Introduction
Testicular injury due to the rotation of the
spermatic cord and its vessels caused by testicular torsion
(T)/detorsion (D) represents an urological emergency (1). Diagnosis and management of TT is a
challenge for physicians (2). Due
to the presence of the various clinical conditions that are covered
in the definitive diagnosis of the disease, detailed anamnesis and
physical examination are very important for the correct diagnosis
of TT. A previous study demonstrated that hemorrhagic infarction
may occur within 2 h from the testicular torsion, while
irreversible damage is likely to occur after 6 h (3). Therefore, time of the diagnosis and
management for T/D is very important to save a viable and
functional testis. Testicular salvage rate has been reported to be
90-100% in 6 h, ~50% in 12 h and >10% in 24 h after D (4,5).
Testicular T/D is responsible for testicular damage
and necrosis due firstly to ischemic (I) injury and secondly to
reperfusion (R) injury after D (6). Post-D I/R injury occurs when blood
circulation is restarted after acute ischemia (7,8).
Testicular reperfusion after testicular D causes more serious
damage compared with ischemia (9).
Oxidative stress is a key factor for testicular damage after I/R
injury due to the excessive production of reactive oxygen species
(ROS) including superoxide anions, hydrogen peroxide, nitric oxide
and hypochlorous acid (10,11).
Previous studies indicate that the increase in ROS and irreversible
damage are associated with an increase in intracellular calcium
(12,13). Oxidative stress is caused by an
imbalance between the oxidative and antioxidative systems and is
responsible for a decrease in cell viability, which is ultimately
caused by lipid peroxidation in the cell membrane, protein
denaturation and DNA damage (11).
Antioxidants control the autoxidation by interrupting the
propagation of free radicals or by inhibiting the formation of free
radicals via different mechanisms. These compounds help in
scavenging the species that initiate the peroxidation, breaking the
autoxidative chain reaction, quenching O2•-,
and preventing the formation of peroxides (14). The primary source of ROS is
considered to be the leukocytes infiltrating into the testicular
tissue (15). Spermatozoa are also
considered to be a further source of ROS (15).
Nanotechnology is currently employed as a tool to
explore the darkest avenues of medical sciences in several ways,
such as in imaging (16), sensing
(17), targeted drug delivery
(18), gene delivery systems
(19) and artificial implants
(20). The new age drugs are
nanoparticles of polymers, metals or ceramics, which can combat
conditions such as cancer (21)
and fight human pathogens such as bacteria. One of the most
promising metal oxide nanoparticles in biological systems is
engineered cerium oxide (CeO2) nanoparticles, also known
as nanoceria. Nanoceria have regenerative antioxidant properties in
oxidative stress. Additionally, nanoceria reduce inflammation and
the autoimmune response (22). The
antioxidant properties of nanoceria are based on its activity as
ROS scavengers that originate due to the presence of cerium ions in
two different oxidation states, Ce3+ and Ce4+
(23). These antioxidant
activities, based on the ratio between Ce3+ and
Ce4+ on the surface of cerium oxide nanoparticles, are
associated with superoxide dismutase mimetic activities, catalase
mimetic activities and nitric oxide and hydroxyl scavenging
properties (24-27).
The present study aimed to investigate the effect of
cerium oxide on pathological and biochemical markers from
testicular tissue after I/R injury in a testicular T and D model,
based on the anti-inflammatory and antioxidant effects previously
emphasized.
Materials and methods
Animals and experimental protocol
A total of 24 Wistar albino, male rats (12 months
old, weighing 250-300 g) were used in the present study, supplied
by Gazi University Experimental Animals Research Center (Ankara,
Turkey), which was approved by the Gazi University Ethics Committee
(approval no. G.U.ET-19-059). Rats were kept in a
temperature-controlled (21±1˚C) and humidity-controlled (45-55%)
room, which was maintained on a 12/12 reversed light cycle. Animals
were fed with a standard pellet and allowed to drink water ad
libitum. All the experimental procedures were performed
according to the guide for the care and use of laboratory animals.
Before each experimental procedures, anesthesia was induced via
intraperitoneal (i.p.) injection of ketamine hydrochloride (50
mg/kg; Ketalar; Parke-Davis Eczacibasi; Pfizer, Inc.) and xylazine
hydrochloride 2% (20 mg/kg; Alfazyne; Ege Vet). During the surgical
procedure, rats were maintained under anesthesia via repetitive
injections of 20 mg.kg-1 ketamine in case of a positive
reaction to surgical stress or intermittent tail pinch. During the
surgical procedure rats were placed on a heating pad in order to
maintain a constant body temperature. Animals were equally and
randomly divided into the follwing four groups: Control,
CeO2, T/D and CeO2-T/D groups.
Control group rats were only subjected to midline
laparotomy. CeO2 group rats underwent surgical left
inguinoscrotal incision and cerium oxide was given via i.p.
injection (0.5 mg/kg) 30 min before the incision period.
In the T/D group, following left inguinoscrotal
incision, animals underwent unilateral testicular T by 720˚
clockwise rotation of the left testis that was subsequently fixed
within the hemiscrotum using a 4/0 atraumatic silk suture. After
120 min of ischemia, rats underwent a spermatic cord D procedure
that was followed by reperfusion for 120 min. Sodium heparin (500
IU/kg) was administered through the peripheral vein in the tail for
the maintenance of reperfusion after occlusion.
In the T/D + CeO2 group, cerium oxide
(Sigma Aldrich; Merck KGaA) was given (i.p 0.5 mg.kg-1)
30 min prior to the ischemic procedure. Following left
inguinoscrotal incision, animals underwent unilateral testicular T
by 720˚ clockwise rotation of the left testis that was subsequently
fixed within the hemiscrotum using a 4/0 atraumatic silk suture.
After 120 min, rats underwent spermatic cord D procedure that was
followed by reperfusion for 120 min. Sodium heparin (500 IU/kg) was
administered through the peripheral vein in the tail for the
maintenance of reperfusion after occlusion.
Following reperfusion, blood samples were collected
from the abdominal aorta. Subsequently, rats were anesthesized
using ketamine (100 mg/kg) and xylazine (10 mg/kg) i.p. injection
and sacrified by taking intracardiac blood with an injector. After
heartbeat and respiration ceased, these were monitored for further
2 min to confirm death. Testicular tissue samples were obtained for
subsequent biochemical and histopathological analyses.
Histopathological analysis
Testicular tissue samples were fixed in 10% neutral
formaldehyde for 48 h at room temperature (RT), dehydrated and
embedded in paraffin. Cross-sections of 4-µm thickness were sliced
from the paraffin blocks using a microtome (Thermo Fisher
Scientific, Inc.). The sections were deparaffinized in xylenes
using three changes for 10 min each at RT and rehydrated in a
descending ethanol series. Tissue specimens were stained with
H&E for 10 min at RT and examined using a Nikon Eclipse 80i
light microscope (Nikon Corporation). Histopathological changes in
the testicular specimens were evaluated according to a four-level
grading system proposed by Cosentino et al (28) (Table
I). Spermatogenesis was quantified based on the profile of the
cells that existed along the seminiferous tubules. A total of 50
seminiferous tubules were evaluated in each specimen and graded
1-10 according to Johnsen's scoring system (Table II) (29). Additionally, diameters (µm) of 50
randomly selected circular seminiferous tubules per specimen were
measured and the mean seminiferous tubular diameter was
calculated.
 | Table ICosentino's et al (17) classification of testicular
damage. |
Table I
Cosentino's et al (17) classification of testicular
damage.
| Score | Features |
|---|
| Grade 1 | Normal testicular
structure with an orderly arrangement of germinal cells |
| Grade 2 | Less orderly,
non-cohesive germinal cells and closely packed seminiferous
tubules |
| Grade 3 | Disordered sloughed
germinal cells with shrunken pyknotic nuclei and impaired borders
of the seminiferous tubules |
| Grade 4 | Seminiferous
tubules tightly surrounded by coagulative necrosis of germinal
cells |
 | Table IIJohnsen scoring system (18). |
Table II
Johnsen scoring system (18).
| Score | Features |
|---|
| 10 | Complete
spermatogenesis with several spermatozoa and regular tubules |
| 9 | Slightly impaired
spermatogenesis with several late spermatids and disorganized
germinal epithelium |
| 8 | Less than five
spermatozoa per tubule with a few late spermatids |
| 7 | No spermatozoa and
late spermatids, several early spermatids |
| 6 | No spermatozoa and
late spermatids, few early spermatids |
| 5 | No spermatozoa or
spermatids, several spermatocytes |
| 4 | No spermatozoa or
spermatids, few spermatocytes |
| 3 | Only
spermatogonia |
| 2 | No germinal cells,
Sertoli cells only |
| 1 | No seminiferous
epithelium |
Immunohistochemistry
The paraffin embedded sections were deparaffinized
and rehydrated in a descending alcohol series. For heat-induced
antigen retrieval, the sections were placed in citrate buffer (pH
6.0) and boiled 3 times for 5 min each using a microwave oven at
700 W. Endogenous peroxidase activity was blocked with 3%
H2O2 and the epitopes were stabilized using
serum blocking solution (Ultra V Block) for 5 min at RT (Thermo
Fisher Scientific, Inc.). Sections were then incubated overnight at
4˚C with PBS containing primary antibodies against Bax (1:100; cat.
no. E-AB-33819; Elabscience Biotechnology, Inc.), Bcl-2 (1:100;
cat. no. E-AB-60012; Elabscience Biotechnology, Inc.), caspase-3
(1:100; cat. no. E-AB-63602; Elabscience Biotechnology, Inc.) and
p53 (1:100; cat. no. E-AB-60866; Elabscience Biotechnology, Inc.).
Following incubation with primary antibody, the sections were
incubated with biotinylated goat anti-polyvalent secondary antibody
and streptavidin peroxidase (cat. no. TP-125-HL; Thermo Fisher
Scientific, Inc.) for 10 min each at RT. PBS was used to wash the
sections between each step. The binding sites of antibody were
visualized using 3,3'-diaminobenzidine (Thermo Fisher Scientific,
Inc.). The sections were counterstained with Harris's hematoxylin
for 30 sec at RT, evaluated under a Nikon Eclipse 80i light
microscope (magnification, x100; Nikon Corporation). ImageJ
analysis software (version 1.52; National Institutes of Health) was
used to assess staining intensity of the antibodies in testis
tissues (30). Average signal
levels in 20 seminiferous tubules in each tissue were measured.
Biochemical evaluations
Testicular tissues were washed with cold NaCl
solution (0.154 M) to discard blood contamination and then
homogenized in a Diax 900 (Heidolph Instruments GmbH and Co KG) at
1,000 rpm for ~3 min. After centrifugation at 10,000 x g for ~60
min at 4˚C, the upper clear supernatant was subjected to further
analysis.
Malondialdehyde (MDA) levels were measured using the
spectrophotometric thiobarbituric acid reactive substances method
developed by Van Ye et al (31) and Hodges et al (32) that is based on the reactivity
towards thiobarbituric acid. MDA reacts with thiobarbituric acid at
90-100˚C and produces a pink dye that has an absorption maximum at
532 nm wavelength. To ensure protein precipitation, the sample was
mixed at room temperature with cold 20% (w/v) trichloroacetic acid
and the precipitate was then centrifuged for 10 min at 1,207 x g at
room temperature. An aliquot of the supernatant was then placed
into an equal volume of 0.6% (w/vol) thiobarbituric acid in a
boiling water bath for 30 min. Following cooling, sample and blank
absorbance were read at 532 nm wavelength and the results expressed
as nmol/mg protein, based on a graph where
1,1,3,3-tetramethoxypropane was used as MDA standard.
Catalase (CAT) activity was measured using the
method developed by Aebi (33)
that is based on the measurement of absorbance decrease due to
H2O2 consumption at 240 nm. The serum
paraoxonase-1 (PON-1) activity was measured based on the hydrolysis
rate of paraoxon (MilliporeSigma) that was measured by monitoring
the increase of absorbance at 405 nm wavelength at 25˚C. The basal
assay mixture included 1.0 mM paraoxon and 1.0 mM CaCl2 in Tris/HCl
buffer (pH 8.0; 100 mM). The definition of 1 unit of paraoxonase
activity was taken as 1 mmol of p-nitrophenol formed per min
(34).
Glutathione S-transferases (GST) activity was
measured using the method described by Habig et al (35). The GST activity method is based on
the measurement of absorbance increase at 340 nm wavelenght due to
the reduction of of 2,4-dinitrophenyl-β-D-glucopyranoside (DNPG).
The PON activity was measured with the method based on the
p-nitrophenol formation in the presence of PON, in which paraoxon
was used as a substrate. For the analysis, the p-nitrophenol was
measured, and formed with paraoxon (diethyl p-nitrophenyl
phosphate, 1 mM) in 50 mM glycine/NaOH (pH 10.5) containing 2 mM
CaCl2 at 25˚C and 412 nm. The molar extinction
coefficient of p-nitrophenol (e=18.290 M/cm) was used in the
calculation of the PON enzyme activity. The results were expressed
in IU/mg protein. Chemicals were purchased from MilliporeSigma.
Statistical analysis
SPSS statistical software, version 24.0 (IBM Corp.)
was used for statistical analyses. The distribution of data was
analysed with the Shapiro-Wilk test and Q-Q plot test. The results
were analysed using the Kruskal-Wallis test followed by Dunn's test
or one-way ANOVA followed by Tukey's test. All quantitative data
are expressed as means±standard deviation. P<0.05 was considered
to indicate a statistically significant difference.
Results
Histopathological findings
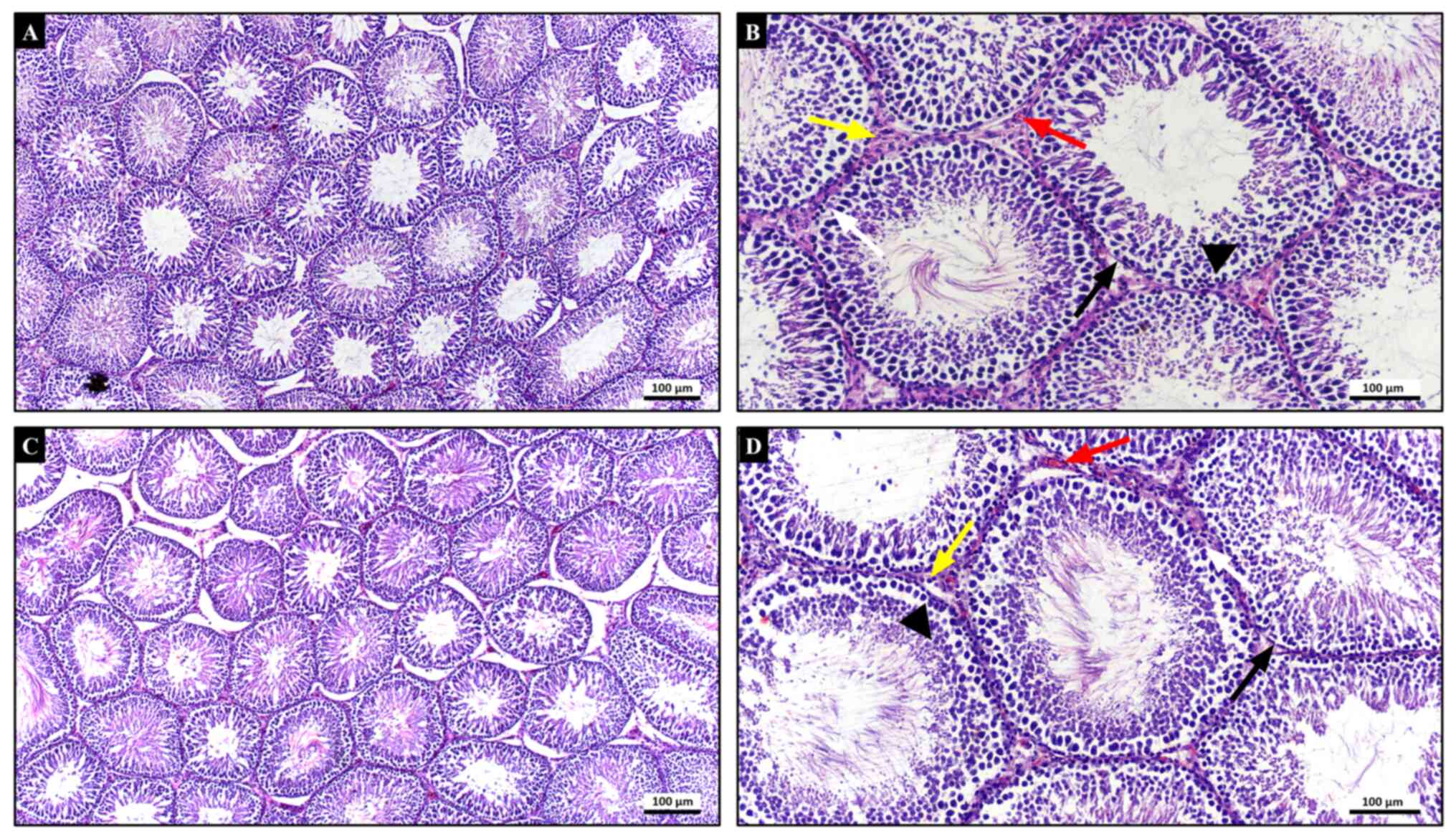
In the histopathological examination of testicular
tissues of both control (Fig. 1A
and B) and CeO2
(Fig. 1C and D) groups, seminiferous tubules bound by
basement membrane had normal morphology. Developing germinal cells
arranged in orderly layers and Sertoli cells with their normal
appearance were lining the seminiferous tubules. Leydig cells and
blood vessels in interstitial connective tissue displayed their
normal histological structure. Therefore, these two groups had a
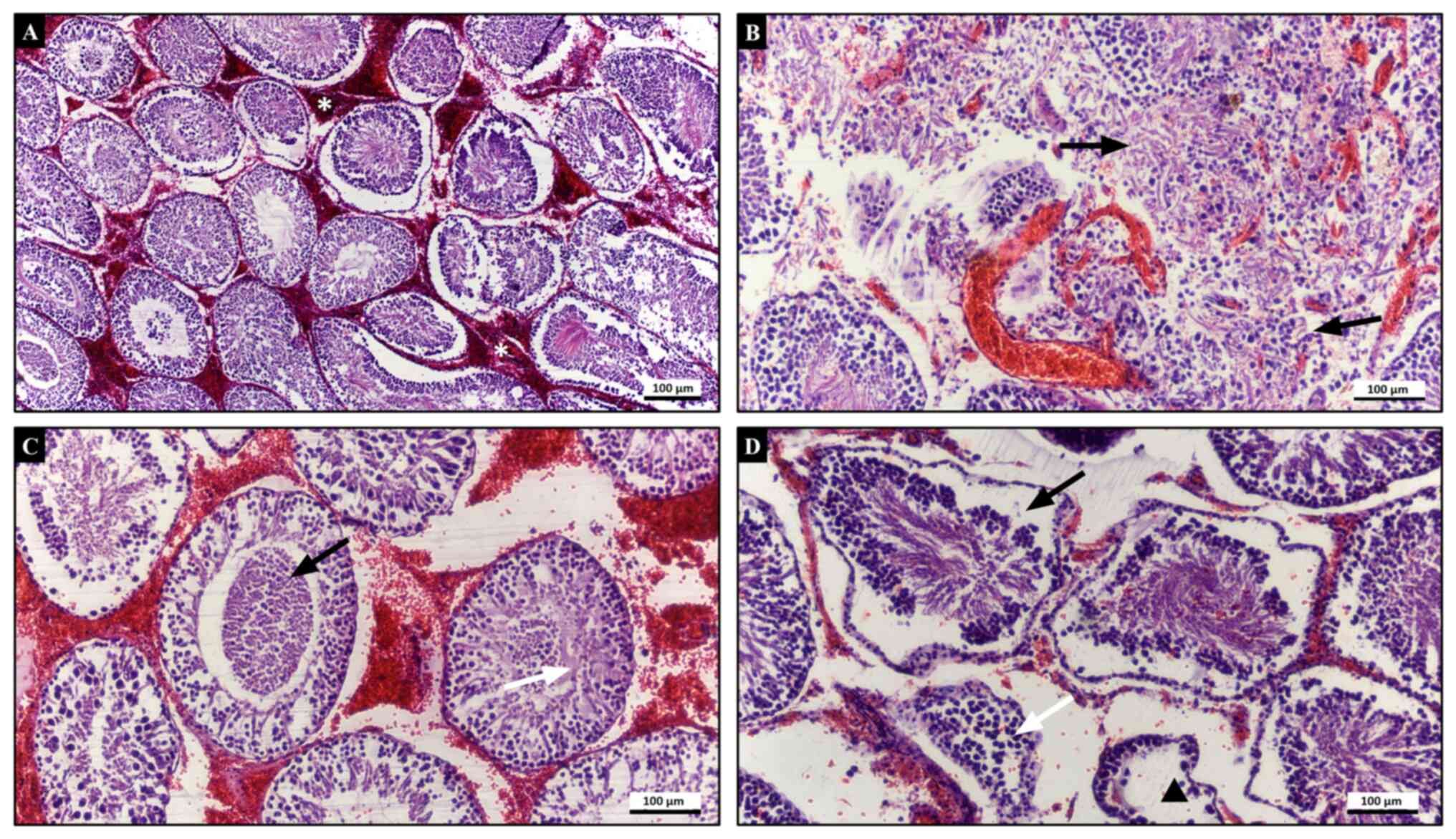
grade 1 testicular damage with a total score of 1. Rats in the T/D
group had severe testicular degenerative changes characterized by
loss of cohesion of germinal cells, sloughed germinal cells within
the seminiferous tubules and coagulative necrosis with loss of
seminiferous tubule epithelium (Fig.
2). Boundaries of some tubules were unclear and germinal cells
were dispersed. Haemorrhage and oedema were prominent in the
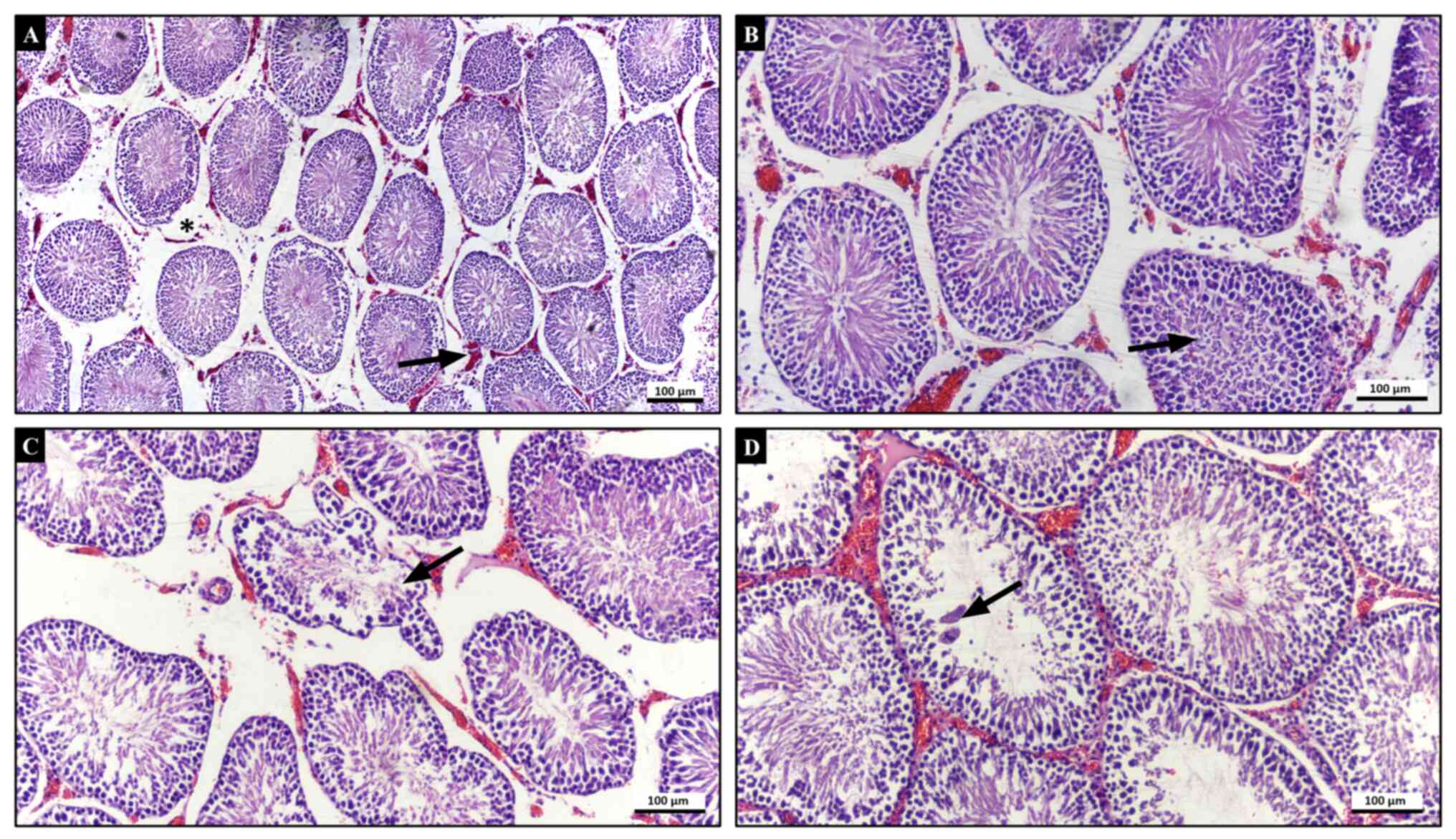
interstitial area. In the T/D + CeO2 group, seminiferous
tubules were relatively intact and germinal cells were more
cohesive compared with those in the T/D group. In some tubules,
degenerated sloughed germinal cells and multinucleated giant cells
were present. Tubular atrophy decreased and only a few tubules
displayed irregularities in boundaries. Haemorrhage and vascular
oedema were also decreased in comparison with that in the T/D group
(Fig. 3). The testicular injury
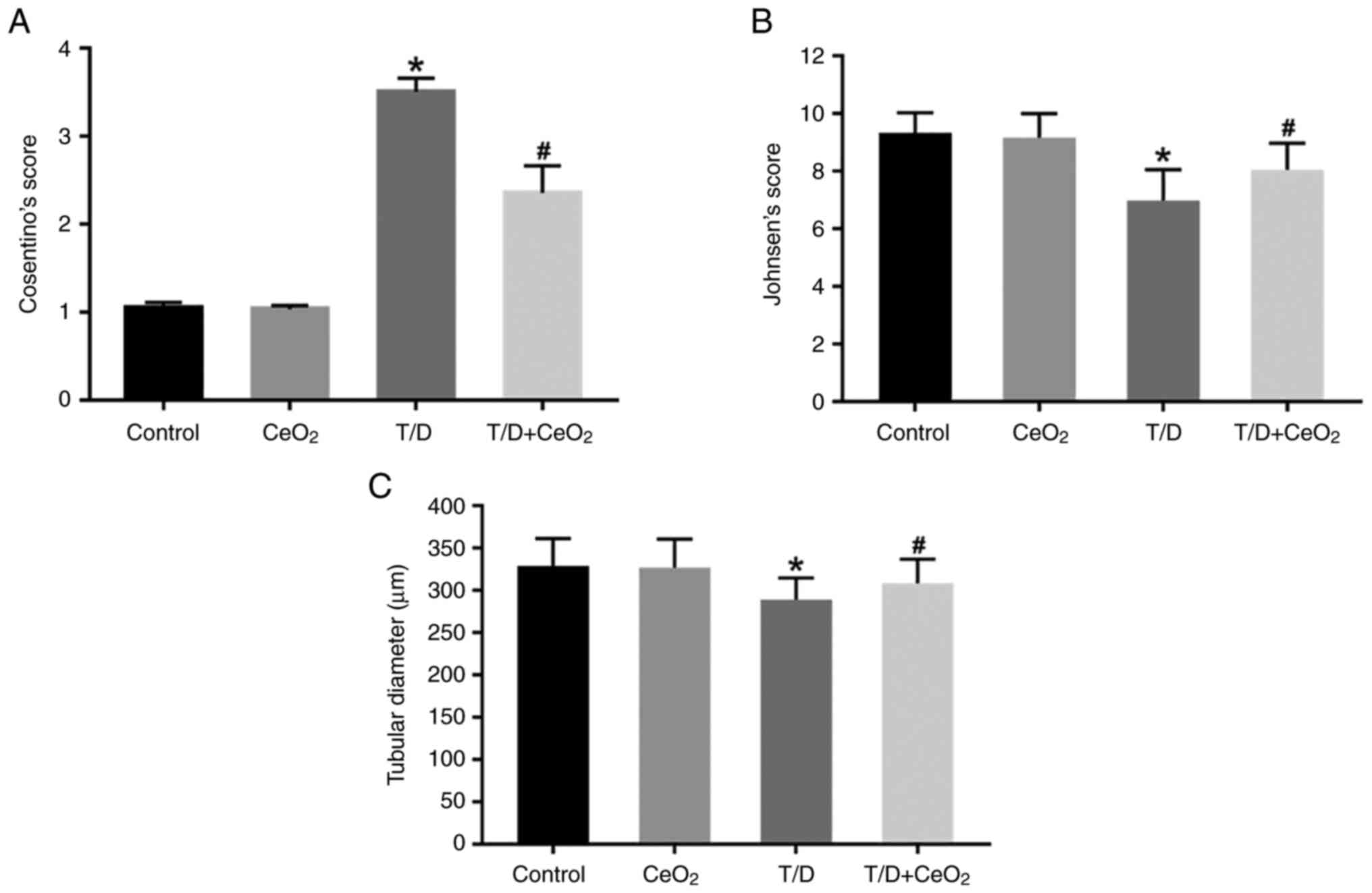
Cosentino's score was significantly increased in the T/D group
(3.51±0.15) compared with the control (1.05±0.06) and
CeO2 group (1.03±0.04) (P<0.0001; Fig. 4A). Rats in the T/D +
CeO2 (2.36±0.31) group demonstrated significantly milder
tissue lesions compared with those in T/D group (P<0.0001;
Fig. 4A) and close to normal
appearance was observed. These findings indicate that
CeO2 treatment ameliorates testicular injury caused by
T/D.
The results of Johnsen's scoring demonstrated that
spermatogenesis was normal in both control (9.33±0.71) and
CeO2 groups (9.17±0.83) (Fig. 4B). In the T/D group, the Johnsen's
score (6.98±1.07) decreased significantly compared with the control
and CeO2 groups (P<0.0001; Fig. 4B). A significant increase in
Johnsen's score was observed in the T/D + CeO2 group
(8.05±0.92) compared with the T/D group (P<0.0001; Fig. 4B). This suggests that
CeO2 helps to maintain spermatogenesis activity at
testicular T/D.
When seminiferous tubular diameter measurements were
evaluated among the groups, a significant decrease was revealed in
T/D group (289.21±25.68) compared with the control (329.05±32.36)
and CeO2 (327.06±33.88) groups (P<0.0001; Fig. 4C). In the T/D + CeO2
group tubule diameter increased significantly (308.52±28.60)
compared to the T/D group (P<0.0001; Fig. 4C), indicating that CeO2
retains the diameter of the seminiferous tubules, which are the
sites where spermatogenesis takes place.
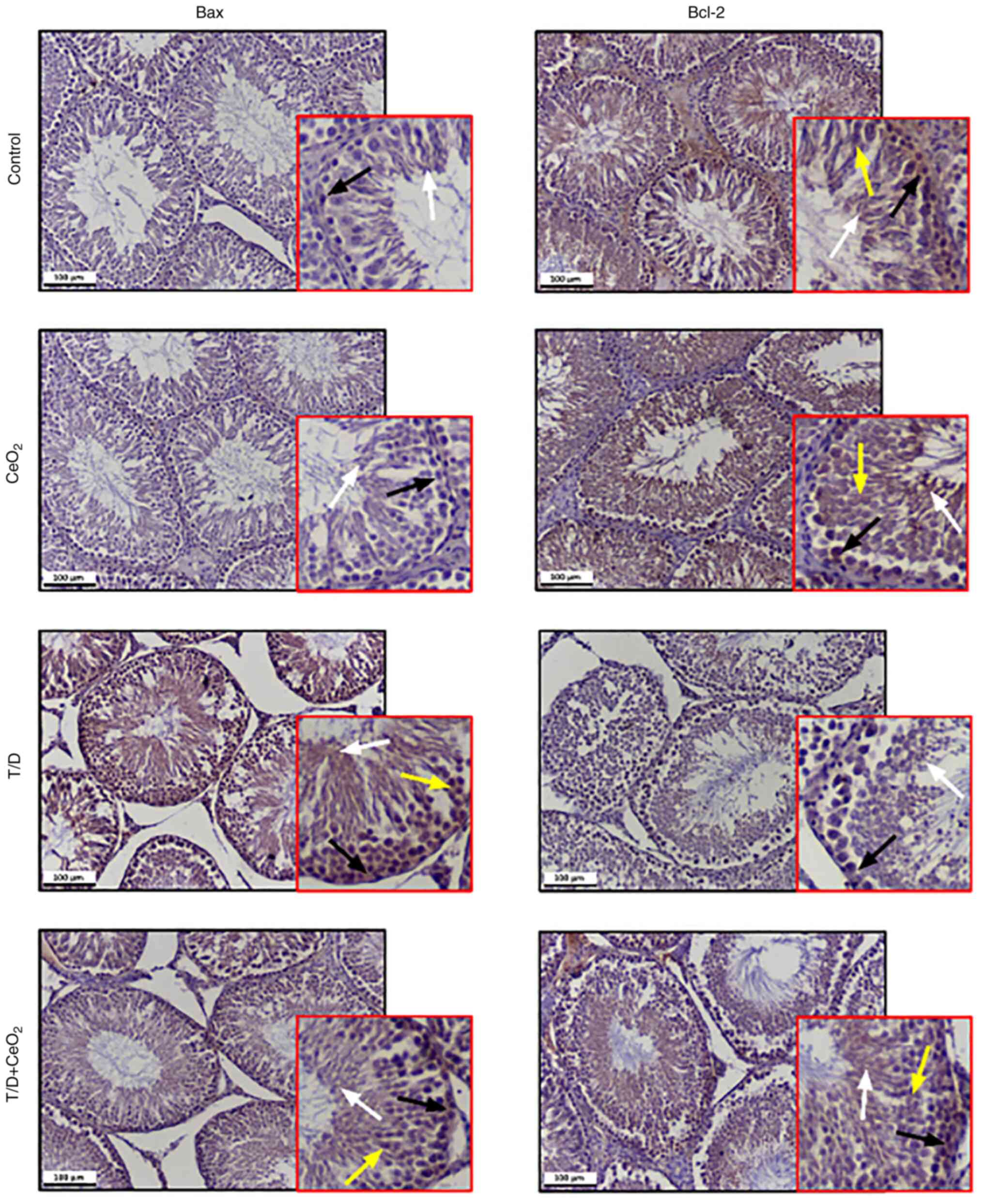
Immunohistochemical expression of
apoptosis-related proteins
Immunohistochemical analysis of the Bax/Bcl-2
expression demonstrated that rats in the control and
CeO2 groups had low Bax expression in a few
spermatogonia and spermatozoa and high Bcl-2 expression in all
germinal cells including spermatogonia, spermatocytes, spermatids
and spermatozoa (Fig. 5). The T/D
group demonstrated increased Bax and decreased Bcl-2 expression
levels in their seminiferous tubules compared with the control and
CeO2 groups (Fig. 5).
Significant downregulation of Bax and upregulation of Bcl-2 was
observed in the T/D + CeO2 group compared to the T/D
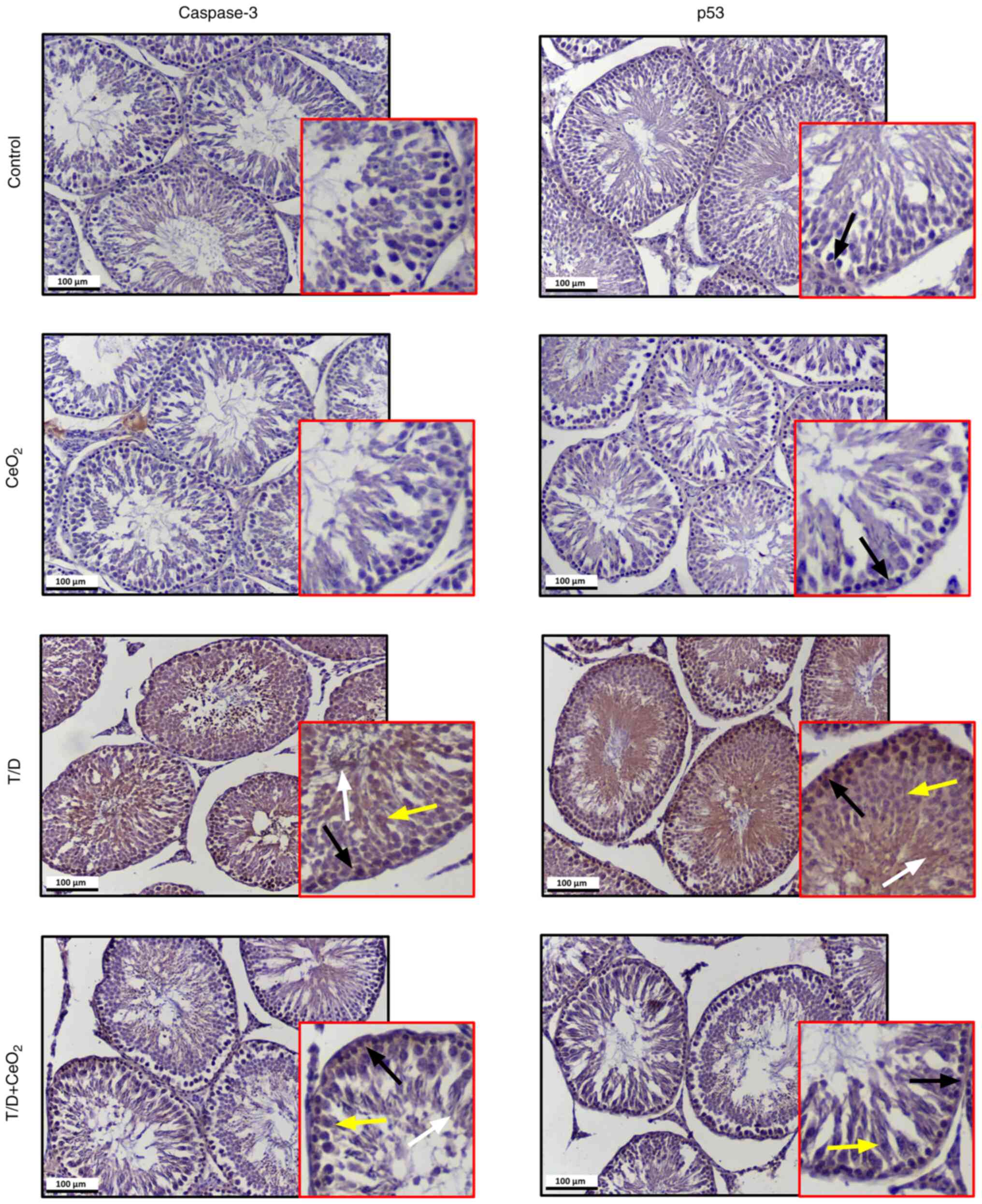
group (Table III). Moreover,
very low expression levels of caspase 3 were observed in some
spermatogonial cells of both control and CeO2 groups
(Fig. 6). The expression of
caspase-3 throughout the seminiferous tubules was increased in the
T/D group compared with the control and CeO2 groups,
while it was reduced in the T/D + CeO2 group compared
with the T/D group (Fig. 6). The
expression of p53 was upregulated in all germinal cells in the T/D
group compared with the control and CeO2 groups that
displayed low expression. The T/D + CeO2 group
demonstrated a significant decrease in the p53 expression level
compared with the T/D group (Fig.
6). Together, these findings suggest that CeO2 is
able to suppress the T/D-induced apoptotic pathway. Table III shows statistical comparison
of the expression levels of apoptosis proteins between groups.
 | Table IIIComparison of staining intensity of
the apoptosis-related proteins between groups. |
Table III
Comparison of staining intensity of
the apoptosis-related proteins between groups.
| Protein | Control (n=6) | CeO2
(n=6) | T/D (n=6) | T/D +
CeO2 (n=6) | Multiple
comparison | P-value |
|---|
| Bax | 24.61±3.67 |
21.81±3.21a,b |
128.68±7.27a |
65.99±6.39a,b | Control vs.
CeO2 | 0.0026 |
| | | | | | Control vs.
T/D | <0.0001 |
| | | | | | Control vs. T/D +
CeO2 | <0.0001 |
| | | | | | T/D vs.
CeO2 | <0.0001 |
| | | | | | T/D vs. T/D +
CeO2 | <0.0001 |
| Bcl-2 | 117.77±8.01 |
107.59±12.32a,b |
32.20±4.43a |
63.16±6.21a,b | Control vs.
CeO2 | 0.0001 |
| | | | | | Control vs.
T/D | <0.0001 |
| | | | | | Control vs.
T/D+CeO2 | <0.0001 |
| | | | | | T/D vs.
CeO2 | <0.0001 |
| | | | | | T/D vs. T/D+
CeO2 | <0.0001 |
| Caspase-3 | 19.34±2.27 |
21.81±2.80b |
78.07±5.45a |
35.60±3.39a,b | Control vs.
CeO2 | 0.1098 |
| | | | | | Control vs.
T/D | <0.0001 |
| | | | | | Control vs.
T/D+CeO2 | <0.0001 |
| | | | | | T/D vs.
CeO2 | <0.0001 |
| | | | | | T/D vs. T/D+
CeO2 | <0.0001 |
| p53 | 36.97±4.77 |
35.42±4.45a,b |
104.75±4.63a |
80.79±6.18a,b | Control vs.
CeO2 | <0.0001 |
| | | | | | Control vs.
T/D | <0.0001 |
| | | | | | Control vs.
T/D+CeO2 | <0.0001 |
| | | | | | T/D vs.
CeO2 | <0.0001 |
| | | | | | T/D vs. T/D+
CeO2 | <0.0001 |
Biochemical analysis
MDA level was significantly increased in the T/D
group compared with the control (P=0.003) and CeO2
(P=0.004) groups in the testicular tissue (Table IV). A significantly decrease in
the MDA level was observed in the T/D + CeO2 group
compared with T/D group (P=0.007; Table IV).
 | Table IVMDA level and CAT, GST and PON-1
enzyme activities. |
Table IV
MDA level and CAT, GST and PON-1
enzyme activities.
| Features | Control (n=6) | CeO2
(n=6) | T/D (n=6) | T/D +
CeO2 (n=6) | Multiple
Comparison | P-value |
|---|
| MDA, nmol/mg
protein | 3.98±1.03 |
4.10±0.48a |
7.06±2.82b |
4.31±0.82a | Control vs.
CeO2 | 0.894 |
| | | | | | Control vs.
T/D | 0.003 |
| | | | | | Control vs. T/D +
CeO2 | 0.720 |
| | | | | | T/D vs.
CeO2 | 0.004 |
| | | | | | T/D vs. T/D +
CeO2 | 0.007 |
| CAT, IU/mg | 238.04±43.40 |
299.19±65.49a |
1,201.68±243.47b |
642.18±117.14a | Control vs.
CeO2 | 0.762 |
| | | | | | Control vs.
T/D | <0.0001 |
| | | | | | Control vs. T/D +
CeO2 | 0.056 |
| | | | | | T/D vs.
CeO2 | <0.0001 |
| | | | | | T/D vs. T/D +
CeO2 | 0.011 |
| GST, mIU/mg | 36.66±8.73 | 38.36±4.52 |
45.65±8.09b |
35.28±2.75a | Control vs.
CeO2 | 0.657 |
| | | | | | Control vs.
T/D | 0.027 |
| | | | | | Control vs. T/D +
CeO2 | 0.719 |
| | | | | | T/D vs.
CeO2 | 0.067 |
| | | | | | T/D vs. T/D +
CeO2 | 0.012 |
| PON-1, U/mg | 3.06±0.77 |
2.72±0.51a |
1.03±0.25b | 2.15±0.56 | Control vs.
CeO2 | 0.659 |
| | | | | | Control vs.
T/D | 0.011 |
| | | | | | Control vs. T/D +
CeO2 | 0.243 |
| | | | | | T/D vs.
CeO2 | 0.029 |
| | | | | | T/D vs. T/D +
CeO2 | 0.171 |
The CAT enzyme activity in the T/D group was
significantly higher compared with that in the control and
CeO2 groups (P<0.0001; Table IV). A significant decrease in CAT
enzyme activity was observed in the T/D + CeO2 group
compared with T/D group (P=0.011; Table IV).
The PON-1 enzyme activity in the T/D group was
significantly lower compared with that in the control (P=0.011) and
CeO2 (P=0.029) groups (Table IV).
GST enzyme activity was revealed to be significantly
increased in the T/D group compared with the control group in the
testicular tissue (P=0.027). A significant decrease in GST enzyme
activity was observed in the T/D + CeO2 group compared
with T/D group (P=0.012; Table
IV).
Discussion
Testicular T can produce germ cell damage, resulting
in subfertility or infertility (36). In the present study, I/R damage was
the main pathological pathway and the present study aimed to
investigate possible treatments for I/R. Some important
pathological processes in damage formation are anoxia, increased
intracellular Ca2+ concentration, leukocyte migration,
increase in proinflammatory cytokines and oxidative stress caused
by ROS (37,38). The balance between oxidative stress
and antioxidant defence is disturbed and a seriesof events that can
cause tissue damage are triggered (39,40).
It has been reported that the activities of various antioxidant
enzymes such as superoxide dismutase (SOD), glutathione peroxidase
and CAT increase during I/R injury (41). Additional damage pathways are
apoptosis and programmed cell death (42,43).
Oxidative stress has been associated with apoptosis in different
cell types, such as germ cells, Sertoli cells and spermatogenic
cells (44). Indeed, apoptosis of
damaged germ cells is a common response to different noxious
stimuli, thereby protecting the next generation of germ cells from
the damaged cell population (45-47).
Several antioxidants such as N-acetylcysteine, growth factors,
carnitine, resveratrol, melatonin, vitamin E have been studied to
reduce reperfusion injury (48-50).
In the present study, the protective effect of nanoceria with
antioxidant and antiapoptotic activity against I/R damage in rat
testicles was investigated.
Nanotechnology is making striking developments in
different areas of human life (51). Nanoceria is a nanoparticle that has
been studied in different oxidative stress models of the testes and
has been reported to reduce cell and tissue damage due to its
antioxidant properties (25,52).
It has been reported that nanoceria reduce tissue damage in lower
limbs and liver ischemia reperfusion models with its antioxidant
effect (53,54). There are studies reporting that
cerium exerts protective and antioxidative effects on testicular
tissue in oxidative stress (44,52,55).
Nanoceria has been reported to show multienzymatic and mimetic
activities, including superoxide oxidase, catalase and oxidase
activities (56). Nonetheless,
studies on male reproductive system in rats and mice have
demonstrated toxicity, disturbing or disrupting the normal activity
and function (55-57).
These negative effects may be caused by excessively high dose of
nanocera, as well as its shape, size, surface charge and the
agglomeration state (58-61).
In the present study, nanoceria was injected in rats
because of the very low absorption of nanoparticles by inhalation
or oral administration (62). The
dose of nanoceria is an important factor. The therapeutic efficacy
of 0.5 mg/kg used in the present study has been demonstrated in
other experimental models (53,63-65).
Ozbal et al (66) reported
that 2 h ischaemia and 2 h reperfusion of the testis causes
testicular damage in rats, such as degenerative changes in testis
tissue, loss of germinal cells maturation, interstitial oedema and
disorganization in the seminiferous tubule. In the present study,
statistically significant changes were observed in biochemical
markers in the T/D group compared with the control group. MDA
level, CAT and GST activities in testicular tissue were revealed to
be significantly higher in the T/D group compared with the control
group. Significant decreases were observed in these three
parameters in the T/D + CeO2 group compared with the T/D
group. PON-1 enzyme activity was significantly decreased in the T/D
group compared with the control group. Although there was a
relative increase in the T/D + CeO2 group compared with
the T/D group, this was not statistically significant. Post-damage
oxidative stress and antioxidant defence markers may differ between
studies.
Although SOD and CAT enzymes, which are in the
antioxidant enzyme group, generally show similar trends in previous
I/R studies, there are also studies with contradictory results.
Islekel et al (67)
reported that SOD activity decreased while CAT activity increased
in a brain ischemia reperfusion model. This can be explained by the
transient substrate induction proposed by Stanimirovic et al
(68) in a study with Mongolian
gerbils. Free radicals produced by ischemia and reperfusion in the
present study may not be enough to affect the three-dimensional
structure of CAT, which is normally retained in peroxisomes. A
small amount of radicals that are not scavenged by other
antioxidant enzymes may diffuse to peroxisomes and cause changes in
the enzyme structure, leading to greater accessibility of the
enzyme to the substrate molecule and an increase in enzyme
activity. It has been reported that I/R damaged tissues have
significantly higher antioxidant enzyme activities and MDA levels
compared with the control (69,70).
A previous study reported an increase in the activities antioxidant
enzymes, while a different study reported that their activities
decreased depending on the degree of I/R damage in the testicular
tissue (71,72). However, other studies have
indicated that lipid peroxidation products increase in I/R damaged
testes (73,74). Moreover, experimental studies on
I/R injury have indicated that antioxidants reduce short-term
damage in testicular T (75,76).
In general. these previous studies have shown that the applied
model is successful in creating oxidative stress and nanoceria can
reduce this stress.
Several biomarkers have been identified to
accurately assess the apoptotic process (77). Bax, bcl-2, caspase-3 and p-53
protein are the most frequently evaluated markers because of their
important roles in the apoptotic pathway. Membrane destabilization
resulting from lipid peroxidation causes mitochondrial cytochrome
c to be released into the cytoplasm (41). The released cytochrome c
facilitates the formation of apoptosomes. This apoptosome activates
initiator caspase-9 and then effector caspase-3, and apoptosis
occurs (78). Among the changes
resulting from ischemic insult in the different parenchymateous
organs, ROS formation and possible apoptotic changes have been the
subject of previous studies (11,12).
Apoptosis is an active form of cell death considered
to occur in adult tissues in a wide range of physiological settings
such as metamorphosis, tissue removal and several other conditions
(79). The mechanism of apoptosis
mainly consists of two core pathways involved in inducing
apoptosis, namely the extrinsic pathway and the intrinsic pathway.
Both of these apoptotic pathways may lead to the same result
(80,81). In relation to testicular functions,
apoptosis of damaged testicular germ cells is a common response to
various testicular toxicants such as ischemic insult, varicocele,
toxic agents and radiation, therefore protecting the next
generations of germ cells from the damaged cell population
(82). Several biomarkers have
been evaluated for their prognostic value in the apoptotic process
and they have been correlated with histological alterations. The
overexpression of proteins that regulate apoptosis, including Bcl-2
and p53, is a useful predictor of histologic alterations in various
pathologies (83). The p53 gene is
regarded as a major tumour-suppressor gene and mutations in the
this gene may result in an altered protein expression that has lost
its suppressive effect (84).
Specific cytoplasmic Bcl-2 antagonizes ischemia-induced apoptotic
pathways by inhibiting the release of cytochrome c from the
mitochondria (85). In response to
DNA damage, p53 mediates the cell cycle, as well as apoptosis
(86). Therefore, as the
regulatory proteins involved in the apoptotic pathway, the
expression levels of Bcl-2 and p53 in testicular tissue undergoing
a possible ischemic period provides a rational point of
investigation.
Another important gene involved in these specific
alterations is Bax (87). The
expression of this gene is regulated by the tumour suppressor p53
and has been indicated to be involved in p53-mediated apoptosis
(88). In the present study, the
immunohistochemical analysis of the apoptotic pathway demonstrated
that rats in the control and CeO2 groups had low Bax
expression and high Bcl-2 expression in their germinal cells. The
T/D group demonstrated increased Bax and decreased Bcl-2 expression
levels in the seminiferous tubules compared with the control and
CeO2 groups. CeO2 treatment led to
downregulation of Bax and upregulation of Bcl-2. Low expression of
caspase 3, an important protease activated during apoptosis
(89), was observed in
spermatogonial cells in both the control and CeO2 groups
compared with the T/D group. The increased caspase-3 expression
along with the seminiferous tubules in the T/D group was
significantly decreased after CeO2 treatment in
T/D+CeO2 group. Expression of apoptosis-inducing p53 was
upregulated in all germinal cells in the T/D group compared with
the control and CeO2 groups. Rats treated with
CeO2 following T/D demonstrated a significant reduction
in p53 expression levels compared with the T/D group. Treatment
with CeO2 reduced the number of apoptotic cells and
immune reactivity. The present results indicated that
CeO2 treatment provided positive results in reducing
apoptosis, which was similar to studies in which the apoptosis
cascade is initiated in different ways such as via toxicity,
hypoxia, cytotoxicity, ionizing radiation and DNA damage (90,91).
In the histopathological evaluation, rats in the T/D
group had severe testicular degenerative changes characterized by
loss of cohesion in germinal cells, shedding of germinal cells
within the seminiferous tubules and coagulative necrosis.
Testicular injury score was significantly increased in this group
compared with the control and CeO2 groups. Rats in the
T/D + CeO2 group demonstrated milder tissue lesions
compared with the T/D group and a near-normal appearance was
observed. Seminiferous tubules were relatively intact and germinal
cells are more adherent. Germinal cells with degenerated shells and
multinucleated giant cells were present in some tubules. Tubular
atrophy was decreased and marginal irregularities were observed in
only a few tubules. Haemorrhage and vascular oedema were also
reduced. Johnsen's scoring results demonstrated that
spermatogenesis was normal in both the control and CeO2
groups, while in the T/D group this was significantly decreased
compared with the control and CeO2 groups. A significant
increase was observed in the Johnsen's score in the T/D +
CeO2 group compared with the T/D group.
Histopathological results and Johnson's scores are consistent with
with the studies from Saleh et al (52) and Mousavi et al (91).
In the present study CeO2 significantly
reduced testicular damage after testicular T/D and increased the
Johnsen's score in histopathological examination. The increased MDA
levels, SOD and GST activities along with decreased PON-1
activities in testicular tissues may reflect cellular oxidative
stress or an involvement of these enzymes in compensatory
mechanisms. CeO2 treatment significantly reduced the
expression of caspase-3 and p53 in the seminiferous tubules. In the
biochemical analysis, a significant decrease in MDA level and CAT
activity, along with a significant decrease in GST enzyme activity,
were detected after CeO2 treatment. The present results
demonstrated that CeO2 had a positive effect after
testicular T/D.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AA, SY and MK were responsible for designing the
study, and analyzing and interpreting the data. CO performed the
study in the laboratory in accordance with the methodology. MA was
responsible for the acquisition, analysis and interpretation of the
data. MA and SY confirm the authenticity of all the raw data. ACG
and MK provided scientific and technical assistance to the
experiments, and critically revised the article for important
intellectual content. SY collected samples and was responsible for
the execution of the project. ACG was responsible for the cellular
and molecular experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was obtained from
Gazi University Experimental Animals Ethics Committee (Ankara,
Turkey; approval no. G.U.ET-19-059).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharp VJ, Kieran K and Arlen AM:
Testicular torsion: Diagnosis, evaluation, and management. American
Family Physician. 88:835–840. 2013.PubMed/NCBI
|
|
2
|
Selbst SM, Friedman MJ and Singh SB:
Epidemiology and etiology of malpractice lawsuits involving
children in US emergency departments and urgent care centers.
Pediatr Emerg Care. 21:165–169. 2005.PubMed/NCBI
|
|
3
|
Bo X, Wang P, Nie Y, Li R, Lu J and Wang
H: Protective effect of hypothermia and vitamin E on spermatogenic
function after reduction of testicular torsion in rats. Exp Ther
Med. 20:796–801. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ringdahl E and Teague L: Testicular
torsion. Am Fam Physician. 74:1739–1743. 2006.PubMed/NCBI
|
|
5
|
Pogorelić Z, Mustapić K, Jukić M, Todorić
J, Mrklić I, Mešštrović J, Jurić I and Furlan D: Management of
acute scrotum in children: A 25-year single center experience on
558 pediatric patients. Can J Urol. 23:8594–8601. 2016.PubMed/NCBI
|
|
6
|
Celik E, Oguzturk H, Sahin N, Turtay MG,
Oguz F and Ciftci O: Protective effects of hesperidin in
experimental testicular ischemia/reperfusion injury in rats. Arch
Med Sci. 12:928–934. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Minutoli L, Antonuccio P, Polito F, Bitto
A, Fiumara T, Squadrito F, Nicotina PA, Arena S, Marini H, Romeo C
and Altavilla D: Involvement of mitogen-activated protein kinases
(MAPKs) during testicular ischemia-reperfusion injury in nuclear
factor-kappaB knock-out mice. Life Sci. 81:413–242. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Akbas H, Ozden M, Kanko M, Maral H, Bulbul
S, Yavuz S, Ozker E and Berki T: Protective antioxidant effects of
carvedilol in a rat model of ischaemia-reperfusion injury. J Int
Med Res. 33:528–536. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Unsal A, Eroglu M, Avci A, Cimentepe E,
Guven C, Derya Balbay M and Durak I: Protective role of natural
antioxidant supplementation on testicular tissue after testicular
torsion and detorsion. Scand J Urol Nephrol. 40:17–22.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chi KK, Zhang WH, Wang GC, Chen Z, He W,
Wang SG, Cui Y, Lu P, Wang XJ and Chen H: Comparison of
intraperitoneal and intraepididymal quercetin for the prevention of
testicular torsion/detorsion-induced injury. Urology. 99:106–111.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Filho DW, Torres MA, Bordin AL,
Crezcynski-Pasa TB and Boveris A: Spermatic cord torsion, reactive
oxygen and nitrogen species and ischemia-reperfusion injury. Mol
Aspects Med. 25:199–210. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nicoud IB, Knox CD, Jones CM, Anderson CD,
Pierce JM, Belous AE, Earl TM and Chari RS: 2-APB protects against
liver ischemia-reperfusion injury by reducing cellular and
mitochondrial calcium uptake. Am J Physiol Gastrointest Liver
Physiol. 293:G623–G30. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vercesi AE, Castilho RF, Kowaltowski AJ,
de Oliveira HCF, de Souza-Pinto NC, Figueira TR and Busanello ENB:
Mitochondrial calcium transport and the redox nature of the
calcium-induced membrane permeability transition. Free Radic Biol
Med. 29:1–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gaschler MM and Stockwell B: Lipid
peroxidation in cell death. Biochem Biophys Res Commun.
482:419–425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Turner TT and Lysiak JJ: Oxidative stress:
A common factor in testicular dysfunction. J Androl. 29:488–498.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chan WC and Nie S: Quantum dot
bioconjugates for ultra sensitive nonisotopic detection. Science.
281:2016–2018. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vaseashta A and Dimova-Malinovska D:
Nanostructured and nanoscale devices, sensors and detectors. Sci
Technol Adv Mater. 6(312)2005.
|
|
18
|
Langer R: Drugs on target. Science.
293:58–59. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Roy K, Mao HQ, Huang SK and Leong KW: Oral
gene delivery with chitosan-DNA nanoparticles generates immunologic
protection in a murine model of peanut allergy. Nat Med.
5(387)1999.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Sachlos E, Gotora D and Czernuszka JT:
Collagen scaffolds reinforced with biomimetic composite nano-sized
carbonate-substituted hydroxyapatite crystals and shaped by rapid
prototyping to contain internal microchannels. Tissue Eng.
12:2479–2487. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Farokhzad OC, Cheng J, Teply BA, Sherifi
I, Jon S, Kantoff PW, Richie JP and Langer R: Targeted
nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo.
Proc Natl Acad Sci USA. 103(6315)2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim J, Kim HY, Song SY, Go SH, Sohn HS,
Baik S, Soh M, Kim K, Kim D, Kim HC, et al: Synergistic oxygen
generation and reactive oxygen species scavenging by manganese
ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis
treatment. ACS Nano. 13:3206–3217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Celardo I, De Nicola M, Mandoli C,
Pedersen JZ, Traversa E and Ghibelli L: Ce3+ Ions
determine redox-dependent anti-apoptotic effect of cerium oxide
nanoparticles. ACS Nano. 5:4537–4549. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Heckert EG, Karakoti AS, Seal S and Self
WT: The role of cerium redox state in the SOD mimetic activity of
nanoceria. Biomaterials. 29:2705–2709. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Moridi H, Hosseini SA, Shateri H,
Kheiripour N, Kaki A, Hatami M and Ranjbar A: Protective effect of
cerium oxide nanoparticle on sperm quality and oxidative damage in
malathion-induced testicular toxicity in rats: An experimental
study. Int J Reprod Biomed. 16:261–266. 2018.PubMed/NCBI
|
|
26
|
Shcherbakov AB, Reukov VV, Yakimansky AV,
Krasnopeeva EL, Ivanova OS, Popov AL and Ivanov VK: CeO2
nanoparticle-containing polymers for biomedical applications: A
review. Polymers (Basel). 13(924)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Singh S, Kumar U, Gittess D, Sakthivel TS,
Babu B and Seal S: Cerium oxide nanomaterial with dual
antioxidative scavenging potential: Synthesis and characterization.
J Biomater Appl. 36:834–842. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cosentino MJ, Nishida M, Rabinowitz R and
Cockett AT: Histopathology of prepubertal rat testes subjected to
various durations of spermatic cord torsion. J Androl. 7:23–31.
1986.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Johnsen SG: Testicular biopsy score
count-a method for registration of spermatogenesis in human testes:
Normal values and results in 335 hypogonadal males. Hormones.
1:2–25. 1970.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Crowe AR and Yue W: Semi-quantitative
determination of protein expression using immunohistochemistry
staining and analysis: An integrated protocol. Bio Protoc.
9(e3465)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Van Ye TM, Roza AM, Pieper GM, Henderson J
Jr, Johnson CP and Adams MB: Inhibition of intestinal lipid
peroxidation does not minimize morphologic damage. J Surg Res.
55:553–558. 1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hodges DM, DeLong JM, Forney CF and Prange
RK: Improving the thiobarbituric acid reactive substances assay for
estimating lipid peroxidation in plant tissues containing
anthocyanin and other interfering compounds. Planta. 207:604–611.
1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Aebi H: Catalase. In: H.U.Bergmeyer (Ed):
Methods of Enzymatic Analysis, Academic Press, New York and London,
pp673-677, 1974.
|
|
34
|
Brites FD, Verona J, Schreier LE, Fruchart
JC, Castro GR and Wikinski RL: Paraoxonase 1 and
platelet-activating factor acetylhydrolase activities in patients
with low hdl-cholesterol levels with or without primary
hypertriglyceridemia. Arch Med Res. 35:235–240. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
36
|
Bodur A, Alver A, Kahraman C, Altay DU and
İnce İ: Investigation of N-acetylcysteine on contralateral testis
tissue injury by experimental testicular torsion: Long-term effect.
Am J Emerg Med. 34:1069–1074. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ustün H, Akgül KT, Ayyildiz A, Yağmurdur
H, Nuhoğlu B, Karagüzel E, Oğüş E and Germiyanoğlu C: Effect of
phospodiesterase 5 inhibitors on apoptosis and nitric oxide
synthases in testis torsion: An experimental study. Pediatr Surg
Int. 24:205–211. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kazez A, Demirbağ M, Ustündağ B, Ozercan
IH and Sağlam M: The role of melatonin in prevention of intestinal
ischemia-reperfusion injury in rats. J Pediatr Surg. 35:1444–1448.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ates B, Yilmaz I, Geckil H, Iraz M,
Birincioglu M and Fiskin K: Protective role of melatonin given
either before ischemia or prior to reperfusion on intestinal
ischemia-reperfusion damage. J Pineal Res. 37:149–152.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Elahi MM, Kong YX and Matata BM: Oxidative
stress as a mediator of cardiovascular disease. Oxid Med Cell
Longev. 2:259–269. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
McCord JM: Oxygen-derived free radicals in
postischemic tissue injury. N Engl J Med. 312:159–163.
1985.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive oxygen species-induced lipid
peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med
Cell Longev. 2019(5080843)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kasahara E, Sato EF, Miyoshi M, Konaka R,
Hiramoto K, Sasaki J, Tokuda M, Nakano Y and Inoue M: Role of
oxidative stress in germ cell apoptosis induced by
di(2-ethylhexyl)phthalate. Biochem J. 365:849–56. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lin WW, Lamb DJ, Wheeler TM, Abrams J,
Lipshultz LI and Kim ED: Apoptotic frequency is increased in
spermatogenic maturation arrest and hypospermatogenic states. J
Urol. 158:1791–1793. 1997.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shin JH, Mori C and Shiota K: Involvement
of germ cell apoptosis in the induction of testicular toxicity
following hydroxyurea treatment. Toxicol Appl Pharmacol.
155:139–149. 1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tesarik J, Greco E, Cohen-Bacrie P and
Mendoza C: Germ cell apoptosis in men with complete and incomplete
spermiogenesis failure. Mol Hum Reprod. 4:757–762. 1998.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Payabvash S, Salmasi AH, Kiumehr S,
Tavangar SM, Nourbakhsh B, Faghihi SH and Dehpour AR: Salutary
effects of N-acetylcysteine on apoptotic damage in a rat model of
testicular torsion. Urol Int. 79:248–254. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Suwalsky M, Villena F and Gallardo MJ: In
vitro protective effects of resveratrol against oxidative damage in
human erythrocytes. Biochim Biophys Acta. 1848:76–82.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Espino J, Bejarano I, Ortiz A, Lozano GM,
García JF, Pariente JA and Rodríguez AB: Melatonin as a potential
tool against oxidative damage and apoptosis in ejaculated human
spermatozoa. Fertil Steril. 94:1915–1957. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ranjbar A, Firozian F, Soleimani Asl S,
Ghasemi H, Taheri Azandariani M, Larki A, Hosseini A and Naserabadi
A: Nitrosative DNA damage after sub-chronic exposure to silver
nanoparticle induces stress nephrotoxicity in rat kidney. Toxin
Rev. 37:327–333. 2017.
|
|
52
|
Saleh H, Nassar AMK, Noreldin AE, Samak D,
Elshony N, Wasef L, Elewa YHA, Hassan SMA, Saati AA, Hetta HF, et
al: Chemo-protective potential of cerium oxide nanoparticles
against fipronil-induced oxidative stress, apoptosis, inflammation
and reproductive dysfunction in male white albino rats. Molecules.
25(3479)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tuncay A, Sivgin V, Ozdemirkan A, Sezen
SC, Boyunaga H, Kucuk A, Gunes I and Arslan M: The effect of cerium
oxide on lung tissue in lower extremity ischemia reperfusion injury
in sevoflurane administered rats. Int J Nanomedicine. 15:7481–7489.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ni D, Wei H, Chen W, Bao Q, Rosenkrans ZT,
Barnhart TE, Ferreira CA, Wang Y, Yao H, Sun T, et al: Ceria
nanoparticles meet hepatic Ischemia-reperfusion injury: The perfect
imperfection. Adv Mater. 31(e1902956)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Artimani T, Amiri I, Soleimani Asl S,
Saidijam M, Hasanvand D and Afshar S: Amelioration of
diabetes-induced testicular and sperm damage in rats by cerium
oxide nanoparticle treatment. Andrologia. 50(e13089)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Charbgoo F, Ahmad MB and Darroudi M:
Cerium oxide nanoparticles: Green synthesis and biological
applications. Int J Nanomedicine. 12:1401–1413. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Adebayo OA, Akinloye O and Adaramoye OA:
Cerium oxide nanoparticle elicits oxidative stress, endocrine
imbalance and lowers sperm characteristics in testes of balb/c
mice. Andrologia. 50:2018.PubMed/NCBI View Article : Google Scholar : doi:
10.1111/and.12920.
|
|
58
|
Alpaslan E, Geilich BM, Yazici H and
Webster TJ: pH-Controlled cerium oxide nanoparticle inhibition of
both gram-positive and gram-negative bacteria growth. Sci Rep.
7(45859)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kalyanaraman V, Naveen SV, Mohana N,
Balaje RM, Navaneethakrishnan KR, Brabu B, Murugan SS and Kumaravel
TS: Biocompatibility studies on cerium oxide nanoparticles-combined
study for local effects, systemic toxicity and genotoxicity via
implantation route. Toxicol Res (Camb). 8:25–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Karakoti AS, Singh S, Kumar A, Malinska M,
Kuchibhatla SV, Wozniak K, Self WT and Seal S: PEGylated nanoceria
as radical scavenger with tunable redox chemistry. J Am Chem Soc.
131:14144–14145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yang X, Pan H, Wang P and Zhao FJ:
Particle-specific toxicity and bioavailability of cerium oxide
(CeO2) nanoparticles to Arabidopsis thaliana. J Hazard
Mater. 322:292–300. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ganji M, Osman H, Karimi J, Hosseini SA,
Moridi H, Hosseini A, Ahmadimoghaddam D and Ranjbar A: Experimental
study of cerium oxide nanoparticles (CeNP) against malathion
induced lung oxidative toxic stress in rats. Iranian J Pharmacol
Ther. 15:1–7. 2017.
|
|
63
|
Tatar T, Polat Y, Comu FM, Kartal H,
Arslan M and Kucuk A: Effect of cerium oxide on erythrocyte
deformability in rat lower extremity ischemia reperfusion injury.
Bratisl Lek Listy. 119:441–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hegazy MA, Maklad HM, Samy DM, Abdelmonsif
DA, El Sabaa BM and Elnozahy FY: Cerium oxide nanoparticles could
ameliorate behavioral and neurochemical impairments in
6-hydroxydopamine induced Parkinson's disease in rats. Neurochem
Int. 108:361–371. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Manne NDPK, Arvapalli R, Graffeo VA,
Bandarupalli VVK, Shokuhfar T, Patel S, Rice KM, Ginjupalli GK and
Blough ER: Prophylactic treatment with cerium oxide nanoparticles
attenuate hepatic ischemia reperfusion injury in sprague dawley
rats. Cell Physiol Biochem. 42:1837–1846. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ozbal S, Ergur BU, Erbil G, Tekmen I,
Bagrıyanık A and Cavdar Z: The effects of α-lipoic acid against
testicular ischemia-reperfusion injury in Rats.
ScientificWorldJournal. 2012(489248)2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Işlekel S, Işlekel H, Güner G and Ozdamar
N: Alterations in superoxide dismutase, glutathione peroxidase and
catalase activities in experimental cerebral ischemia-reperfusion.
Res Exp Med (Berl). 199:167–176. 1999.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Stanimirovic DB, Micic DV, Markovic M,
Spatz M and Mrsulja BB: ‘Therapeutic window’ for multiple drug
treatment of experimental cerebral ischemia in gerbils. Neurochem
Res. 19:189–194. 1994.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Koltuksuz U, Ozen S, Uz E, Aydinç M,
Karaman A, Gültek A, Akyol O, Gürsoy MH and Aydin E: Caffeic acid
phenethyl ester prevents intestinal reperfusion injury in rats. J
Pediatr Surg. 34:1458–1462. 1999.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yildiz Y, Serter M, Ek RO, Ergin K, Cecen
S, Demir EM and Yenisey C: Protective effects of caffeic acid
phenethyl ester on intestinal ischemia-reperfusion injury. Dig Dis
Sci. 54:738–744. 2009.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Erdemir F, Parlaktas BS, Ozyurt H, Boztepe
O, Atis O and Sahin S: Antioxidant effect of melatonin in systemic
circulation of rats after unilateral testicular torsion. Turk J Med
Sci. 38:1–6. 2008.
|
|
72
|
Wei SM, Yan ZZ and Zhou J: Protective
effect of rutin on testicular ischemia-reperfusion injury. J
Pediatr Surg. 46:1419–1424. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Akgür FM, Kilinç K and Aktuğ T:
Reperfusion injury after detorsion of unilateral testicular
torsion. Urol Res. 21:395–399. 1993.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gökçe A, Oktar S, Koc A, Gonenci R,
Yalcinkaya F, Yonden Z and Duru M: Protective effect of
thymoquinone in experimental testicular torsion. Urol Int.
85:461–465. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Blank ML, O'Neill PJ, Steigman CK, Cobb
LM, Wilde RA, Havenstein PJ and Chaudry IH: Reperfusion injury
following testicular torsion and detorsion in prepubertal rats.
Urol Res. 21:389–393. 1993.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Prillaman HM and Turner TT: Rescue of
testicular function after acute experimental torsion. J Urol.
157:340–345. 1997.PubMed/NCBI
|
|
77
|
Ward TH, Cummings J, Dean E, Greystoke A,
Hou JM, Backen A, Ranson M and Dive C: Biomarkers of apoptosis. Br
J Cancer. 99:841–846. 2008.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104.
1999.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ishizuya-Oka A, Hasebe T and Shi YB:
Apoptosis in amphibian organs during metamorphosis. Apoptosis.
15:350–364. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Bejarano I, Rodríguez AB and Pariente JA:
Apoptosis is a demanding selective tool during the development of
fetal male germ cells. Front Cell Dev Biol. 6(65)2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Jairajpuri ZS, Ghai R, Saluja S, Kapur S
and Bhowmik KT: Expression of apoptosis related and proliferative
proteins in malignant lympho-proliferative disorders. Iran J
Pathol. 12:231–240. 2007.PubMed/NCBI
|
|
84
|
Shi Y, Norberg E and
Vakifahmetoglu-Norberg H: Mutant p53 as a regulator and target of
autophagy. Front Oncol. 10(607149)2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Chen X, Ko LJ, Jayaraman L and Prives C:
p53 levels, functional domains, and DNA damage determine the extent
of the apoptotic response of tumor cells. Genes Dev. 10:2438–2451.
1996.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Misao J, Hayakawa Y, Ohno M, Kato S,
Fujiwara T and Fujiwara H: Expression of bcl-2 protein, an
inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in
ventricular myocytes of human hearts with myocardial infarction.
Circulation. 94:1506–1512. 1996.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kumi-Diaka J and Butler A: Caspase-3
protease activation during the process of genistein-induced
apoptosis in TM4 testicular cells. Biol Cell.
92:115–124. 2000.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kolli MB, Manne NDPK, Para R, Nalabotu SK,
Nandyala G, Shokuhfar T, He K, Hamlekhan A, Ma JY, Wehner PS, et
al: Cerium oxide nanoparticles attenuate monocrotaline induced
right ventricular hypertrophy following pulmonary arterial
hypertension. Biomaterials. 35:9951–9962. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Mousavi A, Gharzi A, Gholami M, Beyranvand
F and Takesh M: The therapeutic effect of cerium oxide nanoparticle
on ischaemia/reperfusion injury in rat testis. Andrologia.
53(e14231)2021.PubMed/NCBI
|