Introduction
Photoaging is the phenomenon of skin aging or
accelerated aging due to prolonged sun exposure (1). Photoaging from intense ultraviolet
(UV) rays is more likely to make the skin rough, sagging, wrinkled,
hyperpigmented and even precancerous, benign or malignant (2). In addition to natural aging caused by
the body's natural cell metabolism, photoaging is the second
largest cause of skin aging. UV radiation can affect the formation
of type I collagen, resulting in a relative increase in type III
collagen (3,4), which eventually leads to a decrease
in mature collagen bundles and skin laxity and wrinkles. Components
of the skin's dermal matrix, such as aminoglycans and
proteoglycans, are also implicated in photoaging (5). Sunlight exposure can cleave
aminopolysaccharides and increase their solubility, thereby
affecting their structure and function (6). UV radiation in sunlight does not
directly damage the dermal matrix components. ROS are naturally
generated in various biochemical reactions within organelles such
as the endoplasmic reticulum, mitochondria and peroxisomes. Under
normal physiological conditions, ROS are related to life activities
such as cell signal transduction, cell cycle and cell
proliferation. Abnormally, ROS levels in cells are elevated after
being subjected to UV exposure compared with healthy cells. Due to
the high activity of ROS, cells generate oxidative stress, leading
to dysfunction (7). It is
generally believed that UV irradiation can cause mtDNA damage,
generate oxygen free radicals ROS, further lead to the oxidation of
proteins in the dermal matrix and cause cellular inflammation
(7,8). In turn, the matrix components are
slowly dissolved. Additionally, the connection between UV exposure
and senescence has been reported; that excessive UV exposure leads
more expression of aging-related genes and generation of
β-galactosidase in cells, indicating senescence induced by
excessive UV exposure (9-11).
Centella asiatica is a perennial herb of the
Umbelliferae family (12).
A traditional Chinese medicinal plant with a long history,
Centella asiatica is used externally to remove scars and
reduce inflammation (13). The
main active components of Centella asiatica are asiaticoside,
madecassoside, asiatic acid and madecassolic acid, although their
proportions in the active components has yet to be reported. Most
of the active ingredients in Centella asiatica are
triterpenoids (12). Among them,
asiaticoside is the main active ingredient in Centella
asiatica because of its rich pharmacological activity and broad
clinical effects. According to scientific reports, asiaticoside
demonstrates potential capability to delay skin aging and reduce
wrinkles clinically (14-16).
Therefore, asiaticoside has good application prospects and
potential for development. Asiaticoside may be able to promote
wound healing, scar removal and other skin disease treatments
(17-19).
However, the anti-photoaging effects of asiaticoside on skin and
the potential mechanisms of action remain to be elucidated.
In the present study, the therapeutic effects of
asiaticoside on skin photoaging and possible molecular mechanisms
were investigated by analyzing the modulation of appearance, cell
structure, oxidative stress, pigmentation index and related
proteins in a cellular photoaging model.
Materials and methods
Materials
HaCat cells were purchased from Procell Life Science
& Technology Co., Ltd. (cat. no. CL-0090) and verified by using
short tandem repeat (STR) profiling. Asiaticoside was obtained from
MilliporeSigma. Primers used in this study were synthesized by
Sangon Biotech Co., Ltd. All the antibodies were purchased from
Cell Signaling Technology, Inc.: TGF-β (56E4) Rabbit mAb cat. no.
3709 (1:1,000); Smad2 (D43B4) XP Rabbit mAb cat. no. 5339
(1:1,000); Smad3 (C67H9) Rabbit mAb cat. no. 9523 (1:1,000), p53
(DO-7) Mouse mAb cat. no. 48818 (1:1,000), p21 Waf1/Cip1 (12D1)
Rabbit mAb cat. no. 2947 (1:1,000), MMP-9 (D6O3H) XP Rabbit mAb
cat. no. 13667 and GAPDH (D16H11) XP® Rabbit mAb cat.
no. 5174 (1:2,000).
Cell culture
A human keratinocyte cell line (HaCat cell) was used
in all the experiments. HaCat cells were cultured by using
Dulbecco's modified eagle medium (DMEM) with 10% (v/v) fetal bovine
serum containing 1% (v/v) penicillin and streptomycin (Thermo
Fisher Scientific, Inc.).
Cell counting kit-8 assay (CCK-8)
The viability of HaCat cells after UV exposure and
incubation with asiaticoside in low, medium and high doses was
determined by using CCK assay (Beijing Solarbio Science &
Technology Co., Ltd.) following the manufacturer's protocol
(20,21). In brief, 2x104 cells
were suspended in 200 µl DMEM medium and then seeded in a 96-well
transparent-bottom plate and cultured in an incubator at 37˚C for
24 h. The culture medium in the wells was replaced by fresh medium
before administration of UV exposure (UV-B, 100 mJ/cm2,
λmax=312 nm) and incubation with asiaticoside in
different doses at 37˚C (low concentration: 1 mg/l, medium
concentration: 10 mg/l and high concentration 30 mg/l). At 24, 48
and 72 h after UV exposure, 20 µl CCK solution was added to the
wells and cell viability of various groups were observed by a
microplate reader at 450 nm after treatment of CCK solution with
cells for 1 h. The absorbance of untreated cells (wildtype group,
WT) served as a negative control in this experiment.
Morphological investigation
After UV treatment, the genome of some HaCat cells
was disrupted leading to the activation of stress systems, which in
turn caused changes in cell morphology. The present study
investigated the protective effect of pretreatment of asiaticoside
on cells subjected to UV stimulation by observing cell morphology.
Control HaCat cells (1x105) were seeded to 6-wells plate
and cultured at 37˚C overnight. The culture medium was replaced and
cells were subjected to a UV exposure for 30 min, while
administration of asiaticoside with various doses was implemented 2
h before UV treatment. At 24 h following UV expose, morphological
data of different groups were acquired by using an optical imaging
system (CX41; Olympus Corporation).
Detection of activity of
β-galactosidase
To calculate the activity of senescence-related
β-galactosidase induced by UV exposure and its activity in HaCat
cells following asiaticoside administration in various doses,
senescence β-galactosidase staining kit (Beyotime Institute of
Biotechnology) was used to count the number of β-galactosidase
positive cells. HaCat cells (1x105) were seeded into a
6-well plate and cultured at 37˚C overnight. Following UV exposure
and incubation of asiaticoside in various doses, medium was removed
and cells were rinsed by fresh PBS buffer and then fixed using the
fixative from the kit for 15 min at room temperature. Cells were
cleaned by PBS for three times following fixation and underwent
incubation with staining buffer for overnight at 37˚C atmosphere.
Parafilm was used to cover the plate to prevent evaporation of the
buffer. Images were captured using an optical microscope at x200
magnification (CX41; Olympus Corporation) and data was analyzed by
using ImageJ software (v1.8.0, National Institutes of Health).
Investigation on reactive oxygen
species (ROS)
ROS can be induced when cells encounter stress such
as exposure to heat and UV. To clarify the protective capability of
asiaticoside to UV-induced skin damage, flow cytometry (Beckman
Coulter, Inc.) was used to identify the ratio of ROS positive cells
in the groups treated with asiaticoside in various concentrations.
Cells were stained by 2',7'-dichlorofluorescin diacetate (DCFH-DA)
probe for facilitating flow cytometric cell sorting assay. Briefly,
1x105 HaCat cells were seeded to 6-wells plate and
cultured at 37˚C overnight and then 1 ml DCFH-DA dye which was
diluted to 10 µM/l as a working concentration by using culture
medium without FBS was added to cells following administration of
UV and incubation of asiaticoside for 20 min at 37˚C. Following
staining, cells were rinsed by pre-warmed (37˚C) culture medium for
three times to remove free DCFH-DA dye. ROS-positive cells were
identified and determined by observing the intensity of
fluorescence in the FITC channel and HaCat cells without any
treatments were used as a negative control. FlowJo software
(v10.5.3, FlowJo LLC) and ImageJ (v1.8.0, National Institutes of
Health) were employed to analyze and visualize data.
Determination of superoxide dismutase
(SOD)
SOD is a metalloenzyme widely found in living
organisms and is an important scavenger of oxygen radicals,
catalyzing the dismutation of superoxide anions to produce
H2O2 and O2. SOD is not only a
superoxide anion scavenger but also a major
H2O2 producing enzyme, which has an important
role in the biological antioxidant system. To investigate the
protection capacities of asiaticoside in various doses to
UV-exposed HaCat cells, SOD activity assay kit (Beijing Solarbio
Science & Technology Co., Ltd.) was used following
manufacturer's protocol. In short, 1x105 HaCat cells
were seeded into 6-wells plate and cultured at 37˚C overnight.
Cells were subjected to UV exposure for 30 min, while cells in
asiaticoside treated groups were preincubated with asiaticoside in
different doses for 2 h before UV exposure. Following
administration of asiaticoside and UV, cells were rinsed by
pre-chilled (4˚C) PBS buffer and disassociated by EDTA-trypsin
buffer. Disassociated cells were centrifuged at 800 x g, and the
supernatant was removed before sediments were resuspended by 100 µl
of lysis buffer from the kit. A sonicator was used to split cells
at 4˚C (40% power, 10 sec stopping time after 10 sec of sonication
for 3 times) to release intracellular SOD. Harvested lysates were
subjected to a centrifugation at ~7,100 x g, 4˚C for 10 min and
supernatants mixed with detection buffers. Following water bath at
37˚C for 30 min, mixtures were transferred to 96-wells plate for
data acquisition by using a microplate reader under 562 nm.
TdT-mediated dUTP Nick-End Labeling
(TUNEL) staining
TUNEL staining was performed by using One-step TUNEL
staining kit (Beyotime Institute of Biotechnology) followed
manufacturer's instructions to observe the protective ability of
asiaticoside to UV-induced apoptosis (22). HaCat cells (1x104) were
seeded to confocal dishes and cultured in an incubator for 37˚C
overnight. HaCat cells were fixed by 4% paraformaldehyde at room
temperature for 1 h following UV radiation and administration of
asiaticoside in different concentrations. Next, 50 µl of prepared
staining buffer was added to confocal dish for 1 h at room
temperature to label cells in early apoptosis. Then, DAPI was used
to stain the nucleus following a proper washing procedure. Finally,
TUNEL staining result of HaCat cells was observed and images
acquired by using a confocal microscope (Carl Zeiss AG).
RNA sequencing (RNAseq) analysis
RNAseq was employed to identify and enrich the mRNAs
with great expression in the WT group, UV exposure group and
asiaticoside treatment following UV exposure. The experiment and
data analysis were carried out at Novogene Biotech Co., Ltd. In
brief, RNAs of all the samples were extracted and purified to build
libraries for following sequencing. After the library was
qualified, the different libraries were pooled according to the
effective concentration and the target amount of data from the
machine, then sequenced by the Illumina NovaSeq 6000 (Illumina,
Inc.).
Index of the reference genome was built and
paired-end clean reads were aligned to the reference genome by
using Hisat2 (v2.0.5, http://daehwankimlab.github.io/hisat2/main/). Gene
Ontology (GO) enrichment analysis of differentially expressed genes
was implemented by the clusterProfiler R pack (http://www.R-project.org/). In addition, correlation
of samples in different group was analyzed by using R pack
(https://cran.r-project.org/web/packages/correlation/).
The analytic tool for Gene Set Enrichment Analysis (GSEA) was from
the Broad Institute (http://www.broadinstitute.org/gsea/index.jsp).
Reverse transcription-quantitative
(RT-q) PCR
RNA was extracted from the cells following UV
exposure and appropriate treatment of asiaticoside and the
expression of indicated genes was detected by RT-qPCR following
reverse transcription (23). In
brief, total RNA was extracted from 1x105 HaCat cells by
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
converted to cDNA by PrimeScript RT reagent kit (Takara Bio, Inc.)
according to the manufacturer's instructions. qPCR was carried out
in QuantStudio 6 Flex RT-PCR System (Thermo Fisher Scientific,
Inc.). The reaction conditions were as follows: 95˚C for 10 min; 40
cycles of 95˚C for 15 sec, 60˚C for 30 sec; followed by 72˚C for 5
min. The quantitation values for each target genes were expressed
as 2-∆∆Cq (24). PCR
primers used to amplify the target genes were as follows: Myosin
regulatory light chain interacting protein (MYLIP) Forward:
AAACAACCAGAGCCCTTCACAC, Reverse: CTCCTCCTCGCAGCACACC; MYC
proto-oncogene (MYC) Forward: CTCCACACATCAGCACAACTACG. Reverse:
GTTCGCCTCTTGACATTCTCCTC; centromere protein F (CENPF) Forward:
GGAGTTACAGCAAGCCAAGAATATG, Reverse: TCTGACTCGCCTGGAACGC;
serum/glucocorticoid regulated kinase 1 (SGK1) Forward:
GAACACAACAGCACAACATCCAC, Reverse: AGGCACCACCAGTCCACAG; phytochrome
interacting factor (PIF) Forward: GGCAGGTGTTCAGATGAGGTG, Reverse:
TGAAGCCGCCTCTCGTTGG; DNA topoisomerase II alpha (TOP2A) Forward:
GGGCACCAGCACATCAAAGG, Reverse: GCAGCATCATCTTCAGGACCAG; TGF-β1
Forward: GGCCAGATCCTGTCCAAGC, Reverse GTGGGTTTCCACCATTAGCAC; Smad2
Forward CGTCCATCTTGCCATTCACG; Reverse CTCAAGCTCATCTAATCGTCCTG;
Smad3 Forward TGGACGCAGGTTCTCCAAAC; Reverse CCGGCTCGCAGTAGGTAAC;
P53 Forward CAGCACATGACGGAGGTTGT, Reverse TCATCCAAATACTCCACACGC.
GAPDH was used as housekeeping gene, Forward GGAGCGAGATCCCTCCAAAAT,
Reverse: GGCTGTTGTCATACTTCTCATGG. qPCR data was collected from
three independent experiments.
Western blotting
HaCat cells were harvested after 5 days, exposed to
UV for 30 min in every 12 h and continually treated by asiaticoside
with various concentration. Collected cells were lysed by RIPA
lysis buffer containing protease and phosphatase inhibitors on the
ice bath. After 30 min, lysates were transferred to EP tubes for a
centrifugation at ~15,500 x g at 4˚C for 10 min and the supernatant
was collected for immunoblotting assay. A BCA kit was used to
calculate concentration for samples to achieve normalization of
sample concentrations. The same amount (30 µg) of protein was
subjected to SDS-PAGE with a 10% SDS-PAGE gel, and then transferred
to polyvinylidene fluoride (PVDF) membrane by using a semi-dry
transfer system (Bio-Rad Laboratories, Inc.). PVDF membranes were
blocked with 5% BSA-Tris Buffered Saline with 0.05% Tween-20 (TBST)
buffer at room temperature for 1 h. Primary antibodies were used to
incubate the membrane overnight at 4˚C and HRP coupling secondary
antibody was used to incubate the membrane for 2 h at room
temperature following an appropriate washing procedure, three times
using TBST. The visualization of corresponding proteins was
achieved by using a ECL chemiluminescence detection system and
intensities of florescence were analyzed by ImageJ (v1.8.0,
National Institutes of Health).
Statistical analysis
Data were collected from three independent samples
unless mentioned otherwise and was expressed as means ± standard
deviation. Differences were identified using Dunnett's multiple
comparison test following one-way analysis of variance (ANOVA) and
performed using GraphPad Prism 7 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protective ability of asiaticoside to
damage by UV
UV exposure can break chemical bonds in the DNA,
which may cause misalignment, inversion, deletion or duplication of
bases in the DNA as it is reassembled, thus altering the genetic
information and eventually leading to cell death. To clarify the
protective ability of asiaticoside in UV-damaged cells, CCK-8 assay
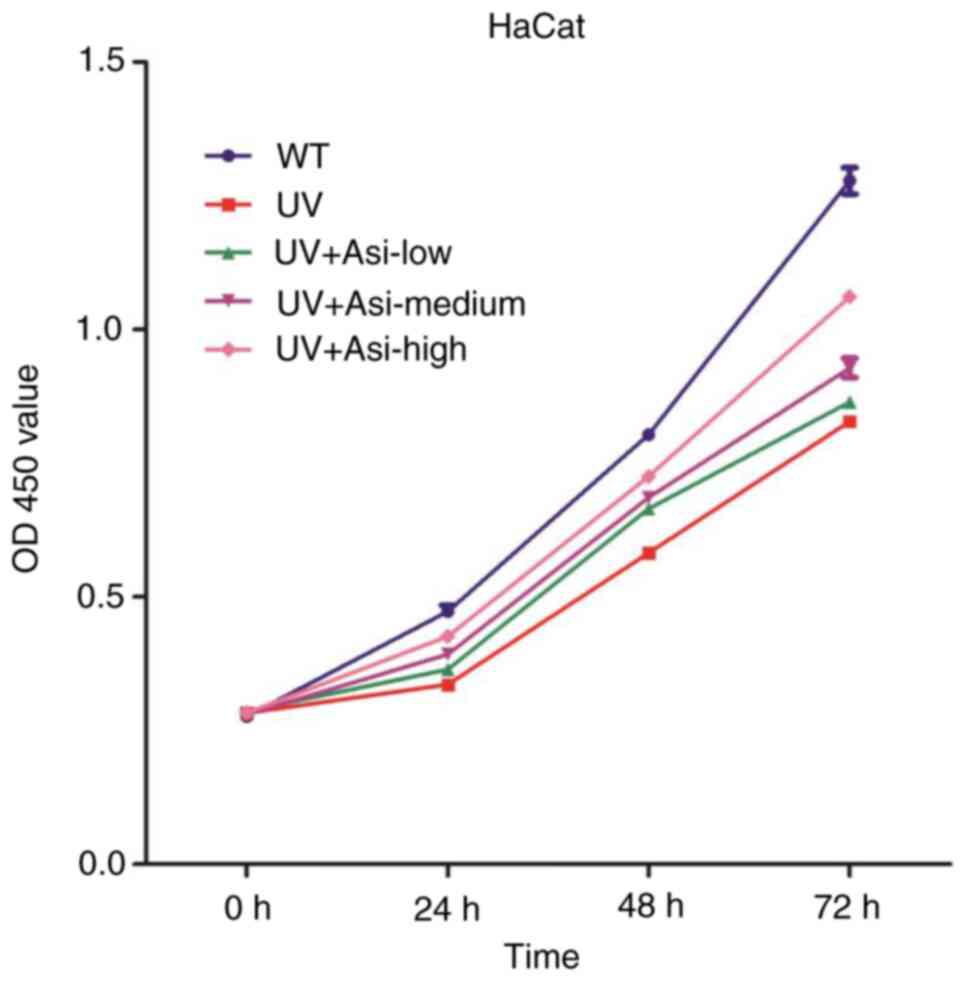
was used to check the cell viability in every 24 h for 3 days.
Viability of cells in the UV exposure group was
impaired which compared with WT group at all of three timepoints.
Treatment of asiaticoside with low-, medium- and high
concentrations achieved improvement of viability at 24, 48 and 72
h. High dose of asiaticoside following UV exposure shown the best
therapeutic result among three kinds of treatment, while low
concentration of asiaticoside had limited function on improvement
of viability (Fig. 1). This data
indicated that asiaticoside is able to protect cell viability from
UV exposure and its ability to protect was concentration
dependent.
Effect of treatment of asiaticoside on
morphology of UV-damaged HaCat cells
It is well-known that excessive radiation of UV can
induce cell apoptosis in cells. During apoptosis, the cell membrane
is unable to maintain normal physiological functions and cytochrome
C in mitochondria is released into the cytoplasm, activating the
classical downstream cysteinyl aspartate specific proteinase
(Caspase)-3 cell death pathway (25). The morphology of cells will be
affected significantly during apoptotic process. Thus, optical
microscopy was employed to identify the variations of WT group and
asiaticoside treated groups to UV exposure.
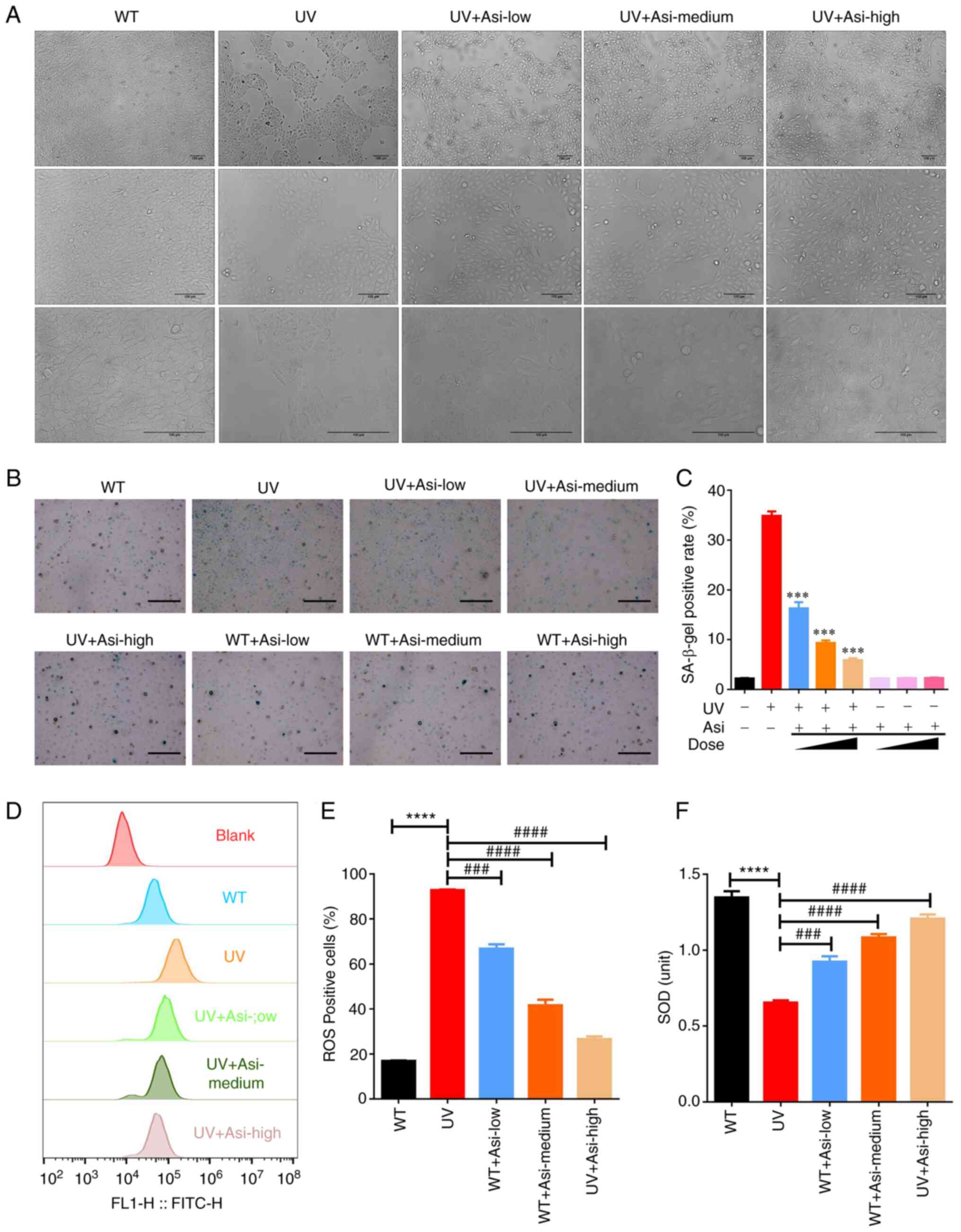
Similar to the result of CCK-8 assay, morphological
results illustrated that a number of cells died and that the area
of confluence was much smaller compared with wildtype group
following UV radiation. However, asiaticoside was able to
ameliorate these effects and maintain more cell colonies even at a
low concentration by preincubation for 2 h before UV exposure. With
increasing concentrations of asiaticoside, the majority of cells
survived radiation and formed more colonies (Fig. 2A). Although damaged cells treated
with a high dose of asiaticoside did not regain as full confluence
as the WT group, their condition greatly improved compared with UV
treated group.
Asiaticoside inhibits activity of
β-galactosidase following UV exposure
The release of β-galactosidase is related to cell
senescence has been widely reported (9,10).
As a main risk factor for photoaging of skin, UV can initiate the
release of β-galactosidase in cells, resulting in activation of p16
protein and its correspondingly signaling pathway, leading to cell
senescence. Thus, detecting activity of β-galactosidase in cells
could testify the protective capability of asiaticoside to
UV-induced β-galactosidase release.
By counting the number of positive cells under the
microscope, the results clearly proved that the UV exposure
promoted the production of β-galactosidase in cells and the ratio
of positive cells increased from 2.2 to 34.8% in the WT group
(Fig. 2B and C). Meanwhile, incubation with
asiaticoside could effectively attenuate the activity of
β-galactosidase and the positive ratios of asiaticoside treated
groups ranges from 16.9% in the low concentration group, 9.3% in
the medium concentration group and 5.9% in the high concentration
group. Treatment with asiaticoside at various concentrations in
control cells did not cause a significant increase of activity of
β-galactosidase, implying the advantage of asiaticoside in its
biosafety to healthy tissues and cells.
Asiaticoside attenuates UV-induced ROS
release
UV exposure generates ROS, which are involved in
body signal transduction and cellular defense mechanisms. Excessive
ROS production leads to the activation of inflammation-related
pathways and the release of inflammatory cytokines (26). The stimulation of cells by chronic
inflammation increases the incidence of cancer (27). As a well-established carcinogenic
factor, UV is a strong health threat to the public. In the present
study, flow cytometric cell sorting was employed to investigate
effect of asiaticoside incubation on ROS generation to UV-exposure
cells.
Comparing fluorescence intensity of ROS-positive
cells in UV treated group with WT group, intracellular ROS was
significantly increased following UV exposure, with the ratio of
ROS-positive cells from 16.8-92.8% (Fig. 2D and E). With the treatment of asiaticoside,
fluorescence intensity of generated ROS was attenuated in the three
groups, achieving 66.7% positive cells in low concentration group,
41.6% positive cells in medium concentration group and 26.5%
positive cells in high concentration group. This result confirmed
that asiaticoside was a strong inhibitor to generation of
UV-induced ROS.
Ability of asiaticoside to recover
activity of SOD
SOD is a natural scavenger of superoxide radicals in
the body, where it can convert harmful superoxide radicals into
hydrogen peroxide, which is eventually harmlessly turned into water
through the action of catalase and peroxidase. Due to its ability
of eliminating superoxide radicals, it could resist cell senescence
induced by UV exposure. SOD activities were investigated by using a
SOD activity assay kit to calculate effect of asiaticoside
incubation on activity of SOD to cells damaged by UV radiation.
As the results showed, activity of SOD was impaired
by UV radiation following UV exposure compared with WT group
(Fig. 2F). Dramatically,
incubation of asiaticoside with various concentrations achieved
successfully rescue of reduction of SOD activity in cells. Along
with the increasing concentration of asiaticoside, SOD activity was
improved compared with UV damaged cells and treatment of high dose
of asiaticoside gained the best therapeutic result among groups
treated by three doses following UV radiation.
Asiaticoside attenuates cellular
apoptosis induced by UV radiation
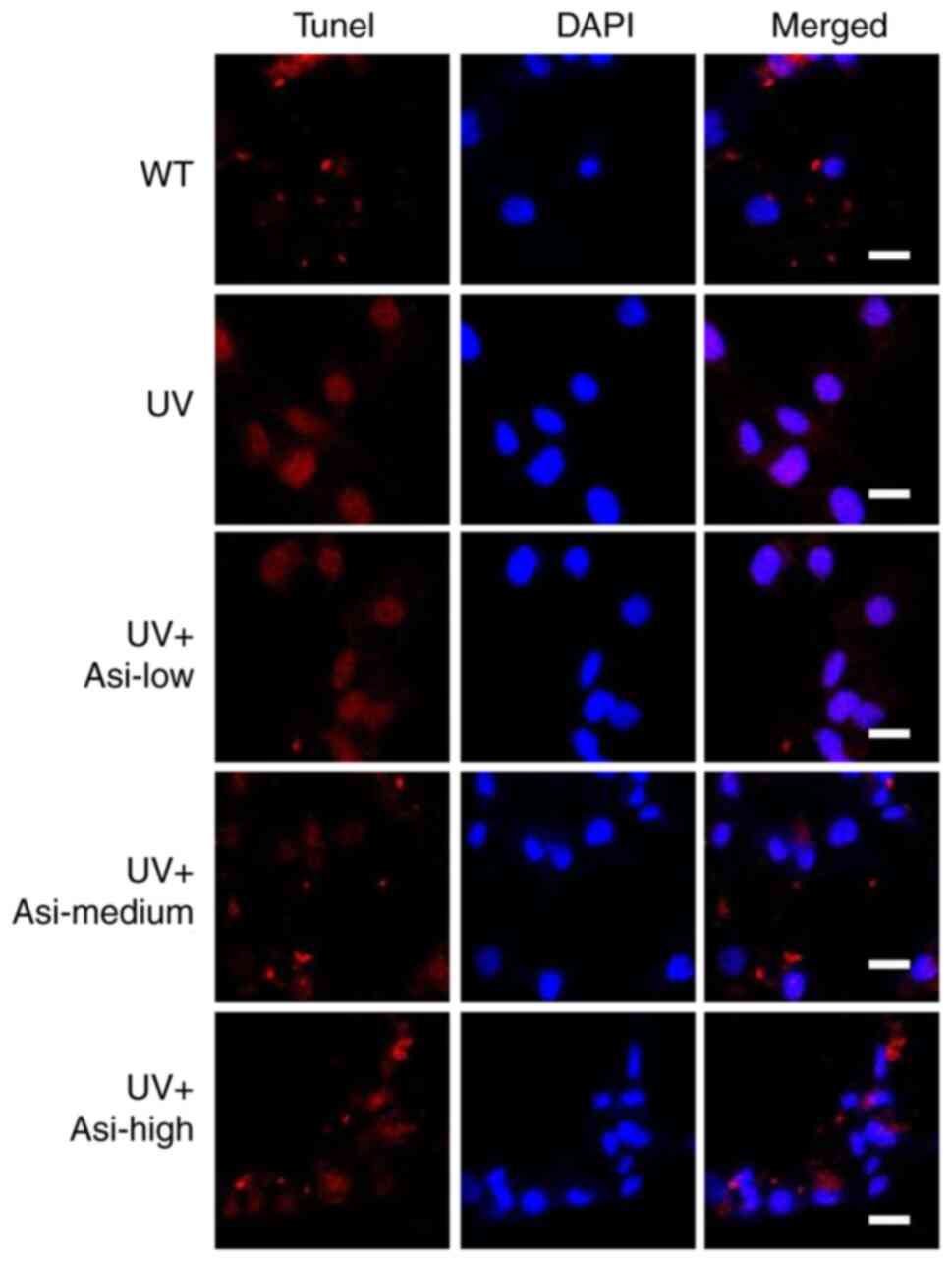
Due to UV damage to genomic structure, damaged cells
initiate apoptosis-related signaling while the broken genome can be
recognized by specific probes and show fluorescent signals. In the
UV group, the nucleus region exhibited a strong signal, indicating
that the cells were severely damaged; however, the irradiated cells
treated with asiaticoside showed a clear difference from the UV
group, with the fluorescence signal being attenuated, demonstrating
the protective effect of asiaticoside on UV-induced injury
(Fig. 3). Notably, the number of
cells undergoing apoptosis decreased with increasing asiaticoside
concentrations.
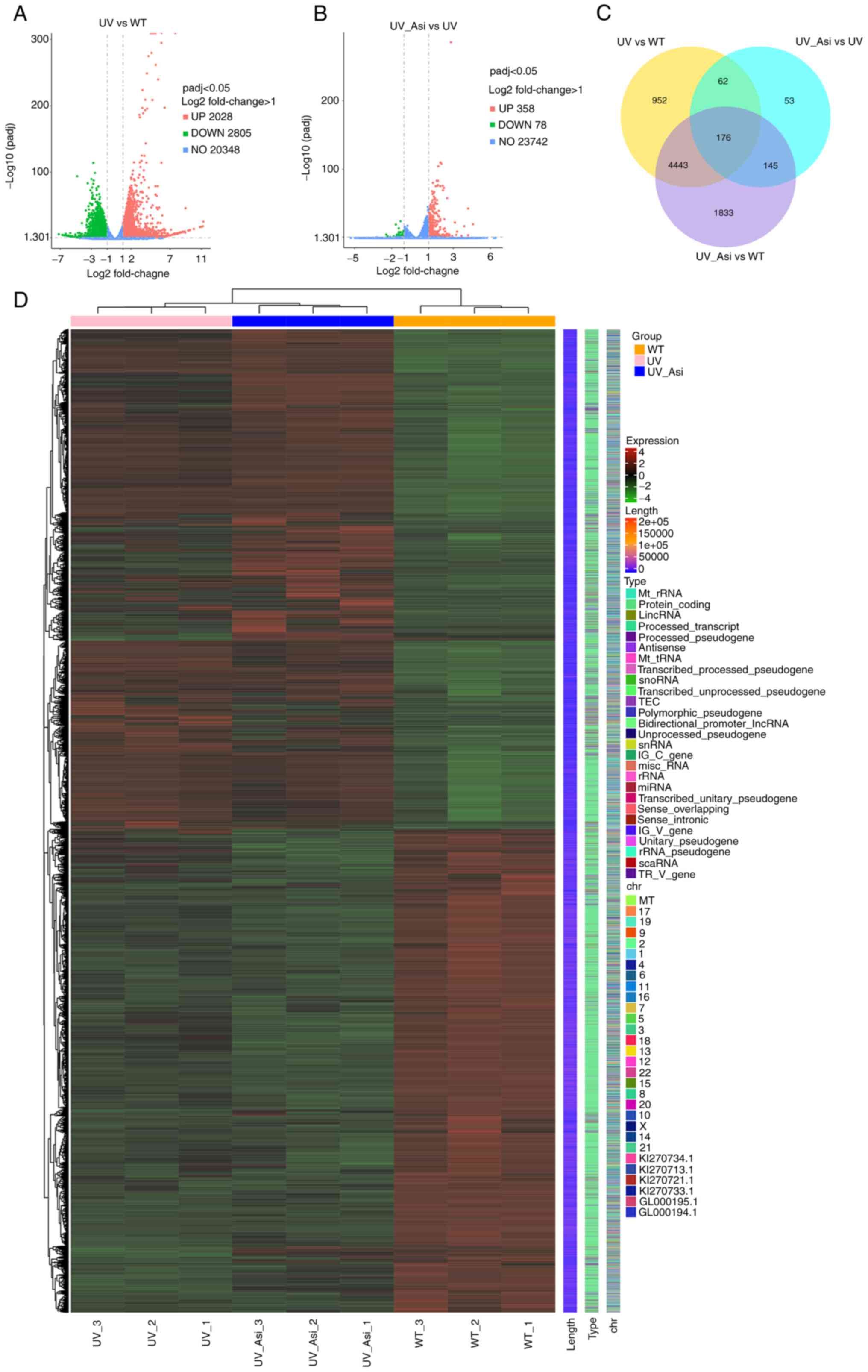
Validation of correlation of samples
and visualization of enriched mRNAs
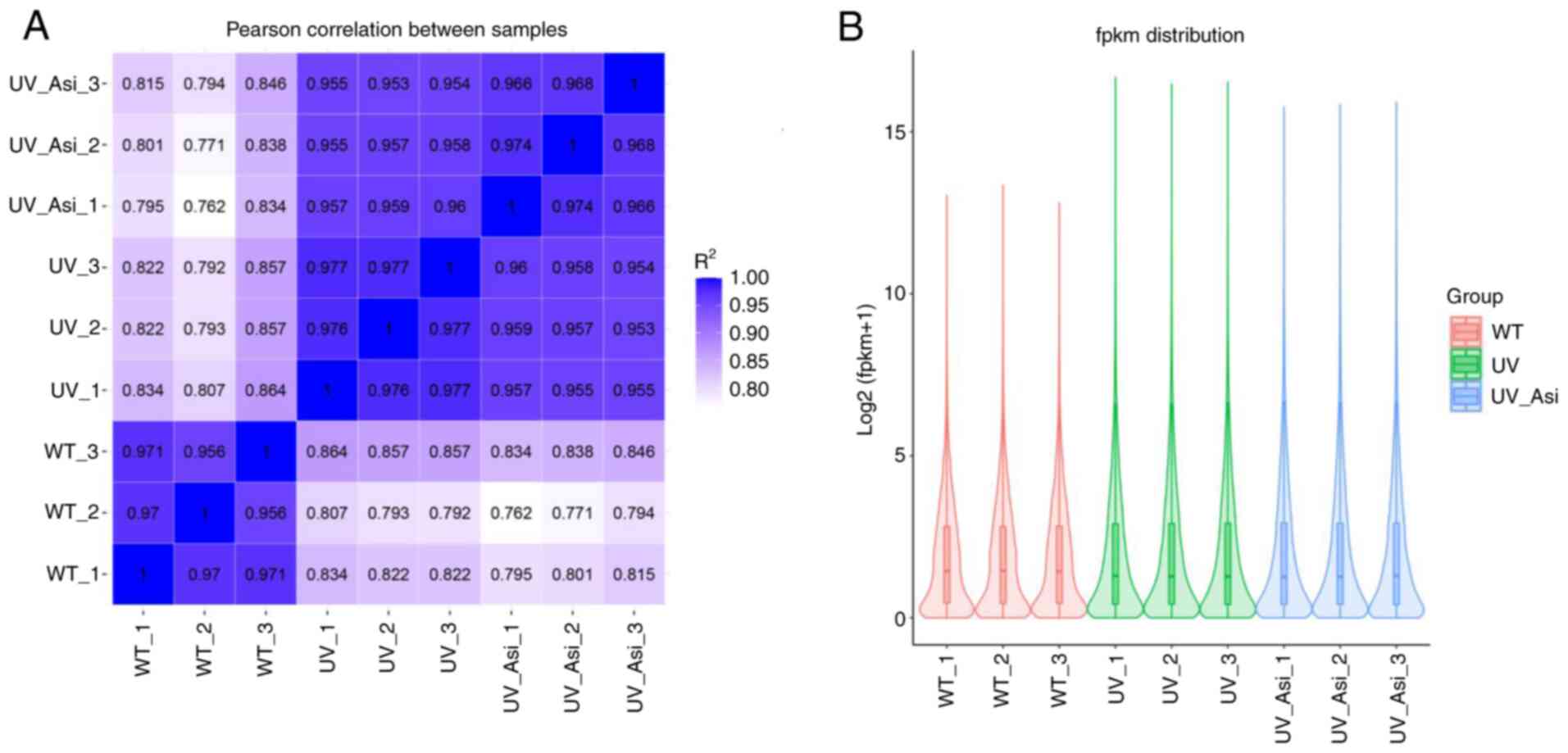
The clustering results of the correlation showed
that the samples in each group were significantly different from
the other groups, indicating the confidence of the sequencing
results (Fig. 4A). In RNA-Seq
analysis, standardization of the number of read counts of genes or
transcripts using Fragments Per Kilobase Million (FPKM) is an
important step for sample analysis to eliminate inter-sample
variation. FPKM results showed the difference in expression of
partial genes occurring following UV exposure and treatment with
asiaticoside compared with the WT group (Fig. 4B). Following visualization of
enriched mRNAs, genes with significant variation were demonstrated.
There were 2,805 downregulated and 2,828 upregulated genes in the
WT group, while there were 78 downregulated and 358 upregulated
genes with treatment of asiaticoside following UV radiation
compared with UV group (Fig. 5A
and B). By categorizing these
differential genes, the number of differential genes between three
groups were shown in the form of a Venn diagram and top ranked
genes based on abundance were expressed in the form of heatmap
(Fig. 5C and D).
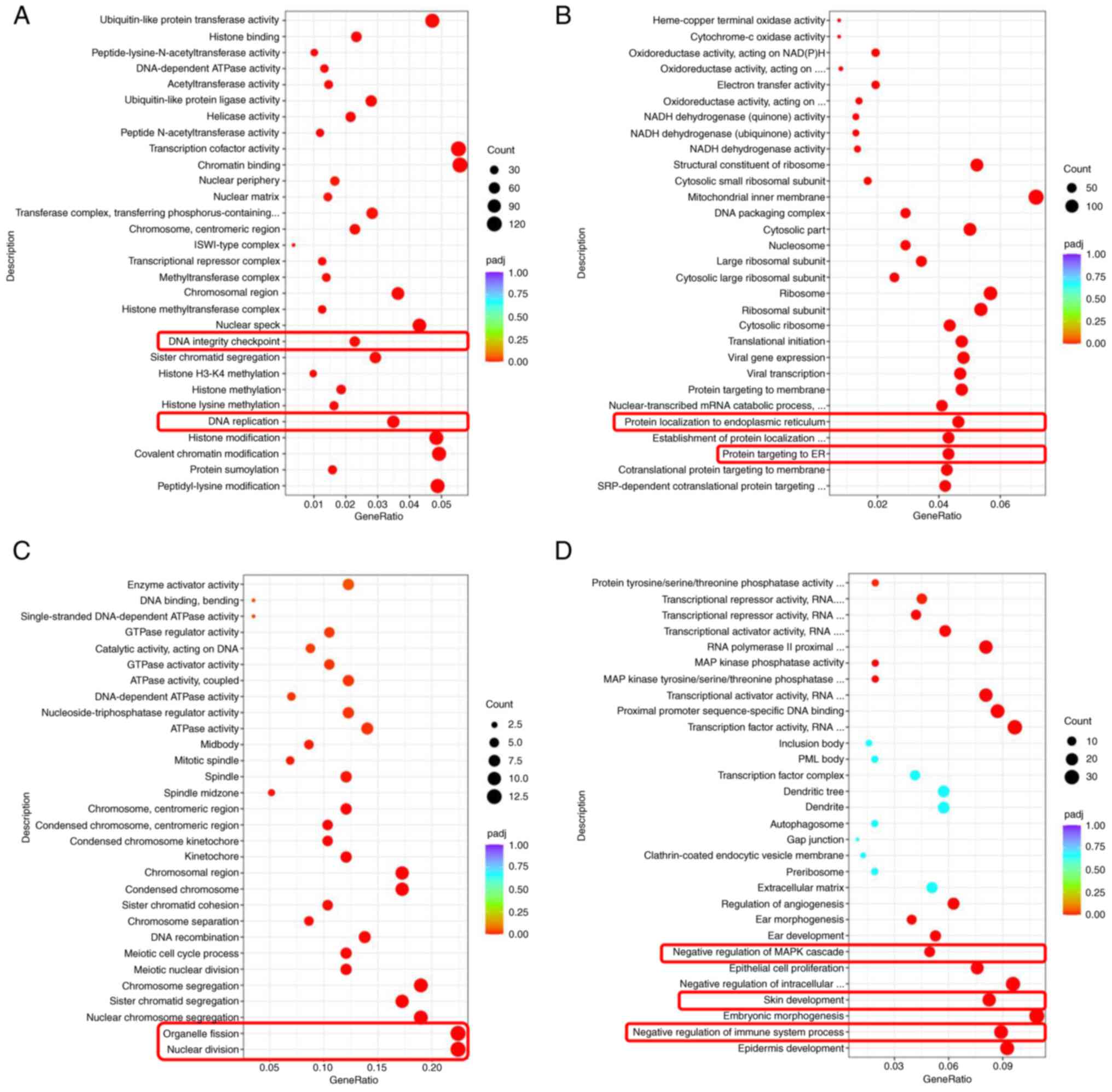
GO enrichment analysis
By using GO analysis, the up- or downregulated vital
activities caused by differential genes were clustered according to
frequency. Following UV radiation, DNA replication and integrity
checkpoints were suppressed, but protein targeting and localization
to endoplasmic reticulum (ER) were upregulated in HaCat cells,
indicating cells were unable to maintain normal genomic integrity
and initiate a stress response through ER (Fig. 6A and B). With the treatment of asiaticoside,
cell fission was inhibited and upregulation of negative regulation
of MAPK cascade was observed (Fig.
6C and D). This result implied
that asiaticoside could regulate cell cycle and control MAPK
pathway in a negative way.
mRNA expressions of UV exposure and
treatment of asiaticoside in HaCat cells
Following intense external stimulation, gene
transcription is regulated in order to maintain normal cellular
homeostasis and in response to transduction of signaling pathways
(1,7). For quantifying transcriptional level
of photoaging-related genes, RT-qPCR was used to determine gene
variation in HaCat cells following UV exposure and treatment with
asiaticoside.
Following UV exposure for 30 min, cells exhibited
significantly suppression of gene transcription to MYLIP, MYC and
SGK1, while inhibition of three genes were induced by UV could be
eased by incubation with asiaticoside (Fig. 7A, B and D).
By contrast, increased transcriptional levels of CENPF, PIF and
TOP2A were downregulated by treatment of asiaticoside, indicating
an effective capability of asiaticoside to anti-photoaging in HaCat
cells (Fig. 7C, E and F).
Since functions of MYC and SGK1 are related to the cell growth,
inflammation and apoptosis, asiaticoside and CENPF, PIF and TOP2A
are related to genomic function, showing that asiaticoside was
capable of regulating multiple cell activities and preventing
damage to the genome from UV exposure. Furthermore, transcriptional
levels of TGF-β1, Smad2, Smad3 and P53 have been determined to
instigate regulatory pattens of asiaticoside in cell growth factor
pathway (28,29) (Fig.
7G-J). As the data suggested, increased expression of afore
mentioned genes was inhibited by administration of asiaticoside at
a high concentration following UV exposure, indicating the
potential of asiaticoside to modulate signaling transduction of
cell growth factor pathway.
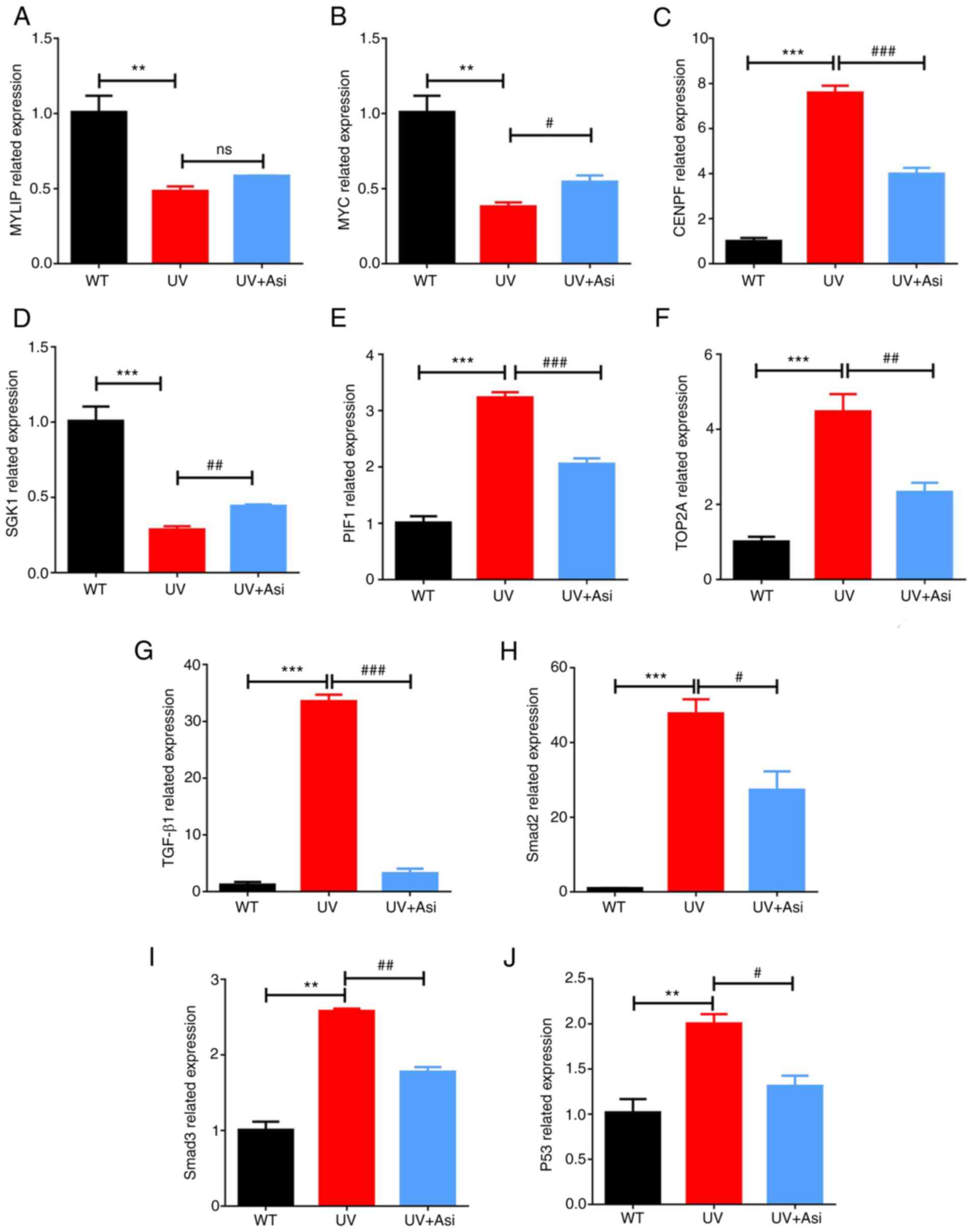 | Figure 7Transcriptional levels of (A) MYLIP;
(B) MYC; (C) CENPF; (D) SGK1; (E) PIF, (F) TOP2A, (G) TGF-β1, (H)
Smad2, (I) Smad3 and (J) P53 mRNA were calculated by RT-qPCR.
*significance of comparison between UV with WT group;
#significance of comparison between treatment of
asiaticoside in various doses with UV group. **P<0.01
and ***P<0.001; #P<0.05,
##P<0.01 and ###P<0.001. MYLIP, myosin
regulatory light chain interacting protein; MYC, MYC
proto-oncogene; CENPF, centromere protein F; SGK1,
serum/glucocorticoid regulated kinase 1; PIF, phytochrome
interacting factor; TOP2A, DNA topoisomerase II alpha. |
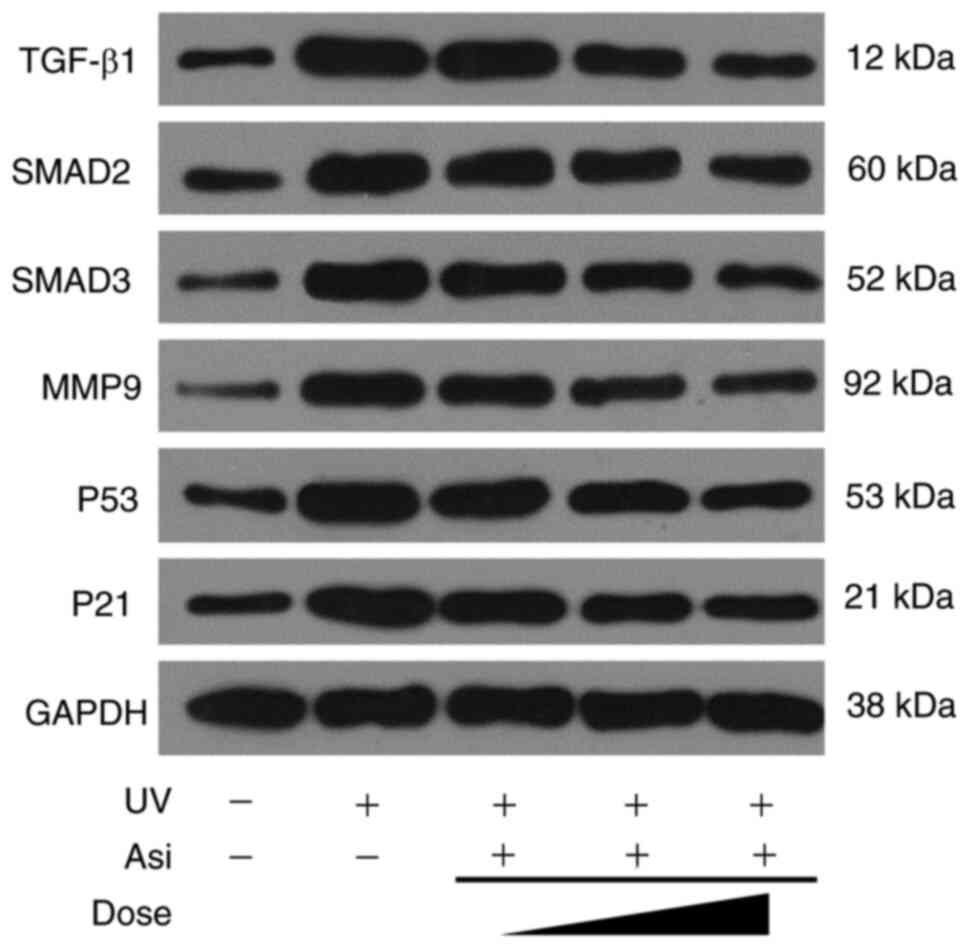
Regulation of Asiaticoside on cell
signaling pathways
As the RNAseq results suggested, asiaticoside
inhibited TGF-β1 family members following incubation with HaCat
cells. However, the detailed signaling transduction of TGF-β1/Smad
pathway remains to be elucidated. Therefore, western blotting was
implemented to clarify the regulation of asiaticoside to
TGF-β1/Smad pathway to understand its potential mechanism.
Cells underwent a stress response following UV
radiation to generate large amounts of TGF-β1, which enters the
cell via the TGF receptor (TGFR) and phosphorylated downstream
proteins Smad2/3 into the nucleus to regulate gene transcription.
However, asiaticoside was able to attenuate ROS generated in
response to oxidative stress and upregulated SOD activity,
resulting in a decrease in intracellular TGF-β1 expression
(Fig. 8). The intensity of
regulation was proportional to the concentration of asiaticoside
treatment and high concentrations of asiaticoside effectively
prevented ROS production, leaving TGF-β1 expression at normal
levels, which in turn affected the amount of downstream Smad2/3
proteins. Meanwhile, the expression of inflammatory protein MM9 was
inhibited by asiaticoside, confirming the existence of a reduced
amount of ROS in asiaticoside-treated cells. At same time,
upregulated expression of cell cycle related proteins p53 and p21,
which respond to DNA damage induced by UV, was significantly
suppressed by incubation with asiaticoside. Due to p53 and p21
being able to stop cell cycle resulting in cell senescence and such
signaling transduction could be inhibited by asiaticoside, the
result of western blotting proved that asiaticoside was capable to
attenuate upregulation of TGF-β1, Smad2 and Smad3 and to reverse
cell senescence.
Discussion
Aging, also known as senescence, is an inevitable
stage in the process of biological life activities. It usually
refers to the gradual process of functional and qualitative decline
of the organism over time as the organism matures under normal
conditions (30). Therefore, aging
is not a disease, but a physiological phenomenon. Skin aging
includes natural aging and photoaging. Photoaging is the damage to
the skin caused by prolonged exposure to sunlight, characterized by
rough skin, thickening, sagging, deep and coarse wrinkles and
localized hyperpigmentation or telangiectasia. Among UV, UVA and
UVB are mainly involved in the pathogenic process of photoaging
(31). UVB irradiation can cause
skin erythema and delayed pigmentation, destroy the skin's
moisturizing ability and make the skin rough and wrinkled.
Long-term UVB irradiation can thicken the skin keratin and even
cause melanoma (32). UVA is the
main spectrum of skin tanning, its photochemical and
photobiological effects are not as obvious as UVB, but the dose of
UVA in sunlight is many times higher than UVB and the penetration
ability is strong, penetrating deep into the skin, so UVA also has
an important impact in causing skin photoaging (33,34).
UVA and UVB can both induce large amounts of ROS in
the skin, causing oxidative damage to cellular structures such as
DNA, proteins or lipids and enhancing oxidative stress on the skin,
which in turn causes deep-seated skin damage (35). Different wavelengths of UV can
induce the skin to produce different types of ROS (30). Among them, UVB mainly stimulates
the production of O2- through the activation of NADPH
oxidase and the respiratory chain reaction (36), while UVA generates
H2O2 through the photosensitization reaction
with internal chromophores such as riboflavin and porphyrin
(37). Therefore, scavenging
intracellular ROS can alleviate skin damage caused by UV and is
also a potential target for preventing photoaging.
Centella asiatica is a traditional Chinese
medicinal plant and its medical value has been affirmed.
Centella asiatica is used as a whole herb, which is bitter
in taste and acrid (15). The main
active components in Centella asiatica are asiaticoside,
madecassoside and asiatic acids, its multiple functions on
regulating cell activities is for attenuating damage from UV
exposure (12). Studies have shown
that extract of Centella asiatica can effectively inhibit
ROS induced by TGF-β1 in HPMC through Nrf2 activation (38,39).
Therefore, the present study investigated the therapeutic effect of
asiaticoside on UV-induced photoaging at the cellular level. It was
found that downregulation of ROS content by asiaticoside also
enhanced SOD activity, improving and restoring UV-induced changes
in skin fibroblast appearance and cell structure. The present study
found that asiaticoside downregulated ROS content and enhanced SOD
activity, improving and restoring UV-induced changes in skin
fibroblast appearance and cell structure. The inhibition of ROS
content and the enhancement of SOD activity by asiaticoside were
dose-dependent within a certain concentration range (40). Antioxidant enzymes such as SOD
synergistically reduce ROS in organisms (41). Thus, the present study demonstrated
that asiaticoside alleviated UV-induced photoaging in dermal cells
by scavenging intracellular excess ROS.
Increased expression of MMPs is one of the major
changes in skin photoaging (42).
Under normal physiological conditions, MMPs cooperate with tissue
inhibitors of metalloproteinases to regulate ECM turnover to
maintain cell stability (43).
These proteins form the connective tissue of the skin's dermis. The
role of MMPs in photoaging was initially discovered by observing
that UV irradiation of human fibroblasts enhanced the expression of
MMP-1, MMP-2, MMP-3, MMP-7, MMP-9 and MMP-36 (42,44).
Therefore, MMPs are considered to be the key molecule of UV-induced
aging (45). UVB radiation further
induces elastic fibrosis and shrinks the extracellular matrix by
inducing elastase and the expression of MMP-1, MMP-3 and MMP-9
(46,47). The present study demonstrated that
the expression level of MMP-9 was significantly reduced in
asiaticoside-treated HaCat cells compared with HaCat cells exposed
to the same UV radiation.
UV-irradiated fibroblasts exhibit senescence
characteristics (48). During
cellular senescence, a major feature is the increased activity of
lysosomal β-galactosidase, also known as Senescence-Associated
β-galactosidase (SA-β-gal) (49).
β-galactosidase is the most frequently used signature molecule to
identify aging in various in vivo and in vitro assays
(50). The present study showed
that UV radiation significantly increased the positive
β-galactosidase in fibroblasts compared with control cells, while
the activity level of β-galactosidase was significantly reduced in
a dose-dependent manner following asiaticoside treatment.
The present study then searched for the molecular
mechanism of asiaticoside in relieving photoaging by RNAseq and
finally focused on the TGF-β1/Smad signaling pathway, which is
involved in a variety of cellular processes in organism and embryo
development, including cell growth, cell differentiation, apoptosis
and cellular homeostasis. Despite the wide range of cellular
processes regulated by the TGF-β signaling pathway, the process is
relatively simple (51,52). Smad protein is a family of 9
species currently known, Smad1-9, all of which have been found to
be involved in the signal transduction of TGF-β. It is the
downstream signal transduction molecule of its receptor, including
receptor type, common mediator type, inhibitory type 3 categories.
Smad complexes need to associate with other transcription factors
to achieve transcriptional activation or repression of effector
genes (53). A central part of
TGF-β1 signaling is the SMAD-dependent canonical pathway: TGF-β
triggers signaling through TGF-β type I receptors (TGF-βRI or ALK5)
and TGF-β type II receptors (TGF-βRII), forming heterozygous
tetramers, which subsequently activate downstream SMAD signaling
proteins (54,55). The receptor-regulated
SMAD/common-partner SMAD (R-SMAD/co-SMAD) complex accumulates in
the nucleus, acts as a transcription factor and participates in the
regulation of target gene expression.
The TGF/Smad pathway has an essential role in both
natural aging and photoaging human skin and collagen is a key
regulator of skin aging and wrinkle formation (56). The present study assessed the
effect of asiaticoside on TGF-β1/Smad signaling pathway by western
blotting. It was found that asiaticoside significantly reduced
UV-mediated upregulation of TGF-β1, Smad2 and Smad3 compared with
the control group.
With the improvement of understanding of
asiaticoside, multiple functions of asiaticoside have been
revealed. Not only capacity of anti-aging in UV damaged cells as
reported in the present study, but also capability against retinal
degradation and ameliorate inflammation through Nrf2/HO-1 pathway
and TLR4/NF-κB pathway, respectively (57,58).
Furthermore, asiaticoside-contained foam dressing, nano-composite
and nanofibrous scaffold have been developed for traumatic
treatment, showing great potential of asiaticoside in wound healing
(14,59,60).
By taking advantage of nanocarriers, novel usages of asiaticoside
could be expanded and more applications related to asiaticoside
might be developed. Thus, more basic research on asiaticoside is
required for a comprehensive understanding of it.
Asiaticoside has significant therapeutic effects on
skin photoaging, ameliorating and restoring UV-induced
intracellular excess ROS in human skin cells and inhibiting the
upregulation of TGF-β1, Smad2 and Smad3 by affecting the
TGF-β1/Smad signaling pathway. Therefore, asiaticoside may be an
effective strategy for the treatment of skin photoaging.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grant no. WX18B15
from Wuhan Municipal Health Commission.
Availability of data and materials
Raw data of RNA sequencing used in this study is
available for downloading and analysis from public database
Sequence Read Archive (https://www.ncbi.nlm.nih.gov/Traces/study/) with
accession ID PRJNA851723. The data presented in this study are
available on request from the corresponding author(s).
Authors' contributions
HJ, XZ and LC contributed to the design and research
methods of this study. HJ performed experiments, analyzed results,
drafted original manuscript and acquired research funding; XZ and
LC reviewed and edited manuscript and administrated the whole
project. XZ and LC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rabe JH, Mamelak AJ, McElgunn PJ, Morison
WL and Sauder DN: Photoaging: Mechanisms and repair. J Am Acad
Dermatol. 55:1–19. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tobin DJ: Introduction to skin aging. J
Tissue Viability. 26:37–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Watson RE, Gibbs NK, Griffiths CE and
Sherratt MJ: Damage to skin extracellular matrix induced by UV
exposure. Antioxid Redox Signal. 21:1063–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Russo PA and Halliday GM: Inhibition of
nitric oxide and reactive oxygen species production improves the
ability of a sunscreen to protect from sunburn, immunosuppression
and photocarcinogenesis. Br J Dermatol. 155:408–415.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hasham R, Choi HK, Sarmidi MR and Park CS:
Protective effects of a Ficus deltoidea (Mas cotek) extract against
UVB-induced photoageing in skin cells. Biotechnology and Bioprocess
Engineering. 18:185–193. 2013.
|
|
6
|
Sun L, Xu C, Lin P, Quigg A, Chin WC and
Santschi PH: . Photo-oxidation of proteins facilitates the
preservation of high molecular weight dissolved organic nitrogen in
the ocean. Marine Chemistry. 229(103907)2021.
|
|
7
|
Liu S, Mizu H and Yamauchi H:
Photoinflammatory responses to UV-irradiated ketoprofen mediated by
the induction of ROS generation, enhancement of cyclooxygenase-2
expression and regulation of multiple signaling pathways. Free
Radic Biol Med. 48:772–780. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wondrak GT, Roberts MJ, Cervantes-Laurean
D, Jacobson MK and Jacobson EL: Proteins of the extracellular
matrix are sensitizers of photo-oxidative stress in human skin
cells. J Invest Dermatol. 121:578–586. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Severino J, Allen RG, Balin S, Balin A and
Cristofalo VJ: Is beta-galactosidase staining a marker of
senescence in vitro and in vivo? Exp Cell Res. 257:162–171.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee BY, Han JA, Im JS, Morrone A, Johung
K, Goodwin EC, Kleijer WJ, DiMaio D and Hwang ES:
Senescence-associated beta-galactosidase is lysosomal
beta-galactosidase. Aging Cell. 5:187–195. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gary RK and Kindell SM: Quantitative assay
of senescence-associated beta-galactosidase activity in mammalian
cell extracts. Anal Biochem. 343:329–334. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gohil KJ, Patel JA and Gajjar AK:
Pharmacological review on centella asiatica: A potential herbal
cure-all. Indian J Pharm Sci. 72:546–556. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
George M, Joseph L and Ramaswamy :
Anti-allergic, anti-pruritic and anti-inflammatory activities of
Centella asiatica extracts. Afr J Tradit Complement Altern Med.
6:554–559. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Namviriyachote N, Muangman P,
Chinaroonchai K, Chuntrasakul C and Ritthidej GC:
Polyurethane-biomacromolecule combined foam dressing containing
asiaticoside: Fabrication, characterization and clinical efficacy
for traumatic dermal wound treatment. Int J Biol Macromol.
143:510–520. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brinkhaus B, Lindner M, Schuppan D and
Hahn EG: Chemical, pharmacological and clinical profile of the East
Asian medical plant Centella aslatica. Phytomedicine. 7:427–448.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao X, Lin J, Sun L and Ren Y: Clinical
effects of dermatix ultra silica gel and asiaticoside cream on
hyperplastic scar tissue in patients after epicanthoplasty. Chinese
J Medical Aesthetics and Cosmetology. 6:508–511. 2019.
|
|
17
|
Shukla A, Rasik AM, Jain GK, Shankar R,
Kulshrestha DK and Dhawan BN: In vitro and in vivo wound healing
activity of asiaticoside isolated from Centella asiatica. J
Ethnopharmacol. 65:1–11. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kimura Y, Sumiyoshi M, Samukawa KI, Satake
N and Sakanaka M: Facilitating action of asiaticoside at low doses
on burn wound repair and its mechanism. Eur J Pharmacol.
584:415–423. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wijeweera P, Arnason JT, Koszycki D and
Merali Z: Evaluation of anxiolytic properties of Gotukola-(Centella
asiatica) extracts and asiaticoside in rat behavioral models.
Phytomedicine. 13:668–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shubhra QTH, Oyane A, Araki H, Nakamura M
and Tsurushima H: Calcium phosphate nanoparticles prepared from
infusion fluids for stem cell transfection: Process optimization
and cytotoxicity analysis. Biomater Sci. 5:972–981. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shubhra QTH, Kardos AF, Feczkó T, Mackova
H, Horák D, Tóth J, Dósa G and Gyenis J: Co-encapsulation of human
serum albumin and superparamagnetic iron oxide in PLGA
nanoparticles: Part I. Effect of process variables on the mean
size. J Microencapsul. 31:147–155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, Ding M, Guo K, Wang Z, Zhang C and
Shubhra QTH: Systemic Co-delivery of drugs by a pH- and
photosensitive smart nanocarrier to treat cancer by
chemo-photothermal-starvation combination therapy. Smart Materials
in Medicine. 3:390–403. 2022.
|
|
23
|
Shubhra QTH, Guo K, Liu Y, Razzak M, Manir
MS and Alam AKM: Dual targeting smart drug delivery system for
multimodal synergistic combination cancer therapy with reduced
cardiotoxicity. Acta Biomater. 131:493–507. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo K, Liu Y, Tang L and Shubhra QTH:
Homotypic biomimetic coating synergizes chemo-photothermal
combination therapy to treat breast cancer overcoming drug
resistance. Chemical Engineering J. 428(131120)2022.
|
|
26
|
Su J, Guo K, Huang M, Liu Y, Zhang J, Sun
L, Li D, Pang KL, Wang G, Chen L, et al: Fucoxanthin, a marine
xanthophyll isolated from conticribra weissflogii ND-8: Preventive
anti-inflammatory effect in a mouse model of sepsis. Front
Pharmacol. 10(906)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo K, Xiao N, Liu Y, Wang Z, Tóth J,
Gyenis J, Thakur VK, Oyane A and Shubhra QTH: Engineering polymer
nanoparticles using cell membrane coating technology and their
application in cancer treatments: Opportunities and challenges.
Nano Materials Science 2021.
|
|
28
|
Ji L, Xu J, Liu J, Amjad A, Zhang K, Liu
Q, Zhou L, Xiao J and Li X: Mutant p53 promotes tumor cell
malignancy by both positive and negative regulation of the
transforming growth factor beta (TGF-beta) pathway. J Biol Chem.
290:11729–11740. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Higgins SP, Tang Y, Higgins CE, Mian B,
Zhang W, Czekay RP, Samarakoon R, Conti DJ and Higgins PJ:
TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal. 43:1–10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cavinato M and Jansen-Durr P: Molecular
mechanisms of UVB-induced senescence of dermal fibroblasts and its
relevance for photoaging of the human skin. Exp Gerontol. 94:78–82.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang SQ, Setlow R, Berwick M, Polsky D,
Marghoob AA, Kopf AW and Bart RS: Ultraviolet A and melanoma: A
review. J Am Acad Dermatol. 44:837–846. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Debacq-Chainiaux F, Borlon C, Pascal T,
Royer V, Eliaers F, Ninane N, Carrard G, Friguet B, de Longueville
F, Boffe S, et al: Repeated exposure of human skin fibroblasts to
UVB at subcytotoxic level triggers premature senescence through the
TGF-beta1 signaling pathway. J Cell Sci. 118:743–758.
2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chainiaux F, Magalhaes JP, Eliaers F,
Remacle J and Toussaint O: UVB-induced premature senescence of
human diploid skin fibroblasts. Int J Biochem Cell Biol.
34:1331–1339. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Georgetti SR, Casagrande R, Vicentini FT,
Baracat MM, Verri WA Jr and Fonseca MJ: Protective effect of
fermented soybean dried extracts against TPA-induced oxidative
stress in hairless mice skin. Biomed Res Int.
2013(340626)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ribeiro FM, Ratti BA, Dos Santos Rando F,
Fernandez MA, Ueda-Nakamura T, de Oliveira Silva Lautenschlager S
and Nakamura CV: Metformin effect on driving cell survival pathway
through inhibition of UVB-induced ROS formation in human
keratinocytes. Mech Ageing Dev. 192(111387)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Graindorge D, Martineau S, Machon C,
Arnoux P, Guitton J, Francesconi S, Frochot C, Sage E and Girard
PM: Singlet oxygen-mediated oxidation during UVA radiation alters
the dynamic of genomic DNA replication. PLoS One.
10(e0140645)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhao J, Shi J, Shan Y, Yu M, Zhu X, Zhu Y,
Liu L and Sheng M: Asiaticoside inhibits TGF-β1-induced
mesothelial-mesenchymal transition and oxidative stress via the
Nrf2/HO-1 signaling pathway in the human peritoneal mesothelial
cell line HMrSV5. Cell Mol Biol Lett. 25(33)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Luo P, Huang Q, Chen S, Wang Y and Dou H:
Asiaticoside ameliorates osteoarthritis progression through
activation of Nrf2/HO-1 and inhibition of the NF-κB pathway. Int
Immunopharmacol. 108(108864)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Proksch E, Schunck M, Zague V, Segger D,
Degwert J and Oesser S: Oral intake of specific bioactive collagen
peptides reduces skin wrinkles and increases dermal matrix
synthesis. Skin Pharmacol Physiol. 27:113–119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen CC, Chiang AN, Liu HN and Chang YT:
EGb-761 prevents ultraviolet B-induced photoaging via inactivation
of mitogen-activated protein kinases and proinflammatory cytokine
expression. J Dermatol Sci. 75:55–62. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci.
17(868)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim MS, Kim YK, Cho KH and Chung JH:
Regulation of type I procollagen and MMP-1 expression after single
or repeated exposure to infrared radiation in human skin. Mech
Ageing Dev. 127:875–882. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Brennan M, Bhatti H, Nerusu KC,
Bhagavathula N, Kang SW, Fisher GJ, Varani J and Voorhees JJ:
Matrix metalloproteinase-1 is the major collagenolytic enzyme
responsible for collagen damage in UV-irradiated human skin.
Photochem Photobiol. 78:43–48. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vedrenne N, Coulomb B, Danigo A, Bonte F
and Desmouliere A: The complex dialogue between (myo)fibroblasts
and the extracellular matrix during skin repair processes and
ageing. Pathol Biol (Paris). 60:20–27. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Qin H, Zhang G and Zhang L: GSK126 (EZH2
inhibitor) interferes with ultraviolet A radiation-induced
photoaging of human skin fibroblast cells. Exp Ther Med.
15:3439–3448. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I and Pereira-Smith
O: A biomarker that identifies senescent human cells in culture and
in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xu YR and Fisher GJ: Ultraviolet (UV)
light irradiation induced signal transduction in skin photoaging. J
Dermatol Sci. 1:S1–S8. 2005.
|
|
52
|
Feczko T, Fodor-Kardos A, Sivakumaran M
and Haque Shubhra QT: In vitro IFN-alpha release from IFN-alpha-
and pegylated IFN-α-loaded poly(lactic-co-glycolic acid) and
pegylated poly(lactic-co-glycolic acid) nanoparticles. Nanomedicine
(Lond). 11:2029–2034. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sun ZW, Hwang E, Lee HJ, Lee TY, Song HG,
Park SY, Shin HS, Lee DG and Yi TH: Effects of Galla chinensis
extracts on UVB-irradiated MMP-1 production in hairless mice. J Nat
Med. 69:22–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hwang E, Lee DG, Park SH, Oh MS and Kim
SY: Coriander leaf extract exerts antioxidant activity and protects
against UVB-induced photoaging of skin by regulation of procollagen
type I and MMP-1 expression. J Med Food. 17:985–995.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Varga J, Rosenbloom J and Jimenez SA:
Transforming growth factor beta (TGF beta) causes a persistent
increase in steady-state amounts of type I and type III collagen
and fibronectin mRNAs in normal human dermal fibroblasts. Biochem
J. 247:597–604. 1987.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Park DW, Lee YG, Jeong YJ, Jeon H and Kang
SC: Preventive effects against retinal degeneration by centella
asiatica extract (CA-HE50) and asiaticoside through apoptosis
suppression by the Nrf2/HO-1 signaling pathway. Antioxidants
(Basel). 10(613)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gao L, Yang M, Cai S, Gao L, Gui C and
Zhang Q: Asiaticoside regulates toll-like receptor 4/nuclear
factor-Kappa B signaling pathway to relieve
lipopolysccharide-lnduced inflammation and apoptosis in ATDC5
cells. Current Topics in Nutraceutical Research. 19(432)2021.
|
|
59
|
Raharjo AB, Putra RDA, Indayaningsih N,
Srifiana Y, Hardiansyah A, Irmawati Y, Widodo H and Destyorini F:
Preparation of polyvinyl alcohol/asiaticoside/chitosan membrane
nano-composite using electrospinning technique for wound dressing.
AIP Conference Proceedings. 2256(030023)2020.
|
|
60
|
Anand S, Rajinikanth PS, Arya DK, Pandey
P, Gupta RK, Sankhwar R and Chidambaram K: Multifunctional
biomimetic nanofibrous scaffold loaded with asiaticoside for rapid
diabetic wound healing. Pharmaceutics. 14(273)2022.PubMed/NCBI View Article : Google Scholar
|