1. Introduction
The human pregnancy is a delicately orchestrated
biological process initiated with fertilization, followed by the
subsequent mitoses of the zygote, leading to the formation of the
blastocyst, and subsequently the differentiated embryo. An
adequately competent blastocyst is implanted in the maternal
endometrium and is comprised of the embryoblast and the enclosing
trophoblast (1). The embryoblast
constitutes the embryonic disc, that will further differentiate
into the three germ layers (ectoderm, mesoderm, endoderm), finally
giving rise to the formation of all human tissues. The cells of the
trophoblast differentiate into syncytiotrophoblast (ST),
participating in maternal-fetal oxygen and nutrient exchange, and
extravillous trophoblast (EVT), invading into the uterine wall and
remodeling the uterine spiral arteries (2). Together with the decidua, form the
placenta, a transitory organ that mediates the maternal-fetal
exchange of oxygen, CO2, and nutrients through an
interface between the maternal and the fetal circulation (3). Apart from its respiratory,
detoxifying, nurturing, and metabolic role subserving the needs of
the growing fetus, the placenta has crucial immunological and
endocrine properties, as it protects the fetus from rejection and
the mother from graft vs. host disease, and massively produces
hormones, such as human chorionic gonadotropin (hCG) and human
placental lactogen (hPL) (3).
It has been previously demonstrated that the
interaction between maternal and fetal cells during implantation
and throughout pregnancy is mediated by the release of exosomes and
other extracellular vesicles both from the embryo and maternal
tissues, especially the placenta (4-6).
Extracellular vesicles (EVs) are enclosed in a lipid bi-layer
membrane and can be subdivided into heterogenous subsets of
populations based on their size and derivation (1,7).
These include apoptotic bodies, with a diameter ranging from 2 to 3
µm, microvesicles, sized 100 nm-1 µm, and exosomes, representing
the smallest population with a diameter of 30 to 120 nm (7). The placenta also releases syncytial
nuclear aggregates with a size of 20 to 100 µm and a largely
unknown function, possibly resulting from dying
syncytiotrophoblasts (8). After
their release into the extracellular space, EVs mediate the
communication between proximal and/or distant cells, by
transferring their origin- and environment-dependent cargo,
including proteins, lipids, coding and non-coding RNAs (such as
miRNAs and lncRNAs) and DNA, thus, inducing biological alterations
in the recipient cells (7). Each
subpopulation of EVs is characterized by distinct surface membrane
markers and cargo, based on their biogenesis and origin, which are
used for their differentiation and classification. More
specifically, the human placenta releases exosomes that distinctly
contain placenta-specific proteins, such as placental alkaline
phosphatase (PLAP) and miRNAs belonging to chromosome 19 miRNA
cluster, thus, facilitating their identification (9).
The regular initiation and continuation of pregnancy
entails a precise regulation of the maternal immune system, in
order not to reject the embryo as a foreign immune tissue, as well
and to protect the mother from the fetus. Exosomes play an
important role in the establishment of maternal immunotolerance to
the fetus by mediating the inter-cellular communication between the
placenta and the maternal peripheral blood T-cells (10). The concentration of
placenta-derived exosomes displays an incremental tendency
throughout a normal pregnancy, starting as early as 6 weeks of
gestation (6). Alterations in
exosome release and bioactivity, by still undefined exact
mechanisms, have been associated with dysregulated maternal-fetal
communication, resulting in pregnancy complications, including
gestational diabetes mellitus, preeclampsia, and preterm birth,
thus posing a serious threat to the survival of the fetus, as well
as having a long-term impact on maternal and fetal health (9).
In recent years, research on the role of exosomes
and exosomal non-coding RNAs in normal and complicated pregnancy
has been extensive. The prospective beneficial research outcomes
include the development of novel and sensitive non-invasive
biomarkers of pregnancy status and potential complications, as well
as of fetal abnormalities. The aim of this review is to update
current knowledge regarding the role of exosomes and exosomal cargo
in normal adolescent and adult pregnancy, as well as in pregnancy
disorders and provide novel perspectives on their potential
applications for the early diagnosis of pregnancy complications.
Although there are no exosome studies that specifically focus on
adolescent pregnancy, there is adequate related information in
studies where the age range of cohorts of pregnant women includes
adolescent and young adult women.
2. Exosomes
Exosome biogenesis
Exosomes, apart from their small size compared to
other EVs, are characterized by a distinct buoyant density
(1.13-1.19 g/ml) and pathway of biogenesis. Exosomes originate from
the endosomal compartment, which participates in vesicle
trafficking (Fig. 1). They are
created by curvature of the membrane of the multivesicular bodies
(MVBs), leading to their initial formation as intraluminal vesicles
(ILVs), enclosed in a lipid bilayer (7). Because of their endosomal origin,
their membrane contains late endosomal markers, such as Tsg101,
CD63, CD9, and CD81, as well as origin cell-specific membrane
markers, which constitute the basis for their discrimination
(9).
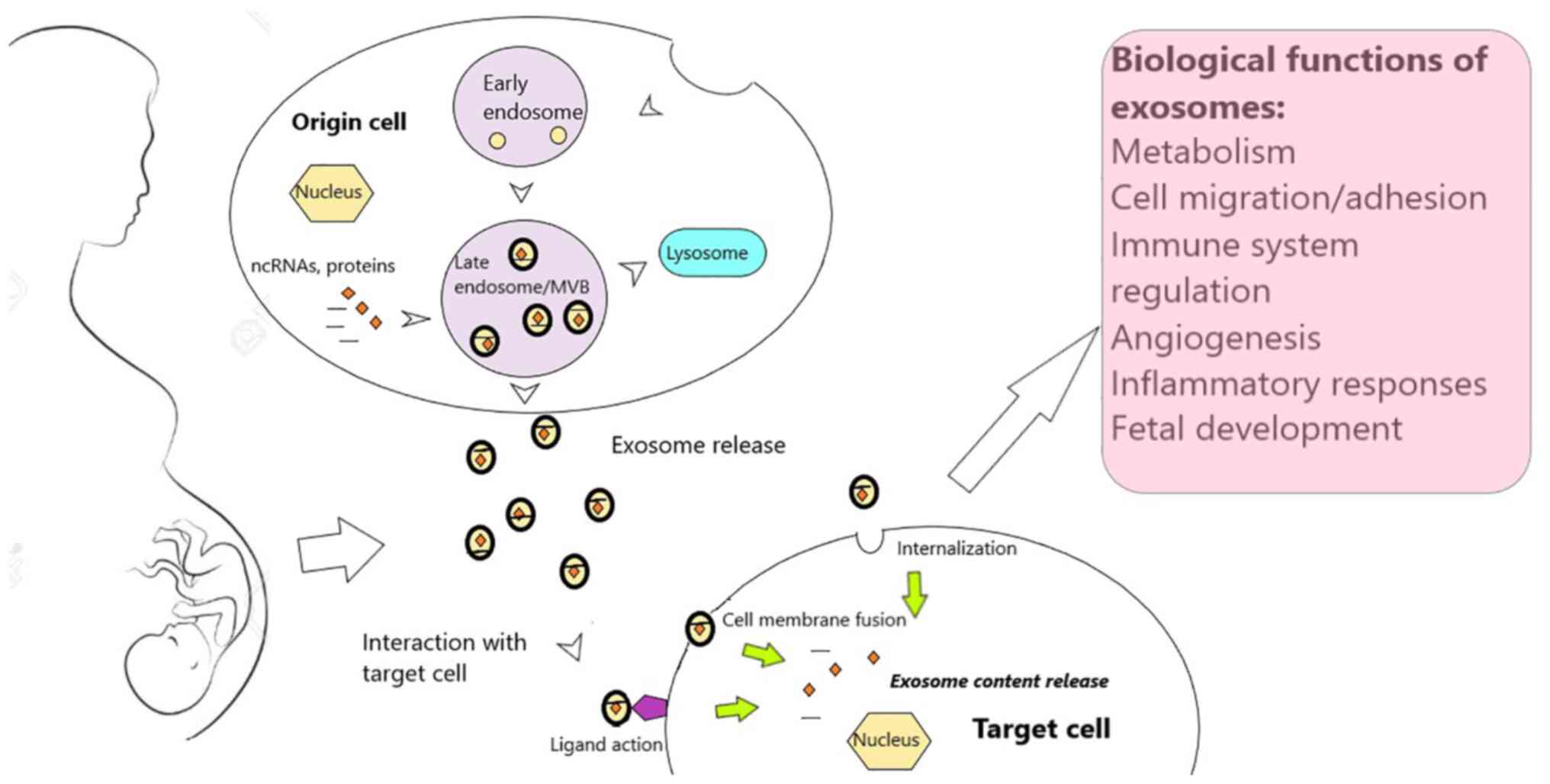 | Figure 1Exosome biogenesis and functions.
Exosomes are generated within the endosomal compartment, where
early endosomes mature to late endosomes and finally form MVBs. The
membrane of the MVBs invaginates to form lipid-bilayer vesicles
with characteristic membrane late endosomal markers, which
incorporate active biomolecules, such as proteins and ncRNAs,
finally leading to the formation of exosomes. Subsequently,
exosomes can be directed to lysosomes for content breakdown or be
released in the extracellular space, where they selectively
interact with target cells via unique surface molecules, and modify
their phenotype. Exosomes can enter target cells via endocytosis or
phagocytosis, or by fusing with the plasma membrane. Alternatively,
they can exert their biologic functions by interacting with target
cell surface receptors, without being internalized. Among the
important biological processes influenced by exosome signaling are
metabolic adaptation, immune and inflammatory reactions, cell
adhesion and migration, angiogenesis, as well as processes required
for regular fetal development. MVBs, multivesicular bodies; ncRNAs,
non-coding RNAs. |
Exosomes can be formed by either of two major
biosynthetic mechanisms, which may or may not involve the endosomal
sorting complex required for transport (ESCRT). The most clearly
defined mechanism relies on the ESCRT complex, which consists of
four subunits. These subunits are consecutively activated to
incorporate ubiquitinylated proteins into ILVs, induce inward
budding of the endosomal membrane and dissociation of the emerging
vesicle from the membrane into MVBs, ultimately leading to exosome
assembly (4). The
ESCRT-independent mechanism is alternatively activated to guide
exosome formation when the components of the ESCRT complex are
consumed, and includes among others the tetraspanin family (CD9,
CD63, CD81, CD82), ceramides, phospholipase D2, -all present in the
exosomes- to guide exosome wrapping and loading of exosomal cargo
(11).
The formation of MVBs can be ensued by direction to
lysosomes for content degradation or to the plasma membrane for
exosome release; however, they can also participate in antigen
presentation by major histocompatibility class II molecules
(MHCII), or be recycled (12).
Vesicle tethering and fusion with the plasma membrane, as well as
exosome release, are regulated by numerous proteins, such as the
distinctive for every cell type Rab GTPases, syntenin, and ALIX,
that have been also identified in placental exosomes, and the
soluble N-ethylmaleimide-sensitive factor attachment protein
receptors (SNARE) (7,13). Ceramide metabolism, intracellular
calcium, endoplasmic reticulum stress, all reflecting the metabolic
state of the originating cell, play a role in exosome biogenesis
and release. Exosome release is subsequently followed by the
selective interaction with target cells, eliciting phenotypic
alterations via the transfer of exosomal cargo. Exosomes contain
specific surface adhesion molecules, that mediate the interaction
with specific recipient cells; e.g., trophoblast exosomes express
fibronectin, syncytin-1, and syncytin-2 that are involved in
selective cell targeting (14).
The uptake of exosomes from the target cells and the delivery of
their content can occur by clathrin-mediated endocytosis or
phagocytosis or by immediate fusion of the exosome membrane with
the plasma membrane. Nevertheless, exosomes can interact with
target cells without being internalized via binding of exosome
membrane proteins, or soluble parts of them, to target cell
membrane receptors (12). However,
the exact mechanisms of exosome uptake remain to be elucidated.
Exosomal Non-coding RNAs
The bioactivity of exosomes is closely correlated to
the cell-to-cell transfer of their unique cargo, comprised of
proteins, lipids, DNA, mRNA and RNAs that do not encode proteins,
i.e. non-coding RNAs (ncRNAs). Intriguingly, ncRNAs appear to
prevail in the total amount of the human genome transcripts and may
orchestrate an intricate network of interactions with other
biomolecules, leading to dynamic gene regulation (7). Commonly, length is used as a
criterion to separate ncRNAs into small ncRNAs, mainly microRNAs
(miRNAs), with a length smaller than 200 ribonucleotides, and long
ncRNAs (lncRNAs), with a length over 200 ribonucleotides (15). Lately, circular RNAs (circRNAs),
have also been described. However, this review will mainly focus on
the description of exosomal miRNAs and lncRNAs.
miRNAs are a subclass of ncRNAs (20-22 nt), that
play a key role in physiological processes, including cell
proliferation, differentiation, and migration, as well as in the
onset and development of various disorders, such as immune
disorders and cancer (16,17). miRNAs, after processing by a
sequence of protein complexes resulting in the formation of the
mature single stranded miRNA, mainly exert their biological
function by inducing post-transcriptional gene silencing, as a key
component of the RNA-induced silencing complex (RISC) (16,17).
RISC binds principally to the 3'-untranslated region (UTR) of
target mRNAs, containing specific recognition sequences, and
destabilizes transcription or hampers translation (18). Furthermore, it has been lately
demonstrated that miRNAs can also induce up-regulation of gene
expression (7). Various cell types
release miRNAs that are shielded from RNase and extreme pH and
temperature alterations, either enclosed in exosomes and other EVs,
or within lipoproteins or even attached to protein complexes while
being in free form (19-21).
Exosomal miRNAs hold great promise for the diagnosis and
therapeutic targeting of various disorders because of their
involvement in the regulation of key physiological and pathological
processes and their relative stability in body fluids, explaining
the reason for intense scientific research in this field
lately.
Another interesting subclass of ncRNAs are the
lncRNAs, with a length that exceeds 200nt, that is however highly
variable (7). LncRNAs result from
the transcription of exonic, intergenic or distal-protein coding
genomic regions, are characterized by 3-polyadenylation, 5-splicing
and prominent thermodynamic stability (22). LncRNAs participate in various
biological processes and their expression patterns are finely
tuned, thus altered levels of specific lncRNAs have been associated
with the onset and progression of various disorders with still
undefined exact mechanisms for most of them (15,23).
Importantly, the nuclear localization of lncRNAs is suggestive of a
principal involvement in epigenetic gene regulation, whereas
cytoplasmic lncRNAs primarily regulate genes
post-transcriptionally. Subsequently, they can interact with other
biomolecules (proteins, DNA, mRNA, ncRNAs) and act as recruiters,
competitors, or miRNA precursors, as well as miRNA sponges, thus
acting on miRNA post-transcriptional gene regulation (15,23,24).
Recently, lncRNAs with still undefined exact mechanisms of loading
were found within exosomes. The enclosure of specific lncRNAs in
exosomes is regulated by the originating cell type and environment,
while they participate in exosomal inter-cellular communication by
transferring information and inducing alterations in neighboring or
distant cells (25). Exosomal
lncRNAs could represent useful potential biomarkers for a number of
disorders, because of their protection from enzymatic degradation
inside exosomes, their higher content compared to other EVs and
their high tissue specificity (24).
Function of exosomes
The predominant function of exosomes that has been
extensively investigated lately is their role in intercellular
communication, mediated by their originating cell- and
environment-dependent biologically active content. This includes
proteins and coding and non-coding RNAs, that transfer important
signals and reflect the originating cell state at the time of
exosome generation (26). Most
cells, including cancer, epithelial, immune and hematopoietic
cells, produce exosomes that upon their release can act proximally
in a paracrine or distally in an endocrine way, the latter by
entering systemic circulation and modifying the expression and
function of recipient cells (1,12).
Intriguingly, exosomes can dispense cells from the need for direct
contact and synchronize epigenetically distant cells by binding of
their ligands to distinct recipient cell receptors concurrently.
Furthermore, they could enrich the target cell membrane with
surface molecules, thus widening cell targeting extent and
providing new adhesion properties to them (20). In this way, exosomes participate in
signal transduction, as well as in direction of harmful material to
lysosomes for removal, to preserve cellular homeostasis in
physiological processes, such as immune and nervous system
regulation, tissue repair, sperm maturation, and, importantly,
maternal immune tolerance during pregnancy (12,27).
Hence, dysregulated exosomal signaling mechanisms in response to
acute stress, could induce recipient cell damage and inflammation,
leading to the onset and progression of pathological situations,
including infections, cancer, neurodegenerative, autoimmune and
cardiometabolic diseases, as well as pregnancy disorders (2,9,26).
Exosomes have been isolated in a number of biological fluids, such
as blood, lymph, urine, breast milk, saliva, lachrymal and mammary
gland secretions, as well as in amniotic and cerebrospinal fluid
(20,25). Their isolation in most biological
fluids and their involvement in multifarious normal and
pathological processes, renders them enticing candidate biomarkers
of health and disease (28).
Placenta-derived exosomes
The establishment of human pregnancy requires a
succession of maternal metabolic adaptations to fetal demands.
Accordingly, the human placenta, as well as the embryo, secrete
exosomes, along with other biomolecules, which ensure an intact
maternal-fetal crosstalk, regulate the maternal immune and vascular
system and possibly participate in placentation (6,9). All
cells forming the placenta, principally syncytiotrophoblast,
produce exosomes (10,29), while placental mesenchymal stem
cells, in vivo and in vitro, release exosomes that promote
endothelial cell migration and vascular tube formation (30,31).
Placental exosomes, similarly to exosomes of
different derivation, transfer specific cargos (proteins, nucleic
acids, lipids), which are indispensable for the mediation of their
biological functions, encompassing the transmission of important
placental information to the mother and the induction of metabolic
modifications. The expression of chromosome 19 miRNA cluster
(C19MC), which represents the largest miRNA gene cluster, occurs
principally in the human placenta, and contains 46 miRNAs
exclusively expressed in the placenta of adolescent and adult
pregnancies (32). The resulting
miRNAs can be subsequently selectively loaded to exosomes and
transported to different cell types (33). Placental miRNAs can be secreted in
a free form or enclosed and shielded from degradation inside
exosomes, with mechanisms that have not been fully elucidated;
however, under normal circumstances exosomal miRNA signature highly
resembles the one of the origin placental cell (33). Placental miRNAs have been detected
in the peripheral blood of pregnant adolescent and adult women
(9,32). In a study investigating the
conditioned medium of chorionic villi of term placentas of
adolescent and adult women for exosomal miRNA content, 456 distinct
miRNAs were detected, most of them pertaining to C19MC cluster, but
also to other non-placental specific families, such as the C14MC
gene cluster and the let-7 family (34). Moreover, C19MC miRNAs expression
profile could play a role in the acquisition of distinct
characteristics between the more invasive EVT and villous
trophoblast cells (VTs); miR-519d targets invasiveness-associated
proteins, thus diminishing cell migration (35).
Microenvironmental factors, such as oxygen tension
and glucose concentration impact, separately and synergistically,
on the formation, secretion, and bioactivity of placental exosomes.
More specifically, metabolic stress accompanying hypoxia augmented
exosome release and altered placental exosome cargo and signaling
to induce EVT invasion and proliferation, which occurs anyway in
presence of low oxygen tension (30). Furthermore, elevated D-glucose
levels correlated positively with primary trophoblast cell exosome
secretion and bioactivity, possibly by enhancing exosome formation
within MVBs, MVB trafficking, exosome exocytosis and altering
exosomal miRNAs (2). Placental
exosomes characterization and distinction can be accomplished by a
set of C19MC miRNAs, and distinct membrane proteins, such as
placental alkaline phosphatase (PLAP), which is derived mostly from
syncytiotrophoblast (9). Lately,
it has been shown that PLAP antibodies could be used to distinguish
and quantify placenta-derived exosomes, possibly providing
important information about the fetus and the placenta status
(36). EVT-derived exosomes are
uniquely characterized by the expression of human leukocyte
antigen-G (HLA-G), possibly involved in maternal immune tolerance
to the fetus (37). For
investigating placenta-derived exosomes, cell cultures of
trophoblasts, chorionic villi explants, placental perfusion, as
well as maternal plasma and urine, are used (1).
3. Exosomes in normal pregnancy
Effective maternal-fetal communication via exosomes
is of great importance for maternal adaptation to pregnancy, fetal
survival, and normal development; however, uncovering their exact
mechanisms of action remains a distant goal, due to the huge
variety of bioactive molecules transported by exosomes. The
peripheral blood of pregnant women contains remarkably more
exosomes than in non-pregnant women (10), which could be identified as early
as the sixth week of pregnancy in peripheral blood (9). Their concentration displays
incremental tendency with the advancement of a normal pregnancy,
peaking towards the end of pregnancy, and is maintained high until
labor (1,2). Placenta-derived exosomes
concentration, as indicated by the presence of PLAP, increases
across gestation (6). The fraction
of placenta-derived exosomes to total exosomes was raised in the
peripheral blood of pregnant women in the final stages of
pregnancy, starting on from mid-gestation (38). Placental exosomes concentration is
normally determined by placental mass (2). The levels of placental miRNAs can
fluctuate throughout pregnancy as well; for example, placental
miRNA-141 plasma levels rise as pregnancy progresses (9) and the concentration of C19MC
gradually declines upon parturition (26).
Exosomes and exosomal miRNA contribute to
embryo-endometrium communication, requisite for effective
implantation and placentation (14,26,39).
Endometrial epithelial cells liberate exosomes, which subsequently
interact with trophoblasts to promote their adhesion to the uterine
cavity by the activation of numerous signaling pathways, including
focal adhesion kinase signaling (40). These exosomes contain miRNAs, such
as miR-30d, that was demonstrated to upregulate adhesion-related
genes (integrins beta-3 and alpha-7, cadherin-5) (41) (Table
I). Tissue remodeling accompanying implantation occurs under
subtle inflammatory conditions and placental EVs may be involved in
this process by regulating cytokine secretion (42). Trophoblast-derived exosomes induce
monocyte migration and differentiation to tissue macrophages and
markedly increase the secretion of cytokines and chemokines,
including IL-1β, IL-6, serpin-E1, granulocyte and
granulocyte/monocyte colony-stimulating factor (G-CSF and GM-CSF,
respectively), and TNFα, that promote the development of the
trophoblast (4). During normal
pregnancy, the communication between the mother and the growing
fetus is accomplished by the exchange of exosomes, produced both
from the mother and the fetus. In a mouse model, maternal exosomes
could translocate to the fetus, overcoming placental barriers
(43).
 | Table IStudies evaluating miRNAs in normal
pregnancy. |
Table I
Studies evaluating miRNAs in normal
pregnancy.
| miRNA | Target | Source | (Refs.) |
|---|
| Embryo
implantation | | | |
|
miR-30d | Adhesion (Itgb3,
Itga7, Cdh5) | EF exosomes | (41) |
| Pregnancy | | | |
|
miR-519d | EVT invasiveness
(CXCL6, NR4A2, FOXL2) | EVT-derived cell
line | (35) |
|
miR-517a-3p | Maternal immune
modulation(NO/cGMP/PRKG1 pathway blockade) | Peripheral blood NK
cells | (48) |
|
miR-210-3p | Endothelial cell
migration | Umbilical serum
exosomes | (49) |
|
miR-376c-3p | | | |
|
miR-151a-5p | | | |
|
miR-296-5p | | | |
|
miR-122-5p | | | |
|
miR-550a-5p | | | |
|
miR-517-3p | Inhibition of viral
replication | Human
trophoblast | (50) |
|
miR-516b-5p | | | |
|
miR-512-3p | | | |
The fetus, as well, releases exosomes that target
uterine and cervical cells and could possibly activate inflammatory
pathways signaling the beginning of labor (2). In pregnant mice, the addition of
exosomes, isolated from other pregnant mice, during late pregnancy
could induce parturition-associated alterations, and such effect
was not observed with the addition of early pregnancy exosomes
(44). Commonly, the decrease of
progesterone levels is essential for the onset of labor;
surprisingly, exosomes promoted the initiation of parturition,
regardless of progesterone levels (45).
The essential state of maternal immune tolerance to
fetal tissues consists of intricate interacting mechanisms
involving various biomolecules, importantly exosomes as well.
Maternal immune cells internalize exosomes of embryonic origin,
which induce an idiosyncratic antigen-specific immunosuppression
(partially mediated by regulatory T cells), thus aiding the embryo
to escape maternal immunosurveillance (1). It has been demonstrated that
trophoblast derived EVs enhance T cell differentiation to
regulatory T cells by transferring the heat shock protein
HSPE1(46). Furthermore, placental
exosomes play a role in the attenuation of effector T-cell response
involved in maternal immunoregulation, protecting the invading
trophoblast from degradation (10). Upon in vitro interaction of
mononuclear and dendritic cells with exosomal proteins,
differentiation of stem cells occurs, promotion of cell migration,
as well as inhibition of activation of natural killer (NK) cells
and macrophages. More specifically, placenta-derived exosomes are
internalized due to their specific ligands, and subsequently
inhibit the expression of the activating NK cell receptor NKG2D and
induce the expression of proapoptotic molecules, such as Fas ligand
(FasL) and tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL), leading to blockade of NK cell activation and
cytotoxicity, and apoptosis of peripheral blood mononuclear cells,
respectively (9,10,47).
Moreover, exosomal miR-517b was found to induce the expression of
TNFα and other apoptosis-promoting ligands (1). It was suggested that miR-517a-3p is
carried by placental exosomes to NK cells during pregnancy, blocks
the NO/cGMP/PRKG1 pathway and possibly activates NF-κB, leading to
maternal immune modulation. Accordingly, miR-517a-3p is absent in
the NK cells of non-pregnant women and remarkably diminishes in the
cells of pregnant women following parturition (48).
Other significant roles of exosomes during normal
pregnancy relate to their potential role in vascular remodeling and
in resistance to viral infections. As the invasion of
cytotrophoblasts progresses, the remodeling of endometrial spiral
arteries ensures competent supply of oxygen and nutrients to the
fetus. Predominantly in presence of low oxygen tension, placental
EVs seem to detect hypoxic conditions and stimulate angiogenesis,
while the embryo releases exosomal miRNAs and vascular endothelial
growth factor A (VEGFA) (1). The
capacity of exosomes to induce endothelial cell migration is higher
in the first trimester of pregnancy compared to the second and
third trimester (9). The
expression of a group of miRNAs, that relate to endothelial cell
migration (miR-210-3p, miR-376c-3p, miR-151a-5p, miR-296-5p,
miR-122-5p, and miR-550a-5p), was found modified in umbilical serum
exosomes (6,49). Interestingly, non-placental cells
upon exposure to trophoblast-derived exosomes enhance their defense
to viral infections, an effect mediated by the transfer of exosomal
miRNAs into trophoblast cells. For instance, miR-517-3p,
miR-516b-5p, and miR-512-3p, belonging to C19MC lead to inhibition
of viral replication and autophagy on target cells (50).
4. Exosomes in pregnancy complications
It has been suggested that exosomal concentration,
content, and function could be related to dysregulated placental
activity and that it may be altered in pregnancy-related disorders
(8,9). Indeed, in comparison to normal
gestation, as well as in comparison to non-pregnant subjects, in
women with pregnancy complications exosome release appears to be
quite prominent (9,16).
Exosomal concentration during normal pregnancy was
associated with trimester of pregnancy and maternal body mass index
(BMI), with obese women characterized by significantly elevated
exosome quantity, while in the latter group exosomes induced a more
marked secretion of inflammatory cytokines (TNF-α, IL-6, IL-8) from
endothelial cells, in comparison to normal weight or overweight
pregnant women (38). Apart from
the BMI, glucose levels and fetal body weight displayed a strong
association with the concentration of placental exosomes during
pregnancy, underscoring the potential role of the latter in
conditioning maternal tissues for gestational metabolic alterations
and their potential predictive use in pregnancy complications
(51). Moreover, alterations of
exosomal miRNA content and exosome-derived cell-free DNA (cfDNA)
were linked to pregnancy disorders (1,52)
(Table II). These observations,
along with their tissue-specificity, suggest that exosomes could
potentially offer a novel type of liquid biopsy, reflecting the
placental status and alerting promptly to various pregnancy
pathologies (26). However, there
is a great heterogeneity of isolation methodologies, and confusion
arises from the lack of standardized methods for vesicle
subpopulation discrimination. Furthermore, most studies use plasma
from the second and third trimesters of pregnancy compared to first
trimester samples, problems that need to be resolved before the
utilization of exosomes in clinical applications (9). Here, we summarize current findings
regarding the potential exosomal contribution to common pregnancy
disorders, including preeclampsia and gestational diabetes
mellitus, as well as preterm birth, and selected fetal
abnormalities.
 | Table IIStudies evaluating miRNAs and lncRNAs
in diverse pregnancy complications. |
Table II
Studies evaluating miRNAs and lncRNAs
in diverse pregnancy complications.
| miRNA/lncRNA | Target | Source | (Refs.) |
|---|
| Early-onset
preeclampsia | | | |
|
miR-210
(↑) | Trophoblast
invasion | Plasma
exosomes | (4) |
|
miR-517-5p
(↑), 423-5p (↑) | Unknown | Plasma | (26) |
| Preeclampsia | | | |
|
miR-155
(↑) | eNOS expression
blockade | Plasma,
placenta | (59,60) |
|
miR-141
(↑) | Trophoblast
invasion | Trophoblast-derived
exosomes | (4) |
|
hsa-miR-486-1-5p,
486-2-5p | Unknown | Plasma total
exosomes and placenta-derived exosomes | (58) |
|
miR-495 (↑),
494 (↑), 136 (↑) | Cell proliferation,
apoptosis | Peripheral blood
and umbilical cord MSCs exosomes | (61) |
|
lncRNA
NONHSAT116812, NONHSAT145880 | Unknown | Plasma,
placenta | (63) |
|
lncRNA
H19 | Trophoblast cell
invasion, migration (↑ FOXO1) | MSCs exosomes | (64) |
| Gestational
diabetes mellitus | | | |
|
miR-518a-5p,
518b, 518c, 518e, 520c-3p, 525-5p | Unknown | Serum exosomes | (1) |
|
miR-125a-3p
(↑), 99b-5p (↑), 197-3p (↑), 22-3p (↑), 224-5p (↑) | Cell migration,
carbohydrate metabolism | Placenta, skeletal
muscle, plasma exosomes | (34) |
|
miR-16-5p
(↑), 17-5p (↑), 20a-5p (↑) | Unknown | Plasma | (70) |
|
lncRNA
MALAT1 (↑) | Unknown | Serum | (71) |
| Preterm birth | | | |
|
miR-515-5p
(↑), 516-5p (↑), 518b (↑), 518f-5p (↑), 519a (↑), 519e-5p (↑),
520a-5p, 520h, 526b-5p (↑) | Unknown | Placenta | (74) |
|
miR-223 (↑),
302b (↓), 548 (↓), 1253 (↓) | Unknown | Plasma | (75) |
| Intrauterine growth
restriction | | | |
|
miR-103a-3p
(↓), 126-3p (↓), 195-5p (↓), 499a-5p (↓) | Unknown | Plasma | (16) |
| Down syndrome | | | |
|
miR-15a (↑),
let-7d (↑), 23a (↑),99a (↑), 142 (↑),
191 (↑), 199 (↑), 3156 (↑) | CNS development,
congenital abnormalities, heart defects | Plasma | (82) |
Exosomes in preeclampsia
Preeclampsia (PE) represents one of the most common
and significant systemic pregnancy disorders, comprising women with
new onset increases of arterial blood pressure, developing mainly
in the third trimester of pregnancy, as a result of various factors
(53). PE prevention and reversal
would be of great importance, given the high prevalence of PE, the
close correlation of PE with intrauterine growth restriction (IUGR)
and premature birth, and its high maternal and neonatal morbidity
and mortality; 76,000 women and 500,000 neonates die each year
globally because of PE (2,54). Although PE pathogenesis has not
been fully elucidated, shallow EVT invasion and deficient spiral
artery remodeling, and resultant placental hypoxia are key
processes involved in abnormal placentation and the development of
PE (4).
There has been intense scientific interest regarding
quantitative and qualitative alterations of placenta-derived
exosomes in pregnancies complicated with PE. As it regards
quantity, the plasma content of placenta-derived exosomes is higher
in early-onset PE (PE occurring before the 33rd pregnancy week),
while it is reduced in late-onset PE (PE after the 34th pregnancy
week), compared to the equivalent weeks of normal pregnancy
(55). Intriguingly, an increase
in the number of total and placental exosomes in the circulation of
women who will later on present with PE, can be observed already
from the first trimester of pregnancy, supporting the attractive
perspective of using placental exosomes as candidate non-invasive
biomarkers of PE (2,29). Exosomes in PE possess a distinct
lipidomic and proteomic profile, probably participating in immune
reactions, vascular regulation, and oxidative stress (9). Pregnancies complicated with PE are
characterized by a unique exosomal protein cargo in comparison with
uncomplicated pregnancies (56).
Interestingly, exosomes seem to be involved in the
pathogenesis of PE. Infusion of exosomes from the plasma of women
with PE to pregnant mice, reduced their body weight, increased
their blood pressure, and disrupted fetal development, an effect
not observed when exosomes from normal pregnancy were injected
(4). In PE, the release of EVs and
remnants from trophoblast cells induced endothelial damage and
vascular inflammation, key processes in the pathogenesis of PE
(56). Endothelial nitric oxide
synthase (eNOS), which is essential for the synthesis of vasoactive
nitric oxide (NO), as well as neprilysin, a vasopeptide-cleaving
enzyme, are incorporated in EVs released from the
syncytiotrophoblast; thus, EVs could serve as indicators of reduced
NO bioactivity and increased risk of hypertension accompanying PE
(1,57). Considering the impact of oxygen
tension on the release of placental exosomes, it seems alluring
that the evaluation of placental exosomes might be used for the
timely detection of asymptomatic women and adolescents susceptible
to PE (58).
The involvement of exosomal miRNAs and proteins in
the pathogenesis of PE in adolescent and adult pregnant women and
their predictive potential are also under evaluation (32). More specifically, exosomal miR-155,
which is upregulated in PE both in the plasma and the placenta,
blocked the expression of eNOS, while the fact that aspirin largely
reversed the occurrence of early onset PE in women at risk was
partially attributed to exosomal miR-155 down-regulation (59). Furthermore, exosomal miR-210 was
markedly upregulated in early-onset PE in comparison to normal
pregnancy, and it has been previously found to interfere with
trophoblast invasion and migration (4), while C19MC miRNAs miR-517a/b and
miR-517c also display distinct expression profiles in PE, possibly
linked to similar biological processes (16). Trophoblast-derived exosomes in PE
contain increased levels of miR-141, also shown to participate in
trophoblast invasion (4). Studies
aiming to identify potential future biomarkers for PE, evaluated
circulating miRNAs altered in the serum of patients who
subsequently presented with PE; 11 up-regulated miRNAs, including
miR-155, as well as 5 down-regulated miRNAs, were detected
(60). It has also been suggested
that exosomal hsa-miR-486-1-5p and hsa-miR-486-2-5p, which are
consistently increased in PE, could be used as biomarkers for the
prediction of PE (58). In another
study, exosomal miR-495, miR-494, and miR-136, which are
upregulated in PE and possibly implicated in the establishment of
PE by reducing cell proliferation and apoptosis, could also be
employed in the clinical setting for the early diagnosis of PE
(61). Furthermore, the expression
of non-exosomal hsa-miR-325 was downregulated in placentas derived
from adolescent and adult women with preeclampsia, suggesting a
potential pathogenetic role, by interfering with oxidative stress
pathways and heat shock protein production (62).
LncRNAs have also been implicated in the modulation
of trophoblast invasion and the establishment of PE (63). In a study evaluating free
circulating lncRNAs profiles in PE, 163 alternatively expressed
lncRNAs were found in the placenta of women with late onset PE and
two lncRNAs (NONHSAT116812 and NONHSAT145880), were significantly
correlated with both early and late onset PE, denoting their
possible use as non-invasive biomarkers of PE (63). Strikingly, exosomes released from
mesenchymal stem cells (MSCs) highly express lncRNA H19, which acts
as a competing endogenous RNA (ceRNA) for miRNA let-7b, thus
upregulating FOXO1 and activating signaling pathways that enhance
trophoblast cell survival, invasion, and migration (64). These findings could offer
entrepreneurial strategies for combating PE.
Exosomes in gestational diabetes
mellitus
Gestational diabetes mellitus (GDM) represents
another severe pregnancy disorder, with an alarming mounting
prevalence parallel to the obesity outbreak (65). GDM can have devastating
consequences, such as premature delivery, perinatal complications,
macrosomia, and future risk of development of type 2 diabetes and
cardiovascular disease, both for the mother and the child (16). GDM is characterized by pathological
glucose metabolism presenting across gestation and/or unprecedented
or unrecognized abnormal glucose tolerance, which to date is
diagnosed by an oral glucose tolerance test (OGTT) at 24-28 weeks
of gestation (15). Normally,
along gestation, insulin sensitivity rises to a peak over the first
and the second trimester to ensure the essential for the
progression of pregnancy energy storage, and subsequently declines,
leading to insulin resistance that shifts glucose and fatty acids
from the mother to the fetus to provide it with adequate nutrients
(15). In case of new onset or
preexisting abnormal maternal insulin resistance, maternal
pancreatic β-cells cannot secrete enough insulin to counterbalance
the high glucose levels present in circulation, leading to maternal
hyperglycemia, hyperinsulinemia, and hypoxia, all impacting
negatively on the maternofetal interface (1). In addition, GDM is characterized by a
more exacerbated proinflammatory condition than the one normally
observed during pregnancy; gestational tissues, as well as adipose
tissue, release proinflammatory cytokines that participate in the
deterioration of insulin resistance (15,66).
Remarkably, it has been demonstrated that an
increased concentration of placental exosomes at 11-14th
gestational week could predict GDM (20). Moreover, GDM was correlated with an
increased concentration of total and placental exosomes in the
maternal blood compared to uncomplicated pregnancies of
corresponding gestational ages, denoting their prospective use in
identifying women at risk for GDM before disease onset (9). However, the contribution of placental
exosomes to total exosomes was reduced compared to normal
pregnancies, suggesting either a dysregulated release from the
placenta or a predominant release of exosomes of a different origin
(2). It has been reported that
placental exosomes could transfer bioactive mediators between
maternal tissues and the placenta that influence immune and
vascular systems, as well as the pancreas and the adipose tissue,
suggesting a potential role in GDM pathogenesis (20). Upon exposure to placental exosomes
derived from adolescent and adult patients with GDM, skeletal
muscle cells of non-diabetic individuals displayed attenuated
insulin-induced migration and glucose uptake; conversely, exosomes
from healthy individuals induced insulin-stimulated glucose uptake
in skeletal muscle cells from GDM patients (34). Hyperglycemia has been demonstrated
to influence first-trimester trophoblast-derived exosomes, with
regards to their concentration and bioactivity, and intensify
proinflammatory cytokine secretion from endothelial cells,
predisposing to disruption of maternal glucose metabolism and GDM
(66). Nevertheless, exosomes were
also found to induce the secretion of the anti-inflammatory IL-4
from endothelial cells, indicating that exosomal modulation of the
inflammatory state could be multidimensional (2). Moreover, the excessive placental
glycogenolysis in GDM, that triggers glucose transport to the
fetus, finally leading to fetal overgrowth, seems to be affected by
adipose tissue-derived exosomes (67). Another indication of the
implication of placental exosomes in the pathophysiology of GDM
arises from their highly elevated content of biologically active
dipeptidyl peptidase-4 (DPP-4) in advanced GDM pregnancies (DPP-4
inhibits pancreatic insulin secretion by cleaving glucagon-like
polypeptide-1, which enhances insulin secretion), and that the
DPP-4-specific inhibitor vildagliptin can inhibit DPP-4 activity in
placental EVs, paving the way for novel therapeutic approaches
(68).
Exosomes in GDM have been shown to have a distinct
composition profile regarding their protein and ncRNA content,
driving scientific interest to their potential applications in the
prediction and treatment of GDM. The differentially expressed
proteins are suggested to participate in energy metabolism, insulin
sensitivity and inflammation; urinary exosomes in GDM overexpressed
damage associated molecular pattern (DAMP) protein S100A9, that may
serve as an indicator of inflammation and immune activation
(69). Existing evidence supports
that exosomes in GDM contain higher levels of miRNAs belonging to
the C19MC cluster (miR-518a-5p, miR-518b, miR-518c, miR-518e,
miR-520c-3p, and miR-525-5p) (1).
Furthermore, exosomal miR-125a-3p, miR-99b-5p, miR-197-3p,
miR-22-3p, and miR-224-5p were increased in the placenta, in
skeletal muscle and in circulation of adolescents and adults with
GDM, thus contributing to the notion that the placental metabolic
function could be reflected in the distinct miRNA content of
placental exosomes (34). Pathway
analysis has demonstrated that the altered miRNAs are involved in
fatty acid metabolism, inflammatory immune reactions, insulin
release and glucose transport, as well as in placentation,
suggesting a role of these miRNAs in peripheral insulin resistance
and the development of GDM (16).
Lately, the potential therapeutic application of exosomes to target
GDM has been proposed (20).
Several studies evaluating free circulating miRNA
profiles have identified specific miRNAs altered in GDM and have
suggested potential pathogenetic mechanisms for their involvement
in insulin resistance and β-cell dysfunction (15), as well as miRNAs that could serve
as predictive biomarkers for GDM development, such as miR-16-5p,
miR-17-5p and miR-20a-5p (70).
Studies evaluating and identifying the involvement of lncRNAs in
the pathogenesis and diagnosis of GDM are scarce at the moment. For
example, lncRNA MALAT1, that has been suggested to participate in
the pathogenesis of diabetic microangiopathy, has been found
elevated in the serum analyzed between the 36th and the 40th week
of pregnancies complicated with GDM compared to normal pregnancies
(71). For a more comprehensive
description of the potential roles of ncRNAs in GDM, see Filardi
et al, 2020(15).
Exosomes in pre-term birth
Every parturition before the completion of the 37th
pregnancy week is defined as a preterm birth. In accordance with
the estimations of the World Health Organization, 15 million babies
are delivered prematurely every year (72). Adverse outcomes of prematurity
account for the most deaths among children younger than five years
old. The etiology of preterm birth is multifactorial; multiple
pregnancies and infections, as well as PE and GDM are among the
causes leading to premature delivery (72). The sequelae of preterm labor, such
as retinopathy of prematurity, respiratory distress, cerebral
palsy, can be devastating for the infant, all contributing to
morbidity and, in the worst-case scenario, mortality; thus, timely
recognition of pregnancies at risk for prematurity is of an
imperative priority (16).
It has been reported that maternal plasma exosome
function could mirror the progression of pregnancy and predict
preterm labor (52). The isolation
of exosomes from maternal serum at the 30th pregnancy week showed a
greatly reduced number of exosomes in gestations that would be
preterm (9). The secretion of IL-2
by activated T-cells was attenuated upon interaction with exosomes
derived from the blood of women with preterm labor, as compared to
interaction with exosomes derived from the blood of women with
normal labor (4). Upon pathway
analysis of exosomal protein expression at normal and premature
deliveries, it has been indicated that inflammatory and endocrine
signaling alterations may destabilize pregnancy homeostasis, while
variations in the protein content of placenta-derived EVs have been
postulated to reveal a predisposal for premature delivery (73). Determination of miRNAs ferried by
exosomes suggests that they display remarkable variations in women
delivering prematurely and that could be possibly used to signal
pre-term birth (16).
Most studies have revealed the potential application
of circulating miRNAs in the plasma of pregnant women as biomarkers
of prematurity; nevertheless, the exosomal transport of these
miRNAs has not been verified (1).
Nine miRNAs pertaining to the C19MC cluster, namely miR-515-5p,
miR-516-5p, miR-518b, miR-518f-5p, miR-519a, miR-519e-5p,
miR-520a-5p, miR-520h, and miR-526b-5p, were increased in pre-term
gestations (74), while Gray et
al reported an increase in miR-223, and a decrease in miR-302b,
miR-548, and miR-1253, in the plasma of women delivering preterm
compared to the ones delivering at term (75). Moreover, placental mRNA and miRNA
expression profiles have been evaluated in association to
pre-pregnancy BMI of adolescent and adult women delivering
extremely prematurely (76). A low
pre-pregnancy BMI in male placentas has been correlated with a
distinct mRNA profile, targeted by miR-4057 and miR-128-1-5p and
involving nutrient metabolism and angiogenesis pathways (76). Another study by Payton et
al, identified a set of placental mRNAs and miRNAs associated
with the birth weight of extremely preterm infants born to
adolescent and adult mothers (77).
Exosomes in fetal abnormalities
Except for maternal pathologic situations, exosomes
have been associated with several fetal disorders. Importantly, the
placental barrier is permeable for maternal exosomes that can
subsequently target fetal tissues. Among important fetal disorders,
intrauterine growth restriction (IUGR) is defined as an incapacity
of a fetus to achieve its growth potential and is accompanied by
considerable metabolic consequences (78). It has been demonstrated that the
number of total and placental exosomes in the maternal plasma of
IUGR pregnancies does not differ significantly with normal
pregnancies (36). Nonetheless,
the contribution of placental to total exosomes was markedly
decreased in pregnancies with IUGR, implying that placental
exosomes could reveal fetal developmental status, while particular
placenta-characteristic non-exosomal miRNAs were downregulated in
IUGR placentas, but not in maternal plasma (79). A study evaluating circulating miRNA
profiles in adolescent and adult pregnant women delivering
selective IUGR monochorionic twins implicates miR-199a-5p in the
pathogenesis of selective IUGR, by interfering with oxidative
stress and placental angiogenesis pathways (80). Furthermore, Baker et al
linked inadequate fetal growth in pregnant, folate-deficient
adolescents, to particular miRNA alterations, namely to the
upregulation of miR-222-3p, miR-141-3p, and miR-34b-5p (81).
Circulating non-exosomal miRNA profiles have been
evaluated for various fetal abnormalities rendering them candidate
non-invasive screening biomarkers for these disorders and potential
ensuing research target of exosome content. More specifically, a
prenatal miRNA profile of women pregnant with fetuses with Down
syndrome has been delineated and includes miR-15a, let-7d, miR-23a,
miR-99a, miR-142, miR-191, miR-199 and miR-3156, which are
upregulated in women pregnant with Down syndrome fetuses (82). Accordingly, a number of
alternatively expressed circulating miRNAs have been identified in
the blood of women pregnant with a fetus with congenital heart
disease (CHD) and these miRNAs have been postulated to regulate
fetal cardiac development (16).
Intriguingly, exosomes derived from diabetic mice, upon injection
to normal pregnant mice, could induce CHD to their fetuses
(83). Furthermore, another study
identified six circulating miRNAs that displayed important
concentration variations in the serum of women carrying fetuses
with neural tube defects compared to normal pregnancies (16). Apparently, these results could have
great implications, as the capability of identifying and monitoring
an increased risk of fetal abnormalities crucial for the
development and survival of the offspring, could pave the way for
non-invasive prenatal diagnosis.
5. Clinical applications
As already described, exosomes and bioactive
exosomal cargo could critically transform current methods of
monitoring pregnancy progression and diagnosing early pregnancy
disorders by representing sensitive, non-invasive biomarkers, or
even future therapeutic targets. The possibility of identifying
promptly increased risk of adverse pregnancy situations, and thus
being capable of preventing them or monitoring them, represents an
alluring perspective. Exosomes and their cargo, especially exosomal
miRNAs, as well as circulating, non-exosomal ncRNAs, have been
extensively investigated for alterations that could lead to the
development of sensitive biomarkers for various pregnancy
disorders. Specific exosomal proteins, including tissue inhibitor
of metalloproteinases 1 (TIMP1), plasminogen activator inhibitor
type 1 (PAI1), and placental growth factor (PIGF), have been
demonstrated to reliably predict PE (84). Nevertheless, placental circulating
RNA biomarkers outweigh protein biomarkers because their
alterations can be identified earlier, while they have a higher
correlation with placental status (62).
It has been demonstrated that therapeutic use of
exosomes as drug carriers could prove to be advantageous, because
of the low immunogenicity of autologous exosomes, their high
stability in circulation, their capability of effective bioactive
cargo transfer in target cells and their limited side effects,
explained by their highly specific action on target cells (20). Remarkably, placenta-derived
exosomes could also be of therapeutic use; exosomes originating
from the mesenchymal stromal cells of the placenta are beneficial
for individuals with Duchenne muscular dystrophy, as they promote
the differentiation and fusion of myocytes and they upregulate
myoblast fibrogenic genes (85).
Moreover, it is expected that analyzing exosomal miRNAs in
GDM-complicated pregnancies will improve our understanding of the
pathogenesis of GDM and pave the way to novel therapeutic
strategies for combatting GDM (20). Possibly, MSC-derived exosomes could
be promising tools in regenerative medicine due to their
immunoregulatory properties, with potential applications in
GDM-associated myopathy and other GDM-associated maternal and fetal
complications. It has been suggested that exosomes derived from
adipose tissue stem cells could represent a novel therapeutic
approach to stress urinary incontinence, observed often at women
after parturition (86).
Furthermore, it has been speculated that trophoblast-derived and
placental MSC-derived exosomes could be employed in the treatment
of PE, considering their angiogenic properties (4). Strikingly, genetic engineering of
MSCs-derived exosomes to inhibit the expression of genes
etiologically correlated with PE and modifying exosomal proteins to
achieve a more targeted delivery of exosomal cargo, holds great
promise for the precise treatment of PE (87).
6. Conclusions and future perspectives
Accumulated evidence supports the view that
exosome-mediated communication plays a key role in an array of
physiological processes required for the regular initiation and
progression of pregnancy. These include maternal metabolic
adaptations to gestation, immune tolerance, and inflammatory
processes, as well as resistance to infections, in all of which
exosomes released from the placenta have been reported to have a
prominent role. Recent studies have started to unveil the exact
pathways of exosome and exosomal cargo contribution to the
interface of maternal and fetal environment across normal
gestation, as well as their alterations in situations of threatened
pregnancy homeostasis in adolescents and adults. Variations in the
concentration, the content, and the biological effects of exosomes
have been implicated in the onset of pregnancy disorders, including
preeclampsia, GDM, preterm birth and fetal anomalies. However,
further studies are needed to improve our understanding of the
exact mechanisms of exosomal participation in the pathogenesis of
these diseases. Moreover, more studies investigating miRNAs and
lncRNAs transferred by exosomes, mainly of placental origin, could
offer a more thorough insight of the roles of exosomes and their
unique cargoes in healthy and pathological states and be of
clinical utility. Furthermore, although the current knowledge on
exosomes and their bioactive cargo is based on studies of pregnant
women with age range that includes adolescent and young adults,
there seems to be a need of studies that specifically focus on
exosomes in adolescent pregnancy that might reveal possibly
unexpected findings.
The distinctive exosomal properties open the way for
the use of exosomes as biomarkers of pregnancy disorders at the
presymptomatic stage, which is a field full of promise. However,
the greatest obstacle encountered in the clinical use of exosomes
is the lack of standardization of pure exosome isolation techniques
and the use of low-efficiency techniques, such as
ultracentrifugation. Other exosome isolation techniques include
polymer precipitation, size-based isolation techniques (mainly
ultrafiltration and size-exclusion chromatography) and
immunoaffinity chromatography (88). Factors as sample type (e.g.,
plasma, urine), the trimester of gestation, the status of the
patient at the time of sample collection, influenced by circadian
rhythms, food intake, etc., should also be considered because they
could influence the accuracy of findings (26). Regarding exosomal miRNA studies,
there is low reproducibility of miRNA profiles, probably because of
the above-mentioned factors, as well as the possible use of
different methods of analysis (miRNA-array or RNA-sequencing).
Therapeutic exosome implementations have been also proposed, given
the capacity of exosome signaling and cargo transfer between
distant tissues, and could consist of either targeting specific
exosomal cargo or loading desired drugs to exosomes for targeted
cargo delivery (20). The use of
exosomes as candidate non-invasive biomarkers and as therapeutic
biomolecules could fundamentally upgrade the current approach of
pregnancy complications diagnosis and management.
Acknowledgements
Not applicable.
Funding
Funding: This work has been co-financed by the European Regional
Development Fund of the European Union and Greek National Funds
through the Operational Program Competitiveness, Entrepreneurship
and Innovation, under the call RESEARCH-CREATE-INNOVATE (grant no.
T2EDK-Milksafe).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
IM performed the literature search, wrote the first
draft of the manuscript and designed the figures and tables. CY
conceived the study, and was responsible for project coordination
and critical revision of the manuscript. KN performed the
literature search and review of the first draft. FB performed
critical revision of the manuscript. GPC performed critical
revision of the manuscript and the final draft. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Czernek L and Düchler M: Exosomes as
messengers between mother and fetus in pregnancy. Int J Mol Sci.
21(4264)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Salomon C and Rice GE: Role of exosomes in
placental homeostasis and pregnancy disorders. Prog Mol Biol Transl
Sci. 145:163–179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tarrade A, Lai Kuen R, Malassiné A,
Tricottet V, Blain P, Vidaud M and Evain-Brion D: Characterization
of human villous and extravillous trophoblasts isolated from first
trimester placenta. Lab Invest. 81:1199–1211. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Burkova EE, Sedykh SE and Nevinsky GA:
Human placenta exosomes: Biogenesis, isolation, composition, and
prospects for use in diagnostics. Int J Mol Sci.
22(2158)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giacomini E, Vago R, Sanchez AM, Podini P,
Zarovni N, Murdica V, Rizzo R, Bortolotti D, Candiani M and Viganò
P: Secretome of in vitro cultured human embryos contains
extracellular vesicles that are uptaken by the maternal side. Sci
Rep. 7(5210)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salomon C, Torres MJ, Kobayashi M,
Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell
MD and Rice GE: A gestational profile of placental exosomes in
maternal plasma and their effects on endothelial cell migration.
PLoS One. 9(e98667)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maligianni I, Yapijakis C, Bacopoulou F
and Chrousos G: The potential role of exosomes in child and
adolescent obesity. Children (Basel). 8(196)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jin J and Menon R: Placental exosomes: A
proxy to understand pregnancy complications. Am J Reprod Immunol.
79(e12788)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mitchell MD, Peiris HN, Kobayashi M, Koh
YQ, Duncombe G, Illanes SE, Rice GE and Salomon C: Placental
exosomes in normal and complicated pregnancy. Am J Obstet Gynecol.
213 (Suppl 4):S173–S181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sabapatha A, Gercel-Taylor C and Taylor
DD: Specific isolation of placenta-derived exosomes from the
circulation of pregnant women and their immunoregulatory
consequences. Am J Reprod Immunol. 56:345–355. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang Y, Liu Y, Liu H and Tang WH:
Exosomes: Biogenesis, biologic function and clinical potential.
Cell Biosci. 9(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vlachakis D, Mitsis Τ, Nicolaides N,
Efthimiadou A, Giannakakis A, Bacopoulou F and Chrousos GP:
Functions, pathophysiology and current insights of exosomal
endocrinology (Review). Mol Med Rep. 23(26)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mincheva-Nilsson L and Baranov V: The role
of placental exosomes in reproduction. Am J Reprod Immunol.
63:520–533. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vargas A, Zhou S, Éthier-Chiasson M, Flipo
D, Lafond J, Gilbert C and Barbeau B: Syncytin proteins
incorporated in placenta exosomes are important for cell uptake and
show variation in abundance in serum exosomes from patients with
preeclampsia. FASEB J. 28:3703–3719. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Filardi T, Catanzaro G, Mardente S, Zicari
A, Santangelo C, Lenzi A, Morano S and Ferretti E: Non-coding RNA:
Role in gestational diabetes pathophysiology and complications. Int
J Mol Sci. 21(4020)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang H, Ma Q, Wang Y and Tang Z: Clinical
application of exosomes and circulating microRNAs in the diagnosis
of pregnancy complications and foetal abnormalities. J Transl Med.
18(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yapijakis C: Regulatory role of MicroRNAs
in brain development and function. Adv Exp Med Biol. 1195:237–247.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK,
Pritchard CC, Gibson DF, Mitchell PS, Bennett CF,
Pogosova-Agadjanyan EL, Stirewalt DL, et al: Argonaute2 complexes
carry a population of circulating microRNAs independent of vesicles
in human plasma. Proc Natl Acad Sci USA. 108:5003–5008.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Floriano JF, Willis G, Catapano F, Lima
PR, Reis FVDS, Barbosa AMP, Rudge MVC and Emanueli C: Exosomes
could offer new options to combat the long-term complications
inflicted by gestational diabetes mellitus. Cells.
9(675)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tabet F, Vickers KC, Cuesta Torres LF,
Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L,
Levin MG, Thacker S, et al: HDL-transferred microRNA-223 regulates
ICAM-1 expression in endothelial cells. Nat Commun.
5(3292)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3(ra8)2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dragomir M, Chen B and Calin GA: Exosomal
lncRNAs as new players in cell-to-cell communication. Transl Cancer
Res. 7 (Suppl 2):S243–S252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mitsis T, Pierouli K, Diakou KL,
Papakonstantinou E, Bacopoulou F, Chrousos GP and Vlachakis D:
Exosomics. EMBnet J. 26(e934)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Karin-Kujundzic V, Sola IM, Predavec N,
Potkonjak A, Somen E, Mioc P, Serman A, Vranic S and Serman L:
Novel epigenetic biomarkers in pregnancy-related disorders and
cancers. Cells. 8(1459)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arenaccio C and Federico M: The
multifaceted functions of exosomes in health and disease: An
overview. Adv Exp Med Biol. 998:3–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Properzi F, Logozzi M and Fais S:
Exosomes: The future of biomarkers in medicine. Biomark Med.
7:769–778. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tannetta D, Masliukaite I, Vatish M,
Redman C and Sargent I: Update of syncytiotrophoblast derived
extracellular vesicles in normal pregnancy and preeclampsia. J
Reprod Immunol. 119:98–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Salomon C, Kobayashi M, Ashman K, Sobrevia
L, Mitchell MD and Rice GE: Hypoxia-induced changes in the
bioactivity of cytotrophoblast-derived exosomes. PLoS One.
8(e79636)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang HC, Liu XB, Huang S, Bi XY, Wang HX,
Xie LX, Wang YQ, Cao XF, Lv J, Xiao FJ, et al: Microvesicles
derived from human umbilical cord mesenchymal stem cells stimulated
by hypoxia promote angiogenesis both in vitro and in vivo. Stem
Cells Dev. 21:3289–3297. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martinez-Fierro ML, Garza-Veloz I,
Gutierrez-Arteaga C, Delgado-Enciso I, Barbosa-Cisneros OY,
Flores-Morales V, Hernandez-Delgadillo GP, Rocha-Pizaña MR,
Rodriguez-Sanchez IP, Badillo-Almaraz JI, et al: Circulating levels
of specific members of chromosome 19 microRNA cluster are
associated with preeclampsia development. Arch Gynecol Obstet.
297:365–371. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Donker RB, Mouillet JF, Chu T, Hubel CA,
Stolz DB, Morelli AE and Sadovsky Y: The expression profile of
C19MC microRNAs in primary human trophoblast cells and exosomes.
Mol Hum Reprod. 18:417–424. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nair S, Jayabalan N, Guanzon D, Palma C,
Scholz-Romero K, Elfeky O, Zuñiga F, Ormazabal V, Diaz E, Rice GE,
et al: Human placental exosomes in gestational diabetes mellitus
carry a specific set of miRNAs associated with skeletal muscle
insulin sensitivity. Clin Sci (Lond). 132:2451–2467.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xie L, Mouillet JF, Chu T, Parks WT,
Sadovsky E, Knöfler M and Sadovsky Y: C19MC microRNAs regulate the
migration of human trophoblasts. Endocrinology. 155:4975–4985.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Miranda J, Paules C, Nair S, Lai A, Palma
C, Scholz-Romero K, Rice GE, Gratacos E, Crispi F and Salomon C:
Placental exosomes profile in maternal and fetal circulation in
intrauterine growth restriction-liquid biopsies to monitoring fetal
growth. Placenta. 64:34–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Adam S, Elfeky O, Kinhal V, Dutta S, Lai
A, Jayabalan N, Nuzhat Z, Palma C, Rice GE and Salomon C: Review:
Fetal-maternal communication via extracellular
vesicles-implications for complications of pregnancies. Placenta.
54:83–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Elfeky O, Longo S, Lai A, Rice GE and
Salomon C: Influence of maternal BMI on the exosomal profile during
gestation and their role on maternal systemic inflammation.
Placenta. 50:60–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chang G, Mouillet JF, Mishima T, Chu T,
Sadovsky E, Coyne CB, Parks WT, Surti U and Sadovsky Y: Expression
and trafficking of placental microRNAs at the feto-maternal
interface. FASEB J. 31:2760–2770. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Greening DW, Nguyen HP, Elgass K, Simpson
RJ and Salamonsen LA: Human endometrial exosomes contain
hormone-specific cargo modulating trophoblast adhesive capacity:
Insights into endometrial-embryo interactions. Biol Reprod.
94(38)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Vilella F, Moreno-Moya JM, Balaguer N,
Grasso A, Herrero M, Martínez S, Marcilla A and Simón C:
Hsa-miR-30d, secreted by the human endometrium, is taken up by the
pre-implantation embryo and might modify its transcriptome.
Development. 142:3210–3221. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mor G, Cardenas I, Abrahams V and Guller
S: Inflammation and pregnancy: The role of the immune system at the
implantation site. Ann N Y Acad Sci. 1221:80–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sheller-Miller S, Choi K, Choi C and Menon
R: Cyclic-recombinase-reporter mouse model to determine exosome
communication and function during pregnancy. Am J Obstet Gynecol.
221:502.e1–502.e12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sheller-Miller S, Trivedi J, Yellon SM and
Menon R: Exosomes cause preterm birth in mice: Evidence for
paracrine signaling in pregnancy. Sci Rep. 9(608)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sheller-Miller S, Lei J, Saade G, Salomon
C, Burd I and Menon R: Feto-maternal trafficking of exosomes in
murine pregnancy models. Front Pharmacol. 7(432)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kovács ÁF, Fekete N, Turiák L, Ács A,
Kőhidai L, Buzás EI and Pállinger É: Unravelling the role of
trophoblastic-derived extracellular vesicles in regulatory T cell
differentiation. Int J Mol Sci. 20(3457)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Stenqvist AC, Nagaeva O, Baranov V and
Mincheva-Nilsson L: Exosomes secreted by human placenta carry
functional Fas ligand and TRAIL molecules and convey apoptosis in
activated immune cells, suggesting exosome-mediated immune
privilege of the fetus. J Immunol. 191:5515–5523. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kambe S, Yoshitake H, Yuge K, Ishida Y,
Ali MM, Takizawa T, Kuwata T, Ohkuchi A, Matsubara S, Suzuki M, et
al: Human exosomal placenta-associated miR-517a-3p modulates the
expression of PRKG1 mRNA in Jurkat cells. Biol Reprod.
91(129)2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jia L, Zhou X, Huang X, Xu X, Jia Y, Wu Y,
Yao J, Wu Y and Wang K: Maternal and umbilical cord serum-derived
exosomes enhance endothelial cell proliferation and migration.
FASEB J. 32:4534–4543. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bayer A, Delorme-Axford E, Sleigher C,
Frey TK, Trobaugh DW, Klimstra WB, Emert-Sedlak LA, Smithgall TE,
Kinchington PR, Vadia S, et al: Human trophoblasts confer
resistance to viruses implicated in perinatal infection. Am J
Obstet Gynecol. 212:71.e1–71.e8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nakahara A, Elfeky O, Garvey C, Guanzon D,
Longo SA and Salmon C: Exosome profiles for normal and complicated
pregnancies-a longitudinal study. Obstet Gynecol. 133(162)2019.
|
|
52
|
Konečná B, Tóthová Ľ and Repiská G:
Exosomes-associated DNA-new marker in pregnancy complications? Int
J Mol Sci. 20(2890)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gintoni I, Adamopoulou M and Yapijakis C:
The angiotensin-converting enzyme insertion/deletion polymorphism
as a common risk factor for major pregnancy complications. In Vivo.
35:95–103. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fox R, Kitt J, Leeson P, Aye CY and
Lewandowski AJ: Preeclampsia: Risk factors, diagnosis, management,
and the cardiovascular impact on the offspring. J Clin Med.
8(1625)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pillay P, Vatish M, Duarte R, Moodley J
and Mackraj I: Exosomal microRNA profiling in early and late onset
preeclamptic pregnant women reflects pathophysiology. Int J
Nanomedicine. 14:5637–5657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Redman CW, Tannetta DS, Dragovic RA,
Gardiner C, Southcombe JH, Collett GP and Sargent IL: Review: Does
size matter? Placental debris and the pathophysiology of
pre-eclampsia. Placenta. 33 (Suppl 1):S48–S54. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Motta-Mejia C, Kandzija N, Zhang W, Mhlomi
V, Cerdeira AS, Burdujan A, Tannetta D, Dragovic R, Sargent IL,
Redman CW, et al: Placental vesicles carry active endothelial
nitric oxide synthase and their activity is reduced in
preeclampsia. Hypertension. 70:372–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Salomon C, Guanzon D, Scholz-Romero K,
Longo S, Correa P, Illanes SE and Rice GE: Placental exosomes as
early biomarker of preeclampsia: Potential role of exosomal
MicroRNAs across gestation. J Clin Endocrinol Metab. 102:3182–3194.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kim J, Lee KS, Kim JH, Lee DK, Park M,
Choi S, Park W, Kim S, Choi YK, Hwang JY, et al: Aspirin prevents
TNF-α-induced endothelial cell dysfunction by regulating the
NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis
in preeclampsia. Free Radic Biol Med. 104:185–198. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Srinivasan S, Treacy R, Herrero T, Olsen
R, Leonardo TR, Zhang X, DeHoff P, To C, Poling LG, Fernando A, et
al: Discovery and verification of extracellular miRNA biomarkers
for non-invasive prediction of pre-eclampsia in asymptomatic women.
Cell Rep Med. 1(100013)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Motawi TMK, Sabry D, Maurice NW and Rizk
SM: Role of mesenchymal stem cells exosomes derived microRNAs;
miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and
evaluation. Arch Biochem Biophys. 659:13–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lázár L, Nagy B, Molvarec A, Szarka A and
Rigó J Jr: Role of hsa-miR-325 in the etiopathology of
preeclampsia. Mol Med Rep. 6:597–600. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang X, Chen Y, Du L, Li X, Li X and Chen
D: Evaluation of circulating placenta-related long noncoding RNAs
as potential biomarkers for preeclampsia. Exp Ther Med.
15:4309–4317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen Y, Ding H, Wei M, Zha W, Guan S, Liu
N, Li Y, Tan Y, Wang Y and Wu F: MSC-secreted exosomal H19 promotes
trophoblast cell invasion and migration by downregulating let-7b
and upregulating FOXO1. Mol Ther Nucleic Acids. 19:1237–1249.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2019. Diabetes Care. 42 (Suppl 1):S13–S28.
2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jayabalan N, Lai A, Ormazabal V, Adam S,
Guanzon D, Palma C, Scholz-Romero K, Lim R, Jansson T, McIntyre HD,
et al: Adipose tissue exosomal proteomic profile reveals a role on
placenta glucose metabolism in gestational diabetes mellitus. J
Clin Endocrinol Metab. 104:1735–1752. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Rice GE, Scholz-Romero K, Sweeney E,
Peiris H, Kobayashi M, Duncombe G, Mitchell MD and Salomon C: The
effect of glucose on the release and bioactivity of exosomes from
first trimester trophoblast cells. J Clin Endocrinol Metab.
100:E1280–E1288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kandzija N, Zhang W, Motta-Mejia C, Mhlomi
V, McGowan-Downey J, James T, Cerdeira AS, Tannetta D, Sargent I,
Redman CW, et al: Placental extracellular vesicles express active
dipeptidyl peptidase IV; levels are increased in gestational
diabetes mellitus. J Extracell Vesicles. 8(1617000)2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ramachandrarao SP, Hamlin AA, Awdishu L,
Overcash R, Zhou M, Proudfoot J, Ishaya M, Aghania E, Madrigal A,
Kokoy-Mondragon C, et al: Proteomic analyses of urine exosomes
reveal new biomarkers of diabetes in pregnancy. Madridge J
Diabetes. 1:11–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Cao YL, Jia YJ, Xing BH, Shi DD and Dong
XJ: Plasma microRNA-16-5p, -17-5p and -20a-5p: Novel diagnostic
biomarkers for gestational diabetes mellitus. J Obstet Gynaecol
Res. 43:974–981. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang Y, Wu H, Wang F, Ye M, Zhu H and Bu
S: Long non-coding RNA MALAT1 expression in patients with
gestational diabetes mellitus. Int J Gynaecol Obstet. 140:164–169.
2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
World Health Organization. Preterm birth.
Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
Accessed June 30, 2021.
|
|
73
|
Menon R, Debnath C, Lai A, Guanzon D,
Bhatnagar S, Kshetrapal P, Sheller-Miller S and Salomon C: Protein
profile changes in circulating placental extracellular vesicles in
term and preterm births: A longitudinal study. Endocrinology.
161(bqaa009)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hromadnikova I, Kotlabova K, Ivankova K
and Krofta L: Expression profile of C19MC microRNAs in placental
tissue of patients with preterm prelabor rupture of membranes and
spontaneous preterm birth. Mol Med Rep. 16:3849–3862.
2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gray C, McCowan LM, Patel R, Taylor RS and
Vickers MH: Maternal plasma miRNAs as biomarkers during
mid-pregnancy to predict later spontaneous preterm birth: A pilot
study. Sci Rep. 7(815)2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Clark J, Eaves LA, Gaona AR, Santos HP Jr,
Smeester L, Bangma JT, Rager JE, O'Shea TM and Fry RC:
Pre-pregnancy BMI-associated miRNA and mRNA expression signatures
in the placenta highlight a sexually-dimorphic response to maternal
underweight status. Sci Rep. 11(15743)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Payton A, Clark J, Eaves L, Santos HP Jr,
Smeester L, Bangma JT, O'Shea TM, Fry RC and Rager JE: Placental
genomic and epigenomic signatures associated with infant birth
weight highlight mechanisms involved in collagen and growth factor
signaling. Reprod Toxicol. 96:221–230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Valsamakis G, Chrousos G and Mastorakos G:
Stress, female reproduction and pregnancy.
Psychoneuroendocrinology. 100:48–57. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Higashijima A, Miura K, Mishima H,
Kinoshita A, Jo O, Abe S, Hasegawa Y, Miura S, Yamasaki K, Yoshida
A, et al: Characterization of placenta-specific microRNAs in fetal
growth restriction pregnancy. Prenat Diagn. 33:214–222.
2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Meng M, Cheng YK, Wu L, Chaemsaithong P,
Leung MB, Chim SS, Sahota DS, Li W, Poon LCY, Wang CC and Leung TY:
Whole genome miRNA profiling revealed miR-199a as potential
placental pathogenesis of selective fetal growth restriction in
monochorionic twin pregnancies. Placenta. 92:44–53. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Baker BC, Mackie FL, Lean SC, Greenwood
SL, Heazell A, Forbes K and Jones RL: Placental dysfunction is
associated with altered microRNA expression in pregnant women with
low folate status. Mol Nutr Food Res. 61(1600646)2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zbucka-Kretowska M, Niemira M,
Paczkowska-Abdulsalam M, Bielska A, Szalkowska A, Parfieniuk E,
Ciborowski M, Wolczynski S and Kretowski A: Prenatal circulating
microRNA signatures of foetal Down syndrome. Sci Rep.
9(2394)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Shi R, Zhao L, Cai W, Wei M, Zhou X, Yang
G and Yuan L: Maternal exosomes in diabetes contribute to the
cardiac development deficiency. Biochem Biophys Res Commun.
483:602–608. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Tan KH, Tan SS, Ng MJ, Tey WS, Sim WK,
Allen JC and Lim SK: Extracellular vesicles yield predictive
pre-eclampsia biomarkers. J Extracell Vesicles.
6(1408390)2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Bier A, Berenstein P, Kronfeld N,
Morgoulis D, Ziv-Av A, Goldstein H, Kazimirsky G, Cazacu S, Meir R,
Popovtzer R, et al: Placenta-derived mesenchymal stromal cells and
their exosomes exert therapeutic effects in Duchenne muscular
dystrophy. Biomaterials. 174:67–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ni J, Li H, Zhou Y, Gu B, Xu Y, Fu Q, Peng
X, Cao N, Fu Q, Jin M, et al: Therapeutic potential of human
adipose-derived stem cell exosomes in stress urinary
incontinence-an in vitro and in vivo study. Cell Physiol Biochem.
48:1710–1722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Pillay P, Moodley K, Moodley J and Mackraj
I: Placenta-derived exosomes: Potential biomarkers of preeclampsia.
Int J Nanomedicine. 12:8009–8023. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and
Li P: Exosome: A review of its classification, isolation
techniques, storage, diagnostic and targeted therapy applications.
Int J Nanomedicine. 15:6917–6934. 2020.PubMed/NCBI View Article : Google Scholar
|















