Introduction
Cardiovascular disease (CVD) poses a significant
threat to the health of middle-aged and elderly individuals and
leads to a decline in quality of life; coronary atherosclerotic
heart disease is an important part of CVD (1). The pathological basis of coronary
atherosclerotic heart disease is the development of coronary
atherosclerosis, in which the production and accumulation of foam
cells are important (2). Foam
cells are fatty macrophages that cause artery hardening and may
further lead to heart disease. Foam cells are the first visible
lesion feature in atherosclerotic diseases (3). Circulating monocytes are recruited to
the damaged vascular endothelium from the blood, penetrate the
subintima through the endothelial space and differentiate into
macrophages. Macrophages oxidize accumulated lipoproteins under the
intima. A large amount of oxidized lipoprotein enters macrophages,
which form foam cells, and smooth muscle cells also participate in
foam cell formation (4). The
accumulation of foam cells forms the inner core and lipid streaks
of coronary atherosclerotic plaques. In the late stage of
atherosclerosis, foam cells undergo necrosis or apoptosis,
resulting in lipid leakage and formation of a necrotic core, which
increases plaque instability. The occurrence and development of
foam cells contributes to the pathophysiological process of
atherosclerosis (5). Therefore,
understanding the mechanism by which foam cells form and reducing
foam cell formation factors may effectively reduce the formation of
atherosclerotic plaques in coronary arteries.
Scavenger receptors (SRs) have an important role in
foam cell formation and are part of a significant mechanism by
which macrophages identify, ingest and engulf lipoprotein oxide
(6). SRs are present on the cell
surface in a variety of molecular forms and are mainly divided into
five types: A, B, C, D and E. CD36 is a member of the class B SR
family and is the main receptor by which macrophages consume
lipoprotein oxide. When oxidized low-density lipoprotein (ox-LDL)
enters macrophages, which form foam cells, CD36 activation is
necessary (7). Since CD36 is not
regulated by negative feedback from intracellular cholesterol
esters during lipoprotein uptake, the formation of tissue foam
cells must be regulated by modulating the regulatory factors
upstream of CD36. Macrophage-produced factors, such as
interleukin-4 and peroxisome proliferator activated receptor-γ
(PPAR-γ), are upstream regulatory factors that may regulate CD36
expression, and PPAR-γ is widely distributed in adipose tissue,
vascular smooth muscle and cardiomyopathy tissue. There it
participates in lipid metabolism, inflammatory reactions and
pathological processes such as those of atherosclerosis (8). Studies have indicated that PPAR-γ
activation inhibits foam cell formation by increasing foam cell
apoptosis while also regulating the activation of ATP-binding
cassette transporter A1 (ABCA1). This occurs by increasing the
expression of liver X receptor-α to affect cholesterol outflow and
delay the formation of atherosclerotic plaques (9). In addition, studies have indicated
that apolipoprotein E (ApoE) has a similar function to that of
ABCA1, which transports excess cholesterol esters from within cells
to the extracellular space, reducing lipid build-up in cells and
delaying foam cell formation (10). Therefore, the aim of the present
study was to provide a basis to find drugs that act on these
targets to prevent foam cell formation.
Zinc finger protein 580 (ZNF580; GenBank ID,
AF184939) is a Cys2-His2 (C2H2) zinc finger protein containing 172
amino acids that was originally cloned by screening a human aortic
cDNA library. The protein contains a highly conserved C-terminus,
three tandem repeated C2H2 zinc finger domains and a proline-rich
N-terminus. The protein structure of ZNF580 is similar to Sp1-like
or Krüppel-like transcription factors, and it is also characterized
by three tandem repeated C2H2 zinc fingers at the C-terminus
(11). Previous studies indicated
that ZNF580 was able to protect against CVD through multiple
signalling pathways: ZNF580 regulates endothelial nitric oxide
synthase expression via the TGF-β1/ALK5/Smad2 pathway and mediates
vascular endothelial inflammation through the
H2O2/NF-κB signalling pathway (12,13).
Single-core macrophages or smooth muscle cells are usually used to
replicate foam cell models; the available cell types are the human
leukaemic monocyte cell line THP-1, rat celiac macrophages and
animal aortic smooth muscle cells, while THP-1 cells are the most
commonly used (14,15). THP-1 is a human mononucleotic cell
line that is induced by phorbol 12-myristate 13-acetate (PMA) to
differentiate into macrophages to build a foam cell model. Oil red
O (ORO) staining is usually performed to confirm or assess the
extent of successful establishment of the foam cell model (16). However, whether ZNF580 affects
atherosclerotic plaque formation has remained to be explored.
Therefore, in the present study, lentiviruses were used to
overexpress or silence ZNF580 to identify genes related to foam
cell formation and clarify the role of ZNF580 in foam cell
formation. The experimental results provide a new theoretical basis
for the treatment of coronary atherosclerotic heart disease.
Materials and methods
Cell culture
THP-1 cells were obtained from the Tianjin Key
Laboratory of Hepatopancreatic Fibrosis and Molecular Diagnosis
& Treatment and cultured in RPMI-1640 culture medium
supplemented with 10% foetal bovine serum (FBS; both from Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin.
THP-1 cells were grown in an incubator with 100% humidity
containing 95% air and 5% CO2 at 37˚C. Depending on cell
growth, the medium was changed once every 2-3 days and cells were
passaged every 3 days. The model was established with
third-generation cells.
Cell transfection
THP-1 cells in the logarithmic growth phase were
uniformly inoculated at 2x105 cells/well in a 6-well
plate and transfected with control small interfering RNA (siRNA).
According to the manufacturer's protocol, THP-1 cells were
transfected with the non-targeting siRNA but with the green
fluorescent protein (negative control; NC; 20 µl/well), a ZNF580
overexpression vector (LV-ZNF580; 20 µl/well) or a ZNF580 silencing
vector (Si-ZNF580; 20 µl/well), (all from Shanghai Gene
Pharma Co., Ltd.) using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.) at 37˚C for 96 h. FBS-free
RPMI-1640 medium was used during transfection. The transfection
efficiency was monitored by fluorescence microscopy. As Si-ZNF580
negative control (Si-NC) and LV-ZNF580 negative control (LV-NC) use
the same vector, they have the same sequences. In order to avoid
the imbalance caused by empty vector, the purpose of LV-NC
insertion of non-targeting siRNA is to parallel the gene skeleton
of overexpressed vectors (17-19).
The sequences for the siRNAs were as follows: Si-NC,
5'-TTCTCCGAACGTGTCACGT-3'; Si-ZNF580, 5'-GGAGCATCATTCCTTCCTTAC-3';
and LV-ZNF580 all from Shanghai GenePharma Co., Ltd.
Cell model
THP-1 cells were inoculated at 1x105
cells/well in a 6-well plate at 37˚C with 5% CO2
for 12 h. After stimulation with 10 ng/ml PMA (MilliporeSigma),
THP-1 cells adhered to the wells and became macrophages. After the
RPMI-1640 medium was replaced with fresh medium, 50 ng/ml ox-LDL
(Thermo Fisher Scientific, Inc.) was added and the cells were
cultured for 24, 48 or 72 h to establish the model (20,21).
Cell grouping
THP-1 cells were grown on 6-well plates and randomly
divided into eight groups at a density of 5x104
cells/well. In the control group, THP-1 cells were grown in an
incubator containing 95% air and 5% CO2 at 37˚C
after stimulation with 10 ng/ml PMA, and no additional treatment
was performed. In the control + ox-LDL (model) group, after 10
ng/ml PMA was added to control group cells, 50 ng/ml ox-LDL was
added and the cells were cultured in an incubator containing 95%
air and 5% CO2 at 37˚C for 72 h. In the
LV-NC group, THP-1 cells were transfected with the relevant siRNA
lacking the ZNF580 target gene but with the green fluorescent
protein, and no additional treatment was performed. In the Si-NC
group, THP-1 cells were transfected with the non-targeting siRNA
vector but with the green fluorescent protein and no additional
treatment was performed. In the LV-ZNF580 group, THP-1 cells were
transfected with 20 µl/well LV-ZNF580 lentivirus and no
additional treatment was performed. In the LV-ZNF580 + ox-LDL
(LV-ZNF580 + model) group, cells treated in the same manner as
those in the LV-ZNF580 group were administered 50 ng/ml ox-LDL and
cultured in an incubator containing 95% air and 5% CO2
at 37˚C for 72 h. In the Si-ZNF580 group, THP-1 cells
were transfected with 20 µl/well Si-ZNF580 lentivirus and no
additional treatment was performed. In the Si-ZNF580 + ox-LDL
(Si-ZNF580 + model) group, cells treated in the same manner as
those in the Si-ZNF580 group were administered 50 ng/ml ox-LDL and
cultured in an incubator containing 95% air and 5% CO2
at 37˚C for 72 h.
Observation of cell morphology and
intracellular lipid levels
ORO staining was used to observe cell morphology and
intracellular lipid levels. The cells in the different groups were
sequentially washed with PBS and deionized water 2-3 times and
incubated with ORO fixative (MilliporeSigma) at 37˚C for 20 min.
After the cells were washed 2-3 times, 0.5% ORO stain was added,
followed by incubation at 37˚C for 20 min, and the cells were then
placed in hematoxylin (cat. no. H3136; MilliporeSigma) solution at
37˚C for 1 min. Finally, the cells were dried in a ventilated place
at 37˚C and cell morphology and intracellular lipid levels were
observed using an optical microscope (Olympus Corporation).
Image-Pro Plus 6.0 software (Media Cybernetics) was used for
quantification of lipid levels.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
A total of 1x105 THP-1 cells per well
were inoculated into 6-well plates and cultured at 37˚C with
5% CO2 for 24 h. RT-qPCR was used to measure the
expression of ABCA1, CD36, ApoE, PPAR-γ and ZNF580 in each group.
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to extract total RNA and a UV spectrophotometer was used to
measure RNA purity. Subsequently, the RNA was reverse-transcribed
into cDNA using a HIFIScript cDNA Synthesis Kit (ComWin Biotech)
according to the manufacturer's protocol. The cDNA templates were
subjected to qPCR with UltraSYBR Mixture Low ROX (CoWin
Biosciences) under the following conditions: 40 cycles of 10 sec at
95˚C, 30 sec at 60˚C and 32 sec at 72˚C
(stepOnePlus system; Thermo Fisher Scientific, Inc.). The
nucleotide sequences of the forward and reverse primers are
provided in Table I. The relative
expression level of each mRNA was calculated using the
2-ΔΔCq method (22).
 | Table ISequences of the primer pairs used
for quantitative PCR. |
Table I
Sequences of the primer pairs used
for quantitative PCR.
|
Primer/direction | Sequence |
|---|
| ABCA1 | |
|
Forward |
5'-ACCCACCCTATGAACAACATGA-3' |
|
Reverse |
5'-GAGTCGGGTAACGGAAACAGG-3' |
| CD36 | |
|
Forward |
5'-GGCTGTGACCGGAACTGTG-3' |
|
Reverse |
5'-AGGTCTCCAACTGGCATTAGAA-3' |
| ApoE | |
|
Forward |
5'-GTTGCTGGTCACATTCCTGG-3' |
|
Reverse |
5'-GCAGGTAATCCCAAAAGCGAC-3' |
| PPAR-γ | |
|
Forward |
5'-GGCCGCAGATTTGAAAGAAG-3' |
|
Reverse |
5'-ATTTCGTTAAAGGCTGACTCTCGTT-3' |
| ZNF580 | |
|
Forward |
5'-GAGGTTACTGCCTTACCCTGG-3' |
|
Reverse |
5'-ACCCAGTTCCGACTGGTTC-3' |
| β-actin | |
|
Forward |
5'-CATGTACGTTGCTATCCAGGC-3' |
|
Reverse |
5'-CTCCTTAATGTCACGCACGAT-3' |
Protein preparation and western blot
analysis
THP-1 cells were washed three times with PBS.
Samples from the different groups were lysed in complete RIPA
buffer (cat. no. R0020; Beijing Solarbio) at 4˚C for 15 min.
The total protein concentrations were determined using a BCA kit
(cat. no. A045-4-2; Nanjing Jiancheng Bioengineering Institute).
Equal amounts of protein (30 µg/lane) were separated by 10%
SDS-PAGE and transferred to nitrocellulose membranes (EMD
Millipore). The membranes were then blocked with Tris-buffered
saline plus Tween-20 containing 5% skimmed milk (cat. no. PH1519;
Phygene Scientific, Inc.)for 3 h at 25˚C, after which the
membranes were incubated with the following primary antibodies
overnight at 4˚C: ZNF580 (cat. no. PA5-62904; 1:1,000
dilution; Thermo Fisher Scientific, Inc.), ApoE (cat. no.
18254-1-AP; 1:2,000 dilution; Proteintech Group, Inc.), CD36 (cat.
no. 18836-1-AP; 1:2,000 dilution; Proteintech Group, Inc.), ABCA1
(cat. no. AB7360; 1:1,000 dilution; Abcam), PPAR-γ (cat. no.
16643-1-AP; 1:2,000 dilution; Proteintech Group, Inc.) and GAPDH
(cat. no. 5174; 1:1,000 dilution; Cell Signalling Technology,
Inc.). The membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7074 V;
1:5,000 dilution; Cell Signalling Technology, Inc.) for 1 h at
25˚C. Signals were observed using ECL substrate (cat. no.
34579; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Band densities were measured using
ImageJ 1.52a software (National Institutes of Health).
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent tests. Statistical
comparisons between groups were performed using an unpaired t-test
or one-way ANOVA followed by Tukey's post-hoc test with SPSS
version 25.0 statistical software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference. All
experiments were repeated at least three times.
Results
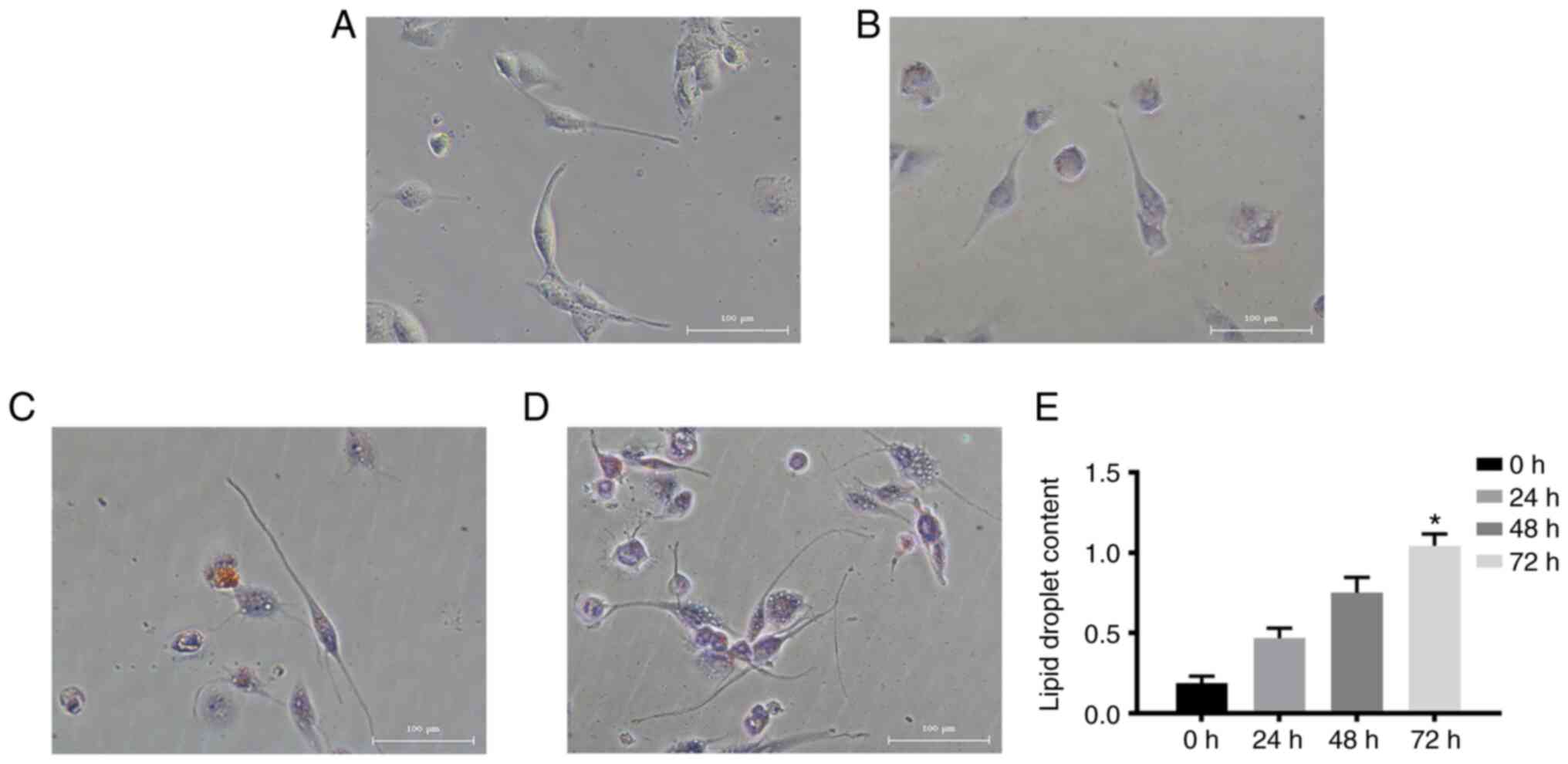
Foam cell model verification
Under normal conditions, THP-1 cells grow in
suspension and the cells are round or oval in shape. After PMA
stimulation, the cells acquired a long spindle-like morphology with
pseudopodia, grew adherently and differentiated into macrophages
(Fig. 1A). When stimulated by 50
ng/ml ox-LDL, macrophages developed into foam cells, and
intracellular lipid droplets were able to be stained with ORO
(Fig. 1B-D). With prolonged ox-LDL
stimulation, the number of ORO-positive cells increased
significantly. Furthermore, the degree of ORO dye aggregation
increased significantly and the colour darkened, suggesting that
the levels of lipid droplets in macrophages increased and foam
cells formed more fully with prolonged ox-LDL incubation time
(Fig. 1E).
Selection of the optimal induction
time for the foam cell model
ORO staining confirmed that it was possible to
successfully establish a foam cell model from ox-LDL-stimulated
macrophages. To determine the optimal stimulation time, 50 ng/ml
ox-LDL was used to stimulate foam cell formation in macrophages for
different durations. The mRNA expression of CD36 increased in the
control group with increasing ox-LDL induction time, and CD36
expression was highest at 72 h, suggesting a time-dependent effect
on CD36 expression in normal macrophages. In the model group, CD36
mRNA expression levels were consistent with those in the control
group, and the growth trends at 24 h were comparable to those at 48
h. However, there were no significant differences in
expression levels between 48 and 72 h. At 72 h, the mRNA expression
of CD36 in the control group was increased compared with that in
the model group and the difference was statistically significant
(P<0.05; Fig. 2A). The mRNA
expression of PPAR-γ did not differ significantly among different
incubation times in the control group, while the mRNA expression of
PPAR-γ increased with prolonged ox-LDL induction time in the model
group; the mRNA expression of PPAR-γ was highest at 72 h and was
significantly higher than that in the control group (P<0.05;
Fig. 2B). The mRNA expression of
the lipid flow-related genes ABCA1 and ApoE was consistent in the
control group and the model group. The mRNA expression levels of
ABCA1 and ApoE increased with prolonged ox-LDL induction time; the
mRNA expression of ABCA1 and ApoE was the highest at 72 h and the
difference between the control and model groups was statistically
significant (P<0.05; Fig. 2C
and D). Therefore, 72 h was chosen
as the optimal time for ox-LDL to induce foam-cell formation in
macrophages.
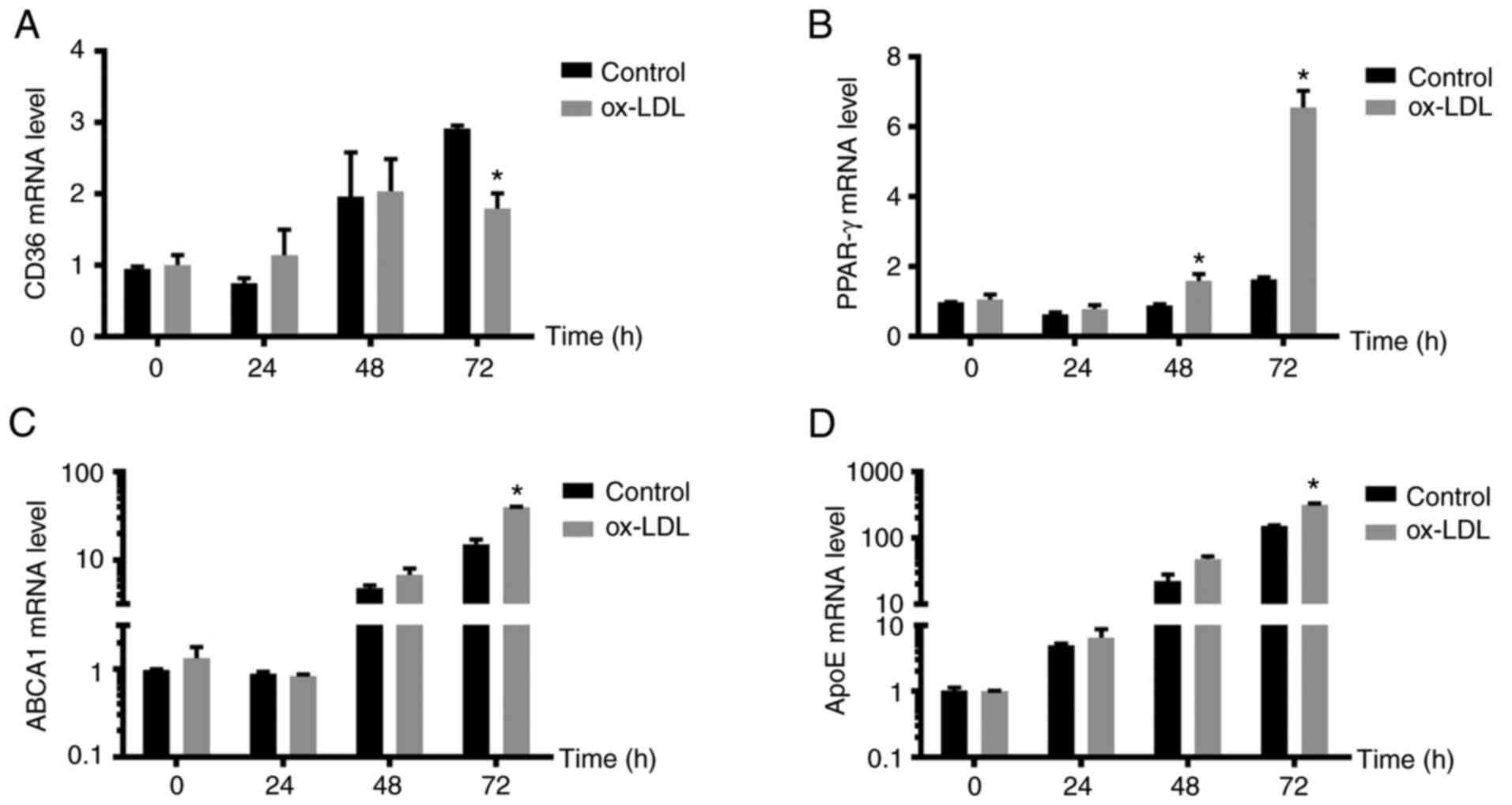 | Figure 2Model foam cells exhibit changes in
lipid metabolism-related mRNA expression at different time-points.
The mRNA levels of lipid metabolism-related genes, including (A)
CD36, (B) PPAR-γ, (C) ABCA1 and (D) ApoE were measured by reverse
transcription-quantitative PCR. The mRNA expression levels of CD36,
PPAR-γ, ABCA1 and ApoE increased with prolonged ox-LDL induction
time compared with those of the control group. At 72 h, the mRNA
expression of CD36 in the control group was 1.5 times that of the
model group and the mRNA expression of PPAR-γ, ABCA1 and ApoE was
highest at 72 h. Therefore, 72 h was chosen as the optimal duration
for ox-LDL to induce macrophages to form foam cells. The expression
levels in the control group at 0 h, normalized to the housekeeping
gene, were set as 1. *P<0.05 compared with the
control group at the same time-point. ox-LDL, oxidized low-density
lipoprotein; PPAR-γ, peroxisome proliferator activated receptor-γ;
ABCA1, ATP-binding cassette transporter A1; ApoE, apolipoprotein
E. |
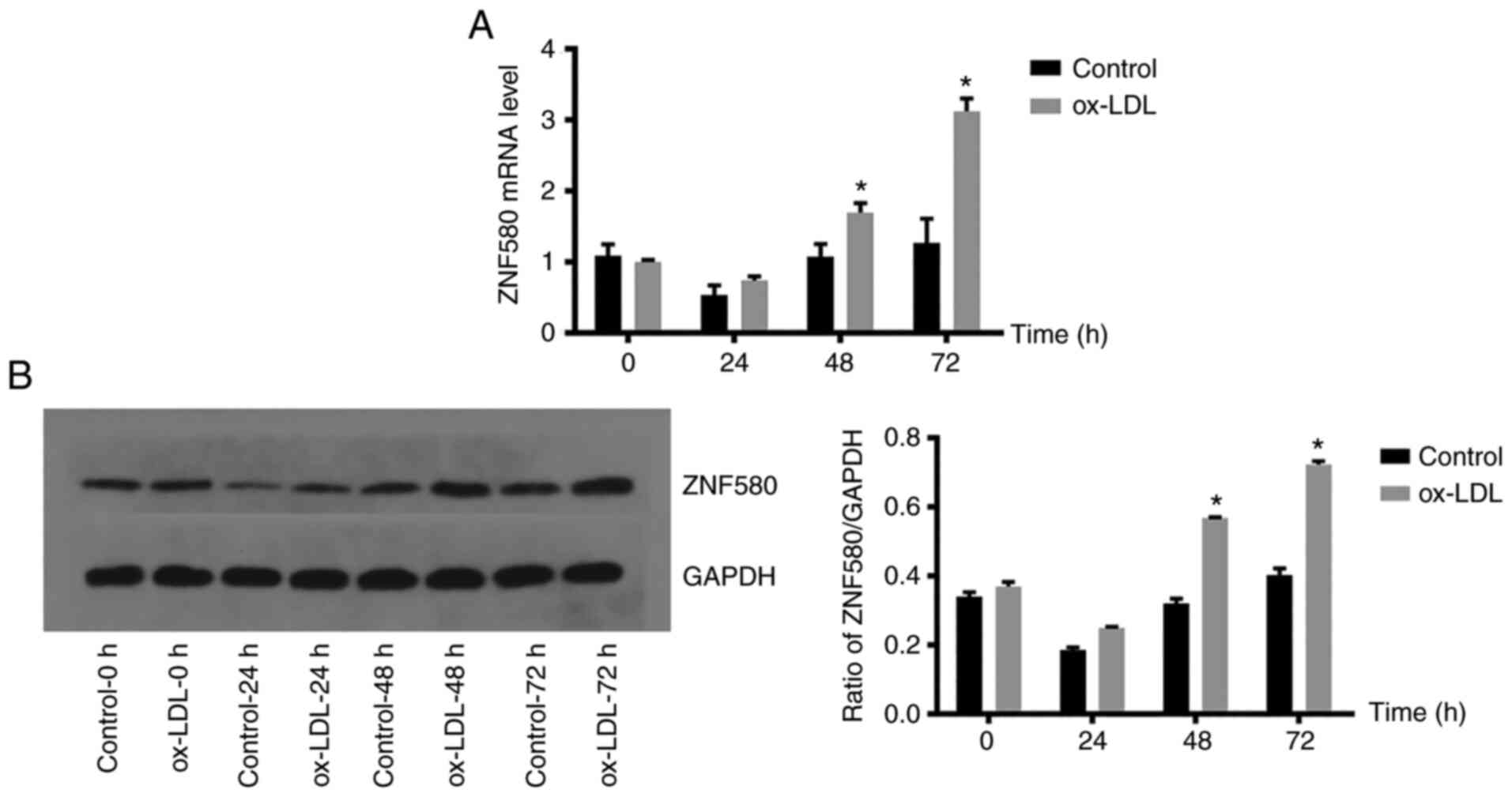
Expression of ZNF580 in the foam cell
model
The expression of ZNF580 in each group was analysed
by RT-qPCR and western blot analysis to examine whether foam cell
formation was associated with ZNF580 expression (Fig. 3). There was no significant
difference between the control group and the model group when the
ox-LDL induction time was 24 h; however, when the induction time
was 48 and 72 h, the mRNA expression level of ZNF580 in the model
group was significantly higher than that in the control group
(P<0.05; Fig. 3A). The results
of the western blot analysis (Fig.
3B) were consistent with those of the RT-qPCR, suggesting an
increase in ZNF580 expression upon foam-cell formation at 72 h.
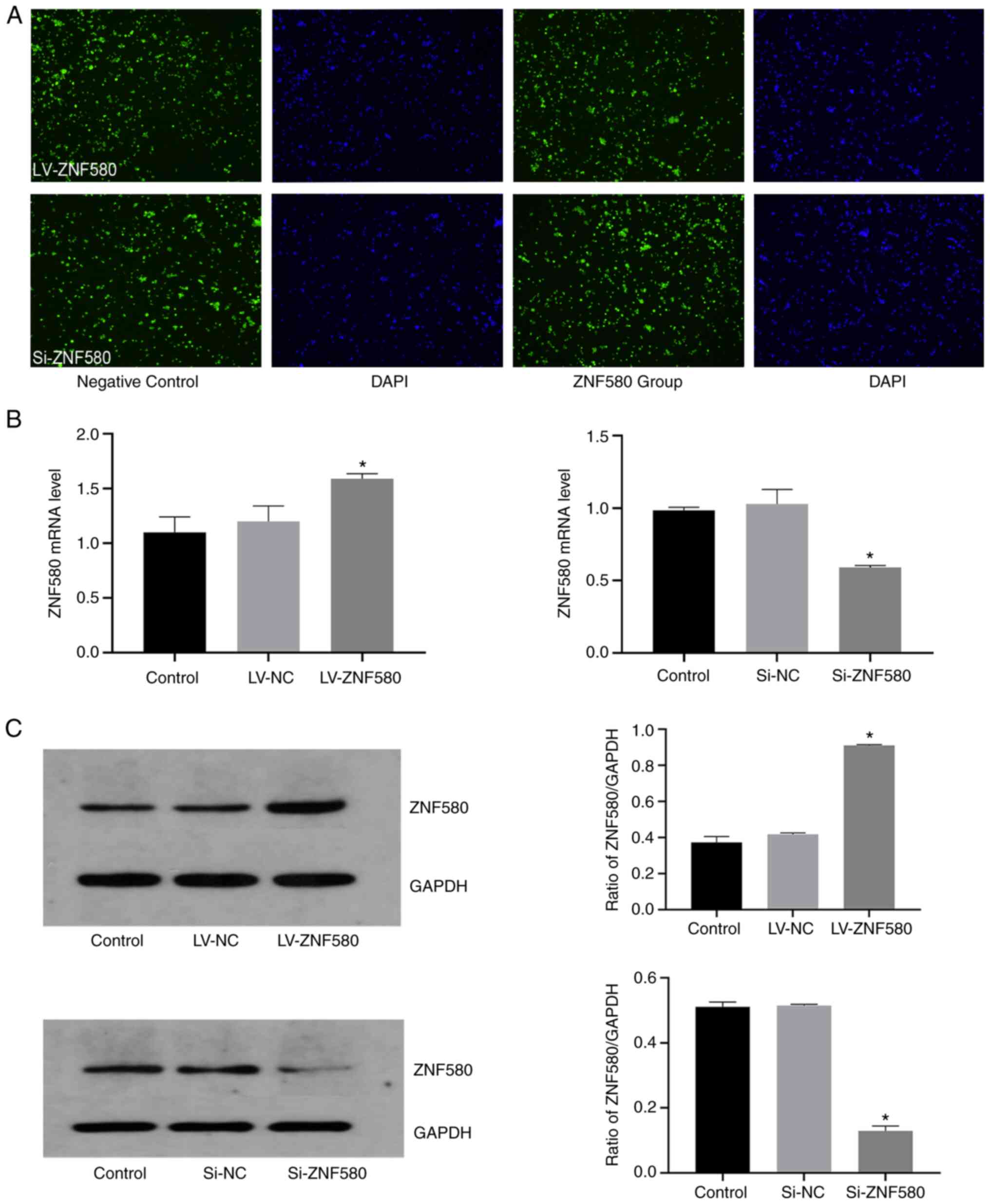
LV-ZNF580 and Si-ZNF580 lentivirus
transfection
Fluorescence microscopy confirmed green fluorescence
in the cytoplasm and around the nuclei of THP-1 cells after
successful transfection, while untransfected cells had no green
fluorescence (Fig. 4A). The
expression of ZNF580 in each group was quantified by RT-qPCR and
western blot analysis to examine whether the cells were
successfully transfected (Fig. 4B
and C). Compared with that in the
LV-NC group, ZNF580 mRNA expression was significantly increased by
LV-ZNF580. Compared with that in the Si-NC group, ZNF580 mRNA
expression was significantly decreased by Si-ZNF580. No differences
were observed between the cells in the control group and the LV-NC
group or the Si-NC group, indicating that the empty vector lacking
ZNF580 target gene but with the green fluorescent protein had no
effect on the cells (P<0.05; Fig.
4B). The protein expression levels of ZNF580 in the different
groups were determined by western blot analysis (Fig. 4C) and the results were consistent
with those regarding the mRNA levels, indicating that THP-1 cells
were successfully transfected with the LV-ZNF580 and Si-ZNF580
lentiviruses.
Establishment of foam cells
transfected with LV-ZNF580 and Si-ZNF580
As indicated by the ORO staining images (Fig. 5A), the LV-ZNF580 model group had
decreased numbers of ORO-stained cells compared to the model group
and the lipid droplet area was smaller than that of the model group
(Fig. 5B). Compared with those in
the model group, the numbers of ORO-stained cells increased in the
Si-ZNF580 model group (Fig. 5A)
and the lipid droplet area was larger than that of the model group
(Fig. 5C), indicating that ZNF580
inhibited foam-cell formation.
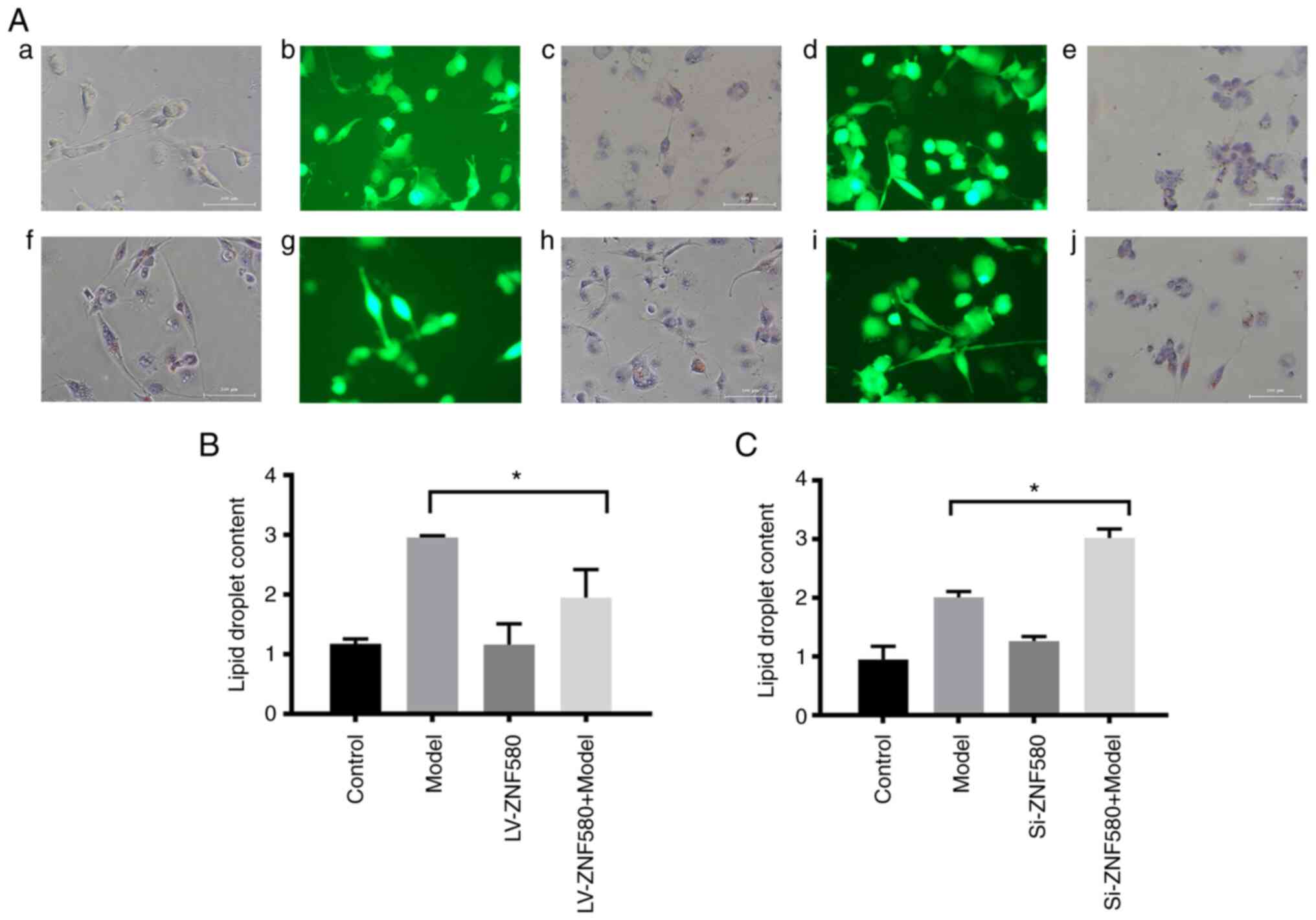 | Figure 5Establishment of foam cells in
LV-ZNF580- and Si-ZNF580-transfected cells. (A) Morphological
changes of cells in the (a) control, (b) LV-ZNF580 (fluorescence
microscopy), (c) LV-ZNF580 (ORO staining), (d) Si-ZNF580
(fluorescence microscopy), (e) Si-ZNF580 (ORO staining), (f) model,
(g) LV-ZNF580 + model (fluorescence microscopy), (h) LV-ZNF580 +
model (ORO staining), (i) Si-ZNF580 + model (fluorescence
microscopy) and (j) Si-ZNF580 + model (ORO staining) groups
(magnification, x100; scale bars, 100 µm). (B) Quantification of
changes in the lipid droplet area in LV-ZNF580 foam cells, as
determined from ORO staining. (C) Quantification of changes in the
lipid droplet area in Si-ZNF580 foam cells, as determined from ORO
staining. *P<0.05 compared with the model group.
ZNF580, zinc finger protein 580; LV-ZNF580, lentivirus for ZNF580
overexpression; Si-ZNF580, lentivirus expressing small interfering
RNA of ZNF580; ORO, oil red O. |
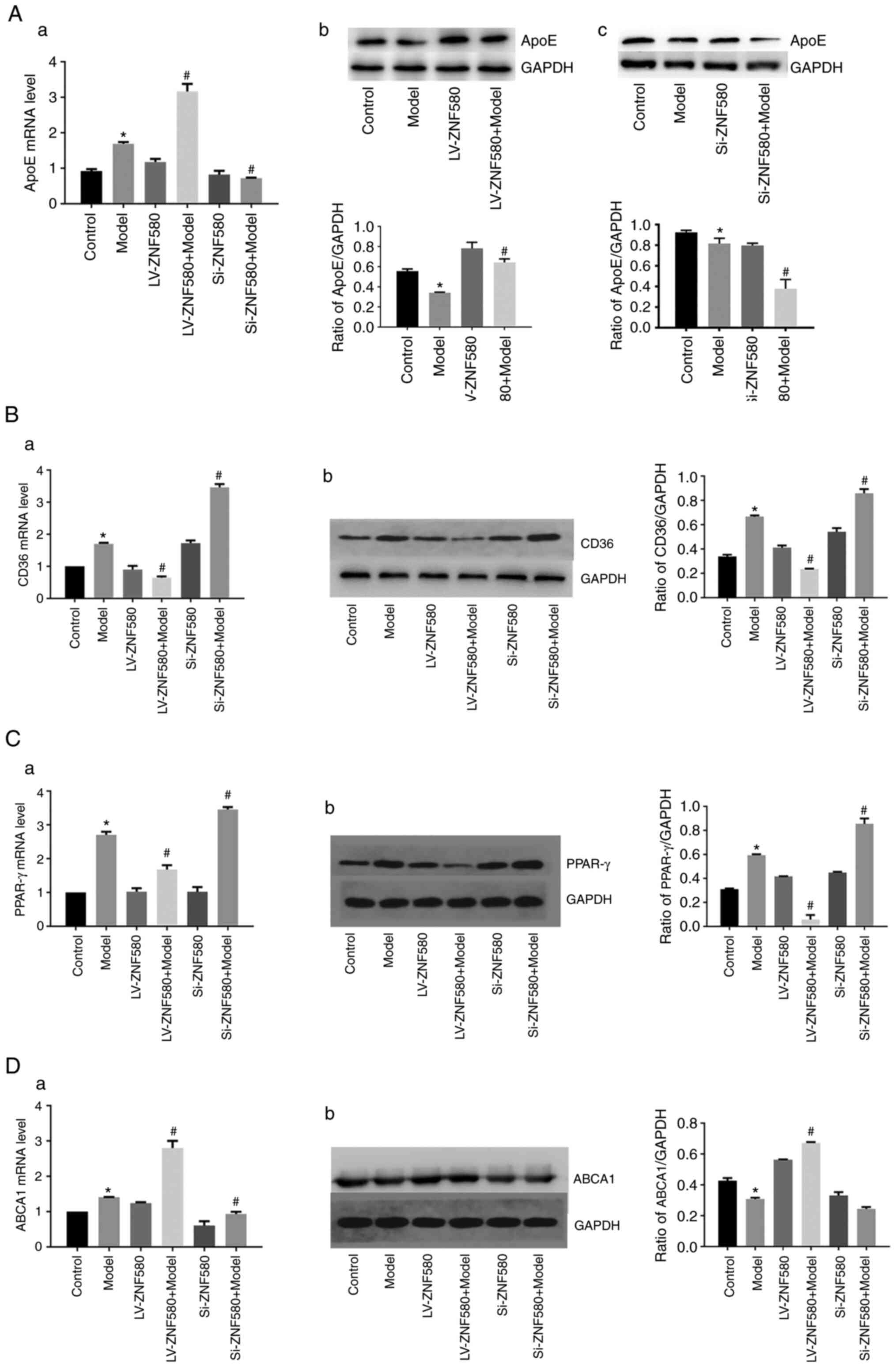
ZNF580 inhibits foam-cell formation by
regulating the expression of lipid-related genes
To determine whether ZNF580 is able to prevent
foam-cell formation by regulating ApoE transcription and increasing
cholesterol outflow (Fig. 6A),
RT-qPCR was performed. The results suggested that the mRNA
expression of ApoE in the model group was higher than that in the
control group. Compared with that in the model group, the mRNA
expression level of ApoE increased significantly in the LV-ZNF580
model group, the mRNA expression level of ApoE decreased
significantly in the Si-ZNF580 model group and the difference was
statistically significant (P<0.05). The western blot results
were consistent with the mRNA levels, indicating that
ZNF580-mediated inhibition of foam-cell formation was associated
with the regulation of ApoE (P<0.05).
The expression of lipid-related genes, such as CD36,
PPAR-γ and ABCA1, in each group was determined using RT-qPCR and
western blot analysis to examine the relationship between ZNF580
and lipid-related genes during foam-cell formation (Fig. 6B-D). Compared with those in the
model group, the mRNA expression levels of CD36 and PPAR-γ
decreased and those of ABCA1 significantly increased in the
LV-ZNF580 model group, while the mRNA expression levels of CD36 and
PPAR-γ increased and those of ABCA1 significantly decreased in the
Si-ZNF580 model group. The western blot results were consistent
with the mRNA levels, indicating that ZNF580-mediated inhibition of
foam-cell formation was associated with the regulation of ABCA1,
CD36 and PPAR-γ.
Discussion
CVD has become the most serious threat to human
health among major diseases and is the leading cause of death
worldwide; thus, the prevention and treatment of CVD has become a
global public health concern. Among CVDs, ischaemic heart disease,
mainly coronary heart disease (CHD), threatens human life (23). The main pathological change in CHD
is coronary atherosclerosis, the pathological basis of which is
atherosclerosis, a chronic inflammatory disease associated with
lipid metabolic disorders. Lipids and other substances circulating
in the blood are deposited in the endometrium of blood vessels,
promoting the growth of local fibrous tissue and causing changes in
the endometrial structure, bulging and expansion of the tube cavity
and constantly forming lipid stripes and necrotic cores under the
influence of persistent chronic inflammation. Lipid stripes and
necrosis kernels begin with foam-cell formation and are the
structural basis for atherosclerosis (24). Therefore, preventing excessive
foam-cell production and accumulation is critical for the treatment
of coronary atherosclerosis. Based on lipid metabolism in
macrophages, the present study examined the receptors and proteins
associated with lipid internalization, fat decomposition and
cholesterol and phospholipid efflux, and explored the possible
correlation between the zinc finger gene ZNF580 and foam-cell
formation.
The class B SR CD36 has an important role in lipid
uptake (25). CD36 is a
single-stranded transmembrane glycoprotein with a long chain that
mostly extends outside the cell; thus, the regulation of
non-negative feedback is not limited by recognition and the intake
of ox-LDL accelerates foam-cell formation (26). After CD36 gene deletion, the
formation of intravascular foam cells was significantly lower than
that in mice without CD36 gene deletion, suggesting that reducing
the expression of CD36 was able to effectively reduce the risk of
developing atherosclerosis (27).
In the present study, ox-LDL-stimulated macrophages had increased
CD36 expression, confirming that CD36 is involved in the process of
foam-cell formation by macrophages. The mRNA expression of CD36
increased with time in ox-LDL-induced macrophages, indicating that
CD36 affected foam-cell formation by macrophages in a
time-dependent manner. Therefore, it is important to effectively
inhibit foam-cell formation to study the regulatory factors that
affect CD36 expression.
PPARs are members of the ligand-activated nuclear
transcription factor superfamily and are divided into three types
(PPAR-α, PPAR-β/δ and PPAR-γ). PPAR-γ is an important regulatory
factor that is able to effectively regulate CD36 expression
(28). It was reported that
inhibiting PPAR-γ activation may effectively reduce the expression
of CD36, thereby reducing the accumulation of lipids in high
sugar-induced THP-1 cells (29).
Therefore, by suppressing the expression of the upstream factor
PPAR-γ, the expression of CD36 may be indirectly reduced. In the
present experiment, RT-qPCR was used to measure the expression
level of PPAR-γ and the results suggested that the trend was
consistent with the trend of CD36 mRNA expression, supporting the
conclusions of the published studies (28,30).
Other studies have also indicated that activating PPAR-γ directly
inhibits the migration and proliferation of single-core macrophages
and indirectly inhibits foam-cell formation (31). The PPAR-γ agonist pyrethroidone is
an important insulin allergen that is widely used to treat insulin
resistance caused by elevated blood sugar. It is able to induce the
expression of lipoprotein esterase to further promote fat breakdown
and reduce plasma cholesterol and triglyceride levels. In addition,
PPAR-γ is able to competitively inhibit the expression of
inflammatory pathways and inflammatory factors to inhibit the
occurrence and expansion of inflammation and reduce inflammation
associated with endothelial damage in blood vessels (32). Therefore, it is urgent to find a
suitable way to modulate PPAR-γ. PPAR-γ activation is also
beneficial in reducing the risk factors associated with
atherosclerotic plaque formation (33). Whether activating or suppressing
PPAR-γ, it is important to find a suitable regulatory point to
treat coronary atherosclerosis.
In the normal state, macrophages remove lipids to
reduce cholesterol in and around the microenvironment and reverse
the transfer of cholesterol to the liver for excretion. They also
use cell surface ABCA1, a transmembrane protein capable of
transporting cholesterol and phospholipids to the outside of cells
in order to form a high-density lipoprotein precursor to achieve
cholesterol reuse and excretion, reduce the accumulation of
cholesterol esters in cells and inhibit foam-cell formation
(34). This protects vascular
walls and cardiovascular health. Furthermore, ABCA1 is able to
delay the progression of atherosclerotic plaques and the erosion of
plaques by inhibiting inflammatory factors involving Toll-like
receptors at the atherosclerotic plaque formation site (35). Therefore, it is critical to explore
the regulatory factors that affect ABCA1 to prevent and treat CHD.
In the present study, RT-qPCR was used to measure gene expression
and ABCA1 mRNA increased with the ox-LDL induction time, indicating
that the cellular demand for cholesterol efflux increased. Another
study confirmed that PPAR-γ activation promoted the transport
efficiency of ABCA1 to facilitate efflux, suggesting that, by
regulating PPAR-γ, the expression of ABCA1, a gene associated with
cholesterol efflux, may be indirectly regulated to reduce
atherosclerotic plaque formation (36).
ApoE is an alkaline protein that is rich in
arginine; is found in high-density lipoproteins, very low-density
lipoproteins and celiac particles; and has an important role in
maintaining the structure of the abovementioned lipoproteins
(37). Furthermore, ApoE is an
independent risk factor for the formation of atherosclerotic heart
disease due to its gene polymorphism, which is involved in various
lipid metabolic processes, such as cholesterol efflux, transport
and storage. Since it was indicated that ApoE deficiency rapidly
leads to hyperlipidaemia in lipid metabolism disorders in the body,
most experiments have modelled atherosclerotic plaques by knocking
out this gene (38). In foam
cells, ApoE has a function similar to that of ABCA1, which is able
to transfer excess cholesterol esters from the cell to the
extracellular space, reduce the accumulation of lipids in foam
cells, and reduce and delay the formation and accumulation of foam
cells. The results suggested that ox-LDL stimulated macrophages,
leading to the upregulation of CD36, which regulated
PPAR-γ-mediated phagocytosis, and an increase in the mRNA
expression of ApoE upon increased ox-LDL uptake by macrophages.
LDL is the most common cholesterol lipoprotein and
is mainly responsible for the transfer of cholesterol to organs and
tissues. As natural LDL cannot induce foam-cell formation in
macrophages, acetylation and other modifications may only induce
mononuclear cells to form macrophages and achieve lipid uptake,
accumulation and eventually foam-cell formation when LDL is
oxidized (39). Oxidation or other
modifications to LDL have a higher risk of progression to
atherosclerotic-like plaques; thus, reducing ox-LDL damage to blood
vessel walls, ox-LDL accumulation in mononuclear cells and
macrophage uptake of ox-LDL are novel strategies to prevent plaque
formation (40).
ZNF580, a nuclear transcription factor that is
similar in protein structure to the Sp/KLF family, is widely
distributed in human organs and regulates downstream target genes
to participate in physiological and pathological processes in the
body in a variety of ways. ZNF580 has an important role in the
disease-causing mechanism of CHD and a previous study suggested
that ZNF580 was able to participate in ischaemic reperfusion damage
mediated by umbilical vein endothelial cell growth
factor-15(41). After
establishment of the experimental foam-cell model, ZNF580 mRNA
levels were measured and the results suggested that the expression
of ZNF580 was higher than that in the normal control group,
indicating that ox-LDL-stimulated macrophages exhibited changes in
ZNF580 expression. To further study the effect of ZNF580 on
foam-cell formation, ZNF580 mRNA and protein levels were measured
in the control group at 24, 48 and 72 h, and the results confirmed
that ZNF580 expression increased with increasing foam-cell
formation over time. This trend was largely the same as that of
CD36, PPAR-γ, ABCA1 and ApoE, which are associated with lipid
uptake and efflux in macrophages. Based on the results of this
experiment, it may be assumed that ZNF580 is able to block
foam-cell formation by affecting relevant lipid regulatory
molecules, possibly in relation to the PPAR-γ-CD36 signalling
pathway. However, the more specific relationship will be further
explored in future work. Lentivirus transfection was then used to
induce high expression or silence the ZNF580 gene in THP-1 cells,
after which the foam-cell model was established and the above
indicators examined. The results suggested that CD36 and PPAR-γ
mRNA expression in the high-expressing ZNF580 group was lower than
that in the model group, and CD36 and PPAR-γ mRNA expression in the
ZNF580 silencing group was significantly higher than that in the
model group. These data indicated that ZNF580 is able to inhibit
the expression of PPAR-γ mRNA, thereby reducing the expression of
the downstream molecule CD36 and LDL intake, hindering the
formation of foam cells. The genes associated with lipid outflow
were examined and it was indicated that high expression of ZNF580
was able to induce an increase in ABCA1 and ApoE mRNA expression
that was significantly higher than that in the model group. In
addition, silencing ZNF580 was able to inhibit the mRNA expression
of ABCA1 and ApoE, which was significantly lower than that in the
model group, suggesting that ZNF580 is able to hinder foam cell
formation by increasing cholesterol efflux. Therefore, it may be
hypothesized that ZNF580 may be situated upstream of PPAR-γ,
directly modulating PPAR-γ and thereby affecting downstream CD36
expression and reducing lipid build-up.
In conclusion, the present study provided new
insight into the utility of ZNF580 in the prevention of
atherosclerosis. High expression of ZNF580 was able to decrease
lipid uptake by macrophages and increase cholesterol efflux, reduce
the possibility of lipid accumulation in foam cells, reduce the
proportion of foam-cell formation, reduce the risk of plaque
formation and delay the progression of atherosclerotic plaques.
However, the present study only carried out cell experiments, which
may be considered a limitation, and future in vivo studies
will be conducted to more fully explore the role of ZNF580 in
atherosclerosis. Based on these findings, ZNF580 may be a potential
target for preventing atherosclerosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Project of
Tianjin Natural Science Foundation (grant no. 16JCZDJC31900),
Fundamental Research Project of Logistics University of People's
Armed Police Force (grant no. WHJ202108) and the Applied Research
Project of Logistics University of People's Armed Police Force
(grant no. WHJ202104).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZBZ, XTQ and MZ designed the experiments and revised
the manuscript. ZBZ, HXC, YCL and MY performed the RT-qPCR assays.
JXC, HWX, YT and YCL performed ORO staining. ZBZ, XTQ, HXC, CL and
MY performed western blot analysis. ZBZ, CL, JYL, JXC and MZ
analysed the datasets and supervised the project. All authors read
and approved the final manuscript and confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
References
|
1
|
Kingstone LL, Currie GM and Torres C: The
pathogenesis, analysis, and imaging methods of atherosclerotic
disease of the carotid artery: Review of the literature. J Med
Imaging Radiat Sci. 43:84–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roy A, Saqib U, Wary K and Baig MS:
Macrophage neuronal nitric oxide synthase (NOS1) controls the
inflammatory response and foam cell formation in atherosclerosis.
Int Immunopharmacol. 83(106382)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grajeda-Iglesias C and Aviram M: Specific
amino acids affect cardiovascular diseases and atherogenesis via
protection against macrophage foam cell formation: Review article.
Rambam Maimonides Med J. 9(e0022)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ghodsian N, Yeandle A and Gieseg SP: Foam
cell formation but not oxLDL cytotoxicity is inhibited by CD36 down
regulation by the macrophage antioxidant 7,8-dihydroneopterin. Int
J Biochem Cell Biol. 133(105918)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chávez-Sánchez L, Garza-Reyes MG,
Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV and
Blanco-Favela F: The role of TLR2, TLR4 and CD36 in macrophage
activation and foam cell formation in response to ox-LDL in humans.
Hum Immunol. 75:322–329. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maréchal L, Laviolette M, Rodrigue-Way A,
Sow B, Brochu M, Caron V and Tremblay A: The CD36-PPARγ pathway in
metabolic disorders. Int J Mol Sci. 19(1529)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ozasa H, Ayaori M, Iizuka M, Terao Y,
Uto-Kondo H, Yakushiji E, Takiguchi S, Nakaya K, Hisada T, Uehara
Y, et al: Pioglitazone enhances cholesterol efflux from macrophages
by increasing ABCA1/ABCG1 expressions via PPARγ/LXRα pathway:
findings from in vitro and ex vivo studies. Atherosclerosis.
219:141–150. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Du M, Yang L, Liu B, Yang L, Mao X, Liang
M and Huang K: Inhibition of NFAT suppresses foam cell formation
and the development of diet-induced atherosclerosis. FASEB J.
35(e21951)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang X, Su B, Gao B, Zhou J, Ren XK, Guo
J, Xia S, Zhang W and Feng Y: Cascaded bio-responsive delivery of
eNOS gene and ZNF580 gene to collaboratively treat hindlimb
ischemia via pro-angiogenesis and anti-inflammation. Biomater Sci.
8:6545–6560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luo Y, Zhao Y, Li X, Zhao J and Zhang W:
ZNF580 mediates eNOS expression and endothelial cell
migration/proliferation via the TGF-β1/ALK5/Smad2 pathway. Mol Cell
Biochem. 393:199–207. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
DangLi R, HeKong W, JiQin L, MingHua Z and
WenCheng Z: ROS-induced ZNF580 expression: A key role for
H2O2/NF-κB signaling pathway in vascular endothelial inflammation.
Mol Cell Biochem. 359:183–191. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu HJ, Wang XL, Zhang L, Qiu Y, Li TJ, Li
R, Wu MC, Wei LX and Rui YC: Inhibitions of vascular endothelial
growth factor expression and foam cell formation by EGb 761, a
special extract of Ginkgo biloba, in oxidatively modified
low-density lipoprotein-induced human THP-1 monocytes cells.
Phytomedicine. 16:138–145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu Y, Luo N, Lopes-Virella MF and Garvey
WT: The adipocyte lipid binding protein (ALBP/aP2) gene facilitates
foam cell formation in human THP-1 macrophages. Atherosclerosis.
165:259–269. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cao H, Jia Q, Yan L, Chen C, Xing S and
Shen D: Quercetin suppresses the progression of atherosclerosis by
regulating MST1-Mediated autophagy in ox-LDL-Induced RAW264.7
macrophage foam cells. Int J Mol Sci. 20(6093)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu JX: The effects of ZNF580
overexpression on the balloon-injury model of Rat (unpublished PhD
thesis). Hebei Medical University, 2013.
|
|
18
|
Yu HL: The role of ZFP580 in ROS mediated
the process of myocardial oxidative stress injury (unpublished PhD
thesis). Hebei Medical University, 2014.
|
|
19
|
Zhao Y: The effects of ZNF580 on the
expression of e NOS induced by TGF-β in endothelial cell
(unpublished PhD thesis). Hebei Medical University, 2013.
|
|
20
|
Daigneault M, Preston JA, Marriott HM,
Whyte MK and Dockrell DH: The identification of markers of
macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PLoS One. 5(e8668)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hamilton TA, Major JA and Chisolm GM: The
effects of oxidized low density lipopmteins on inducible mouse
macmphage gene expression are gene and stimulus dependent. J Clin
Invest. 95:2020–2027. 1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(-Delta Delta C(T) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: Systematic analysis of population health data.
Lancet. 367:1747–1757. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gonzalez L and Trigatti BL: Macrophage
apoptosis and necrotic core development in atherosclerosis: A
rapidly advancing field with clinical relevance to imaging and
therapy. Can J Cardiol. 33:303–312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Greenberg ME, Sun M, Zhang R, Febbraio M,
Silverstein R and Hazen SL: Oxidized phosphatidylserine-CD36
interactions play an essential role in macrophage-dependent
phagocytosis of apoptotic cells. J Exp Med. 203:2613–2625.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Luan Y and Griffiths HR: Ceramides reduce
CD36 cell surface expression and oxidised LDL uptake by monocytes
and macrophages. Arch Biochem Biophys. 450:80–99. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Coburn CT, Knapp FF Jr, Febbraio M, Beets
AL, Silverstein RL and Abumrad NA: Defective uptake and utilization
of long chain fatty acids in muscle and adipose tissues of CD36
knockout mice. J Biol Chem. 275:32523–32529. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhuang JL, Liu YY, Li ZZ, Zhuang QZ, Tang
WZ, Xiong Y and Huang XZ: Amentoflavone prevents ox-LDL-induced
lipid accumulation by suppressing the PPARγ/CD36 signal pathway.
Toxicol Appl Pharmacol. 431(115733)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tan YL, Zeng Y, Mo ZC, He PP, Ou YX, Yao
F, Xie W, Tang CK and Yi GH: Regulation of PPAR γ on the expression
of CD36 and lipid accumulation induced by high glucose in THP-1
macrophages. Chin J Mod Med. 24:18–23. 2014.(In Chinese).
|
|
30
|
Wu XH, Cheng B, Guo XJ, Wu QQ, Sun S and
He P: PPARα/γ signaling pathways are involved in chlamydia
pneumoniae-induced foam cell formation via upregulation of SR-A1
and ACAT1 and downregulation of ABCA1/G1. Microb Pathog.
161(105284)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li AC, Binder CJ, Gutierrez A, Brown KK,
Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum
JL, et al: Differential inhibition of macrophage foam-cell
formation and atherosclerosis in mice by PPAR-α,β/δ and γ. J
Clin Invest. 114:1564–1576. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bayliak MM, Dmytriv TR, Melnychuk AV,
Strilets NV, Storey KB and Lushchak VI: Chamomile as a potential
remedy for obesity and metabolic syndrome. EXCLI J. 20:1261–1286.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ahmadian M, Suh JM, Hah N, Liddle C,
Atkins AR, Downes M and Evans RM: PPARγ signaling and metabolism:
The good, the bad and the future. Nat Med. 19:557–566.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li K, Yao W, Zheng X and Liao K: Berberine
promotes the development of atherosclerosis and foam cell formation
by inducing scavenger receptor A expression in macrophage. Cell
Res. 19:1006–1017. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu X, Owen JS, Wilson MD, Li H, Griffiths
GL, Thomas MJ, Hiltbold EM, Fessler MB and Parks JS: Macrophage
ABCA1 reduces MyD88-dependent toll-like receptor trafficking to
lipid rafts by reduction of lipid raft cholesterol. J Lipid Res.
51:3196–3206. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu XH, Chen JJ, Deng WY, Xu XD, Liu QX,
Shi MW and Ren K: Biochanin A mitigates atherosclerosis by
inhibiting lipid accumulation and inflammatory response. Oxid Med
Cell Longev. 2020(8965047)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang J, Shi Q, Hu Y and Li X: Silibinin
augments the effect of clopidogrel on atherosclerosis in diabetic
ApoE deficiency mice. Clin Hemorheol Microcirc. 80:353–361.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Siest G, Pillot T, Régis-Bailly A,
Leininger-Muller B, Steinmetz J, Galteau MM and Visvikis S:
Apolipoprotein E: An important gene and protein to follow in
laboratory medicine. Clin Chem. 41:1068–1086. 1995.PubMed/NCBI
|
|
39
|
Di Pietro N, Formoso G and Pandolfi A:
Physiology and pathophysiology of ox-LDL uptake by vascular wall
cells in atherosclerosis. Vascul Pharmacol. 84:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu W, Wei Z, Dong J, Duan F, Chen K, Chen
C, Liu J, Yang X, Chen L, Xiao H and Liu A: Global metabolomics
reveals the metabolic dysfunction in ox-LDL induced
macrophage-derived foam cells. Front Pharmacol.
8(586)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Meng FP: The role and molecular mechanism
of zinc finger gene ZFP580/ZNF580 in GDF-15 attenuating
ischemia/reperfusion induced no reflow injury (unpublished PhD
thesis). Hebei Medical University, 2016.
|