Introduction
Cardiovascular disease is the number one cause of
death (~7.8%) worldwide, thus posing a significant threat to human
health (1-3).
Atherosclerosis is a cardiovascular disease that involves the
biochemical narrowing of the diameter of blood vessels through the
development of fatty streaks and plaques (4); and is characterized by the interaction
between carbohydrates, lipids and genetic factors (5-7).
Usai MV et al indicated that atherosclerosis is closely
associated with the metabolic disorder of blood lipids (8); however, the mechanisms of
atherosclerosis remain largely unknown. It is important to
understand the mechanisms behind high fat diet-induced
atherosclerosis to identify potential drugs that could relieve
atherosclerosis.
Resveratrol (RV) is a multifunctional biological
polyphenol that serves therapeutic roles in multiple types of human
cardiovascular disease (9). A
previous study demonstrated that RV exhibited neuroprotective
effects in ischemic injury in rats through improving brain energy
metabolism and alleviating oxidative stress (10). A study revealed that RV presented
anti-oxidative and anti-inflammatory effects in the prevention or
inhibition of age-related cardiovascular disease (11). In one study, the protective
cardiovascular effects of RV were directly linked to improved heart
contraction, which could be a potential target for the development
of new clinical therapies for patients (12). Furthermore, a dietary and clinical
perspective study observed that RV demonstrated cardioprotective
benefits in the primary and secondary prevention of cardiovascular
disease (13), with another study
reporting that RV may be a potential agent for the prevention and
treatment of cardiovascular disease (14); however, the potential RV-mediated
mechanism remains largely understood.
Lipoprotein oxidation and oxidative processes in
general serve important roles in the pathogenesis of
atherosclerosis. Disorders of lipid metabolism manifest as
elevations in the levels of plasma lipids and lipoprotein
fractions, which in turn results in the development of
cardiovascular disease (15). The
Atherogenic Index of Plasma is a marker of cardiovascular disease
(16). Several studies have
demonstrated that total cholesterol (TC), triglyceride (TG), low
density lipoprotein cholesterin (LDL-c) and high-density
lipoprotein cholesterin (HDL-c) levels are responses to increased
thickness of vessel walls in patients with atherosclerosis
(17,18). A previous study indicated that RV
may reduce serum cholesterol levels through downregulation of
HMG-CoAreductase (HMGR) mRNA expression levels in hamsters fed a
high-fat diet (HFD) (19). Total
panax notoginsenosides are the main ingredients found in the root
of Panax notoginseng (Burk) F.H. Chen, which belongs to the
Araliaceae family. They are useful in preventing the development of
atherosclerosis in apolipoprotein E-knockout mice by downregulating
CD40 and matrix metalloproteinase-9 (MMP-9) expression (20). By contrast, it was demonstrated that
increased expression levels of tumor necrosis factor (TNF)-α and
C-reactive protein (CRP) promoted the development of early
atherosclerosis by increasing the transcytosis of LDL across
endothelial cells (21-23).
In addition, activation of the mitochondrial-related AMP
kinase/PI3K/AKT/endothelial nitric oxide synthase signaling pathway
was observed to improve endothelial function and alleviate
atherosclerosis (24). mTOR
inhibitors have also been demonstrated to prevent lipid storage,
increase LDL-c levels and activate lipolysis, which further lead to
a decreased risk of developing atherosclerosis (25); however, the association between RV
and the PI3K/AKT/mTOR signaling pathway have not been well
investigated in umbilical vein endothelial cells (UVECS) in
atherosclerosis.
Thus, the aim of the present study was to
investigate the effects of RV treatment in atherosclerosis murine
model. The regulatory effects of RV treatment on the expression of
inflammatory markers, the AIP, 3-hydroxy-3-methyl-glutaryl-Coa
(HMG-CoA) reductase activity and marker enzymes were analyzed. This
study also indicated that RV treatment may mediate the
PI3K/AKT/mTOR signaling pathway in UVECS through the administration
of a PI3K inhibitor prior to RV treatment.
Materials and methods
Animal studies
The present study was carried out in strict
accordance with the guidelines set by the Committee of the People's
Hospital of Weifang. All animal experiments were approved by the
Ethics Committee of the People's Hospital of Weifang (approval no.
TPH20170812S08). A total of 36 Apolipoprotein E-deficient
(ApoE-/-) mice (age, 6 weeks; sex, male; body weight,
18-23 g) were purchased from the Laboratory Animal Science Center
at Peking University People's Hospital (Beijing, China). The mice
were housed in a 12-h light/dark cycle at 24-26˚C and 60±10%
humidity. All animals had access to food and water ad
libitum.
To establish an atherosclerosis model, all
ApoE-/- mice were fed a HFD, consisting of 21% fat from
lard and 1.25% (wt/wt) cholesterol for 8 weeks. Subsequently, the
ApoE-/- mice were randomly divided into 2 groups
(n=12/group), a PBS and RV group and received an intraperitoneal
injection of PBS or RV (50 mg/kg/day), respectively, for 5 weeks.
At the end of experiment, all the mice were sacrificed following
anesthesia with sodium pentobarbital (35 mg/kg) and cervical
dislocation.
Immunohistochemistry (IHC)
staining
Following euthanasia, the heart, aortic trunk and
right apex of the left ventricular myocardium were dissected from
the mice. The tissues were rinsed with pre-cooled 0.9% saline,
fixed in 4% paraformaldehyde for 2 h at room temperature and
embedded in paraffin. Paraffin-embedded tissue samples were cut
into 5-µm serial sections. The tissue sections were subsequently
deparaffinized in xylene at room temperature and rehydrated in a
descending ethanol series. Sections were then blocked with 3%
hydrogen peroxide for 10 min at 25˚C to inhibit endogenous
peroxidase activity. Tissue sections were incubated with the
following primary antibodies for 12 h at 4˚C: Anti-MMP-9 (1:4,000;
cat. no. ab38898; Abcam) and anti-CD40L (1:4,000; cat. no. ab2391;
Abcam). Following the primary antibody incubation, the sections
were incubated with an Alexa Fluor 488-conjugated secondary
antibody (1:2,000; ab150077; Abcam) at 25˚C for 2 h. The slides
were observed under an Olympus IX73 microscope (magnification,
x100; Olympus Corporation). The quantification of expression levels
was performed using Quantity One version 3.2 software (Bio-Rad
Laboratories, Inc.).
Oil Red O staining
To quantify the area of the atherosclerotic lesions
in the aortic sinus, Oil Red O staining was used. Slices (5 µm)
were fixed with formalin for 10 min, rinsed with 60% isopropanol
for 5 min at room temperature and stained with freshly prepared Oil
Red O (1.0%) at room temperature. Subsequently, slides were stained
with alum hematoxylin at room temperature. The average area of the
atherosclerotic lesions in the aortic sinus were quantified by
determining the percentage of positively-stained Oil-Red O areas
using Image-Pro Plus software Version 1.1 (Media Cybernetics,
Inc.).
Biochemical analysis
Blood (5 ml) was obtained in week 1-5 and
subsequently centrifuged at 2,000 x g for 10 min at 4˚C to obtain
the serum. TC, TG, HDL-c, aspartate transaminase (AST; cat. no.
EZ-0506; Assay Biotechnology Company, Inc.), alanine transaminase
(ALT; cat. no. ab263882, Abcam), alkaline phosphatase (ALP, cat.
no. KA1294 Amyjet Scientific, Inc.), lactate dehydrogenase (LDH;
cat. no. A55954-050; EpiGentek Group, Inc.), creatine phosphokinase
(CPK; cat. no. A55954-020; EpiGentek Group, Inc.), TNF-α (cat. no.
ab208348; Abcam), and CRP cat. no. 256398; Abcam), expression
levels were measured using commercial kits according to the
manufacturer's protocol.
LDH and CPK activity measurement
Serum (200 µl) from mice was obtained as previously
described above. LDH and CPK activity was estimated using a fully
automated XL-640 clinical chemistry analyzer (Transasia
Bio-Medicals).
Cell culture and reagents
UVECS were isolated from the ApoE-/-
atherosclerosis model mice as described previously (26). UVECS were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and maintained in a
humidified atmosphere at 37˚C and 5% CO2. For
experiments, cells were treated with 1 mg/ml PI3K inhibitor
(PI3KIR; cat. no. 526559; Sigma-Aldrich; Merck KGaA) and/or 1 mg/ml
RV (cat. no. R5010; Sigma-Aldrich; Merck KGaA) for 6 h at 37˚C for
further analysis. Cells treated with PBS were used as control.
Cell transfection
DNA sequences of PI3K were amplified and
cloned into the pcDNA3.1 plasmid expression vector (Invitrogen;
Thermo Fisher Scientific, Inc.) to generate PI3K
overexpressing (PI3KOR) plasmids. Empty plasmids were used as the
control. In brief, 1x106 UVECS were cultured in 6-well
plates for 24 h at 37˚C and subsequently transfected with 100 nM
PI3KOR or the empty plasmid using a Lipofectamine® 2000
transfection kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The cells were collected for subsequent
experimentation following 72 h of transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from
1x107-treated UVECS using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The purity of the RNA was determined using
an RNA 6000 nano-bioanalyzer (Agilent Technologies, Inc.). Total
RNA was reverse transcribed into cDNA using the SuperScript™ II
Reverse Transcriptase kit (Invitrogen; Thermo Fisher Scientific,
Inc.) for 2 h at 42˚C, according to the manufacturer's protocol.
qPCR was subsequently performed using 12.5 µl 2X real-time PCR
SYBR®-Green master mix, 1.5 µl cDNA template, 2 µl primers and 9 µl
0.1% DEPC H2O (Fast SYBR-Green Master Mix; Invitrogen; Thermo
Fisher Scientific, Inc.). The following primer pairs were used for
the qPCR: PI3K forward, 5'-ATTACGCTAGTTACACTGCA-3' and reverse,
5'-TGGACCTGGCCATCGACTGA-3'; AKT forward,
5'-GCCACCATGAATGAGGTGAAT-3' and reverse,
5'-GCGTATGACAAAGGTGTTGGG-3'; mTOR forward,
5'-ACTCGCTTCTATGACCAACTGA-3' and reverse,
5'-TTTCCATGACAACTGGGTCATTG-3'; and β-actin forward,
5'-CAACGAGCGGTTCAGGTGT-3' and reverse, 5'-TGGAGTTGAAGGTGGTCTCGT-3'.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95˚C for 30 sec; 45 cycles of 95˚C for 30
sec, 56.8˚C for 30 sec and 72˚C for 30 sec. Expression levels were
normalized to the internal reference gene β-actin. Expression
levels were quantified using the 2-ΔΔCq method (27).
Western blot analysis
Total protein was extracted from arterial tissue
(1.0 g) using RIPA lysis buffer (Thermo Fisher Scientific, Inc.).
Total protein was quantified using a bicinchoninic acid assay kit
(Thermo Fisher Scientific, Inc.) and 40 µg protein/lane was
separated using 15% SDS-PAGE. The separated proteins were
subsequently transferred onto a nitrocellulose membrane and blocked
with 5% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at 37˚C. The
membranes were subsequently incubated with the following primary
antibodies for 12 h at 4˚C: Anti-PI3K (1:2,000; cat. no. ab232997;
Abcam), anti-phosphorylated (p)-PI3K (1:1,000; cat. no. ab182651;
Abcam), anti-AKT (1:2,000; cat. no. ab185633; Abcam), anti-p-AKT
(1:2,000; cat. no. ab133458; Abcam), anti-mTOR (1:2,000; cat. no.
ab32028; Abcam) and anti-β-actin (1:1,000; cat. no. ab8226; Abcam).
Following the primary antibody incubation, membranes were incubated
with HRP-conjugated goat anti-rabbit IgG secondary antibody
(1:5,000; ab6721; Abcam) for 24 h at 4˚C. Protein bands were
visualized using the Pierce™ ECL Western Blotting
Substrate (cat. no. 32209; Invitrogen; Thermo Fisher Scientific,
Inc.). Protein expression was quantified using Quantity-One version
3.2 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was performed using SPSS version 13.0 software
(SPSS Inc.). Statistical differences among groups were determined
using Student's t-test or one-way ANOVA followed by Tukey's post
hoc analysis. All data are presented as the mean ± SEM. P<0.05
was considered to indicate a statistically significant
difference.
Results
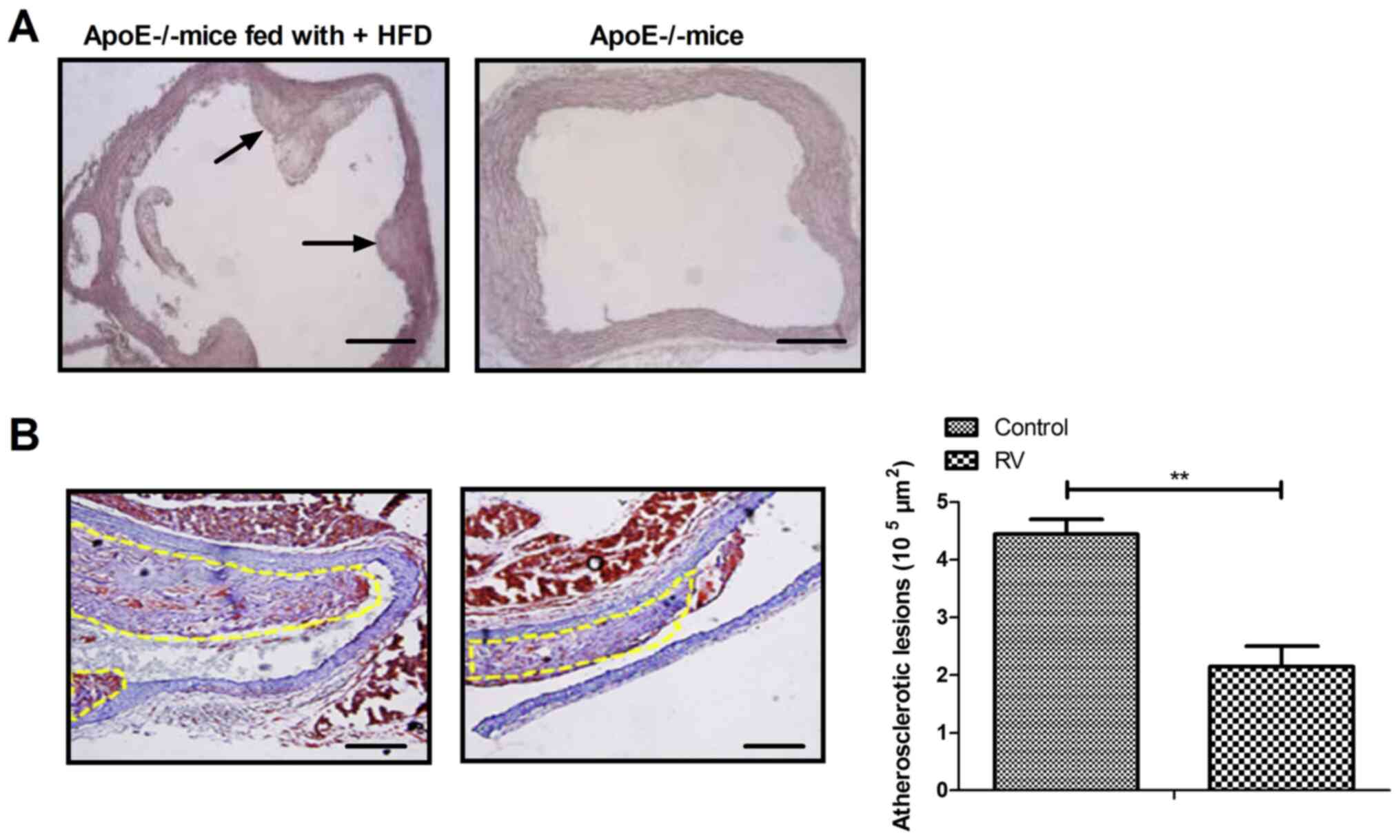
Pathological characteristics of
ApoE-/-atherosclerosis model mice
To verify the role of RV treatment in
ApoE-/- mice, the characteristics of the
arteriosclerotic lesions were investigated. Fibrous caps of
atherosclerotic lesions were observed in the aortic sinus of
ApoE-/- mice fed the high fat diet (Fig. 1A); however, no significant lesion
was observed in the aortic sinus intima of ApoE-/- mice
without high fat diet. RV treatment significantly decreased the
formation of atherosclerotic plaques in atherosclerosis model mice
compared to the control group (Fig.
1B). These results indicated that RV may improve the
pathological characteristics of atherosclerosis in
ApoE-/- mice.
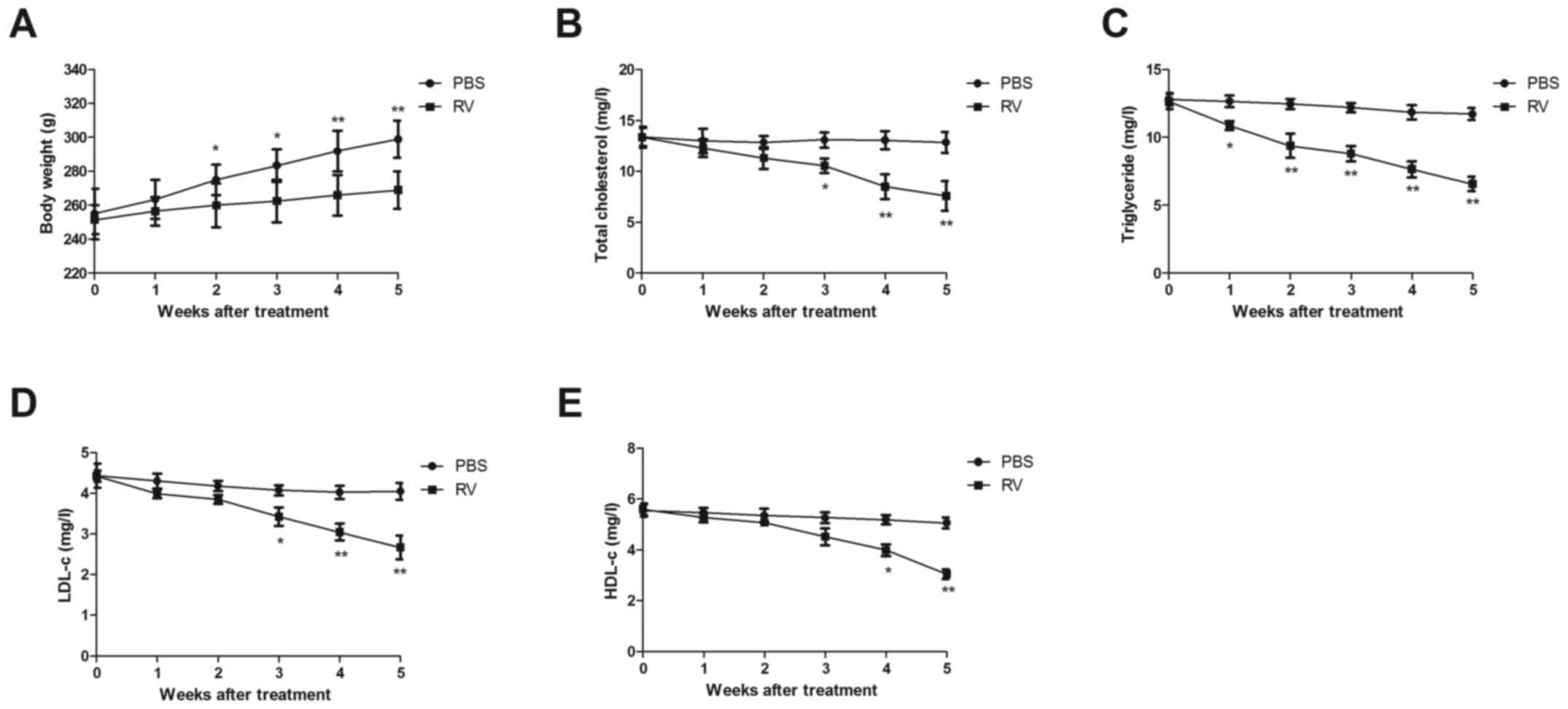
RV treatment increases body weight and
improves blood lipid levels in atherosclerosis model mice
Changes in the body weight of atherosclerosis model
mice were analyzed in the RV and control treatment group. The body
weight of mice was significantly decreased from 2 weeks to the end
of experiment in the RV group compared with the control group
(Fig. 2A). Furthermore, RV
treatment significantly reduced the TC, TG, LDL-c and HDL-c levels
compared with the PBS group in atherosclerosis model mice following
5 weeks of treatment (Fig.
2B-E).
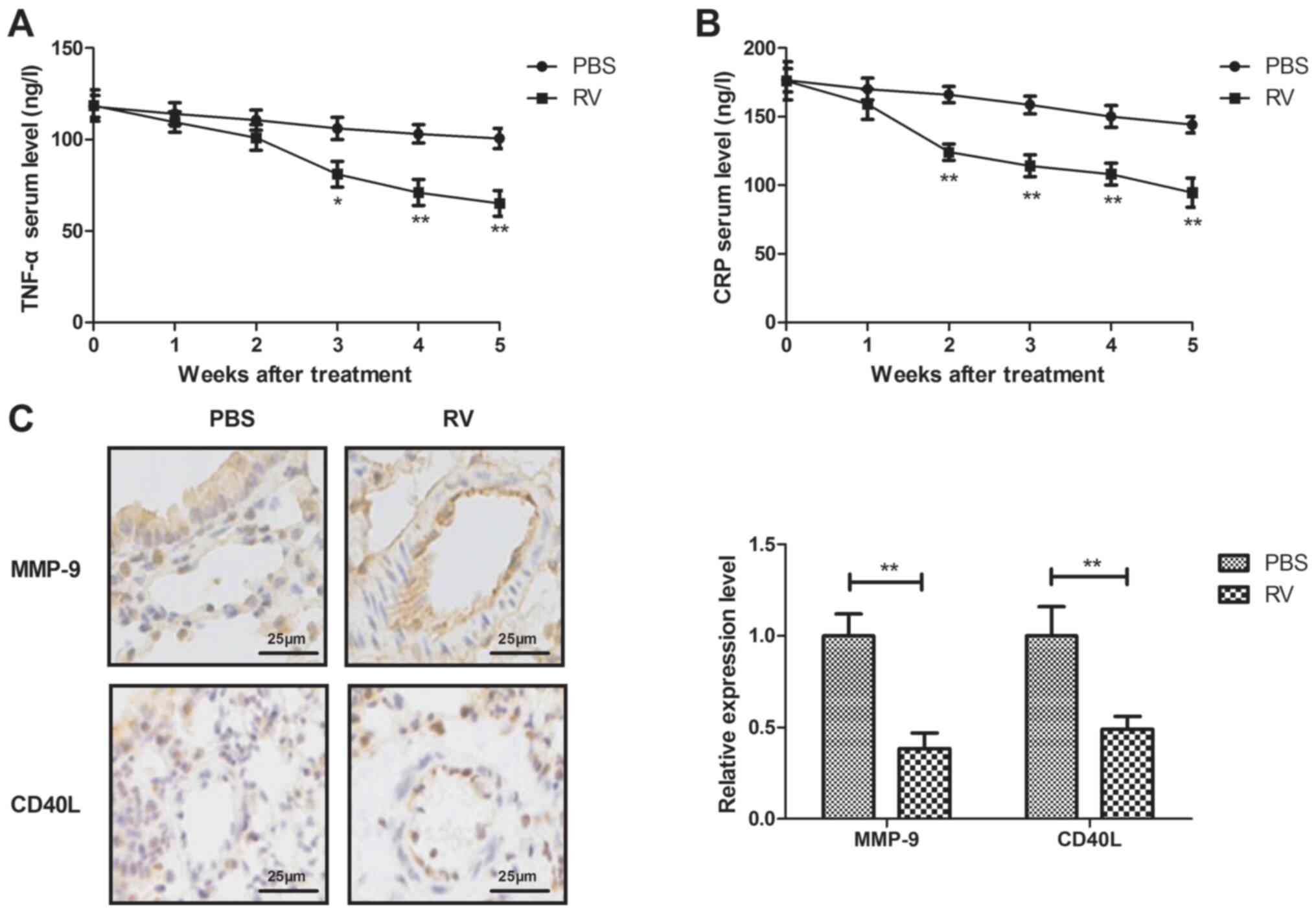
RV treatment decreases serum levels of
inflammatory cytokines in atherosclerosis model mice
The anti-inflammatory effects of RV were
investigated in atherosclerosis model mice. RV treatment
significantly decreased the serum levels of TNF-α and CRP compared
with the control group (Fig. 3A and
B). In addition, RV treatment
significantly decreased MMP-9 and CD40L expression levels in
arterial lesion tissue compared with the control group (Fig. 3C).
RV treatment demonstrates
anti-atherosclerotic activity in atherosclerosis model mice
RV treatment significantly decreased HMG-CoA
reductase activity compared with the control group (Fig. 4A). LDH and CPK activities were also
significantly decreased by 5 weeks post-treatment in the RV group
compared with the control group (Fig.
4B and C). In addition, serum
levels of ALT, AST and ALP were significantly decreased in the RV
group compared to the control group (Fig. 4D-F). These results indicated that RV
treatment may exhibit anti-atherosclerotic activity in
atherosclerosis model mice.
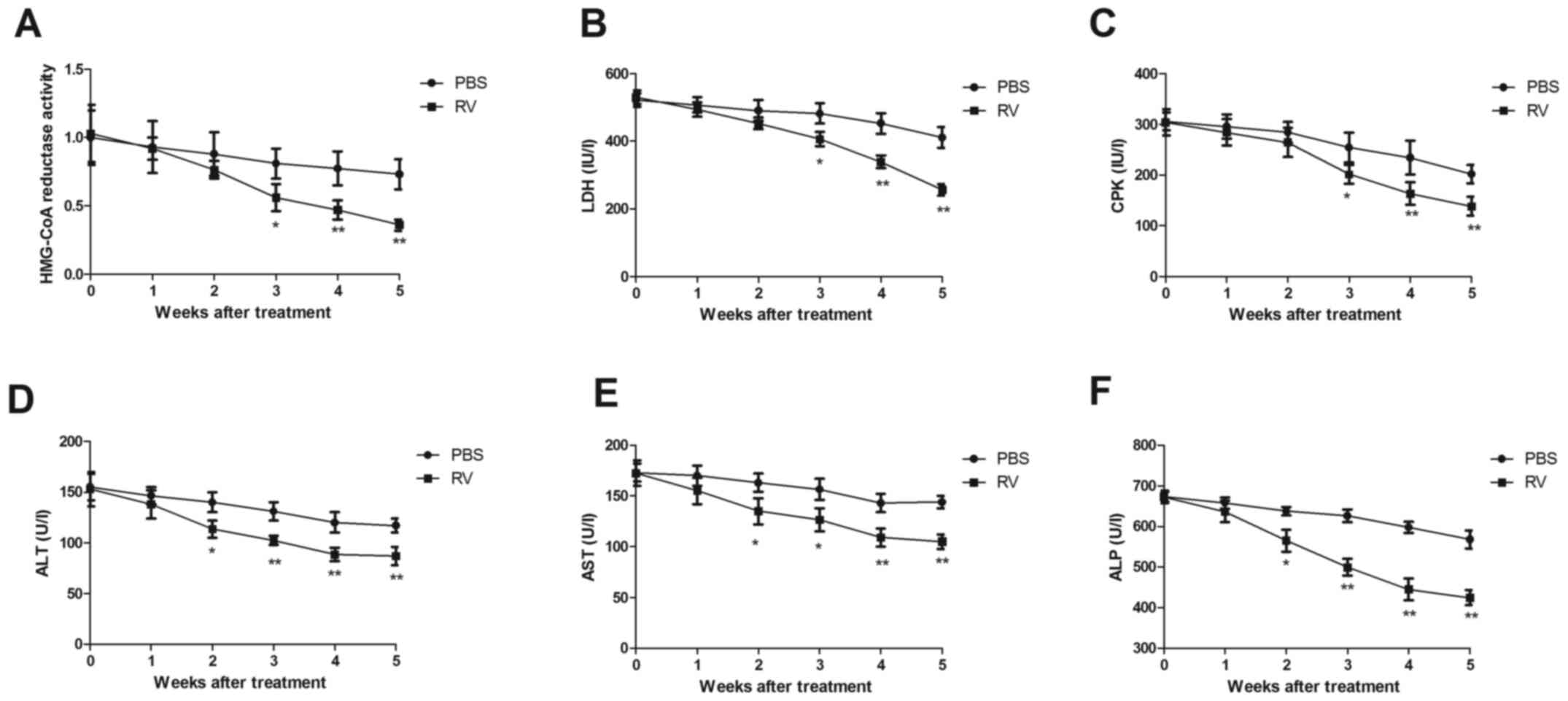 | Figure 4RV treatment promotes
anti-atherosclerotic activity in atherosclerosis model mice. (A-F)
Effects of RV treatment on (A) HMG-CoA reductase activity, (B) LDH
levels, (C) CPK activity, (D) serum ALT levels, (E) serum AST
levels and (F) serum ALP levels compared with the control.
*P<0.05 and **P<0.01. RV, resveratrol;
LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ALT,
alanine transaminase; AST, aspartate transaminase; ALP, alkaline
phosphatase. |
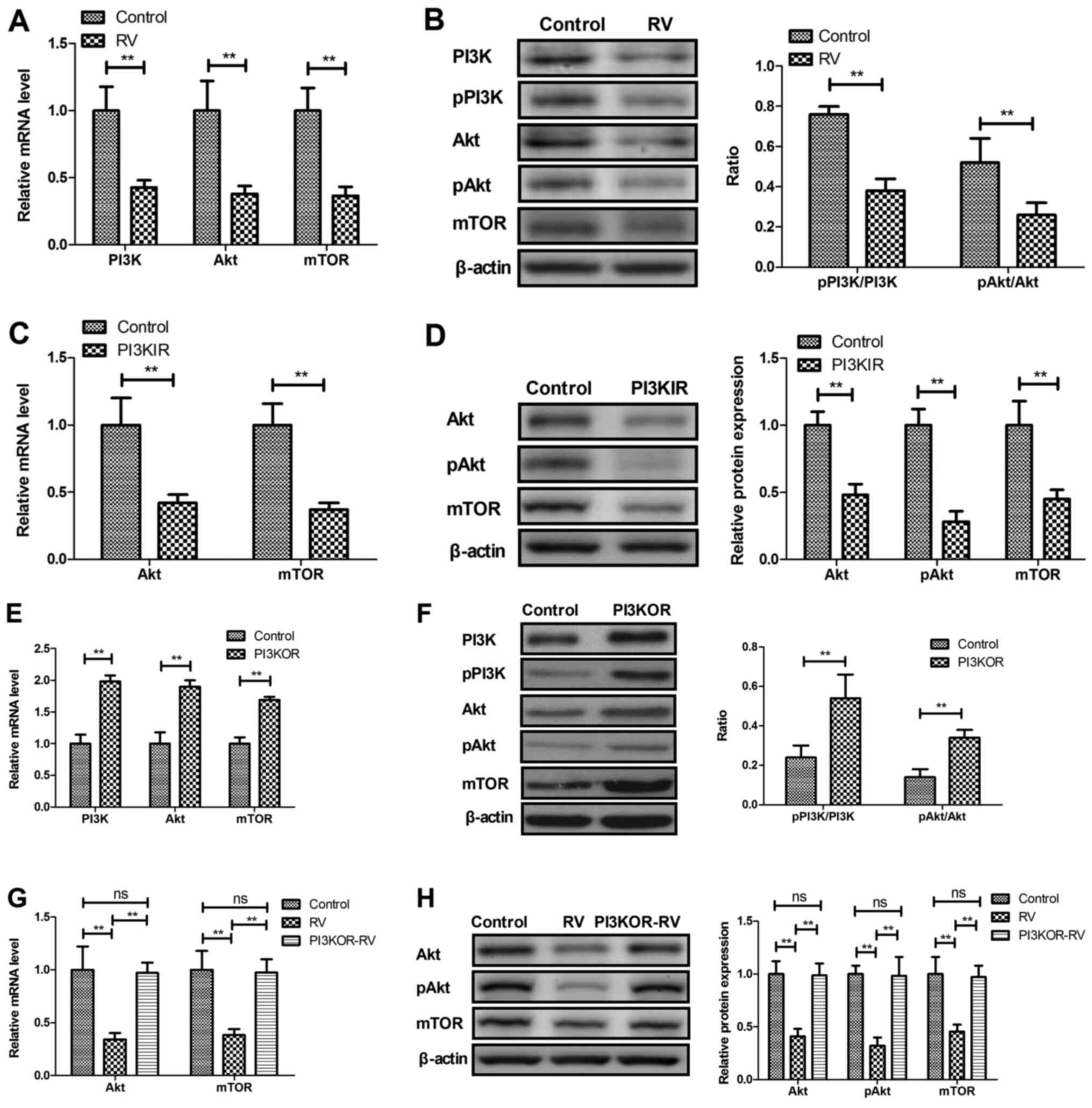
RV decreases PI3K/AKT/mTOR signaling
in UVECs
The potential RV-mediated mechanism was investigated
in UVECs isolated from atherosclerosis model mice. RV treatment
significantly decreased the mRNA and protein expression levels of
PI3K, AKT and mTOR compared with the control group (Fig. 5A and B). UVECs treated with the PI3KIR
demonstrated significantly decreased mRNA and protein expression
levels of AKT and mTOR compared with the control group (Fig. 5C and D). In addition, PI3K overexpression in
UVECs using PI3KOR was observed to significantly increase the mRNA
and protein expression levels of PI3K, AKT and mTOR compared with
the control group (Fig. 5E and
F), whereas PI3KOR-transfected
UVECs significantly reversed the RV-mediated decrease in mRNA and
protein expression levels of AKT and mTOR (Fig. 5G and H).
Discussion
RV was previously demonstrated to reduce the
expression levels of inflammatory cytokines in atherosclerosis
model rabbits (28). In the present
study, the therapeutic effects of RV on atherosclerosis model mice
was analyzed and the relationship between RV and the PI3K/AKT/mTOR
signaling pathway in UVECS obtained from atherosclerosis model mice
was also investigated. The results demonstrated that RV treatment
increased the expression levels of PI3K, AKT and mTOR, as well as
the ratios between phosphorylated and total proteins in UVECS and
increased the serum levels of TC, TG, LDL-c and HDL-c in
atherosclerosis model mice. The study also observed that RV
promoted anti-inflammatory effects, which may contribute to the
anti-atherosclerotic activity of RV.
A previous study reported that RV treatment
protected against TNF-α-induced injury in human UVECs through
promoting sirtuin-1-induced repression of NF-kB and p38 MAPK
(29). Verschuren et al
(30) demonstrated that RV
treatment decreased CRP expression levels, which improved lipid
metabolism and reduced atherosclerotic lesion development in female
transgenic mice. Results from the present study observed that RV
treatment decreased TNF-α and CRP serum levels in atherosclerosis
model mice. In addition, HMG-CoA reductase inhibitors reduced
chronic subacute inflammation in atherosclerosis induced by dietary
cholesterol (31). The present
study found that RV treatment decreased the body weight of
atherosclerotic mice by measuring pathological improvement of
atherosclerosis. A previous study reported that the association
between liver function and coronary atherosclerosis may be more
complex than appreciated (32), and
TG and LDL-c levels were found at higher levels in patients with
atherosclerosis (33). In this
study, RV treatment decreased the serum levels of TC, TG, LDL-c and
HDL-c in atherosclerotic model mice compared to the control group.
It was also reported that RV treatment decreased HMG-CoA reductase
activity and marker enzymes, including LDH, CPK, AST, ALT and ALP
in atherosclerosis model mice, which suggested that atherosclerosis
may be associated with liver dysfunction.
RV has demonstrated numerous pharmacological
effects, including acting as an antioxidant, an anti-inflammatory
agent, eliminating free radicals, exhibiting an anti-tumorigenic
role, regulating lipids and regulating glucose metabolism (34-36).
Data from the current study observed that RV treatment improved
lipid metabolism compared with the control group in atherosclerosis
model mice. MMP-9 serum levels are consistently associated with
markers of carotid atherosclerosis and lesion vulnerability
(37); and the present study
demonstrated that RV treatment could significantly decrease MMP-9
and CD40L expression levels in arterial lesion tissue compared to
the control group. Notably, the in vivo experiments
demonstrated the protective role of RV against atherosclerotic
lesions in atherosclerosis model mice. Clinically, HDL-c is
response to statin treatment by improving carotid intima-media
thickness, which is closely related to a regression of
atherosclerosis (38). Data have
supported that serum levels of LDL-c can be used to predict the
severity of coronary atherosclerosis (39). Consistently, our data found that RV
treatment significantly reduced the degree of atherosclerosis. In
addition, RV treatment increased the metabolism of hyperlipidemia
in HFD-fed atherosclerosis model mice, which further led to an
increase in anti-atherosclerotic activity and may prevent
cardiovascular complications. Thus, it was hypothesized that
decreasing HDL-c levels in the serum may have a negative effect of
RV in the treatment of HFD-fed atherosclerosis; however, further
studies should be performed to identify the anti-atherosclerotic
mechanism of RV in oxidized (ox)-LDL-induced human endothelial
cells.
The mTOR inhibitor, everolimus, has been proven to
prevent the development of atherosclerosis in LDLR-/-
mice, even in the presence of severe hypercholesterolemia (40). A previous study also suggested that
the increased activation of the PI3K/AKT signaling pathway
attenuated ox-LDL-induced endothelial cell apoptosis (41); and similarly, another study
demonstrated that targeting the PI3K/AKT/mTOR signaling pathway in
vascular endothelial cells represented a potential therapeutic
target for the treatment of atherosclerosis (42). Notably, the activation of the
PI3K/AKT/mTOR signaling pathway exerted a protective role against
atherosclerosis (43). In addition,
previous studies have reported that inhibiting the PI3K/AKT/mTOR
pathway alleviated ox-LDL-induced apoptosis of human endothelial
cells, which further prevented atherosclerosis development
(44-46).
Results in the present study were consistent with the majority of
these previous studies; RV treatment downregulated the
PI3K/AKT/mTOR signaling pathway in UVECS obtained from
atherosclerosis model mice. In addition, it was demonstrated that
PI3K overexpression increased and abolished RV-regulated AKT and
mTOR expression in UVECS; however, further investigations are
required to determine the therapeutic efficacy of RV in patients
with atherosclerosis. In addition, future studies should aim to
analyze the dissociation constant/inhibition constant (KD/KI)
between downstream molecules of RV. In conclusion, the results from
the present study indicated that RV may improve atherosclerosis
through the PI3K/AKT/mTOR signaling pathway. This study provided
evidence for the application of RV in the treatment of
atherosclerosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The analyzed datasets used and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
WJ performed the experiments and data analysis. JS
and ZH performed experiments and collected data. BS designed the
experiments and wrote the original manuscript. All authors read and
approved the final manuscript. WJ, JS, ZH and BS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The People's Hospital of Weifang.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gandhi S, Chen S, Hong L, Sun K, Gong E,
Li C, Yan LL and Schwalm JD: Effect of mobile health interventions
on the secondary prevention of cardiovascular disease: Systematic
review and meta-analysis. Can J Cardiol. 33:219–231.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang Y, Cai X, Mai W, Li M and Hu Y:
Association between prediabetes and risk of cardiovascular disease
and all cause mortality: Systematic review and meta-analysis. BMJ.
355(i5953)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aziz M, Ali SS, Das S, Younus A, Malik R,
Latif MA, Humayun C, Anugula D, Abbas G, Salami J, et al:
Association of subjective and objective sleep duration as well as
sleep quality with non-invasive markers of sub-clinical
cardiovascular disease (CVD): A systematic review. J Atheroscler
Thromb. 24:208–226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sedighi M, Bahmani M, Asgary S, Beyranvand
F and Rafieian-Kopaei M: A review of plant-based compounds and
medicinal plants effective on atherosclerosis. J Res Med Sci.
22(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Milic NM, Milin-Lazovic J, Weissgerber TL,
Trajkovic G, White WM and Garovic VD: Preclinical atherosclerosis
at the time of pre-eclamptic pregnancy and up to 10 years
postpartum: Systematic review and meta-analysis. Ultrasound Obstet
Gynecol. 49:110–115. 2017.PubMed/NCBI View Article : Google Scholar : (In En,
Spanish).
|
|
6
|
Zanoli L, Signorelli SS, Inserra G and
Castellino P: Subclinical atherosclerosis in patients with
inflammatory bowel diseases: A systematic review and meta-analysis.
Angiology. 68(463)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu GC, Leng RX, Lu Q, Fan YG, Wang DG and
Ye DQ: Subclinical atherosclerosis in patients with inflammatory
bowel diseases: A systematic review and meta-analysis. Angiology.
68:447–461. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Usai MV, Bosiers MJ, Bisdas T, Torsello G,
Beropoulis E, Kasprzak B, Stachmann A and Stavroulakis K: Surgical
versus endovascular revascularization of subclavian artery
arteriosclerotic disease. J Cardiovasc Surg (Torino). 61:53–59.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Petrovski G, Gurusamy N and Das DK:
Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci.
1215:22–33. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li H, Yan Z, Zhu J, Yang J and He J:
Neuroprotective effects of resveratrol on ischemic injury mediated
by improving brain energy metabolism and alleviating oxidative
stress in rats. Neuropharmacology. 60:252–258. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Csiszar A: Anti-inflammatory effects of
resveratrol: Possible role in prevention of age-related
cardiovascular disease. Ann N Y Acad Sci. 1215:117–122.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang H, Yang YJ, Qian HY, Zhang Q, Xu H
and Li JJ: Resveratrol in cardiovascular disease: What is known
from current research? Heart Fail Rev. 17:437–448. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tomé-Carneiro J, Gonzálvez M, Larrosa M,
Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, Tomás-Barberán FA,
García-Conesa MT and Espín JC: Resveratrol in primary and secondary
prevention of cardiovascular disease: A dietary and clinical
perspective. Ann NY Acad Sci. 1290:37–51. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ruan BF, Lu XQ, Song J and Zhu HL:
Derivatives of resveratrol: Potential agents in prevention and
treatment of cardiovascular disease. Curr Med Chem. 19:4175–4183.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kattoor AJ, Pothineni NVK, Palagiri D and
Mehta JL: Oxidative stress in atherosclerosis. Curr Atheroscler
Rep. 19(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Niroumand S, Khajedaluee M,
Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G and
Dadgarmoghaddam M: Atherogenic index of plasma (AIP): A marker of
cardiovascular disease. Med J Islam Repub Iran.
29(240)2015.PubMed/NCBI
|
|
17
|
Henrot P, Foret J, Barnetche T, Lazaro E,
Duffau P, Seneschal J, Schaeverbeke T, Truchetet ME and Richez C:
Assessment of subclinical atherosclerosis in systemic lupus
erythematosus: A systematic review and meta-analysis. Joint Bone
Spine. 85:155–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yong WC, Sanguankeo A and Upala S:
Association between sarcoidosis, pulse wave velocity, and other
measures of subclinical atherosclerosis: A systematic review and
meta-analysis. Clin Rheumatol. 37:2825–2832. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cho IJ, Ahn JY, Kim S, Choi MS and Ha TY:
Resveratrol attenuates the expression of HMG-CoA reductase mRNA in
hamsters. Biochem Biophys Res Commun. 367:190–194. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu G, Wang B, Zhang J, Jiang H and Liu F:
Total panax notoginsenosides prevent atherosclerosis in
apolipoprotein E-knockout mice: Role of downregulation of CD40 and
MMP-9 expression. J Ethnopharmacol. 126:350–354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu
G, Li W, Chi J, Ouyang C, Zheng T, et al: TNF-α promotes early
atherosclerosis by increasing transcytosis of LDL across
endothelial cells: Crosstalk between NF-κB and PPAR-γ. J Mol Cell
Cardiol. 72:85–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Piechota W: Correlation of
high-sensitivity CRP concentration with the extent of coronary
atherosclerosis in men with symptoms of ischemic heart disease. Pol
Merkur Lekarski. 18:511–515. 2005.PubMed/NCBI(In Polish).
|
|
23
|
Egorova MO: Increased serum level of the
acute inflammation phase parameter CRP and the high level of low
density lipoprotein cholesterol-factors of increased risk of
development of atherosclerosis and its complications (a literature
review). Klin Lab Diagn. 3-6:2002.PubMed/NCBI(In Russian).
|
|
24
|
Xing SS, Yang XY, Zheng T, Li WJ, Wu D,
Chi JY, Bian F, Bai XL, Wu GJ, Zhang YZ, et al: Salidroside
improves endothelial function and alleviates atherosclerosis by
activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway.
Vascul Pharmacol. 72:141–152. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kurdi A, Martinet W and De Meyer GRY: mTOR
inhibition and cardiovascular diseases: Dyslipidemia and
atherosclerosis. Transplantation. 102 (Suppl 1):S44–S46.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hu Q, Chai J, Liu L, Hou Y, Wang Y, Li B
and Yang H: Isolation, culture, and identification of canine
umbilical vein vascular endothelial cells. Zhongguo Xiu Fu Chong
Jian Wai Ke Za Zhi. 27:460–463. 2013.PubMed/NCBI(In Chinese).
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song R, Li WQ, Dou JL, Li L, Hu Y, Guo J,
Lu D, Zhang G and Sun L: Resveratrol reduces inflammatory cytokines
via inhibiting nuclear factor-κB and mitogen-activated protein
kinase signal pathway in a rabbit atherosclerosis model. Zhonghua
Xin Xue Guan Bing Za Zhi. 41:866–869. 2013.PubMed/NCBI(In Chinese).
|
|
29
|
Pan W, Yu H, Huang S and Zhu P:
Resveratrol protects against TNF-α-induced injury in human
umbilical endothelial cells through promoting sirtuin-1-induced
repression of NF-KB and p38 MAPK. PLoS One.
11(e0147034)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Verschuren L, Wielinga PY, van
Duyvenvoorde W, Tijani S, Toet K, van Ommen B, Kooistra T and
Kleemann R: A dietary mixture containing fish oil, resveratrol,
lycopene, catechins, and vitamins E and C reduces atherosclerosis
in transgenic mice. J Nutr. 141:863–869. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kleemann R and Kooistra T: HMG-CoA
reductase inhibitors: Effects on chronic subacute inflammation and
onset of atherosclerosis induced by dietary cholesterol. Curr Drug
Targets Cardiovasc Haematol Disord. 5:441–453. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Doganer YC, Rohrer JE, Aydogan U, Agerter
DC, Cayci T and Barcin C: Atherosclerosis and liver function tests
in coronary angiography patients. West Indian Med J. 64:333–337.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lin JH, Lin YF, Wang WJ, Lin YF, Chueh SJ,
Wu VC, Chu TS and Wu KD: Taiwan Primary Aldosteronism Investigation
(TAIPAI) Study Group. Plasma aldosterone concentration as a
determinant for statin use among middle-aged hypertensive patients
for atherosclerotic cardiovascular disease. J Clin Med.
7(382)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Agarwal R and Agarwal P: Targeting
extracellular matrix remodeling in disease: Could resveratrol be a
potential candidate? Exp Biol Med (Maywood). 242:374–383.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vilar-Pereira G, Carneiro VC, Mata-Santos
H, Vicentino ARR, Ramos IP, Giarola NLL, Feijó DF, Meyer-Fernandes
JR, Paula-Neto HA, Medei E, et al: Resveratrol reverses functional
chagas heart disease in mice. PLoS Pathog.
12(e1005947)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Naia L, Rosenstock TR, Oliveira AM,
Oliveira-Sousa SI, Caldeira GL, Carmo C, Laço MN, Hayden MR,
Oliveira CR and Rego AC: Comparative mitochondrial-based protective
effects of resveratrol and nicotinamide in Huntington's disease
models. Mol Neurobiol. 54:5385–5399. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Silvello D, Narvaes LB, Albuquerque LC,
Forgiarini LF, Meurer L, Martinelli NC, Andrades ME, Clausell N,
dos Santos KG and Rohde LE: Serum levels and polymorphisms of
matrix metalloproteinases (MMPs) in carotid artery atherosclerosis:
Higher MMP-9 levels are associated with plaque vulnerability.
Biomarkers. 19:49–55. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ishigaki Y, Kono S, Katagiri H, Oka Y and
Oikawa S: NTTP investigators. Elevation of HDL-C in response to
statin treatment is involved in the regression of carotid
atherosclerosis. J Atheroscler Thromb. 21:1055–1065.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang Y, Wu NQ, Li S, Zhu CG, Guo YL, Qing
P, Gao Y, Li XL, Liu G, Dong Q and Li JJ: Non-HDL-C is a better
predictor for the severity of coronary atherosclerosis compared
with LDL-C. Heart Lung Circ. 25:975–981. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mueller MA, Beutner F, Teupser D, Ceglarek
U and Thiery J: Prevention of atherosclerosis by the mTOR inhibitor
everolimus in LDLR-/-mice despite severe hypercholesterolemia.
Atherosclerosis. 198:39–48. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu XL, Zhao CH, Yao XL and Zhang H:
Quercetin attenuates high fructose feeding-induced atherosclerosis
by suppressing inflammation and apoptosis via ROS-regulated
PI3K/AKT signaling pathway. Biomed Pharmacother. 85:658–671.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lv J, Yang L, Guo R, Shi Y, Zhang Z and Ye
J: Ox-LDL-induced microRNA-155 promotes autophagy in human
endothelial cells via repressing the Rheb/mTOR pathway. Cell
Physiol Biochem. 43:1436–1448. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yao X, Yan C, Zhang L, Li Y and Wan Q:
LncRNA ENST00113 promotes proliferation, survival, and migration by
activating PI3K/Akt/mTOR signaling pathway in atherosclerosis.
Medicine (Baltimore). 97(e0473)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Che J, Liang B, Zhang Y, Wang Y, Tang J
and Shi G: Kaempferol alleviates ox-LDL-induced apoptosis by
up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in
human endothelial cells. Cardiovasc Pathol. 31:57–62.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhai C, Cheng J, Mujahid H, Wang H, Kong
J, Yin Y, Li J, Zhang Y, Ji X and Chen W: Selective inhibition of
PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage
and vulnerability of atherosclerotic plaque. PLoS One.
9(e90563)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tang F and Yang TL: MicroRNA-126
alleviates endothelial cells injury in atherosclerosis by restoring
autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem
Biophys Res Commun. 495:1482–1489. 2018.PubMed/NCBI View Article : Google Scholar
|