Introduction
Immune check point inhibitors (ICI) are associated
with immune related adverse events (irAEs) involving multiple
endocrinological organs (1).
Hypophysitis and thyroid abnormalities are the most common
endocrine irAEs reported to date (2). Οverall, the incidence of hypophysitis
is up to 17% in patients treated with ICI with male predominance.
The mean age at onset is approximately 60 years old and the mean
time to onset of the diagnosis at aproximately 10.5 weeks. The
prevalence of hypophysitis depends on the type and the dose of ICI;
70% of cases were due to cytotoxic T-lymphocyte protein 4 (CTLA-4)
blockade, 23% to programmed cell death protein 1 (PD-1) blockade,
or in 2% of the cases to its ligand (PD-L1) blockade, and in 3.9%
to combination therapy (CTLA-4 and PD-1) (2-4).
At present, the CTLA-4 antibodies (Abs), ipilimumab, PD-1 Abs
nivolumab, pembrolizumab, cemiplimab and PD-L1 Abs atezolizumab,
avelumab, and durvalumab are Food and Drug Administration (FDA)-
and European Medicines Agencies (EMA)-approved (5,6).
In a systematic review and meta-analysis including
data from 38 randomized clinical trials comprising 7,551 patients
investigating the use of ICIs in the treatment of various cancer
types, hypophysitis incidence ranged from 1.5 to 13.3% in patients
treated with CTLA-4 Abs and 0.3-3% in those with PD-1Abs. Recently,
isolated cases reports have described the diagnosis of central
diabetes insipidus (CDI) due to dysfunction of posterior
pituitary/hypothalamus in patients treated with ICI (1,7-16).
According to data from the WHO global database of
individual case safety reports (17) between January 2011 and March 2019,
a total of 6,089 ICI-related endocrine AEs were reported, of which
1,144 (18.8%) were pituitary events, including hypophysitis
(n=835), hypopituitarism (n=268), pituitary enlargement (n=28), and
other (n=13), while CDI was reported in 7 out of 1,072 (0.7%) of
the registered hypophysitis/hypopituitarism cases.
Herein, we report the case of a patient diagnosed
with simultaneous anterior and posterior hypophysitis
(panhypophysitis) induced by nivolumab and discuss the emerging
difference in the incidence of hypophysitis/CDI among subclasses of
ICIs and the related pathogenic mechanisms.
Case report
A 53-year-old female patient was followed at Laikon
General Hospital for metastatic melanoma of the left tibial treated
with multiples surgeries due to local recurrences. A treatment with
nivolumab-a PD-L1 Ab-was introduced at January 2021. The patient
received 240 mg flat dose by intravenous infusion every 2 weeks and
achieved a partial response (RECIST 1.1) within 6 months, based on
computerised tomography (CT) scanning. Her other routine medication
included venlafaxine. A thorough baseline work-up revealed normal
electrolyte, hepatic, and renal function at the initiation of
immunotherapy and before every session.
However, 6 months after the initiation of nivolumab
she presented with extreme fatigue necessitating a precipitating
hormonal work-up which revealed deficiency of the corticotrope and
thyreotrope axis. The detailed biochemical work-up is shown in
Table I. A replacement treatment
with hydrocortisone (25 mg/24 h) and thyroxine (50 µg/24 h) was
initiated with prompt clinical improvement. Two months after the
diagnosis of the anterior pituitary deficiency the patient was
complaining for frequent nocturia (three to four times with
increased volume each night), fatigue, polydipsia, and polyuria.
Biochemical analyses showed normal 24-h urinary collection and
blood levels of sodium, potassium and calcium as well as glucose
levels. A 24-h urinary collection showed an important water
diuresis of 5.3 lt/day with low urinary osmolality 184 mOsm/kg
(500-800) and urine specific gravity of 1,002. Plasma osmolality
was also found increased at 309 mOsm/Kg (280-295) indicating a
possible diagnosis of DI. Of note, the patient denied any use of
non-steroid anti-inflammatory drugs or other over-the counter
medications. Moreover, a recent cerebral CT performed in the
context of the staging for the melanoma was normal without
suspicion of secondary metastases. The patient refused initially
the hospitalisation for further functional test. Thus, we decided
to perform the measurement of baseline copeptin levels which was
found low at 2,4 pmol/l being in favour of CDI (Table II).
 | Table IBaseline biochemical parameters of the
patient at diagnosis and post-treatment of DI. |
Table I
Baseline biochemical parameters of the
patient at diagnosis and post-treatment of DI.
| Biochemical
parameter | Onset of DI
diagnosis | After the treatment
of DI | Normal range |
|---|
| Blood | | | |
|
Sodium,
mmol/l | 143 | 139 | 136-143 |
|
Potassium,
mmol/l | 4.9 | 4.4 | 3.7-4.9 |
|
Calcium,
mmol/l | 9.4 | 9.6 | 8-10 |
|
Creatinine,
mg/dl | 1.11 | 1.03 | 0.7-1.2 |
|
Osmolality,
mOsmol/kg H2O | 309.92 | 294 | 280-295 |
| Urine | | | |
|
Urine
specific gravity | 1.004 | 1.020 | 1.010-1.030 |
|
Osmolality,
mOsmol/kg H2O | 184 | 757 | 500-800 |
|
Sodium,
mEq/24 h | 175 | nd | 40-200 |
|
Potassium,
mEq/24 h | 87 | nd | 25-120 |
|
Calcium,
mEq/24 h | 138 | nd | 100-300 |
| Serum | | | |
|
TSH,
µIU/ml | 0.98 | 0.99 | 0.27-4.7 |
|
FT4,
ng/dl | 0.80 | 1.13 | 0.7-2 |
|
ACTH,
pg/ml | <3.0 | <2.9 | 7.0-64 |
|
Prolactin,
ng/ml | 31.0 | 32 | 4.8-23.3 |
|
Cortisol,
µg/dl | 1.04 | 0.7 | 6.2-19.4 |
|
LH,
IU/l | 49.7 | 51 | 7.7-58.5 |
|
FSH,
IU/l | 87.9 | 89 | 25.8-134.8 |
 | Table IIDifferential diagnosis of the
polyuria syndrome based on the water deprivation test and copeptin
levels. |
Table II
Differential diagnosis of the
polyuria syndrome based on the water deprivation test and copeptin
levels.
| Biochemical
parameters | Normal | Central DI | NDI | Primary
polydipsia | Partial CDI |
|---|
| Baseline urinary
osmolality, (mOsm/kg) | >300 | <300 | <300 | 300-800 | 300-800 |
| Urinary osmolality
after water derivationa, mOsm/kg | 800-1,200 | <300 | <300 | 300-800 | 300-800 |
| Urine osmolality
after administration of desmopressin, mOsm/kg | | Increase
>50% | No response | Normal | Increase
<50% |
| Baseline copeptin
levelsb, pmol/l | Normal | <4.9 | >21.4 | Normal | Normal/low |
Following these results, the patient eventually
accepted to be hospitalised and a water deprivation test followed
by desmopressin (DDAVP) administration test was performed (Table III) (18). The weight, blood pressure, urinary
and plasma osmolality were measured at baseline at the initiation
of the test (at 8.00 am) as well as during the phase of dehydration
(every 2 h). The water deprivation test was interrupted after 6 h
due to hypernatremia at 146 mmol/l and patient's intolerance with
symptoms of dizziness (orthostatic symptoms with systolic blood
pressure at 105 mmHg in decubitus and 90 mmHg in the upright
position). Urinary osmolality as well as plasma osmolality remained
unchanged during the 6 h water deprivation excluding a primary
polydipsia syndrome. We then administrated 2 µg of desmopressin
(DDAVP) intravenously with immediate amelioration of clinical
symptoms of polyuria-polydipsia and an increase of the urinary
osmolality from 327 to 716 mOsm/kg (39%) in favor of partial CDI
(19), (Table II). The results of the water
deprivation test are shown in Table
IV.
 | Table IIIDescription of the water deprivation
test. |
Table III
Description of the water deprivation
test.
| Steps to
follow | Parameters or
criteria to evaluate |
|---|
| Before any
measurement: Correction of any electrolyte abnormalities, including
serum potassium and calcium and discontinuation of any medications
that can affect urine output for at least 24 h | Diuretics, SGLT-2
inshibitors, DDAVP, carbamazepine, chlorpropamide, glucocorticoids
and non-steroidal anti-inflammatory drugs; smoking and
caffeine |
| Baseline
measurements (every 2 h) | Weight, blood
pressure, heart rate prior to initiation of dehydration, plasma
osmolality, serum sodium, urine osmolality; urine output and urine
osmolality, serum sodium and plasma osmolality |
| Criteria of
discontinuation | i) Loss of >3%
of body weight; ii) elevation of serum sodium to above normal
limits (≥146-150 mmol/l); and iii) orthostatic hypotension or
orthostatic symptoms or intractable thirst |
| Administration of
DDVAP (2 µg intravenous or intramuscular) | When DDVAP is
administrated: i) Dehydration phase is completed for 8 h; or ii)
two consecutive urine osmolality measurements do not differ by
>10% and there is loss of 2% body weight; or iii) premature
termination of dehydration phase due to loss of >3% of body
weight, elevation of serum sodium to above normal limits, or
intractable |
| Measurement
post-DDAVP administration | Urine and
serum/plasma measurements are obtained hourly for 1-2 h after the
injection. In patients with complete forms of DI, the test can be
performed in <8 h while in those with partial DI the test could
last longer (even 18 h) |
 | Table IVWater deprivation test/DDAVP
administration in the present case. |
Table IV
Water deprivation test/DDAVP
administration in the present case.
| Sampling time
(t) | Weight, kg | Serum osmolality,
mOsmol/kg H2O | Urine osmolality,
mOsmol/kg H2O |
|---|
| 08:00 | 84.5 | 304.73 | 351 |
| 09:00 | | | 356 |
| 10:00 | 83.5 | 306.59 | 291 |
| 11:00 | | | 294 |
| 12:00 | 84.5 | 306.76 | 334 |
| 13:00 | | | 332 |
| 14:00 | 83.5 | 304.07 | 327 |
| After 2 µcg DDAVP
IV administration | | | |
| 15:00 (t=0) | | 305 | 457 |
| 15:30 (t=+30
min) | 84.5 | 303 | na |
| 16:00 (t=+60
min) | | 300 | 683 |
| 16:30 (t=+90
min) | 84.4 | 303 | na |
| 17:00 (t=+120
min) | | 299 | 716 |
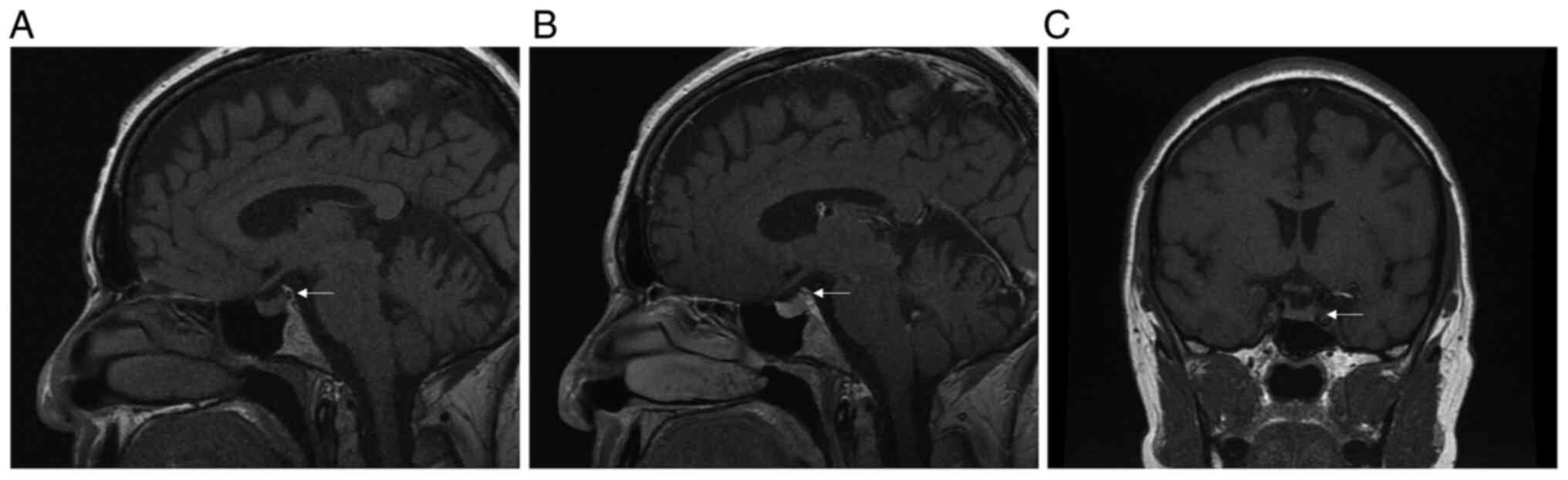
Pituitary magnetic resonance imaging (MRI) did not
show signs of hypophysitis (Fig.
1), however, the posterior pituitary bright spot was absent in
the non-contrast T1 sagittal sequalae (Fig. 1A).
The patient was started a replacement treatment with
oral desmopressin (DDAVP) at 60 mg once daily with titration of the
dose increasing to twice daily with evident improvement of her
polyuria, nycturia, and polydipsia and re-initiation of nivolumab.
Panhypopituitarism including CDI persisted after 6 months of follow
up.
Literature review
Systematic review of the
literature
To identify studies and determine their eligibility,
a systematic research was conducted in the PubMed Database on June
10, 2022. Research included the following keywords: ‘diabetes
insipidus’, ‘immunotherapy’, ‘immune check-point inhibitors’,
‘posterior hypophysitis’, ‘pituitary’. The above keywords were also
combined with the Boolean operators AND and OR. PICOT (population,
intervention, comparison, outcomes, time) criteria were used in
order the irrelevant articles to be excluded. Articles that do not
align with the PICOT format were dismissed. More specifically,
studies including population without malignancy (irrelevant
population) or population presenting CDI induced by other causes
than immunotherapy (irrelevant intervention) as well as studies
including ICI-treated patients with hypophysitis without data on
CDI (irrelevant outcome) were also excluded. Additionally, we
excluded not original studies (Reviews or systematic
reviews/meta-analysis) or in vitro/in vivo studies.
Finally eligible for inclusion in our analysis were studies on
humans with malignancy treated with immunotherapy and presenting
ICI-induced DI. Four of the investigators (PP, DM, MM and AK)
independently examined all potentially eligible titles and
abstracts. Full manuscripts were obtained as necessary to finalise
eligibility (studies which were available only as abstracts were
excluded). Reference lists of eligible studies were also searched
through to identify additional studies. Only English language
papers published were selected. Research strategy is illustrated in
the flow diagram (Fig. 2).
Results
PubMed research revealed 583 English written
reports; n=13 of them concerned in vitro or animal studies.
From the remaining 570 studies, we further excluded n=283 not
original papers (reviews, systematic reviews or meta-analyses)
providing no data on clinical cases with ICI-induced DI. Based on
full text of the rest 287 original studies and cases reports, n=139
were excluded as they included either not relevant population or
not relevant treatment (n=121 articles studied adults and n=12
children without malignancy presenting DI induced by other causes
besides immunotherapy and n=6 articles studied patients presenting
DI post-COVID vaccination), further n=135 articles were excluded
because although they included patients with malignancy treated
with ICI, they provided data for other pituitary deficiencies but
not DI (not relevant outcome). Finally, we ended-up to only 13
cases reports reporting data on DI among ICI treated patients (Flow
diagram). A total of 14 patients presenting with DI
post-immunotherapy with predominance of the male sex (11 males vs.
1 female, in 2 patients sex was not specified) were described
(Table V), (1,7-16,20,21).
All patients had been treated with ICIs for solid malignancies
except two cases treated for Hodgkin lymphoma and acute myeloid
leukaemia. Five patients had been treated with CTLA-4 Abs
monotherapy, 6 with PDL-1 Abs monotherapy and the rest 3 with
combined therapies (CTLA-4Abs and PDL-1Abs). The median time from
the initiation of immunotherapy to DI onset varied from immediate
after the first cycle of PD-1 Ab (sintilimab) to 270 days
post-initiation of PD-L1 Ab (atezolizumab). Five patients presented
isolated injury of the posterior pituitary with maintenance of the
secretion of the anterior pituitary. In 7 patients, CDI was
associated with deficiency of the anterior pituitary
(panhypopituitarism) from which, in one case treated with
atezolizumab, there was a strong suspicion for hypothalamitis based
on imaging findings (hypothalamic mass). In 5 cases, CDI was either
transient or prolonged (varying from 5 days to 5 months) whether in
3 cases was chronic (more than 6 months of duration). In the
majority of cases in which MRI's data were available, pituitary
image was normal (n=5) or showed an adenomatous lesions with or
without stalk thickening (n=2). Interestingly, none of the patients
with available imaging data presented absence of the bright spot on
the MRI.
 | Table VCases in the literature presenting
with ICI-induced central DI. |
Table V
Cases in the literature presenting
with ICI-induced central DI.
| First author/s,
year | Age, years | Sex | Malignancy | Drug | ICI category | Dysfunction of
pituitary | Dysfunction of
hypothalamus | Median time to
onset of DI, days | Duration of DI | MRI findings | Grade of AE | Follow-up,
days | (Refs.) |
|---|
| Dillard et
al, 2010 | 50 | M | Adenocarcinoma of
prostate | Ipilimumab | CTLA-4 Ab |
Panhypopituitarism | No | 84 | 3 weeks | Normal | III | ND | (7) |
| Nallapanemi et
al, 2014 | 62 | M | Melanoma | Ipilimumab | CTLA-4 Ab |
Panhypopituitarism | No | 121 | 5 months | ND | II | 180 | (8) |
| Gunawan et
al, 2018 | 52 | M | Melanoma | Ipilimumab +
nivolumab | CTLA-4 Ab (+) PD-1
Ab | Isolated posterior
pituitary | No | 28 | ND | ND | I | ND | (9) |
| Zhao et al,
2018 | 73 | M | Merkel cell
carcinoma | Avelumab | PD-L1 Ab | Isolated posterior
pituitary | No | 112 | 6 weeks | Normal | I | 240 | (1) |
| Tshuma et
al, 2018 | 74 | F | Bladder cancer | Atezolizumab | PD-L1 Ab |
Panhypopituitarism | Yes | 270 | ΝD | Hypothalamic
mass | I | 365 | (10) |
| Deligiorgi et
al, 2020 | 71 | M | Adenocarcinoma of
the lung | Nivolumab | PD-L1 Ab | Isolated posterior
pituitary | No | 90 | ND | Normal | IV | a | (11) |
| Barnabei et
al, 2020 | 64 | M | Melanoma | Ipilimumab | CTLA-4 Ab |
Panhypopituitarism | No | 60 | 5 days | Normal | I | 1,230 | (12) |
| Grami et al,
2020 | 30 | M | Acute myeloid
leukemia | Ipilimumab +
nivolumab | CTLA-4 Ab (+) PD-1
Ab |
Panhypopituitarism | No | ND | ND | ND | III | ND | (13) |
| Brilli et
al, 2020 | 68 | M | Mesothelioma | Tremelimumab and
durvalumab | CTLA-4 Ab (+) PD-L1
Ab | Isolated posterior
pituitary | No | 60 | Persisted | Normal | ND | 570 | (16) |
| Yu et al,
2021 | 60 | M | Hodgkin
lymphoma | Sintilimab | PD-1Ab | Isolated posterior
pituitary | No | Immediate | 3 months | Nodular signal | II | 90 | (14) |
| Fosci et al,
2021 | 62 | M | Hypopharynx
cancer | Nivolumab | PD-1 Ab |
Panhypopituitarism | No | 35 | 50
daysb | Stalk enlarged | I | 24 | (15) |
| Terán et al,
2022 | 46 | M | Adenocarcinoma of
the lung | Nivolumab | PD-1 Ab |
Panhypopituitarism | No | 62 | ND | ND | I | ND | (20) |
| Amereller et
al, 2022 | 2 cases | 1F/1M | ND | Ipilimumab | CTLA-4 Ab | ND | ND | ND | ND | ND | ND | ND | (21) |
Discussion
This is the case of a 53 year old woman treated with
nivolumab for metastatic melanoma, presenting with a syndrome of
polyuria-polydipsia, 6 months post-initiation of immunotherapy and
2 months after the diagnosis of the anterior pituitary deficiency
(insufficiency of the corticotrope and thyreotrope axes). The
diagnosis of partial CDI was retained based on biochemical findings
that included inappropriately low urine osmolality for serum
osmolality increased less than 50% after desmopressin
administration in combination with low baseline copeptin levels.
CDI induced by nivolumab treatment was confirmed through the
medical history of the patient, the pituitary MRI and the water
deprivation tests which allowed to exclude nephrogenic DI (NDI) and
primary polydipsia.
DI is a rare condition that affects one in 25,000
persons (14,22). CDI is the most common form of DI
and is generally the result of hypothalamic-neurohypophysial
dysfunction leading to inadequate arginine vasopressin (AVP)
secretion from the posterior pituitary or inadequate production
from the hypothalamus (19). The
majority of the causes of CDI are acquired (idiopathic and
iatrogenic) whereas inherited/familial CDI causes account for
approximately 1% of cases (19).
CDI develops when more than 80% of the AVP-secreting neurons are
damaged. The less common NDI is caused by a partial or complete
resistance of AVP receptors to vasopressin. Some of the commonest
NDI etiologies are electrolytic disturbances including hypokalemia
and hypercalcemia. In our patient, both the potassium and calcium
levels were within the normal limits.
In our patient the exclusion of metastatic disease
was also challenging. Indeed, the posterior pituitary is most
frequently affected by metastases, due to its vascularisation by
the inferior hypophyseal artery (23). Besides, its small size compared to
the anterior pituitary explain why the same volume of metastatic
tissue can produce earlier symptoms compared to the adenohypophysis
damage (24). In our case
pituitary MRI did not show any evidence of metastatic disease,
stalk thickening or posterior pituitary mass.
Secondary hypophysitis related to ICI has a reported
incidence ranging from 8 to 13% in patients treated with CTLA-4 Abs
therapy (25) and from 8.5 to 9.0%
in patients treated with PD-1 Abs therapy (26). Unlike other forms of hypophysitis
(lymphocytic, granulomatous, xanthomatous, and plasmacytic), the
ICIs-associated hypophysitis is more common in males (27) and typically occurs after a period
of 2 to 3 months post-immunotherapy as in our case. Older age and
male sex are potential risk factors (27). Moreover, ACTH and thyrotropin
deficiency are the most common abnormalities an observation
confirmed in our case; however it can also affect sex hormones,
growth hormone, and prolactin.
Patients treated with ICIs rarely develop CDI
secondary to an autoimmune process involving the
hypothalamo-posterior pituitary region. Dysregulation of the
posterior pituitary-hypothalamic axis induced by ICI has been
reported in 13 case reports that are available in the current
literature (summarized in Table
II), (1,7-16,20,21).
Almost all patients developed CDI with a substantial delay from
treatment administration, ranging from 28 to 270 days, except in
one case where CDI developed immediately after sintilimab, a PD-1
inhibitor (14). From the 14
patients presenting with CDI with available data on their
treatment, 5 had been treated with monotherapy with CTLA-4 Abs, 6
with monotherapy with PD1-Abs whereas 3 cases had been treated with
combination treatment (CTLA-4 Abs and PD-1 Abs). Regarding our
case, this is the fourth case of nivolumab-induced CDI published in
the literature (11,15,20)
and the first female patient presenting with nivolumab-induced CDI.
In two other cases CDI was induced by a combination treatment with
nivolumab and ipilimumab (9,13).
The pathophysiological mechanism for ICI-induced CDI
remains unclear and may be linked to multiple pathways (28). Prior works suggested that type II
and type IV hypersensitivity reactions as well as ectopic pituitary
CTLA-4 expression may be associated with anti-CTLA-4
treatment-related hypophysitis (29,30).
PD-1 may also be expressed in pituitary cells or lymphocytes and
PD-L1 was expressed in pituitary adenomas (31).
In many cases of patients with suspected DI, the
diagnosis may be obvious based on serum and urinary osmolalities.
If the serum osmolality is greatly increased, with concomitant low
urinary osmolality, no further testing may be necessary. The
diagnostic challenge arises when there are symptoms of polyuria and
polydipsia with inappropriate normal or ‘almost normal’ serum
osmolality or sodium levels or when NDI or primary polydipsia
should be excluded. In such cases, dynamic test such as water
deprivation test is required since direct measurement of plasma AVP
is seldomly performed because of its rapid clearance. However, yet
even under optimal conditions water deprivation test often require
long periods of observation, and still is of low sensitivity (86%)
and specificity (70%) (19,32).
Recently, copeptin-C-terminal peptide of
pro-vasopressin-levels, either baseline or after stimulation (with
hypertonic saline infusion or with L-arginine stimulation), has
proven to be the most convenient and accurate way for the diagnosis
of DI. Copeptin is co-secreted with AVP and is a surrogate of its
secretion as it is a more stable compound (33,34).
Baseline copeptin levels <4.6 pmol/l are diagnostic of CDI,
whereas levels >21.4 pmol/l are diagnostic of NDI with 100%
sensitivity and specificity (19,33,35).
If baseline levels are intermediate the diagnosis could be either
CDI or PP; in that case stimulated copeptin levels are required
(36-38).
A randomized multicenter prospective study is currently being
carried out (clinical trials.gov NCT03572166) in order to confirm
the arginine-stimulated copeptin cut-off levels. Unfortunately,
copeptin measurement has not been routinely used in most
laboratories.
In the MRI, CDI generally manifests as a pituitary
‘bright spot’ absence with or without enlargement (2-3 mm) of the
pituitary stalk, although this finding alone is not necessarily
sufficient to support CDI diagnosis. The posterior pituitary bright
spot is a manifestation of stored vasopressin and although it is
missing in 20% of the general population (39), its absence on MRI is consistent
with CDI. In our patient no bright spot was apparent arguing in
favour of the CDI. On the contrast, no other abnormality was
observed on the MRI on the rest of the pituitary gland in favour of
hypophysitis which may presents a mild-to-moderate diffuse
enlargement of the pituitary gland (40).
In conclusion, the recent widespread use of ICIs in
oncology could explain why clinicians should be aware of the
potential risk for developing CDI. For normoglycemic patients
presenting with persistent polyuria/polydipsia syndrome during ICI
therapy and in particular anti-PD-1/PD-L1, testing for DI via serum
and urine specific osmolalities, urine specific gravity, and, if
needed, a water deprivation test are required. Patients' symptoms
of CDI can be easily controlled with DDAVP. As ICI are relatively
new agents, rare side effects such as DI should be reported to the
Food and Drug Administration adverse event reporting system (FAERS)
to better understand their side effects and effective management of
drug related adverse events.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HG and AA conceived and designed the study. MM, DM,
AA, PP, AK and DZ collected and interpreted all relevant clinical
and laboratory data. AA, PP and HG prepared the manuscript. HG and
AA confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao C, Tella SH, Del Rivero J,
Kommalapati A, Ebenuwa I, Gulley J, Strauss J and Brownell I:
Anti-PD-L1 treatment induced central diabetes insipidus. J Clin
Endocrinol Metab. 103:365–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Husebye ES, Castinetti F, Criseno S,
Curigliano G, Decallonne B, Fleseriu M, Higham CE, Lupi I, Paschou
SA, Toth M, et al: Endocrine-related adverse conditions in patients
receiving immune checkpoint inhibition-an ESE clinical practice
guideline. Eur J Endocrinol. (EJE-22-0689)(2022): (Epub ahead of
print).
|
|
3
|
Di Dalmazi G, Ippolito S, Lupi I and
Caturegli P: Hypophysitis induced by immune checkpoint inhibitors:
A 10-year assessment. Expert Rev Endocrinol Metab. 14:381–398.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fernandes S, Varlamov EV, McCartney S and
Fleseriu M: A novel etiology of hypophysitis: Immune checkpoint
inhibitors. Endocrinol Metab Clin North Am. 49:387–399.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vaddepally RK, Kharel P, Pandey R, Garje R
and Chandra AB: Review of indications of FDA-approved immune
checkpoint inhibitors per NCCN guidelines with the level of
evidence. Cancers (Basel). 12(738)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mutter CM, Smith T, Menze O, Zakharia M
and Nguyen H: Diabetes insipidus: Pathogenesis, diagnosis, and
clinical management. Cureus. 13(e13523)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dillard T, Yedinak CG, Alumkal J and
Fleseriu M: Anti-CTLA-4 antibody therapy associated autoimmune
hypophysitis: Serious immune related adverse events across a
spectrum of cancer subtypes. Pituitary. 13:29–38. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nallapaneni NN, Mourya R, Bhatt VR,
Malhotra S, Ganti AK and Tendulkar KK: Ipilimumab-induced
hypophysitis and uveitis in a patient with metastatic melanoma and
a history of ipilimumab-induced skin rash. J Natl Compr Cancer
Netw. 12:1077–1081. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gunawan F, George E and Roberts A:
Combination immune checkpoint inhibitor therapy nivolumab and
ipilimumab associated with multiple endocrinopathies. Endocrinol
Diabetes Metab Case Reports. 2018:17–0146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tshuma N, Glynn N, Evanson J, Powles T and
Drake WM: Hypothalamitis and severe hypothalamic dysfunction
associated with anti-programmed cell death ligand 1 antibody
treatment. Eur J Cancer. 104:247–249. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deligiorgi MV, Siasos G, Vergadis C and
Trafalis DT: Central diabetes insipidus related to anti-programmed
cell-death 1 protein active immunotherapy. Int Immunopharmacol.
83(106427)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barnabei A, Carpano S, Chiefari A,
Bianchini M, Lauretta R, Mormando M, Puliani G, Paoletti G,
Appetecchia M and Torino F: Case report: Ipilimumab-induced
panhypophysitis: An infrequent occurrence and literature review.
Front Oncol. 10(582394)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grami Z, Manjappachar N and Reddy DR: 323:
Diabetes insipidus in checkpoint inhibitor treatment and acute
myeloid leukemia. Crit Care Med. 48(144)2020.
|
|
14
|
Yu M, Liu L, Shi P, Zhou H, Qian S and
Chen K: Anti-PD-1 treatment-induced immediate central diabetes
insipidus: A case report. Immunotherapy. 13:1255–1260.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fosci M, Pigliaru F, Salcuni AS, Ghiani M,
Cherchi MV, Calia MA, Loviselli A and Velluzzi F: Diabetes
insipidus secondary to nivolumab-induced neurohypophysitis and
pituitary metastasis. Endocrinol Diabetes Metab Case Rep.
2021:20–0123. 2021.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
16
|
Brilli L, Calabrò L, Campanile M, Pilli T,
Agostinis C, Cerase A, Maio M and Castagna MG: Permanent diabetes
insipidus in a patient with mesothelioma treated with
immunotherapy. Arch Endocrinol Metab. 64:483–486. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bai X, Chen X, Wu X, Huang Y, Zhuang Y,
Chen Y, Feng C and Lin X: Immune checkpoint inhibitor-associated
pituitary adverse events: An observational, retrospective,
disproportionality study. J Endocrinol Invest. 43:1473–1483.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gubbi S, Hannah-Shmouni F, Koch CA,
Verbalis JG, Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder
WW, Dhatariya K (eds), et al: Diagnostic testing for diabetes
insipidus. In: Endotext [Internet]. MDText.com, Inc., South
Dartmouth, MA, 2000.
|
|
19
|
Christ-Crain M, Winzeler B and Refardt J:
Diagnosis and management of diabetes insipidus for the internist:
An update. J Intern Med. 290:73–87. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Terán Brage E, Heras Benito M, Navalón
Jiménez MB, Vidal Tocino R, del Barco Morillo E and Fonseca Sánchez
E: Severe hyponatremia masking central diabetes insipidus in a
patient with a lung adenocarcinoma. Case Rep Oncol. 15:91–98.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Amereller F, Deutschbein T, Joshi M,
Schopohl J, Schilbach K, Detomas M, Duffy L, Carroll P, Papa S and
Störmann S: Differences between immunotherapy-induced and primary
hypophysitis-a multicenter retrospective study. Pituitary.
25:152–158. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Di Iorgi N, Napoli F, Allegri AE, Olivieri
I, Bertelli E, Gallizia A, Rossi A and Maghnie M: Diabetes
insipidus-diagnosis and management. Horm Res Paediatr. 77:69–84.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Di Nunno V, Mollica V, Corcioni B,
Fiorentino M, Nobili E, Schiavina R, Golfieri R, Brunocilla E,
Ardizzoni A and Massari F: Clinical management of a pituitary gland
metastasis from clear cell renal cell carcinoma. Anticancer Drugs.
29:710–715. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Javanbakht A, D'Apuzzo M, Badie B and
Salehian B: Pituitary metastasis: A rare condition. Endocr Connect.
7:1049–1057. 2018.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
25
|
Faje A: Immunotherapy and hypophysitis:
Clinical presentation, treatment, and biologic insights. Pituitary.
19:82–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ryder M, Callahan M, Postow MA, Wolchok J
and Fagin JA: Endocrine-related adverse events following ipilimumab
in patients with advanced melanoma: A comprehensive retrospective
review from a single institution. Endocr Relat Cancer. 21:371–381.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Byun DJ, Wolchok JD, Rosenberg LM and
Girotra M: Cancer immunotherapy-immune checkpoint blockade and
associated endocrinopathies. Nat Rev Endocrinol. 13:195–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bellastella G, Maiorino MI, Bizzarro A,
Giugliano D, Esposito K, Bellastella A and De Bellis A:
Revisitation of autoimmune hypophysitis: Knowledge and
uncertainties on pathophysiological and clinical aspects.
Pituitary. 19:625–642. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iwama S, De Remigis A, Callahan MK, Slovin
SF, Wolchok JD and Caturegli P: Pituitary expression of CTLA-4
mediates hypophysitis secondary to administration of CTLA-4
blocking antibody. Sci Transl Med. 6(230ra45)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Caturegli P, Di Dalmazi G, Lombardi M,
Grosso F, Larman HB, Larman T, Taverna G, Cosottini M and Lupi I:
Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein
4 blockade: Insights into pathogenesis from an autopsy series. Am J
Pathol. 186:3225–3235. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mei Y, Bi WL, Greenwald NF, Du Z, Agar NY,
Kaiser UB, Woodmansee WW, Reardon DA, Freeman GJ, Fecci PE, et al:
Increased expression of programmed death ligand 1 (PD-L1) in human
pituitary tumors. Oncotarget. 7(76565)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Priya G, Kalra S, Dasgupta A and Grewal E:
Diabetes insipidus: A pragmatic approach to management. Cureus.
13(e12498)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Christ-Crain M and Fenske W: Copeptin in
the diagnosis of vasopressin-dependent disorders of fluid
homeostasis. Nat Rev Endocrinol. 12:168–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Christ-Crain M, Hoorn EJ, Sherlock M,
Thompson CJ and Wass J: Endocrinology in the time of COVID-19-2021
updates: The management of diabetes insipidus and hyponatraemia.
Eur J Endocrinol. 185:G35–G42. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Garrahy A, Moran C and Thompson CJ:
Diagnosis and management of central diabetes insipidus in adults.
Clin Endocrinol (Oxf). 90:23–30. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fenske W, Refardt J, Chifu I, Schnyder I,
Winzeler B, Drummond J, Ribeiro-Oliveira A Jr, Drescher T, Bilz S,
Vogt DR, et al: A copeptin-based approach in the diagnosis of
diabetes insipidus. N Engl J Med. 379:428–439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Winzeler B, Cesana-Nigro N, Refardt J,
Vogt DR, Imber C, Morin B, Popovic M, Steinmetz M, Sailer CO,
Szinnai G, et al: Arginine-stimulated copeptin measurements in the
differential diagnosis of diabetes insipidus: A prospective
diagnostic study. Lancet. 394:587–595. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Timper K, Fenske W, Kühn F, Frech N, Arici
B, Rutishauser J, Kopp P, Allolio B, Stettler C, Müller B, et al:
Diagnostic accuracy of copeptin in the differential diagnosis of
the polyuria-polydipsia syndrome: A prospective multicenter study.
J Clin Endocrinol Metab. 100:2268–2274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brooks BS, el Gammal T, Allison JD and
Hoffman WH: Frequency and variation of the posterior pituitary
bright signal on MR images. Am J Roentgenol. 153:1033–1038.
1989.PubMed/NCBI
|
|
40
|
Corsello SM, Barnabei A, Marchetti P, De
Vecchis L, Salvatori R and Torino F: Endocrine side effects induced
by immune checkpoint inhibitors. J Clin Endocrinol Metab.
98:1361–1375. 2013.PubMed/NCBI View Article : Google Scholar
|