1. Introduction
The majority of immunocompromised individuals with
uncontrolled diabetes, haematological malignancies, renal failure,
chemotherapy, long-term steroid use, diabetes with post-COVID-19
infection or acquired immunodeficiency syndrome are susceptible to
mucormycosis, a rare opportunistic fungal disease, which has lately
become increasingly prevalent (1,2). In
1885, Paltauf (3) published the
first description of mucormycosis. Since then it has been
considered as the most lethal and quickly progressing form of
fungal infection in humans, initiated by a fungus of the
saprophytic variety such as Mucor and Rhizopus. The
most common causes of fungus-related illness are rotting fruits and
vegetables, although fungi spores can also spread disease when
inhaled from dust or air conditioning units (4).
Rhizopus is the most frequent source of
rhinocerebral mucormycosis and the genera Absidia, Mucor and
Mucorale also contribute to the disease (5). Mucorale can penetrate the
vascular system, preventing arterial blood flow, causing thrombosis
and ischemia. Due to soft- and hard-tissue necrosis, the infection
quickly spreads to adjacent tissues. Dentists and medical
professionals can help with early identification and treatment of
mucormycosis due to intraoral presentation being amongst the
earliest clinical symptoms of cranial, rhino and ocular
mucormycosis (6). The two most
frequent causes of oral mucormycosis are direct wound infection and
palatal mucormycosis, which are primarily disseminated by inhaling
fungal spores through the nasal and paranasal sinuses (7). In the majority of cases, this
progresses to a systemic fungal infection, often with a poor
prognosis. Mucormycosis is difficult to identify due to its
radiographic resemblance to aspergillosis, in addition to a paucity
of screening methods (8).
Therefore, it is essential in medicine to create diagnostic tests
that are precise, quick, specific and sensitive. Despite a number
of notable recent improvements, multiple fundamental diagnostic
techniques employed in the initial detection of mucormycosis have
remained unchanged. Serology, lateral flow devices, radiography and
CT imaging, histology, microscopy and in vitro fungal
culture are still employed extensively (9).
In situations where sophisticated diagnostic tools
are unavailabile, several of these diagnostic procedures can be
transformed into point-of-care testing. These essential processes
are being supplemented by elevated biomolecule alternative
technologies, such as DNA sequencing-based techniques and
matrix-assisted laser desorption ionization time of flight mass
spectrometry (10). Microscopy and
histology are the foundational components of diagnosis. Molecular
tests can also be recommended as a helpful addition to conventional
diagnostic techniques for the detection and identification of
mucormycosis.
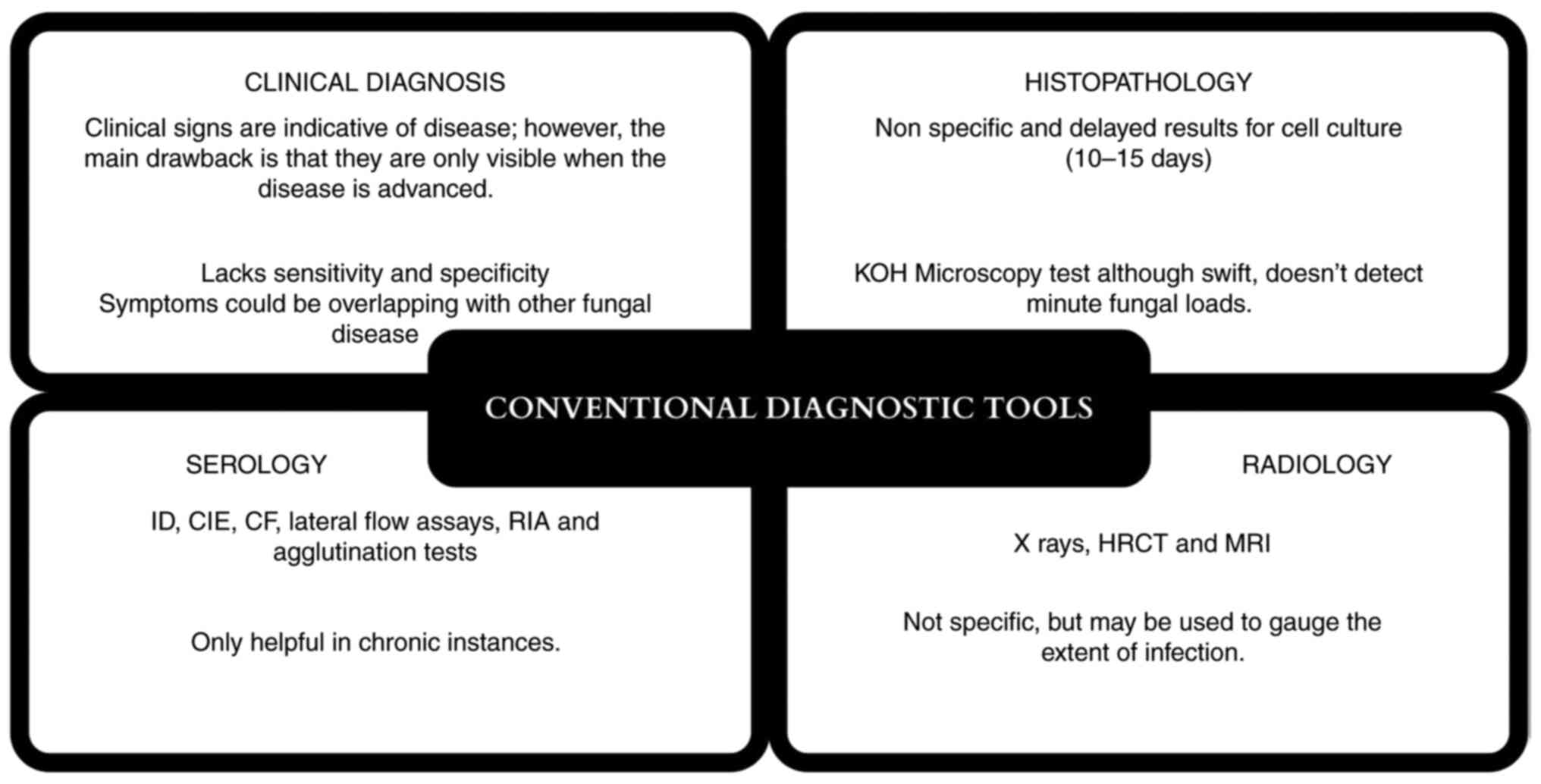
2. Conventional diagnostic tools
Clinical diagnosis
Clinical diagnosis is frequently used in medical
terminology, although it can be challenging for doctors to diagnose
mucormycosis. The sensitivity and specificity of a clinical
diagnosis are subpar. Tissue necrosis is the most suggestive
clinical sign of mucormycosis. Despite this, after the disease has
progressed to an advanced level, it helps to raise suspicion, start
laboratory testing and reveal the clinical indicators of the
condition (11). The primary
manifestations of mucormycosis are dermal, respiratory and
rhinocerebral mucormycosis of which the following are the clinical
signs: i) Oral ulceration, which is accompanied by pain and
swelling in the face; ii) black lesions on the bridge of the nose;
iii) nasal discharge containing blood; iv) paranasal sinus
infection, which can spread to the mouth; v) perforations in the
palate; vi) paraesthesia; and vii) facial cellulitis (12).
However, the symptoms listed above can overlap with
those of other systemic disorders such as invasive aspergillosis,
fusariosis, nocardiosis, Wegener granulomatosis and other
malignancies, thus making clinical diagnosis a non-specific
procedure (13). The clinical
signs that are crucial in arriving at a clinical diagnosis for
mucormycosis include some pertinent indicators that should not be
overlooked, such as cranial nerve palsy, diplopia, sinus pain,
periorbital swelling, orbital apex syndrome and palatal ulcers.
These indicators are considered hallmarks for the diagnosis of
mucormycosis (14). The
disadvantages of conventional diagnostic tools are summarised in
Fig. 1.
Histopathology
The current gold-standard diagnostic methods for
mucormycosis include microscopy, cell culture studies and
histopathology (15). The
foundation of microscopy is the identification and isolation of the
fungus responsible for the disease. Multiple specimens may be
examined for microscopy depending on the clinical symptoms and
infection location; however, tissue biopsy is still the preferable
method (7). Histopathological
staining, including Grocott's methenamine silver (GMS) and periodic
acid-Schiff (PAS) staining, offers enhanced outlines of the fungal
wall. However, compared with GMS, PAS offers superior visualisation
of surrounding tissues. Hence, it is more specific for mucormycosis
(16).
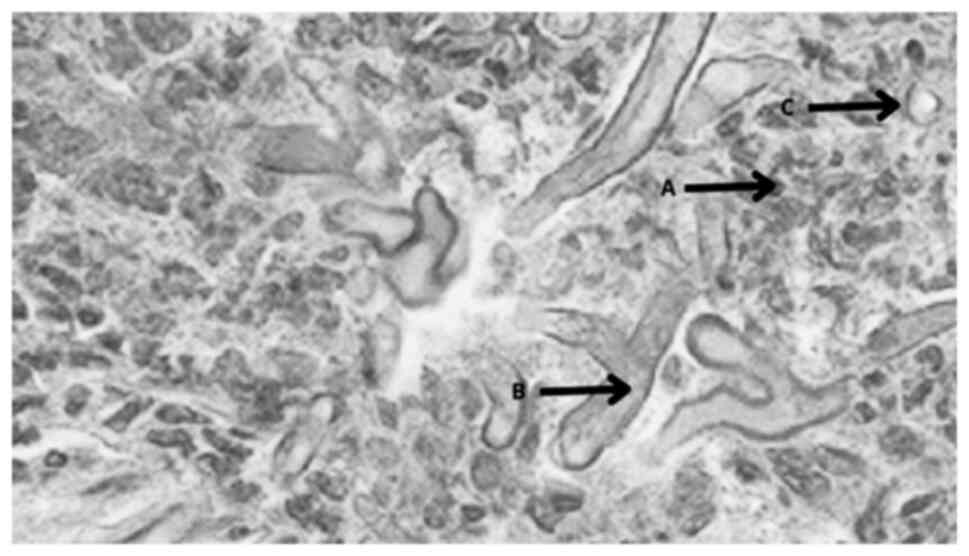
Typical histopathological images of mucormycosis
comprise fungal septate or pauciseptate hyphae (Fig. 2). Histopathological diagnostics, in
addition to direct microscopy, aid in the differentiation of a
fungal infection from a culture contaminant. However, one
significant disadvantage is that it can only provide morphological
diagnosis and does not provide information regarding the
specificity of the infecting organism (17).
Radiology
Preferred imaging techniques include contrast
enhanced MRI and conventional CT. Imaging is necessary for a
variety of reasons, including early diagnosis, initiation of
antifungal therapy and monitoring of treatment response. Due to its
improved contrast resolution in soft-tissue and marrow
abnormalities, MRI is the gold standard while CT is often used in
conjunction. The key symptom of black turbinate is a lack of
contrast enhancement of invading mucosa due to small artery
occlusion; an example of this is rhinocerebral mucormycosis.
Radiography does offer signs of the type and quantity of infection,
which can assist and guide biopsy sampling. However, radiography
may not allow for the exact identification of the causative fungal
agent or even a conclusive diagnosis of a fungal aetiology
(18). The existence of major
nodules (>1 cm) or perinodular halos throughout chest
radiographs can show fungal infections invading blood vessels. A
reverse halo accompanied by rapid tissue invasion or multiple
nodules accompanied by lung effusion indicates infection by
Mucorale mould. These characteristics can be indicative of
fungal aetiology (19). The
reverse halo sign on a CT scan is another symptom of mucormycosis
and can be seen within the first week of illness in 94% of cases,
as reported by Legouge et al (20) thus suggesting that CT imaging is a
sensitive radiographic technique for the early diagnosis of
mucormycosis.
Serology
Antibodies to fungi are identified using serology as
a diagnostic tool. Serology has undergone extended use in the
detection of fungal infections and is a commonly used technique.
Lateral flow tests, radio-immunosorbent assays, enzyme
immunoassays, immunodiffusion, counter-immunoelectrophoresis,
complement fixation (CF), immunoassays using antibodies and
agglutination techniques are some of the technologies used to
identify antibodies in the blood or saliva (21). Future molecular technologies may be
used to enhance serological techniques, but they will require
direct tissue collection, standardisation, technological
advancements and cost reduction (22). A monoclonal antibody (2DA6) was
examined by Burnham-Marusich et al (23) using sandwich ELISA and was found to
have high reactivity with purified fucomannan of the Mucor
species. However, lateral flow immunoassay (LFIA) has been
demonstrated to be more convenient in comparison to ELISA, as it
can be used to test serum, urine and tissues more easily.
Some disadvantages of serological investigations
include the technique being time intensive, such as CF, in addition
to being technically challenging. Immunocompromised patients may
have a lower antibody response that can also limit the utility of
the test. The difficulty of serology to discriminate between
current and previous infection also makes interpretation of
serological tests unreliable (24).
3. Advanced diagnostic techniques
Advanced serological tests
ELISA, immunoblots and immuno-diffusion tests have
all been used to diagnose mucormycosis in the past, with varying
degrees of success. Serological approaches for detecting specific
antigens, as well as antisera targeted at specific fungal antigens,
have recently improved the specificity and sensitivity of these
types of tests. For ~70 years, the precipitation in gel technique
has been widely used. These tests are frequently employed with
in-house antigens produced from fungal cultures to detect different
forms of immunoglobulin over time. Employing an enzyme-linked
immune-spot (ELISpot) assay, specific Mucorales T cells were
recently observed in invasive mucormycosis (24). More research will need to be
carried out to discover if these specific T cells can be employed
as diagnostic surrogates (22).
Burnham-Marusich et al (23) tested the monoclonocal 2DA6 antibody
in the ELISA for new serological test targets and found it to be
strongly reactive with distilled Mucor species.
Despite the high sensitivity of various serological
tests, there are some disadvantages to be aware of such as test
specificity, which has been demonstrated to be decreased by cross
reactivity. Early identification of infection-induced antibody
response may be challenging, since its manifestation in the
peripheral blood can take 4-8 weeks. To avoid producing
false-negative results, precise titre cut-off values are required.
When dealing with a disease that is still in its early stages, this
is especially true (25). Despite
these shortcomings, serology diagnostic tests remain affordable,
non-invasive and instantly offer information that can help doctors
make more accurate and timely diagnoses (26).
Nucleic acid-based diagnostics
PCR methods have been improved and used in a variety
of situations for the diagnosis of fungal infections. Examples of
molecular assays include: i) Multiplex PCR; ii) nested PCR; iii)
reverse transcription-quantitative (RT-qPCR); iv) PCR based on
internal transcribed spacer regions and ribosomal DNA; v)
PCR-ELISA; vi) conventional PCR; and vii) direct DNA sequencing
(27). This variety of techniques
offers notable benefits in terms of diagnostic specificity, as
primers may be constructed to recognise specific illnesses;
nevertheless, there are concerns in terms of responsiveness and
reproducibility, notably in the fabrication of false-negative
findings (28).
Traditional PCR is quick and can increase
sensitivity; however, as there are no standardised PCR techniques
that have been Food and Drug Agency approved for Mucorales
detection, results might differ from lab to lab. This truth is
generally acknowledged, even in advanced molecular labs where PCR
methods are often used and attempts are made to standardise diverse
testing components. Therefore, modified nested PCR techniques have
been created for improved specificity and sensitivity (29). This is achieved by running samples
through two sequential PCR reactions with two sets of primers,
which enables the detection of fungal DNA with 100% specificity at
a mass as low as 1 fg (24).
However, this is highly dependent on sample type and concentration,
and is particularly prone to contamination. MucorGenius
(PathoNostics; ADT India) is a fast RT-qPCR test kit that detects
fungal nucleic acid sequences to help in early identification
despite low loads. It is a pan-Mucorale test, as it can
detect five different species of fungus that can aid in the early
and prompt detection of mucormycosis (30).
4. Future diagnostic tools
Biosensors
As stated by The International Union of Pure and
Applied Chemistry (IUPAC), biosensors are integrated
receptor-transducer systems that can offer selective quantitative
or semi-quantitative analytical information utilising a biological
recognition element. The three primary components of sensors and
biosensors are: i) A transducer that generates an electrical
signal; ii) an identification element that identifies a particular
analyte or a group of analytes; and iii) a signal processor
(Fig. 3). Analytical tools that
can translate chemical, physical or biological data are known as
sensors. In the medical field, there are 14 important types of
biosensors. One such type is a wearable biosensor, which has been
used to improve patient quality of life (9). Illness surveillance, aiding early
detection, chronic disease therapy and, specifically, fungal
identification are all essential applications of biosensors
(31).
Electrochemical bio-sensors have been used to detect
fungi such as Candida albicans and A. fumigatus. The
relevant electrochemical biosensors for these fungi use
membrane-bound impedance spectroscopy and chitosan-stabilised gold
nano-particles (32). Optical
biosensors to detect Candida species were developed in the
study by Cai et al (33),
which used Mannan on the cell surface to bind to the
hydrogel Con-A. For fungal biomarker detection, optical biosensor
platforms use a very flexible and ultrasensitive transducer.
Whispering Gallery Mode makes use of a micro optical biosensor that
can identify bacterial cell molecules and may be tweaked to detect
certain fungus biomarkers (9).
Fungal diagnostic research is expected to gain a lot from current
and upcoming developments in bio-sensor technology, which employ a
range of methodologies not yet used in medical mycology (9) .
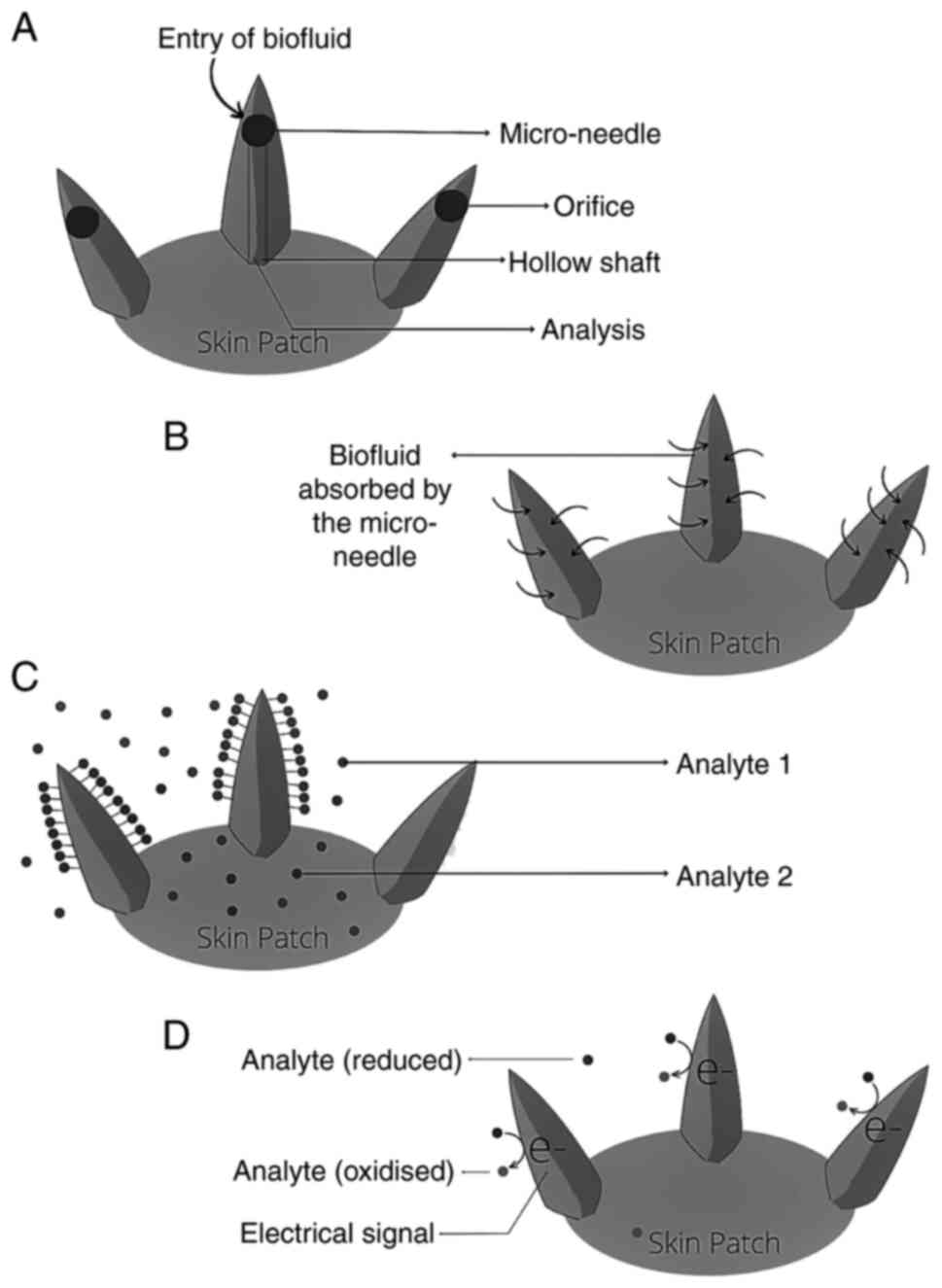
Micro-needle-based diagnostics
Micro-needles are microscopic needles with a typical
length of <1 mm and a width of 100 µm. These micro-projections
can be shaped into different geometries, such as conical,
pyramidal, cylindrical or even fang-like shapes, with or without a
lumen, to enable effective skin penetration and bio-analysis
(Fig. 4) (31). A micro-needle array is made up of
hundreds of these micro-projections. As the micro-needles avoid
contact with blood vessels and nerve endings, the devices produce
no discomfort and are widely accepted by patients. Silicon, metals,
polymers, ceramics, glass and, more recently, nanocomposite
materials have all been used to create micro-needle devices
(34). Historically, infectious
illnesses, such as tuberculosis, were diagnosed using micro-needle
based platforms (35).
There are various micro-needle based diagnostic
systems that have been developed to collect or detect biomarkers in
the skin. These include analyte capture micro-needles, micro-needle
sensing systems, micro-needles for blood or interstitial fluid
extraction, and combinations of these (34). Since the technological limitations
are analogous, research into micro-needle-based diagnostics for
communicable diseases can benefit from the specialized knowledge
acquired via research on other diseases, even though not all
techniques have been expressly proved for infectious illness
detection (34). Since integrated
lab-on-a-chip transdermal drug delivery devices may overcome
bottlenecks and accessibility problems that afflict centralised
test facilities, they have the potential to speed up a diagnosis.
This makes the notion of such devices attractive to researchers.
This is particularly true in the field of infectious illnesses,
where there are already challenging requirements for transportation
of individuals and samples, and other logistics.
5. Conclusions
The deadly fungal illness known as mucormycosis is
initiated by saprophytic fungi Mucor or Rhizopus.
Ingestion, inoculation or inhalation of fungus spores are all
possible routes to infection. Mucormycosis is particularly common
in individuals with diabetes, autoimmune illnesses, organ
transplantation, haematological malignancies and weakened immune
systems (14). The mortality rate
of mucormycosis, particularly invasive mucormycosis, is
>90% (34).
Early detection of mucormycosis is critical in
preventing mortality and the spread of the disease. Clinical
diagnosis, radiography and serology are all traditional diagnostic
methods with limited diagnostic utility, thus making histology and
microscopy key techniques in forming the majority of diagnoses.
Furthermore, depending on the observer's experience,
interpretation of diagnostic results can vary, potentially leading
to misdiagnosis (36). As a
result, advanced serological assays such as ELISA, immunoblotting,
immune-diffusion and ELISpot are required. Mucor-specific T
lymphocytes are detected in the peripheral blood using the ELISpot
assay. The ELISpot assay helps to reduce the percentage of patients
with invasive mucormycosis who are treated with high-dose
antifungal drugs only on the basis of clinical signs (27,37).
Furthermore, nucleic acid diagnostics such as conventional PCR,
RT-qPCR, PCR-ELISA, multiplex PCR, direct DNA sequencing and the
MucorGenius rapid RT-qPCR test kit, a pan-Mucorale test, aid
in the early detection of the fungus even when the fungal load is
minimal (24). The most noteworthy
benefit of this test is that it can detect five species of
Mucor families, with blood and biopsy tissue serving as
biomarker specimens (38).
Biosensors and their components, as well as their functioning
principles and types, have been suggested as future diagnostic
tools that are species-specific and aid in the detection of
specific fungal biomarkers. Biosensors enable continuous
monitoring, which might be used to assess therapy effectiveness
(9,39).
Future production and development of fungal
biosensors for clinical use will require specific biomarkers,
ideally from clinical samples, and superior immobilisation of the
markers on the sensing surface. It is necessary to consider if it
is possible to modify a suitable bio-fluid or biomarker for
biosensor detection. Micro-needle diagnostics facilitate the
detection of infectious diseases and expedite the diagnostic
procedure. Micro-needles (long micro-needles) with functionalized
bacterial encapsulation have been mixed with Bacillus
subtilis, which is naturally present on human skin and widely
used for food preparation, for effective fungal infection therapy
(40). A range of antifungal
medications that may specifically bind to proteins on the fungal
cell are continuously produced and secreted by the encapsulated
B. subtilis. Consistent production and release of different
antifungal medications that can attach to molecules on the yeast
cell surface-associated proteins and destroy the cell membranes may
also help to prevent drug resistance (41).
Invasive fungal infections are regularly diagnosed
using traditional diagnostic procedures. While the techniques used
are capable of detecting fungal infections, they lack sensitivity
and specificity in detecting the fungus. Newer diagnostic tests and
methodologies, such as ELISA and RT-qPCR, have improved the
diagnostic approaches available (25). The present study reviewed the
traditional, present and future diagnostic tools for mucoromycosis,
which assist in making an accurate diagnosis and initiating
treatment as soon as possible to limit disease spread and
mortality. To avoid fatal effects, mucormycosis must be detected as
soon as possible. The diagnosis of mucormycosis is still difficult
and although molecular approaches are advancing, histopathology,
direct inspection and culture remain important tools. Direct
culture and inspection continue to be needed as diagnostic tools,
even if advanced diagnostic techniques have acquired approval for
confirmation when applied to tissues. The importance of modern
diagnostic procedures is at the forefront for the identification of
mucormycosis at an earlier stage. The encouraging results of PCR
methods based on the detection of Mucorale DNA in the blood
is a promising approach for screening tests in high-risk patients
(7).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
The manuscript was written by DP and CMA. The
original manuscript was proofread and revised by PSGP, RRR, GK, SRN
and SS. The manuscript was referenced by RRR, GK and SRN. The
figures were created by RRR. All authors read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosenberg SW and Lepley JB: Mucormycosis
in leukemia. Oral Surg Oral Med Oral Pathol. 54:26–32.
1982.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abramson E, Wilson D and Arky RA:
Rhinocerebral phycomycosis in association with diabetic
ketoacidosis. Report of two cases and a review of clinical and
experimental experience with amphotericin B therapy. Ann Intern
Med. 66:735–742. 1967.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Paltauf A: Mycosis mucorina. Arch für
Pathol Anat und Physiol und für Klin Med. 102:543–564. 1885.
|
|
4
|
Manjunatha BS, Das N, Sutariya RV and
Ahmed T: Mucormycosis of the hard palate masquerading as carcinoma.
Clin Pract. 2(e28)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Prabhu RM and Patel R: Mucormycosis and
entomophthoramycosis: A review of the clinical manifestations,
diagnosis and treatment. Clin Microbiol Infect. 10 (Suppl
1):S31–S47. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Janjua OS, Shaikh MS, Fareed MA, Qureshi
SM, Khan MI, Hashem D and Zafar MS: Dental and oral manifestations
of COVID-19 related mucormycosis: Diagnoses, management strategies
and outcomes. J fungi (Basel). 8(44)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Skiada A, Pavleas I and
Drogari-Apiranthitou M: Epidemiology and diagnosis of mucormycosis:
An update. J fungi (Basel). 6(265)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pilmis B, Alanio A, Lortholary O and
Lanternier F: Recent advances in the understanding and management
of mucormycosis. F1000Res. 7(F1000)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hussain KK, Malavia D, Johnson EM,
Littlechild J, Winlove CP, Vollmer F and Gow NAR: Biosensors and
diagnostics for fungal detection. J fungi (Basel).
6(349)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singhal N, Kumar M, Kanaujia PK and Virdi
JS: MALDI-TOF mass spectrometry: An emerging technology for
microbial identification and diagnosis. Front Microbiol.
6(791)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gupta MK, Kumar N, Dhameja N, Sharma A and
Tilak R: Laboratory diagnosis of mucormycosis: Present perspective.
J Family Med Prim Care. 11:1664–1671. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Petrikkos G, Skiada A, Lortholary O,
Roilides E, Walsh TJ and Kontoyiannis DP: Epidemiology and clinical
manifestations of mucormycosis. Clin Infect Dis. 54 (Suppl
1):S23–S34. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bhandari J, Thada PK and Nagalli S:
Rhinocerebral Mucormycosis. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2022.
|
|
14
|
Corzo-León DE, Chora-Hernández LD,
Rodríguez-Zulueta AP and Walsh TJ: Diabetes mellitus as the major
risk factor for mucormycosis in Mexico: Epidemiology, diagnosis,
and outcomes of reported cases. Med Mycol. 56:29–43.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Goel A, Kini U and Shetty S: Role of
histopathology as an aid to prognosis in rhino-orbito-cerebral
zygomycosis. Indian J Pathol Microbiol. 53:253–257. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guarner J and Brandt ME: Histopathologic
diagnosis of fungal infections in the 21st century. Clin Microbiol
Rev. 24:247–280. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sravani T, Uppin SG, Uppin MS and Sundaram
C: Rhinocerebral mucormycosis: Pathology revisited with emphasis on
perineural spread. Neurol India. 62:383–386. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Groppo ER, El-Sayed IH, Aiken AH and
Glastonbury CM: Computed tomography and magnetic resonance imaging
characteristics of acute invasive fungal sinusitis. Arch
Otolaryngol Head Neck Surg. 137:1005–1010. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Passi N, Wadhwa AC and Naik S:
Radiological spectrum of invasive mucormycosis in COVID-19. BJR
Case Rep. 7(20210111)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Legouge C, Caillot D, Chrétien ML, Lafon
I, Ferrant E, Audia S, Pagès PB, Roques M, Estivalet L, Martin L,
et al: The reversed halo sign: Pathognomonic pattern of pulmonary
mucormycosis in leukemic patients with neutropenia? Clin Infect
Dis. 58:672–678. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dadwal SS and Kontoyiannis DP: Recent
advances in the molecular diagnosis of mucormycosis. Expert Rev Mol
Diagn. 18:845–854. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Richardson M and Page I: Role of
serological tests in the diagnosis of mold infections. Curr Fungal
Infect Rep. 12:127–136. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Burnham-Marusich AR, Hubbard B, Kvam AJ,
Gates-Hollingsworth M, Green HR, Soukup E, Limper AH and Kozel TR:
Conservation of mannan synthesis in fungi of the zygomycota and
ascomycota reveals a broad diagnostic target. mSphere.
3(e00094)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lackner N, Posch W and Lass-Flörl C:
Microbiological and molecular diagnosis of mucormycosis: From old
to new. Microorganisms. 9(1518)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Skiada A, Lass-Floerl C, Klimko N, Ibrahim
A, Roilides E and Petrikkos G: Challenges in the diagnosis and
treatment of mucormycosis. Med Mycol. 56:93–101. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Potenza L, Vallerini D, Barozzi P, Riva G,
Forghieri F, Zanetti E, Quadrelli C, Candoni A, Maertens J, Rossi
G, et al: Mucorales-specific T cells emerge in the course of
invasive mucormycosis and may be used as a surrogate diagnostic
marker in high-risk patients. Blood. 118:5416–5419. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
White PL: Recent advances and novel
approaches in laboratory-based diagnostic mycology. Med Mycol.
57:S259–S266. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sue MJ, Yeap SK, Omar AR and Tan SW:
Application of PCR-ELISA in molecular diagnosis. Biomed Res Int.
2014(653014)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Baldin C, Soliman SSM, Jeon HH, Alkhazraji
S, Gebremariam T, Gu Y, Bruno VM, Cornely OA, Leather HL, Sugrue
MW, et al: PCR-based approach targeting mucorales-specific gene
family for diagnosis of mucormycosis. J Clin Microbiol.
56(e00746)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pandey M, Xess I, Sachdev J, Yadav U,
Singh G, Pradhan D, Xess AB, Rana B, Dar L, Bakhshi S, et al:
Development of a sensitive and specific novel qPCR assay for
simultaneous detection and differentiation of mucormycosis and
aspergillosis by melting curve analysis. Front Fungal Biol. 2:1–11.
2022.
|
|
31
|
Asdaq SMB, Rajan A, Damodaran A, Kamath
SR, Nair KS, Zachariah SM, Sahu RK, Fattepur S, Sreeharsha N, Nair
A, et al: Identifying mucormycosis severity in indian COVID-19
patients: A nano-based diagnosis and the necessity for critical
therapeutic intervention. Antibiot (Basel). 10(1308)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dutta P, Lu YJ, Hsieh HY, Lee TY, Lee YT,
Cheng CM and Fan YJ: Detection of candida albicans using a
manufactured electrochemical sensor. Micromachines.
12(166)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cai D, Xiao M, Xu P, Xu YC and Du W: An
integrated microfluidic device utilizing dielectrophoresis and
multiplex array PCR for point-of-care detection of pathogens. Lab
Chip. 14:3917–3924. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dixon RV, Skaria E, Lau WM, Manning P,
Birch-Machin MA, Moghimi SM and Ng KW: Microneedle-based devices
for point-of-care infectious disease diagnostics. Acta Pharm Sin B.
11:2344–2361. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Samson R and Dharne M: COVID-19 associated
mucormycosis: Evolving technologies for early and rapid diagnosis.
3 Biotech. 12(6)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hu L, Zhou P, Zhao W, Hua H and Yan Z:
Fluorescence staining vs. routine KOH smear for rapid diagnosis of
oral candidiasis-A diagnostic test. Oral Dis. 26:941–947.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Potenza L, Vallerini D, Barozzi P, Riva G,
Gilioli A, Forghieri F, Candoni A, Cesaro S, Quadrelli C, Maertens
J, et al: Mucorales-specific T cells in patients with hematologic
malignancies. PLoS One. 11(e0149108)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Caramalho R, Madl L, Rosam K, Rambach G,
Speth C, Pallua J, Larentis T, Araujo R, Alastruey-Izquierdo A,
Lass-Flörl C and Lackner M: Evaluation of a novel mitochondrial
pan-mucorales marker for the detection, identification,
quantification, and growth stage determination of mucormycetes. J
fungi (Basel). 5(98)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang F, Zhang X, Chen G and Zhao Y: Living
bacterial microneedles for fungal infection treatment. Res
(Washington DC). 2020(2760594)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dannaoui E: Recent developments in the
diagnosis of mucormycosis. J fungi (Basel). 8(457)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sharma A and Goel A: Mucormycosis: Risk
factors, diagnosis, treatments, and challenges during COVID-19
pandemic. Folia Microbiol (Praha). 67:363–387. 2022.PubMed/NCBI View Article : Google Scholar
|