Introduction
Lipoblastoma is a benign tumor of the embryonic
white fat with a prevalence of ~0.6% of benign soft tissue tumors
(1). It is the second most common
childhood adipocyte tumor after lipoma and typically occurs in boys
younger than three years of age (2,3).
Lipoblastomas are often present in the extremities, trunk and head
and neck, but they may also appear in the mediastinum,
retroperitoneum, perineum and parotid gland (4). Most lipoblastomas are ≤5 cm in size,
but larger sizes occasionally occur. Magnetic resonance imaging is
the preferred modality for assessing tumor location, size,
composition, adjacent organs and surgical resection site (5). Although preoperative imaging is
useful in assessing the extent of the tumor, it cannot
differentiate between various adipose tissue tumors because there
are no pathological imaging features associated with lipoblastoma
(6). Treatment typically involves
complete surgical removal and preservation of the vital organs. The
disease is a benign lesion with a good prognosis, with no reports
of malignant transformation and metastasis. Although there is no
risk of metastasis, it may relapse in the late stage with
incomplete resection (1).
Lipoblastomas typically exhibit a simple
pseudodiploid or hyperdiploid karyotype. The most common type is
one or more additional copies of chromosome 8, which contains
structural abnormalities at 8q11-13 (including translocation,
insertion, inversion, or circular chromosomes), resulting in
pleiomorphic adenoma gene 1 (PLAG1) rearrangement (2).
PLAG1 is primarily composed of five exons,
and codes from exon 4 produce a protein composed of 500 amino
acids. Its oncogenic effect is associated with upregulation of
multiple direct target genes, including growth factors, growth
factor-binding proteins, growth factor receptors and cell
cycle-associated proteins, such as insulin-like growth factor 2,
vascular endothelial growth factor and mitogen-activated protein
kinase (7-11).
PLAG1 acts as a transcriptional regulator and is not
expressed in adult tissue (12,13).
It is hypothesized that this is due to the presence of negative
control elements that inhibit PLAG gene expression) in exon 1 of
PLAG1 in adults; however, ectopic PLAG1 in tumors
results in loss of these elements and overexpression of the coding
region (14). Tumors caused by
overexpression of PLAG1 include salivary gland pleomorphic
adenoma, lipoblastoma, hepatoblastoma and acute myeloid leukemia
(15). Detection of PLAG1
rearrangement is helpful in the diagnosis of pleomorphic adenoma.
Andreasen et al (16) found
that 16 (76.2%) of 21 pleomorphic adenomas showed copy-neutral
PLAG1 rearrangements. Four other markers also serve a role
in the development of lipoblastoma including CD34, S-100, desmin,
and p16. It has been reported that the CD34+
fibroblastic stem cell may be the putative cell of origin for
lipoblastoma (17). S-100-positive
mono- and multi-vacuolar lipoblasts indicate tumor origin in
adipose tissue (18). Aberrant
immunoreactivity to desmin has been described in non-adipose
mesenchymal tumors, except those with myogenic or myofibroblast
differentiation (17,19). In the study by Kubota et al
(20), PLAG1-positive spindle
cells expressed desmin, but not other myogenic markers. Combining
the immunological features of desmin, spindle cell morphology and
ultrastructural features (invaginated nuclei, well-developed rough
endoplasmic reticula, pinocytotic vesicles and desmin filaments),
it was inferred that spindle cells in lipoblastoma may exhibit
myofibroblastic differentiation. PLAG1 overexpression and
activation are key events in lipoblastoma and this pathway is
independent of p16. However, alterations in the retinoblastoma
pathway during liposarcoma development serve a key role in
overexpression of p16 (21,22).
P16 immunohistochemistry has been reported to show high negative
predictive value from lipoblastoma (87%) and benign adipocytic
lesions (93%) in differentiating liposarcoma (23,24).
Therefore, malignancy is unlikely in the absence of p16
expression.
Morphologically, lipoblastoma is primarily composed
of primitive spindle mesenchymal cells and adipocytes at various
stages of maturation. In particular, it contains lobules of mature
adipocytes of different sizes divided by fiber septa, with a fine
capillary network and mucinous stroma. In immunohistochemistry,
lipoblasts are usually S-100-positive and P16-negative, while CD34
and desmin expression is usually observed in primitive mesenchymal
cells; these properties serve as a useful diagnostic marker
(20,25). However, because lipoblastoma has
its own characteristic genetic changes, fluorescence in situ
hybridization (FISH) detection of PLAG1 fragmentation and
rearrangement may be useful in diagnosis of lipoblastoma (26,27).
Furthermore, lipoblastoma often shows atypical
morphology or occur in young and adult age groups and are easily
misdiagnosed as lipoma, fibrous hamartoma of infancy, myxoid
liposarcoma, primitive myxoid mesenchymal tumor of infancy and
superficial angiomyxoma (28-30).
Therefore, as a cytogenetic detection method, PLAG1 FISH is
required to aid the diagnosis by pathologists. Currently, reports
on this disease are mostly limited to case reports (4,5,8,26).
Therefore, to gain a deeper understanding of the occurrence and
development of lipoblastoma, changes in molecular structure and the
effect of fusion genes on prognosis, the present study collected 36
cases of lipoblastoma in Shengjing Hospital of China Medical
University over the past 7 years. Combined with evidence from
international literature (1,3-6,8,17-20,25,26),
the present study summarized clinical features, morphological
changes, immunophenotype, FISH detection, diagnosis and
differential diagnosis, to improve the accuracy of diagnosis and
decrease the rate of misdiagnosis and missed diagnosis.
Materials and methods
Case selection
A total of 36 cases of lipoblastoma that were
surgically resected and diagnosed at Shengjing Hospital of China
Medical University, Shenyang, China, between January 2015 and
January 2021 were collected. There were 22 males and 14 females,
aged between 7 days and 33 years. Inclusion criteria included
histopathologically confirmed lipoblastoma or lipoblastomatosis
with hematoxylin- and eosin-stained sections, paraffin blocks and
clinical data (age, sex, tumor location, size, surgery,
complications and follow-up) available for all cases. Cases with
missing IHC (S-100, CD-34, P-16 and desmin) analysis and cases with
poorly preserved tissue blocks were excluded. The Institutional
Review Committee of the hospital approved the study (ethical
approval no. 2022PS104J). The need for written informed consent was
waived in view of the retrospective nature. All cases were reviewed
by two pediatric pathologists and lipoblastoma was classified into
three subtypes: Classic, lipoma-like, and myxoid, as previously
described (17,31).
Immunohistochemistry
All specimens were surgically excised, fixed in 3.7%
neutral formaldehyde at room temperature for 24 h, routinely
embedded in paraffin, sliced to 4-5 um thickness and stained with
hematoxylin and eosin for ~45 min at room temperature by Roche
fully automated system (VENTANA HE600; Roche) and histological
characteristics were observed under a light microscope (DM 2500;
Leica GmbH). The lesion area was selected and 3-µm-thick
formalin-fixed paraffin-embedded sections were subjected to
immunohistochemical staining with S-100, CD34 (cytoplasmic), desmin
(cytoplasmic), and P16 using Envision kit (Elabscience
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. Paraffin-embedded tissues were submerged three times
in xylene for 5 min each. The sections were washed in 95% ethanol
for 2 min, 90% ethanol for 2 min, 85% ethanol for 2 min, 80%
ethanol for 2 min, 75% ethanol for 2 min and distilled water for 2
min to remove the xylene. The sections were then placed in EDTA PH
9.0 Antigen Repair Solution (E-IR-R104, Elabscience Biotechnology
Co., Ltd.) for 20 min at 95 ˚C. After cooling to room temperature,
the sections were removed. Place in distilled water for 5 min.
Submerge sections in 3% H2O2 (E-IR-R115,
Elabscience) for 10 min at room temperature. Rinse well with
distilled water. Rinse 3 times with PBS buffer for 5 min each. Drop
ready-to-use goat serum (E-IR-R217A, Elabscience) and block for 30
min at room temperature. Primary antibody was added at 4˚C for 12
h. Rinse 3 times with PBS buffer for 5 min each. Add ready-to-use
polyperoxidase-anti-mouse/rabbit IgG (E-IR-R214B, Elabscience) at
room temperature for 30 min. Rinse 3 times with PBS buffer for 5
min each. Add freshly prepared 20x diluted to 1x DAB developer
(E-IR-R214D, Elabscience) for approximately 2-3 min. The sections
were submerged in Mayer hematoxylin stain for approximately 5 min,
washed with water to remove the stain, rapidly fractionated with 1%
ethanol hydrochloride for 30 seconds, rinsed with tap water, and
returned to blue for 1 min. Sections were sequentially placed in
75% ethanol for 1 min, 80% ethanol for 1 min, 90% ethanol for 1
min, 95% ethanol for 1 min, anhydrous ethanol twice for 3 min each,
submerged in xylene twice for 5 min, and xylene (second) for 5 min,
and the tissue was sealed by adding neutral gum dropwise on a
coverslip. Primary antibodies were as follows: S-100 rabbit
polyclonal (cat. no. ZA-0225, ready-to-use), CD34 rabbit monoclonal
(cat. no. ZM-0046, ready-to-use), P16 mouse monoclonal (cat. no.
ZM-0205, ready-to-use) and desmin rabbit monoclonal (cat. no.
ZA-0610, ready-to-use), purchased from Zhongshan Jinqiao
Biotechnology Co., Ltd. Staining intensity and positive area were
independently interpreted by two pathologists.
FISH
FISH detected the breakage and rearrangement of
PLAG1 (8q12; gene ID: 5324). A PLAG1 mRNA probe (cat. no. CL-003;
HealthCare Biotechnology Co., Ltd.) was designed and synthesized
using cDNA as a template. The length of the probe was 1,090 kb.
Total RNA was extracted from 50 mg lipoblastoma tissue stored at
-80˚C using RNAiso Plus (cat. no. 9108; Takara Biotechnology Co.,
Ltd.) and RNase-free (cat. no. 2270A; Takara Biotechnology Co.,
Ltd.), followed by PrimeScript™ RT kit (dNTP and RNase Inhibitor
are included) (cat. no. RR037A; Takara Biotechnology Co., Ltd) for
RT using PLAG1 forward primer: GCCGCAACAAGTGGTGACCTC; reverse
primer: CCAGACGACTTGCCTGCATGAG. Thermocycling: pre-denaturation
95˚C, 5 min; 95˚C, 15 sec, PCR reaction 60˚C for 1 min, 35 cycles;
solubility curve phase 95˚C, 15 sec, 50˚C, 1 min, 95˚C, 30 sec. The
target gene cDNA fragment was inserted into a plasmid (cat. no.
3340; Takara Biotechnology Co., Ltd.) containing a specific RNA
polymerase (cat. no. 2520A; Takara Biotechnology Co., Ltd.)
promoter sequence, and the recombinant plasmid was then amplified,
purified. The plasmid template was cleaved with restriction enzyme
(cat. no. 1060A/B; Takara Biotechnology Co., Ltd.) to linearize.
And then under the action of RNAase (cat. no. RR420Q; Takara
Biotechnology Co., Ltd.), starting from the promoter site, the cDNA
was used as a template for in vitro transcription. In the
in vitro transcription reaction system, nucleotide feedstock
with digoxigenin labeling is provided, and the labeled RNA probe is
obtained after in vitro transcription. The nucleotide
sequences were PLAG1-red end: CTD-2245E20, CTD-2124P3, CTD-2359C13;
PLAG1-green end: CTD-2283H1, RP11-22I14, CTD-2005O24, CTD-2344J3,
CTD-2266F8, CTD-2130C19. Paraffin tissue sections of 3 µm thickness
were selected and baked at 65˚C for 2 h. Sections were dewaxed:
xylene 10 min x2 times, 100, 85, 70% gradient ethanol rehydration
(5 min/cylinder), deionized water rinsing. Sections were placed in
distilled water at 100˚C for 25 min, covered with pepsin (20 µg/ml;
cat. no. 9001-75-6; Guangzhou LBP Medicine Science and Technology
Co., Ltd.), incubated at 37˚C for 20 min, and then incubated with
2x saline-sodium citrate (SSC; cat. no. 6132-04-3; Guangzhou LBP
Medicine Science and Technology Co., Ltd.) for 10 min x2 times at
room temperature, fixed in ethanol gradient dehydration and dried.
Using a ThermoBrite in situ hybridizer (S500-24; Leica), the
probe was co-denatured with the tissue in hybridization buffer at
85˚C for 5 min and hybridized at 42˚C for 16 h. Gradient washes
were performed under SSC buffer (2X SSC at 37˚C for 1 min, 1X SSC
at 37˚C for 10 min, 0.5X SSC at 37˚C for 10 min). The slides were
placed in pre-warmed 0.3% NP-40/0.4X SSC at 68˚C for 2 min. The
sections were removed and immersed in pre-warmed deionized water at
37˚C for 1 min. The slides were dried naturally in the dark. No
blocking reagent was applied in this experiment. DAPI re-staining
agent (5 µg/ml; cat. no. 220401; HealthCare Biotechnology Co.,
Ltd.) was dropped onto the hybridized area, immediately covered
with a coverslip, and then observed under a fluorescence microscope
at 100x magnification (Axio Imager.A2; ZEISS). PLAG1 breaks were
considered positive if ≥10% of at least 100 cells (positive
control, salivary gland tumors from HealthCare Biotechnology Co.,
Ltd.) showed PLAG1 breaks. The results were analyzed using Isis
software version 5.9.1 (MetaSystems).
Statistical analysis
SPSS 26.0 software (IBM Corp.) was used for
statistical analysis of clinical pathological data. Values are
expressed as median. Continuous variables that conformed to normal
distribution were analyzed by one-way ANOVA with LSD method for
post hoc tests and continuous variables that did not conform to
normal distribution were analyzed by Kruskal-Wallis rank sum test.
Fisher's exact test was used for categorical variables. P<0.05
was considered to indicate a statistically significant difference.
Disease-free survival curves were constructed using the
Kaplan-Meier method.
Results
Clinical features
Clinicopathological characteristics of lipoblastoma
series (n=36) are shown in Tables
I and II. The study included
22 males and 14 females (male to female ratio, 1.6:1.0) with age
ranging from 7 days to 33 years (median, 1.5 years; mean, 3.2
years). A total of 28 and eight patients were aged ≤3 and >3
years (8/36, 22%), respectively. The median ages of patients with
classic, lipoma-like and myxoid subtypes were 10 months (range, 7
days to 3 years), 2 years (range, 10 months to 7 years) and 4 years
(range, 3 months to 33 years), respectively. There was a
significant difference in age between the groups. The sites
included the extremities (n=12, 33%), head and neck (n=6, 17%),
trunk (n=16, 44%) and perineum (n=2, 6%); in most cases, the site
was the abdomen (8/36 cases, 22%). Tumor size ranged from 1.7 to
16.2 cm (median, 4.7 cm; mean, 5.5 cm), without statistically
significant differences between subtypes. In our case, lipoblastoma
was more frequent in males, in patients no older than 3 years of
age, and in the extremities. Patients are slightly older in the
mucinous subtype.
 | Table ICharacteristics of 36 patients with
lipoblastoma. |
Table I
Characteristics of 36 patients with
lipoblastoma.
| Characteristic | Total (n=36) | Classic subtype
(n=15) | Lipoma-like subtype
(n=13) | Myxoid subtype
(n=8) | P-value |
|---|
| Male | 22 | 9 | 8 | 5 | 1.00a |
| Age, months | | | | | 0.01b |
|
Mean | 38.98 | 15.08 | 33.08 | 93.38 | |
|
Median
(range) | 18.00
(0.23-396) | 10 (0.23-36) | 24 (10-84) | 48 (3-396) | |
| Location | | | | | 0.91a |
|
Extremities | 12 | 4 | 5 | 3 | |
|
Head and
neck | 6 | 4 | 1 | 1 | |
|
Trunk | 16 | 6 | 6 | 4 | |
|
Perineum | 2 | 1 | 1 | 0 | |
| Size, cm | | | | | 0.24c |
|
Mean | 5.54 | 4.58 | 6.46 | 5.85 | |
|
Median
(range) | 4.70
(1.70-16.20) |
4.00(2.50-8.20) | 5.60
(1.70-16.20) | 5.10
(2.20-10.50) | |
| S-100+ | 29 | 12 | 10 | 7 | 1.00a |
| CD-34+ | 36 | 15 | 13 | 8 | - |
| P-16+ | 8 | 5 | 0 | 3 | 0.03a |
| Desmin+ | 26 | 9 | 10 | 7 | 0.40a |
| FISH
PLAG1+ | 24 | 11 | 8 | 5 | 1.00a |
 | Table IIClinicopathological characteristics
of lipoblastoma. |
Table II
Clinicopathological characteristics
of lipoblastoma.
| A, Classic subtype
(n=15) |
|---|
| Case | Sex/age | Location | Size, cm | PLAG1
FISH | Complete
resection | Follow-up,
months |
|---|
| 2 | M/7 m | Left armpit | 5.6x5.4 | +, 69% | Yes | AWOD, 78 |
| 3 | M/10 m |
Retroperitoneum | 4.8x4.8 | +, 30% | Yes | Lost to
follow-up |
| 4 | F/6 m | Right neck | 2.8x2.2 | +, 39% | Yes | AWOD, 74 |
| 8 | F/7 m | Right armpit | 8.0x5.0 | +, 28% | Yes | Lost to
follow-up |
| 10 | M/1 y | Neck | 4.0x3.0 | -, 2% | Yes | AWOD, 64 |
| 12a | F/7 d | Abdominal wall | 4.2x3.5 | -, 2% | Yes | Lost to
follow-up |
| 15 | M/3 y | Left scapular
region | 8.2x5.2 | +, 35% | Yes | Lost to
follow-up |
| 16a | M/10 m | Armpit | 3.1x3.1 | +, 33% | Yes | AWOD, 54 |
| 18 | F/1 y | Supraclavicular
fossa | 2.5x2.0 | -, 6% | Yes | AWOD, 53 |
| 19 | M/8 m | Left maxilla | 3.0x2.0 | -, 3% | Yes | AWOD, 54 |
| 20 | M/10 m | Mediastinum | 4.0x4.0 | +, 39% | Yes | Lost to
follow-up |
| 22 | M/1 y | Perineum | 3.8x2.7 | +, 35% | Yes | AWOD, 39 |
| 25 | M/2 y | Left armpit | 5.7x5.0 | +, 32% | Yes | AWOD, 31 |
| 31 | F/3 y | Left chest
wall | 2.8x2.2 | +, 57% | Yes | AWOD, 11 |
| 32 | F/3 y | Right upper
mediastinum | 6.2x5.2 | +, 45% | No | AWOD, 5 |
| B, Lipoma-like
subtype (n=13) |
| Case | Sex/age | Location | Size, cm | PLAG1
FISH | Complete
resection | Follow-up,
months |
| 7a | M/2 y | Left chest
wall | 6.0x4.0 | -, 1% | No | AWOD, 68 |
| 11 | M/2 y | Scrotum | 1.7x1.3 | +, 22% | Yes | AWOD, 63 |
| 13 | M/5 y | Abdominal
cavity | 5.6x4.6 | +, 35% | Yes | AWOD, 57 |
| 14a | F/3 y | Right parotid
gland | 3.4x3.3 | ND | Yes | AWOD, 57 |
| 17 | M/3 y | Right upper
arm | 7.2x5.0 | -, 5% | Yes | Relapse after 54
months |
| 21 | F/1 y | Right popliteal
fossa | 9.7x5.0 | +, 42% | Yes | AWOD, 43 |
| 23b | M/5 y | Mesentery | 3.0x3.0 | ND | NO | Relapse after 51
months |
| 26 | M/3 y | Mesentery | 16.2x13.8 | +, 31% | Yes | AWOD, 29 |
| 27 | M/1 y | Right thigh | 5.4x3.3 | +, 23% | Yes | AWOD, 26 |
| 29 | F/10 m | Abdomen | 7.7x6.5 | ND | Yes | AWOD, 18 |
| 30 | F/7 y | Right foot | 4.5x2.2 | +, 42% | Yes | AWOD, 16 |
| 34 | M/2 y | Left hip | 4.1x2.6 | +, 26% | Yes | AWOD, 3 |
| 35 | F/1 y | Mesentery | 9.5x9.4 | +, 45% | Yes | AWOD, 3 |
| C, Myxoid subtype
(n=9) |
| Case | Sex/age | Location | Size, cm | PLAG1
FISH | Complete
resection | Follow-up,
months |
| 1 | M/4 y | Right shoulder | 4.6x4.4 | +, 32% | Yes | Lost to
follow-up |
| 5a | F/3 m | Behind the right
ankle | 2.2x1.8 | +, 41% | Yes | Lost to
follow-up |
| 6a | M/11 y | Right sole | 3.8x2.8 | +, 50% | Yes | AWOD, 71 |
| 9 | M/8 y | Left thigh | 10.0x5.0 | -, 2% | Yes | Lost to
follow-up |
| 24a | M/1 y | Back | 7.1x2.3 | ND | Yes | AWOD, 32 |
| 28 | M/33 y | Mediastinum | 10.5x3.5 | -, 2% | No | Relapse after 15
months |
| 33 | F/4 y | Right thoracic
cavity | 5.6x3.4 | +, 82% | Yes | AWOD, 3 |
| 36 | F/1 y | Neck | 3.0x2.9 | +, 47% | Yes | AWOD, 2 |
Myxoid lipoblastomas were yellow-to-white, medium in
texture and partly translucent. Classic and lipomatous
lipoblastomas appeared more homogeneous, with a yellow appearance
and medium texture, accompanied by fibrous septa and localized
hemorrhage. Histologically, all subtypes of lipoblastoma exhibited
fibrous septa (data not shown). Classic lipoblastoma (n=15) had
lobular architecture and variable amounts of mature adipose tissue,
with scattered lipoblasts, spindle cells and myxoid stroma
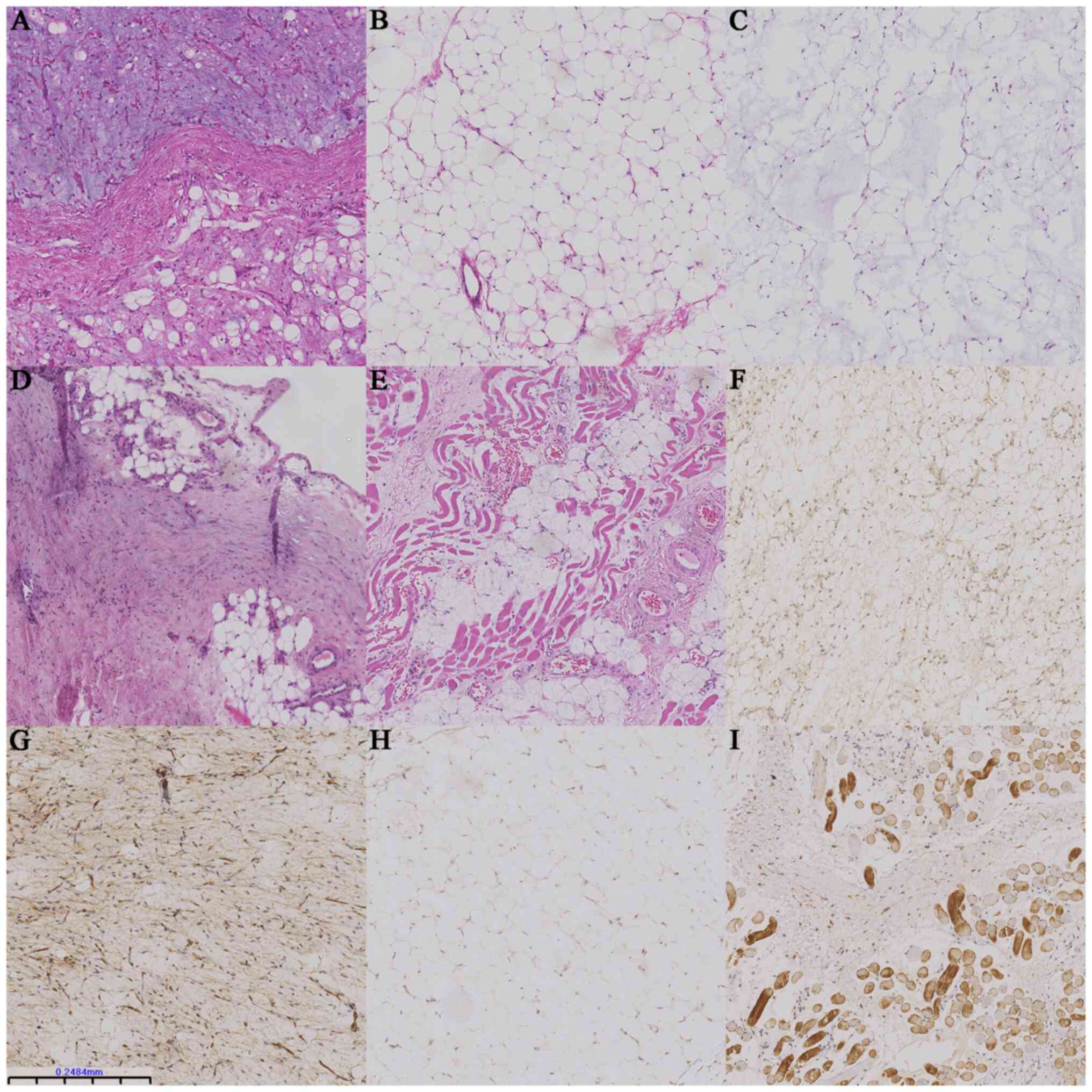
accounting for <50% of the section (Fig. 1A). This subtype presented with
mature banded morphology and a small amount of plexiform vascular
tissue with a predominantly myxoid background. The lipoma-like
subtype (n=13) consisted primarily of mature adipocytes of varying
sizes surrounded by fibrous tissue with few lipoblasts or myxoid
stroma (Fig. 1B). Myxoid
lipoblastomas (n=8) were cytologically diverse, with a myxoid
background accounting for >50% of the entire morphology,
including a myxoid pool, stellate- and spindle-shaped primitive
mesenchymal cells, a small number of adipocyte components, no
pathological mitosis (Fig. 1C) and
vascular plexiform, resembling myxoid liposarcoma.
Molecular characteristics
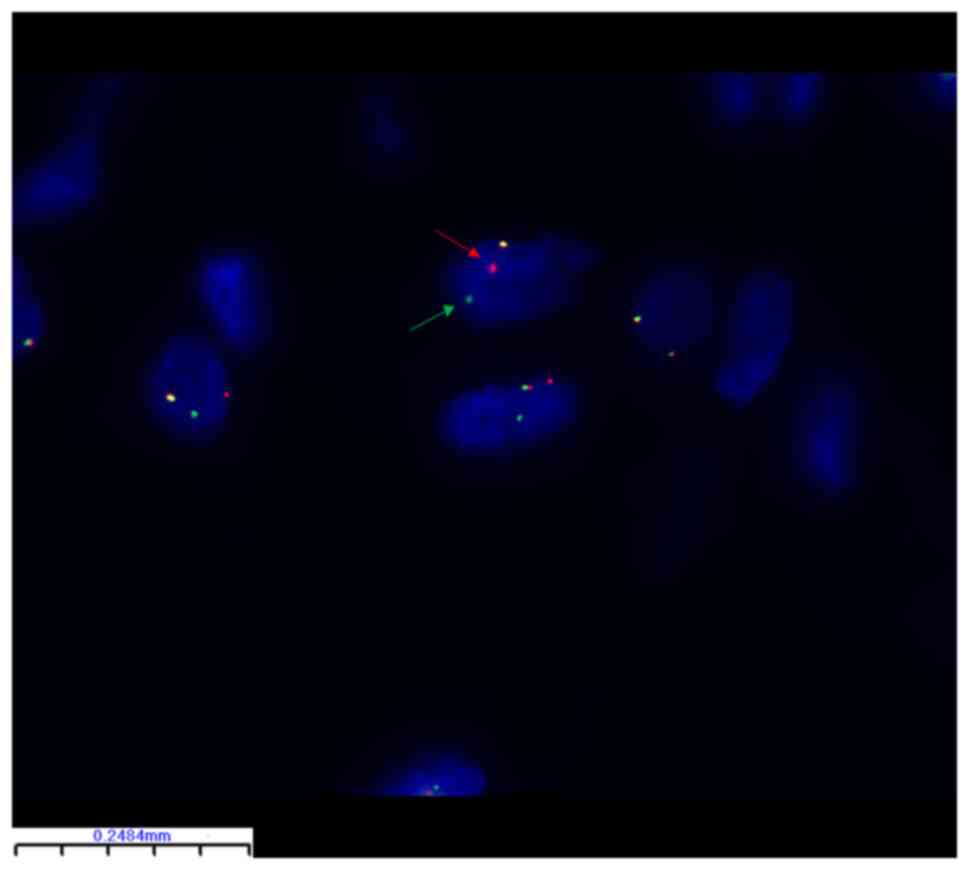
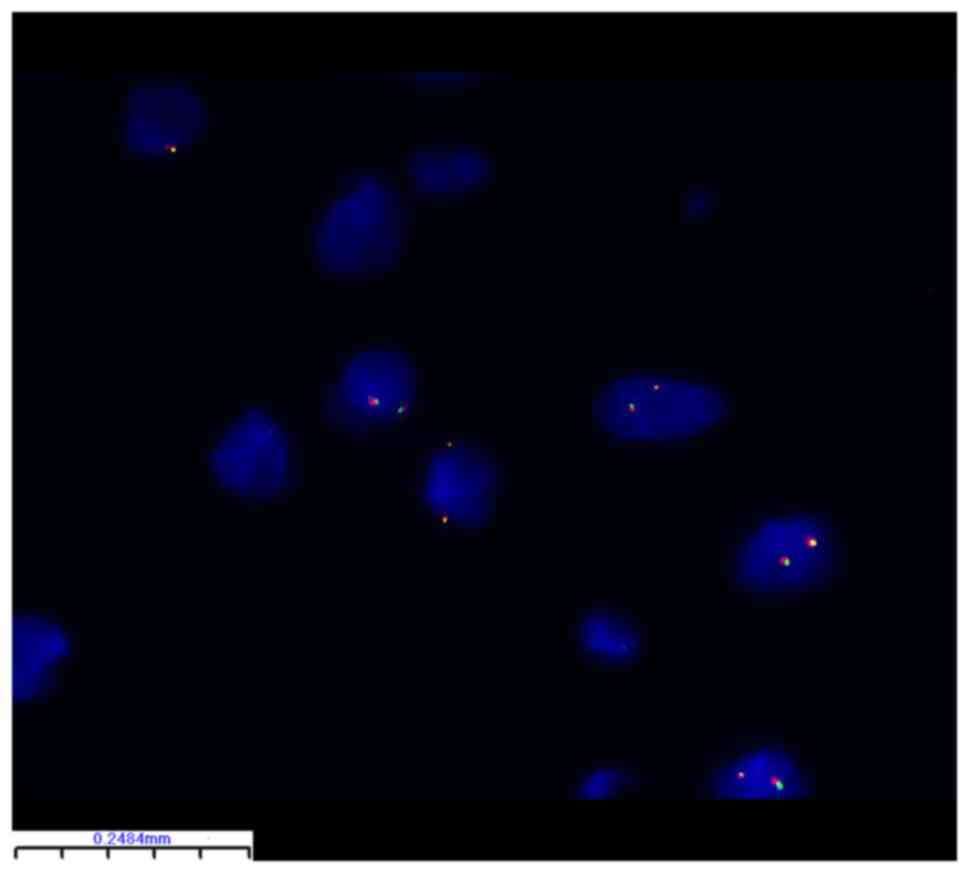
FISH identified PLAG1 breakage and
rearrangement in 24 of 32 (75%) lipoblastoma samples (Table II). There were no statistically
significant differences between the subgroups (Table I). Representative FISH images are
shown in Figs. 2 and 3. The PLAG1 gene breakage probe
uses orange-red dye to label PLAG1 3' end region (460 kb)
and green dye to label the PLAG1 5' end region (580 kb) with
the 50 kb PLAG1 gene in the middle. In Fig. 2, orange-red and green signal are
separated in the tumor cells, which represents breakage and
rearrangement of PLAG1 in this tumor cell. By contrast, in
Fig. 3, tumor cells did not
undergo breakage and rearrangement of PLAG1 so the
orange-red and green signals were not separated. In the classic and
myxoid subgroups of lipoblastoma, FISH-negative detection rates
were 27 and 29%, respectively, compared with 20% in the lipoma-like
subtype. Therefore, the highest probability of
PLAG1-positive rearrangement was detected using FISH in the
lipoma-like subgroup. Also, two of three relapsed patients tested
negative for PLAG1 fragmentation and rearrangement. One
patient failed the trial. Although S-100, CD-34, P-16 and desmin
IHC were not associated with recurrence in patients with
lipoblastoma patients by Kaplan-Meier analysis (Fig. S1A-D), patients with positive PLAG1
fragmentation and rearrangement had a risk of relapse of only 0.57%
of those with negative PLAG1. Patients who were positive for PLAG1
fragmentation and rearrangement had a 99.49% lower risk of
recurrence than those who were negative (Fig. S1E). However, there were only 3
patients with recurrence in the present study, including one
patient who was not detected, which requires further verification
with large samples.
Immunohistochemical
characteristics
Immunohistochemical results are presented in
Table SI. S-100-positive mono- or
multivesicular lipoblasts were observed (29/36, 81%), with
occasional mature adipocytes (Fig.
1F). CD34 (36/36, 100%) staining was diffusely strong in the
cytoplasm of primitive spindle mesenchyme and peripheral vessels
and abundant in blood vessels, highlighting a zonal pattern
(Fig. 1G). Most tumor cells were
negative while only few were positive for P16 (8/36, 22%; Fig. 1H). Desmin (26/36, 72%) staining was
positive in primitive mesenchymal cells and striated muscle tissue
in the dense fibrous septum (Fig.
1I). However, P16 IHC was less likely to be expressed in the
lipoma-like subtype than in the other two subtypes (Table I).
Follow-up
In total, 28 cases were clinically followed up
(range, 2-84 months; median, 41 months), of which 3 patients
relapsed, 8 were lost to follow-up and others were alive without
disease.
Discussion
Here, 36 lipoblastoma cases underwent morphological,
S-100, CD34, desmin and P16 immunohistochemical and PLAG1
FISH analysis. Other studies with similar sample size have mainly
focused on the morphology and immunophenotyping (19,32).
However, the present study attempted to diagnose at the molecular
level. Similar to other cases (17-20),
the majority of the present cases (28/36, 78%) were detected within
three years of age. By contrast with the large series studied by
Coffin et al (19) (n=59),
who reported a range of clinical associations in up to 17% of
lipoblastomas, the present study did not identify any syndromic
association, except for one case with complications of subcutaneous
emphysema (case 20). According to other reports, more than
one-fifth of lipoblastomas occur in patients >3 years of age,
consistent with the proportion reported in the present study
(17,31). The present findings differ from
those of other studies showing that lipoblastoma occurs most
frequently in extremities, since lipoblastomas in the present cases
occurred most frequently in the trunk (19,32-35).
The patients were predominantly male and exhibited median tumor
size of 4.7 cm. In certain studies, the recurrence rate in children
reached 25% (1,6); here, only 3 (8%) recurrences
occurred, 2 of which recurred because the tumor was too close to
the adjacent organ with incomplete resection (in case 23, following
recurrence the patient returned for further resection and was
diagnosed with lipoma-like lipoblastoma, which matured into
lipoma). The reason may be that the infant had relapsed after 51
months and the lipoblasts had matured and progressed. In another
case (case 17), recurrence occurred in situ in the
superficial subcutaneous fascia of the right upper arm 54 months
after complete resection. The longest (case 17) and shortest (case
28) intervals between recurrences were 54 and 15 months,
respectively.
Notably, recurrence occurred in two of four patients
(50%; at 1 and 33 years of age, respectively) with incomplete tumor
resection; three patients (case 7, 23, and 32) were young and one
(case 28) was older. It was hypothesized that patients with
incompletely resected tumors are more likely to relapse regardless
of age.
The clinical outcome primarily depends on the
completeness of resection, with a high recurrence rate in
incompletely resected tumors. Close follow-up for at least five
years is recommended (6). A total
of seven cases had lipoblastomatosis characterized by a diffuse
growth pattern, including two classic and lipoma-like subtypes and
three myxoid subtypes; these histological characteristics are
similar to those of lipoblastomas (34). Similar to intramuscular lipoma,
residual skeletal muscle tissue can be observed in
lipoblastomatosis, but without recurrence (5). Dao et al (36) conducted a meta-analysis and found
that lipoblastomatosis has a higher risk of recurrence than
localized lipoblastoma (odds ratio=5.1; 95% confidence interval,
1.9-15.9).
The primary genetic feature of lipoblastoma is
chromosomal translocation (minor inversions, insertion and circular
chromosomes), resulting in the rearrangement of PLAG1, which
encodes a zinc finger transcription factor that is expressed during
fetal life and is present at low levels or absent in most adult
organs (9). In lipoblastoma, exons
2 or 3 of PLAG1 most commonly combine with exon 1 at the
N-terminus of partner genes (the fourth exon is the coding region
containing the initiation codon) (12) so that the entire coding sequence is
retained under transcriptional control of a more active promoter
(37), resulting in subsequent
upregulation (14,38). In a mouse model, overexpression of
PLAG1 was found to induce tumorigenesis (39). Here, 75% of cases showed
PLAG1 breakage and rearrangement, greater than the 60-70%
reported in the literature (31,34,38).
Coiled-Coil-Helix-Coiled-Coil-Helix Domain Containing 7
(CHCHD7)-PLAG1 fusion, which also results in promoter
swap and PLAG1 activation, has been reported as a recurrent
event in salivary gland pleomorphic adenoma (40-42).
Two studies reported that hyaluronan synthase 2 (HAS2)
(8q24.1) and serine and arginine rich splicing factor 3
(SRSF3) (6p21.3-p21.1) fusion genes are prone to local
recurrence in lipoblastoma (9,28).
At present, RNA next-generation sequencing (NGS) serves a key role
in the classification and diagnosis of soft tissue tumors,
including defining the tumors, especially in cases where the
morphology is atypical and immunohistochemistry cannot indicate the
specific tumor type (31). It has
been suggested that even if FISH and immunohistochemistry results
are negative, the tumor may still be a lipoblastoma because high
mobility group at-hook 2 (HMGA2) is an upstream regulatory
activator of PLAG1 (43).
In the absence of chromosome 8 abnormality involving the
PLAG1 locus, HMGA2 induces activation and
overexpression of PLAG1; therefore, PLAG1 FISH and
immunohistochemistry results may be negative in cases with
HMGA2 rearrangement.
PLAG1 expression has been observed in the
absence of PLAG1 rearrangements, suggesting an alternative
mechanism underlying alterations in genes upregulates PLAG1
expression, such as increasing the PLAG1 copy number, as
proposed previously (38,44). Thus, the diagnostic role of
PLAG1 immunohistochemistry is limited, particularly in
morphologically difficult cases of mature lipoblastoma, where only
sparsely positive immature adipocytes and spindle cells are
typically identified; this finding is consistent with the results
of Lopez-Nunez et al (17).
However, if PLAG1 FISH is positive in adipose tumor, an
accurate diagnosis can be made using a combination of morphology
and immunohistochemistry. Therefore, it was hypothesized that when
lipoblastoma is suspected, FISH detection of PLAG1 may
replace PLAG1 immunohistochemistry.
Moreover, NGS not only detects PLAG1 or
HMGA2 rearrangements with high sensitivity but also
identifies PLAG1-associated fusion genes. Numerous fusion
gene partners [dead-box helicase 6 (DDX6), klf transcription
factor 10 (KLF10), kat8 regulatory nsl complex subunit 1
like (KANSL1L), hyaluronan synthase 2 (HAS2),
collagen type III alpha 1 chain (COL3A1), rad51 paralog b
(RAD51L1), collagen type I alpha 2 chain, ras-related
protein rab-2a (RAB2A), CHCHD7, serine and arginine
rich splicing factor 3 (SRSF3), heterogeneous nuclear
ribonucleoprotein c (HNRNPC), protein-l-isoaspartate
(d-aspartate) o-methyltransferase domain containing 1
(PCMTD1), tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein zeta (YWHAZ), ctd small
phosphatase 2 (CTDSP2), protein phosphatase 2 regulatory
subunit α, boc cell adhesion associated, oncogene regulated, zinc
finger e-box binding homeobox 2, runx1 partner transcriptional
co-repressor 1, Versican, peptidase inhibitor 15 and eukaryotic
translation elongation factor 1 alpha 1] with PLAG1 have
been identified in lipoblastoma (8,14,17,31,37,44-52).
These fusions lead to overexpression of PLAG1 (17,37).
Although only the YWHAZ promoter is fused to PLAG1,
it does not contribute to protein expression (49). The rearrangement of PLAG1 [a
transcriptional activator of Insulin Like Growth Factor 2
(IGF2)] in lipoblastoma activates the MAPK and
PI3K/AKT signaling pathways by directly increasing
expression of IGF2 and promoting the differentiation of
CD34-positive primitive mesenchymal cells into lipoblasts (14,53).
Pleomorphic adenoma and lipoblastoma with PLAG1
rearrangement differ in their partner genes (13,54).
In salivary gland tumors, the primary targets of translocation are
DNA-binding transcription factors (PLAG1 and HMGA2)
involved in growth factor signaling and cell cycle regulation as
well as co-activators of Notch [mastermind like transcriptional
coactivator 2, (MAML2)] and cAMP [target of rapamycin
complex 1, (TORC1)] signaling pathways. These fusion genes,
which are also involved in molecular tumor pathways, serve a key
role in tumorigenesis. Therefore, timely identification of the
associated genetic alterations and molecular classification of
these childhood lesions are important for proper follow-up,
evaluation of potential recurrence and avoidance of secondary
surgery.
The histological diagnosis of lipoblastoma with
typical morphological features is relatively straightforward in
infancy and early childhood. In first-born infants, tumors are
predominantly composed of primitive mesenchymal cells with
extensive myxoid stromal and minimal lipoblastoid components;
therefore, primitive myxoid mesenchymal tumor of infancy (PMMTI)
should be considered, which exhibits a more aggressive behavior
(55). PMMTI has typical cellular
atypia, strong mitotic activity and distinct round cellular
component compared with lipoblastoma, as reported by Warren et
al (50). PMMTI can overlap
with undifferentiated myxoid lipoblastoma, which resembles myxoid
lipoblastoma. Notably, RNA NGS has been used to assess internal
tandem repeats of BCL6 corepressor, a characteristic change in
PMMTI (56). Lipoblastoma-like
tumors of the vulva (LLTV) occur in adults, mostly commonly at age
17-46 years. Most often, LLTV presents as a unilateral, severely
myxoid, gelatinous, well-circumscribed, lobulated vulvar mass. The
morphology includes lobulated and varied proportions of mature
adipocytes; mild lipoblasts; spindle cells with short, thick nuclei
and prominent branching vessels with minimal nuclear atypia in a
diffuse myxoid background. In LLTV, mitosis without necrosis is
rare. Therefore, it also shows histological overlap with
lipoblastoma (57). Here, two
cases of lipoblastoma occurred in the perineal region (cases 11 and
22); both were males aged <2 years. FISH showed PLAG1
rearrangement; therefore, LLTV was excluded. In children, the
morphological appearance of a lipoma-like subtype requires
differential diagnoses, including lipoma, lipofibromatosis, fibrous
hamartoma of infancy, atypical lipomatous tumor/well-differentiated
liposarcoma and non-neoplastic lesions with post-traumatic fat
necrosis (such as post-traumatic pseudolipoma) (Table III). Microscopically,
lipofibromatosis may have a spindle cell component in fascicular
cells, lack lipoblasts and show more invasive growth in the
surrounding tissue (58). In cases
of post-traumatic fat necrosis, morphology is characterized by
chronic inflammatory changes, fat necrosis and hematoma formation.
A clinical history is helpful for diagnosis (59). Although lipoblastoma can be
differentiated from liposarcoma based primarily on age, when
lipoblasts mature, especially in older adults or deep in the limbs,
mediastinum, abdominal pelvis or retroperitoneum, they tend to be
larger. In these cases, tumors present with extensive myxoid or
lipomatous morphology and rare lipoblasts, especially focal
adipocytes and stromal cells, with nuclear atypia and local
invasiveness. In such cases, other diseases such as myxoid
liposarcoma and atypical lipomatous tumor/well-differentiated
liposarcoma should be considered (58,60).
With fine-needle biopsy specimens, it is difficult to
histologically distinguish lipoblastoma, which may require further
molecular detection (61).
(PLAG1 and DDIT3) and (PLAG1 and MDM2)
genetic fusion testing may confirm this diagnosis. In addition,
insulin-induced lipoatrophy should be considered if there is a
clinical history of diabetes or malnutrition, as these cases may
exhibit myxoid changes and pseudolipoblasts (62). The morphology of the classic
subtype of lipoblastoma is more typical (31). In case 34, lipoblastoma with
fibroblast morphology which is the fourth class of lipoblastoma
(31) was focally present but
surrounded by classic lipoblastoma. Thus, it was classified as a
classic subtype. The fifth class of lipoblastoma, consisting
primarily of multivacuolar lipoblasts, some of which have central
nucleus and granular eosinophilic cytoplasm lacking a myxoid
component, was not observed in the present study. The tumor was
named lipoblastoma with hibernoma-like features (38). Studies have reported that P16
effectively differentiates benign lesions from liposarcoma
(24,63). It is necessary to combine
CD34-positive primitive mesenchymal cells with a more
characteristic histological morphology in lipoblastoma with
clinical information and biological behavior to diagnose
lipoblastoma. Clinical features, histological morphology,
biological behavior, immunohistochemical expression, and genetic
changes of the 12 tumors that are difficult to differentiate from
lipoblastoma are summarized in Table
III.
 | Table IIIClinicopathological features of the
differential diagnoses of lipoblastoma. |
Table III
Clinicopathological features of the
differential diagnoses of lipoblastoma.
| A, Lipoblastoma
with predominant myxoid morphology |
|---|
| Diagnosis | Features | First author, year
(Refs.) |
|---|
| Myxoid
liposarcoma | Myxoid liposarcoma
is rare in children, usually occurs in 30-60-year-old adults.
Histomorphologically, mature adipocytes in the lobules are
concentrated in the periphery, whereas in lipoblastoma, maturation
occurs in the center of the lobule and the periphery is composed of
more primitive cells, lacking the enrichment phenomenon cells
around blood vessels. The focal nuclei are atypic and pleomorphic,
with pathological mitosis. A characteristic pulmonary edema
morphology is observed due to the pooling of matrix mucins. Tumor
cells express S-100 protein and NY-ESO-1 to varying degrees and are
negative for CD34, desmin, keratin, SMA, MDM2 and CDK4. In this
tumor, rearrangement of chromosome 12q13 (CHOP/DDIT3) with
translocation partners 16p11 (FUS-TLS) or 22p11 (EWSR1) results in
disruption of the CHOP gene on chromosome 12. | Coffin,
2012(58) |
| PMMTI | PMMTI primarily
occurs in infants, mainly on the trunk, neck, and extremities.
Histomorphologically, there are mainly round cell components,
primitive mesenchymal cells and myxoid matrix,
curvilinear-plexiform vascular morphology, pseudolipoblasts,
cellular atypia, and frequent mitosis. S-100 expression is negative
on histochemistry. BCOR-ITD or YWHAE gene rearrangement and lack of
PLAG1 gene rearrangement are seen in this tumor. | Warren,
2021(47); Deen, 2013(52); Andersson, 2019(53) |
| Invasive
angiomyxoma | Invasive
angiomyxoma primarily occurs in young and middle-aged females.
Histomorphologically, there are spindle and asteroid cell with
unclear boundaries, thin-walled or thick-walled blood vessels of
varying sizes distributed in a myxoid matrix rich in slender
collagen fibers, atypical tumor cells, small round or oval nuclear,
and no mitosis. Tumor cells express vimentin, desmin, ER, and PR;
most nuclei express HMGA2; CD34 and S-100 are not expressed. HMGA2
(12q14.3) rearrangement is seen in this tumor. | Yang, 2021(28) |
| LLTV | LLTV primarily
occurs in adult females, primarily in the vulva or groin.
Histomorphologically, there are lobular appearance, mature
adipocytes interspersed with spindle cells, lipoblasts, myxoid
matrix, branching vessels, and no banded mature morphology. On
histochemistry the tumor loses nuclear expression of RB protein
(usually mosaic morphology) and is negative for S-100, PLAG1, and
HMGA2 expression. The tumor has no DDIT3T fusion and no evidence of
PLAG1 gene alteration, and associates with chromosomal 13q
alteration. | Stenman,
2005(54) |
| Lipoatrophy | Lipoatrophy
primarily occurs in adults with a clinical history of
insulin-dependent diabetes or starvation (malnutrition or
anorexia). Histomorphologically, there are presence of lobular
architecture, myxoid changes, and pseudolipoblasts. | Jermendy,
2000(62) |
| Superficial
angiomyxoma | Superficial
angiomyxoma is rare in infants and young children, more common in
middle-aged people (median age 40 years). Histomorphologically,
there are abundant myxoid matrix, sparse spindle or asteroid cells,
and slender capillaries, lobulated structures with indistinct
boundaries. Lack of lipoblasts at different developmental stages
are key to differential diagnosis. Tumor cells express CD34; some
cells are positive for SMA, MSA, and desmin; S-100 is not
expressed. | Iwashita,
2020(29) |
| B, Lipoblastoma
with predominant mature lipoma-like morphology |
| Diagnosis | Features | First author, year
(Refs.) |
|
Lipofibromatosis | Lipofibromatosis
primarily occurs in infants (congenital subgroup) to 14 years of
age. Histomorphologically, there are infiltration in skeletal
muscle, spindle cell components forming long thin fascicles, mature
adipose tissue, and absent of lipoblasts. Spindle cells express
vimentin, CD34, and a-SMA to varying degrees and can express S-100
focally, but not desmin. Activation of EGFR (HER1), EGFR, ROS1,
RET, or PDGFRB may indicate pathogenesis. | Mirkovic,
2015(57) |
| ALT/WDLPS | ALT/WDLPS primarily
occurs in middle-aged and elderly; peak age of onset is 50-60
years. Histomorphologically, there are focally seen nuclear
heterogeneity or hyperchromasia. Neoplastic adipocytes and atypical
stromal cells express MDM2, CDK4, and P16. MDM2 gene amplification
is the gold standard for identifying benign fatty tumors and
ALT/WDLPS. | Alaggio,
2009(60) |
| Post-traumatic
pseudolipoma | Post-traumatic
pseudolipoma can occurs in all age groups. Histomorphologically,
there are foam cells, adipocytes surrounding necrotic tissue,
showing pseudoadenoid structures, multinucleated giant cells with
hyperchromatic nuclei, and interstitial inflammatory cells.
Adipocytes express S-100, histiocytes express CD68. Diagnosis of
the disease requires a combination of medical history and gross
structure. | Aust, 2007(59) |
| Lipoma | Lipoma primarily
occurs in adults. Histomorphologically, there are rarely seen
lobulated structures, lipoblasts, and areas of mucinous stroma with
plexiform capillaries. Lipomas can be misdiagnosed when they are
associated with extensive myxoid degeneration. Tumor cells express
S-100 and HMGA2; no express MDM2 and CDK4. | Abdul-Ghafar,
2018(25) |
| Fibrous hamartoma
of infancy | Fibrous hamartoma
of infancy primarily occurs in infants, is usually less than 2
years old, and is more common in boys. Histomorphologically, there
are characteristic organ growth structures, including bland
fibroblasts/myofibroblasts, primitive mesenchymal cells, and mature
adipose tissue, without lipoblasts/pseudolipoblasts. CD34 is only
diffusely expressed in collagenized pseudoangioma-like (or
neurofibromatous) areas; mature adipose tissue express S-100. EGF
expression is rarely detected. EGFR exon 20 insertion/repeat
mutation are seen in this tumor. | Al-Ibraheemi,
2017(30) |
| Hibernoma | Hibernoma primarily
occurs in young and middle-aged people (20-40 years old). Brown
adipocyte component is more prominent, with a central nucleus and
abundant fine-grained eosinophilic cytoplasm. Tumor cells express
S100 in varying degrees, and the spindle cell subtype express
CD34. | Gisselsson,
2001(38) |
In clinical practice, rapid FISH detection is
favored by pathologists for soft tissue tumors because of its
rapidity, low cost and high diagnostic efficiency. Here, the
frequency of PLAG1 rearrangement (75%) in lipoblastoma was
higher than that reported in previous studies (17,38);
however, 4 lipoblastomas were not successfully detected. Quality
control review was performed to rule out factors that were more
likely to be associated with short formalin fixation time, too old
specimens and DNA degradation. In addition, the present study
lacked a control group for other adipose tumors and only tested
PLAG1 for lipoblastoma.
In the classic and myxoid subgroups of lipoblastoma,
FISH-negative detection rates were 27 and 29%, respectively,
compared with 20% in the lipoma-like subtype. The higher negative
detection rate in the first two groups indicated that in certain
cases, lipoblastoma may not result in cytogenetic segregation of
the target gene, which leads to undetected rearrangements by FISH
(64). RNA NGS may improve
diagnostic accuracy. Therefore, RNA NGS is necessary, especially
for uncommon age groups or morphologically atypical populations.
Lipoblastomas mature into regular lipomas or are similar to
fibrolipoma (25,34). Here, subjects with a median age of
up to three years had the lipoma-like subtype. Whether older
individuals have a higher tendency to develop the lipoma-like
subtype or mature lipoma requires further experimental
verification, clinical observation and molecular characterization
to clarify the occurrence and development of the disease.
Patients with lipoblastoma are usually within the
age of three years; however, the disease cannot be ruled out in
patients older than three years and may also occur in adults with
mature adipose tissue with prominent lobular structures.
Immunochemistry showing S-100-positive single or multivesicular
adipocytes and CD34- and desmin-positive primitive mesenchymal
cells as well as FISH results indicating PLAG1 gene breakage
and rearrangement are key to diagnosis and differential
diagnosis.
Supplementary Material
Role of biomarker expression in
assessing the prognosis of patients with lipoblastoma, including
(A) S-100, (B) CD34, (C) P16, (D) Desmin and (E) fluorescence in
situ hybridization PLAG1. PLAG1, pleiomorphic adenoma
gene 1.
Immunohistochemical expression.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81601692), the Technology
Research from the Department of Education of Liaoning Province
(grant no. JCZR2020013) and 345 Talent Project of Shengjing
Hospital of China Medical University (grant no. M0367).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WZ collected and analyzed data and wrote the paper.
WZ, SZ, ZY and YZ performed immunohistochemistry and FISH. ZW
conceived and supervised the study. WZ and ZW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Committee of Shengjing Hospital of China Medical University
(approval no. 2022PS104J). The requirement for written informed
consent was waived due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Speer AL, Schofield DE, Wang KS, Shin CE,
Stein JE, Shaul DB, Mahour GH and Ford HR: Contemporary management
of lipoblastoma. J Pediatr Surg. 43:1295–1300. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Putra J and Al-Ibraheemi A: Adipocytic
tumors in children: A contemporary review. Semin Diagn Pathol.
36:95–104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spătaru RI, Cîrstoveanu C, Iozsa DA,
Enculescu A, Tomescu LF and Șerban D: Lipoblastoma: Diagnosis and
surgical considerations. Exp Ther Med. 22(903)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jandali D, Heilingoetter A, Ghai R, Jeffe
J and Al-Khudari S: Large parotid gland lipoblastoma in a teenager.
Front Pediatr. 6(50)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Besouw MT, Verlinde PF, Uyttebroeck AM and
Renard MM: Lipoblastoma and lipoblastomatosis: Especially in
children. Ned Tijdschr Geneeskd. 155(A3467)2011.PubMed/NCBI(In Dutch).
|
|
6
|
McVay MR, Keller JE, Wagner CW, Jackson RJ
and Smith SD: Surgical management of lipoblastoma. J Pediatr Surg.
41:1067–1071. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Voz ML, Mathys J, Hensen K, Pendeville H,
Van Valckenborgh I, Van Huffel C, Chavez M, Van Damme B, De Moor B,
Moreau Y and Van de Ven WJM: Microarray screening for target genes
of the proto-oncogene PLAG1. Oncogene. 23:179–191. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nitta Y, Miyachi M, Tomida A, Sugimoto Y,
Nakagawa N, Yoshida H, Ouchi K, Tsuchiya K, Iehara T, Konishi E, et
al: Identification of a novel BOC-PLAG1 fusion gene in a case of
lipoblastoma. Biochem Biophys Res Commun. 512:49–52.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Juma AR, Damdimopoulou PE, Grommen SV, Van
de Ven WJ and De Groef B: Emerging role of PLAG1 as a regulator of
growth and reproduction. J Endocrinol. 228:R45–R56. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen KS, Stroup EK, Budhipramono A,
Rakheja D, Nichols-Vinueza D, Xu L, Stuart SH, Shukla AA, Fraire C,
Mendell JT and Amatruda JF: Mutations in microrna processing genes
in wilms tumors derepress the IGF2 regulator PLAG1. Genes Dev.
32:996–1007. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patz M, Pallasch CP and Wendtner CM:
Critical role of micrornas in chronic lymphocytic leukemia:
Overexpression of the oncogene PLAG1 by deregulated mirnas. Leuk
Lymphoma. 51:1379–1381. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Van Dyck F, Declercq J, Braem CV and Van
de Ven WJ: PLAG1, the prototype of the plag gene family:
Versatility in tumour development (Review). Int J Oncol.
30:765–774. 2007.PubMed/NCBI
|
|
13
|
Kas K, Voz ML, Röijer E, Aström AK, Meyen
E, Stenman G and Van de Ven WJ: Promoter swapping between the genes
for a novel zinc finger protein and beta-catenin in pleiomorphic
adenomas with t(3;8)(P21;Q12) translocations. Nat Genet.
15:170–174. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hibbard MK, Kozakewich HP, Dal Cin P,
Sciot R, Tan X, Xiao S and Fletcher JA: PLAG1 fusion oncogenes in
lipoblastoma. Cancer Res. 60:4869–4872. 2000.PubMed/NCBI
|
|
15
|
Matsuyama A, Hisaoka M and Hashimoto H:
PLAG1 expression in mesenchymal tumors: An immunohistochemical
study with special emphasis on the pathogenetical distinction
between soft tissue myoepithelioma and pleomorphic adenoma of the
salivary gland. Pathol Int. 62:1–7. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Andreasen S, von Holstein SL, Homøe P and
Heegaard S: Recurrent rearrangements of the Plag1 and HMGA2 genes
in lacrimal gland pleomorphic adenoma and carcinoma ex pleomorphic
adenoma. Acta Ophthalmol. 96:e768–e771. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lopez-Nunez O, Alaggio R, Ranganathan S,
Schmitt L, John I, Church AJ and Picarsic J: New molecular insights
into the pathogenesis of lipoblastomas: Clinicopathologic,
immunohistochemical, and molecular analysis in pediatric cases. Hum
Pathol. 104:30–41. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou J, Li X, Cai Y, Wang L and Yang SD:
Undifferentiated myxoid lipoblastoma with PLAG1 gene rearrangement
in infant. Pathol Res Pract. 216(152765)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Coffin CM, Lowichik A and Putnam A:
Lipoblastoma (Lpb): A clinicopathologic and immunohistochemical
analysis of 59 cases. Am J Surg Pathol. 33:1705–1712.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kubota F, Matsuyama A, Shibuya R, Nakamoto
M and Hisaoka M: Desmin-positivity in spindle cells:
Under-recognized immunophenotype of lipoblastoma. Pathol Int.
63:353–357. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sabah M, Cummins R, Leader M and Kay E:
Aberrant expression of the Rb pathway proteins in soft tissue
sarcomas. Appl Immunohistochem Mol Morphol. 14:397–403.
2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Louis-Brennetot C, Coindre JM, Ferreira C,
Pérot G, Terrier P and Aurias AL: The CDKN2A/CDKN2B/CDK4/CCND1
pathway is pivotal in well-differentiated and dedifferentiated
liposarcoma oncogenesis: An analysis of 104 tumors. Genes
Chromosomes Cancer. 50:896–907. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
He M, Aisner S, Benevenia J, Patterson F,
Aviv H and Hameed M: P16 immunohistochemistry as an alternative
marker to distinguish atypical lipomatous tumor from deep-seated
lipoma. Appl Immunohistochem Mol Morphol. 17:51–56. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gonzalez RS, McClain CM, Chamberlain BK,
Coffin CM and Cates JM: Cyclin-dependent kinase inhibitor 2a (P16)
distinguishes well-differentiated liposarcoma from lipoma.
Histopathology. 62:1109–1111. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abdul-Ghafar J, Ahmad Z, Tariq MU, Kayani
N and Uddin N: Lipoblastoma: A clinicopathologic review of 23 cases
from a major tertiary care center plus detailed review of
literature. BMC Res Notes. 11(42)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
de Saint Aubain Somerhausen N, Coindre JM,
Debiec-Rychter M, Delplace J and Sciot R: Lipoblastoma in
adolescents and young adults: Report of six cases with fish
analysis. Histopathology. 52:294–298. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Creytens D: A contemporary review of
myxoid adipocytic tumors. Semin Diagn Pathol. 36:129–141.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang X, Zhang L, Zhao W, Zhang Y and Yu J:
Invasive angiomyxoma diagnosed by transvaginal ultrasound: A case
report. Ann Palliat Med. 10:5870–5874. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iwashita W, Kurabayashi A, Tanaka C,
Naganuma S, Kawamura T, Aki F and Furihata M: Superficial
angiomyxoma of the nipple in a Japanese woman: A case report and
review of literature. Int J Surg Pathol. 28:683–687.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Al-Ibraheemi A, Martinez A, Weiss SW,
Kozakewich HP, Perez-Atayde AR, Tran H, Parham DM, Sukov WR,
Fritchie KJ and Folpe AL: Fibrous hamartoma of infancy: A
clinicopathologic study of 145 cases, including 2 with sarcomatous
features. Mod Pathol. 30:474–485. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fritchie K, Wang L, Yin Z, Nakitandwe J,
Hedges D, Horvai A, Mora JT, Folpe AL and Bahrami A: Lipoblastomas
presenting in older children and adults: Analysis of 22 cases with
identification of novel PLAG1 fusion partners. Mod Pathol.
34:584–591. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mentzel T, Calonje E and Fletcher CD:
Lipoblastoma and lipoblastomatosis: A clinicopathological study of
14 cases. Histopathology. 23:527–533. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chung EB and Enzinger FM: Benign
lipoblastomatosis. An analysis of 35 cases. Cancer. 32:482–492.
1973.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Collins MH and Chatten J:
Lipoblastoma/lipoblastomatosis: A clinicopathologic study of 25
tumors. Am J Surg Pathol. 21:1131–1137. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Han JW, Kim H, Youn JK, Oh C, Jung SE,
Park KW, Lee SC and Kim HY: Analysis of clinical features of
lipoblastoma in children. Pediatr Hematol Oncol. 34:212–220.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dao D, Najor AJ, Sun PY, Farrokhyar F,
Moir CR and Ishitani MB: Follow-up outcomes of pediatric patients
who underwent surgical resection for lipoblastomas or
lipoblastomatosis: A single-institution experience with a
systematic review and meta-analysis. Pediatr Surg Int. 36:341–355.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yoshida H, Miyachi M, Ouchi K, Kuwahara Y,
Tsuchiya K, Iehara T, Konishi E, Yanagisawa A and Hosoi H:
Identification of COL3A1 and RAB2A as novel translocation partner
genes of PLAG1 in lipoblastoma. Genes Chromosomes Cancer.
53:606–611. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gisselsson D, Hibbard MK, Dal Cin P, Sciot
R, His BL, Kozakewich HP and Fletcher JA: PLAG1 alterations in
lipoblastoma: Involvement in varied mesenchymal cell types and
evidence for alternative oncogenic mechanisms. Am J Pathol.
159:955–962. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Declercq J, Van Dyck F, Braem CV, Van
Valckenborgh IC, Voz M, Wassef M, Schoonjans L, Van Damme B, Fiette
L and Van de Ven WJM: Salivary gland tumors in transgenic mice with
targeted plag1 proto-oncogene overexpression. Cancer Res.
65:4544–4553. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Asp J, Persson F, Kost-Alimova M and
Stenman G: CHCHD7-PLAG1 and TCEA1-PLAG1 gene fusions resulting from
cryptic, intrachromosomal 8q rearrangements in pleomorphic salivary
gland adenomas. Genes Chromosomes Cancer. 45:820–828.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Matsuyama A, Hisaoka M, Nagao Y and
Hashimoto H: Aberrant PLAG1 expression in pleomorphic adenomas of
the salivary gland: A molecular genetic and immunohistochemical
study. Virchows Arch. 458:583–592. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Asahina M, Saito T, Hayashi T, Fukumura Y,
Mitani K and Yao T: Clinicopathological effect of PLAG1 fusion
genes in pleomorphic adenoma and carcinoma ex pleomorphic adenoma
with special emphasis on histological features. Histopathology.
74:514–525. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Klemke M, Müller MH, Wosniok W, Markowski
DN, Nimzyk R, Helmke BM and Bullerdiek J: Correlated expression of
HMGA2 and PLAG1 in thyroid tumors, uterine leiomyomas and
experimental models. PLoS One. 9(e88126)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Krsková L, Němečková T, Balko J, Brož P
and Vícha A: Novel ZEB2-PLAG1 fusion gene identified by RNA
sequencing in a case of lipoblastoma. Pediatr Blood Cancer.
68(e28691)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Morerio C, Rapella A, Rosanda C, Tassano
E, Gambini C, Romagnoli G and Panarello C: PLAG1-HAS2 fusion in
lipoblastoma with masked 8q intrachromosomal rearrangement. Cancer
Genet Cytogenet. 156:183–184. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gerhard-Hartmann E, Vokuhl C, Roth S,
Steinmüller T, Rosenfeldt M, Zamò A, Rosenwald A, Appenzeller S,
Ernestus K and Maurus K: The histological and molecular spectrum of
lipoblastoma: A case series with identification of three novel gene
fusions by targeted rna-sequencing. Pathol Res Pract.
226(153591)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Warren M, Tiwari N, Sy S, Raca G, Schmidt
RJ and Pawel B: Plag1 immunohistochemical staining is a surrogate
marker for plag1 fusions in lipoblastomas. Pediatr Dev Pathol.
25:134–140. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Brčić I, Igrec J, Halbwedl I, Viertler C
and Liegl-Atzwanger B: Expanding the spectrum of PLAG1-rearranged
lipoblastomas arising in patients over 45, with identification of
novel fusion partners. Mod Pathol. 35:283–285. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chung CT, Antonescu CR, Dickson BC, Chami
R, Marrano P, Fan R, Shago M, Hameed M and Thorner PS: Pediatric
fibromyxoid soft tissue tumor with plag1 fusion: A novel entity?
Genes Chromosomes Cancer. 60:263–271. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Warren M, Turpin BK, Mark M, Smolarek TA
and Li X: Undifferentiated myxoid lipoblastoma with PLAG1-HAS2
fusion in an infant; morphologically mimicking primitive myxoid
mesenchymal tumor of infancy (PMMTI)-diagnostic importance of
cytogenetic and molecular testing and literature review. Cancer
Genet. 209:21–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Morerio C, Nozza P, Tassano E, Rosanda C,
Granata C, Conte M and Panarello C: Differential diagnosis of
lipoma-like lipoblastoma. Pediatr Blood Cancer. 52:132–134.
2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Deen M, Ebrahim S, Schloff D and Mohamed
AN: A novel PLAG1-RAD51L1 gene fusion resulting from a
t(8;14)(Q12;Q24) in a case of lipoblastoma. Cancer Genet.
206:233–237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Andersson MK, Åman P and Stenman G:
IGF2/IGF1R signaling as a therapeutic target in MYB-positive
adenoid cystic carcinomas and other fusion gene-driven tumors.
Cells. 8(913)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Stenman G: Fusion oncogenes and tumor type
specificity-insights from salivary gland tumors. Semin Cancer Biol.
15:224–235. 2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Alaggio R, Ninfo V, Rosolen A and Coffin
CM: Primitive myxoid mesenchymal tumor of infancy: A
clinicopathologic report of 6 cases. Am J Surg Pathol. 30:388–394.
2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Santiago T, Clay MR, Allen SJ and Orr BA:
Recurrent bcor internal tandem duplication and BCOR or BCL6
expression distinguish primitive myxoid mesenchymal tumor of
infancy from congenital infantile fibrosarcoma. Mod Pathol.
30:884–891. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mirkovic J and Fletcher CD:
Lipoblastoma-like tumor of the vulva: Further characterization in 8
new cases. Am J Surg Pathol. 39:1290–1295. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Coffin CM and Alaggio R: Adipose and
myxoid tumors of childhood and adolescence. Pediatr Dev Pathol.
15:239–254. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Aust MC, Spies M, Kall S, Gohritz A,
Boorboor P, Kolokythas P and Vogt PM: Lipomas after blunt soft
tissue trauma: Are they real? Analysis of 31 Cases. Br J Dermatol.
157:92–99. 2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Alaggio R, Coffin CM, Weiss SW, Bridge JA,
Issakov J, Oliveira AM and Folpe AL: Liposarcomas in young
patients: A study of 82 cases occurring in patients younger than 22
years of age. Am J Surg Pathol. 33:645–658. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ferreira J, Esteves G, Fonseca R, Martins
C, André S and Lemos MM: Fine-needle aspiration of lipoblastoma:
Cytological, molecular, and clinical features. Cancer Cytopathol.
125:934–939. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jermendy G, Nádas J and Sápi Z:
‘Lipoblastoma-like’ lipoatrophy induced by human insulin:
Morphological evidence for local dedifferentiation of adipocytes?
Diabetologia. 43:955–956. 2000.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cappellesso R, d'Amore ES, Dall'Igna P,
Guzzardo V, Vassarotto E, Rugge M and Alaggio R:
Immunohistochemical expression of P16 in lipoblastomas. Hum Pathol.
47:64–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen S, Deniz K, Sung YS, Zhang L, Dry S
and Antonescu CR: Ewing sarcoma with ERG gene rearrangements: A
molecular study focusing on the prevalence of fus-erg and common
pitfalls in detecting EWSR1-ERG fusions by fish. Genes Chromosomes
Cancer. 55:340–349. 2016.PubMed/NCBI View Article : Google Scholar
|