Introduction
Age-related hearing loss (ARHL) is characterized by
bilaterally symmetric auditory dysfunction caused by aging and
degeneration of the auditory system, the severity of which is
associated with increasing age (1,2).
ARHL is commonly characterized by decreased hearing ability for
high frequencies that gradually include lower ones (2). It has been reported by the World
Health Organization that >460 million individuals worldwide have
hearing disorders, while by 2025 70-80% of individuals aged >65
years may have ARHL (3,4). Accumulating evidence has suggested
that the development of ARHL is associated with social isolation,
depression, anxiety and cognitive impairment (5,6).
Therefore, early detection, intervention and delay of the
occurrence and development of ARHL are of importance for the
elderly population.
The ubiquitin-proteasome system is a key pathway
involved in the degradation of cell protein and maintenance of
normal deubiquitinating enzyme (DUB)-dependent cellular function
(7-9).
Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1, also called
PARK5), belonging to the DUB family, is primarily involved in
protein stability in cells via regulating the ubiquitin/proteasome
pathway (10). Emerging evidence
has indicated that UCHL1 is aberrantly expressed in age-associated
diseases, including Parkinson's and Alzheimer's disease (11,12).
More importantly, a recent study demonstrated that UCHL1 is
downregulated in the cochlea of ARHL mice (13). Nonetheless, the effects of UCHL1 on
ARHL remain unclear.
Specificity protein 1 (Sp1) is a member of the
SP/Krüppel-like factor (KLF) transcription factor family, which is
widely expressed in human cells under normal conditions and is
involved in numerous cell processes, including cell proliferation,
apoptosis, differentiation and transformation (14). Recent literature has elucidated
that Sp1 mediates cochlear cell apoptosis in hearing loss models
(15,16). Furthermore, it has been also
reported that NF-κB participates in the pathogenesis of ARHL via
interaction with immune-associated genes (17).
The present study aimed to evaluate the role of
UCHL1 in ARHL and reveal the association between UCHL1, Sp1 and
NF-κB signaling in ARHL.
Materials and methods
Bioinformatics analysis
The PROMO database (alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3/)
was used to predict the association between UCHL1 promoter and
Sp1.
Cell culture and treatment
Murine cochlea hair cells (HEI-OC1; cat. no.
BFN60808695), obtained from BLUEFBIO, were cultured in DMEM
supplemented with 10% FBS (both Shanghai ExCell Biology, Inc.) at
33˚C with 10% CO2. To establish an in vitro ARHL
model, HEI-OC1 cells were exposed to 1 mM hydrogen peroxide
(H2O2) for 2 h at 33˚C, as previously
described (18,19).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from HEI-OC1 cells (6-well
plates at a density of 6x104 cells per well) using
TRIzol® reagent (Invitrogen) and cDNA was synthesized
using the AffinityScript cDNA synthesis kit (Agilent Technologies)
according to the manufacturer's instructions. qPCR was performed on
a LightCycler 480 PCR instrument (Roche Diagnostics) using the
AceQ® qPCR SYBR Green Master Mix (Vazyme Biotechnology
Co. Ltd.). The following thermocycling conditions were used for
qPCR: Pre-denaturation at 95˚C for 1 min, followed by 40 cycles of
denaturation at 95˚C for 15 sec, annealing at 60˚C for 40 sec and
extension at 72˚C for 15 sec. The changes in gene expression levels
were assessed using the 2-ΔΔCq method (20). The following primer pairs were used
for qPCR: UCHL1, forward, 5'-AGGGACAGGAAGTTAGCCCTA-3' and reverse,
5'-AGCTTCTCCGTTTCAGACAGA-3'; Sp1 forward,
5'-CCTGGCATCCCACCAGAGTA-3' and reverse, 5'-GTGCAAGGAGCTGATCCCAA-3'
and β-actin forward, 5'-GTTGGAGCAAACATCCCCCA-3' and reverse,
5'-CGCGACCATCCTCCTCTTAG-3'.
Western blot analysis
Total protein was extracted from HEI-OC1 cells
(1x106 cells) using RIPA lysis buffer (Shanghai Yisheng
Biotechnology Co., Ltd.) and the protein concentration was
determined using a BCA protein assay kit (Beijing Solarbio Science
& Technology Co., Ltd.). The proteins were transferred onto
PVDF membranes (30 µg/lane) following separation by SDS-PAGE on a
10% gel. Following blocking with 5% skimmed milk at room
temperature for 1 h, membranes were first incubated with primary
antibodies at 4˚C overnight and then with a goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (cat. no.
ab205718; 1:2,000; Abcam) for 1 h at room temperature. The ECL
Western Blot kit (Jiangsu CoWin Biotech Co., Ltd.) was used to
develop the immunoreactive signals and protein band intensity was
calculated using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.). The primary antibodies were as follows:
Anti-UCHL1 (cat. no. ab108986; 1:1,000; Abcam), anti-B cell
lymphoma-2 (Bcl-2; cat. no. ab182858; 1:2,000; Abcam), anti-Bcl-2
associated X (Bax; cat. no. ab32503; 1:1,000; Abcam), anti-p16
(cat. no. ab51243; 1:1,000; Abcam), anti-p21 (cat. no. ab109199;
1:1,000; Abcam), anti-Sp1 (cat. no. ab227383; 1:1,000; Abcam),
anti-phosphorylated (p)-NF-κB p65 (cat. no. ab76302; 1:1,000;
Abcam), anti-NF-κB p65 (cat. no. ab32536; 1:1,000; Abcam) and
anti-β-actin (cat. no. ab8227; 1:1,000; Abcam).
Plasmid transfection
The recombinant plasmids pcDNA3.1-UCHL1 (Oe-UCHL1)
and pcDNA3.1-Sp1 (Oe-Sp1), as well as the empty vector pcDNA3.1
negative control (Oe-NC), were obtained from Sangon Biotech Co.,
Ltd. HEI-OC1 cells seeded into six-well plates at a density of
5x105 cells/well were transfected with 5 µg plasmids
using Lipofectamine® 2000 (Life Technologies; Thermo
Fisher Scientific, Inc.) at 37˚C for 48 h, according to the
manufacturer's recommendation. The subsequent experiments were
performed at 48 h following cell transfection.
Cell Counting Kit-8 (CCK-8) assay
To assess cell viability, a CCK-8 kit (Abnova) was
used according to the manufacturer's instructions. Briefly, HEI-OC1
cells were seeded into 96-well plates at a density of
5x103 cells/well and treated with 10 µl CCK-8 solution
at 37˚C for 2 h. Subsequently, optical density (OD) at a wavelength
of 450 nm was measured using a microplate reader (Infinite M200;
Tecan Group, Ltd.).
Evaluation of superoxide dismutase
(SOD), glutathione peroxidase (GSH-Px) and reactive oxygen species
(ROS)
HEI-OC1 cells were seeded into 96-well plates
(5x103 cells/well). Following cell lysis in 300 µl lysis
buffer (0.1% SDS, 0.5% Triton X-100, 20 mM Tris-HCl, pH 8.1),
protein concentration was measured using a BCA protein assay kit
(Bio Basic, Inc.). Assay kits for SOD (cat. no. A001-1-2) and
GSH-Px (cat. no. A005-1-2) were obtained from Nanjing Jiancheng
Bioengineering Institute and used according to the manufacturer's
instructions. The OD values at a wavelength of 450 nm were
determined using a microplate reader (Infinite M200; Tecan Group,
Ltd.). To measure ROS accumulation, HEI-OC1 cells seeded into
24-well plates were treated with 10 µΜ dichloro-dihydro-fluorescein
diacetate (MilliporeSigma) at 37˚C for 30 min in the dark followed
by washing with serum-free DMEM three times. ROS generation was
assessed by flow cytometry (Merck KGaA) using FACSAria (BD
Biosciences) using the corresponding kit (cat. no. ab113851; Abcam)
according to the manufacturer's instructions. The data were viewed
in FlowJo software (version 10; FlowJo, LLC).
TUNEL assay
HEI-OC1 cells were fixed with 4% paraformaldehyde
for 30 min at room temperature and were then treated with 0.1%
Triton X-100 for 5 min at room temperature. TUNEL assay was
performed using TUNEL reagent (cat. no. MK1013; Wuhan Boster
Biological Technology, Ltd.) for 60 min at 37˚C according to the
manufacturer's instructions. Cell nuclei were labeled with 10 mg/ml
DAPI for 5 min at room temperature in the dark and images were
captured from four random fields under a fluorescence microscope
(LSM800; Carl Zeiss AG) after Antifade Mounting Medium (Beyotime
Institute of Biotechnology) was added to the sections.
Senescence-associated-β-galactosidase
(SA-β-gal) staining
Briefly, HEI-OC1 cells were seeded in six-well
plates at a density of 5x104 cell/well. HEI-OC1 cells at ~80%
confluency plated into 6-well plates were treated with 4%
formaldehyde for 15 min at room temperature. Following washing with
PBS, cells were incubated with SA-β-gal staining solution (cat. no.
K320-250; BioVision, Inc.) overnight at 37˚C without
CO2. Finally, stained cells from 3 random fields of view
were observed under a light microscope (Carl Zeiss AG).
Luciferase reporter assay
Wild-type (WT; 5'-CCCGCCCCG-3') or mutant (MUT;
5'-CAAAAAAAC-3') UCHL1 promoter were cloned into the pGL3 Basic
vector (Promega Corporation). Cells (5x105) were seeded
in 24-well plates for 24 h at 37˚C and were transfected with these
plasmids as well as with Oe-Sp1 and Oe-NC using
Lipofectamine® 2000 (Life Technologies; Thermo Fisher
Scientific, Inc.) at 37˚C. After 48 h transfection, the luciferase
activity was measured using the Dual-Luciferase Reporter Gene Assay
kit (Shanghai Qcbio Science & Technologies Co., Ltd.) according
to the manufacturer's instructions. The relative luciferase
activity was normalized to that of Renilla luciferase.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed using the ChIP kit (Abcam).
Briefly, 1% formaldehyde was added to HEI-OC1 cells for 10 min at
room temperature. The fixed cells were washed twice with
phosphate-buffered saline and were lysed using a lysis buffer (0.1%
SDS, 0.5% Triton X-100, 20 mM Tris-HCl, pH 8.1) that contained 150
mM NaCl and a protease inhibitor, after which chromatin fragments
were obtained using sonication using a 10 sec on and 10 sec off
mode for 12 cycles at 4˚C. Following centrifugation at 13,000 x g
for 10 min at 4˚C, the DNA fragments were incubated with antibodies
against Sp1 (cat. no. ab227383; 1:200: Abcam) or IgG (cat. no.
ab6702; 1:40; Abcam) for 2 h at 4˚C. The abundance of Sp1 on the
UCHL1 promoter was measured by PCR as aforementioned. The sequence
of oligonucleotides flagging the Sp1 binding site in the UCHL1
promoter was 5'-CCCGCCCCC-3'.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 8 software (GraphPad Software, Inc.).
Continuous variables are expressed as the mean ± standard deviation
from three independent experiments. The differences between two
groups were compared using unpaired Student's t test, while those
between multiple groups were by one-way ANOVA followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
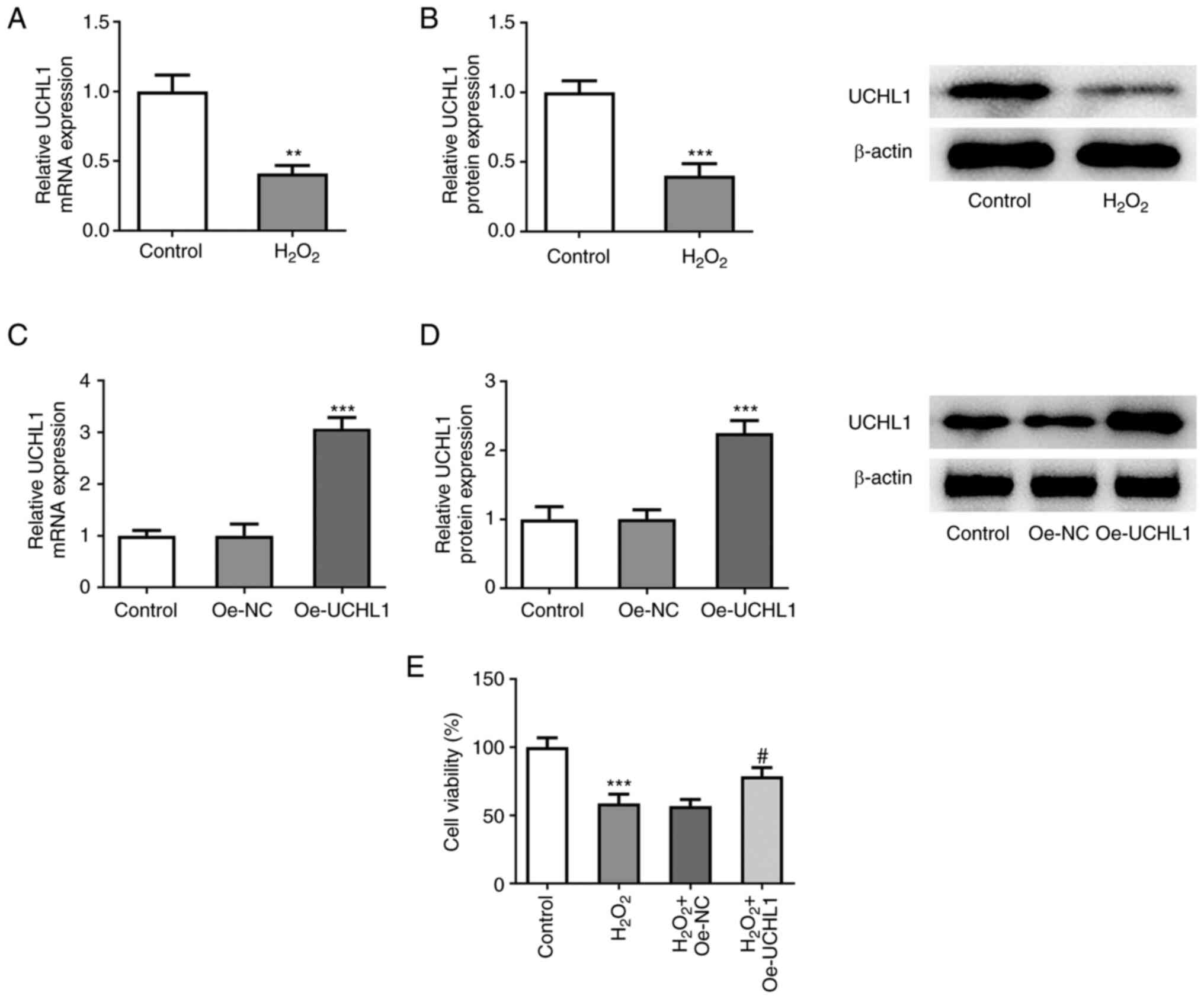
UCHL1 overexpression enhances the
viability of H2O2-treated HEI-OC1 cells
To evaluate the role of UCHL1 in
H2O2-induced HEI-OC1 cells, expression levels
of UCHL1 were measured. RT-qPCR and western blot analysis showed
that UCHL1 was downregulated in H2O2-treated
HEI-OC1 cells (Fig. 1A and
B). To determine the effect of
UCHL1 on H2O2-treated HEI-OC1 cells, UCHL1
was overexpressed following cell transduction with Oe-UCHL1
plasmid. The overexpression efficiency was verified via RT-qPCR and
western blot analysis (Fig. 1C and
D). Furthermore, CCK-8 assay
demonstrated that treatment with H2O2
diminished HEI-OC1 cell viability. However, UCHL1 overexpression
increased the viability of HEI-OC1 cells exposed to
H2O2. These findings indicated that UCHL1
protected HEI-OC1 cells from H2O2-triggered
cell injury.
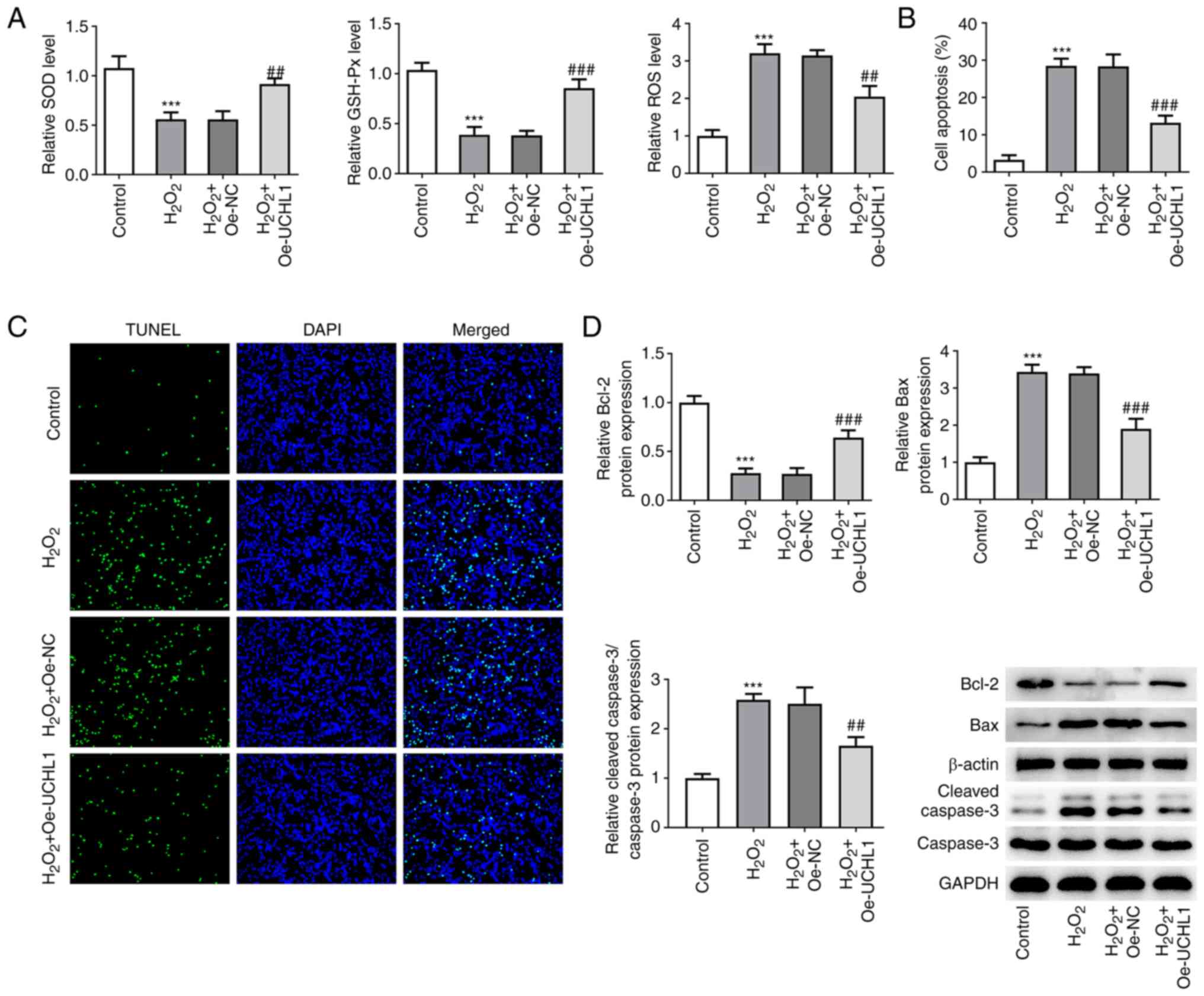
UCHL1 overexpression mitigates
H2O2-mediated oxidative injury and apoptosis
in HEI-OC1 cells
Contents of the oxidative stress-related markers
SOD, GSH-Px and ROS were measured using the corresponding kits. The
results revealed that exposure to H2O2
decreased SOD and GSH-Px levels but increased ROS activity.
However, UCHL1 overexpression increased SOD and GSH-Px levels and
attenuated ROS activity in H2O2-treated
HEI-OC1 cells (Fig. 2A).
Furthermore, TUNEL assay showed that UCHL1 ameliorated
H2O2-induced HEI-OC1 cell apoptosis (Fig. 2B and C). In addition, western blot analysis
revealed that cell exposure to H2O2
downregulated Bcl-2 and upregulated Bax, which were reversed by
UCHL1 overexpression (Fig. 2D).
Overall, these results suggested that UCHL1 exerted an inhibitory
effect on H2O2-triggered oxidative stress and
apoptosis in HEI-OC1 cells.
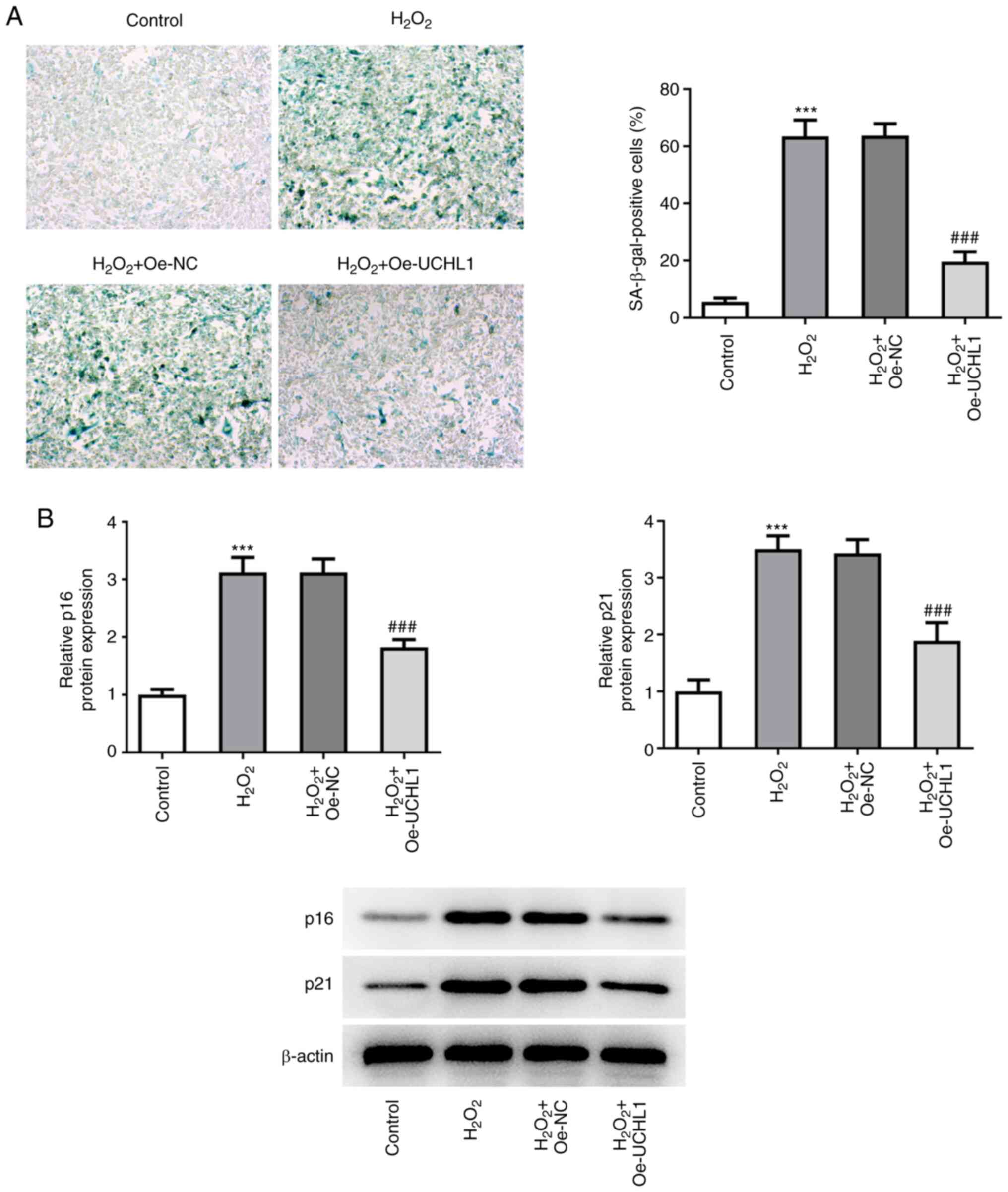
UCHL1 overexpression inhibits
H2O2-induced HEI-OC1 cell senescence
SA-β-gal staining illustrated that the increased
number of SA-β-gal-positive H2O2-treated
HEI-OC1 cells was decreased following UCHL1 overexpression
(Fig. 3A). In addition, UCHL1
overexpression suppressed the H2O2-mediated
enhanced expression levels of p16 and p21 (Fig. 3B). These results indicated that the
H2O2-mediated HEI-OC1 cell senescence was
attenuated by UCHL1 overexpression.
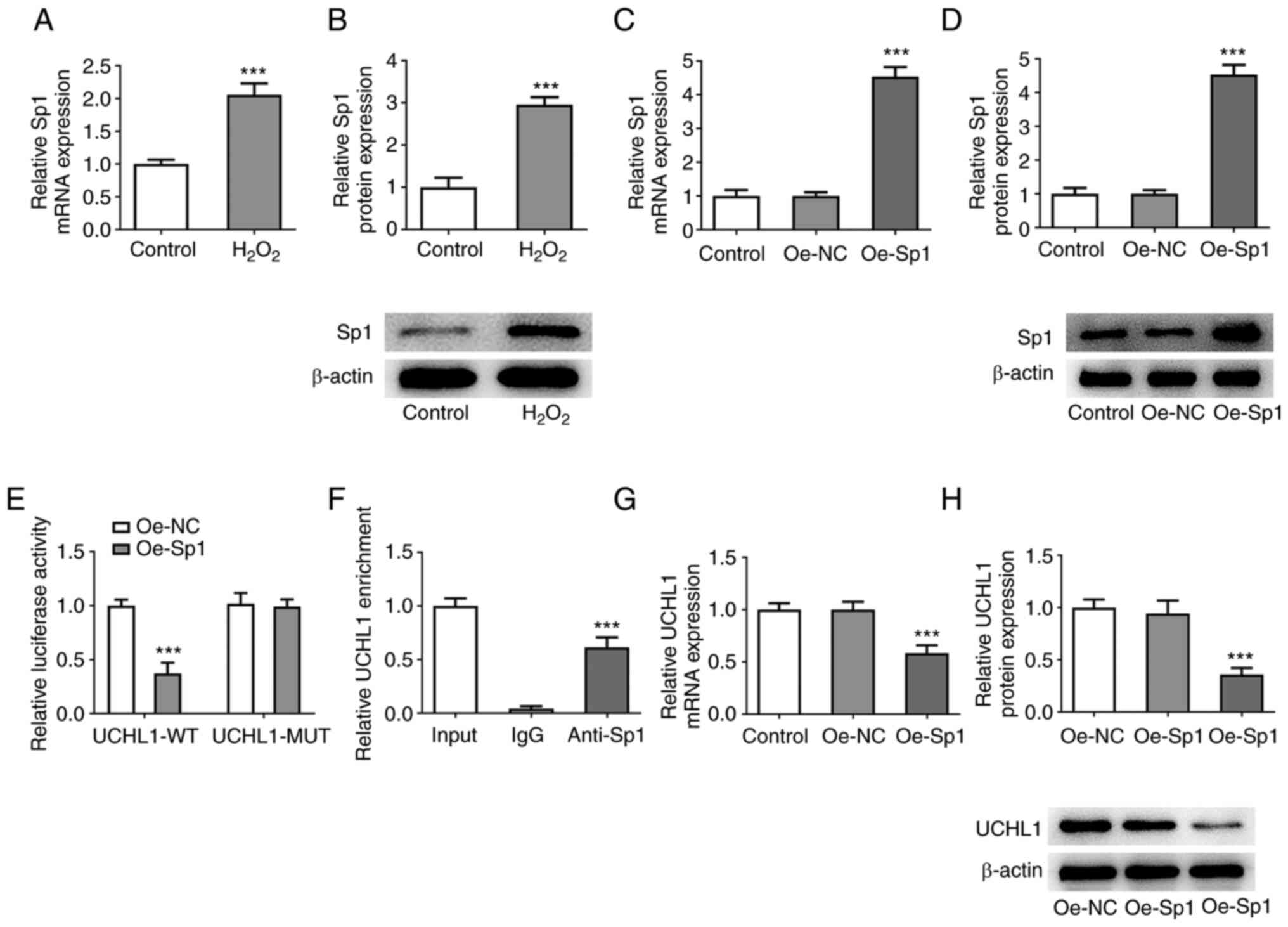
Sp1 suppresses transcription of
UCHL1
The PROMO database revealed that the UCHL1 promoter
interacted with the Sp1 transcription factor. RT-qPCR and western
blot analysis revealed that Sp1 was upregulated in
H2O2-induced HEI-OC1 cells (Fig. 4A and B). Following Sp1 overexpression by cell
transduction with Oe-Sp1 plasmid (Fig.
4C and D), luciferase reporter
assay showed that Sp1 overexpression diminished the luciferase
activity of UCHL1-WT compared with UCHL1-MUT (Fig. 4E). ChIP assay showed that UCHL1 was
precipitated following incubation of nuclear extracts with Sp1
antibody (Fig. 4F). Additionally,
Sp1 overexpression decreased expression levels of UCHL1 (Fig. 4G and H). Collectively, the aforementioned
findings demonstrated that Sp1 was a transcriptional inhibitor of
UCHL1.
Sp1 overexpression abrogates the
protective effect of UCHL1 on H2O2-induced
HEI-OC1 cell injury
To uncover the association between Sp1 and UCHL1 in
H2O2-treated HEI-OC1 cells, functional
experiments were performed in H2O2-induced
HEI-OC1 cells co-transduced with Oe-UCHL1 and Oe-Sp1 plasmids.
CCK-8 assay revealed that UCHL1 restored the suppressed viability
of H2O2-induced HEI-OC1 cells. However, this
effect was reversed by Sp1 overexpression (Fig. 5A). The enhanced SOD and GSH-Px
activity, as well as the reduced ROS levels mediated by UCHL1
overexpression in H2O2-induced HEI-OC1 cells,
were restored following Sp1 overexpression (Fig. 5B). In addition, the attenuated
H2O2-induced HEI-OC1 cell apoptosis mediated
by UCHL1 overexpression was further increased in cells
co-transfected with Oe-Sp1 plasmid (Fig. 5C). This was further verified by
western blot analysis, showing that Sp1 overexpression abrogated
the enhanced Bcl-2 and decreased Bax expression levels in
H2O2-exposed HEI-OC1 cells co-transduced with
Oe-UCHL1 plasmid (Fig. 5D).
Additionally, SA-β-gal staining and western blot analysis
demonstrated that the decreased number of SA-β-gal-positive cells,
as well as p61 and p21 downregulation triggered by UCHL1
overexpression in H2O2-induced HEI-OC1 cells,
were reversed by Sp1 overexpression (Fig. 5E and F). Notably, UCHL1 overexpression restored
the H2O2-induced p-NF-κB p65 upregulation,
which was further abolished by Sp1 elevation (Fig. 5G). Taken together, these findings
indicated that UCHL1, negatively regulated by Sp1, promoted
H2O2-mediated cell injury, oxidative stress,
apoptosis and senescence, and modulated NF-κB signaling in HEI-OC1
cells.
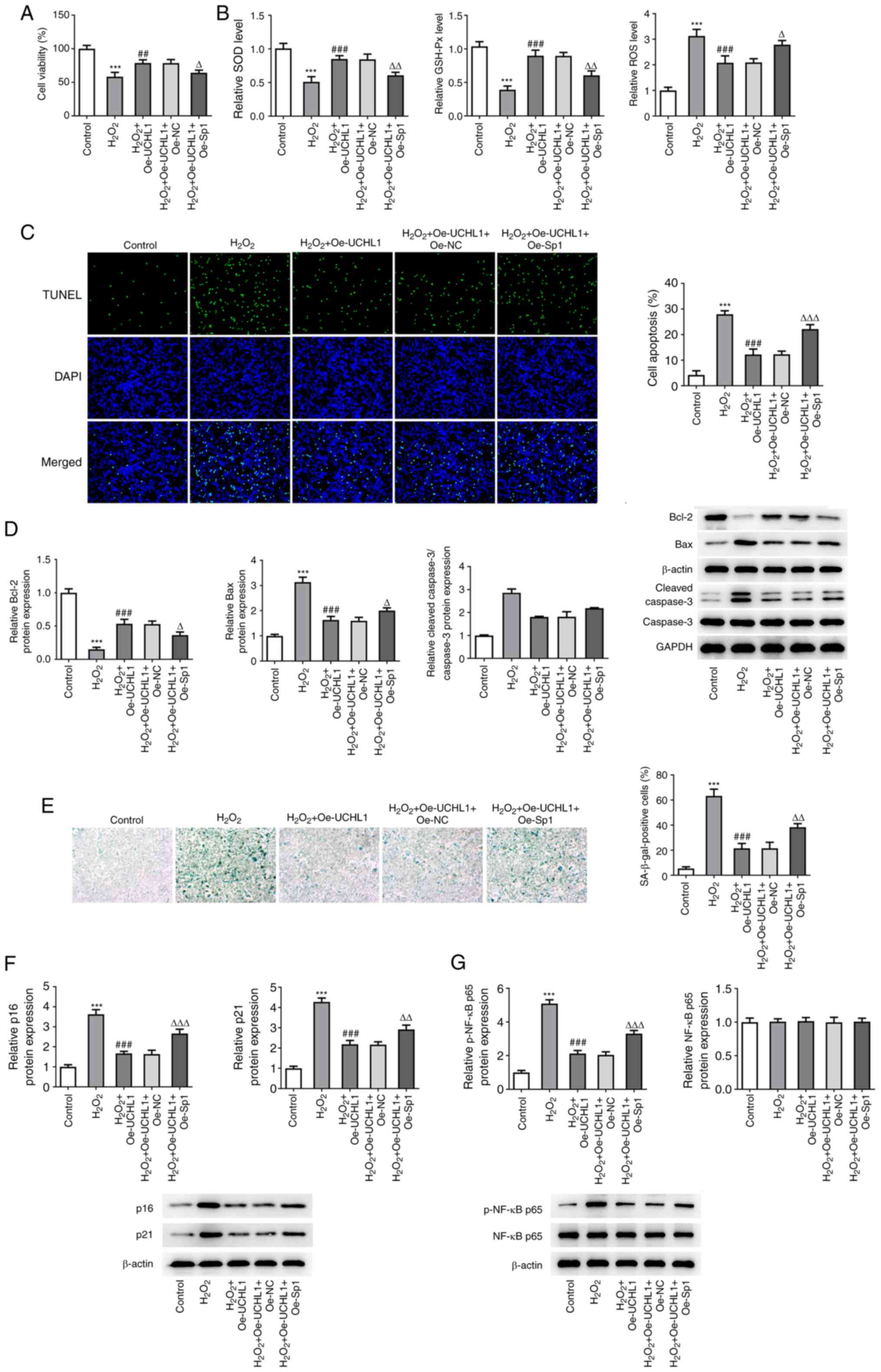 | Figure 5Overexpression of Sp1 abrogates the
protective role of UCHL1 in H2O2-induced
HEI-OC1 cell injury. (A) Viability of
H2O2-exposed HEI-OC1 cells evaluated by CCK-8
assay. (B) Detection of the levels of oxidative stress markers
using corresponding kits. (C) TUNEL assay of apoptosis of
H2O2-exposed HEI-OC1 cells. Magnification,
x200. (D) Western blot analysis of expression of
apoptosis-associated factors. (E) SA-β-gal staining analysis of
cell senescence. Western blot analysis of expression of (F)
senescence- and (G) NF-κB signaling-associated factors.
***P<0.001 vs. control. ##P<0.01 and
###P<0.001 vs. H2O2.
∆P<0.05, ∆∆P<0.01 and
∆∆∆P<0.001 vs. H2O2 + Oe-UCHL1
+ Oe-NC. UCHL1, ubiquitin carboxyl-terminal hydrolase L1; Sp1,
specificity protein 1; SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase; ROS, reactive oxygen species; Bcl-2, B cell
lymphoma-2; p-, phosphorylated; Oe-NC, negative control; SA-β-gal,
senescence-associated β-galactosidase. |
Discussion
ARHL is a common clinical condition with complicated
pathogenesis (21). Globally, more
than 500 million people have ARHL (18). Cochlear hair cells are
mechanoreceptors of the auditory system and their loss is a
predominant factor contributing to hearing loss (22). Apoptosis is a type of programmed
cell death and accelerated cochlear hair cell apoptosis is
considered a key factor leading to ARHL (23). Oxidative stress promotes cell or
tissue damage caused by the imbalance between ROS production and
elimination (24). A growing body
of evidence has suggested that oxidative stress is associated with
hearing loss and cochlear hair cell injury (23,25).
A previous study suggested that cell senescence is a permanent and
inevitable state of cell cycle arrest caused by ROS-mediated
oxidative stress injury (26).
H2O2 has been used to induce premature
senescence in vascular endothelial cells (27) and keratinocytes (28). Therefore, in the present study, 1
mM H2O2 was utilized to induce cochlear hair
cell senescence. Cell apoptosis, oxidative stress and senescence
were investigated to uncover the underlying mechanism of ARHL.
UCHL1 is a key component of the ubiquitin-dependent
protein degradation system, which is a highly conserved pathway
involved in removal of damaged or misfolded proteins to prevent
protein accumulation and maintain normal cell function (29). A recent study showed that UCHL1 is
downregulated in the cochlea of ARHL mice (13). Additionally, UCHL1 regulates
expression of ubiquitin proteasome system (UPS)-associated proteins
to modify the aging process in the auditory cortex (30). Another study demonstrated that
UCHL1 silencing facilitates autophagy-dependent auditory cell death
following treatment with gentamicin (31). The results of the present study
also revealed that UCHL1 was downregulated in
H2O2-treated murine cochlea hair cells
(HEI-OC1). Furthermore, UCHL1 overexpression effectively mitigated
the loss of cell viability triggered by exposure of HEI-OC1 cells
to H2O2. ROS comprise oxygen radicals, the
level of which may reflect the degree of oxidative stress (32). It has been reported that the
antioxidant enzymes SOD and GSH-Px eliminate excess ROS levels
during the metabolic process to maintain balance (33). As expected, in the present study,
cell exposure to H2O2 reduced SOD and GSH-Px
levels but enhanced ROS activity. However, these effects were
restored after UCHL1 overexpression, suggesting that UCHL1 could
protect HEI-OC1 cells against H2O2-induced
oxidative injury. Similarly, H2O2-induced
HEI-OC1 cell apoptosis was also restored by UCHL1 overexpression.
This was further supported since Bcl-2 downregulation and Bax
upregulation in H2O2-treated HEI-OC1 cells
were both reversed by UCHL1 overexpression. Furthermore, p16 and
p21 serve a key role in regulating cellular senescence (34). Here, UCHL1 overexpression
alleviated H2O2-induced cell senescence and
decreased the H2O2-enhanced p16 and p21
expression levels in HEI-OC1 cells.
As a widely investigated transcription factor, Sp1
activates or inactivates the transcription of several genes
encompassing putative CG-rich Sp-binding sites in their promoters
(35-36). Bioinformatics analysis using the
PROMO database predicted that Sp1 bound to the UCHL1 promoter.
Additionally, Sp1 was upregulated in
H2O2-induced HEI-OC1 cells. The strong
affinity of Sp1 with the promoter region of UCHL1 was verified by
luciferase reporter and ChIP assays. The results demonstrated that
UCHL1 expression was negatively regulated by Sp1. Emerging evidence
has suggested that Sp1 is involved in diverse biological events,
including embryonic development, cell proliferation, death,
senescence and angiogenesis (36).
Consistent with the aforementioned findings, the experimental data
of the current study also demonstrated that the effects of UCHL1 on
viability, oxidative stress, apoptosis and senescence of
H2O2-induced HEI-OC1 cells were counteracted
by Sp1 overexpression.
NF-κB signaling is key in the development of ARHL
(17). Furthermore, UCHL1 is
considered a downstream protein of NF-κB signaling (37) and is involved in numerous human
diseases by regulating NF-κB signaling (38,39).
Xue et al (40)
demonstrated that Sp1 is a regulator of NF-κB signaling in
osteoarthritis. The results of the present study showed that the
activation of NF-κB signaling mediated by exposure of HEI-OC1 cells
to H2O2 was inhibited by UCHL1
overexpression. This effect of UCHL1 on NF-κB signaling was
abrogated by Sp1 overexpression.
In conclusion, the present study suggested that
UCHL1, negatively regulated by Sp1, could promote
H2O2-mediated cell injury, oxidative stress,
apoptosis and senescence and inhibit NF-κB signaling in an in
vitro model of ARHL. To the best of our knowledge, this is the
first study to report the role of UCHL1, as well as the association
between UCHL1 and Sp1 in an H2O2-induced ARHL
model in cochlear hair cells. Taken together, the results of the
present study supported the efficacy of a novel targeted therapy
for ARHL based on an UCHL1-mediated molecular mechanism. However,
further studies in an in vivo animal model should be
performed to verify the role of UCHL1 in ARHL and changes in the
expression levels of UCHL1 and Sp1. In addition, how changes in
expression of downstream factors of NF-kB signaling regulate UCHL1
expression should be further investigated.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Fund (grant nos. 81960190 and 81700919) and the Natural
Science Foundation of Jiangxi Province (grant nos. 20192BAB215026
and 20171BBG70007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and LL designed the study. LL, KX, XB and ZW
performed the research. XT and XC analyzed the data. XC and LL
drafted the manuscript. XC and LL confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He ZH, Li M, Zou SY, Liao FL, Ding YY, Su
HG, Wei XF, Wei CJ, Mu YR and Kong WJ: Protection and prevention of
age-related hearing loss. Adv Exp Med Biol. 1130:59–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Patel R and McKinnon BJ: Hearing loss in
the elderly. Clin Geriatr Med. 34:163–174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ding N, Lee S, Lieber-Kotz M, Yang J and
Gao X: Advances in genome editing for genetic hearing loss. Adv
Drug Deliv Rev. 168:118–133. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sprinzl GM and Riechelmann H: Current
trends in treating hearing loss in elderly people: A review of the
technology and treatment options-a mini-review. Gerontology.
56:351–358. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kamil RJ, Betz J, Powers BB, Pratt S,
Kritchevsky S, Ayonayon HN, Harris TB, Helzner E, Deal JA, Martin
K, et al: Association of hearing impairment with incident frailty
and falls in older adults. J Aging Health. 28:644–660.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rutherford BR, Brewster K, Golub JS, Kim
AH and Roose SP: Sensation and psychiatry: Linking age-related
hearing loss to late-life depression and cognitive decline. Am J
Psychiatry. 175:215–224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matějíková J, Kubiczková L, Sedlaříková L,
Potáčová A, Hájek R and Sevčíková S: Degradation of proteins by
ubiquitin proteasome pathway. Klin Onkol. 26:251–256.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thibaudeau TA and Smith DM: A practical
review of proteasome pharmacology. Pharmacol Rev. 71:170–197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Farshi P, Deshmukh RR, Nwankwo JO,
Arkwright RT, Cvek B, Liu J and Dou QP: Deubiquitinases (DUBs) and
DUB inhibitors: A patent review. Expert Opin Ther Pat.
25:1191–1208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mi Z, Liu H, Rose ME, Ma X, Reay DP, Ma J,
Henchir J, Dixon CE and Graham SH: Abolishing UCHL1's hydrolase
activity exacerbates TBI-induced axonal injury and neuronal death
in mice. Exp Neurol. 336(113524)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tramutola A, Di Domenico F, Barone E,
Perluigi M and Butterfield DA: It is all about (U)biquitin: Role of
altered ubiquitin-proteasome system and UCHL1 in Alzheimer disease.
Oxid Med Cell Longev. 2016(2756068)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ng ASL, Tan YJ, Lu Z, Ng EY, Ng SYE, Chia
NSY, Setiawan F, Xu Z, Keong NCH, Tay KY, et al: Plasma ubiquitin
C-terminal hydrolase L1 levels reflect disease stage and motor
severity in Parkinson's disease. Aging (Albany NY). 12:1488–1495.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Su Z, Xiong H, Liu Y, Pang J, Lin H, Zhang
W and Zheng Y: Transcriptomic analysis highlights cochlear
inflammation associated with age-related hearing loss in C57BL/6
mice using next generation sequencing. PeerJ.
8(e9737)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
O'Connor L, Gilmour J and Bonifer C: The
role of the ubiquitously expressed transcription factor Sp1 in
tissue-specific transcriptional regulation and in disease. Yale J
Biol Med. 89:513–525. 2016.PubMed/NCBI
|
|
15
|
Xie L, Zhou Q, Chen X, Du X, Liu Z, Fei B,
Hou J, Dai Y and She W: Elucidation of the
Hdac2/Sp1/miR-204-5p/Bcl-2 axis as a modulator of cochlear
apoptosis via in vivo/in vitro models of acute hearing loss. Mol
Ther Nucleic Acids. 23:1093–1109. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang B, Wan L, Sun P, Zhang L, Han L,
Zhang H, Zhang J, Pu Y and Zhu B: Associations of genetic variation
in E3 SUMO-protein ligase CBX4 with noise-induced hearing loss. Hum
Mol Genet. 31:2109–2120. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Uraguchi K, Maeda Y, Takahara J, Omichi R,
Fujimoto S, Kariya S, Nishizaki K and Ando M: Upregulation of a
nuclear factor-kappa B-interacting immune gene network in mice
cochleae with age-related hearing loss. PLoS One.
16(e0258977)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Y, Lv Z, Liu Y, Cao H, Yang J and
Wang B: PIN1 protects hair cells and auditory HEI-OC1 cells against
senescence by inhibiting the PI3K/Akt/mTOR pathway. Oxid Med Cell
Longev. 2021(9980444)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin H, Xiong H, Su Z, Pang J, Lai L, Zhang
H, Jian B, Zhang W and Zheng Y: Inhibition of DRP-1-dependent
mitophagy promotes cochlea hair cell senescence and exacerbates
age-related hearing loss. Front Cell Neurosci.
13(550)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fortunato S, Forli F, Guglielmi V, De
Corso E, Paludetti G, Berrettini S and Fetoni AR: A review of new
insights on the association between hearing loss and cognitive
decline in ageing. Acta Otorhinolaryngol Ital. 36:155–166.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liberman MC: Noise-induced and age-related
hearing loss: New perspectives and potential therapies. F1000Res.
6(927)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kamogashira T, Fujimoto C and Yamasoba T:
Reactive oxygen species, apoptosis, and mitochondrial dysfunction
in hearing loss. Biomed Res Int. 2015(617207)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017(8416763)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fujimoto C and Yamasoba T: Oxidative
stresses and mitochondrial dysfunction in age-related hearing loss.
Oxid Med Cell Longev. 2014(582849)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wei W and Ji S: Cellular senescence:
Molecular mechanisms and pathogenicity. J Cell Physiol.
233:9121–9135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luo Y, Zou P, Zou J, Wang J, Zhou D and
Liu L: Autophagy regulates ROS-induced cellular senescence via p21
in a p38 MAPKα dependent manner. Exp Gerontol. 46:860–867.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pattison JS, Osinska H and Robbins J: Atg7
induces basal autophagy and rescues autophagic deficiency in
CryABR120G cardiomyocytes. Circ Res. 109:151–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schmidt MF, Gan ZY, Komander D and Dewson
G: Ubiquitin signalling in neurodegeneration: Mechanisms and
therapeutic opportunities. Cell Death Differ. 28:570–590.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y, Huang X, Zhao XY, Hu YJ, Sun HY
and Kong WJ: Role of the ubiquitin C-terminal hydrolase
L1-modulated ubiquitin proteasome system in auditory cortex
senescence. ORL J Otorhinolaryngol Relat Spec. 79:153–163.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim YJ, Kim K, Lee YY, Choo OS, Jang JH
and Choung YH: Downregulated UCHL1 accelerates gentamicin-induced
auditory cell death via autophagy. Mol Neurobiol. 56:7433–7447.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kudryavtseva AV, Krasnov GS, Dmitriev AA,
Alekseev BY, Kardymon OL, Sadritdinova AF, Fedorova MS, Pokrovsky
AV, Melnikova NV, Kaprin AD, et al: Mitochondrial dysfunction and
oxidative stress in aging and cancer. Oncotarget. 7:44879–44905.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mosa KA, El-Naggar M, Ramamoorthy K,
Alawadhi H, Elnaggar A, Wartanian S, Ibrahim E and Hani H: Copper
nanoparticles induced genotoxicty, oxidative stress, and changes in
superoxide dismutase (SOD) gene expression in cucumber (cucumis
sativus) plants. Front Plant Sci. 9(872)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sikora E, Bielak-Żmijewska A and Mosieniak
G: What is and what is not cell senescence. Postepy Biochem.
64:110–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vizcaíno C, Mansilla S and Portugal J: Sp1
transcription factor: A long-standing target in cancer
chemotherapy. Pharmacol Ther. 152:111–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Beishline K and Azizkhan-Clifford J: Sp1
and the ‘hallmarks of cancer’. FEBS J. 282:224–258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang H, Mao X, Sun Y, Hu R, Luo W, Zhao
Z, Chen Q and Zhang Z: NF-κB upregulates ubiquitin C-terminal
hydrolase 1 in diseased podocytes in glomerulonephritis. Mol Med
Rep. 12:2893–2901. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang Z, Liu N and Chen X, Zhang F, Kong
T, Tang X, Yang Q, Chen W, Xiong X and Chen X: UCHL1 regulates
inflammation via MAPK and NF-κB pathways in LPS-activated
macrophages. Cell Biol Int. 45:2107–2117. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gong Z, Ye Q, Wu JW, Zhou JL, Kong XY and
Ma LK: UCHL1 inhibition attenuates cardiac fibrosis via modulation
of nuclear factor-κB signaling in fibroblasts. Eur J Pharmacol.
900(174045)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xue H, Yu P, Wang WZ, Niu YY and Li X: The
reduced lncRNA NKILA inhibited proliferation and promoted apoptosis
of chondrocytes via miR-145/SP1/NF-κB signaling in human
osteoarthritis. Eur Rev Med Pharmacol Sci. 24:535–548.
2020.PubMed/NCBI View Article : Google Scholar
|