Introduction
As the fourth leading cause of disability globally,
type 2 diabetes mellitus (T2DM) has become a major public health
concern and increasing global health burden (1). Previous evidence has estimated that
the global prevalence of diabetes mellitus in 2019 was 9.3% (463
million individuals), and it will rise to 10.2% by 2030, even to
10.9% by 2045(2). Therefore, it is
necessary to plan effective national prevention and control
programs for T2DM management and reducing its disease
complications.
The development of T2DM and the occurrence of
related complications are chronic processes. Identification of
diagnostic biomarkers for glucose metabolism disorder are critical
to prevent and control the occurrence and development of T2DM at
the earliest stage. MicroRNAs (miRNAs) are a family of noncoding
single-strand RNA molecules (19 to 25 nucleotides) that play an
important role both in physiological and pathological pathways by
regulating posttranscriptional silencing of target genes (3,4).
Several previous studies have showed the different expression
levels of circulating miRNAs that have been identified to associate
with T2DM and related metabolic diseases, such as obesity and
insulin resistance (IR) (5-9),
and different research indicated specific upregulated/downregulated
miRNAs expression in patients with T2DM or related metabolic
diseases. In those miRNAs with altered expression, some are
regulating glucose and lipid metabolism in liver such as
miR-103/107 and miR-122, and miR-375 and miR-126 are promising
clinical biomarkers for T2DM (10). After reviewing the original
articles and systematic reviews on the relationship between miRNAs
and T2DM or related metabolic parameters, combined with the
differentially expressed candidate plasma exosome miRNAs in our
established discovery study, miR-126 and miR-122 were screened to
be further discussed since they have been widely studied in recent
years.
miR-126 and miR-122 has been proposed to play a
central role in the regulation of the blood glucose metabolism and
the T2DM development (4,11-13).
For instance, the plasma miRNA profiles in patients with T2DM
revealed dysregulated endothelial miR-126(6), which could regulate the glucose
homeostasis through its target insulin receptor substrate (14). Moreover, animal experiments showed
that the fasting blood glucose (FBG) level increased significantly
after the birth in the mouse with miR-126 knockdown compared with
the wild-type mouse (15). In
addition, a prior study suggested that miR-122-5p expression was
associated with homeostasis model assessment of insulin resistance
(HOMA-IR) index and could predict the presence of IR (a determinant
underlying the pathophysiology of T2DM) in adolescents with obesity
(16). Although increasing studies
have been performed to examine the potential associations of
miR-126 and miR-122 expression with T2DM and related glucose
metabolism parameters, inconsistent results of existing studies
remained. For example, evidence has shown a lower expression level
of miR-126 in prevalent T2DM compared with healthy controls
(6,11,17),
whereas one recent study published in 2021 indicated that
miR-126-3p was positively associated with T2DM risk and higher FBG
level (18). Therefore, this
meta-analysis systematically and quantitatively evaluated the
relationship between circulating miR-126 and miR-122 expression
levels and the risks of T2DM as well as related glucose metabolism
parameters.
Materials and methods
Search strategy
The present study was performed based on the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guideline (PRISMA 2020 statement) (http://www.prisma-statement.org/). A literature search
strategy was developed according to the four elements of the study
question (participants, intervention, comparison and outcome) and
the study design. PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com), Web of Science (https://clarivate.com/webofsciencegroup/solutions/web-of-science/),
Cochrane Library databases (https://www.cochranelibrary.com/) and Chinese National
Knowledge Infrastructure (CNKI, https://www.cnki.net/) electronic databases were used
to identify eligible original articles published up to May 3, 2022.
In addition, the Chinese Biomedical Literatures database (CBM,
http://www.sinomed.ac.cn/index.jsp),
Wanfang Digital Periodicals (https://www.wanfangdata.com.cn/index.html), and
Chinese Science and Technology Periodicals database (VIP,
http://qikan.cqvip.com/) were also used to
identify additional records (3 studies). The search terms used in
these databases are presented in Table SI. Eligible original studies
include those published in English or Chinese and those conducted
on humans.
Study selection, quality assessment
and data extraction
The selection process of the studies, quality
assessment process, and data extraction from the included studies
were conducted by two authors (YH and YL) independently in the
current meta-analysis. Moreover, controversies regarding the
eligibility of the paper were solved through discussion by these
two authors (YH and YL), rather than adjudication by a third
author. Papers meeting the following criteria were potentially
eligible: i) case-control studies or cohort studies investigating
the relationship between circulating miR-126 and miR-122 level and
the T2DM risk or the correlation with FBG/HOMA-IR index; ii)
cross-sectional study with a clear case group and healthy controls
at the data analysis stage; iii) study outcomes including T2DM and
its complications, obesity, IR [with results expressed as relative
ratio (RR), odds ratio (OR), or hazard ratio (HR) and 95%
confidence interval (CI)], FBG level and HOMA-IR index [with
results expressed as correlation coefficient (r)]; iv) the quality
score of the studies assessed by the Newcastle Ottawa Scale (NOS)
was at least 6. The excluded criteria were: i) duplication of
studies (repeating data that were already reported by other
included articles), including articles published by the same
authors in different years using the same data; ii) articles were
letters, comments, reviews, chapters or conference abstracts only;
iii) study outcomes were other diseases which were not associated
with diabetes or its complications; iv) articles where the required
data was not available or the effect estimates were abnormal; v)
studies with the NOS score less than 6 (low-quality).
NOS was used to assess the quality of the included
studies, which included selection, comparability and outcome of
three categories, with a full score of 9 stars (a maximum of one
star for items within the selection and outcome categories, but a
maximum of two stars for the items within the comparability
category) (19). Furthermore, the
following information was extracted from the selected studies by
two authors (YH and YL) independently: First author; publication
year; study type; study country; sample size and average age of
study subjects; definitions and diagnostic criteria of T2DM and
related metabolic diseases; assessment of circulating miR-126 and
miR-122; the OR/RR/HR and 95% CI of circulating miR-126 related to
T2DM; the correlation coefficient (r, 95% CI) of circulating
miR-126 and miR-122 with FBG or HOMA-IR index; and covariates of
the adjusted model. If there were multiple adjusted models in an
original study, the relevant data in the model with the most
adjusted covariates were extracted.
Statistical analysis
This meta-analysis aimed to examine the relationship
of miR-126 and miR-122 expression level with T2DM risk, in which
effect estimates were expressed as OR (case-control studies), RR or
HR (cohort studies). HR and RR were roughly considered equivalent
to OR (20). For the effect
estimate on T2DM when the independent variable was not changed by
one unit, it has been uniformly converted as the independent
variable increased by one unit [OR convert=EXP (LN (OR
original)/original unit change x1-unit change)]. The
method recommended by Hamling et al (21) was used to convert effect estimates
when the reference group of miR-126 or miR-122 used in the present
study was not the lowest level. The random effect model was used to
estimate the OR (95% CI) of the included studies (22), and captured differences between the
statistical parameters across studies by introducing study-specific
effects in the model (23). In
addition, the correlation coefficient (r, 95% CI) was assessed
between miR-126 and miR-122 expression and FBG level as well as
HOMA-IR index, and the corresponding effect estimates expressed as
r (95% CI). The r extracted from the selected studies is converted
to Fisher's Z value and standard Error (SE) [ZCOR=0.5 x
LN(1+r)/(1-r); SE=1/√(n-3)] (24),
then merged and converted to r value (25), and the data were pooled with the
random effect model based on the inverse variance method.
Heterogeneity among the included studies was
assessed by the Q test and I2 statistic that describes
the proportion attributed to heterogeneity in total variation in
study estimates (I2<50% and P>0.05 were considered
as no heterogeneity) (26,27). In addition, subgroup analyses were
conducted to examine the potential sources of heterogeneity by
sample size (<100 vs. ≥100), source of participants (Asia,
Africa, Europe, North America, South America, Oceania), average age
of subjects (<60 vs. ≥60; <30 vs. ≥30) and study types
(descriptive, analytical or experimental studies). Sensitivity
analyses were performed to examine the influence of individual
studies on the pooled estimates by excluding one study at a time
(28). Potential publication bias
was assessed by the Begg's funnel plot and Egger's tests, and it
was considered as no publication bias if P>0.05 (29,30).
All statistical analyses were performed using Stata version 13.1
software (Stata Corp LLC), and R software (version 3.6.1; R
Development Core Team).
Results
Literature search and characteristics
of included studies
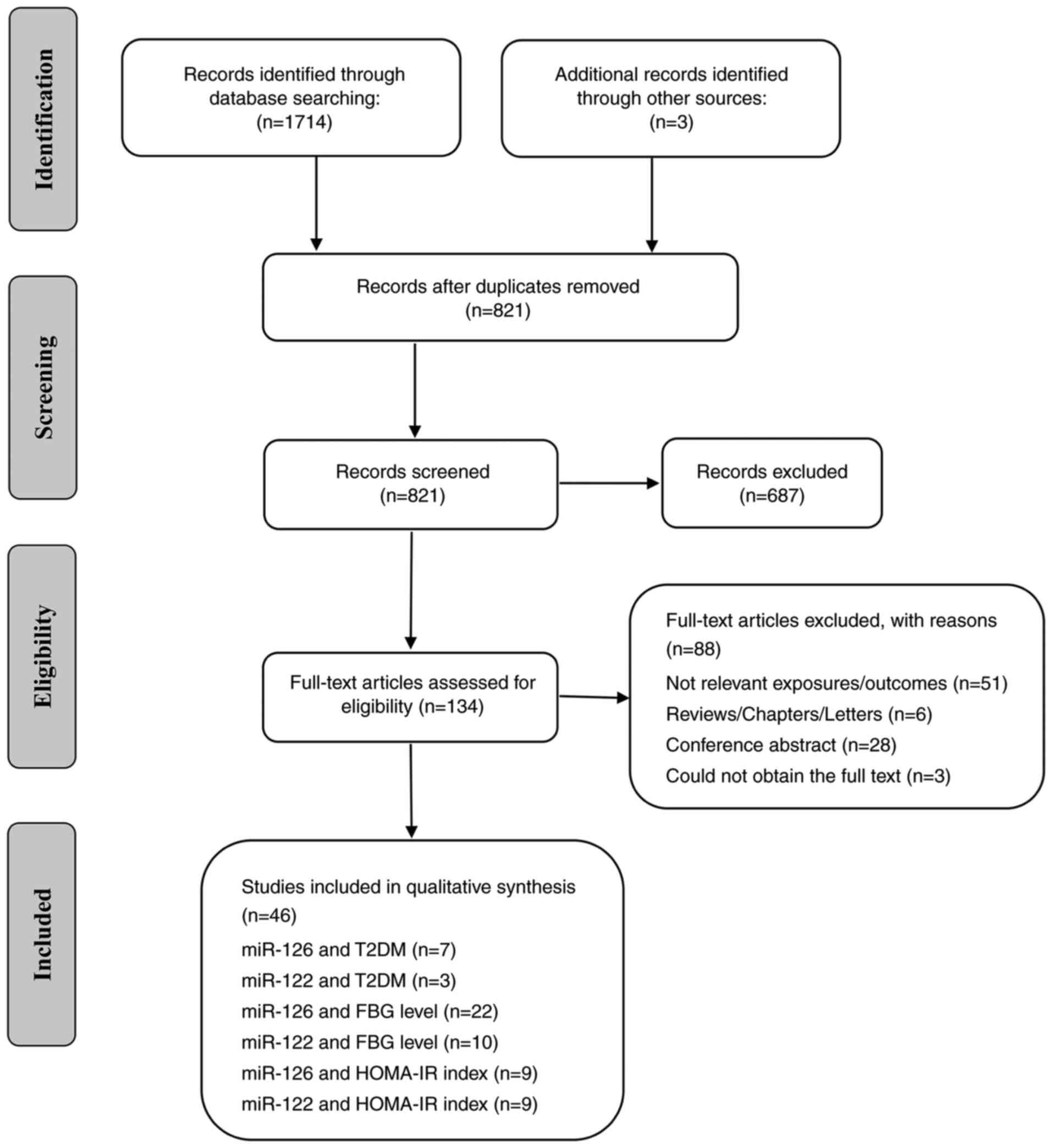
A total of 1717 relevant studies for miR-126 and
miR-122 were searched in the aforementioned 8 databases. After
removing duplicates, screening the title/abstract, and assessing
the full-text article, 46 studies were included in the final
analyses about the relationships of circulating miR-126 and miR-122
level with T2DM risk, FBG level and HOMA-IR index (Fig. 1; flowchart for study selection).
The NOS scores of the included studies are presented in Table SII, and the scores of all studies
were at least 6.
The characteristics of the included studies on the
relationship of circulating miR-126 and miR-122 expression levels
with T2DM risks, of which the first 7 studies were about miR-126
expression and the risk of T2DM, and the last 3 studies were about
miR-122 expression and the risk of T2DM are presented in Table I. Of the 7 studies about miR-126
with the risk of T2DM, 4 were conducted in Asia (2 in Kingdom of
Bahrain and 2 in China), 2 were performed in Europe (1 in Italy and
1 in Sweden) and 1 was in Africa (the Republic of South Africa).
The average age of the participants was >45 years. The sample
size of the studies was not less than 100 except for two study in
Asia (5,31). Of the 3 studies about miR-122 with
the risk of T2DM, 1 was conducted in Asia (China), 1 was performed
in Europe (Italy) and 1 was in North America (USA). The average age
of the participants was >50 years. And the sample size of the
studies was not less than 100. In addition, the characteristics of
the included studies involving correlation of circulating miR-126
and miR-122 expression with FBG level and HOMA-IR index are
revealed in Table SIII. Of the 38
studies, 21 were conducted in Asia, 6 in Europe, 5 in North
America, 4 in Africa, 1 in South America and 1 in Oceania. In
addition, 5 of the studies involved subjects younger than 18 and
nearly half of the studies (18/38) had sample sizes of less than
100. Similarly, as revealed in Table
SII, all studies on relationship of miR-126 and miR-122 with
T2DM-related metabolic parameters were of high quality (with NOS
scores ranging from 6 to 9).
 | Table ICharacteristics of the included
studies for association of miR-126 and miR-122 with T2DM risk. |
Table I
Characteristics of the included
studies for association of miR-126 and miR-122 with T2DM risk.
| First Author
(year) | Country | Sample, n
(male/female) | Age, years | Diagnosis and
assessment of T2DM | Biological
Sample | miRNAs
assessment | Adjusted
variables | (Refs.) |
|---|
| Zhang (2015) | China | 40 (22/18) | 59.23±10.14 | FPG ≥7.0 mmol/L or
2 h PG ≥11.1 mmol/L in | Plasma samples | miR-126
RT-qPCR | Univariate logistic
regression. analyses | (5) |
| Zampetaki
(2010) | Italy | 160 (60/100) | 40 to 79 years old
66.3±8.9 | OGTT WHO
criteria | Plasma samples | miR-126 qPCR | Adjusted for age,
sex, social status, Fam-TD, BMI, waist-to-hip ratio, smoking
status, alcohol consumption, physical activity, and hs-CRP. | (6) |
| Liu (2014) | China | 298 (145/153) | 48.58±6.94 | World Health
Organization criteria | Serum samples | miR-126
RT-qPCR | Adjusted for age,
gender, BMI and some biochemical indicators. | (11) |
| Al-Kafaji
(2016) | Kingdom of
Bahrain | 102 (49/53) | 59.06±8.34 | World Health
Organization criteria | Plasma | miR-126
RT-qPCR | Adjusted for age,
gender, BMI and BP, FG and HbA1c, triglyceride. and LDL | (17) |
| Weale (2021) | The Republic of
South Africa | 1066 (303/763) | 46.36±15.41 | WHO criteria | Whole blood | miR-126
RT-qPCR | Included age, sex,
BMI, SBP, triglycerides, HDL-C and LDL-C. | (18) |
| Al-Kafaji
(2017) | Kingdom of
Bahrain | 90 (44/46) | 57.00±10.44 | World Health
Organization criteria | Plasma | miR-126
RT-qPCR | Multivariate
logistic regression. analysis | (31) |
| Wang (2014) | Sweden | 152 (83/69) | 55.76±5.80 | FPG ≥7.0 mmol/l
and/or a 2-h PG in the OGTT of | Plasma samples | miR-126 RT-PCR | Adjusted for sex,
age, WC, Fam-TD and sedentary lifestyle. | (32) |
| Willeit (2017) | Italy | 910 | 63±11 | 1997 American
Diabetes Association criteria | Serum samples | miR-122
RT-qPCR | Adjusted for age,
sex, socioeconomic status, smoking, physical activity, and alcohol
consumption. | (12) |
| Ezaz (2020) | USA | 132 (81/51) | 50.6 | - | Serum samples | miR-122
RT-qPCR | Univariate logistic
regression. analyses | (33) |
| Nie (2022) | China | 188 (82/106) | 64.45±6.97 | 2020 American
Diabetes Association criteria | Plasma samples | miR-122
RT-qPCR | Adjusted for age,
sex, BMI, smoking status, drinking status, regular exercise,
education and. Fam-TD | (34) |
Association of circulating miR-126 and
miR-122 with T2DM
When 7 studies involving a total of 2,570
participants (484 T2DM cases) were included in the meta-analysis
for association of miR-126 with T2DM risk (5,6,11,17,18,31,32),
the pooled result showed there was negative association between the
expression level of miR-126 and T2DM risk, but did not reach
statistical significance (OR: 0.85, 95% CI: 0.67-1.08; Fig. S1). And significant heterogeneity
was observed between these studies (I2=99.3%,
P<0.001). Besides, in order to detect potential sources of
heterogeneity between these studies, subgroup analyses (Table SIV) were performed, which revealed
that heterogeneity between these 7 studies was not attributable to
study source, study sample size, average age of study subjects, or
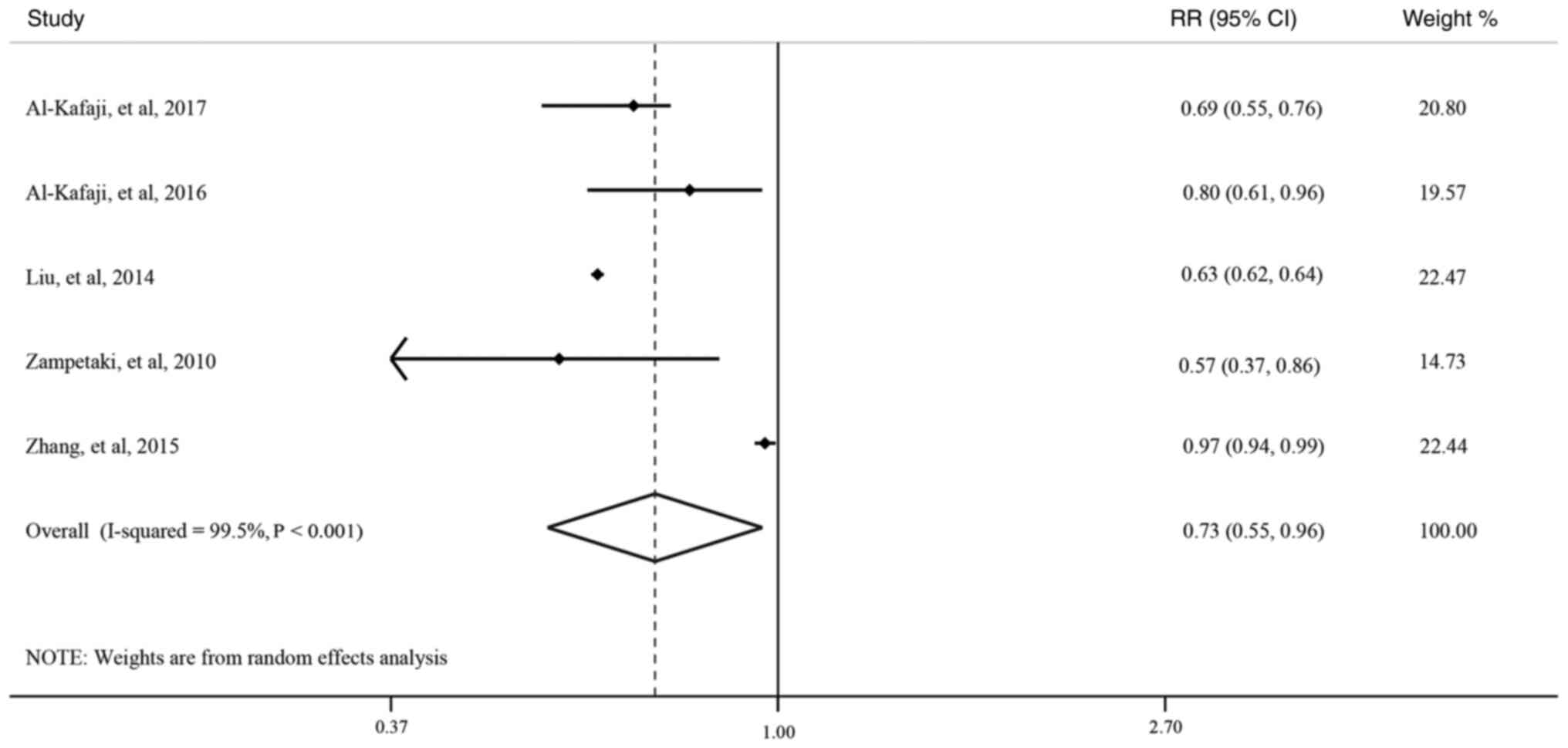
study types. However, after the exclusion of the 2 cross-sectional
studies (18,32), the pooled results of the remaining
5 analytical epidemiological studies (4 case-control studies and 1
cohort study) showed that the association of miR-126 expression
level with T2DM risk had a significant negative association
(OR=0.73, 95% CI: 0.55-0.96; Fig.
2), and the heterogeneity was decreased to 55.3% (P=0.107) in
studies with sample size more than 100 (Table SV). In addition, there was no
evidence of publication bias with either Egger's test (P=0.731,
Table SVI) or Begg's funnel plot
(P=1.000; Fig. S2). Sensitivity
analyses demonstrated that the exclusion of one study conducted in
China (11) had significant effect
on the current pooled results.
For the association of miR-122 with T2DM, there are
only 3 relevant studies to date (12,33,34).
The Bruneck study (followed up over up to 15 years) found per one
standard deviation (SD) increasement of log miR-122 was obviously
associated with higher T2DM risk in the multivariable model
(RR=1.37, 95% CI: 1.03-1.82, P=0.021) (12). Another study conducted in China
indicated that miR-122-5p was linked to increased T2DM risk after
adjustment for important covariates (OR=1.36, 95% CI: 1.14-1.62)
(34). However, the study of Ezaz
et al (33), revealed that
there was no significant association between miR-122 expression and
diabetes in patients with non-alcoholic fatty liver disease
(β=0.293, P=0.1). More future studies are needed to provide related
evidence.
Correlation between circulating
miR-126 and miR-122 and FBG level
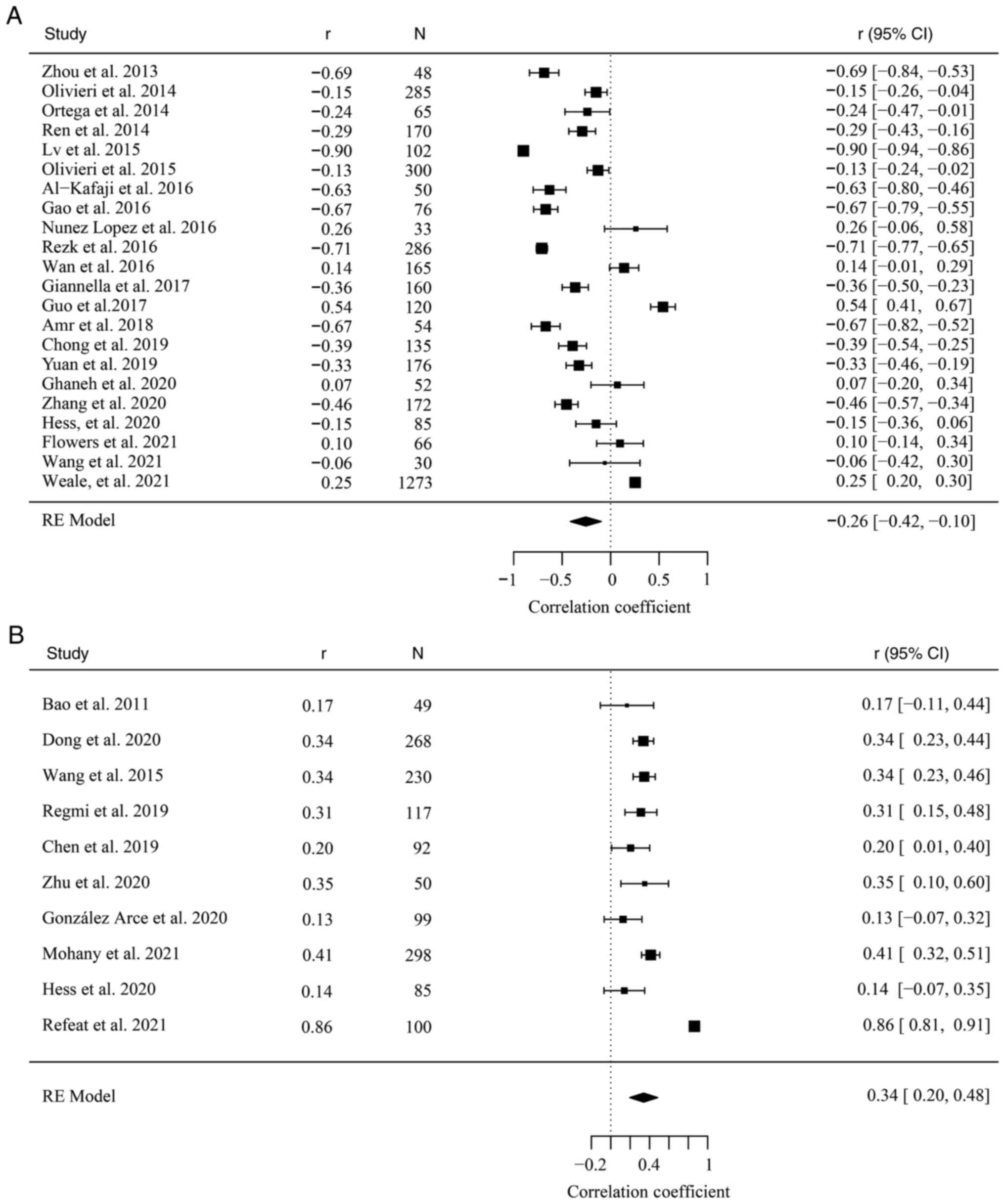
A total of 22 studies involving a total of 3,903
participants were included in the meta-analysis for correlation of
miR-126 with FBG level (4,8,17,18,35-52).
The pooled result revealed significant inverse correlation between
miR-126 expression and FBG level with the random effect model
(r=-0.26, 95% CI: -0.42, -0.10; Fig.
3A). However, significant heterogeneity was observed between
these studies (I2=97.92%, P<0.001), but there was no
evidence of publication bias with either Egger's test (P=0.067,
Table SVI) or Begg's funnel plot
(Fig. S3A). Subgroup analyses
(Table SVII) were conducted to
examine the potential sources of heterogeneity, which showed that
significant correlations were still observed in the Asian and
European studies, and the heterogeneity was decreased to 53.51%
(P=0.08) in European studies. Moreover, no heterogeneity was
observed in 2 descriptive studies or 2 experimental studies. The
heterogeneity may be attributed to different study source and study
types of included studies.
In addition, 10 studies involving a total of 1,388
participants were included in the meta-analysis for correlation of
miR-122 with FBG level (13,51,53-60),
and the pooled result demonstrated significant positive correlation
between miR-122 expression and FBG level with the random effect
model (r=0.34, 95% CI: 0.20, 0.48; Fig. 3B). However, significant
heterogeneity was observed between these studies
(I2=92.01%, P<0.001). Besides, the Begg's funnel plot
shows obvious asymmetry which suggested the presence of a potential
publication bias (Fig. S3B), and
that was also suggested by the Egger's test (P=0.001, Table SVI). Subgroup analyses (Table SVIII) indicated that significant
correlation persisted in the Asian and African studies, and no
heterogeneity was observed in studies with sample size less than
100 and moderate heterogeneity was observed in studies with
participants younger than 30 years. The heterogeneity may be
attributed to different sample size of included studies and the
average age of included subjects.
Correlation between circulating
miR-126 and miR-122 and HOMA-IR index
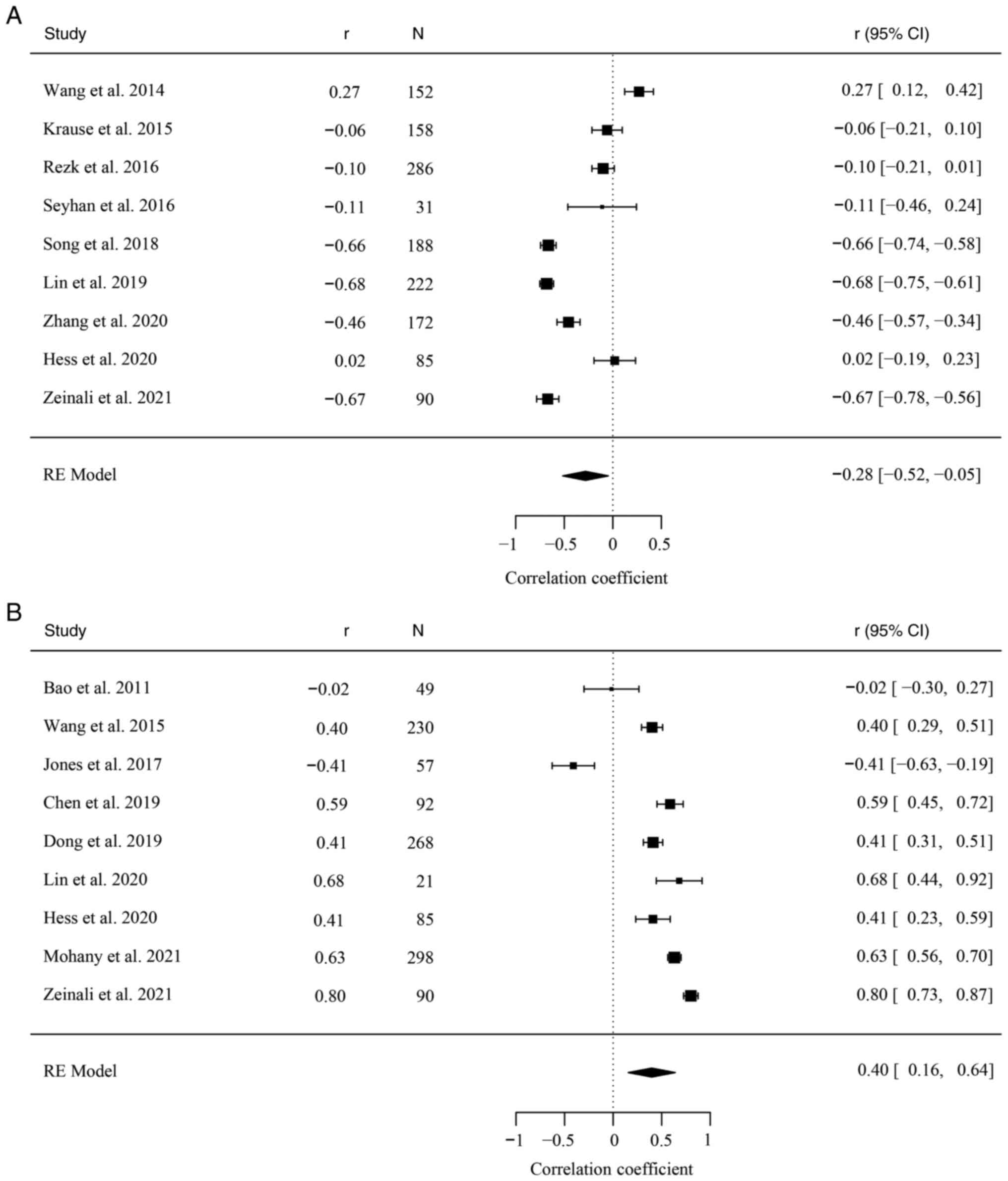
A total of 9 studies involving a total of 1,384
subjects were included in the meta-analysis for correlation of
miR-126 with HOMA-IR index (4,32,37,51,61-65).
The pooled result revealed significant inverse correlation between
miR-126 expression and HOMA-IR index with the random effect model
(r=-0.28, 95% CI: -0.52, -0.05; Fig.
4A). However, significant heterogeneity was observed between
these studies (I2=97.10%, P<0.001), and heterogeneity
was still significant when the most influential study was excluded
(32) (r=-0.35, 95% CI: -0.57,
-0.14; I2=96.22%, P<0.001). Furthermore, there was no
evidence of publication bias with the Egger's test (P=0.098;
Table SVI), and the Begg's funnel
plot suggested roughly symmetry (Fig.
S3C). On subgroup analyses (Table SIX), significant correlations
persisted in the Asian and African studies, as well as in
participants with age ≥60 years, and no heterogeneity was observed
in studies with participants aged no less than 60 years. The
heterogeneity may be due to the average age of subjects in
different included study.
A total of 1,190 subjects were involved in the 9
included studies in the meta-analysis for correlation of miR-122
expression with HOMA-IR index (9,16,51,53-56,58,65),
and positive correlation for miR-122 and HOMA-IR index was found in
the pooled result with the random effect model (r=0.40, 95% CI:
0.16, 0.64; Fig. 4B). However,
significant heterogeneity was observed between these studies
(I2=97.29%, P<0.001), and heterogeneity was still
significant when the most influential study was excluded (9) (r=0.50, 95% CI: 0.35, 0.66;
I2=93.01%, P<0.001). The Begg's funnel plot revealed
asymmetry (Fig. S3D) and there
was evidence of publication bias with the Egger's test (P=0.044,
Table SVI). On subgroup analyses
(Table SX), significant
correlations persisted in the Asian studies, studies with
participants ≥100, as well as in participants with age <30
years, but not in the Oceanian, North American, European studies
and those with sample size <100 and aged ≥30 years.
Discussion
To the best of our knowledge, original research or
reviews on relationship of miRNAs with T2DM risk are abundant, but
limited studies have systematically examined the associations of
miR-126 and miR-122 expression level with the T2DM risks. Moreover,
no study has explored the correlations between miR-126 and miR-122
expression and the FBG level or HOMA-IR index to date. Therefore,
this meta-analysis pooled the results of the existing relevant
studies and drew quantitative conclusions.
In the present study, negative association was found
between circulating miR-126 expression and the T2DM risk, and the
association was statistically significant after the exclusion of
the 2 cross-sectional studies. Regarding the relationship of
miR-126 level with T2DM risk, the results of the current available
studies were inconsistent. The study types may partly account for
the inconsistent results. For example, no statistically significant
association was identified between circulating miR-126 expression
and the T2DM risk among the pooled result from 7 studies. However,
after the exclusion of the 2 cross-sectional studies (18,32),
the pooled result were statistically significant, and the
heterogeneity was decreased in studies with sample size more than
100.
Besides, marked differences were observed in miRNA
profiles as well as T2DM prevalence across racial and ethnic groups
(66,67). For differences of the miRNA
profiles among different ethnic groups, for example, miR-146a and
miR-15 were significantly lower in subjects from Mexico compared
with that from the US (49).
Additionally, for differences in the T2DM prevalence across racial
and ethnic groups, for instance, the diagnosed diabetes prevalence
among American Indians/Alaska Natives is almost twice that of
Caucasian Americans in the United States (14.7% vs. 7.5%) (68). In Europe, compared with Caucasian
European populations, Latin American, East and Southeast Asian,
sub-Saharan African, Middle Eastern and North African, and South
Asian populations are 1.3-3.7 times as likely to develop T2DM
(69). Moreover, previous studies
have shown that significant association of higher miR-144
expression with T2D risk was observed in Swedes (OR=2.43, P=0.035),
but not in Iraqis (P=0.169) (32).
Furthermore, strongest association of miR-126-3p with FBG level was
observed in Hispanic men, and a moderate correlation was found
between miR-192 and FBG in Black women (66). Therefore, it was hypothesized that
ethnicity of the study population may account for the inconsistent
results. More studies are needed in the future to support and
verify the findings of the current meta-analysis.
Except for the negative association of miR-126
expression with T2DM risk, negative correlations were also found
between circulating miR-126 expression and FBG level as well as
HOMA-IR index (two important indicators of T2DM) in the present
meta-analysis. Some established evidence suggested similar results.
For example, the study of Zhang et al (70) showed that miR-126 expression level
was significantly reduced among the participants with medium or
high FBG compared with those with low FBG (both P<0.05).
Moreover, one previous study conducted in Italian population with
different degrees of glucose condition found that FBG level was
significantly associated with the reduced expression level of
miR-126-3p (β=-0.286; P=0.003) (47). Besides, prior studies in China
demonstrated that miR-126 expression was low in patients with newly
diagnosed T2DM, which may be involved in the development and
progression of IR and T2DM (37,63,65,71).
These findings indicated that miR-126 could be used as a biomarker
of low FBG level and HOMA-IR index, as well as the low risk of the
T2DM development. Of course, the development of T2DM may require
the intricate interactions of multiple miRNAs, genetics,
environment and lifestyle.
Meanwhile, positive correlations were observed
between miR-122 expression and FBG level as well as HOMA-IR index
in the present meta-analysis, which suggested that miR-122 could be
involved in IR and may be used as a potential biomarker of T2DM
risk. For the association of miR-122 with T2DM, relevant studies
are limited to date. Previous studies from the Bruneck Study and
China found that miR-122 was obviously associated with increased
T2DM risk (P<0.05) (12,34).
Moreover, previous studies showed that miR-122 expression level was
significantly increased in patients with T2DM when compared with
healthy control, and miR-122 was positively associated with FBG
(P<0.05) (13,57,58).
Moreover, evidence suggested that elevated circulating miR-122 was
an independent risk factor for IR in young adults (OR=3.379 for
1-SD unit increase of miR-122 level, P<0.05) (56), and correlation was also observed in
both the general children and those obese (r=0.586, P<0.001;
r=0.442, P=0.002, respectively) (54). The finding was also supported by a
previous systematic review (72).
Evidence has shown that circulating miRNAs are
packaged inside proteins or extracellular vesicles (such as
exosomes and microvesicles) to avoid being degraded by RNase
(73). Islet endothelial
progenitor cells-derived microvesicles miR-126 may sustain
revascularization and β-cell function of human pancreatic islets
(74). In addition, a previous
study indicated that lean mice treated with exosomes transfected
with obesity-associated miRNAs mimics (including miR-122) for 4
weeks were glucose intolerant and IR (75). However, there was no study
exploring the associations of plasma exosomal miR-126 and miR-122
with T2DM to date. Future studies are needed to be conducted to
assess these associations.
Previous studies demonstrated that miRNAs were
involved in multiple metabolic pathways, including insulin
signaling, adipokine expression, adipogenesis and lipid metabolism.
For the potential mechanisms of miR-126 expression with the
metabolic diseases, established epidemiological studies have
suggested some pathways (74,76-79).
On one hand, abnormal islet β cell function is the basis of
dysglycemia and IR. A previous study revealed that miR-126 carried
by microvesicles derived from islet endothelial progenitor cells
may sustain revascularization and β-cell function of human
pancreatic islets (74). On the
other hand, endothelial dysfunction commonly occurs in the earliest
stages of the diseases and is associated with T2DM (76). Moreover, previous studies have
shown that miR-126 was highly enriched in endothelial cells and
played a pivotal role in maintaining endothelial homeostasis and
vascular integrity through regulating vascular endothelial growth
factor, Angiopoietin-1 and vascular cell adhesion protein-1
expression levels (77-79).
In addition, for the pathways of miR-122 expression with the
metabolic diseases, previous study showed that miR-122 may promote
the development and progression of IR directly through inhibiting
the IGF-1R/PI3K/AKT pathway (55),
which was related to multiple physiological processes including
diseases such as diabetes. Moreover, miR-122 directly targets
protein-tyrosine phosphatase 1B and regulates the
insulin/insulin-like growth factor signaling pathway (80). Besides, miR-122 has also been
implicated in glucose homeostasis by indirect effects on
AMP-activated kinase and glucose 6-phosphatase, a key regulatory
enzyme of hepatic gluconeogenesis (81). Further studies are warranted to
explore the potential mechanism.
Several subgroup analyses were conducted to discover
the potential sources of the high heterogeneity of the included
studies. When the study types were limited to analytical
epidemiological studies, moderate heterogeneity was observed for
the association between miR-126 and T2DM risk in studies with a
sample size of 100 or more. Besides, no heterogeneity was found in
North American studies and a moderate heterogeneity was observed in
European studies for the correlation of miR-126 with FBG; no
heterogeneity was observed in North American and European studies
for the correlation of miR-122 with FBG. This may be explained by
different sources of limited study subjects and different ethnicity
or lifestyle in different countries as well as varied quality
levels of included studies. In addition, significant association
and no heterogeneity were observed in group with participants less
than 100 for the correlation of miR-122 with FBG. For average age,
heterogeneity was significantly reduced in participants aged ≥60
years for the correlation of miR-126 with HOMA-IR, which may be
explained by the fact that metabolic disease was a chronic
condition that was highly age-dependent (82). Besides, the high heterogeneity may
be explained by the quality of studies or the ethnicity (different
countries) of the study population to a certain degree. More
numbers of studies with high quality and different ethnicity are
needed to explore this association in the future.
There were several limitations to the present
meta-analyses that should be considered. Firstly, due to the
limitations of the quantity, quality and data extraction of the
papers, some studies exploring the association between individual
miRNA and T2DM were not included. Secondly, the numbers of included
analytical studies are relatively limited in the association of
miR-126 or miR-122 expression with T2DM risk. More relevant and
available analytical epidemiological studies are needed in the
future to make more definitive conclusions. Thirdly, high
heterogeneity was found due to the difference of population
demographics from different regions (such as ethnicity, lifestyle),
the varied quality of studies, different age of recruited
participants and inconsistent covariate adjustment in each study.
Fourthly, exposure-response relationship could not conduct in the
current meta-analyses due to limited eligible studies and
categories of cases. Future studies were encouraged to assess the
dose-response relationship between miR-126 and miR-122 expression
and T2DM risk as well as related metabolic parameters. Finally,
some important factors such as family history of diabetes and
dietary intake were not adjusted in some included studies, and it
may remain other unmeasured and residual confounders in original
studies.
In conclusion, the current meta-analyses suggested
that high miR-126 expression was negatively correlated to lower
T2DM development risk, FBG level and HOMA-IR index in the general
healthy population. Besides, miR-122 expression was related to
higher FBG levels and HOMA-IR index in the general healthy
population. The findings have notable clinical and public health
implications for screening and control of glucose metabolic
disorders, IR and T2DM development. However, more multicenter
studies with large sample sizes are needed to draw the firm
conclusions.
Supplementary Material
Association of microRNA-126 expression
with type 2 diabetes mellitus risk in 7 epidemiological studies (5
analytical and 2 cross-sectional studies). The pooled estimates
were obtained using a random-effects model, and the dotted lines
represent the corresponding pooled results. RR, relative
ratio.
Begg's funnel plot for the association
of microRNA-126 with type 2 diabetes mellitus risk. Asymmetry
suggested the presence of a potential publication bias.
Begg's funnel plot for the association
of miR-126 and miR-122 with FBG level and HOMA-IR index. (A) A
total of 22 studies for the association of miR-126 with FBG level.
(B) A total of 10 studies for the association of miR-122 with FBG
level. (C) A total of 9 studies for the association of miR-126 with
HOMA-IR index. (D) A total of 9 studies for the association of
miR-122 with HOMA-IR index. Asymmetry suggested the presence of a
potential publication bias. miR, microRNA; FBG, fasting blood
glucose; HOMA-IR, homeostasis model assessment of insulin
resistance.
Systematic literature review of
databases: search terms and strategy.
Quality assessment of the included
studies according to Newcastle-Ottawa Scale scores.
Characteristics of the included
studies for correlation of miR-126 and miR-122 with FBG level and
HOMA-IR index.
Subgroup analyses to examine the
association of miR-126 with T2DM.
Subgroup analyses to examine the
association of miR-126 with T2DM in 5 analytical studies.
Results of the Egger’s tests for the
association of miR-126 and miR-122 with T2DM, FBG level and HOMA-IR
index.
Subgroup analyses to examine the
correlation of microRNAR-126 with fasting blood glucose level.
Subgroup analyses to examine the
correlation of microRNA-122 with fasting blood glucose level.
Subgroup analyses to examine the
correlation of microRNA-126 with homeostasis model assessment of
insulin resistance index.
Subgroup analyses to examine the
correlation of miR-122 with homeostasis model assessment of insulin
resistance index.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Foundation of
National Key Program of Research and Development of China (grant
no. 2016YFC0900803), the China Postdoctoral Science Foundation
(grant no. 2020M672297), the National Natural Science Foundation of
China (grant nos. 82003543, 81573243 and 81602925), the Henan
Natural Science Foundation of China (grant no. 182300410293), the
Science and Technology Foundation for Innovation Talent of Henan
Province (grant no. 164100510021), the Science and Technology
Innovation Talents Support Plan of Henan Province Colleges and
Universities (grant no. 14HASTIT035) and the High-level Personnel
Special Support Project of Zhengzhou University (grant no.
ZDGD13001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and XL conceived and designed the present study.
YH and YL carried out literature search, study selection, quality
assessment and data extraction. ZhiZ, PL, and YZ confirm the
authenticity of all the raw data. YH, YZ and LN performed
statistical analysis, and were assisted by ZhiZ and PL. YH, XL and
YL wrote the manuscript. JH, WH, ZM, ZheZ and CW interpreted the
data and revised the manuscript. The final version of the
publication was written by YH, XL, YL and YZ. XL had primary
responsibility for final content. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2017 Disease and Injury Incidence and
Prevalence Collaborators. Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: A systematic
analysis for the global burden of disease study 2017. Lancet.
392:1789–1858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the international diabetes federation diabetes atlas, 9(th)
edition. Diabetes Res Clin Pract. 157(107843)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rezk NA, Sabbah NA and Saad MS: Role of
MicroRNA 126 in screening, diagnosis, and prognosis of diabetic
patients in Egypt. IUBMB Life. 68:452–458. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Zhang T, Li L, Shang Q, Lv C, Wang C and
Su B: Circulating miR-126 is a potential biomarker to predict the
onset of type 2 diabetes mellitus in susceptible individuals.
Biochem Biophys Res Commun. 463:60–63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zampetaki A, Kiechl S, Drozdov I, Willeit
P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E,
et al: Plasma microRNA profiling reveals loss of endothelial
miR-126 and other microRNAs in type 2 diabetes. Circ Res.
107:810–817. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guay C and Regazzi R: Circulating
microRNAs as novel biomarkers for diabetes mellitus. Nat Rev
Endocrinol. 9:513–521. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nunez Lopez YO, Garufi G and Seyhan AA:
Altered levels of circulating cytokines and microRNAs in lean and
obese individuals with prediabetes and type 2 diabetes. Mol
Biosyst. 13:106–121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jones A, Danielson KM, Benton MC, Ziegler
O, Shah R, Stubbs RS, Das S and Macartney-Coxson D: miRNA
signatures of insulin resistance in obesity. Obesity (Silver
Spring). 25:1734–1744. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mao Y, Mohan R, Zhang S and Tang X:
MicroRNAs as pharmacological targets in diabetes. Pharmacol Res.
75:37–47. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu Y, Gao G, Yang C, Zhou K, Shen B,
Liang H and Jiang X: The role of circulating microRNA-126
(miR-126): A novel biomarker for screening prediabetes and newly
diagnosed type 2 diabetes mellitus. Int J Mol Sci. 15:10567–10577.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Willeit P, Skroblin P, Moschen AR, Yin X,
Kaudewitz D, Zampetaki A, Barwari T, Whitehead M, Ramírez CM,
Goedeke L, et al: Circulating MicroRNA-122 is associated with the
risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes.
66:347–357. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Regmi A, Liu G, Zhong X, Hu S, Ma R, Gou
L, Zafar MI and Chen L: Evaluation of serum microRNAs in patients
with diabetic kidney disease: A nested case-controlled study and
bioinformatics analysis. Med Sci Monit. 25:1699–1708.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tao H, Wang MM, Zhang M, Zhang SP, Wang
CH, Yuan WJ, Sun T, He LJ and Hu QK: MiR-126 suppresses the
glucose-stimulated proliferation via IRS-2 in INS-1 β cells. PLoS
One. 11(e0149954)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu Y, Li Y, Chen C, Zhu S, Guo M, Liu S,
Zheng J, Qin N and Xu L: Identification of miR-126 knockdown mouse
and the change of blood glucose. Zhong Nan Da Xue Xue Bao Yi Xue
Ban. 40:12–17. 2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
16
|
Lin H, Tas E, Børsheim E and Mercer KE:
Circulating miRNA signatures associated with insulin resistance in
adolescents with obesity. Diabetes Metab Syndr Obes. 13:4929–4939.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Kafaji G, Al-Mahroos G, Al-Muhtaresh
HA, Skrypnyk C, Sabry MA and Ramadan AR: Decreased expression of
circulating microRNA-126 in patients with type 2 diabetic
nephropathy: A potential blood-based biomarker. Exp Ther Med.
12:815–822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Weale CJ, Matshazi DM, Davids SFG,
Raghubeer S, Erasmus RT, Kengne AP, Davison GM and Matsha TE:
MicroRNAs-1299, -126-3p and -30e-3p as potential diagnostic
biomarkers for prediabetes. Diagnostics (Basel).
11(949)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. Ottawa Health Research Institute, Ottawa, ON,
1999.
|
|
20
|
Liu F, Chen G, Huo W, Wang C, Liu S, Li N,
Mao S, Hou Y, Lu Y and Xiang H: Associations between long-term
exposure to ambient air pollution and risk of type 2 diabetes
mellitus: A systematic review and meta-analysis. Environ Pollut.
252:1235–1245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hamling J, Lee P, Weitkunat R and Ambühl
M: Facilitating meta-analyses by deriving relative effect and
precision estimates for alternative comparisons from a set of
estimates presented by exposure level or disease category. Stat
Med. 27:954–970. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials revisited. Contemp Clin Trials. 45:139–145.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cheung MW and Cheung SF: Random-effects
models for meta-analytic structural equation modeling: Review,
issues, and illustrations. Res Synth Methods. 7:140–155.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nakagawa S and Cuthill IC: Effect size,
confidence interval and statistical significance: A practical guide
for biologists. Biol Rev Camb Philos Soc. 82:591–605.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei X, Zhang Y, Tao H, Tang H, Chen H and
Zeng X: Meta analysis of correlation coefficient data using meta
package and metafor package of R software. Chin J Evid-Based Med.
15:855–860. 2015.(In Chinese).
|
|
26
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558.
2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kulinskaya E and Dollinger MB: Commentary
on ‘Misunderstandings about Q and ‘Cochran's Q test’ in meta
analysis’. Stat Med. 35:501–502. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu X, Luo X, Liu Y, Sun X, Han C, Zhang
L, Wang B, Ren Y, Zhao Y, Zhang D, et al: Resting heart rate and
risk of metabolic syndrome in adults: A dose-response meta-analysis
of observational studies. Acta Diabetol. 54:223–235.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994.PubMed/NCBI
|
|
31
|
Al-Kafaji G, Al-Mahroos G, Al-Muhtaresh
HA, Sabry MA, Razzak RA and Salem AH: Circulating
endothelium-enriched microRNA-126 as a potential biomarker for
coronary artery disease in type 2 diabetes mellitus patients.
Biomarkers. 22:268–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Sundquist J, Zöller B, Memon AA,
Palmér K, Sundquist K and Bennet L: Determination of 14 circulating
microRNAs in Swedes and Iraqis with and without diabetes mellitus
type 2. PLoS One. 9(e86792)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ezaz G, Trivedi HD, Connelly MA, Filozof
C, Howard K, Parrish ML, Kim M, Herman MA, Nasser I, Afdhal NH, et
al: Differential associations of circulating MicroRNAs with
pathogenic factors in NAFLD. Hepatol Commun. 4:670–680.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nie H, Hu H, Li Z, Wang R, He J, Li P, Li
W, Cheng X, An J, Zhang Z, et al: Associations of plasma metal
levels with type 2 diabetes and the mediating effects of microRNAs.
Environ Pollut. 292(118452)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yuan JF: Serum miRNA expression and
clinical value in patients with type 2 diabetes mellitus
complicated with coronary atherosclerosis (Master thesis). Jiangsu
University, R541.4; R587.1, 2019 (In Chinese).
|
|
36
|
Wan SJ, Wang C, Wang J, Niu DM, Zhang CN
and Wang JJ: Study on serum levels of miR-16, miR-126 and miR-221
and their clinical significance in type 2 diabetes patients with or
without microvascular complications. J Modern Laboratory Medicine.
31:9–13. 2016.
|
|
37
|
Zhang YD, Gao QC, Zhao R, Pu YF, Li SH and
Wei JF: Correlation between serum miR-126, VEGF levels and insulin
resistance in patients with newly diagnosed Type 2 Diabetes
Mellitus. Int J Lab Med. 41:935–938. 2020.(In Chinese).
|
|
38
|
Zhou N, Liang J, Teng F, Zou CY, Yang MQ,
Qi L and Song HD: Effect of abnormal glucose metabolism on
expression of serum microRNA in patients with coronary heart
disease. Chin J Geriatr Heart Brain Vessel Dis. 15:14–17. 2013.(In
Chinese).
|
|
39
|
Wang L, Zeng N, Xie WP, Jiang YL, Li Z,
Tang LZ, Long CD and Wu BL: Effect of intensive blood glucose and
blood pressure control on exosomal effect microRNA in patients with
diabetic foot. Chin Youjiang Med J. 49:97–102. 2021.(In
Chinese).
|
|
40
|
Lv YB and Pan ZQ: The impact of abnormal
glucose metabolism on mir-126 in progenitor cells to patients with
coronary heart disease. J Clin Cardiol (China). 31:1061–1064.
2015.(In Chinese).
|
|
41
|
Gao W, Liu YZ, Wang LJ, Fang BH and Lei
NY: Expression of miR-126 in PBMCs of diabetic retinopathy
patients. Chin J Pract Ophthalmol. 34:322–325. 2016.(In
Chinese).
|
|
42
|
Chong XJ, Yu QY and Yang LX: Expression
and clinical significance of miR-126 and sVCAM-1 in patients with
type 2 diabetic nephropathy. Hebei Med J. 41:334–337+342. 2019.(In
Chinese).
|
|
43
|
Ren Y, Huang T and Shi X: The change of
expression level of circulating miRNA-126 in patients with type 2
diabetes and its relative factors. Chin J Diabet. 22:633–636.
2014.(In Chinese).
|
|
44
|
Ortega FJ, Mercader JM, Moreno-Navarrete
JM, Rovira O, Guerra E, Esteve E, Xifra G, Martinez C, Ricart W,
Rieusset J, et al: Profiling of circulating microRNAs reveals
common microRNAs linked to type 2 diabetes that change with insulin
sensitization. Diabetes Care. 37:1375–1383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Olivieri F, Spazzafumo L, Bonafè M,
Recchioni R, Prattichizzo F, Marcheselli F, Micolucci L, Mensà E,
Giuliani A, Santini G, et al: MiR-21-5p and miR-126a-3p levels in
plasma and circulating angiogenic cells: Relationship with type 2
diabetes complications. Oncotarget. 6:35372–35382. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Olivieri F, Bonafè M, Spazzafumo L, Gobbi
M, Prattichizzo F, Recchioni R, Marcheselli F, La Sala L, Galeazzi
R, Rippo MR, et al: Age- and glycemia-related miR-126-3p levels in
plasma and endothelial cells. Aging (Albany NY). 6:771–787.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Giannella A, Radu CM, Franco L, Campello
E, Simioni P, Avogaro A, de Kreutzenberg SV and Ceolotto G:
Circulating levels and characterization of microparticles in
patients with different degrees of glucose tolerance. Cardiovasc
Diabetol. 16(118)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ghaneh T, Zeinali F, Babini H, Astaraki S
and Hassan-Zadeh V: An increase in the expression of circulating
miR30d-5p and miR126-3p is associated with intermediate
hyperglycaemia in Iranian population. Arch Physiol Biochem.
1-8(1839105)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Flowers E, Ramirez-Mares JD,
Velazquez-Villafana M, Rangel-Salazar R, Sucher A, Kanaya AM,
Aouizerat BE and de la Vega Monroy ML: Circulating microRNAs
associated with prediabetes and geographic location in Latinos. Int
J Diabetes Dev Ctries. 41:570–578. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Amr KS, Abdelmawgoud H, Ali ZY, Shehata S
and Raslan HM: Potential value of circulating microRNA-126 and
microRNA-210 as biomarkers for type 2 diabetes with coronary artery
disease. Br J Biomed Sci. 75:82–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hess AL, Larsen LH, Udesen PB, Sanz Y,
Larsen TM and Dalgaard LT: Levels of circulating miR-122 are
associated with weight loss and metabolic syndrome. Obesity (Silver
Spring). 28:493–501. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guo XL, Chen Y, Ma WG, Wang QY, Du Y and
Li SS: Clinical significance of miR-126 as marker in the diagnosis
of type 2 diabetic kidney disease. J Clin Nephrol. 17:361–365.
2017.(In Chinese).
|
|
53
|
Bao W: Heme oxygenase-1, plasma ferritin
levels and type 2 diabetes. PhD dissertation, Huazhong University
of Science and Technology, R587.1, 2011 (In Chinese).
|
|
54
|
Chen Q, Zhang YD, Wu SN, Chen YX, Liu XJ
and Wei HY: Correlation between serum microRNA-122 and insulin
resistance in obese children. Zhongguo Dang Dai Er Ke Za Zhi.
21:910–914. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
55
|
Dong L, Hou X, Liu F, Tao H, Zhang Y, Zhao
H and Song G: Regulation of insulin resistance by targeting the
insulin-like growth factor 1 receptor with microRNA-122-5p in
hepatic cells. Cell Biol Int. 43:553–564. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang R, Hong J, Cao Y, Shi J, Gu W, Ning
G, Zhang Y and Wang W: Elevated circulating microRNA-122 is
associated with obesity and insulin resistance in young adults. Eur
J Endocrinol. 172:291–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhu JH, Wang JL, Feng ZX, Chen AL, Huang J
and Du WS: Expression and clinical significance of miR-122 in
patients with diabetes mellitus and chronic hepatitis B. Lab Med
Clin Pract. 17:582–585. 2020.
|
|
58
|
Mohany KM, Al Rugaie O, Al-Wutayd O and
Al-Nafeesah A: Investigation of the levels of circulating miR-29a,
miR-122, sestrin 2 and inflammatory markers in obese children
with/without type 2 diabetes: A case control study. BMC Endocr
Disord. 21(152)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
González-Arce LM, Lara-Riegos JC,
Pérez-Mendoza GJ, Rubí-Castellanos R, Vega-Marcín M,
Valencia-Pacheco G, Torres-Romero JC and González-Herrera L: High
expression levels of circulating microRNA-122 and microRNA-222 are
associated with obesity in children with Mayan ethnicity. Am J Hum
Biol. 33(e23540)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Refeat MM, Hassan NAM, Ahmad IH, Mostafa
ERM and Amr KS: Correlation of circulating miRNA-33a and miRNA-122
with lipid metabolism among Egyptian patients with metabolic
syndrome. J Genet Eng Biotechnol. 19(147)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Krause BJ, Carrasco-Wong I, Dominguez A,
Arnaiz P, Farías M, Barja S, Mardones F and Casanello P: Micro-RNAs
Let7e and 126 in plasma as markers of metabolic dysfunction in 10
to 12 years old children. PLoS One. 10(e0128140)2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Seyhan AA, Nunez Lopez YO, Xie H, Yi F,
Mathews C, Pasarica M and Pratley RE: Pancreas-enriched miRNAs are
altered in the circulation of subjects with diabetes: a pilot
cross-sectional study. Sci Rep. 6(31479)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Song B, Fu L and Liu J: Association
between serum miR-126 and non-alcoholic fatty liver disease in
patients with newly diagnosed type 2 diabetes mellitus. Chin J
Diabet. 26:812–816. 2018.(In Chinese).
|
|
64
|
Lin MP and Wang JJ: Expression of
microRNA-126 and microRNA-155 in patients of coronary heart disease
combined with impaired glucose tolerance and intervention of
acarbose. J Clin Intern Med. 36:108–112. 2019.(In Chinese).
|
|
65
|
Zeinali F, Zarch SMA, Jahan-Mihan A,
Kalantar SM, Mehrjardi MYV, Fallahzadeh H, Hosseinzadeh M,
Rahmanian M and Mozaffari-Khosravi H: Circulating microRNA-122,
microRNA-126-3p and microRNA-146a are associated with inflammation
in patients with pre-diabetes and type 2 diabetes mellitus: A case
control study. PLoS One. 16(e0251697)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Flowers E, Kanaya AM, Zhang L and
Aouizerat BE: The role of racial and ethnic factors in microRNA
expression and risk for type 2 diabetes. Front Genet.
13(853633)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Spanakis EK and Golden SH: Race/ethnic
difference in diabetes and diabetic complications. Curr Diab Rep.
13:814–23. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Centers for Disease Control and Prevention
(CDC): National Diabetes Statistics Report. CDC Atlanta, GA, 2020.
https://www.cdc.gov/diabetes/data/statistics-report/.
Accessed May 3, 2022.
|
|
69
|
Meeks KA, Freitas-Da-Silva D, Adeyemo A,
Beune EJ, Modesti PA, Stronks K, Zafarmand MH and Agyemang C:
Disparities in type 2 diabetes prevalence among ethnic minority
groups resident in Europe: A systematic review and meta-analysis.
Intern Emerg Med. 11:327–340. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhang T, Lv C, Li L, Chen S, Liu S, Wang C
and Su B: Plasma miR-126 is a potential biomarker for early
prediction of type 2 diabetes mellitus in susceptible individuals.
Biomed Res Int. 2013(761617)2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ryu HS, Park SY, Ma D, Zhang J and Lee W:
The induction of microRNA targeting IRS-1 is involved in the
development of insulin resistance under conditions of mitochondrial
dysfunction in hepatocytes. PLoS One. 6(e17343)2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Oses M, Sanchez JM, Portillo MP, Aguilera
CM and Labayen I: Circulating miRNAs as biomarkers of obesity and
obesity-associated comorbidities in children and adolescents: A
systematic review. Nutrients. 11(2890)2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Heishima K, Ichikawa Y, Yoshida K, Iwasaki
R, Sakai H, Nakagawa T, Tanaka Y, Hoshino Y, Okamura Y, Murakami M,
et al: Circulating microRNA-214 and -126 as potential biomarkers
for canine neoplastic disease. Sci Rep. 7(2301)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Cantaluppi V, Biancone L, Figliolini F,
Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R,
Salizzoni M, Tetta C, et al: Microvesicles derived from endothelial
progenitor cells enhance neoangiogenesis of human pancreatic
islets. Cell Transplant. 21:1305–1320. 2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Castaño C, Kalko S, Novials A and Párrizas
M: Obesity-associated exosomal miRNAs modulate glucose and lipid
metabolism in mice. Proc Natl Acad Sci USA. 115:12158–12163.
2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Jamwal S and Sharma S: Vascular
endothelium dysfunction: A conservative target in metabolic
disorders. Inflamm Res. 67:391–405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wang Y and Yan H: MicroRNA-126 contributes
to niaspan treatment induced vascular restoration after diabetic
retinopathy. Sci Rep. 6(26909)2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Nigi L, Grieco GE, Ventriglia G, Brusco N,
Mancarella F, Formichi C, Dotta F and Sebastiani G: MicroRNAs as
regulators of insulin signaling: Research updates and potential
therapeutic perspectives in type 2 diabetes. Int J Mol Sci.
19(3705)2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Willeit P, Skroblin P, Kiechl S,
Fernández-Hernando C and Mayr M: Liver microRNAs: potential
mediators and biomarkers for metabolic and cardiovascular disease?
Eur Heart J. 37:3260–3266. 2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Dedov I, Shestakova M, Benedetti MM, Simon
D, Pakhomov I and Galstyan G: Prevalence of type 2 diabetes
mellitus (T2DM) in the adult Russian population (NATION study).
Diabetes Res Clin Pract. 115:90–95. 2016.PubMed/NCBI View Article : Google Scholar
|