Introduction
With many unsuccessful attempts in designing novel
therapeutic approaches, advanced peritoneal metastasis (PM) remains
one of the key challenges in current surgical oncology. Many known
concepts have demonstrated only little improvement, especially
regarding the outcome of advanced, unresectable PM (1-5).
Hyperthermic intraperitoneal chemotherapy (HIPEC) in combination
with cytoreductive surgery (CRS) has raised hopes for a potentially
curative treatment in patients with limited disease progression
(6). Until today, effective
hyperthermic intraperitoneal chemotherapy remains limited to
temperatures of 42-43˚ Celsius (C). During HIPEC procedures,
hyperthermic liquid chemotherapy is introduced into the abdominal
cavity with the aim to cover organ surfaces, displaying only little
distribution inhomogeneity (6).
The inflow temperature, medium perfusate temperature and core body
temperatures usually remain at around 40˚C (7). The relatively low temperature
gradient between the HIPEC solution and core body temperature
decreases the risk of overheating abdominal organs which could
otherwise cause severe complications. A recent study by
Goldenshluger et al (8)
demonstrated, that increases in core body temperature were a
positive predictor of postoperative complications in HIPEC
procedures. To avoid complications associated with the application
of heated fluid solutions, we believe that replacing a liquid-based
heating system by an air-based heating system can be of great
significance. The sensitivity of cancer cells to increasing
hyperthermia has been extensively demonstrated (8-11),
and hyperthermia has shown to increase the response rate of cancer
cells to chemo- and radiotherapy (12-14).
However, water-based solutions restrict any further temperature
increase in hyperthermic solutions. H2O has a
heat-capacity of 4.186 kj/liter˚C which is the highest
heat-capacity of any known substance. According to the rules of
thermodynamics, high heat-capacities cause the transfer of
significant heat-energy to any objects in close proximity. In
contrast to water, air has a much lower heat capacity of around
0.718 kj/kg˚C, considering a density of 1.127 kg/m3 at
40˚C and atmospheric pressure. Therefore, any close-range objects
should retain their temperature for a longer time when surrounded
by a medium with an over 5000-fold decreased heat capacity. In
fact, we assume that there is a large temperature gradient between
hyperthermic air and the superficial tissues. Thus, only the
superficial layer is exposed to higher temperatures whereas deeper
tissues remain unaffected. By means of this study, we aim to
evaluate the feasibility of extreme hyperthermia for potential
intraperitoneal treatment. To our knowledge, this was the first
study to ever explore the hyperthermic signature, physical and
structural effects on the peritoneal tissue of extreme hyperthermia
as well as its feasibility in clinical applications. Our aim was to
develop a reliable and sensitive model which incorporates important
aspects such as regular heat transfer and heat conduction in an
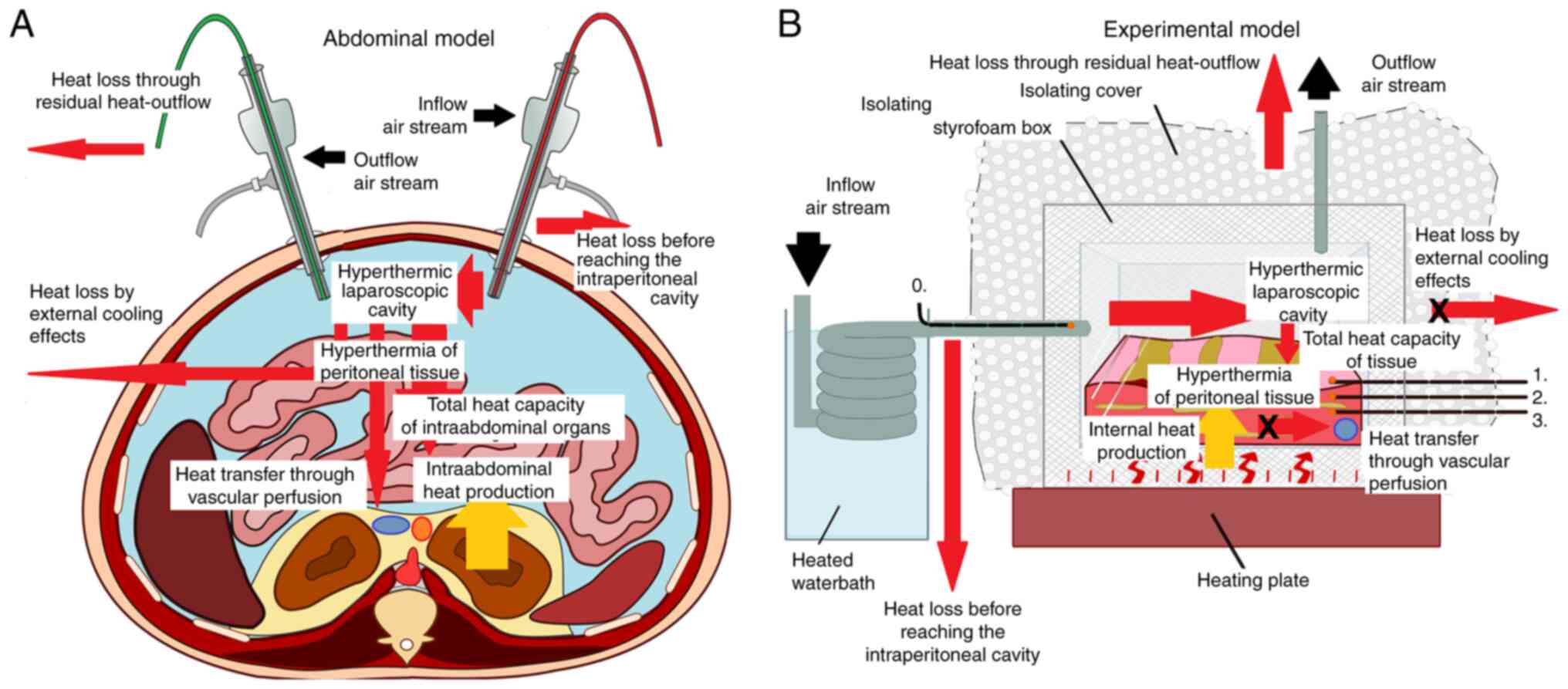
anatomical model (Fig. 1A). For
research purposes, this model has been standardized for further
analyses (Fig. 1B). After initial
evaluation of this model, we also conducted the first in
vivo experiment to evaluate the time and spatial heat signature
in gas-based intraperitoneal hyperthermia beyond 43˚C.
Material and methods
Abdominal model and cavitary heat
exposure
Tissue experiments were performed in an ex
vivo model using commercially available porcine tissue samples
(local pork supplier, Zerniki Wielkie). Fresh postmortem swine
parietal peritoneum samples (5x5x8 cm) were placed at the bottom of
a sealed and heat-isolated box (Fig.
1B). Two trocars, one of 5 mm and one of 12 mm diameter (Kii
Balloon Blunt Tip System; Applied Medical Resources Corporation,
Rancho Santa Margarita, USA) were placed at the side and top of the
box, respectively. The styropor box was additionally isolated with
bubble wrap. The heat isolated box was placed in a warm water bath
(Lighted Tissue Bath XH-1003, Gabe Court Manassas, USA). Sensitive
miniature temperature probes (Digital thermometer, FisherbrandTM
Tracebale, Pittsburgh, USA) were placed at multiple sites in the
box (Fig. 1B). One was placed in
the incoming tube (Probe 0), a second close to the peritoneal
surface (Probe 1) and two further probes (Probe 2 at 2 mm and Probe
3 at 5 mm penetration) were placed within the peritoneum. The
incoming airflow was kept constant at 15 liters per minute (l/min).
Prior to entering the box, the air was directed through a
separately heated water bath to regulate incoming air temperature
at this flow rate. By means of an underlying heater, the
temperature in the box was kept constant at an equilibrium of 37˚C.
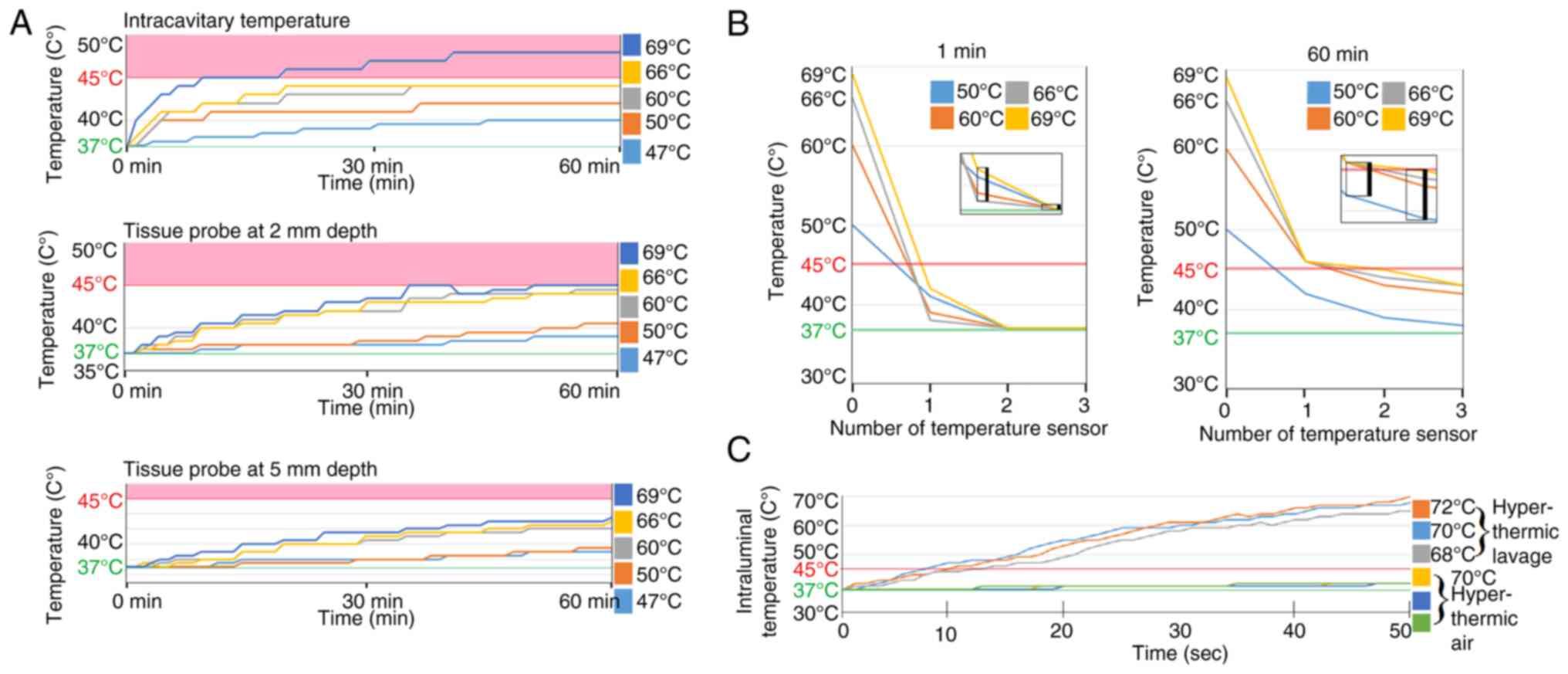
All temperature probes indicated a stable temperature for 5 min
before experiments were conducted. Experiments were conducted three
times for each temperature. The following temperatures were
applied: 47˚C, 50˚C, 60˚C, 66˚ and 69˚C. Temperature increases at
probes 1, 2 and 3 were measured for 1 h at an airflow of 15 l/min
(Fig. 2A and B).
Close-range tissue heat exposure
Fresh postmortem small intestinal samples (12 cm
length) were placed at the bottom of the box. The head of the
temperature probe was placed inside the small intestinal lumen.
Both sides of the lumen were closed. One group was treated with
heated 0.9% saline at 68˚C, 70˚C and 72˚C by pouring the saline
solution into the box. In the second group, the airstream was
heated to 70˚C, directed through a tube and impacted the small
intestinal samples at 1 cm distance and a flow rate of 15 l/min for
a total of 50 sec. The temperature increase was measured using
temperature probes (Fig. 2C).
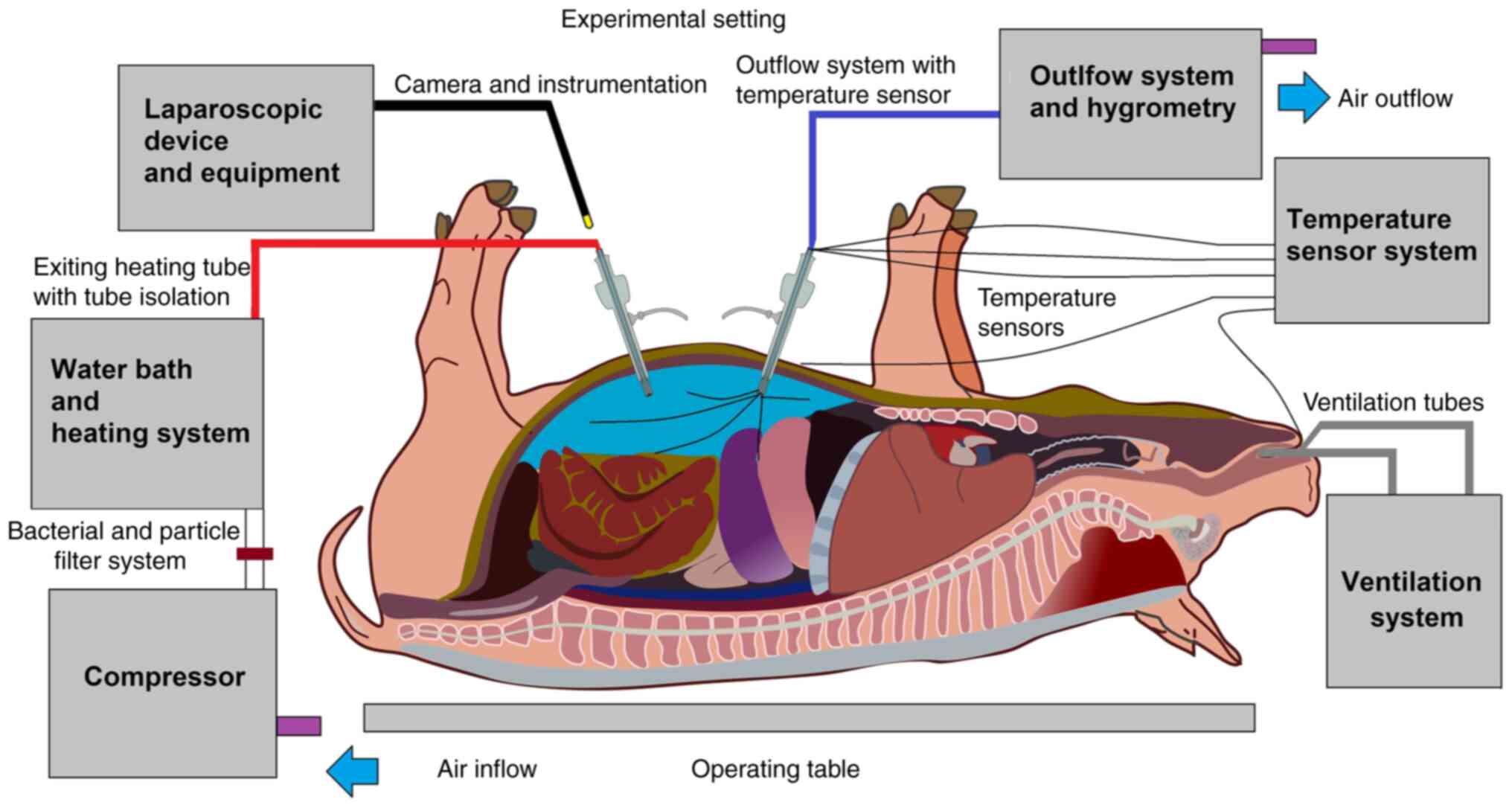
In vivo swine model
The data used for this study is part of a larger
in vivo study protocol on hyperthermia and dehydration. All
animals received humane care in compliance with the Guide for the
Care and Use of Laboratory Animals as published by the National
Institutes of Health. For this study, data from three 65-day-old,
ca. 50 kg swine were used. The swine received a diagnostic
laparoscopy without surgical intervention, under a high-flow air
stream at 15 l/min at 48˚C (Swine A), 49˚C (Swine B) and 50˚C
(Swine C). Swine were premedicated prior to laparoscopy with an
intramuscular injection of midazolam (0.3 mg/kg, WZF Polfa S.A.,
Poland), medetomidine (0.02 mg/kg, Cepetor 1 mg/ml, CP-Pharma
Handelsgesellschaft, Germany) and ketamine (9 mg/kg, Ketamina 100
mg/ml, Biowet Puławy sp. z o.o., Poland) mixture. Anesthesia was
performed with Propofol at 1 mg/kg. Swine were intubated and
further anesthesia was continued with isoflurane 1%. Additional
analgesia was provided with fentanyl 2 µg/kg and crystalloid fluid
at 0.2-0.3 µg/kg/min. Swine were placed in supine position. An
infra-umbilical mini laparotomy was performed and another at about
8 cm distance to the first one. A 10 mm trocar
(Kii®Balloon Blunt Tip System, Applied Medical, Rancho
Santa Margarita, CA, USA) was inserted through the infra-umbilical
trocar while multiple 5 mm trocars were placed at the other sites
(Fig. 2) after insufflation. The
abdominal cavity was insufflated with filtered room air through a
tube entering the central 10 mm trocar. An initial diagnostic
check-up was made via laparoscopic imaging via a 5 mm camera system
(Karl Storz 5 mm/30˚ Laparoscope/Tuttlingen, Germany) through a 5
mm trocar. After visual confirmation, and placement of multiple
temperature sensors within the abdominal cavity the high-flow air
stream was started at 15 l/min for a total of 45 min. Several
temperature sensors were also placed outside the abdominal cavity.
The temperature development was monitored continuously throughout
the laparoscopic procedure. A total number of 9 temperature sensors
were placed for the experiment. The location of these sensors was:
(1) the inside of the inflow tube,
(2) the inside of the outflow
tube. One sensor was placed in the upper right quadrant (3), one in the upper left quadrant
(4), and one in the lower abdomen
(5). One sensor was placed
directly on the peritoneum in the lower left quadrant (6). One sensor (7) was placed in the cystohepatic
triangle, another was placed and taped on the skin of the
abdomen/periumbilical (8) and a
final one was placed in the esophageal area by the
anesthesiologist.
Cell cultures
Human colorectal cancer cell line HT-29 was obtained
from CLS (Cell Lines Service GmbH, Eppelheim, Germany). HT-29 cells
were grown in Dulbecco's modified Eagle's medium (DMEM-high
glucose, Sigma-Aldrich, Poznan, Poland) and supplemented with 10%
heat-inactivated fetal bovine serum (FBS, Gibco, Thermo Fisher
Scientific, Poland), 2 mmol/l glutamine, 100 IU/ml penicillin, and
100 µg/ml streptomycin (Sigma-Aldrich) in a humidified 5%
CO2 incubator (NuAire CO2 Incubator,
Biogenet, Warszawa, Poland) at 37˚C. Cells
(1.4x105/well) were seeded in 24-well plates (TC Plate
24 Well, Standard, F, Sarstedt AG & Co. KG, Germany) and
incubated for 48 h.
In vitro short-interval
hyperthermia
Cells were seeded in 24-well plates at a
concentration of 2x105 cells/well in 1 ml of medium.
After 48 h of incubation, medium was changed for 10 sec and
replaced with 2 ml of heated medium at the following temperatures:
37˚C (control), 42˚C, 45˚C, 50˚C, 55˚C, 60˚C, 65˚C, 70˚C. After 10
sec medium was again replaced with 1 ml of medium heated to 37˚C
and cells were incubated for another 24 h. For positive control,
Oxaliplatin was used at a concentration of 1.2 mg/ml and added to
the well for 1 h, then standard medium was applied and incubated
for a further 23 h. Next, cytotoxicity and viability testing were
performed. Before these experiments were conducted, a previous
experiment was performed to ensure that heated medium maintained
its temperature when placed in a 24 well plate for 10 sec (data not
shown).
Analysis of in vitro effects of
short-term hyperthermia using viability testing and cytotoxicity
assay
An MTS test (colorimetric CellTiter 96®
AQueous One Solution assay, Promega, Poland) was used to measure
cell viability following heat or oxaliplatin treatment. The test
was performed according to the manufacturer's instruction. Medium
was removed from each well and replaced by 0.3 ml of fresh DMEM.
Next, after 1 h of incubation at 37˚C and 5% CO2, an
MTS-based reagent was added to each well and absorbance was
detected at 490 nanometer (nm) using a microplate reader (Tecan,
Basel, Switzerland). Cells treated with medium heated to 37˚C were
used as control. The percentage of viability was referenced to
control for all groups. The extent of cytotoxicity caused by heat
or oxaliplatin, respectively, was measured by release of lactate
dehydrogenase (LDH) into the supernatants using Pierce LDH
Cytotoxicity Assay Kit (Thermo Scientific). 50 µl of medium was
taken from each well. The test was performed according to the
manufacturer's protocol. Cytotoxicity levels were calculated as the
percentage of LDH released from test samples cells compared to LDH
released by lysis buffer treated cells and normalized to the
spontaneous release from control cells. As reference, color
reaction was measured spectrophotometrically on a microplate reader
(Tecan, Basel, Switzerland) at 490 and 680 nm.
Statistical analysis
Tissue experiments have been performed three
different times at the following inflow temperatures: 47˚C, 50˚C,
60˚C, 66˚C and 69˚C. The presented colored tissue curves represent
the mean of these three temperature measurements for each exposed
temperature. Cell experiments were repeated three different times.
Each well was considered a single value, corresponding to the
subgroups, meaning six wells were exposed to the same conditions in
each experiment. A one-way ANOVA was used to compare independent
groups. A post-hoc (Bonferroni) test was performed to confirm
significance levels. P<0.05 was considered to indicate a
statistically significant difference. Data are presented as the
mean standard deviation unless otherwise indicated.
Results
Temperature in the experimental
cavity
Data from the cavity probe showed a slow mean
temperature increase when insufflation was performed at lower
(47˚C) vs. higher temperatures (69˚C). After about 30 min, the
temperature increase reached a plateau, or barely increased any
further. This plateau is assumed to be the maximum achievable
cavitary temperature. Our reference temperature of 45˚C is
surpassed by the highest medium insufflation temperature of 69˚C.
Data from the superficial tissue samples showed a slow medium
temperature increase when insufflation was performed compared to
data from the intracavitary sample. The mean temperature
continuously increases and does not seem to reach a clear plateau
within the observed timeframe. Our reference temperature at 45˚C is
achieved but not surpassed by the highest insufflation temperature
of 69˚C. Data from the deepest cavity sample (3) showed an even slower mean temperature
increase compared to the previous two probes (1 and 2). Here again,
the temperature increased to a plateau after about 30 min. This
indicated that the maximum cavitary temperature was achieved. Our
temperature reference of 45˚C was surpassed by the highest
insufflation temperature of 69˚C.
Temperature measured at various time
points and locations in the experimental model
A major temperature difference is detected when
comparing air temperature of the incoming tube (0) with the cavity
temperature. This difference decreases after one hour of
hyperthermic insufflation. A further temperature decrease is noted
when comparing sensors at the position 0 and 1 with those located
at points 2 and 3. The temperature jump from point 0 to 1 is quite
drastic while the jump from point 1 to 2 is less intense.
Furthermore, the temperature at the furthest point 3 seems to
stabilize within a temperature range above 37˚C but still below
45˚C. At 60 min, it seems as if a temperature equilibrium is
reached within the tissue despite the large temperature difference
at the incoming tube 0 (Fig.
3).
Results of short-term hyperthermia on
small intestine via air and lavage
The performed experiments show that there is a
significant difference in heat conduction between different media.
While hyperthermic lavage rapidly heats up the entire tissue,
exposure to hyperthermic air only slowly heats up deeper tissues.
In the observed timeframe of 50 sec, the hyperthermic air curve
appeared nearly flat despite exposure to high temperatures of 70˚C
from the hot applied air stream (Fig.
2C). The direct hyperthermic temperature of 70˚C corresponds to
the outer wall temperature of the small intestine and the inner
wall temperature as recorded in Fig.
2C. A large temperature gradient can be created and maintained
which leads to high surface temperatures while deeper tissues
retain their original temperature.
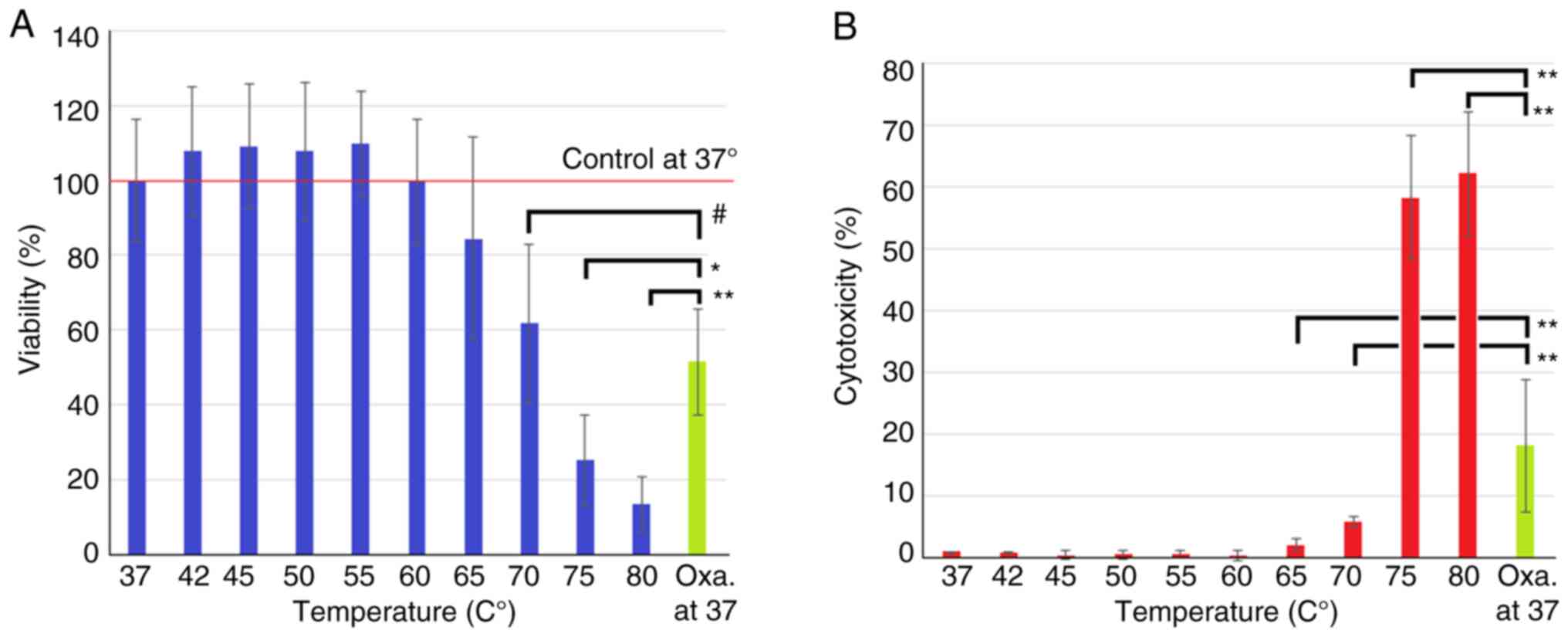
Analyzing short-term in vitro
hyperthermia on colon cancer cells using viability and cytotoxicity
assays
The performed viability test shows that in a
short-term exposure of 10 sec, significant effects on viability can
be observed with temperatures of 70˚C and higher (Fig. 3A). Temperatures below 60˚C have no
statistically significant effect. Viability decreases with
temperatures of 65˚C and higher. Observed effects on viability
increase with each temperature jump. Temperatures at 70˚C have
similar effects on viability as oxaliplatin treatment. Temperatures
beyond that, namely at 75˚ and 80˚C, outweigh the effects of
Oxaliplatin treatment by far. The performed cytotoxicity results
are similar to results in the viability tests. While temperatures
below 60˚C do not seem to affect cytotoxicity (Fig. 3B), with temperatures of 65˚C and
higher rapidly increasing signs of cytotoxicity were observed. At
temperatures between 65˚ and 70˚C, cytotoxicity is significantly
lower compared to oxaliplatin treatment. Temperatures between 75˚
and 80˚C have proven to be more toxic than oxaliplatin
application.
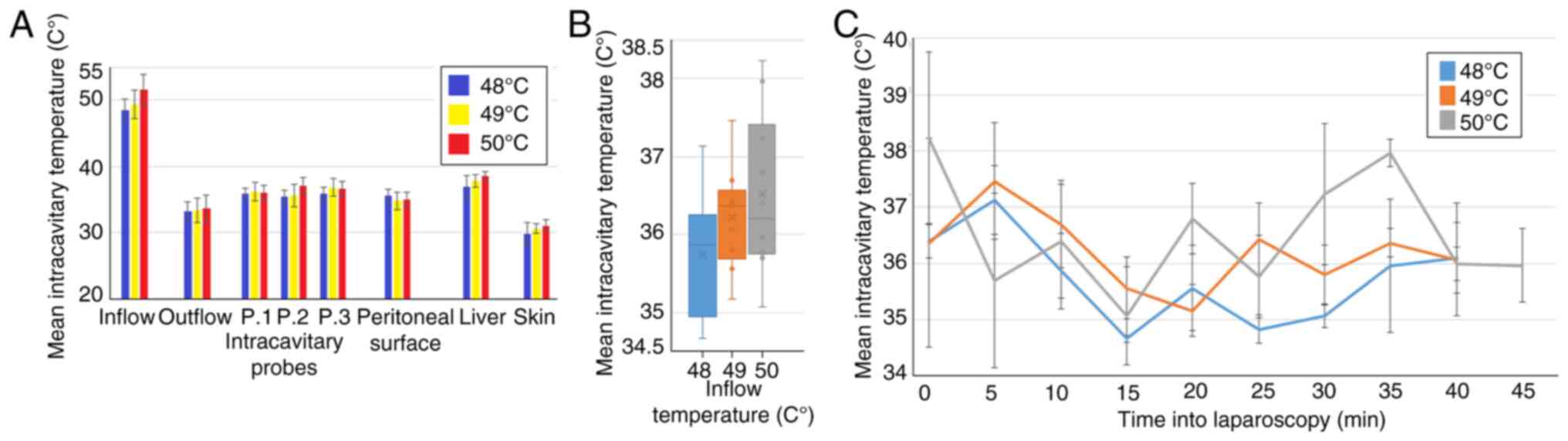
Intraoperative temperature development
during laparoscopy
From a technical point of view, the application of a
high-flow constant airstream in the peritoneal cavity was possible
(Fig. 4). No intraoperative or
postoperative complications were observed. The total duration of 45
min under high flow was well tolerated. The mean intraoperative
temperatures did not exceed 40˚C except at the inflow trocar
(Fig. 5A). The highest measured
mean temperature was at the cystohepatic triangle. No significant
difference was observable within the applied temperatures in swine
A, B and C at the measured sites. The mean intracavitary
temperatures remained at around 35.9±0.95˚C (A), 36.2±0.99˚C (B),
36.5±1.3˚C (C) (Fig. 5B). No
indications of critical intracavitary peaks were observed (Fig. 5C). The temperature fluctuations
measured within the cavity remained within 3˚C. There were no
postoperative problems within the observed time frame of 7 days
post-surgery after which an autopsy was performed.
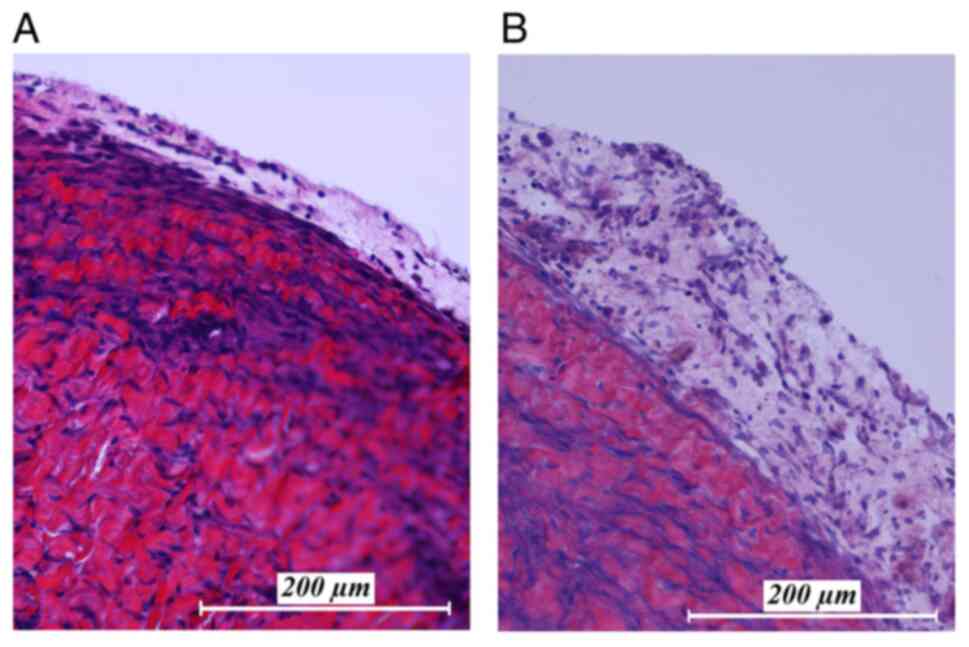
Histopathological examination of
peritoneal tissue after high temperature exposure
After autopsy of the swine tissue samples were
removed from multiple location of the peritoneum, areas that were
exposed to the hyperthermic laparoscopic space were compared to
unexposed peritoneal samples in the same swine (Fig. 6). Hematoxylin and eosin staining
shows changes after one week after intraperitoneal hyperthermia.
Peritoneal edema and an increase of white blood cell infiltration
in the peritoneum was detected in the peritoneal tissue exposed to
the laparoscopic cavity. Peritoneal tissue samples which were not
exposed to the cavity did not show any specific changes, nor did
they present signs of edema or infiltration by white blood
cells.
Discussion
The concept of applying new physical principles and
incorporating them into treatments for peritoneal metastases (PM)
(15,16) and other surface malignancies
(17-19)
has been promising. Many concepts including irradiation (20-22),
high-intensity ultrasound (23-25)
and nanoparticles (26) or new
substances (27,28) have been previously investigated for
potential clinical use. Beside radiation, hyperthermia is probably
the second most widely applied physical principle which is added to
chemotherapeutic procedures. In fact, hyperthermia has demonstrated
great efficacy in enhancing antitumoral effects when combined with
chemotherapy or radiation, without causing disproportionate
additional side effects (29-31).
Until now, hyperthermia was usually applied via water-based fluid
solutions, which display their own set of limitations due to the
unique physical properties of water. However, based on the
different physical properties of air, the same limitations do not
apply when changing the carrier medium from water to air. The
presented data indicates that a hyperthermic medium e.g. air could
probably be introduced into a body cavity at temperatures exceeding
far beyond 43˚C, without significantly heating up the abdominal
cavity itself. Also, the core body temperature remains stable even
at a high flow at 15 l/min with temperatures ranging between 48˚ to
50˚C. By means of our cell-model, we could demonstrate that
hyperthermia is indeed cytotoxic. However, during the short period
of exposure, HT-29 cells seemed to display high-resistance to
temperatures below 60˚C. The changes observed on the peritoneal
tissue are an indication that gas-based hyperthermia affects the
peritoneal surface even if no major temperature peaks can be
detected. The possible impact of this observation in PM treatment
must be further studied. Until today, very limited in vivo
data were available on hyperthermic insufflation beyond 43˚C during
laparoscopy. In fact, the clinical research community has just
developed some awareness on how the insufflation temperature may be
a potentially relevant factor during laparoscopic procedures.
Therefore, multiple studies in the last 10 years have tried to
analyze the effect of normothermic, humidified CO2 vs.
cold dry CO2 which is currently the standard in
laparoscopic procedures. For instance, the use of normothermic,
humidified CO2 for pneumoperitoneum in laparoscopic
procedures seems to be associated with reduced postoperative pain,
lower risk of postoperative hypothermia, and lower analgesic
requirements (32,33). A meta-analysis of current data
performed by Dean et al (33) indicated that heated, humidified
CO2 insufflation during laparoscopic abdominal surgery
can potentially improve intraoperative maintenance of normothermia
when compared with cold dry CO2. For decades, the
applied, relatively mild hypothermia induced by CO2 gas
at room temperature (31) has been
regarded as unproblematic. In fact, dry cold CO2 will
probably remain the worldwide standard in laparoscopy for the
foreseeable future. With the core body temperature at around 38˚C,
laparoscopy causes a temperature difference of 10-11˚C for
procedures lasting up to hours. This de-facto hypothermic
insufflation can be used as an example that an intracavitary
temperature deviation of 10-11˚C and beyond may be well tolerated,
possibly even in a hyperthermic setting. However, it has been
recognized that there is still no experience and only little
understanding of the effects of a hypo- or hyperthermic
capnoperitoneum due to the physical challenges created by air as a
carrier medium with an extremely low-heat capacity and unique
physical qualities (30). However,
more basic research should be conducted to improve the
understanding and management of air-based hyperthermia.
Furthermore, this novel concept should be studied further and
evaluated. Possibly some skepticism and prejudice will have to be
overcome to adapt to the thought that large volumes of air heated
beyond 43˚C can be applied within the abdominal cavity without
causing any measurable systemic side effects. Additionally, further
studies are required to investigate if extreme hyperthermia can
serve as an independent therapeutic option for PM treatment or
whether it is rather more favorably applied as an add-on therapy in
a setting with novel concepts of intraperitoneal chemotherapies
(34-37).
Intraperitoneal hyperthermia beyond temperatures of 43˚C is
possible and might serve as a tool to revolutionize PM treatment by
creating and temperature gradient along the peritoneal surface to
reduce PM progression (38,39).
However, applicational, biological and technical aspects of this
novel approach must be further analyzed.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by institutional funds from the
participating departments. No specific funds or grants were
applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST designed the study and drafted the manuscript.
AMM designed the study, performed lab analysis and data
acquisition. AD designed the study, performed lab analysis and data
acquisition. TK made substantial contributions to conception and
design, acquisition of data, drafted the manuscript and critically
revised it for important intellectual content. JN designed the
study, drafted the manuscript and critically revised it for
important intellectual content. KZ designed the study, performed
lab analysis and data acquisition. ZK performed lab analysis and
data acquisition. PP designed the study, performed lab analysis and
data acquisition. BL designed the study, performed lab analysis and
data acquisition. PK performed histology, lab analysis and data
acquisition. SL performed data acquisition and drafted the
manuscript. HL performed data analyses and critically revised it
for important intellectual content. WK performed data analyses and
critically revised the manuscript for important intellectual
content. VK supervised the study, performed lab analyses,
conceptualized the study and drafted the manuscript. ST, TK, VK and
AMM confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were approved (approval no. 030/2021/P2)
by the local Board on Animal Welfare at Wroclaw University of
Environmental and Life Sciences, Wroclaw, Poland.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aoyagi T, Terracina KP, Raza A and Takabe
K: Current treatment options for colon cancer peritoneal
carcinomatosis. World J Gastroenterol. 20:12493–12500.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Glehen O, Kwiatkowski F, Sugarbaker PH,
Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F,
Quenet F, et al: Cytoreductive surgery combined with perioperative
intraperitoneal chemotherapy for the management of peritoneal
carcinomatosis from colorectal cancer: A multi-institutional study.
J Oncol. 22:3284–3292. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chu DZ, Lang NP, Thompson C, Osteen PK and
Westbrook KC: Peritoneal carcinomatosis in nongynecologic
malignancy. A prospective study of prognostic factors. Cancer.
63:364–367. 1989.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Köhne CH, Cunningham D, Di Costanzo F,
Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer
M, Wils J, et al: Clinical determinants of survival in patients
with 5-fluorouracil-based treatment for metastatic colorectal
cancer: Results of a multivariate analysis of 3825 patients. Ann
Oncol. 13:308–317. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Verwaal VJ, van Ruth S, de Bree E, van
Sloothen GW, van Tinteren H, Boot H and Zoetmulder FA: Randomized
trial of cytoreduction and hyperthermic intraperitoneal
chemotherapy versus systemic chemotherapy and palliative surgery in
patients with peritoneal carcinomatosis of colorectal cancer. J
Clin Oncol. 21:3737–3743. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sugarbaker PH: Peritoneal metastases from
gastrointestinal cancer. Curr Oncol Rep. 20(62)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rettenmaier MA, Mendivil AA, Gray CM,
Chapman AP, Stone MK, Tinnerman EJ and Goldstein BH:
Intra-abdominal temperature distribution during consolidation
hyperthermic intraperitoneal chemotherapy with carboplatin in the
treatment of advanced stage ovarian carcinoma. Int J Hyperthermia.
31:396–402. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldenshluger M, Zippel D, Ben-Yaacov A,
Dux J, Yalon T, Zendel A, Rayman S, Mor E, Berkenstadt H,
Fogel-Grinvald H, et al: Core body temperature but not
intraabdominal pressure predicts postoperative complications
following closed-system hyperthermic intraperitoneal chemotherapy
(HIPEC) administration. Ann Surg Oncol. 25:660–666. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
de Andrade Mello P, Bian S, Savio LEB,
Zhang H, Zhang J, Junger W, Wink MR, Lenz G, Buffon A, Wu Y and
Robson SC: Hyperthermia and associated changes in membrane fluidity
potentiate P2X7 activation to promote tumor cell death. Oncotarget.
8:67254–67268. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li DY, Tang YP, Zhao LY, Geng CY and Tang
JT: Antitumor effect and immune response induced by local
hyperthermia in B16 murine melanoma: Effect of thermal dose. Oncol
Lett. 4:711–718. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zivanovic O, Chi DS, Filippova O, Randall
LM, Bristow RE and O'Cearbhaill RE: It's time to warm up to
hyperthermic intraperitoneal chemotherapy for patients with ovarian
cancer. Gynecol Oncol. 151:555–561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Muckle DS and Dickson JA: The selective
inhibitory effect of hyperthermia on the metabolism and growth of
malignant cells. Br J Cancer. 25:771–778. 1971.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Seifert G, Budach V, Keilholz U, Wust P,
Eggert A and Ghadjar P: Regional hyperthermia combined with
chemotherapy in paediatric, adolescent and young adult patients:
Current and future perspectives. Radiat Oncol.
11(65)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sugarbaker PH: Laboratory and clinical
basis for hyperthermia as a component of intracavitary
chemotherapy. Int J Hyperthermia. 23:431–442. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khosrawipour T, Schubert J, Kulas J,
Migdal P, Arafkas M, Bania J and Khosrawipour V: Creating
nanocrystallized chemotherapy: The differences in pressurized
aerosol chemotherapy (PAC) via intracavitary (IAG) and
extracavitary aerosol generation (EAG) regarding particle
generation, morphology and structure. J Cancer. 11:1308–1314.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khosrawipour V, Reinhard S, Martino A,
Khosrawipour T, Arafkas M and Mikolajczyk A: Increased tissue
penetration of doxorubicin in pressurized intraperitoneal aerosol
chemotherapy (PIPAC) after High-Intensity Ultrasound (HIUS). Int J
Surg Oncol. 2019(6185313)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schubert J, Khosrawipour T, Pigazzi A,
Kulas J, Bania J, Migdal P, Arafkas M and Khosrawipour V:
Evaluation of Cell-detaching Effect of EDTA in combination with
oxaliplatin for a possible application in HIPEC after cytoreductive
surgery: A preliminary in-vitro study. Curr Pharm Des.
25:4813–4819. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Plociennik M, Nowak K, Fahr C, Chaudhry H and Khosrawipour T:
Feasibility and characteristics of pressurized aerosol chemotherapy
(PAC) in the bladder as a therapeutical option in early-stage
urinary bladder cancer. In Vivo. 32:1369–1372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khosrawipour V, Mikolajczyk A, Paslawski
R, Plociennik M, Nowak K, Kulas J, Arafkas M and Khosrawipour T:
Intrathoracic aerosol chemotherapy via spray-catheter. Mol Clin
Oncol. 12:350–354. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khosrawipour V, Bellendorf A, Khosrawipour
C, Hedayat-Pour Y, Diaz-Carballo D, Förster E, Mücke R, Kabakci B,
Adamietz IA and Fakhrian K: Irradiation does not increase the
penetration depth of doxorubicin in normal tissue after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model.
In Vivo. 30:593–597. 2016.PubMed/NCBI
|
|
21
|
Khosrawipour V, Giger-Pabst U,
Khosrawipour T, Pour YH, Diaz-Carballo D, Förster E, Böse-Ribeiro
H, Adamietz IA, Zieren J and Fakhrian K: Effect of irradiation on
tissue penetration depth of doxorubicin after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex-vivo
model. J Cancer. 7:910–914. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khosrawipour V, Khosrawipour T,
Hedayat-Pour Y, Diaz-Carballo D, Bellendorf A, Böse-Ribeiro H,
Mücke R, Mohanaraja N, Adamietz IA and Fakhrian K: Effect of
Whole-abdominal irradiation on penetration depth of doxorubicin in
normal tissue after pressurized intraperitoneal aerosol
chemotherapy (PIPAC) in a post-mortem swine model. Anticancer Res.
37:1677–1680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Grzesiak J, Chaudhry H, Pigazzi A and Khosrawipour T: Effect of
liposomal doxorubicin in pressurized intra-peritoneal aerosol
chemotherapy (PIPAC). J Cancer. 9:4301–4305. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mikolajczyk A, Khosrawipour T, Kulas J,
Migdal P, Arafkas M, Nicpon J and Khosrawipour V: The structural
effect of high intensity ultrasound on peritoneal tissue: A
potential vehicle for targeting peritoneal metastases. BMC Cancer.
20(481)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mikolajczyk A, Khosrawipour T, Martino A,
Kulas J, Pieczka M, Zacharski M, Nicpon J and Khosrawipour V:
Enabling microparticle imprinting to achieve penetration and local
endurance in the peritoneum via high-intensity ultrasound (HIUS)
for the treatment of peritoneal metastasis. Int J Surg Oncol.
2020(9679385)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mikolajczyk A, Khosrawipour V, Kulas J,
Kocielek K, Migdal P, Arafkas M and Khosrawipour T: Release of
doxorubicin from its liposomal coating via high intensity
ultrasound. Mol Clin Oncol. 11:483–487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mikolajczyk A, Khosrawipour V, Lau H, Li
S, Migdal P, Labbe MK, Kielan W, Nicpon J, Stieglitz S and
Khosrawipour T: Exploring the potential of taurolidine in inducing
mobilisation and detachment of colon cancer cells: A preliminary
in-vitro study. BMC Pharmacol Toxicol. 23(38)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schubert J, Khosrawipour V, Chaudhry H,
Arafkas M, Knoefel WT, Pigazzi A and Khosrawipour T: Comparing the
cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the
proliferation of in vitro colon carcinoma cells following
pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J
Surg Oncol. 17(93)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cihoric N, Tsikkinis A, van Rhoon G,
Crezee H, Aebersold DM, Bodis S, Beck M, Nadobny J, Budach V, Wust
P and Ghadjar P: Hyperthermia-related clinical trials on cancer
treatment within the urihttp://ClinicalTrials.govsimpleClinicalTrials.gov
registry. Int J Hyperthermia. 31:609–614. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kok HP, Wust P, Stauffer PR, Bardati F,
van Rhoon GC and Crezee J: Current state of the art of regional
hyperthermia treatment planning: A review. Radiat Oncol.
10(196)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sajid MS, Mallick AS, Rimpel J, Bokari SA,
Cheek E and Baig MK: Effect of heated and humidified carbon dioxide
on patients after laparoscopic procedures: A meta-analysis. Surg
Laparosc Endosc Percutan Tech. 18:539–546. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dean M, Ramsay R, Heriot A, Mackay J,
Hiscock R and Lynch AC: Warmed, humidified CO2
insufflation benefits intraoperative core temperature during
laparoscopic surgery: A meta-analysis. Asian J Endosc Surg.
10:128–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Binda MM: Humidification during
laparoscopic surgery: Overview of the clinical benefits of using
humidified gas during laparoscopic surgery. Arch Gynecol Obstet.
292:955–971. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khosrawipour T, Schubert J, Khosrawipour
V, Chaudhry H, Grzesiak J, Arafkas M and Mikolajczyk A: Particle
stability and structure on the peritoneal surface in pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) analysed by electron
microscopy: First evidence of a new physical concept for PIPAC.
Oncol Lett. 17:4921–4927. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schubert J, Khosrawipour T, Reinhard S,
Arafkas M, Martino A, Bania J, Pieczka M, Pigazzi A and
Khosrawipour V: The concept of foam as a drug carrier for
intraperitoneal chemotherapy, feasibility, cytotoxicity and
characteristics. Sci Rep. 10(10341)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Chaudhry H, Pigazzi A and Khosrawipour T: Particle stability during
pressurized intra-peritoneal aerosol chemotherapy (PIPAC).
Anticancer Res. 38:4645–4649. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Diakun A, Khosrawipour T,
Mikolajczyk-Martinez A, Nicpoń J, Kiełbowicz Z, Prządka P, Liszka
B, Kielan W, Zielinski K, Migdal P, et al: The onset of in-vivo
dehydration in gas-based intraperitoneal hyperthermia and its
cytotoxic effects on colon cancer cells. Front Oncol.
12(927714)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Diakun A, Khosrawipour T,
Mikolajczyk-Martinez A, Kuropka P, Nicpoń J, Kiełbowicz Z, Prządka
P, Liszka B, Li S, Lau H, et al: In-vivo thermodynamic exploration
of gas-based intraperitoneal hyperthermia. Front Oncol.
12(92572)2022.PubMed/NCBI View Article : Google Scholar
|