CDC, Atlanta, GA, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
Updated July 9, 2021.
|
|
23
|
Deer RR, Rock MA, Vasilevsky N, Carmody L,
Rando H, Anzalone AJ, Basson MD, Bennett TD, Bergquist T, Boudreau
EA, et al: Characterizing long COVID: Deep phenotype of a complex
condition. EBioMedicine. 74(103722)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brodin P: Immune determinants of COVID-19
disease presentation and severity. Nat Med. 27:28–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stefanou MI, Palaiodimou L, Bakola E,
Smyrnis N, Papadopoulou M, Paraskevas GP, Rizos E, Boutati E,
Grigoriadis N, Krogias C, et al: Neurological manifestations of
long-COVID syndrome: A narrative review. Ther Adv Chronic Dis.
13(20406223221076890)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Soriano JB, Murthy S, Marshall JC, Relan P
and Diaz JV: WHO Clinical Case Definition Working Group on
Post-COVID-19 Condition. A clinical case definition of
post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis.
22:e102–e107. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rajan S, Khunti K, Alwan N, Steves C,
Greenhalgh T, MacDermott N, Sagan A and McKee M: In the wake of the
pandemic. Preparing for Long COVID Policy Brief.
(39)2021.PubMed/NCBI
|
|
28
|
Fernández-de-Las-Peñas C, Palacios-Ceña D,
Gómez-Mayordomo V, Cuadrado ML and Florencio LL: Defining
Post-COVID symptoms (Post-Acute COVID, Long COVID, Persistent
Post-COVID): An integrative classification. Int J Environ Res
Public Health. 18(2621)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Davis HE, Assaf GS, McCorkell L, Wei H,
Low RJ, Re'em Y, Redfield S, Austin JP and Akrami A: Characterizing
long COVID in an international cohort: 7 months of symptoms and
their impact. EClinicalMedicine. 38(101019)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Al-Aly Z, Xie Y and Bowe B:
High-dimensional characterization of post-acute sequelae of
COVID-19. Nature. 594:259–264. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lopez-Leon S, Wegman-Ostrosky T, Perelman
C, Sepulveda R, Rebolledo PA, Cuapio A and Villapol S: More than 50
long-term effects of COVID-19: A systematic review and
meta-analysis. Sci Rep. 11(16144)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lamontagne SJ, Winters MF, Pizzagalli DA
and Olmstead MC: Post-acute sequelae of COVID-19: Evidence of mood
& cognitive impairment. Brain Behav Immun Health.
17(100347)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jesuthasan A, Massey F, Manji H, Zandi MS
and Wiethoff S: Emerging potential mechanisms and predispositions
to the neurological manifestations of COVID-19. J Neurol Sci.
428(117608)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dąbrowska E, Galińska-Skok B and
Waszkiewicz N: Depressive and neurocognitive disorders in the
context of the inflammatory background of COVID-19. Life (Basel).
11(1056)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Flores G: SARS-COV-2 (COVID-19) has
neurotropic and neuroinvasive properties. Int J Clin Pract.
75(e13708)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang
Y, Hu P, Guo L, Liu M, Xu J, et al: 1-year outcomes in hospital
survivors with COVID-19: A longitudinal cohort study. Lancet.
398:747–758. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lagadinou M, Kostopoulou E, Karatza A,
Marangos M and Gkentzi D: The prolonged effects of COVID-19. A New
‘Threat’? Eur Rev Med Pharmacol Sci. 25:4611–4615. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shanbehzadeh S, Tavahomi M, Zanjari N,
Ebrahimi-Takamjani I and Amiri-Arimi S: Physical and mental health
complications post-COVID-19: Scoping review. J Psychosom Res.
147(110525)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mazza MG, De Lorenzo R, Conte C, Poletti
S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F,
Rovere-Querini P, et al: Anxiety and depression in COVID-19
survivors: Role of inflammatory and clinical predictors. Brain
Behav Immun. 89:594–600. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mazza MG, Palladini M, De Lorenzo R,
Magnaghi C, Poletti S, Furlan R, Ciceri F, Rovere-Querini P and
Benedetti F: Persistent psychopathology and neurocognitive
impairment in COVID-19 survivors: Effect of inflammatory biomarkers
at three-month follow-up. Brain Behav Immun. 94:138–147.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Huang C, Huang L, Wang Y, Li X, Ren L, Gu
X, Kang L, Guo L, Liu M, Zhou X, et al: 6-month consequences of
COVID-19 in patients discharged from hospital: A cohort study.
Lancet. 397:220–232. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Taquet M, Geddes JR, Husain M, Luciano S
and Harrison PJ: 6-month neurological and psychiatric outcomes in
236 379 survivors of COVID-19: A retrospective cohort study using
electronic health records. Lancet Psychiatry. 8:416–427.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bourmistrova NW, Solomon T, Braude P,
Strawbridge R and Carter B: Long-term effects of COVID-19 on mental
health: A systematic review. J Affect Disord. 299:118–125.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Young AS, Klap R, Shoai R and Wells KB:
Persistent depression and anxiety in the United States: Prevalence
and quality of care. Psychiatr Serv. 59:1391–1398. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Renaud-Charest O, Lui LMW, Eskander S,
Ceban F, Ho R, Di Vincenzo JD, Rosenblat JD, Lee Y,
Subramaniapillai M and McIntyre RS: Onset and frequency of
depression in post-COVID-19 syndrome: A systematic review. J
Psychiatr Res. 144:129–137. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
De Lorenzo R, Cinel E, Cilla M, Compagnone
N, Ferrante M, Falbo E, Patrizi A, Castellani J, Magnaghi C,
Calvisi SL, et al: Physical and psychological sequelae at three
months after acute illness in COVID-19 survivors. Panminerva Med:
Jun 1, 2021 (Epub ahead of print). doi:
10.23736/S0031-0808.21.04399-8.
|
|
47
|
Sykes DL, Holdsworth L, Jawad N,
Gunasekera P, Morice AH and Crooks MG: Post-COVID-19 Symptom
Burden: What is Long-COVID and how should we manage it? Lung.
199:113–119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Morin L, Savale L, Pham T, Colle R,
Figueiredo S, Harrois A, Gasnier M, Lecoq AL, Meyrignac O and Noel
N: Four-month clinical status of a cohort of patients after
hospitalization for COVID-19. JAMA. 325:1525–1534. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Boari GEM, Bonetti S, Braglia-Orlandini F,
Chiarini G, Faustini C, Bianco G, Santagiuliana M, Guarinoni V,
Saottini M, Viola S, et al: Short-term consequences of
SARS-CoV-2-related pneumonia: A follow up study. High Blood Press
Cardiovasc Prev. 28:373–381. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gautam N, Madathil S, Tahani N, Bolton S,
Parekh D, Stockley J, Goyal S, Qureshi H, Yasmin S, Cooper BG, et
al: Medium-term outcome of severe to critically ill patients with
Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin
Infect Dis. 74:301–308. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Benedetti F, Palladini M, Paolini M,
Melloni E, Vai B, De Lorenzo R, Furlan R, Rovere-Querini P, Falini
A and Mazza MG: Brain correlates of depression, post-traumatic
distress, and inflammatory biomarkers in COVID-19 survivors: A
multimodal magnetic resonance imaging study. Brain Behav Immun
Health. 18(100387)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fernández-de-Las-Peñas C, Gómez-Mayordomo
V, de-la-Llave-Rincón AI, Palacios-Ceña M, Rodríguez-Jiménez J,
Florencio LL, Velasco-Arribas M, Fuensalida-Novo S, Cigarán-Méndez
M, Ambite-Quesada S, et al: Anxiety, depression and poor sleep
quality as long-term post-COVID sequelae in previously hospitalized
patients: A multicenter study. J Infect. 83:496–522.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pilotto A, Cristillo V, Cotti Piccinelli
S, Zoppi N, Bonzi G, Sattin D, Schiavolin S, Raggi A, Canale A,
Gipponi S, et al: Long-term neurological manifestations of
COVID-19: prevalence and predictive factors. Neurol Sci.
42:4903–4907. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Méndez R, Balanzá-Martínez V, Luperdi SC,
Estrada I, Latorre A, González-Jiménez P, Bouzas L, Yépez K,
Ferrando A, Reyes S, et al: Long-term neuropsychiatric outcomes in
COVID-19 survivors: A 1-year longitudinal study. J Intern Med.
291:247–251. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang X, Wang F, Shen Y, Zhang X, Cen Y,
Wang B, Zhao S, Zhou Y, Hu B, Wang M, et al: Symptoms and Health
Outcomes Among Survivors of COVID-19 Infection 1 Year After
Discharge From Hospitals in Wuhan, China. JAMA Netw Open.
4(e2127403)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Matalon N, Dorman-Ilan S, Hasson-Ohayon I,
Hertz-Palmor N, Shani S, Basel D, Gross R, Chen W, Abramovich A and
Afek A: Trajectories of post-traumatic stress symptoms, anxiety,
and depression in hospitalized COVID-19 patients: A one-month
follow-up. J Psychosom Res. 143(110399)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alemanno F, Houdayer E, Parma A, Spina A,
Del Forno A, Scatolini A, Angelone S, Brugliera L, Tettamanti A and
Beretta L: COVID-19 cognitive deficits after respiratory assistance
in the subacute phase: A COVID-rehabilitation unit experience. PLoS
One. 16(e0246590)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Halpin SJ, McIvor C, Whyatt G, Adams A,
Harvey O, McLean L, Walshaw C, Kemp S, Corrado J and Singh R:
Postdischarge symptoms and rehabilitation needs in survivors of
COVID-19 infection: A cross-sectional evaluation. J Med Virol.
93:1013–1022. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
de Graaf MA, Antoni ML, Ter Kuile MM,
Arbous MS, Duinisveld AJF, Feltkamp MCW, Groeneveld GH, Hinnen SCH,
Janssen VR, Lijfering WM, et al: Short-term outpatient follow-up of
COVID-19 patients: A multidisciplinary approach. EClinicalMedicine.
32(100731)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Albu S, Zozaya NR, Murillo N,
García-Molina A, Chacón CAF and Kumru H: What's going on following
acute COVID-19? Clinical characteristics of patients in an
out-patient rehabilitation program. NeuroRehabilitation.
48:469–480. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
van den Borst B, Peters JB, Brink M,
Schoon Y, Bleeker-Rovers CP, Schers H, van Hees HWH, van Helvoort
H, van den Boogaard M, van der Hoeven H, et al: Comprehensive
health assessment 3 months after recovery from acute coronavirus
disease 2019 (COVID-19). Clin Infect Dis. 73:e1089–e1098.
2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Rass V, Beer R, Schiefecker AJ, Kofler M,
Lindner A, Mahlknecht P, Heim B, Limmert V, Sahanic S, Pizzini A,
et al: Neurological outcome and quality of life 3 months after
COVID-19: A prospective observational cohort study. Eur J Neurol.
28:3348–3359. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yuan B, Li W, Liu H, Cai X, Song S, Zhao
J, Hu X, Li Z, Chen Y, Zhang K, et al: Correlation between immune
response and self-reported depression during convalescence from
COVID-19. Brain Behav Immun. 88:39–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wong AW, Shah AS, Johnston JC, Carlsten C
and Ryerson CJ: Patient-reported outcome measures after COVID-19: A
prospective cohort study. Eur Respir J. 56(200327)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Tomasoni D, Bai F, Castoldi R, Barbanotti
D, Falcinella C, Mulè G, Mondatore D, Tavelli A, Vegni E, Marchetti
G and d'Arminio Monforte A: Anxiety and depression symptoms after
virological clearance of COVID-19: A cross-sectional study in
Milan, Italy. J Med Virol. 93:1175–1179. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wu C, Hu X, Song J, Yang D, Xu J, Cheng K,
Chen D, Zhong M, Jiang J, Xiong W, et al: Mental health status and
related influencing factors of COVID-19 survivors in Wuhan, China.
Clin Transl Med. 10(e52)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Romero-Duarte Á, Rivera-Izquierdo M,
Guerrero-Fernández de Alba I, Pérez-Contreras M, Fernández-Martínez
NF, Ruiz-Montero R, Serrano-Ortiz Á, González-Serna RO,
Salcedo-Leal I, Jiménez-Mejías E and Cárdenas-Cruz A: Sequelae,
persistent symptomatology and outcomes after COVID-19
hospitalization: The ANCOHVID multicentre 6-month follow-up study.
BMC Med. 19(129)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kaseda ET and Levine AJ: Post-traumatic
stress disorder: A differential diagnostic consideration for
COVID-19 survivors. Clin Neuropsychol. 34:1498–1514.
2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Giannopoulou I, Galinaki S, Kollintza E,
Adamaki M, Kympouropoulos S, Alevyzakis E, Tsamakis K, Tsangaris I,
Spandidos DA, Siafakas N, et al: COVID-19 and post-traumatic stress
disorder: The perfect ‘storm’ for mental health (Review). Exp Ther
Med. 22(1162)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Naidu SB, Shah AJ, Saigal A, Smith C,
Brill SE, Goldring J, Hurst JR, Jarvis H, Lipman M and Mandal S:
The high mental health burden of ʻLong COVIDʼ and its association
with on-going physical and respiratory symptoms in all adults
discharged from hospital. Eur Respir J. 57(2004364)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Venturelli S, Benatti SV, Casati M, Binda
F, Zuglian G, Imeri G, Conti C, Biffi AM, Spada MS, Bondi E, et al:
Surviving COVID-19 in Bergamo province: A post-acute outpatient
re-evaluation. Epidemiol Infect. 149(e32)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Evans RA, McAuley H, Harrison EM, Shikotra
A, Singapuri A, Sereno M, Elneima O, Docherty AB, Lone NI, Leavy
OC, et al: Physical, cognitive, and mental health impacts of
COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre,
prospective cohort study. Lancet Respir Med. 9:1275–1287.
2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Bellan M, Soddu D, Balbo PE, Baricich A,
Zeppegno P, Avanzi GC, Baldon G, Bartolomei G, Battaglia M,
Battistini S, et al: Respiratory and psychophysical sequelae among
patients with COVID-19 four months after hospital discharge. JAMA
Network Open. 4(e2036142)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Horn M, Wathelet M, Fovet T, Amad A,
Vuotto F, Faure K, Astier T, Noël H, Henry M and Duhem S: Is
COVID-19 associated with posttraumatic stress disorder? J Clin
Psychiatry. 82(9886)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Tarsitani L, Vassalini P, Koukopoulos A,
Borrazzo C, Alessi F, Di Nicolantonio C, Serra R, Alessandri F,
Ceccarelli G, Mastroianni CM, et al: Post-traumatic stress disorder
among COVID-19 survivors at 3-month follow-up after hospital
discharge. J Gen Intern Med. 36:1702–1707. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
De Lorenzo R, Conte C, Lanzani C,
Benedetti F, Roveri L, Mazza MG, Brioni E, Giacalone G, Canti V,
Sofia V, et al: Residual clinical damage after COVID-19: A
retrospective and prospective observational cohort study. PLoS One.
15(e0239570)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Poyraz BÇ, Poyraz CA, Olgun Y, Gürel Ö,
Alkan S, Özdemir YE, Balkan İİ and Karaali R: Psychiatric morbidity
and protracted symptoms after COVID-19. Psychiatry Res.
295(113604)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nalbandian A, Sehgal K, Gupta A, Madhavan
MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat
TS, et al: Post-acute COVID-19 syndrome. Nat Med. 27:601–615.
2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Sun LL, Wang J, Wang YS, Hu PF, Zhao ZQ,
Chen W, Ning BF, Yin C, Hao YS, Wang Q, et al: Symptomatic features
and prognosis of 932 hospitalized patients with coronavirus disease
2019 in Wuhan. J Dig Dis. 22:271–281. 2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Augustin M, Schommers P, Stecher M, Dewald
F, Gieselmann L, Gruell H, Horn C, Vanshylla K, Cristanziano VD,
Osebold L, et al: Post-COVID syndrome in non-hospitalised patients
with COVID-19: A longitudinal prospective cohort study. Lancet Reg
Health Eur. 6(100122)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Shang YF, Liu T, Yu JN, Xu XR, Zahid KR,
Wei YC, Wang XH and Zhou FL: Half-year follow-up of patients
recovering from severe COVID-19: Analysis of symptoms and their
risk factors. J Intern Med. 290:444–450. 2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Grandner MA: Sleep, health, and society.
Sleep Med Clin. 12:1–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Freeman D, Sheaves B, Waite F, Harvey AG
and Harrison PJ: Sleep disturbance and psychiatric disorders.
Lancet Psychiatry. 7:628–637. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ceban F, Ling S, Lui LMW, Lee Y, Gill H,
Teopiz KM, Rodrigues NB, Subramaniapillai M, Di Vincenzo JD, Cao B,
et al: Fatigue and cognitive impairment in Post-COVID-19 Syndrome:
A systematic review and meta-analysis. Brain Behav Immun.
101:93–135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Townsend L, Dyer AH, Naughton A, Kiersey
R, Holden D, Gardiner M, Dowds J, O'Brien K, Bannan C, Nadarajan P,
et al: Longitudinal analysis of COVID-19 patients shows
age-associated T cell changes independent of ongoing Ill-health.
Front Immunol. 12(676932)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Fernández-de-Las-Peñas C, Palacios-Ceña D,
Gómez-Mayordomo V, Rodríuez-Jiménez J, Palacios-Ceña M,
Velasco-Arribas M, Guijarro C, de-la-Llave-Rincón AI,
Fuensalida-Novo S, Elvira-Martínez CM, et al: Long-term post-COVID
symptoms and associated risk factors in previously hospitalized
patients: A multicenter study. J Infect. 83:237–279.
2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
González-Hermosillo JA, Martínez-López JP,
Carrillo-Lampón SA, Ruiz-Ojeda D, Herrera-Ramírez S, Amezcua-Guerra
LM and Martínez-Alvarado MDR: Post-acute COVID-19 symptoms, a
potential Link with Myalgic Encephalomyelitis/Chronic fatigue
syndrome: A 6-month survey in a Mexican cohort. Brain Sci.
11(760)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Menges D, Ballouz T, Anagnostopoulos A,
Aschmann HE, Domenghino A, Fehr JS and Puhan MA: Burden of
post-COVID-19 syndrome and implications for healthcare service
planning: A population-based cohort study. PLoS One.
16(e0254523)2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Stavem K, Ghanima W, Olsen MK, Gilboe HM
and Einvik G: Prevalence and determinants of fatigue after COVID-19
in Non-hospitalized subjects: A Population-based study. Int J
Environ Res Public Health. 18(2030)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Peghin M, Palese A, Venturini M, De
Martino M, Gerussi V, Graziano E, Bontempo G, Marrella F, Tommasini
A, Fabris M, et al: Post-COVID-19 symptoms 6 months after acute
infection among hospitalized and non-hospitalized patients. Clin
Microbiol Infect. 27:1507–1513. 2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Liang L, Yang B, Jiang N, Fu W, He X, Zhou
Y, Ma WL and Wang X: Three-month Follow-up study of survivors of
coronavirus disease 2019 after discharge. J Korean Med Sci.
35:e418. 2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Daroische R, Hemminghyth MS, Eilertsen TH,
Breitve MH and Chwiszczuk LJ: Cognitive impairment after COVID-19-A
review on objective test data. Front Neurol.
12(699582)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Rogers JP, Chesney E, Oliver D, Pollak TA,
McGuire P, Fusar-Poli P, Zandi MS, Lewis G and David AS:
Psychiatric and neuropsychiatric presentations associated with
severe coronavirus infections: A systematic review and
meta-analysis with comparison to the COVID-19 pandemic. Lancet
Psychiatry. 7:611–627. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Méndez R, Balanzá-Martínez V, Luperdi SC,
Estrada I, Latorre A, González-Jiménez P, Feced L, Bouzas L, Yépez
K, Ferrando A, et al: Short-term neuropsychiatric outcomes and
quality of life in COVID-19 survivors. J Intern Med. 290:621–631.
2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Desforges M, Le Coupanec A, Dubeau P,
Bourgouin A, Lajoie L, Dubé M and Talbot PJ: Human coronaviruses
and other respiratory viruses: Underestimated opportunistic
pathogens of the central nervous system? Viruses.
12(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Li CW, Syue LS, Tsai YS, Li MC, Lo CL,
Tsai CS, Chen PL, Ko WC and Lee NY: Anosmia and olfactory tract
neuropathy in a case of COVID-19. J Microbiol Immunol Infect.
54:93–96. 2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Lin E, Lantos JE, Strauss SB, Phillips CD,
Campion TR Jr, Navi BB, Parikh NS, Merkler AE, Mir S, Zhang C, et
al: Brain imaging of patients with COVID-19: Findings at an
academic institution during the height of the outbreak in New York
City. AJNR Am J Neuroradiol. 41:2001–2008. 2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Lu S, Wei N, Jiang J, Wu L, Sheng J, Zhou
J, Fang Q, Chen Y, Zheng S, Chen F, et al: First report of
manic-like symptoms in a COVID-19 patient with no previous history
of a psychiatric disorder. J Affect Disord. 277:337–340.
2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Najjar S, Najjar A, Chong DJ, Pramanik BK,
Kirsch C, Kuzniecky RI, Pacia SV and Azhar S: Central nervous
system complications associated with SARS-CoV-2 infection:
Integrative concepts of pathophysiology and case reports. J
Neuroinflammation. 17(231)2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Muus C, Luecken MD, Eraslan G, Waghray A,
Heimberg G, Sikkema L, Kobayashi Y, Vaishnav ED, Subramanian A,
Smilie C, et al: Integrated analyses of single-cell atlases reveal
age, gender, and smoking status associations with cell
type-specific expression of mediators of SARS-CoV-2 viral entry and
highlights inflammatory programs in putative target cells. bioRxiv:
https://doi.org/10.1101/2020.04.19.049254.
|
|
101
|
Rhea EM, Logsdon AF, Hansen KM, Williams
LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA and Erickson
MA: The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in
mice. Nat Neurosci. 24:368–378. 2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Gheblawi M, Wang K, Viveiros A, Nguyen Q,
Zhong JC, Turner AJ, Raizada MK, Grant MB and Oudit GY:
Angiotensin-converting Enzyme 2: SARS-CoV-2 receptor and regulator
of the renin-angiotensin system: Celebrating the 20th anniversary
of the discovery of ACE2. Circ Res. 126:1456–1474. 2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Inoue Y, Tanaka N, Tanaka Y, Inoue S,
Morita K, Zhuang M, Hattori T and Sugamura K: Clathrin-dependent
entry of severe acute respiratory syndrome coronavirus into target
cells expressing ACE2 with the cytoplasmic tail deleted. J Virol.
81:8722–8729. 2007.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Wrapp D, Wang N, Corbett KS, Goldsmith JA,
Hsieh CL, Abiona O, Graham BS and McLellan JS: Cryo-EM structure of
the 2019-nCoV spike in the prefusion conformation. Science.
367:1260–1263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ,
Du P, Wei D, Zhang Y, Sun XX, Gong L, et al: CD147-spike protein is
a novel route for SARS-CoV-2 infection to host cells. Signal
Transduct Target Ther. 5(283)2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Dantzer R: Neuroimmune Interactions: From
the Brain to the immune system and vice versa. Physiol Rev.
98:477–504. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Ye Q, Wang B and Mao J: The pathogenesis
and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect.
80:607–613. 2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Park MD: Macrophages: A Trojan horse in
COVID-19? Nat Rev Immunol. 20(351)2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Cameron MJ, Bermejo-Martin JF, Danesh A,
Muller MP and Kelvin DJ: Human immunopathogenesis of severe acute
respiratory syndrome (SARS). Virus Res. 133:13–19. 2008.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Miller AH and Raison CL: The role of
inflammation in depression: From evolutionary imperative to modern
treatment target. Nat Rev Immunol. 16:22–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Ozbay F, Johnson DC, Dimoulas E, Morgan
CA, Charney D and Southwick S: Social support and resilience to
stress: From neurobiology to clinical practice. Psychiatry
(Edgmont). 4:35–40. 2007.PubMed/NCBI
|
|
112
|
Smith SM and Vale WW: The role of the
hypothalamic-pituitary-adrenal axis in neuroendocrine responses to
stress. Dialogues Clin Neurosci. 8:383–395. 2006.PubMed/NCBI View Article : Google Scholar
|
|
113
|
de Pablos RM, Herrera AJ, Espinosa-Oliva
AM, Sarmiento M, Muñoz MF, Machado A and Venero JL: Chronic stress
enhances microglia activation and exacerbates death of nigral
dopaminergic neurons under conditions of inflammation. J
Neuroinflammation. 11(34)2014.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Schoenfeld TJ and Gould E: Stress, stress
hormones, and adult neurogenesis. Exp Neurol. 233:12–21.
2012.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Futtrup J, Margolinsky R, Benros ME, Moos
T, Routhe LJ, Rungby J and Krogh J: Blood-brain barrier pathology
in patients with severe mental disorders: A systematic review and
meta-analysis of biomarkers in case-control studies. Brain Behav
Immun Health. 6(100102)2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Tremblay ME, Madore C, Bordeleau M, Tian L
and Verkhratsky A: Neuropathobiology of COVID-19: The role for
glia. Front Cell Neurosci. 14(592214)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu
H, Shi C and Hu S: The landscape of cognitive function in recovered
COVID-19 patients. J Psychiatr Res. 129:98–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Mazza MG, Lucchi S, Tringali AGM, Rossetti
A, Botti ER and Clerici M: Neutrophil/lymphocyte ratio and
platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog
Neuropsychopharmacol Biol Psychiatry. 84:229–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Escartin C, Galea E, Lakatos A,
O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra
A, Carmignoto G, Agarwal A, et al: Reactive astrocyte nomenclature,
definitions, and future directions. Nat Neurosci. 24:312–325.
2021.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Lee MH, Perl DP, Nair G, Li W, Maric D,
Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, et al:
Microvascular Injury in the Brains of patients with Covid-19. N
Engl J Med. 384:481–483. 2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Dietz AG, Goldman SA and Nedergaard M:
Glial cells in schizophrenia: A unified hypothesis. Lancet
Psychiatry. 7:272–281. 2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Verkhratsky A, Rodríguez JJ and Steardo L:
Astrogliopathology: A central element of neuropsychiatric diseases?
Neuroscientist. 20:576–588. 2014.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Barbic F, Heusser K, Minonzio M, Shiffer
D, Cairo B, Tank J, Jordan J, Diedrich A, Gauger P, Zamuner RA, et
al: Effects of prolonged head-down bed rest on cardiac and vascular
baroreceptor modulation and orthostatic tolerance in healthy
individuals. Front Physiol. 10(1061)2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Beck L, Baisch F, Gaffney FA, Buckey JC,
Arbeille P, Patat F, Ten Harkel AD, Hillebrecht A, Schulz H,
Karemaker JM, et al: Cardiovascular response to lower body negative
pressure before, during, and after ten days head-down tilt bedrest.
Acta Physiol Scand Suppl. 604:43–52. 1992.PubMed/NCBI
|
|
125
|
Goldstein DS, Vernikos J, Holmes C and
Convertino VA: Catecholaminergic effects of prolonged head-down bed
rest. J Appl Physiol (1985). 78:1023–1029. 1995.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Kamiya A, Michikami D, Fu Q, Iwase S,
Hayano J, Kawada T, Mano T and Sunagawa K: Pathophysiology of
orthostatic hypotension after bed rest: Paradoxical sympathetic
withdrawal. Am J Physiol Heart Circ Physiol. 285:H1158–H1167.
2003.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Guilmot A, Maldonado Slootjes S, Sellimi
A, Bronchain M, Hanseeuw B, Belkhir L, Yombi JC, De Greef J, Pothen
L, Yildiz H, et al: Immune-mediated neurological syndromes in
SARS-CoV-2-infected patients. J Neurol. 268:751–757.
2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Follmer C: Viral infection-induced gut
dysbiosis, neuroinflammation, and α-synuclein aggregation: Updates
and perspectives on COVID-19 and neurodegenerative disorders. ACS
Chem Neurosci. 11:4012–4016. 2020.PubMed/NCBI View Article : Google Scholar
|
|
129
|
McAlpine LS, Zubair AS, Maran I, Chojecka
P, Lleva P, Jasne AS, Navaratnam D, Matouk C, Schindler J, Sheth
KN, et al: Ischemic stroke, inflammation, and endotheliopathy in
COVID-19 patients. Stroke. 52:e233–e238. 2021.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Wang Z, Yang Y, Liang X, Gao B, Liu M, Li
W, Chen Z and Wang Z: COVID-19 associated ischemic stroke and
hemorrhagic stroke: Incidence, potential pathological mechanism,
and management. Front Neurol. 11(571996)2020.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Lu Y, Li X, Geng D, Mei N, Wu PY, Huang
CC, Jia T, Zhao Y, Wang D, Xiao A and Yin B: Cerebral
Micro-structural changes in COVID-19 patients-an MRI-based 3-month
Follow-up study. EClinicalMedicine. 25(100484)2020.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Sulzer D, Antonini A, Leta V, Nordvig A,
Smeyne RJ, Goldman JE, Al-Dalahmah O, Zecca L, Sette A, Bubacco L,
et al: COVID-19 and possible links with Parkinson's disease and
parkinsonism: From bench to bedside. NPJ Parkinsons Dis.
6(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Guedj E, Campion JY, Dudouet P, Kaphan E,
Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P,
Ceccaldi M, et al: 18F-FDG brain PET hypometabolism in
patients with long COVID. Eur J Nucl Med Mol Imaging. 48:2823–2833.
2021.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Attademo L and Bernardini F: Are dopamine
and serotonin involved in COVID-19 pathophysiology? Eur J
Psychiatry. 35:62–63. 2021.PubMed/NCBI View Article : Google Scholar
|















