Introduction
Food allergy (FA) is an rapidly growing public
health problem worldwide (1-4).
In recent years, the incidence of FA has been increasing (5). FA can cause a series of allergic
disease complications such as hives, asthma and diarrhea, which can
put human health at risk (6,7).
Food allergens are usually some proteins that can cause
hypersensitive responses. The main allergic responses are
attributed to only a limited variety of proteins that are
considered the main allergens of foods (8). Among the limited proteins, ovalbumin
(OVA), which accounts for 54% of the protein in egg white, is a
major cause of allergic reactions in humans, especially in children
(9,10).
A number of studies have demonstrated that most FAs
are immunoglobulin E (IgE)-mediated type 1 hypersensitivity
reactions, which depend on antigen-specific differentiation of
helper T cell (Th) 2 cells in the sensitization phase and
degranulation and cytokine production of mast cells and basophils
in the effect phase (11-13).
MAPK signaling serves an important role in the cell
differentiation, cell activation, cell proliferation, degranulation
and cell migration of various immune cells (14). MAPK signaling is involved in mast
cell regulating the production of cytokines in response to specific
extracellular stimuli and then initiates biological reactions
(15). Among the kinases, p38
participates in the production of proinflammatory cytokines by
regulating the expression of NF-κB (16).
Bisdemethoxycurcumin (BDMC) is an ingredient derived
from turmeric in addition to curcumin, which has been shown to have
effects on food allergies and allergic rhinitis (17-19).
BDMC is a relatively stable component in vivo and is more
readily absorbed into the cell nucleus than curcumin (20,21).
BDMC possesses anticancer, antioxidant and antibacterial properties
(22-24).
Additionally, BDMC has been shown to have inhibitory effects on
mice with OVA-induced allergic rhinitis in our previous study
(25). In the present study, the
effects of BDMC were evaluated in a murine model of FA.
Materials and methods
Mice
In total, 36 female BALB/c mice weighing 18-22 g
were purchased from Liaoning Changsheng Biotechnology Co., Ltd.
Mice were housed in an air-conditioned room (temperature 25±2˚C,
relative humidity 55±5%) with a 12 h light/dark cycle and ad
libitum food and water. All animal experiments were approved by
the Institutional Animal Care and Use Committee of Jilin University
(approval no. 20200050).
Induction of FA mice
To induce FA, 36 mice were divided into the Control
group, OVA group, BDMC low-dose group (100 mg/kg) and BDMC
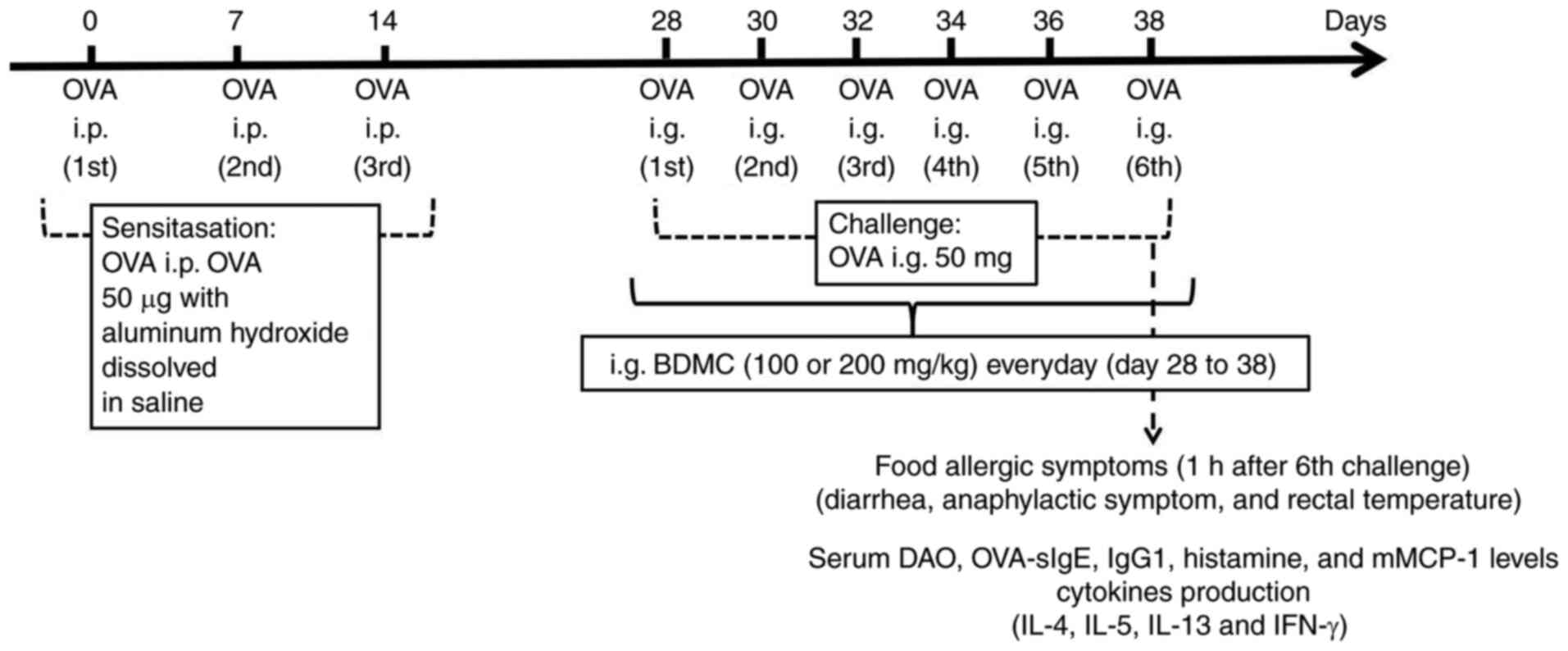
high-dose group (200 mg/kg; n=9/group). As previously described
(17,18) and depicted in Fig. 1, the mice in all groups except the
Control group were intraperitoneally (i.p.) injected with 50 µg OVA
(MilliporeSigma) in 50 µl aluminum hydroxide (2 mg; Beijing
Solarbio Science & Technology Co., Ltd.) dissolved in saline on
days 0, 7 and 14 and the Control group was administered i.p. saline
injections of the same amount. From day 28-38, all groups except
the Control group were challenged intragastrically (i.g.) with 50
mg OVA, which was dissolved in 250 µl phosphate buffered saline
(PBS) every other day for a total of six times and the Control
group was given the same solvent. Mice were starved for 3-4 h
before each intragastric challenge to ensure that the OVA antigen
could quickly pass through the stomach without being destroyed by
gastric acid.
Drug treatment
Following our previous research (25), drug treatment groups were orally
treated with BDMC (100 and 200 mg/kg; MilliporeSigma) in 1% carboxy
methyl cellulose (CMC) every day from day 28-38. The Control group
and OVA group were given only 1% CMC. The mice were anesthetized
with an intraperitoneal injection of 50 mg/kg pentobarbital sodium
and sacrificed by cervical dislocation 1 h after the sixth
challenge.
Evaluation of allergic symptoms
Diarrhea scores were estimated as: 0, normal stools;
1, a little wet, unshaped stools; 2, a small amount of wet and
unshaped stools with moderate perianal staining of the coat; and 3,
severe and watery stools with severe perianal staining of the coat
(17). Anaphylactic symptoms were
evaluated according to a scoring system from previous reports
(26). Symptom scores were rated
as follows: 0, no symptoms; 1, scratching around the nose and head;
2, swelling around the eyes and mouth; 3, wheezing, difficult
respiration, cyanosis around the mouth and tail; 4, no activity
after stimulation or tremors and convulsions; and 5, death. Rectal
temperature was measured by a thermometer within 60 min and the
rectal temperature change of the mice in each group was also
calculated.
Histological analysis
The jejunum tissues were fixed in 10% neutral
formalin for 4 h at room temperature (RT) and embedded in paraffin.
The slides (4-µm thick) were stained with hematoxylin and eosin
(HE) for 30 sec at RT. The numbers of inflammatory infiltrates in
individual samples were measured in a blinded manner. The number of
inflammatory cells in the five fields with the most infiltrates for
each mouse was calculated in a blinded manner. Images were obtained
using a light microscope (Leica Microsystems, Inc.; x400
magnification) for detection of inflammatory cell infiltration.
Enzyme-linked immunosorbent assay
The levels of OVA-specific immunoglobulin
(OVA-sIg)E, IgG1, histamine, mouse mast cell protease-1 (mMCP-1),
diamine oxidase (DAO) and cytokines (IL-4, IL-5, and IL-13) and
interferon-γ (IFN-γ) in serum were analyzed by commercially
available ELISA kits (Shanghai Enzyme-linked Biotechnology Co.,
Ltd.), according to the manufacturer's instructions. OVA-sIgE (cat.
no. ml063583), OVA-sIgG1 (cat. no. ml037615), histamine (cat. no.
ml001877), mMCP-1 (cat. no. ml037840), DAO (cat. no. ml002199-C),
IL-4 (cat. no. ml063156-J), IL-5 (cat. no. ml063157) and IL-13
(cat. no. ml063157).
Western blot analysis
Total proteins were resolved from the mouse
intestinal jejunum tissue with RIPA buffer (Genstar Biosolutions
Co., Ltd.) supplemented with protease inhibitors, including PMSF
(Genstar Biosolutions Co., Ltd.). The protein concentrations were
measured using the BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Protein (~20-40 µg) was electrophoresed on 10%
SDS-PAGE and then electroporated with a PVDF membrane. The
separated proteins were transferred to PVDF membranes (Beyotime
Institute of Biotechnology), which then were put into TBS-T
(Tris-buffered saline, 0.1% Tween 20) solution containing 5%
skimmed milk and shaken for 2 h at room temperature to avoid
non-specific binding. Then, the membranes were incubated with
primary antibodies (1:1,000) at 4˚C overnight and then incubated
with specific HRP-conjugated secondary antibodies (1:4,000) for 1 h
at room temperature. Finally, the signals were visualized using an
enhanced chemiluminescence reagent. The densitometry was calculated
using ImageJ (version no. 20150116; National Institutes of Health).
Anti-p38 (cat. no. bs-33423M), anti-phosphorylated (p)-p38 (cat.
no. bs-0636R), anti-JNK (cat. no. bs-0636R), anti-p-JNK (cat. no.
bs-4163R), anti-ERK (cat. no. bsm-33337M), anti-p-ERK (cat. no.
bs-3016R), anti-IκBα (cat. no. bs-1287R), anti-p-IκBα (cat. no.
bs-5514R), anti-NF-κB p65 (cat. no. bs-23216R), anti-p-NF-κB p65
(cat. no. bs-0982R) primary antibodies were purchased from BIOSS;
anti-β-actin (cat. no. AA128) primary antibody was purchased from
Beyotime Institute of Biotechnology. Goat anti-mouse IgG (cat. no.
NC-AP124P) and goat anti-rabbit IgG (cat. no. NC-AP1332P) secondary
antibodies were purchased from Changchun Changsheng Life
Sciences.
Statistical analysis
Data analyses were performed using the SPSS 20.0
statistical software package (IBM Corp.) and GraphPad Prism 6.2
software (GraphPad Software, Inc.). The experimental data were
expressed as the mean ± standard deviation (SD) or individual
values. One-way analysis of variance was used to evaluated
significant differences between multiple groups, followed by
Dunnett's post hoc test using GraphPad Prism software (version 5.0;
GraphPad Software, Inc.). For diarrhea and symptom scores,
Kruskall-Wallis followed by Dunn's multiple comparison test was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
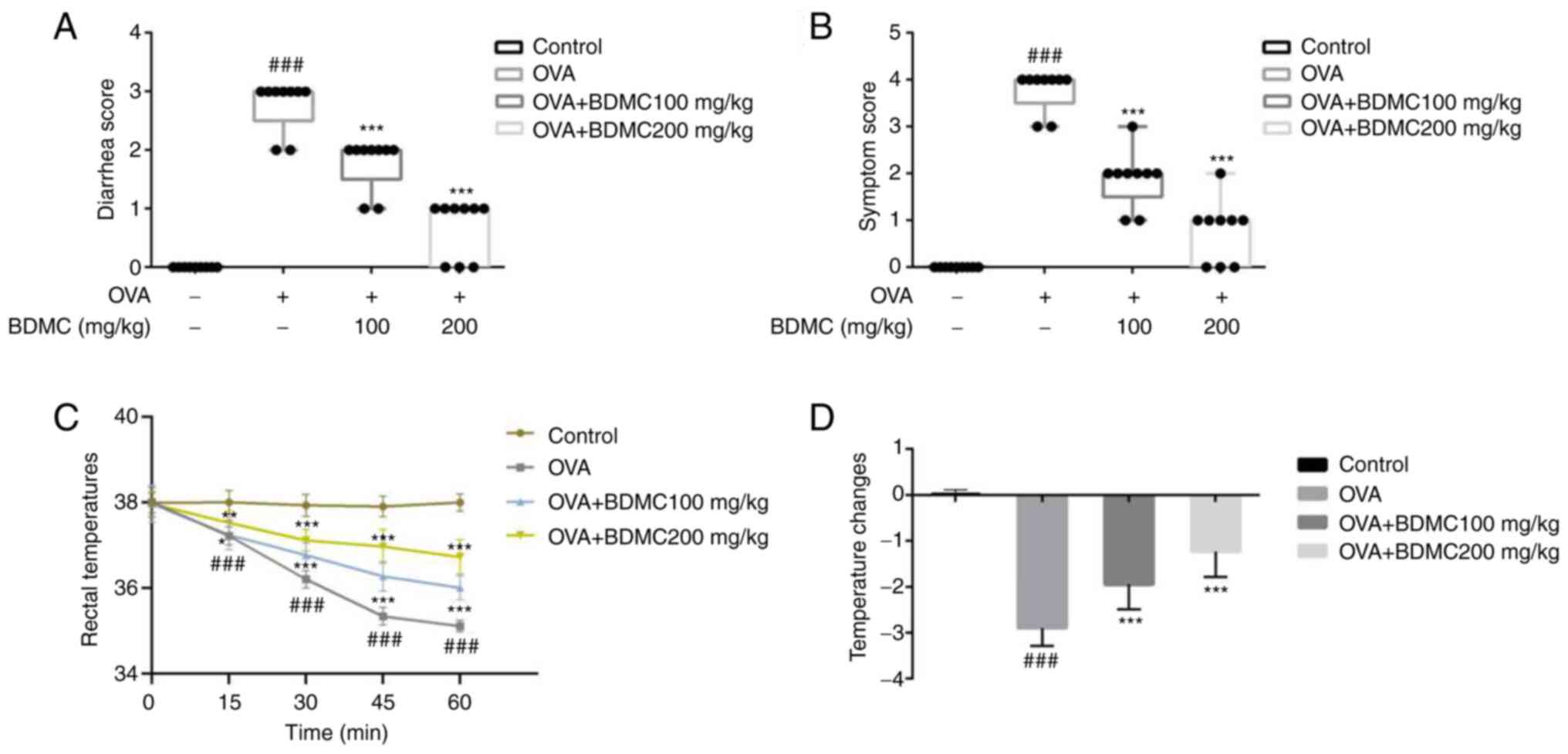
Effect of BDMC on FA symptoms
The results showed that while OVA group mice
exhibited severe profuse diarrhea compared with the Control group,
the BDMC low-dose group, BDMC high-dose group mice showed a lower
diarrhea score compared with the OVA group (Fig. 2A). Similarly, OVA group mice
exhibited severe anaphylaxis reactions compared with the Control
group. In contrast, the anaphylaxis symptom scores of the mice in
the BDMC low-dose group and the BDMC high-dose group were reduced
(Fig. 2B). The results showed that
the rectal temperature of mice in the OVA group decreased by 3.01˚C
within 60 min compared with the Control group. Furthermore,
decreased rectal temperature was significantly suppressed by BDMC
in a dose-dependent manner (Fig.
2C). The results also indicated that the rectal temperature
changes in the OVA group was significantly decreased, while
treatment with BDMC attenuated the decrease in rectal temperature
in mice (Fig. 2D).
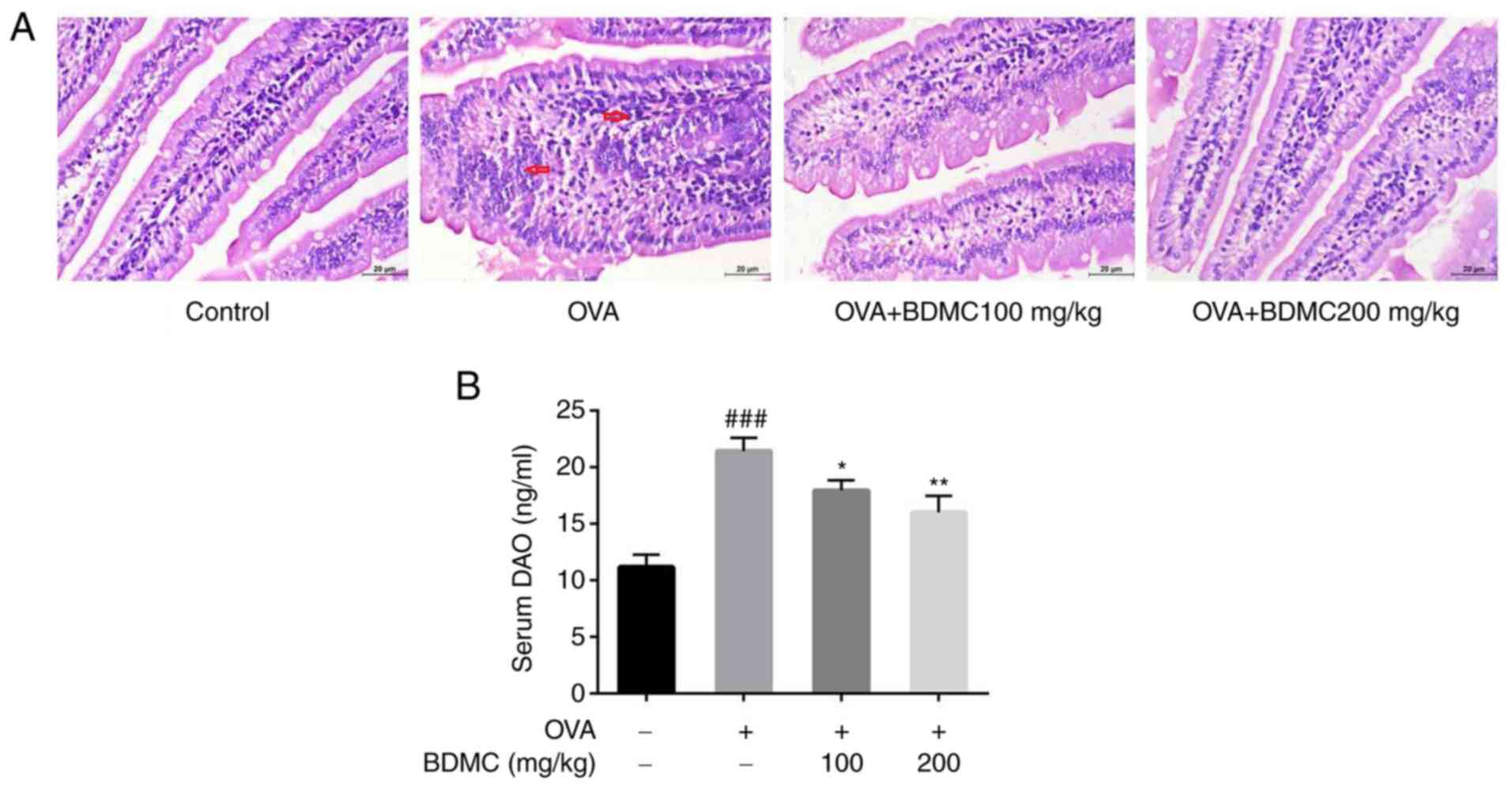
Effect of BDMC on the histology of
jejunum tissue and DAO levels in serum
For improved understanding of the effect of BDMC on
the intestinal tissue in FA mice, HE staining of the jejunum tissue
of each group of mice was performed. As shown in Fig. 3A, the OVA group showed shorter and
blunt villi and obvious inflammatory cell infiltration and
intestinal villus edema compared with the Control group. However,
intestinal villus edema was relieved, inflammatory cell
infiltration was significantly improved and intestinal tissue
morphology was relatively complete when treated with different
doses of BDMC. Similarly, the level of DAO in serum of OVA group
mice was significantly higher than the Control group. Compared with
the OVA group, the level of DAO in serum in both the BDMC low-dose
and high-dose groups exhibited a significant decrease (Fig. 3B).
Effect of BDMC on the levels of
OVA-sIgE, OVA-sIgG1, histamine and mMCP-1 in serum
Compared with the Control group, mice in the OVA
group produced higher levels of OVA-sIgE and OVA-sIgG1. BDMC
treatment significantly reduced the levels of serum OVA-sIgE
(Fig. 4A) and OVA-sIgG1 (Fig. 4B). Additionally, compared with
those in the Control group, histamine (Fig. 4C) and mMCP-1 (Fig. 4D) levels in serum in the OVA group
were significantly increased and their levels were decreased
significantly after the administration of BDMC.
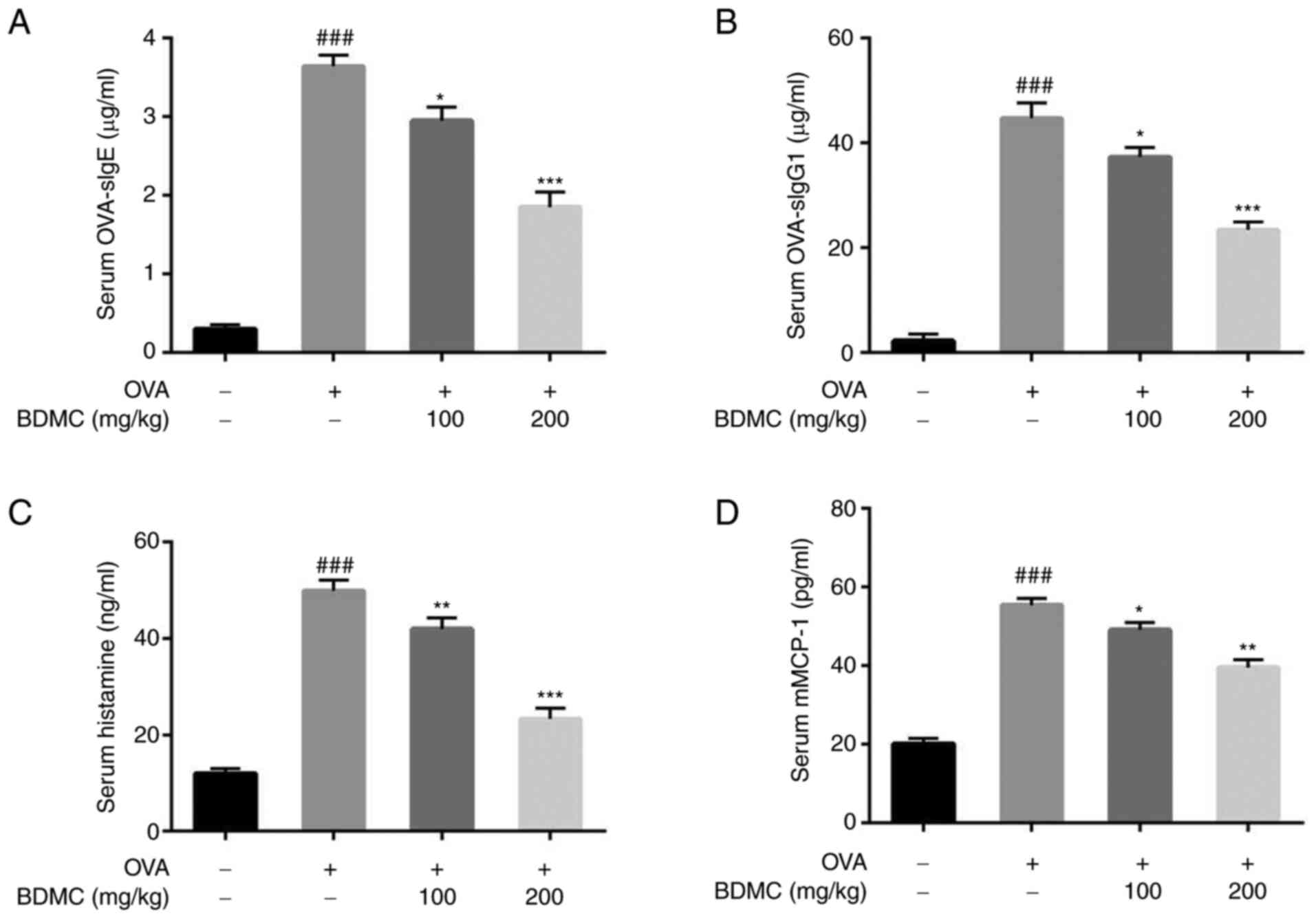 | Figure 4Effect of BDMC on OVA-sIgE,
OVA-sIgG1, histamine, mMCP-1 levels in serum. Level of serum (A)
OVA-sIgE, (B) OVA-sIgG1, (C) histamine and (D) mMCP-1 of mice in
all groups. The data are presented as the mean ± standard
deviation. Data are representative of 3 independent experiments.
n=9/group. ###P<0.001 vs. Control group;
*P<0.05, **P<0.01,
***P<0.001 vs. OVA group. BDMC, bisdemethoxycurcumin;
OVA-sIg, OVA-specific immunoglobulin; mMCP-1, mouse mast cell
protease-1; OVA, ovalbumin. |
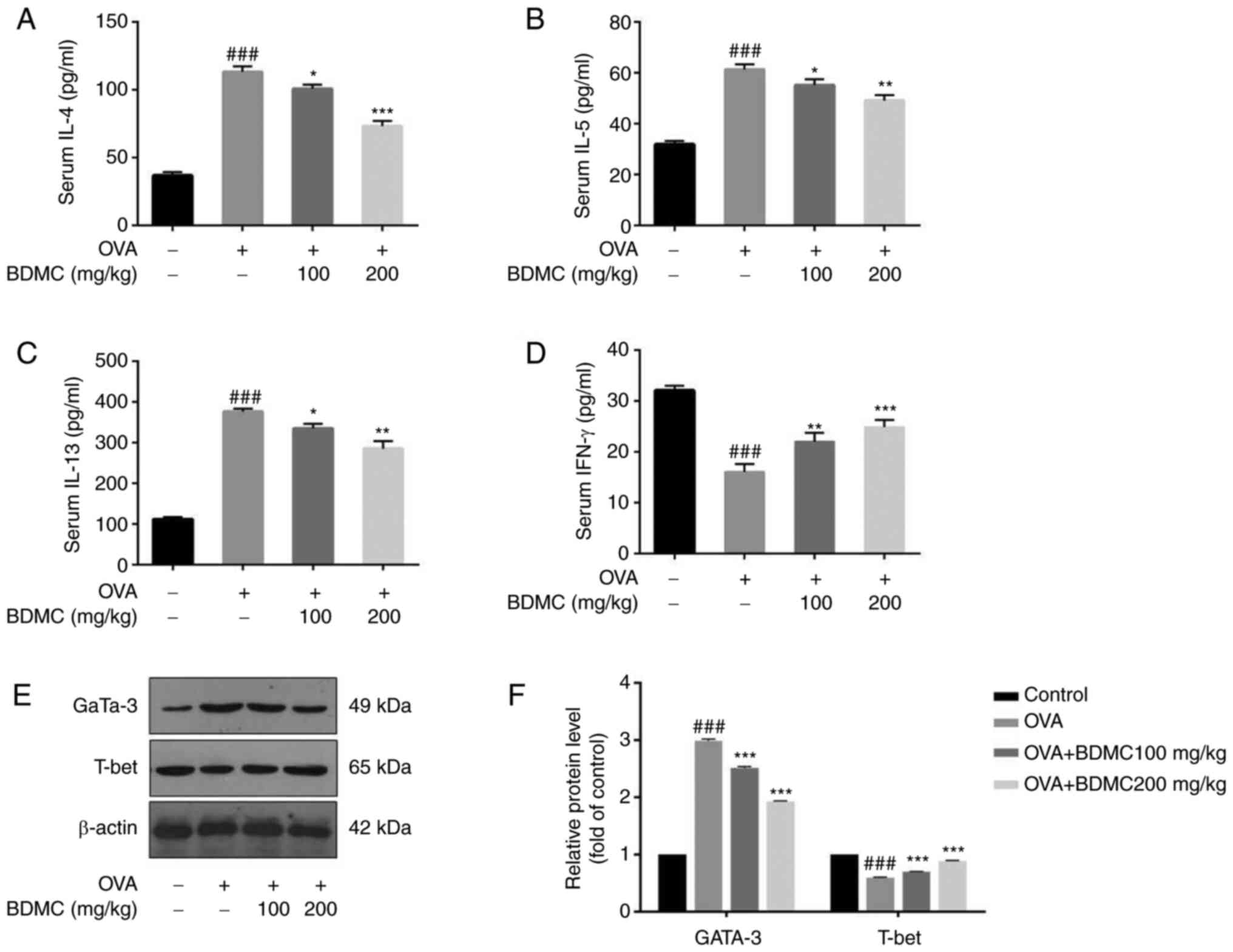
Effect of BDMC on the levels of
cytokines in serum
In the OVA group, Th2 cytokines (IL-4, IL-5 and
IL-13) were increased and oral treatment with different doses of
BDMC significantly reduced its levels (Fig. 5A, B, C).
T-helper cells (Th)1 cytokine, such as IFN-γ, was decreased in the
OVA group and its level was increased after BDMC treatment
(Fig. 5D). In particular, the BDMC
high-dose group (200 mg/kg) suppressed the production of IL-4, IL-5
and IL-13 and increased IFN-γ production.
In addition, the key transcription factors T-bet for
the Th1 immune response and Gata-3 for the Th2 immune response were
checked at the protein level and the results were similar to the
results of Th1/Th2 cytokines. The protein level of Gata-3 in the
OVA group was upregulated, while the protein level of T-bet was
significantly downregulated compared with the Control group.
However, after BDMC treatment, the protein level of Gata-3 was
downregulated, while the protein level of T-bet was upregulated in
a dose-dependent manner (Fig. 5E
and F).
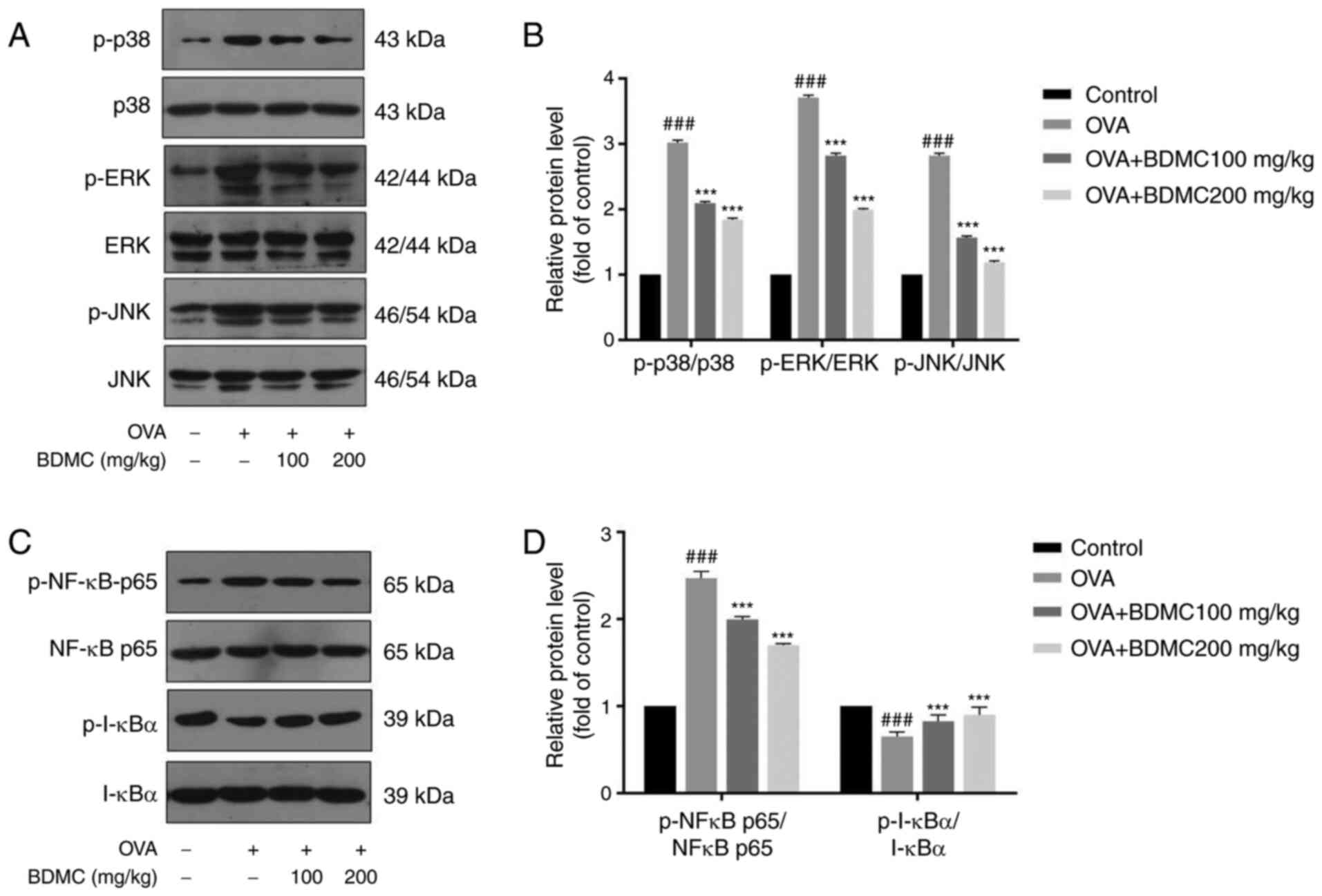
Effect of BDMC on the activation of
MAPK and NF-κB pathway
The expression levels of the related proteins of
MAPK pathway such as p-p38, p38, p-JNK, JNK, p-ERK and ERK were
evaluated by western blot analysis (Fig. 6A and B). The results demonstrated that the
levels of p-p38, p-JNK and p-ERK in the OVA group were markedly
upregulated compared with those in the Control group. When treated
with different doses of BDMC, their expression levels were
dose-dependently downregulated. Additionally, the expression levels
of the NF-κB pathway-related proteins p-NF-κBp65 and p-IκBα were
evaluated (Fig. 6C and D). Similarly, the results showed that the
protein expression level of p-NF-κBp65 in the OVA group was
upregulated and the protein expression level of p-IκBα was
downregulated compared with the Control group. Particularly, BDMC
reversed their expression levels.
Discussion
Our previous study found that BDMC possesses
inhibitory effects on mice with OVA-induced allergic rhinitis and
other allergic diseases (25), but
its anti-food allergies have not been studied. FA is a type of
immune adverse reaction caused by food, which includes a range of
disorders such as IgE-mediated anaphylaxis, a decrease in rectal
temperature and gastrointestinal adverse reactions (27,28).
FA symptoms have previously been reported to be the result of a
complex immune response involving the systemic, gastrointestinal
and mucosal immune systems (29,30).
Anaphylactic symptoms and rectal temperature are associated with
systemic immune responses and diarrhetic symptoms are related to
the gastrointestinal immune system (30). In the present study, the diarrhea
scores and symptom scores were significantly reduced following BDMC
treatment. The administration of different doses of BDMC also
significantly reduced the rectal temperature of mice with FA.
Therefore, the results indicated that BDMC has an important effect
on systemic immunity and the gastrointestinal immunity.
According to previous reports, mice with FA exhibit
intestinal tissue inflammatory cell infiltration and severe edema
of intestinal tissue (31,32). In line with these studies, the HE
staining results of jejunum tissue in the present study confirmed
that BDMC can reduce inflammatory cell infiltration and intestinal
villus edema to maintain the integrity of the intestinal tissue
structure. In particular, DAO is one of the most important enzymes
that is regarded as an indicator of changes in intestinal
permeability in FA mice (33).
OVA-induced FA mice have been reported to have elevated expression
levels of DAO (34). Consistent
with these studies, the results of the present study showed that
BDMC could reduce the level of serum DAO of FA mice to maintain the
integrity of the intestinal mucosa.
FA is mainly a hypersensitivity response mediated by
IgE that binds primarily to the high-affinity IgE receptor (FcεRI)
and cross-links with mast cells allergen. Allergen-stimulated mast
cells cause their degranulation; then, histamine and mMCP-1 are
released, causing allergic symptoms (32,35).
Other immunoglobulins (such as IgG1) also serve a key role in the
basic regulatory mechanism of allergic inflammation (36). In addition, histamine is a key
mediator that can cause allergic symptoms in FA mice and mMCP-1 is
one of the mast cell proteases found in mucosal mast cells
(37-39).
Previous studies have shown that OVA-sIgE, OVA-sIgG1, histamine and
mMCP-1 levels are significantly increased in mice with FA (40-42).
Consistent with these studies, the results of the present study
demonstrated that BDMC could decrease the levels of OVA-sIgE,
OVA-sIgG1, histamine and mMCP-1 in serum to exert effects against
food allergies.
Following ingestion of food allergens, T cells from
intestinal-associated lymphoid tissues, spleen and many other
immune tissues are activated to differentiate into other Th cell
lineages (43,44). Among them, immune cells including
CD4+ T cells secret cytokines (IL-4, IL-5, IL-13 and
IFN-γ) to maintain the immune balance (45,46).
In particular, it is reported that serum IL-4, IL-5 and IL-13
levels are higher and IFN-γ levels are lower in FA mice (17). Based on the above studies, of the
present study indicated that BDMC not only significantly increased
the levels of IL-4, IL-5 and IL-13 but also significantly decreased
the level of IFN-γ.
Regarding protein expression, T-bet, the key nuclear
transcription factor of the Th1-type immune response and Gata-3,
the key nuclear transcription factor of the Th2-type immune
response, have been reported (26). The results of the present study
also demonstrated that BDMC significantly increased the expression
of T-bet and decreased the expression level of Gata-3 in FA mice.
Therefore, the data demonstrated that BDMC improves the Th1/Th2
response balance to attenuates FA symptoms.
Additionally, the activation of basophils and mast
cells includes a complex network of signaling pathways and
molecules, including a type of tyrosine kinases and MAPKs (47). p38 MAPK is important for human
allergic reactions (48).
Furthermore, NF-κB activation typically requires the
phosphorylation of IκB by the IκB kinase complex, which results in
IκB degradation and subsequent translocation of NF-κB to the
nucleus, which serves an important role of innate immune defense
(49,50). It has also been reported that BDMC
is able to inhibit cytokine secretion and the activation of NF-κB
and the breakdown of IκB and improve human mast cell inflammation
by inhibiting MAPK and NF-κB pathways (51). Based on the above studies, the
present study demonstrated that BDMC could inhibit the activation
of the MAPK signaling pathway and nuclear translocation of NF-κB in
FA mice. The significance of this result is crucial for the
treatment of FA.
In conclusion, the present study shows that BDMC has
inhibitory effects on mice with OVA-induced food allergy. The
effectiveness of the mechanism underlying BDMC may involve
regulating the Th1/Th2 balance and inhibiting the activation of the
MAPK and NF-κB pathway in FA mice. This discovery may have
important implications for the treatment and further research of
FA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW and TH conceived and designed the study. YW, PZ
and JZ collected and analyzed the data. YW and TH confirm the
authenticity of all the raw data and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Institutional Animal Care and Use Committee of Jilin University
(approval no. 20200050).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prescott S and Allen KJ: Food allergy:
Riding the second wave of the allergy epidemic. Pediatr Allergy
Immunol. 22:155–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johnston LK, Chien KB and Bryce PJ: The
immunology of food allergy. J Immunol. 192:2529–2534.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sicherer SH: Epidemiology of food allergy.
J Allergy Clin Immunol. 127:594–602. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sicherer SH and Sampson HA: Food allergy:
Recent advances in pathophysiology and treatment. Annu Rev Med.
60:261–277. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Venter C and Arshad SH: Epidemiology of
food allergy. Pediatr Clin North Am. 58:327–349. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Krogulska A, Polakowska E,
Wąsowska-Królikowska K, Małachowska B, Młynarski W and Borowiec M:
Decreased FOXP3 mRNA expression in children with atopic asthma and
IgE-mediated food allergy. Ann Allergy Asthma Immunol. 115:415–421.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kucuk ZY, Strait R, Khodoun MV, Mahler A,
Hogan S and Finkelman FD: Induction and suppression of allergic
diarrhea and systemic anaphylaxis in a murine model of food
allergy. J Allergy Clin Immunol. 129:1343–1348. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gendel SM: Bioinformatics and Food
Allergens. J AOAC Int. 87:1417–1422. 2004.PubMed/NCBI
|
|
9
|
Bernhisel-Broadbent J, Dintzis HM, Dintzis
RZ and Sampson HA: Allergenicity and antigenicity of chicken egg
ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children
with egg allergy and in mice. J Allergy Clin Immunol. 93:1047–1059.
1994.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Van Asperen P, Kemp AS and Mellis CM: Skin
test reactivity and clinical allergen sensitivity in infancy. J
Allergy Clin Immunol. 73:381–386. 1984.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sampson HA: Food allergy. Part 1:
Immunopathogenesis and clinical disorders. J Allergy Clin Immunol.
103 (5 Pt 1):717–728. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu W, Freeland DMH and Nadeau KC: Food
allergy: Immune mechanisms, diagnosis and immunotherapy. Nat Rev
Immunol. 16:751–765. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumar S, Verma AK, Das M and Dwivedi PD:
Molecular mechanisms of IgE mediated food allergy. Int
Immunopharmacol. 13:432–439. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Duan W and Wong WS: Targeting
mitogen-activated protein kinases for asthma. Curr Drug Targets.
7:691–698. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li L, Zhang XH, Liu GR, Liu C and Dong YM:
Isoquercitrin suppresses the expression of histamine and
pro-inflammatory cytokines by inhibiting the activation of MAP
Kinases and NF-κB in human KU812 cells. Chin J Nat Med. 14:407–412.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou Y, Yang Q, Xu H, Zhang J, Deng H, Gao
H, Yang J, Zhao D and Liu F: MiRNA-221-3p Enhances the secretion of
interleukin-4 in mast cells through the phosphatase and tensin
Homolog/p38/Nuclear Factor-kappaB pathway. PLoS One.
11(e0148821)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shin HS, See HJ, Jung SY, Choi DW, Kwon
DA, Bae MJ, Sung KS and Shon DH: Turmeric (Curcuma longa)
attenuates food allergy symptoms by regulating type 1/2 helper T
cells (Th1/Th2) balance in a mouse model of food allergy. J
Ethnopharmacol. 175:21–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kinney SR, Carlson L, Ser-Dolansky J,
Thompson C, Shah S, Gambrah A, Xing W, Schneider SS and Mathias CB:
Curcumin ingestion inhibits mastocytosis and suppresses intestinal
anaphylaxis in a murine model of food allergy. PLoS One.
10(e0132467)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang N, Li H, Jia J and He M:
Anti-inflammatory effect of curcumin on mast cell-mediated allergic
responses in ovalbumin-induced allergic rhinitis mouse. Cell
Immunol. 298:88–95. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gordon ON, Luis PB, Ashley RE, Osheroff N
and Schneider C: Oxidative transformation of demethoxy-and
bisdemethoxycurcumin: Products, mechanism of formation, and
poisoning of human topoisomerase IIα. Chem Res Toxicol. 28:989–996.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ramezani M, Hatamipour M and Sahebkar A:
Promising Anti-tumor properties of Bisdemethoxycurcumin: A
naturally occurring curcumin analogue. J Cell Physiol. 233:880–887.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu JH, Yang HP, Zhou XD, Wang HJ, Gong L
and Tang CL: Role of Wnt inhibitory factor-1 in inhibition of
bisdemethoxycurcumin mediated epithelial-to-mesenchymal transition
in highly metastatic lung cancer 95D cells. Chin Med J (Engl).
128:1376–1383. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li YB, Gao JL, Zhong ZF, Hoi PM, Lee SM
and Wang YT: Bisdemethoxycurcumin suppresses MCF-7 cells
proliferation by inducing ROS accumulation and modulating
senescence-related pathways. Pharmacol Rep. 65:700–709.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haukvik T, Bruzell E, Kristensen S and
Tønnesen HH: A screening of curcumin derivatives for antibacterial
phototoxic effects Studies on curcumin and curcuminoids. XLIII.
Pharmazie. 66:69–74. 2011.PubMed/NCBI
|
|
25
|
Fu M, Fu S, Ni S, Wang D and Hong T:
Inhibitory effects of bisdemethoxycurcumin on mast cell-mediated
allergic diseases. Int Immunopharmacol. 65:182–189. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee D, Kim HS, Shin E, Do SG, Lee CK, Kim
YM, Lee MB, Min KY, Koo J, Kim SJ, et al: Polysaccharide isolated
from Aloe vera gel suppresses ovalbumin-induced food allergy
through inhibition of Th2 immunity in mice. Biomed Pharmacother.
101:201–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sicherer SH and Sampson HA: Food allergy:
Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy
Clin Immunol. 133:291–307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Berin MC: Pathogenesis of IgE-mediated
food allergy. Clin Exp Allergy. 45:1483–1496. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sicherer SH and Sampson HA: Food allergy.
J Allergy Clin Immunol. 125 (Suppl 2):S116–S125. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kweon MN, Yamamoto M, Kajiki M, Takahashi
I and Kiyono H: Systemically derived large intestinal CD4(+) Th2
cells play a central role in STAT6-mediated allergic diarrhea. J
Clin Invest. 106:199–206. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Yamaki K and Yoshino S: Remission of food
allergy by the Janus kinase inhibitor ruxolitinib in mice. Int
Immunopharmacol. 18:217–224. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bischoff S and Crowe SE: Gastrointestinal
food allergy: New insights into pathophysiology and clinical
perspectives. Gastroenterology. 128:1089–1113. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wollin A, Wang X and Tso P: Nutrients
regulate diamine oxidase release from intestinal mucosa. Am J
Physiol. 275:R969–R975. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang S, Han S, Chen J, Li X and Che H:
Inhibition effect of blunting Notch signaling on food allergy
through improving TH1/TH2 balance in mice.
Ann Allergy Asthma Immunol. 118:94–102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454.
2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ciprandi G, Marseglia GL, Castagnoli R,
Valsecchi C, Tagliacarne C, Caimmi S and Licari A: From IgE to
clinical trials of allergic rhinitis. Expert Rev Clin Immunol.
11:1321–1333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang M, Takeda K, Shiraishi Y, Okamoto M,
Dakhama A, Joetham A and Gelfand EW: Peanut-induced intestinal
allergy is mediated through a mast cell-IgE-FcepsilonRI-IL-13
pathway. J Allergy Clin Immunol. 126:306–316, 316.e1-12.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hua X and He SH: Roles of histamine and
its receptors in allergic and inflammatory bowel diseases. World J
Gastroenterol. 11:2851–2857. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Maintz L and Novak N: Histamine and
histamine intolerance. Am J Clin Nutr. 85:1185–1196.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yamamoto T, Fujiwara K, Yoshida M,
Kageyama-Yahara N, Kuramoto H, Shibahara N and Kadowaki M:
Therapeutic effect of kakkonto in a mouse model of food allergy
with gastrointestinal symptoms. Int Arch Allergy Immunol.
148:175–185. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Brandt EB, Strait RT, Hershko D, Wang Q,
Muntel EE, Scribner TA, Zimmermann N, Finkelman FD and Rothenberg
ME: Mast cells are required for experimental oral allergen-induced
diarrhea. J Clin Invest. 112:1666–1677. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Matsui T, Yamashita H, Mori M, Tanaka H
and Inagaki N: Eppikajutsuto protects against food allergy induced
by ovalbumin in a murine model. Int Arch Allergy Immunol.
173:71–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang C, Collins M and Kuchroo VK: Effector
T cell differentiation: Are master regulators of effector T cells
still the masters? Curr Opin Immunol. 37:6–10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cook PC, Jones LH, Jenkins SJ, Wynn TA,
Allen JE and MacDonald AS: Alternatively activated dendritic cells
regulate CD4+ T-cell polarization in vitro and in vivo.
Proc Natl Acad Sci USA. 109:9977–9982. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yamane H and Paul WE: Cytokines of the
γ(c) family control CD4+ T cell differentiation and
function. Nat Immunol. 13:1037–1044. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Manise M, Holtappels G, Van Crombruggen K,
Schleich F, Bachert C and Louis R: Sputum IgE and cytokines in
asthma: Relationship with sputum cellular profile. PLoS One.
8(e58388)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gilfillan AM and Tkaczyk C: Integrated
signalling pathways for mast-cell activation. Nat Rev Immunol.
6:218–230. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118(Pt 16):3569–3572. 2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Azzolina A, Bongiovanni A and Lampiasi N:
Substance P. induces TNF alpha and IL-6 production through NF kappa
B in peritoneal mast cells. Biochim Biophys Acta. 1643:75–83.
2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schuliga M: NF-kappaB signaling in chronic
inflammatory airway disease. Biomolecules. 5:1266–1283.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kong R, Kang OH, Seo YS, Zhou T, Kim SA,
Shin DW and Kwon DY: MAPKs and NF-κB pathway inhibitory effect of
bisdemethoxycurcumin on phorbol-12-myristate-13-acetate and
A23187-induced inflammation in human mast cells. Mol Med Rep.
17:630–635. 2018.PubMed/NCBI View Article : Google Scholar
|