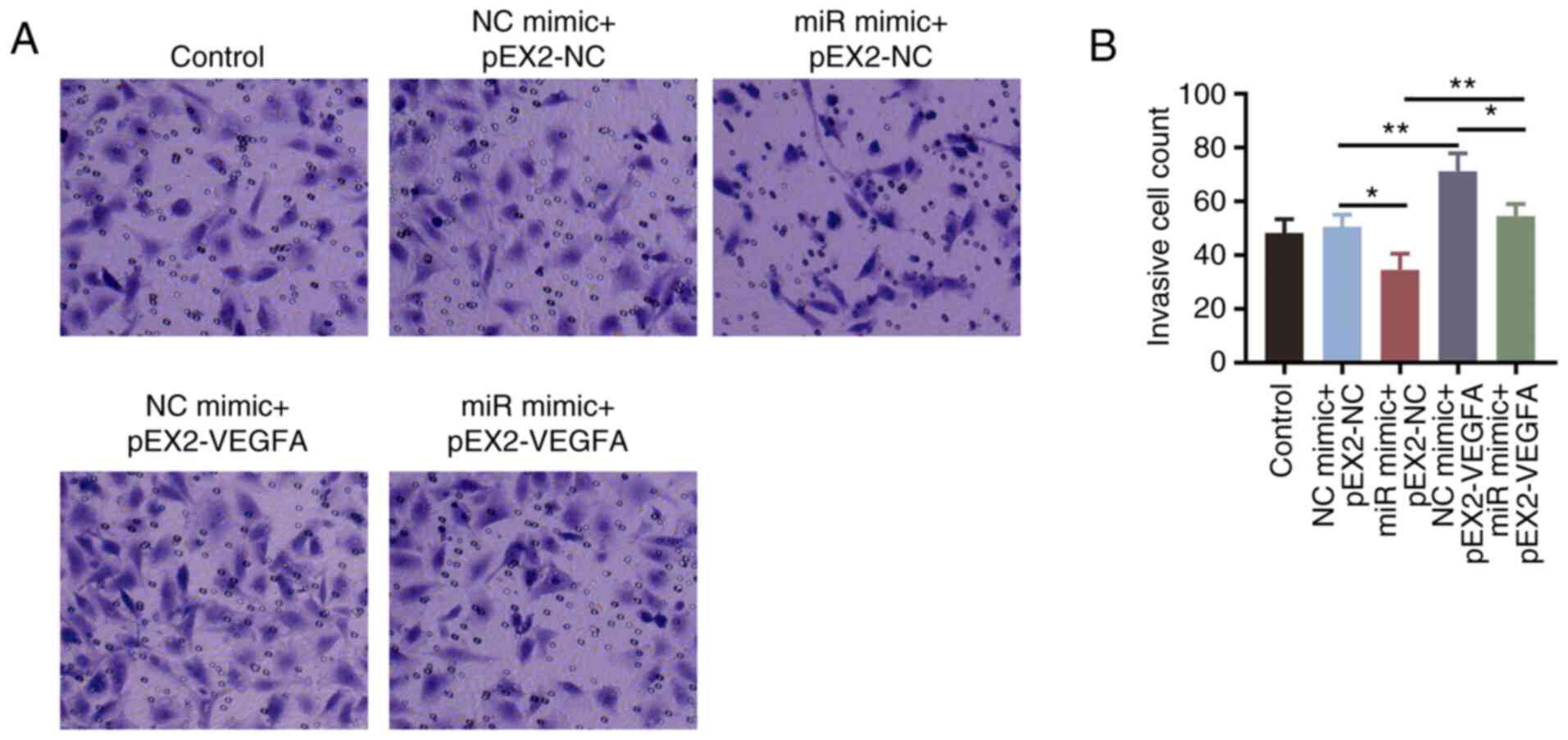
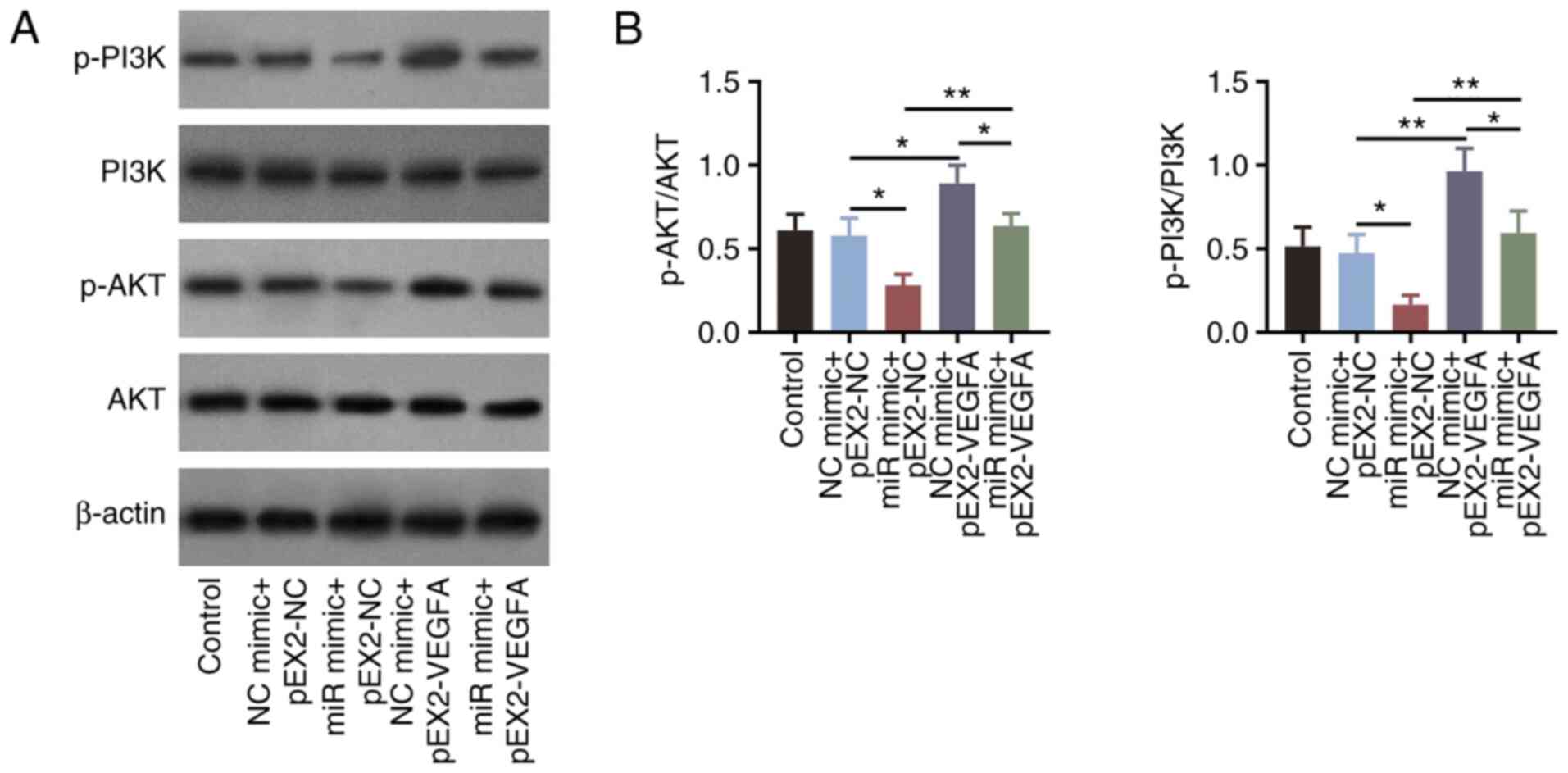
|
1
|
Jung HL: Update on infantile hemangioma.
Clin Exp Pediatr. 64:559–572. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sebaratnam DF, Rodriguez Bandera AL, Wong
LF and Wargon O: Infantile hemangioma. Part 2: Management. J Am
Acad Dermatol. 85:1395–1404. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Valdebran M and Wine Lee L:
Hemangioma-related syndromes. Curr Opin Pediatr. 32:498–505.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
DeHart A and Richter G: Hemangioma: Recent
advances. F1000Res. 8(F1000)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ji Y, Chen S, Wang Q, Xiang B, Xu Z, Zhong
L, Yang K, Lu G and Qiu L: Intolerable side effects during
propranolol therapy for infantile hemangioma: frequency, risk
factors and management. Sci Rep. 8(4264)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kowalska M, Debek W and Matuszczak E:
Infantile hemangiomas: An update on pathogenesis and treatment. J
Clin Med. 10(4631)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rolle K, Piwecka M, Belter A, Wawrzyniak
D, Jeleniewicz J, Barciszewska MZ and Barciszewski J: The sequence
and structure determine the function of mature human miRNAs. PLoS
One. 11(e0151246)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chi Y, Jin Q, Liu X, Xu L, He X, Shen Y,
Zhou Q, Zhang J and Jin M: miR-203 inhibits cell proliferation,
invasion, and migration of non-small-cell lung cancer by
downregulating RGS17. Cancer Sci. 108:2366–2372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin W, Zhu X, Yang S, Chen X, Wang L,
Huang Z, Ding Y, Huang L and Lv C: MicroRNA-203 inhibits
proliferation and invasion, and promotes apoptosis of osteosarcoma
cells by targeting Runt-related transcription factor 2. Biomed
Pharmacother. 91:1075–1084. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han N, Li H and Wang H: MicroRNA-203
inhibits epithelial-mesenchymal transition, migration, and invasion
of renal cell carcinoma cells via the inactivation of the PI3K/AKT
signaling pathway by inhibiting CAV1. Cell Adh Migr. 14:227–241.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma M, Zhang J, Gao X, Yao W, Li Q and Pan
Z: miR-361-5p Mediates SMAD4 to promote porcine granulosa cell
apoptosis through VEGFA. Biomolecules. 10(1281)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang T, Qian Y, Yuan C, Wu Y, Qian H, Lu
H, Hu C and Li W: Propranolol suppresses proliferation and
migration of HUVECs through regulation of the miR-206/VEGFA axis.
Biomed Res Int. 2021(7629176)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Geng A, Luo L, Ren F, Zhang L, Zhou H and
Gao X: miR-29a-3p inhibits endometrial cancer cell proliferation,
migration and invasion by targeting VEGFA/CD C42/PAK1. BMC Cancer.
21(843)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Makkeyah SM, Elseedawy ME, Abdel-Kader HM,
Mokhtar GM and Ragab IA: Vascular endothelial growth factor
response with propranolol therapy in patients with infantile
hemangioma. Pediatr Hematol Oncol. 39:215–224. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Oszajca K, Szemraj J, Wyrzykowski D,
Chrzanowska B, Salamon A and Przewratil P: Single-nucleotide
polymorphisms of VEGF-A and VEGFR-2 genes and risk of infantile
hemangioma. Int J Dermatol. 57:1201–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sanaei MJ, Razi S, Pourbagheri-Sigaroodi A
and Bashash D: The PI3K/Akt/mTOR pathway in lung cancer; oncogenic
alterations, therapeutic opportunities, challenges, and a glance at
the application of nanoparticles. Transl Oncol.
18(101364)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Barzegar Behrooz A, Talaie Z, Jusheghani
F, Los MJ, Klonisch T and Ghavami S: Wnt and PI3K/Akt/mTOR survival
pathways as therapeutic targets in glioblastoma. Int J Mol Sci.
23(1353)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ji Y, Chen S, Li K, Li L, Xu C and Xiang
B: Signaling pathways in the development of infantile hemangioma. J
Hematol Oncol. 7(13)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu Z, Liu X, Guo J, Zhuo L, Chen Y and
Yuan H: Knockdown of lncRNA MEG8 inhibits cell proliferation and
invasion, but promotes cell apoptosis in hemangioma, via
miR-203-induced mediation of the Notch signaling pathway. Mol Med
Rep. 24(872)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu X, Bai S, Li L, Tian P, Wang S, Zhang
N, Shen B, Du J and Liu S: MiR-200c-3p increased HDMEC
proliferation through the notch signaling pathway. Exp Biol Med
(Maywood). 246:897–905. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jin W, Chen L, Gao F, Yang M, Liu Y and
Wang B: Down-regulation of miR-556-3p inhibits hemangioma cell
proliferation and promotes apoptosis by targeting VEGFC. Cell Mol
Biol (Noisy-le-grand). 66:204–207. 2020.PubMed/NCBI
|
|
23
|
Wu Y, Li H, Xie J, Wang F, Cao D and Lou
Y: miR1395p affects cell proliferation, migration and adipogenesis
by targeting insulinlike growth factor 1 receptor in hemangioma
stem cells. Int J Mol Med. 45:569–577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuan X, Xu Y, Wei Z and Ding Q: CircAP2A2
acts as a ceRNA to participate in infantile hemangiomas progression
by sponging miR-382-5p via regulating the expression of VEGFA. J
Clin Lab Anal. 34(e23258)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang T, Zhang FL, Zhao Y, Guo DD and Yang
R: Effects of miR-125b-5p on the proliferation and apoptosis of
human hemangioma endothelial cells HemES and its mechanism.
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 37:247–253. 2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
26
|
Zong M, Feng W, Wan L, Yu X and Yu W:
miR-203 affects esophageal cancer cell proliferation, apoptosis and
invasion by targeting MAP3K1. Oncol Lett. 20:751–757.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen H, Kong M, Chen Y, Jiang Y, Wen M and
Zhang X: Prognostic significance of miR-203 and ZEB1 expression in
early-stage hepatocellular carcinoma. J Cancer. 12:4810–4818.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin J, Wang L, Gao J and Zhu S: MiR-203
inhibits estrogen-induced viability, migration and invasion of
estrogen receptor alpha-positive breast cancer cells. Exp Ther Med.
14:2702–2708. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang Z, Huang L, Liu L, Wang L, Lin W,
Zhu X, Su W and Lv C: Knockdown of microRNA-203 reduces cisplatin
chemo-sensitivity to osteosarcoma cell lines MG63 and U2OS in vitro
by targeting RUNX2. J Chemother. 33:328–341. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Iyer S and Acharya KR: Tying the knot: The
cystine signature and molecular-recognition processes of the
vascular endothelial growth factor family of angiogenic cytokines.
FEBS J. 278:4304–4322. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ye X, Gaucher JF, Vidal M and Broussy S: A
structural overview of vascular endothelial growth factors
pharmacological ligands: From macromolecules to designed
peptidomimetics. Molecules. 26(6759)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang S, Ren L, Shen G, Liu M and Luo J:
The knockdown of MALAT1 inhibits the proliferation, invasion and
migration of hemangioma endothelial cells by regulating
MiR-206/VEGFA axis. Mol Cell Probes. 51(101540)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cai C, Böttcher MC, Werner JA and Mandic
R: Differential expression of VEGF121, VEGF165 and VEGF189 in
angiomas and squamous cell carcinoma cell lines of the head and
neck. Anticancer Res. 30:805–810. 2010.PubMed/NCBI
|
|
35
|
Xu L, Shen B, Chen T and Dong P: miR-203
is involved in the laryngeal carcinoma pathogenesis via targeting
VEGFA and Cox-2. Onco Targets Ther. 9:4629–4637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y,
Zhu J, Cui F, Zhao W and Shi H: miR-203 suppresses tumor growth and
angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol
Biochem. 32:64–73. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dimri M and Satyanarayana A: Molecular
signaling pathways and therapeutic targets in hepatocellular
carcinoma. Cancers (Basel). 12(491)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tang ZL, Zhang K, Lv SC, Xu GW, Zhang JF
and Jia HY: LncRNA MEG3 suppresses PI3K/AKT/mTOR signalling pathway
to enhance autophagy and inhibit inflammation in TNF-α-treated
keratinocytes and psoriatic mice. Cytokine.
148(155657)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shen C, Shyu DL, Xu M, Yang L, Webb A,
Duan W and Williams TM: Deregulation of AKT-mTOR signaling
contributes to chemoradiation resistance in lung squamous cell
carcinoma. Mol Cancer Res. 20:425–433. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Owusu IA, Passalacqua KD, Mirabelli C, Lu
J, Young VL, Hosmillo M, Quaye O, Goodfellow I, Ward VK and Wobus
CE: Akt plays differential roles during the life cycles of acute
and persistent murine norovirus strains in macrophages. J Virol.
96(e0192321)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Amin RM, Hiroshima K, Miyagi Y, Kokubo T,
Hoshi K, Fujisawa T and Nakatani Y: Role of the PI3K/Akt, mTOR, and
STK11/LKB1 pathways in the tumorigenesis of sclerosing hemangioma
of the lung. Pathol Int. 58:38–44. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ou JM, Qui MK, Dai YX, Dong Q, Shen J,
Dong P, Wang XF, Liu YB and Fei ZW: Combined blockade of AKT/mTOR
pathway inhibits growth of human hemangioma via downregulation of
proliferating cell nuclear antigen. Int J Immunopathol Pharmacol.
25:945–953. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang X, Li X, Lin Q and Xu Q:
Up-regulation of microRNA-203 inhibits myocardial fibrosis and
oxidative stress in mice with diabetic cardiomyopathy through the
inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene.
715(143995)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Czechowicz JA, Benjamin T, Bly RA, Ganti
SN, Balkin DM, Perkins JA, Frieden IJ and Rosbe KW: Airway
hemangiomas in PHACE syndrome: A multicenter experience.
Otolaryngol Head Neck Surg. 165:182–186. 2021.PubMed/NCBI View Article : Google Scholar
|