Introduction
Burn injuries are a severe form of trauma, and they
are a global public health problem that causes an estimated 265,000
deaths each year in China (1).
Burn injuries are not a single pathophysiological event; they are
destructive injuries that lead to structural and functional
deficits in various organ systems (2). Cardiac dysfunction induced by burn
injury, such as cardiogenic shock, which often appears early after
a burn injury, contributes to multiple organ failure, sepsis and
death (3,4). Despite the significant advances in
the treatment of patients with burn injuries, systemic and burn
wound-related complications are still common (5-7).
Therefore, it is important to further explore the pathogenesis of
burn complications and to find therapeutic targets and pathways for
burn injury treatment.
Exosomes are small extracellular vesicles that can
serve as carriers of proteins, DNA and RNA. Exosomes, as important
mediators of cell-to-cell communication, have attracted much
attention (8). Growing evidence
has revealed that substances carried by exosomes can be transported
into target cells (9,10). They have notable roles in multiple
biological processes and signaling pathways, including cell
proliferation, migration, invasion and apoptosis (11). A previous study has demonstrated
that microRNA-181c in exosomes derived from human umbilical cord
mesenchymal stem cells reduces burn-induced inflammation (12). A previous study also indicated that
S100 calcium-binding protein A9 (S100A9) is highly expressed in the
serum exosomes isolated from patients with burn injuries (13). S100A9 is a small calcium-binding
protein that is hypothesized to be an alarmin released by stressed
cells. It is also an endogenous danger signal that promotes and
exacerbates inflammatory responses (14). However, the detailed function of
S100A9 in burn injury progression remains unclear.
Inflammation, as a self-protective mechanism of the
host organ from pathogens, plays a notable role in infectious and
non-infectious burn injuries (15). Inflammatory responses are necessary
to initiate tissue repair and immune response modulation to improve
the recovery of patients with severe burn injuries (16). Pyroptosis is a type of programmed
cell death mediated by the gasdermin family. It is accompanied by
inflammatory and immune responses (17), and can also regulate cell death
depending on the enzymatic activity of inflammatory proteases
(18,19), which are members of the
cysteine-dependent aspartate-specific protease (caspase) family.
Gasdermin D (GSDMD) is an important pyroptosis substrate.
Caspase-1, which belongs to the caspase family, has been reported
to be widely expressed in humans and mice, and it is closely
associated with cell pyroptosis (20-22).
NLRs are proteins that elicit an inflammatory
response through extracellular and intracellular changes. Among
them, NLR family pyrin domain containing 3 (NLRP3) is the most
characteristic inflammasome. As a redox-sensitive cytosolic sensor,
NLRP3 recruits and triggers the formation of adaptor protein
apoptosis-associated speck-like proteins; it also activates
pro-caspase-1, which processes pre-IL-18 and pre-IL-1β into
maturity (23,24). Furthermore, the biochemical
function of NLRP3 is to activate caspase-1, which leads to the
maturation of IL-1β and IL-18, thereby inducing cell pyroptosis
(25). However, the roles of serum
exosomes in cell pyroptosis and its associated underlying
mechanisms in burn injuries remain unclear. Therefore, the detailed
function of S100A9 in this process needs to be further
explored.
In the present study, exosomes were isolated from
the serum of patients with burn injuries and were used to treat
human myocardial cells. Afterwards, S100A9 antibodies and CY-09, an
NLRP3 inhibitor, were introduced to explore the underlying
molecular mechanisms. These findings provide evidence towards novel
therapeutic targets and pathways for burn injury treatment.
Materials and methods
Isolation and characterization of
serum exosomes
Patients with burn injuries (III-degree burns) were
recruited from Seventh People's Hospital Affiliated to Shanghai
University of Traditional Chinese Medicine (Shanghai, China) from
June 2017 to October 2019, including (27 males and 13 females; age
range, 37-67 years; mean age, 52.1±6.0 years) and 5 ml of venous
blood was collected from each patient. The present research
protocol was approved by the Ethics Committee of Shanghai Seventh
People's Hospital (approval no. 2018-HIRB-046), and written
informed consent was obtained from all patients. The venous blood
was centrifuged at 1,409 x g for 8 min at 4˚C and the serum was
obtained. Differential centrifugation was used to isolate exosomes
from the serum. First, the serum was centrifuged at 10,000 x g for
30 min at 4˚C. Afterwards, the supernatant was transferred to a new
5-ml ultrahigh speed centrifuge tube and centrifuged at 17,000 x g
for 2 h at 4˚C. This step was repeated three times. After removing
the supernatant, the sediment was resuspended in 200 µl of
phosphate-buffered saline, filtered using a 0.22-um filter and
stored at -80˚C for further analysis.
The concentration of exosomes was measured using a
BCA Protein Concentration Assay kit (Wuhan Boster Biological
Technology, Ltd.), following the manufacturer's instructions. The
morphology of exosomes was visualized using transmission electron
microscopy (TEM; FEI Tecnai 12; Philips Healthcare), as previously
described (26). Afterwards, based
on the method of Yin et al (27), the protein expression levels of
CD63, CD9 and TSG101, which are specific proteins, were determined
using western blotting with their corresponding antibodies as
described below.
Cell culture
Human myocardial cell AC16 cells were obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. The cells were cultured in Dulbecco's Modified Eagle
Medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100
µg/ml streptomycin in an incubator set at 37˚C with 5%
CO2.
Cellular uptake of exosomes in AC16
cells
Serum exosomes were labeled with PKH67 using a PKH67
Green Fluorescent Cell Linker kit (Sigma-Aldrich; Merck KGaA),
following the manufacturer's protocols. Briefly, exosomes diluted
in 1.5 ml of Diluent C were incubated with 6 µl of PKH67 dye at
room temperature. After being incubated for 5 min, the exosomes
were incubated with 3 ml of ultracentrifuged FBS for 1 min at
37°C to allow the binding of the excess dye. After being
washed with 15 ml of keratinocyte serum-free medium (KSFM;
Invitrogen; Thermo Fisher Scientific, Inc.), the mixture was
centrifuged at 100,000 x g for 75 min at 4˚C, and the sediment
(PKH67-labeled exosomes) was resuspended with KSFM for use.
The AC16 cells were seeded into a 24-well plate at a
density of 3x104 per well and were cultured overnight at
37°C. Thereafter, 10 µg/ml PKH67-labeled exosomes were
added to the cells. After 24 h of incubation at 37˚C,
the AC16 cells were fixed with 4% paraformaldehyde for 10 min at
37˚C. After washing, the cells were stained with
4,6-diamidino-2-phenylindole and were observed under a fluorescence
microscope (magnification, x400; Olympus IX71; Olympus
Corporation).
Enzyme-linked immunosorbent assay
(ELISA)
The expression levels of IL-1β and IL-18 in the
cells were examined using a rat IL-18 ELISA kit (cat. no.
E-EL-R0567c) and rat IL-1β ELISA kit (cat. no. E-EL-R0012c) (both
from Elabscience Biotechnology, Inc.), following the manufacturer's
protocol. Briefly, IL-1β and IL-18 antibodies were applied to the
AC16 cells at 37˚C for 2 h. After rinsing off the washing solution,
secondary antibodies were applied. Subsequently, the stop solution
was added, A microplate absorbance reader (Tecan Group, Ltd.) was
used to measure the absorbance at 450 nm. Standard curves were
applied to calculate the concentrations of analytes. Each reaction
was carried out three times.
Evaluation of pyroptosis by flow
cytometry
The AC16 cells in different treatments as indicated
below were harvested and washed with ice-cold PBS. Afterwards, the
AC16 cells were stained with activated caspase-1 antibodies
(FLICA® 660 Caspase-1 Assay; cat. no. 9122; Bio-Rad
Laboratories, Inc.) and propidium iodide (PI; Thermo Fisher
Scientific, Inc.). Cell pyroptosis (activated capase-1 and PI
double-positive) was analyzed using flow cytometry (BD
Biosciences).
Western blotting
Total AC16 cell lysates were prepared using a
radioimmunoprecipitation buffer containing a proteinase inhibitor
(Beyotime Institute of Biotechnology). Protein levels were
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Protein samples (20 µg per lane) were separated by
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
were transferred to nitrocellulose membranes (MilliporeSigma).
After being blocked with 5% skim milk at room temperature for 2 h,
the membranes were incubated with primary antibodies overnight at
4˚C (Table SI). After being
washed with PBST (0.05% Tween) for three times, the membranes were
incubated with HRP-conjugated rabbit secondary antibodies
(1:10,000; cat. no. ZB-2301; OriGene Technologies, Inc.) at room
temperature for 1 h. Finally, band intensity was semi-quantified
via densitometry analysis using Quantity-One software v4.62
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using Prism 7.0
(GraphPad Software, Inc.). Data are presented as the mean ±
standard deviation of the three replicates. Comparisons among
multiple independent groups were analyzed using Kruskal-Wallis test
followed by Dunn's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
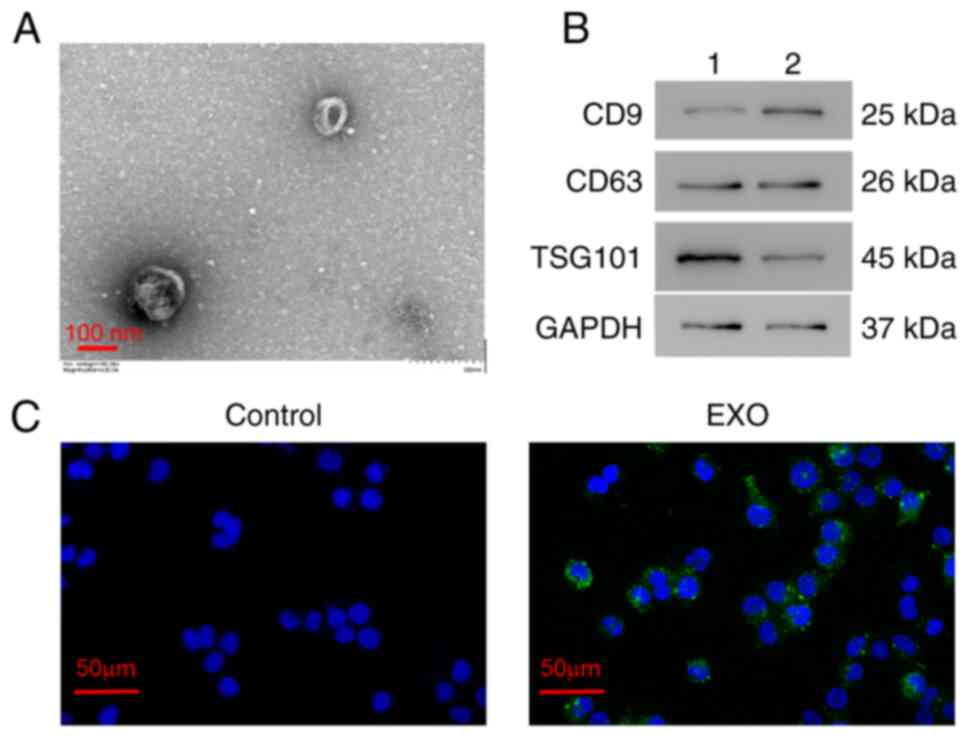
Identification of exosomes
To identify the exosomes isolated from the serum of
patients with burn injuries, TEM and western blotting were
performed. TEM results revealed that exosomes exhibited a
cup-shaped morphology with a diameter of ~100-nm (Fig. 1A). Western blotting revealed that
exosome markers CD9, CD63 and TSG101 were all expressed in the
serum exosomes (Fig. 1B). These
results indicated that exosomes were successfully extracted from
the serum of patients with burn injuries using a differential
centrifugation method.
Serum exosomes were also labeled with PKH67 (green
fluorescence) and were co-cultured with AC16 cells for 24 h. Most
AC16 cells exhibited a green fluorescence (Fig. 1C). These results indicated that the
serum exosomes extracted from patients with burn injuries could be
taken up by human myocardial AC16 cells.
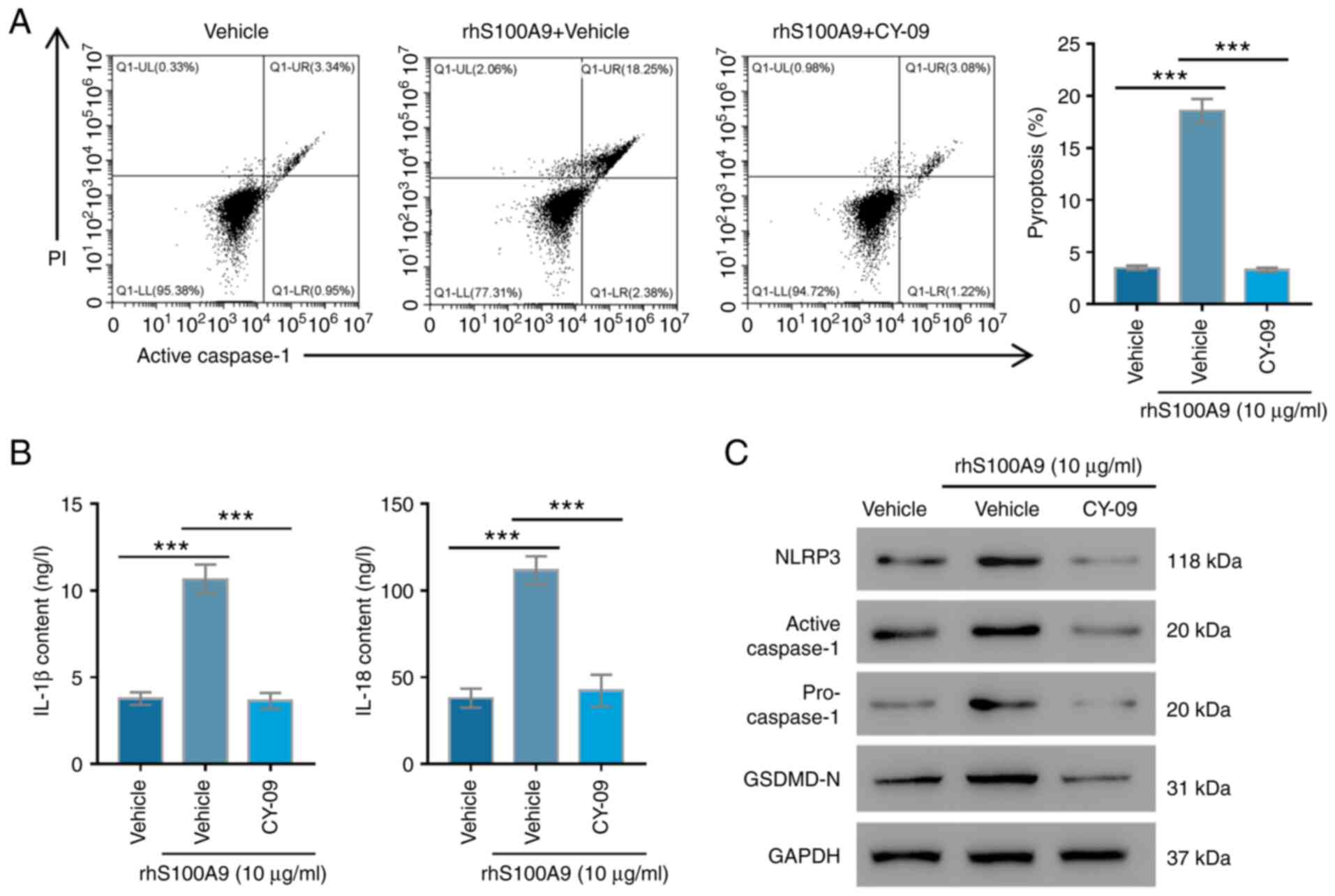
Serum exosomes from patients with burn
injuries promote AC16 cell pyroptosis
To further investigate the effects of serum exosomes
from patients with burn injuries on AC16 cell pyroptosis, active
caspase-1 levels were measured. The pyroptosis of AC16 cells with
exosome treatment was significantly increased with increasing
culture duration (P<0.01; Fig.
2A). Increasing culture duration also significantly elevated
the IL-18 and IL-1β contents in exosome-treated AC16 cells
(P<0.01; Fig. 2B). Furthermore,
western blotting revealed that S100A9, active caspase-1, NLRP3,
pro-caspase-1 and GSDMD-N expression levels were all markedly
upregulated in AC16 cells treated with serum exosomes after being
cultured for 12, 24 and 48 h compared with those in cells treated
with exosomes for 0 h (Fig. 2C).
These results indicated that the serum exosomes from patients with
burn injuries might promote the pyroptosis of human myocardial
cells by upregulating the expression levels of S100A9, NLRP3,
caspase-1 and GSDMD-N.
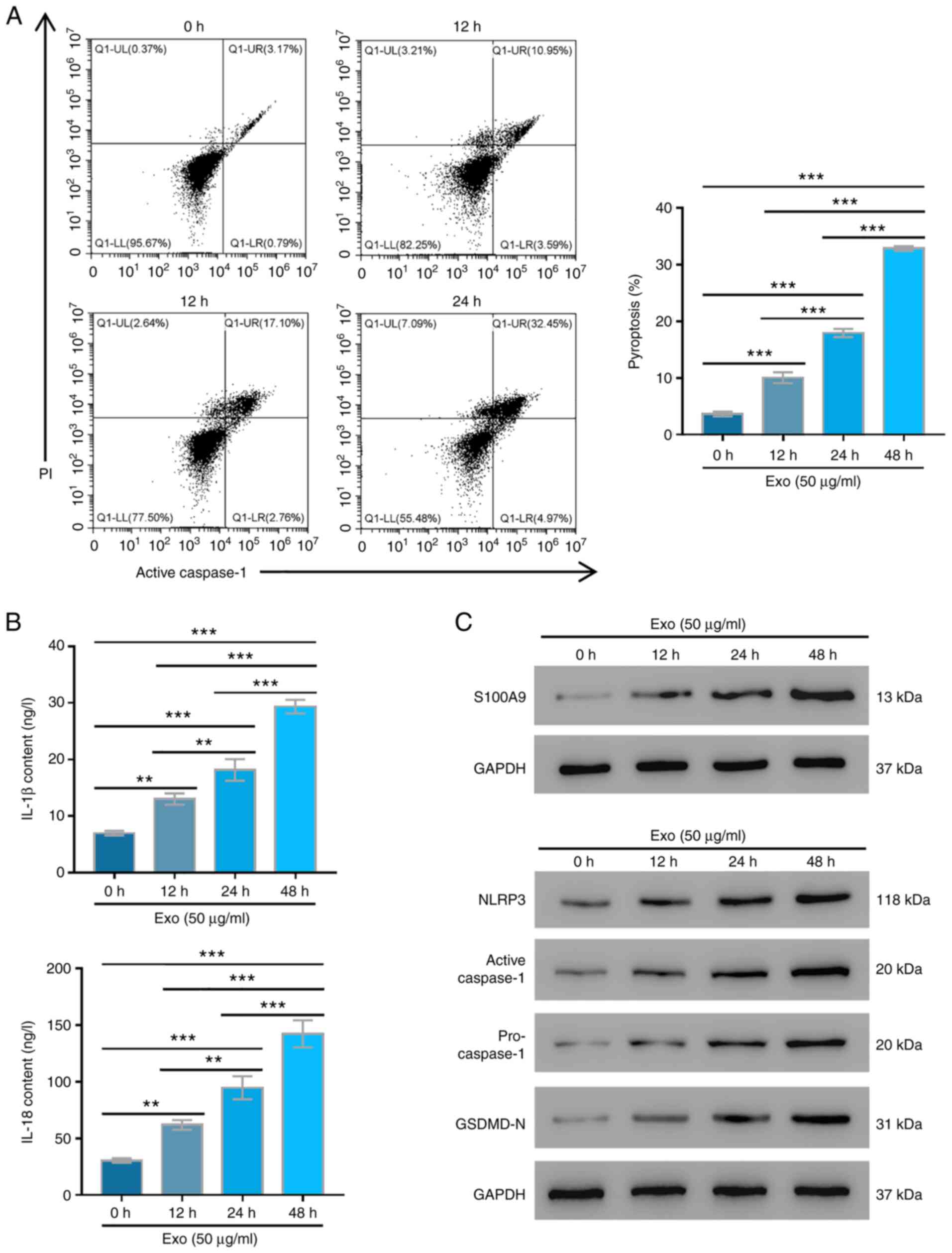 | Figure 2Serum exosomes extracted from patients
with burn injuries promote pyroptosis and S100A9 expression. AC16
cells were treated with 50 µg/ml exosomes for 0, 12, 24 and 48 h.
(A) Cell pyroptosis was tested by flow cytometry in three
independent experiments with three parallel samples. (B) IL-1β and
IL-18 contents were determined using ELISA kits in three
independent experiments with three parallel samples. (C) S100A9,
NLRP3, active caspase-1, pro-caspase-1 and GSDMD-N expression
levels were measured using western blotting in three independent
experiments with three parallel samples. **P<0.01,
***P<0.001. Exo, exosomes; S100A9, S100
calcium-binding protein A9; NLRP3, NLR family pyrin domain
containing 3; GSDMD, Gasdermin D. |
Exosome-induced pyroptosis is reversed
by anti-S100A9 antibodies
To explore the role of S100A9 in burn injuries,
anti-S100A9 antibodies were used to treat exosome-induced cell
injury, after which cell pyroptosis was detected. Relative to the
control group, the pyroptosis of cells only treated with exosomes
was significantly increased (P<0.001; Fig. 3A). However, after being treated
with different concentrations of anti-S100A9, pyroptosis was
significantly lower and gradually decreased with increasing
anti-S100A9 concentrations (P<0.001; Fig. 3A). The changes in IL-18 and IL-1β
contents of AC16 cells under different treatments presented a
similar trend with those of cell pyroptosis in different groups
(Fig. 3B). Western blotting
results revealed that active caspase-1, GSDMD-N, NLRP3 and
pro-caspase-1 expression levels were markedly upregulated after
exosome treatment compared with the control group, whereas
different anti-S100A9 concentrations markedly suppressed their
expressions. The inhibitory effects became more obvious with
increasing anti-S100A9 antibody concentrations (Fig. 3C). The results suggested that
S100A9 inhibition could reverse the pyroptosis of human myocardial
cells induced by exosomes isolated from patients with burn
injuries.
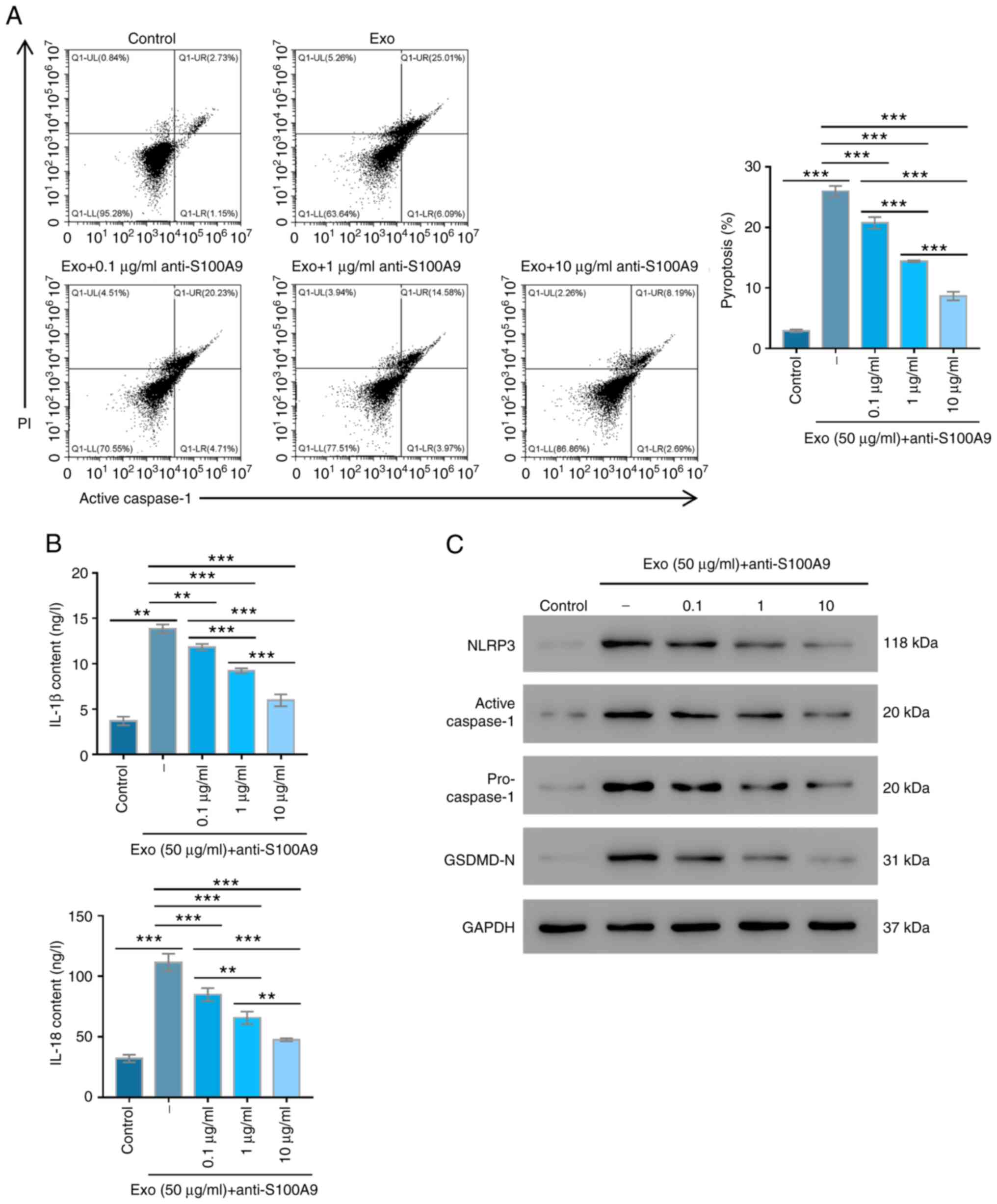 | Figure 3Anti-S100A9 protect AC16 cells from
serum exosome-induced pyroptosis. (A) Pyroptosis was tested in AC16
cells treated with 50 µg/ml exosomes supplemented with 0.1, 1, or
10 µg/ml anti-S100A9 antibodies by flow cytometry in three
independent experiments with three parallel samples. (B) IL-1β and
IL-18 contents of AC16 cells under different treatments were
determined by ELISA in three independent experiments with three
parallel samples. (C) S100A9, NLRP3, active caspase-1,
pro-caspase-1 and GSDMD-N expression levels were measured using
western blotting in three independent experiments with three
parallel samples. **P<0.01, ***P<0.001.
Exo, exosomes; S100A9, S100 calcium-binding protein A9; NLRP3, NLR
family pyrin domain containing 3; GSDMD, Gasdermin D. |
NLRP3 inflammasome is involved in
S100A9-induced AC16 cell pyroptosis
NLRP3 is a key factor in recruiting caspase-1, and
this allows the cleavage of pro-IL-1β to its mature form (25). Therefore, the effects of NLRP3 on
S100A9-mediated AC16 cell pyroptosis were further studied. It was
revealed that recombinant S100A9 significantly enhanced the
pyroptosis of AC16 cells (P<0.001), whereas CY-09, an NLRP3
inhibitor, significantly reversed these effects (P<0.001;
Fig. 4A). The IL-18 and IL-1β
contents were significantly higher in the recombinant S100A9 group
compared with in the control group (P<0.001); whereas their
contents in the CY-09 group were similar compared with the control
group (P>0.05, Fig. 4B).
Furthermore, the expression levels of active caspase-1, NLPR3,
pro-caspase-1 and GSDMD-N were markedly elevated in the recombinant
S100A9 group compared with the control group, while these were
markedly reduced after the CY-09 treatment (Fig. 4C). These results revealed that
NLRP3 suppression could alleviate the pyroptosis of human
myocardial cells induced by S100A9.
Discussion
Burn injuries are one of the most debilitating
traumas that can inflict humans, seriously affecting the
individuals' health and mental health, and puts a huge burden on
healthcare systems (2). Exosomes,
as carriers of bioactive substances, have been reported to play
important roles in various diseases, including cardiovascular and
central nervous system-related diseases, and cancer progression
(26-29).
S100A9 is highly expressed in the exosomes extracted from the serum
of patients with burn injuries, and it may be a potent stimulator
of inflammatory responses (30).
The present study revealed that burn injury-associated exosomes
significantly promoted the pyroptosis of AC16 cells, and that this
was significantly reversed by S100A9 antibodies. Moreover, CY-09
could restore the pyroptosis caused by recombinant S100A9. These
findings indicated that burn injury-associated exosomes promoted
the pyroptosis of AC16 cells by enhancing the activity of the
S100A9-NLRP3 axis.
Previous studies have demonstrated that exosomes can
serve as a natural carrier system that can transport RNA, DNA and
proteins and participate in multiple cellular functions (31,32).
In the current study, exosomes were successfully isolated from the
serum of patients with burn injuries; these promoted S100A9
expression and AC16 cell pyroptosis. A previous study has indicated
that S100A9 activates the NLRP3 inflammasome and promotes IL-1β
release (33). Another study has
indicated that S100A9 induces the formation of NLRP3 inflammasomes,
activates caspase-1 and produces IL-1β and IL-18, leading to
pyroptosis in myelodysplastic syndrome cells (34). Thus, it can be concluded that burn
injury-associated exosomes promote AC16 cell pyroptosis by
regulating the S100A9/NLRP3 pathway.
S100A9, a member of the S100 protein family, can
increase the metabolism of the cytoskeleton, enhance the migration
of phagocytes and inhibit the development of microbes (35-37).
S100A9 overexpression was observed in a number of
inflammation-related diseases, including sepsis, acute
pancreatitis, inflammatory bowel disease and myocardial infraction
(38). Isaacs et al
(39) indicated that tasquinimod
can bind with S100A9, inhibiting the interaction between S100A9 and
Toll-like receptor 4 and repressing the TNF-α release. Furthermore,
S100A9 has been identified to be a novel clinical target for
cardiovascular diseases, including aneurysms and acute coronary
syndrome (40). To the best of our
knowledge, this is the first study to show that S100A9 secreted by
burn injury-associated exosomes could regulate the progression of
human myocardial cell pyroptosis and its potential value as a novel
target for burn injury therapy. However, the lack of data to obtain
in in vivo assays limited the significance of these
findings. Therefore, further examination should be conducted in
vivo. Nevertheless, the present data illustrated the notable
role of S100A9 and its potential role as a target for burn injury
treatment.
In conclusion, burn injury-associated exosomes
containing S100A9 can promote myocardial cell pyroptosis by
upregulating the expression levels of NLRP3, caspase-1 and GSDMD-N
involved in cardiogenic shock after a burn injury. These findings
could help improve understanding of pyroptosis after a burn injury
and provide novel insights for the S100A9/NLRP3 pathway as a novel
therapeutic approach.
Supplementary Material
Antibody list.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Shanghai Chinese and
Western Medicine Clinical Cooperation Pilot Construction
Project-Water Burning Injury [grant no. ZY(2018-2020)-FWTX-1106],
the Training Program for Discipline Leaders of Shanghai Pudong New
Area Health Planning Commission (Burn Surgery; grant no.
PWRd2017-09) and the General Program of Pudong New Area Municipal
Health Commission of Shanghai (grant no. PW2017A-18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX and AW designed the experiments. AW, NZ, YC and
QJ performed the experiments. AW and YC confirm the authenticity of
all the raw data. AW, NZ and SX wrote and edited the manuscript.
All authors read and approved the final the manuscript.
Ethics approval and consent to
participate
The present research protocol was approved by the
Ethics Committee of Shanghai Seventh People's Hospital (approval
no. 2018-HIRB-046), and written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duan WQ, Xu XW, Cen Y, Xiao HT, Liu XX and
Liu Y: Epidemiologic investigation of burn patients in Sichuan
Province, China. Med Sci Monit. 25:872–879. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abdullahi A, Amini-Nik S and Jeschke MG:
Animal models in burn research. Cell Mol Life Sci. 71:3241–3255.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fozzard HA: Myocardial injury in burn
shock. Ann Surg. 154:113–119. 1961.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kulp GA, Herndon DN, Lee JO, Suman OE and
Jeschke MG: Extent and magnitude of catecholamine surge in
pediatric burned patients. Shock. 33:369–374. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gauglitz GG, Herndon DN and Jeschke MG:
Insulin resistance postburn: Underlying mechanisms and current
therapeutic strategies. J Burn Care Res. 29:683–694.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mecott GA, Al-Mousawi AM, Gauglitz GG,
Herndon DN and Jeschke MG: The role of hyperglycemia in burned
patients: Evidence-based studies. Shock. 33:5–13. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rivers EP, Katranji M, Jaehne KA, Brown S,
Abou Dagher G, Cannon C and Coba V: Early interventions in severe
sepsis and septic shock: A review of the evidence one decade later.
Minerva Anestesiol. 78:712–724. 2012.PubMed/NCBI
|
|
8
|
Chen G, Xie M, Dai Z, Wan P, Ye H, Zeng X
and Sun Y: Kudingcha and fuzhuan brick tea prevent obesity and
modulate gut microbiota in high-fat diet fed mice. Mol Nutr Food
Res. 62(e1700485)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He C, Zheng S, Luo Y and Wang B: Exosome
theranostics: Biology and translational medicine. Theranostics.
8:237–255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and
Li P: Exosome: A review of its classification, isolation
techniques, storage, diagnostic and targeted therapy applications.
Int J Nanomedicine. 15:6917–6934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L and Yu D: Exosomes in cancer
development, metastasis, and immunity. Biochim Biophys Acta Rev
Cancer. 1871:455–468. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li X, Liu L, Yang J, Yu Y, Chai J, Wang L,
Ma L and Yin H: Exosome derived from human umbilical cord
mesenchymal stem cell mediates MiR-181c attenuating burn-induced
excessive inflammation. EBioMedicine. 8:72–82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qin D, Yang W, Pan Z, Zhang Y, Li X and
Lakshmanan S: Differential proteomics analysis of serum exosomein
burn patients. Saudi J Biol Sci. 27:2215–2220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vogl T, Stratis A, Wixler V, Völler T,
Thurainayagam S, Jorch SK, Zenker S, Dreiling A, Chakraborty D,
Fröhling M, et al: Autoinhibitory regulation of S100A8/S100A9
alarmin activity locally restricts sterile inflammation. J Clin
Invest. 128:1852–1866. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Idrovo JP, Boe DM, Kaahui S, Yang WL and
Kovacs EJ: Hepatic inflammation after burn injury is associated
with necroptotic cell death signaling. J Trauma Acute Care Surg.
89:768–774. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fontaine M, Lepape A, Piriou V, Venet F
and Friggeri A: Innate danger signals in acute injury: From bench
to bedside. Anaesth Crit Care Pain Med. 35:283–292. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xia X, Wang X, Cheng Z, Qin W, Lei L,
Jiang J and Hu J: The role of pyroptosis in cancer: pro-cancer or
pro-‘host’? Cell Death Dis. 10(650)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin J, Shou X, Mao X, Dong J, Mohabeer N,
Kushwaha KK, Wang L, Su Y, Fang H and Li D: Oxidized low density
lipoprotein induced caspase-1 mediated pyroptotic cell death in
macrophages: Implication in lesion instability? PLoS One.
8(e62148)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miao EA, Leaf IA, Treuting PM, Mao DP,
Dors M, Sarkar A, Warren SE, Wewers MD and Aderem A:
Caspase-1-induced pyroptosis is an innate immune effector mechanism
against intracellular bacteria. Nat Immunol. 11:1136–1142.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Yuan J, Najafov A and Py BF: Roles of
caspases in necrotic cell death. Cell. 167:1693–1704.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Franchi L, Muñoz-Planillo R and Núñez G:
Sensing and reacting to microbes through the inflammasomes. Nat
Immunol. 13:325–332. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Martinon F, Mayor A and Tschopp J: The
inflammasomes: Guardians of the body. Annu Rev Immunol. 27:229–265.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu Q, Li Q, Niu X, Zhang G, Ling X, Zhang
J, Wang Y and Deng Z: Extracellular vesicles secreted by human
urine-derived stem cells promote ischemia repair in a mouse model
of hind-limb ischemia. Cell Physiol Biochem. 47:1181–1192.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yin J, Zeng A, Zhang Z, Shi Z, Yan W and
You Y: Exosomal transfer of miR-1238 contributes to
temozolomide-resistance in glioblastoma. EBioMedicine. 42:238–251.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gritsenko A, Yu S, Martin-Sanchez F,
Diaz-Del-Olmo I, Nichols EM, Davis DM, Brough D and Lopez-Castejon
G: Priming is dispensable for NLRP3 inflammasome activation in
human monocytes in vitro. Front Immunol. 11(565924)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367(eaau6977)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang S, Song R, Wang Z, Jing Z, Wang S and
Ma J: S100A8/A9 in inflammation. Front Immunol.
9(1298)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Emanueli C, Shearn AI, Angelini GD and
Sahoo S: Exosomes and exosomal miRNAs in cardiovascular protection
and repair. Vascul Pharmacol. 71:24–30. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi L, Zhao Y, Fei C, Guo J, Jia Y, Wu D,
Wu L and Chang C: Cellular senescence induced by S100A9 in
mesenchymal stromal cells through NLRP3 inflammasome activation.
Aging (Albany NY). 11:9626–9642. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Basiorka AA, McGraw KL, Eksioglu EA, Chen
X, Johnson J, Zhang L, Zhang Q, Irvine BA, Cluzeau T, Sallman DA,
et al: The NLRP3 inflammasome functions as a driver of the
myelodysplastic syndrome phenotype. Blood. 128:2960–2975.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Viemann D, Barczyk K, Vogl T, Fischer U,
Sunderkötter C, Schulze-Osthoff K and Roth J: MRP8/MRP14 impairs
endothelial integrity and induces a caspase-dependent and
-independent cell death program. Blood. 109:2453–2460.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Anceriz N, Vandal K and Tessier PA: S100A9
mediates neutrophil adhesion to fibronectin through activation of
beta2 integrins. Biochem Biophys Res Commun. 354:84–89.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ryckman C, Vandal K, Rouleau P, Talbot M
and Tessier PA: Proinflammatory activities of S100: Proteins
S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and
adhesion. J Immunol. 170:3233–3242. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hermani A, Hess J, De Servi B, Medunjanin
S, Grobholz R, Trojan L, Angel P and Mayer D: Calcium-binding
proteins S100A8 and S100A9 as novel diagnostic markers in human
prostate cancer. Clin Cancer Res. 11:5146–5152. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Isaacs JT, Dalrymple SL, Rosen DM, Hammers
H, Olsson A and Leanderson T: Anti-cancer potency of tasquinimod is
enhanced via albumin-binding facilitating increased uptake in the
tumor microenvironment. Oncotarget. 5:8093–8106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schiopu A and Cotoi OS: S100A8 and S100A9:
DAMPs at the crossroads between innate immunity, traditional risk
factors, and cardiovascular disease. Mediators Inflamm.
2013(828354)2013.PubMed/NCBI View Article : Google Scholar
|