Introduction
Traumatic brain injury (TBI) can be caused by
anything from a blow to the head to penetrating brain damage
induced by external forces (1).
TBI has a global annual incidence of >294/100,000, and is more
common in older adolescents (15-19 years) and the elderly (≥65
years) (2). Notably, TBI is mainly
caused by falling injuries, blows and car accidents, and often
results in impairment of consciousness, movement, sensation,
language, vision, hearing and memory, and even death (3). Secondary brain injury after TBI
occurs as a result of cerebral blood vessel and brain parenchymal
damage, and involves oxidative stress, inflammatory response,
excitatory toxicity, imbalanced calcium homeostasis, increased
vascular permeability, blood-brain barrier (BBB) damage, brain
edema and other pathological processes (4). BBB damage can aggravate cerebral
edema, disrupt ion balance and induce immune cell infiltration,
leading to cell death (5). To the
best of our knowledge, the specific pathway involved in early brain
injury and BBB permeability changes post-TBI remains unclear.
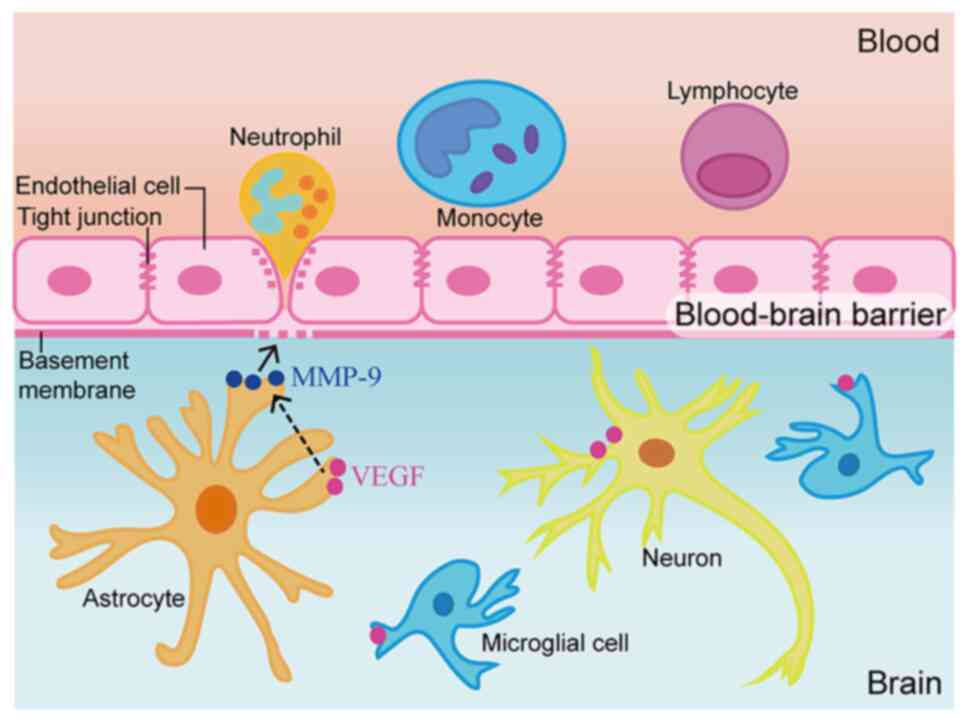
The BBB separates the blood from the brain, and
mostly comprises vascular endothelial cells, pericytes, astrocytes
and basement membrane (6). In
healthy individuals, BBB integrity heavily depends on the
capability of the aforementioned cells to prevent blood-derived
factors and immune cells from entering the brain tissue while
maintaining the highly restricted environment of the brain
(7). BBB breakdown allows excess
water to accumulate in the brain, leading to edema, or swelling
(8). Regulatory molecules,
including MMPs, also induce BBB damage by affecting the transport
system of vascular endothelial cells, leading to cerebral edema
(9).
As zinc-dependent proteolytic enzymes, MMPs
represent multifunctional endopeptidases with several functions
under physiological and pathological conditions. In the brain, MMPs
are essential for tissue generation, neural network remodeling and
BBB integrity, and constitute the main proteases involved in
extracellular matrix degradation (10). MMP-9 is a subtype of MMPs, also
referred to as gelatin enzyme B, which is closely related to
changes in BBB permeability (11).
Various cell types contribute to MMP-9 synthesis and secretion,
including endothelial cells, glial cells, neurons, mesenchymal
cells, T cells, macrophages, neutrophils and eosinophils (12). The upregulation of MMPs following
brain damage leads to enhanced BBB permeability and mediates
cerebral edema formation (10). It
has been suggested that VEGF inhibition in early cerebral ischemia
may serve an anti-cerebral ischemia role partially via MMPs
(13).
VEGF is a growth factor that induces angiogenesis
and increases vascular permeability, which can indirectly promote
the development of brain edema and tightly regulate angiogenesis
(14). VEGF inhibition has been
reported to attenuate vascular permeability and reduce vasogenic
edema in acute cerebral ischemia (15). It has been demonstrated that
blocking VEGF with bevacizumab can attenuate the brain
injury-induced disruption of tight junction proteins (16) and block the increase of brain edema
in the rat brain (17). In
addition, although MMP-9 upregulation enhances the destruction of
tight junction proteins, whether MMP-9-induced damage to tight
junction proteins is related to VEGF upregulation in TBI remains
unclear. The present study aimed to investigate whether VEGF
affects BBB integrity by controlling MMP-9 secretion, in order to
provide additional evidence for the application of VEGF inhibitors
in the treatment of TBI.
Materials and methods
Study design and grouping
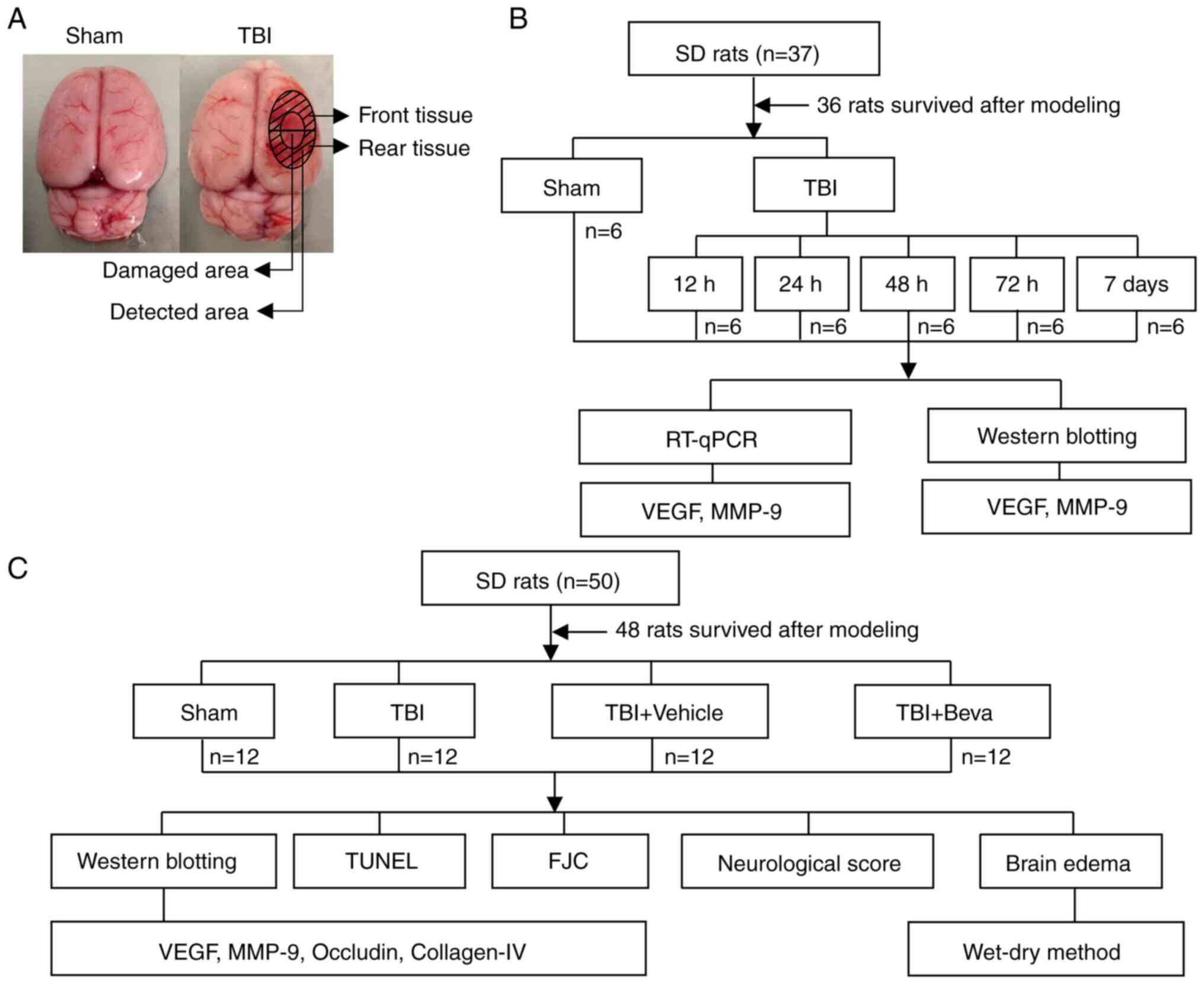
Two distinct assays were performed (Fig. 1). Experiment 1 assessed the time
courses of VEGF and MMP-9 post-TBI. A total of 36 rats underwent
randomization into the Sham, TBI 12 h, TBI 24 h, TBI 48 h, TBI 72 h
and TBI 7 days groups (n=6/group). After rats were sacrificed and
tissues were collected, reverse transcription-quantitative PCR
(RT-qPCR) and western blotting (WB) were performed to measure the
mRNA and protein expression levels of VEGF and MMP-9, respectively
(Fig. 1B). Brain tissue samples
were collected around the injured area. WB was performed on the
tissue collected from the front of the damaged area and tissue from
the rear was used for RT-qPCR (Fig.
1A).
In Experiment 2, 48 rats were randomly assigned to
the Sham, TBI, TBI + Vehicle and TBI + bevacizumab (TBI + Beva)
groups (n=12/group), to assess the roles of VEGF and MMP-9 in TBI
brain injury. A total of 24 h after TBI, which was performed based
on Experiment 1, animal euthanasia was performed, followed by the
collection of injured brain tissues. Specimens from six rats were
assessed by WB of VEGF, MMP-9, occludin and collagen-IV protein
expression, and TUNEL and Fluoro-Jade C (FJC) staining of neuronal
apoptosis and necrosis. Tissue from the front of the damaged area
was used for WB, and tissue from the rear was used for TUNEL and
FJC assays (Fig. 1A). The
remaining six rats per group were selected for analyzing brain
edema. In addition, six rats per group were randomly selected for
neurological score assessment, which was performed 24 h after TBI
and before euthanasia (Fig. 1C).
All experiments strictly used the blinded method.
Animals
A total of 87 male Sprague-Dawley rats (weight,
300-350 g; age, 8 weeks) were provided by JOINN Laboratories
(China) Co., Ltd., including 84 that were analyzed (one rat in
Experiment 1 and two rats in Experiment 2 died during anesthesia or
modeling). Rats were maintained under a 12-h light/dark cycle with
constant temperature (25˚C) and humidity (50%), as well as free
access to food and drinking water. All experimental protocols were
approved by the Animal Ethics and Welfare Committee of Zhangjiagang
TCM Hospital Affiliated to Nanjing University of Chinese Medicine
(approval no. 2020-14-1; Zhangjiagang, China) and conformed to the
guidelines on the care and use of animals outlined by the National
Institutes of Health (18).
The rats were sacrificed upon reaching humane
endpoints. The reasons for the death of rats during anesthesia or
modeling were likely caused by: i) Intraperitoneal injection of
sodium pentobarbital into the internal organs; ii) shock due to
excessive bleeding in the sagittal sinus when the bone window was
opened; and iii) heart rate and breathing not recovered after the
40-g weight was dropped. The humane endpoints of the study included
dyspnea, cyanosis, persistent convulsions and severe hypothermia
that could not be recovered by warming measures. Death in rats was
verified as heart rate and breathing arrest.
Establishment of the TBI rat
model
Experimental TBI in rats was established as
described in a previous report (19). Rats underwent intraperitoneal
anesthesia with 1% sodium pentobarbital at 40 mg/kg followed by
fixation onto a stereotaxic apparatus (Shanghai Yuyan Instruments
Co., Ltd.).
A bone drill was used to make a 5-mm diameter
parietal window at the right of the midline and behind the coronal
suture, keeping the dura intact. Next, a 4-mm diameter and 5-mm
height copper weight was placed in the bone window. This was
followed by dropping a 40-g steel rod from a height of 25 cm onto
the copper weight to cause trauma. A short pause in the heart rate
and breathing of the rats indicated successful modeling. After
disinfection and suturing, the rats were allowed to recover in a
warm room. Rats in the Sham group were also submitted to the
aforementioned procedure, without dropping the steel rod. Important
parameters were monitored during modeling, including heart rate,
respiration, body temperature and body weight. These parameters
were monitored at 9 a.m. every day after the TBI model was
established until the rats were sacrificed at the indicated time
points (12, 24, 48, 72 h and 7 days after TBI).
Drug injection
In the TBI + Beva group, bevacizumab (10 mg/kg, in
saline solution; Selleck Chemicals) was intraperitoneally injected
once immediately after TBI (16,20).
The TBI + Vehicle group animals were intraperitoneally administered
equal amounts of saline.
Tissue collection and sectioning
Sodium pentobarbital (1%, 40 mg/kg) anesthesia was
performed at the indicated time points post-injury (12, 24, 48, 72
h and 7 days after TBI). The animals underwent perfusion with 200
ml 0.9% normal saline via the heart before being sacrificed by the
intraperitoneal injection of sodium pentobarbital (2%; 150 mg/kg).
Cortical specimens around the injury area (3 mm from the edge of
the injury site in the TBI group or the same location in Sham
animals) were obtained and placed on ice. A portion of the
specimens from experiment 1 and experiment 2 underwent snap
freezing and storage at -80˚C for WB and RT-qPCR, whereas the
remaining samples from experiment 2 were fixed with 4% formalin
overnight at room temperature, embedded in paraffin and cut into
5-µm sections using a paraffin slicer (SLEE medical GmbH) for TUNEL
and FJC staining. Two blinded pathologists extracted and selected
the tissue samples.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from the
brain tissue around the injury. Total RNA (1 µg) underwent RT. qPCR
was carried out on a QuantStudio™ Dx RT-PCR Instrument (Thermo
Fisher Scientific, Inc.) with SYBR™ Green Master Mix (Thermo Fisher
Scientific, Inc.). Thermocycling conditions were as follows:
Denaturation at 95˚C for 2 min; followed by 40 amplification cycles
at 95˚C for 15 sec, 60˚C for 15 sec and 72˚C for 60 sec; final
extension at 72˚C for 10 min, and hold at 4˚C. The
2-∆∆Cq method was utilized to analyze data that were
normalized to GAPDH expression (21). The assays were performed in
triplicate. The sequences of primers used for RT-qPCR are presented
in Table I.
 | Table IPrimer sequences for quantitative
PCR. |
Table I
Primer sequences for quantitative
PCR.
| Primer name | Primer
sequence | Product size,
bp |
|---|
| VEGF | F:
5'-ACGGGCCTCTGAAACCATGAA-3' | 121 |
| | R:
5'-TTTCTGCTCCCCTTCTGTCGT-3' | |
| MMP-9 | F:
5'-GCCGGGAACGTATCTGGAAA-3' | 177 |
| | R:
5'-GGTTGTGGAAACTCACACGC-3' | |
| GAPDH | F:
5'-TGTGAACGGATTTGGCCGTA-3' | 208 |
| | R:
5'-GATGGTGATGGGTTTCCCGT-3' | |
WB
WB was performed based on a previous method
(22). Brain tissue homogenization
was performed in tissue protein extraction reagent (CoWin
Biosciences) containing protease inhibitors on ice for 20 min. The
homogenates were cleared by centrifugation at 12,000 x g for 20 min
at 4˚C. Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific,
Inc.) was utilized for protein quantification. Equal amounts of
total protein (30 µg/lane) were separated by 10% SDS-PAGE and
transferred onto a PVDF membrane (MilliporeSigma). Membranes were
blocked with QuickBlock™ Blocking Buffer (Beyotime Institute of
Biotechnology) at room temperature for 1 h. Subsequently, membranes
were incubated overnight at 4˚C with primary antibodies (Table II) and then with goat anti-rabbit
(dilution, 1:5,000; cat. no. 65-6120; Invitrogen; Thermo Fisher
Scientific, Inc.) or anti-mouse IgG-HRP (dilution, 1:5,000; cat.
no. 62-6520; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Finally, Immobilon™ Western Chemiluminescent
HRP Substrate (MilliporeSigma) was used to detect immunoblots and
an imaging system (Bio-Rad Laboratories, Inc.) was used for
visualization. ImageJ (version 1.8.0; National Institutes of
Health) was utilized for semi-quantification.
 | Table IIPrimary antibodies used for western
blotting. |
Table II
Primary antibodies used for western
blotting.
| Antibody
against | Catalogue
number | Supplier | Host species | Dilution |
|---|
| VEGF | ab214424 | Abcam | Rabbit
monoclonal | 1:1,000 |
| MMP-9 | ab76003 | Abcam | Rabbit
monoclonal | 1:3,000 |
| Occludin | sc-271842 | Santa Cruz
Biotechnology, Inc. | Mouse
monoclonal | 1:500 |
| Collagen-Ⅳ | ab6586 | Abcam | Rabbit
polyclonal | 1:500 |
| GAPDH | PA1-987 | Invitrogen; Thermo
Fisher Scientific, Inc. | Rabbit
polyclonal | 1:10,000 |
Neurological score assessment
Neurological score evaluation was performed 24 h
post-TBI using the modified Garcia test (23,24).
The scoring system consisted of seven components: i) Spontaneous
activity; ii) axial sensation; iii) vibrissae proprioception; iv)
symmetry of limb movement; v) lateral turning; vi) forelimb
outstretching; and vii) climbing. Every subtest was scored from 0
to 3, with a maximum score of 21. The higher the score, the lower
the nerve damage.
TUNEL assay
The TUNEL assay was performed to measure neuronal
apoptosis using a kit purchased from Beyotime Institute of
Biotechnology, according to the manufacturer's instructions. After
dewaxing in xylene for 5 min twice, anhydrous ethanol for 5 min,
90% ethanol for 2 min, 70% ethanol for 2 min and distilled
H2O for 2 min (all at room temperature),
paraffin-embedded sections were treated for 20 min with DNase-free
proteinase K (20 µg/ml) at 37˚C. Subsequently, the sections were
incubated for 1 h TUNEL working solution at 37˚C in the dark. DAPI
Fluoromount-G™ (Shanghai Yeasen Biotechnology Co., Ltd.) was
utilized for counterstaining for 10 min at room temperature before
observation under a fluorescence microscope (Olympus Corporation).
The apoptotic index was determined as (TUNEL-positive cells)/(total
cells) x100.
FJC staining
Necrosis was measured via FJC staining using a kit
from Biosensis Pty Ltd., according to the manufacturer's
instructions. After dewaxing in xylene for 5 min twice, anhydrous
ethanol for 5 min, 90% ethanol for 2 min, 70% ethanol for 2 min and
distilled H2O for 2 min (all at room temperature),
paraffin-embedded sections were transferred to solution B (1:9,
potassium permanganate:distilled water) and incubated for 10 min at
room temperature. Subsequently, the sections were incubated with
solution C (1:9, FJC solution:distilled water) for 30 min at room
temperature in the dark and washed with distilled water. After
drying at 60˚C for 10 min, specimens underwent soaking in xylene
for 5 min at room temperature. Finally, sealing was performed using
neutral balsam (Shanghai Yeasen Biotechnology Co., Ltd.) before
observation under a fluorescence microscope (Olympus Corporation)
to count the FJC-positive cells.
Brain edema assessment
Brain edema assessment was performed using the
wet-dry method (25). After the
rat brains were collected, they were divided into ipsilateral and
contralateral sides, and immediately weighed to assess the wet
weights. Subsequently, brain tissues were dried at 100˚C for 24 h
and dry weights were obtained. The percentage of water content in
the brains was quantified as [(wet weight-dry weight)/wet weight]
x100.
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, Inc.) was
used for statistical analysis. Data distribution was analyzed using
the Shapiro-Wilk test and all data were normally distributed except
for the neurological score data. The neurological score is shown as
the median and interquartile range, and other data are presented as
the mean ± standard deviation. One-way ANOVA followed by Dunnett's
post hoc test was performed to compare experimental groups with the
Sham group in Experiment 1. One-way ANOVA followed by Tukey's post
hoc test was performed to compare the groups in Experiment 2.
Kruskal-Wallis test followed by Dunn's post hoc test was used to
analyze the neurological scores. P<0.05 was considered to
indicate a statistically significant difference.
Results
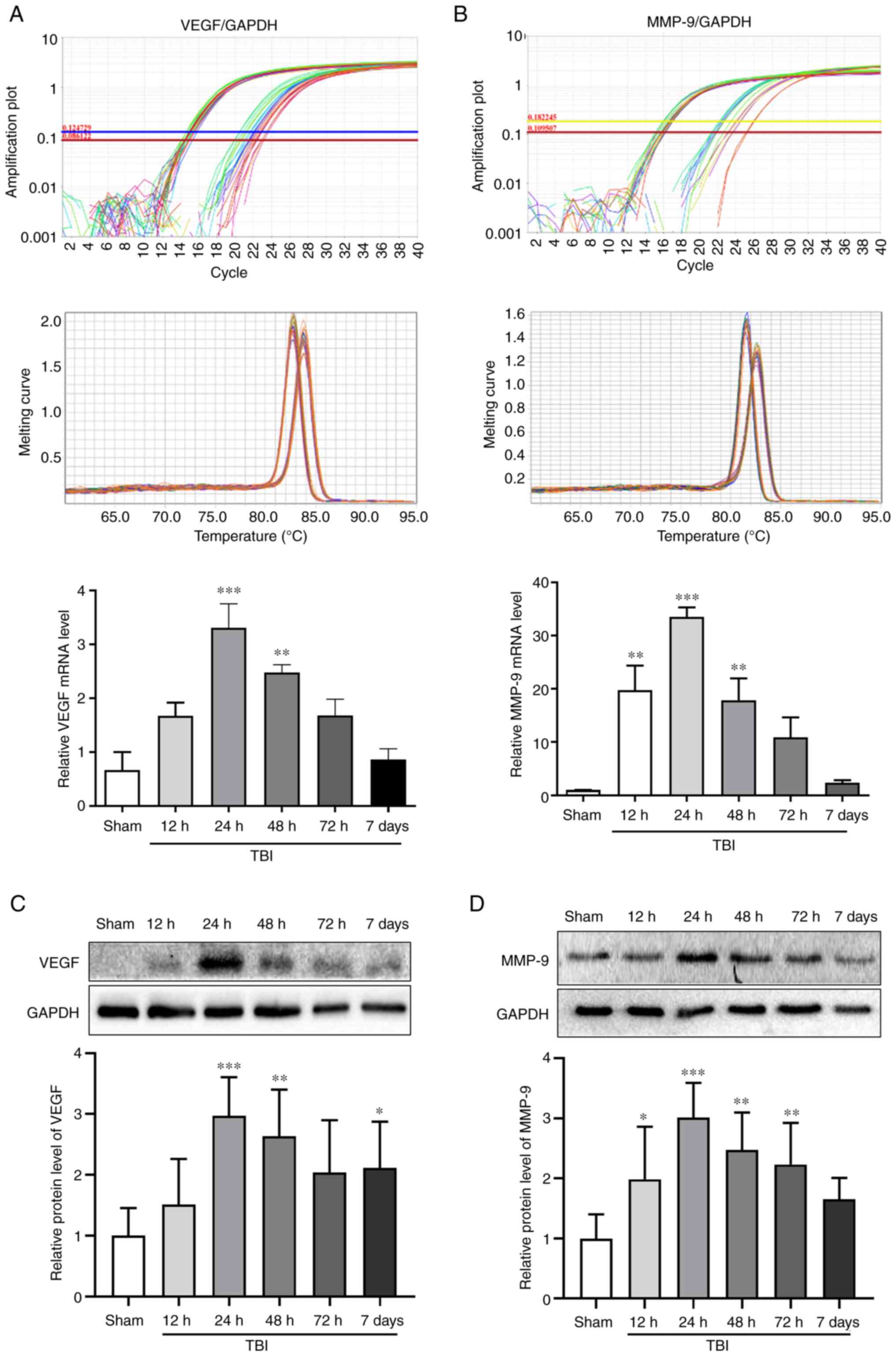
VEGF and MMP-9 expression in the rat
brains after TBI
The mRNA and protein expression levels of VEGF and
MMP-9 were assessed in the Sham group, and at 12, 24, 48 and 72 h
and 7 days after TBI by RT-qPCR and WB, respectively (Fig. 2). The mRNA and protein expression
levels of VEGF peaked at 24 h after TBI (P<0.001; Fig. 2A and C). The changes in the mRNA and protein
expression levels of MMP-9 exhibited an increase detected 12 h
after TBI and peaking at 24 h (P<0.001; Fig. 2B and D). Notably, 24 h (P<0.001) and 48 h
(P<0.01) after TBI, the expression levels of VEGF and MMP-9 were
significantly increased compared with those in the Sham group. The
time point of 24 h after TBI, which was associated with the maximum
expression of VEGF and MMP-9, was selected to investigate the
effects of bevacizumab.
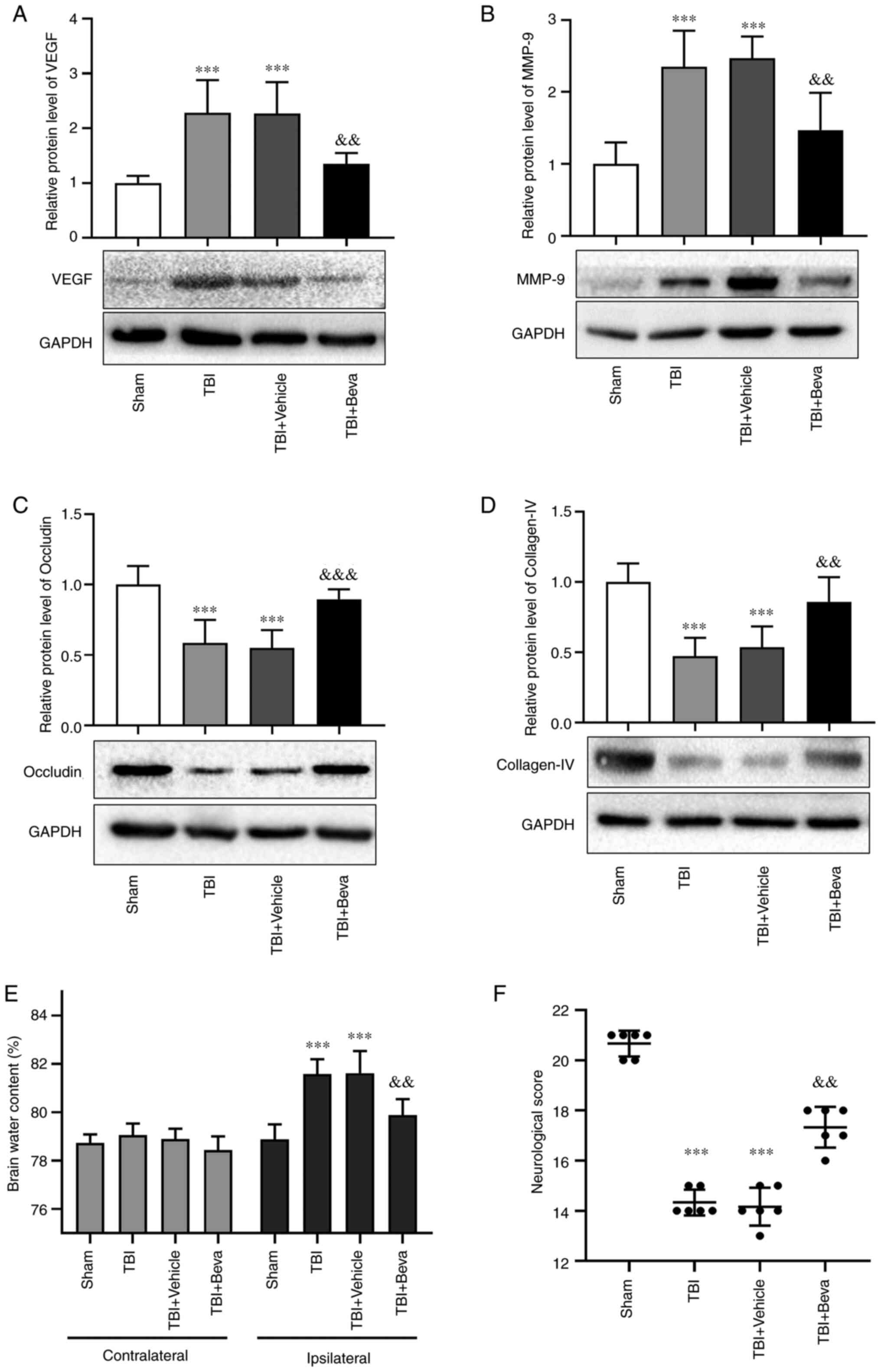
Effects of bevacizumab on VEGF and
MMP-9 expression after TBI
Bevacizumab was injected following TBI. Compared
with in the Sham group, the protein expression levels of VEGF and
MMP-9 were markedly increased in the TBI group (P<0.001;
Fig. 3A and B), which was consistent with the results
of Experiment 1. Their levels in the TBI and TBI + Vehicle groups
were comparable. Furthermore, the expression levels of VEGF and
MMP-9 in the TBI + Beva group were significantly lower than those
in the TBI + Vehicle group (P<0.01; Fig. 3A and B). These results indicated that
bevacizumab administration may reduce the expression of VEGF and
MMP-9 after TBI.
BBB integrity in rats with TBI upon
bevacizumab administration
The expression levels of the tight junction proteins
occludin and collagen-IV were assessed as indicators of BBB
degradation (Fig. 3C and D). The expression levels of occludin and
collagen-IV were significantly decreased in the TBI group compared
with those in the Sham group (P<0.001). Their levels in the TBI
and TBI + Vehicle groups were comparable. Furthermore, the TBI +
Beva group exhibited significantly increased expression levels of
occludin (P<0.001) and collagen-IV (P<0.01) compared with in
the TBI + Vehicle group, indicating that BBB damage was ameliorated
after bevacizumab administration. Additionally, brain edema was
assessed using the wet-dry method (Fig. 3E). Compared with in the Sham group,
brain water levels were increased on the ipsilateral side as a
result of TBI (P<0.001). Bevacizumab injection significantly
reduced brain edema in the damaged hemisphere after TBI compared
with in the TBI + Vehicle group (P<0.01). Brain edema on the
ipsilateral side of injury was generally higher than that on the
contralateral side. However, brain edema on the contralateral side
showed no significant changes among the Sham group and all of the
TBI groups (P>0.05).
Neurological scores in TBI rats
following bevacizumab administration
The modified Garcia test was used to reflect
neurological scores at 24 h post-TBI (Fig. 3F). Neurological scores were
significantly decreased in the TBI group compared with those in the
Sham group (P<0.001). The scores in the TBI and TBI + Vehicle
groups were comparable. Neurological scores in the TBI + Beva group
were markedly improved in comparison with the TBI + Vehicle group
(P<0.01).
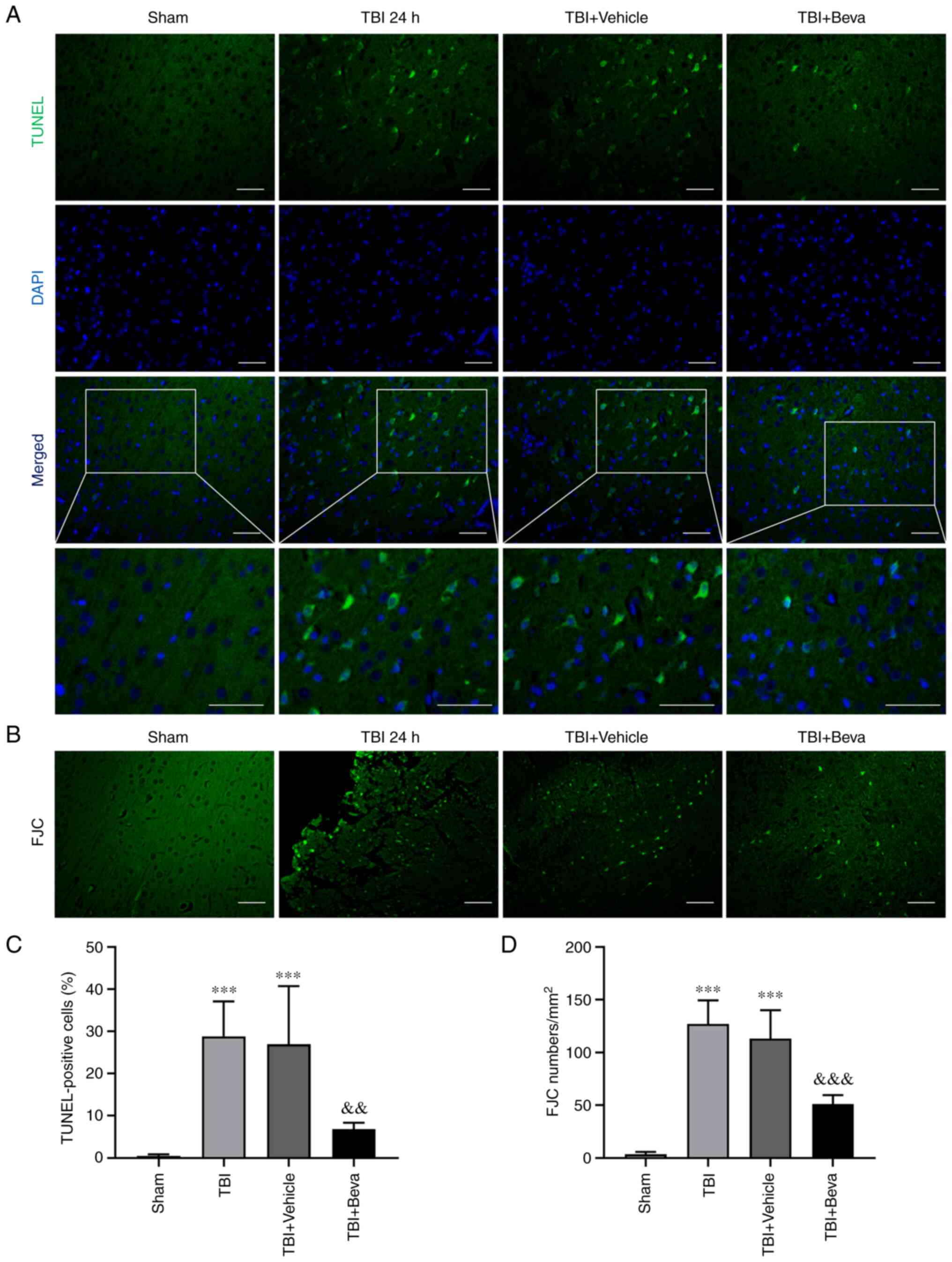
Neuronal apoptosis and necrosis in
rats with TBI after bevacizumab intervention
The TUNEL assay (Fig.
4A and C) and FJC staining
(Fig. 4B and D) were performed to assess neuronal
apoptosis and necrosis, respectively. The TBI group exhibited
significantly increased neuronal apoptosis compared with in the
Sham group (P<0.001; Fig. 4C),
whereas it was comparable to the TBI + Vehicle group (P>0.05).
Neuronal apoptosis was significantly decreased in the TBI + Beva
group compared with that in the TBI + Vehicle group (P<0.01;
Fig. 4C). Neuronal necrosis was
significantly increased in the TBI group in comparison with the
Sham group (P<0.001), whereas it was significantly decreased in
the TBI + Beva group (P<0.001; Fig.
4D), which was consistent with the results of TUNEL assay.
Discussion
VEGF is an important factor that induces vascular
permeability, and contributes to BBB destruction and brain edema
(26). In the present study, the
effects of VEGF inhibition on BBB protection and neuroprotection
were investigated in a rat model of TBI. The results showed that
the mRNA and protein expression levels of VEGF were increased
post-TBI and reached their maximum level at 24 h post-TBI.
Bevacizumab, also known as Avastin, can bind to VEGF and block its
biological activity and is commonly used as a clinical antitumor
agent (27). Bevacizumab has been
reported to serve an important role in brain tumor-related brain
edema, particularly in the treatment of brain radiation necrosis
(28,29). However, it remains unclear as to
whether there is an effect on TBI-induced brain edema. In the
present study, bevacizumab was administered immediately after TBI
to inhibit the expression of VEGF and to investigate whether the
inhibition of VEGF ameliorated BBB damage by reducing the
expression of MMP-9. The present results demonstrated that after
bevacizumab injection, the expression levels of VEGF were decreased
compared with those in the TBI group, whereas brain edema was
ameliorated. These findings indicated that VEGF may promote
cerebral edema in the TBI rat model. Moreover, the inhibition of
VEGF by bevacizumab may improve brain edema after TBI.
The occurrence of brain edema after TBI and BBB
dysfunction are directly correlated (30). Previous studies have shown that the
degradation of the tight junction protein occludin has a critical
function in the loss of BBB integrity (31,32).
Collagen-IV represents the main constituent of the basement
membrane of the BBB and its degradation may lead to defects in
basement membrane function, which may serve an essential role in
the pathogenetic mechanism of cerebral damage (33,34).
The present study investigated the BBB integrity by measuring the
levels of occludin and collagen-IV. The protein expression levels
of occludin and collagen-IV were decreased following TBI compared
with those in the Sham group, with reduced integrity of the BBB.
However, following inhibition of VEGF by bevacizumab, the
expression levels of occludin and collagen-IV were increased
compared with those in the TBI group. The injection of bevacizumab
may increase the levels of tight junction proteins and possibly
repair the basement membrane of the BBB after TBI, as also
suggested by the improvement in brain edema.
Previous findings have suggested that MMPs can
damage tight junctions and the basement membrane in the BBB,
aggravating brain edema (35). Our
previous study also confirmed that MMP-9 is upregulated in a rat
model of TBI and the specific MMP-9 suppressor SB-3CT can reverse
the increase in MMP-9 and brain edema, and reduce nerve apoptosis
and necrosis (36). The present
study used bevacizumab to inhibit VEGF following TBI, which led to
the downregulation of MMP-9, upregulation of occludin and
collagen-IV, and improved BBB integrity compared with in the TBI
group.
Damage to the BBB can aggravate brain edema, disrupt
ion balance and induce immune cell infiltration, leading to cell
death (7,37). In the present study, TUNEL and FJC
staining were used to assess apoptosis and necrosis in the brain
tissue surrounding the TBI area, respectively. The present results
showed increased levels of TUNEL- and FJC-positive cells with
increasing VEGF and MMP-9 levels post-TBI. Upon bevacizumab
administration, the levels of TUNEL- and FJC-positive cells were
significantly lower than those in the TBI group, suggesting that
bevacizumab treatment may be helpful in improving secondary nerve
injury after TBI.
The mechanism of TBI leading to secondary brain
injury is very complex. As aforementioned, VEGF was revealed to be
significantly increased in the brain tissue surrounding the TBI
area, resulting in a corresponding increase in MMP-9 levels. MMP-9
increases brain water content by regulating BBB permeability, leads
to neuron necrosis and apoptosis, and causes neurological
dysfunction (36). In the present
study, inhibition of VEGF with bevacizumab reduced TBI-induced
brain edema and may inhibit secondary brain injury; this effect may
partly be regulated by occludin and collagen-IV through the MMP-9
pathway (Fig. 5). This result
indicated that VEGF suppression in early TBI may have great
potential in the treatment of secondary brain injury post-TBI. The
possible mechanism of action was explored in the present study to a
certain extent in terms of improved brain edema after TBI.
The present study has some limitations. Firstly,
since female rats have estrus and menstrual cycles, which can add
an additional variable to scientific experiments and data analysis,
only male rats were used in the present study. The effect of this
will be considered in future studies. Secondly, the present study
only used animal experiments to investigate the effect of
bevacizumab on the expression of VEGF/MMP-9 after TBI, as well as
on brain edema and neurological function, whereas the specific
mechanism of action was not verified in vitro. Future
studies will be performed to investigate the mechanism of action of
bevacizumab in the treatment of brain edema after TBI in
vitro. Thirdly, the half-life of bevacizumab is estimated to be
20 days and there is a potential risk of it affecting wound healing
(38); therefore, further
validation studies are needed to assess the clinical application of
bevacizumab in patients with TBI. The present study explored the
effect of bevacizumab on brain edema from the perspective of BBB
integrity in an animal model of TBI, with the aim of providing
experimental guidance for the possible future clinical application
of bevacizumab in the treatment of TBI.
In conclusion, VEGF was upregulated after TBI in
rats, which damaged BBB integrity by activating MMP-9 and may
aggravate secondary brain injury. Additionally, inhibition of VEGF
exerted neuroprotective effects. The present findings indicated
that VEGF may represent a critical target for the prevention and
control of secondary brain injury after TBI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Zhangjiagang Health
Youth Science and Technology Project (grant no. ZJGQNKJ202011), the
Zhangjiagang Science and Technology Support Project (grant nos.
ZKS2020, ZKS2029) and the Suzhou Science and Technology Development
Project (grant nos. SKJY2021001 and SKJY2021003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BD and HS contributed to study conception and
design. MW, YG, LJ, MZ and HG performed experiments. MW and YG
drafted the manuscript. HS and YG generated figures and performed
data analysis. HS and BD confirm the authenticity of all the raw
data. MW and BD revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments involving animals received ethical
approval from the Animal Ethics and Welfare Committee of
Zhangjiagang TCM Hospital Affiliated to Nanjing University of
Chinese Medicine (approval no. 2020-14-1). No human subjects were
assessed in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Georges A and M Das J: Traumatic Brain
Injury. In: StatPearls [Internet]. StatPearls Publishing, Treasure
Island, FL, 2021.
|
|
2
|
Nguyen R, Fiest KM, McChesney J, Kwon CS,
Jette N, Frolkis AD, Atta C, Mah S, Dhaliwal H, Reid A, et al: The
International incidence of traumatic brain injury: A systematic
review and meta-analysis. Can J Neurol Sci. 43:774–785.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Capizzi A, Woo J and Verduzco-Gutierrez M:
Traumatic brain injury: An overview of epidemiology,
pathophysiology and medical management. Med Clin North Am.
104:213–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sivandzade F, Alqahtani F and Cucullo L:
Traumatic brain injury and blood-brain barrier (BBB): Underlying
pathophysiological mechanisms and the influence of cigarette
smoking as a premorbid condition. Int J Mol Sci.
21(2721)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fang Y, Gao S, Wang X, Cao Y, Lu J, Chen
S, Lenahan C, Zhang JH, Shao A and Zhang J: Programmed Cell Deaths
and Potential Crosstalk With Blood-Brain Barrier Dysfunction After
Hemorrhagic Stroke. Front Cell Neurosci. 14(68)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Daneman R and Prat A: The blood-brain
barrier. Cold Spring Harb Perspect Biol. 7(a020412)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bhowmick S, D'Mello V, Caruso D,
Wallerstein A and Abdul-Muneer PM: Impairment of
pericyte-endothelium crosstalk leads to blood-brain barrier
dysfunction following traumatic brain injury. Exp Neurol.
317:260–270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jha RM, Kochanek PM and Simard JM:
Pathophysiology and treatment of cerebral edema in traumatic brain
injury. Neuropharmacology. 145(Pt B):230–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Blixt J, Svensson M, Gunnarson E and
Wanecek M: Aquaporins and blood-brain barrier permeability in early
edema development after traumatic brain injury. Brain Res.
1611:18–28. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rempe RG, Hartz AMS and Bauer B: Matrix
metalloproteinases in the brain and blood-brain barrier: Versatile
breakers and makers. J Cereb Blood Flow Metab. 36:1481–1507.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang S, An Q, Wang T, Gao S and Zhou G:
Autophagy- and MMP-2/9-mediated Reduction and Redistribution of
ZO-1 Contribute to Hyperglycemia-increased Blood-brain barrier
permeability during early reperfusion in stroke. Neuroscience.
377:126–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dang B, Duan X, Wang Z, He W and Chen G: A
therapeutic target of cerebral hemorrhagic stroke: Matrix
metalloproteinase-9. Curr Drug Targets. 18:1358–1366.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang HT, Zhang P, Gao Y, Li CL, Wang HJ,
Chen LC, Feng Y, Li RY, Li YL and Jiang CL: Early VEGF inhibition
attenuates blood-brain barrier disruption in ischemic rat brains by
regulating the expression of MMPs. Mol Med Rep. 15:57–64.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma C, Zhou J, Xu X, Wang L, Qin S, Hu C,
Nie L and Tu Y: The construction of a radiation-induced brain
injury model and preliminary study on the effect of human
recombinant endostatin in treating radiation-induced brain injury.
Med Sci Monit. 25:9392–9401. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kimura R, Nakase H, Tamaki R and Sakaki T:
Vascular endothelial growth factor antagonist reduces brain edema
formation and venous infarction. Stroke. 36:1259–1263.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Deng Z, Zhou L, Wang Y, Liao S, Huang Y,
Shan Y, Tan S, Zeng Q, Peng L, Huang H and Lu Z: Astrocyte-derived
VEGF increases cerebral microvascular permeability under high salt
conditions. Aging (Albany NY). 12:11781–11793. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pishko GL, Muldoon LL, Pagel MA, Schwartz
DL and Neuwelt EA: Vascular endothelial growth factor blockade
alters magnetic resonance imaging biomarkers of vascular function
and decreases barrier permeability in a rat model of lung cancer
brain metastasis. Fluids Barriers CNS. 12(5)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|
|
19
|
Gao F, Li D, Rui Q, Ni H, Liu H, Jiang F,
Tao L, Gao R and Dang B: Annexin A7 levels increase in rats with
traumatic brain injury and promote secondary brain injury. Front
Neurosci. 12(357)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu CS, Wang ZF, Dai LM, Chu SH, Gong LL,
Yang MH and Li ZQ: Induction of proline-rich tyrosine kinase 2
activation-mediated C6 glioma cell invasion after anti-vascular
endothelial growth factor therapy. J Transl Med.
12(148)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gong Y, Wu M, Shen J, Tang J, Li J, Xu J,
Dang B and Chen G: Inhibition of the NKCC1/NF-κB signaling pathway
decreases inflammation and improves brain edema and nerve cell
apoptosis in an SBI rat model. Front Mol Neurosci.
14(641993)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Feng D, Wang B, Wang L, Abraham N, Tao K,
Huang L, Shi W, Dong Y and Qu Y: Pre-ischemia melatonin treatment
alleviated acute neuronal injury after ischemic stroke by
inhibiting endoplasmic reticulum stress-dependent autophagy via
PERK and IRE1 signalings. J Pineal Res. 62(e12395)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Garcia JH, Wagner S, Liu KF and Hu XJ:
Neurological deficit and extent of neuronal necrosis attributable
to middle cerebral artery occlusion in rats. Statistical
validation. Stroke. 26:627–634; discussion 635. 1995.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gong Y, Wu M, Gao F, Shi M, Gu H, Gao R,
Dang BQ and Chen G: Inhibition of the pSPAK/pNKCC1 signaling
pathway protects the bloodbrain barrier and reduces neuronal
apoptosis in a rat model of surgical brain injury. Mol Med Rep.
24(717)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang ZG, Zhang L, Jiang Q, Zhang R,
Davies K, Powers C, Bruggen Nv and Chopp M: VEGF enhances
angiogenesis and promotes blood-brain barrier leakage in the
ischemic brain. J Clin Invest. 106:829–838. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab (Avastin(R)) in
cancer treatment: A review of 15 years of clinical experience and
future outlook. Cancer Treat Rev. 86(102017)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhuang H, Shi S, Yuan Z and Chang JY:
Bevacizumab treatment for radiation brain necrosis: Mechanism,
efficacy and issues. Mol Cancer. 18(21)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Voss M, Wenger KJ, Fokas E, Forster MT,
Steinbach JP and Ronellenfitsch MW: Single-shot bevacizumab for
cerebral radiation injury. BMC Neurol. 21(77)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu J, Li X, Yin J, Hu Y, Gu Y and Pan S:
Glycocalyx degradation leads to blood-brain barrier dysfunction and
brain edema after asphyxia cardiac arrest in rats. J Cereb Blood
Flow Metab. 38:1979–1992. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim KA, Kim D, Kim JH, Shin YJ, Kim ES,
Akram M, Kim EH, Majid A, Baek SH and Bae ON: Autophagy-mediated
occludin degradation contributes to blood-brain barrier disruption
during ischemia in bEnd.3 brain endothelial cells and rat ischemic
stroke models. Fluids Barriers CNS. 17(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Y, Li X, Qiao S, Yang D, Li Z, Xu J,
Li W, Su L and Liu W: Occludin degradation makes brain
microvascular endothelial cells more vulnerable to reperfusion
injury in vitro. J Neurochem. 156:352–366. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee WH, Warrington JP, Sonntag WE and Lee
YW: Irradiation alters MMP-2/TIMP-2 system and collagen type IV
degradation in brain. Int J Radiat Oncol Biol Phys. 82:1559–1566.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cottarelli A, Corada M, Beznoussenko GV,
Mironov AA, Globisch MA, Biswas S, Huang H, Dimberg A, Magnusson
PU, Agalliu D, et al: Fgfbp1 promotes blood-brain barrier
development by regulating collagen IV deposition and maintaining
Wnt/β-catenin signaling. Development. 147(dev185140)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rempe RG, Hartz AMS, Soldner ELB, Sokola
BS, Alluri SR, Abner EL, Kryscio RJ, Pekcec A, Schlichtiger J and
Bauer B: Matrix metalloproteinase-mediated blood-brain barrier
dysfunction in epilepsy. J Neurosci. 38:4301–4315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu MY, Gao F, Yang XM, Qin X, Chen GZ, Li
D, Dang BQ and Chen G: Matrix metalloproteinase-9 regulates the
blood brain barrier via the hedgehog pathway in a rat model of
traumatic brain injury. Brain Res. 1727(146553)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Alluri H, Wiggins-Dohlvik K, Davis ML,
Huang JH and Tharakan B: Blood-brain barrier dysfunction following
traumatic brain injury. Metab Brain Dis. 30:1093–1104.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bergsland E and Dickler MN: Maximizing the
potential of bevacizumab in cancer treatment. Oncologist. 9 (Suppl
1):S36–S42. 2004.PubMed/NCBI View Article : Google Scholar
|