Introduction
Gynecological pelvic abscess is a common infectious
disease in clinical practice that includes pyometra, ovarian cyst
infection and fallopian tube abscess. Surgery for gynecologic
cancer with lymphadenectomy and pelvic radiotherapy can induce
lymphatic congestion and produce lymphoceles. Infection is
sometimes complicated, resulting in abscess (1). Such cases are treated with
antibiotics and drainage or surgery, similarly to gut-derived
pelvic abscess; however, few studies have focused on gynecological
pelvic abscess. This condition develops in the deep pelvis;
therefore, attention should be paid to the indication for a
surgical procedure and percutaneous drainage. A culture test is
needed to choose antibiotics to treat the infection, but it is
difficult to collect specimens. Furthermore, invasive approaches
are required for specimen collection in many patients who are not
improved by conservative treatment, but the results of the culture
tests are modified by antibiotic therapy. Therefore, tests are
needed that allow identification of appropriate antibiotics and
predict the clinical course before treatment.
Magnetic resonance imaging (MRI) detects differences
in radiofrequency changes in protons in a magnetic field in free
water compared to that of protons in other molecules, including
proteins. The amounts of substances containing protons can be
estimated from the changes in radiofrequency using magnetic
resonance spectroscopy (MRS), which is used in clinical practice to
detect necrosis and recurrence after radiotherapy for head tumors
(2-4).
This technology may also be applicable to pelvic tumors, including
identification of pathogenic bacteria and prediction of clinical
course using MRS signals from abscesses.
The ‘true causative bacteria of the abscess’ can be
difficult to detect due to prior antimicrobial agents, as culture
specimens may be collected after treatment has started. Recent
innovative genome sequencing technology, which is referred to as
next-generation sequencing (NGS), enables analyses of bacterial
flora using 16S ribosomal DNA. NGS is frequently used to detect
true pathogenic bacteria in abscesses (5,6) and
can also detect dead bacteria if 16S ribosomal DNA remains, leading
to identification of pathogenic bacteria after antibiotic therapy.
In this exploratory study, we examined the utility of MRS for
treatment of gynecological pelvic abscess using detection of
pathogenic bacteria with NGS. This is the first report in the world
using NGS and MRS simultaneously.
Materials and methods
Patients
The subjects were patients diagnosed with
gynecological pelvic abscess (ovarian abscess, fallopian tube
abscess, postoperative cyst of the pouch of Douglas, and infectious
lymphocele) and treated in our hospital from July 2015 to September
2016. Inclusion criteria were those who were hospitalized and
treated in our hospital for a diagnosis of pelvic abscess during
the above period. Exclusion criteria were i) those for whom MRI or
MRS imaging was not possible or sufficient signal could not be
obtained, ii) those for whom close examination revealed disease
other than pelvic abscess, and iii) those for whom the patient did
not give consent to participate in the study. Treatment was
provided based on normal clinical decision-making and the disease
course was recorded. Most patients received antimicrobials prior to
or from the time of admission, had MRI and MRS imaging after
admission, and subsequently underwent surgical intervention if
considered necessary. A culture test and NGS analyses of bacterial
flora were performed using specimens collected in surgery and
percutaneous drainage, and lactic acid in abscess fluid was
determined. All patients gave written informed consent after
receiving an oral explanation of the study. The study was approved
by the research ethics committee of Keio University School of
Medicine (approval no. 20140406; Tokyo, Japan) and is registered in
the UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctr/index-j.htm; registration
no. UMIN000016705 on March 11, 2015).
MRI and MRS
MRI and MRS were performed with a 3T clinical
scanner equipped with a 32-channel body coil (Discovery MR750 3.0T,
GE Healthcare, Waukesha, WI). Before MRS, routine clinical MRI was
performed, including collection of fast spin echo (FSE) T2-weighted
images (T2WI) in the axial and sagittal planes (TR/TE=4,000/100
msec), FSE T1-weighted images (T1WI, TR/TE=500/6 msec), and axial
diffusion-weighted images (DWI, TR/TE=6,000/60 msec, b=0 and 1,500
s/mm2). MRS was performed before surgery or drainage
using a point-resolved spectroscopic sequence (PRESS)
(TR/TE=2,000/144 msec, spectral width=2,500 Hz, number of
points=2,048, total number of scans=96) with an automated shimming
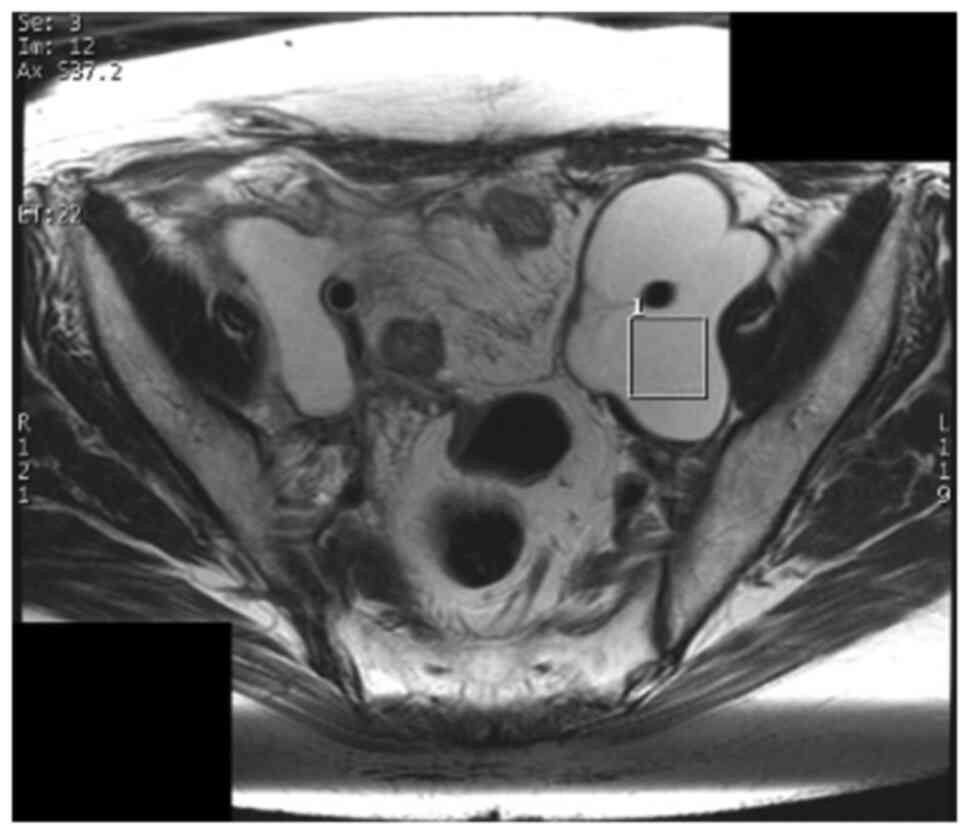
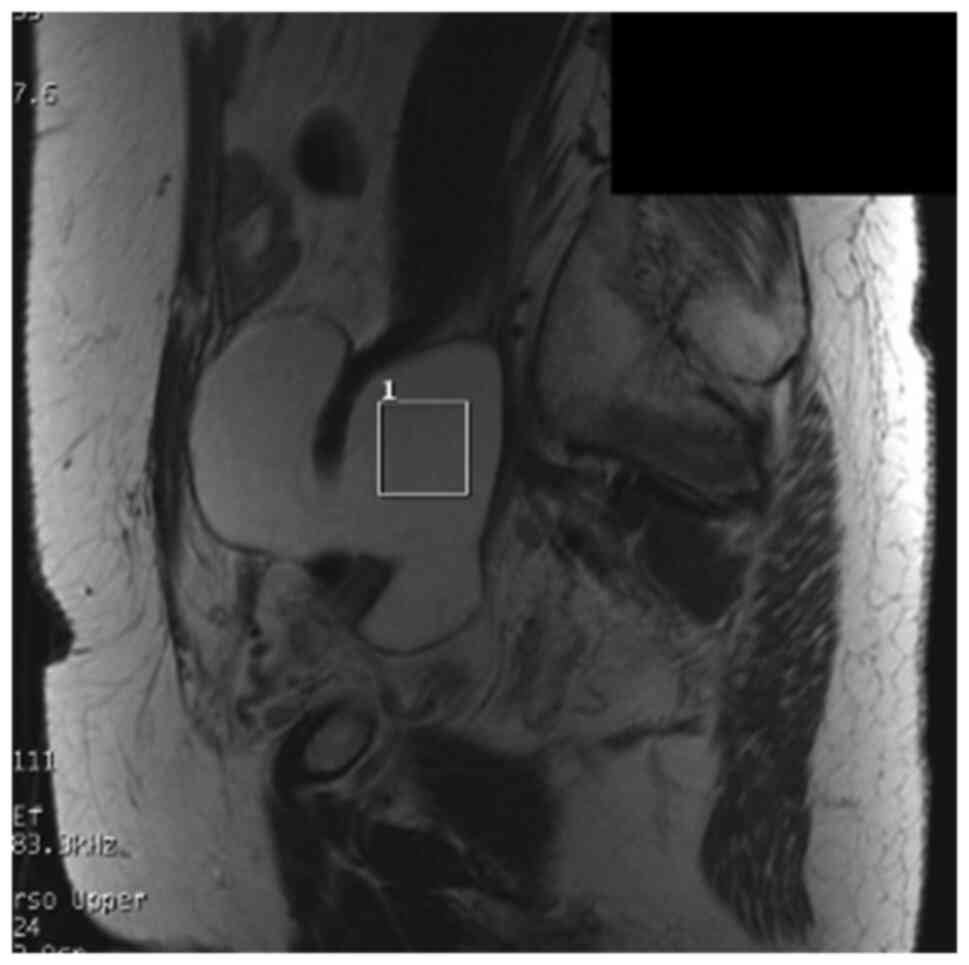
method. A region of interest (ROI) for data acquisition of size
20x20x20 mm3 was placed in the center of the abscess,
with reference to previously obtained T2WIs in the axial and
sagittal planes (Figs. 1 and
2). In general, lesions in the
pelvis show little respiratory variability. To obtain signal
intensity and to minimize errors, a cube with 20 mm long sides was
set as ROI inside the abscess, which was considered to be a
homogenous area, without including wall.
Following MRS, a 3D-fat suppressed gradient echo
sequence (TR/TE=4.4/2.2 msec) was obtained. In patients in whom
Gd-based contrast material was not contraindicated, Gadoteridol
(Bracco Diagnostics Inc, Princeton, NJ) (0.1 mmol/kg body weight)
was transvenously injected and 3D-fat suppressed T1WIs were
obtained in the axial, sagittal and coronal planes.
Postprocessing of MRS data
MRS data were analyzed using Spectroscopy Analysis
by General Electric (SAGE) on the scanner console (GE Healthcare).
An MRS spectrum was generated for each receiver coil; therefore, 32
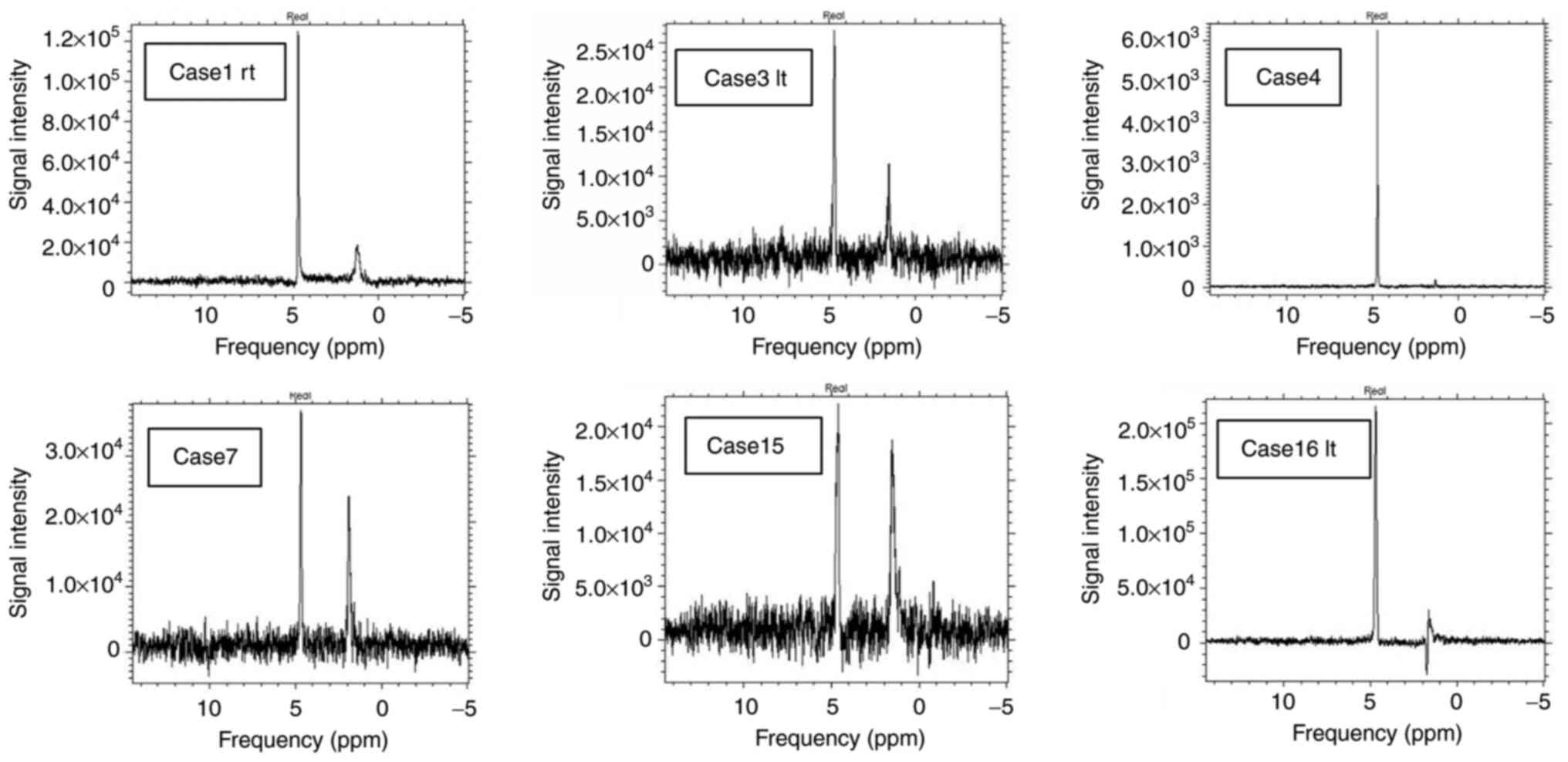
MRS images were generated in every case. MRS spectra were
classified into three categories based on the peak height at a
chemical shift of about 1.3 ppm, which is representing lipid or
lactate. The peak level was classified into three classes, in
comparison with the noise level by visual estimation: that is,
twofold higher than the average noise level (++), higher than the
average noise level but lower than a twofold higher noise level
(+), and the same as the average noise level (-), respectively,
based on the criteria proposed by Okada et al (7). Okada's criteria were not designed for
abscesses, but were used to qualitatively categorize the signal
strength of MRS, a quantitative and continuous variable, in
analyzing the results of this exploratory study.
Next-generation sequencing
Purulent drainage samples were stored at -80˚C until
DNA extraction. Genomic DNA was isolated using a NucleoSpin
Microbial DNA kit (Macherey-Nagel). The extraction protocol was
performed according to the manufacturer's instructions. DNA samples
were extracted from approximately 500 µl of purulent contents of
each abscess, but the DNA concentration of the sample from Case 11
was too low for subsequent analysis. Thus, extraction was performed
again using all the remaining sample from Case 11.
Sequencing of the 16S rRNA gene
Two steps of PCR were used for the purified DNA
samples to obtain sequence libraries. The first PCR was performed
for amplification using a 16S (V3-V4) Metagenomic Library
Construction Kit for NGS (Takara Bio Inc.) with primer pair 341F
(5'-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGC AG-3') and
806R
(5'-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3'),
corresponding to the V3-V4 region of the 16S rRNA gene. N, W, H and
V were mixed base codes (N for A, C, G, T; W for A, T; H for A, C,
T; V for A, C, G). The accession number of the gene used in
designing primers was Gene ID 948332 (rrsA, Escherichia coli
str. K-12 substr. MG1655; https://www.ncbi.nlm.nih.gov/gene/?term=948332). These
pair primers are universal primers in popular use (8,9). The
second PCR was performed to add the index sequences for the
Illumina sequencer with a barcode sequence using the Nextera XT
Index Kit (Illumina, San Diego, CA). The prepared libraries were
subjected to sequencing of 250 paired-end bases using the MiSeq
Reagent Kit v.3 on the MiSeq (Illumina) at Takara Bio. In
processing of sequence data, operational taxonomic unit (OTU)
definition, taxonomy assignment, and an OTU BLAST search were
performed using CD-HIT-OTU 0.0.1, QIIME ver. 1.8, and the DDBJ 16S
rRNA database, respectively.
Statistical analysis
Fisher's exact test and Student's t-test was used
for statistical analysis. P<0.05 was considered statistically
significant. Data were analyzed using SPSS (version 25).
Results
Background of the patients
MRS was performed in 17 patients, including 4 with
fallopian tube abscess, one with ovarian abscess (endometriotic
ovarian cyst), 9 with infectious lymphocele, and 2 with a Douglas
cyst. One patient was excluded from the study because her pelvic
lesion was found to be a tumor of recurrent cervical cancer, rather
than an abscess. Twelve patients received antimicrobials prior to
or from the time of admission, and had MRI and MRS imaging after
admission. In four cases, no antimicrobials were administered prior
to MRI and MRS imaging. All cases were assessed for indications for
surgery or percutaneous drainage after MRS and MRI, which were
performed if considered necessary. Consequently, 13 of the 16
patients underwent surgical intervention. Three patients with
infectious lymphocele were improved rapidly with only conservative
treatment with antimicrobial agents, they were determined that
drainage was not indicated. In MRS, peaks other than that for
H2O (4.7 ppm) were found in 6 of 16 patients. Peaks at
around 1-2 ppm found in 5 cases suggested the presence of lipid
(cases 1, 3, 4, 7 and 15). An inverted peak at 1.33 ppm found in
one case suggested the presence of lactate acid (case 16). MRS
signals for (+) and (++) cases are shown in Fig. 3 and those for MRS (-) cases are
shown in Fig. 4.
The treatment plan was determined by normal clinical
decision-making, and not by a prescribed study protocol. Attending
doctors considered that infectious lymphocele needed drainage only
when not improved by antibiotics, and fallopian tube or ovarian
abscess usually needed a surgical procedure due to its severity.
Thus, all patients with a fallopian tube or ovarian abscess or a
Douglas cyst underwent surgery or drainage and abscess fluids were
collected. Six of 9 patients with infectious lymphocele underwent
drainage, and three were improved by conservative therapy with
antibiotics alone. Age, diagnosis, lesion type, immunosuppressive
conditions, results of blood culture, antibiotic treatment before
imaging, tumor size on MRI, MRS signals, apparent diffusion
coefficient (ADC) values, treatment before drainage, properties of
tumor fluid, lactic acid levels, results of culture tests, and
bacterial strains identified by NGS are shown in Tables I and II, in which cases are sorted by disease,
rather than by onset date.
 | Table IBackground and clinical data. |
Table I
Background and clinical data.
| | Treatment before
imaging | |
|---|
| Case | Age, years | Diagnosis | Location |
Immunosuppression | Blood culture | Antibiotics | Period, days | Size on MRI (WxLxH),
mm | MRS signal | ADC, 10-3
mm2/sec |
|---|
| 1 | 45 | Fallopian tube
abscess | Bilateral | None | Negative | CMZ | 5 | Lt 64x63x65 | - | 0.8878 |
| | | | | | | | | Rt: 55x63x78 | ++ | 0.9604 |
| 2 | 36 | Fallopian tube
abscess | Lt | None | Negative | CZOP | 1 | 77x85x85 | - | 1.107 |
| 3 | 41 | Fallopian tube
abscess | Bilateral | None | Negative | CZOP, VCM, AZT,
MNZ | 5 | Lt: 41x41x62 Rt:
57x32x70 | ++ | (2-L) 0.4831,
1.084 |
| | | | | | | | | | - | (2-L) 0.4896,
0.7472 |
| 4 | 44 | Fallopian tube
abscess | Rt | None | Negative | PIPC/TAZ, MINO,
MEPM | 10 | 58x58x51 | + | 1.138 |
| 5 | 41 | Ovarian abscess
(endometriotic cyst) | Rt | None | Negative | CMZ | 1 | 43x50x41 | - | 0.8564 |
| 6 | 35 | Douglas cyst
(intestinal perforation) | | Postoperative | Bacteriodes fragilis
group | CZOP | 3 | 63x74x78 | - | (3-L) 1.197, 2.106,
3.006 |
| 7 | 34 | Douglas' abscess | | Postoperative | Negative | None | 0 | 70x32x30 | ++ | (3-L) 2.637, 1.77,
1.518 |
| 8 | 58 | Infectious
lymphocele | Rt pelvis | None | Negative | CPFX, CZOP | 1 | 26x29x67 | - | 2.686 |
| 9 | 65 | Infectious
lymphocele | Lt pelvis | None | Negative | CZOP | 2 | 57x32x66 | - | 2.692 |
| 10 | 35 | Infectious
lymphocele | Para-aortic | During CTx | Escherichia coli | CZOP | 2 | 61x51x100 | - | NA |
| 11 | 52 | Infectious
lymphocele | Rt pelvis | During CTx | Negative | None | | 88x57x87 | - | 2.685 |
| 12 | 39 | Infectious
lymphocele | Lt pelvis | None | Negative | CFPX(OA),
CVA/AMPC | 15 | 30x52x42 | - | 2.292 |
| 13 | 63 | Infectious
lymphocele | Bilateral
pelvis | Postoperative | Negative | CZOP, PIP/TAZ | 6 | Lt: 78x120x85 | - | NA |
| | | | | | | | | Rt: 110x76x210 | - | NA |
| 14 | 43 | Infectious
lymphocele | Rt | None | Negative | None | 0 | 21x26x50 | - | 2.941 |
| 15 | 29 | Infectious
lymphocele | Lt pelvis | None | Negative | CZOP | 1 | 23x42x36 | ++ | 2.625 |
| 16 | 60 | Infectious
lymphocele | Bilateral | During CTx | Negative | None | 0 | Lt: 40x63x71 | + | (2-L) |
| | | | | | | | | Rt: 64x40x92 | | 3.062, 3.121 |
| | | | | | | | | | NA | 3.117 |
 | Table IICulture results and bacteria
identified in next-generation sequencing. |
Table II
Culture results and bacteria
identified in next-generation sequencing.
| Case | Intervention | Location | Properties of
abscess sample | Lactate, mg/dl | Culture
results | NGS results |
|---|
| 1 | Operation | Lt | Red-brown,
purulent | 107 |
Peptotereptococcus tetradius | Anaerococcus
tetradius |
| | | Rt | Red-brown,
purulent | 100 | Negative | Anaerococcus
tetradius |
| 2 | Operation | | Red, transparent,
including floaters | 81.6 | Prevotella
bivia; Peptostrepto. Anaerobius; Fusovacterium nucleatum;
Anaerobic GPR; Anaerobic GPC | Prevotella
bivia; Fusobacterium necrophorum subsp. funduliforme;
Peptostreptococcus stomatis; Bilophila wadsworthia; Olsenella
sp. Marseille-P2936 |
| 3 | Operation | Lt | Red-brown,
purulent | 140 | Escherichia
coli | Escherichia
coli |
| | | Rt | Red-brown,
purulent | 133 | Escherichia
coli | Escherichia
coli |
| 4 | Operation | | White,
purulent | 88.2 | Negative | Sneathia
sanguinegens |
| 5 | Drainage | | Reddish white,
purulent | 273 | Escherichia
coli | Escherichia
coli |
| 6 | Operation | | Red,
transparent | 149 | Enterococcus
faecalis; Bacteroides fragillis group; Bacteroides eggerthii;
Peptostreptococcus magnus; Anaerobic GPR; Anaerobic GPR | Faecalibacterium
prausnitzii; Bacteroides sp. MC_16; Bilophila wadsworthia;
Parabacteroides sp.; Bacteroides ovatus V975;
Sutterella massiliensis; Bacteroides vulgatus |
| 7 | Drainage | | Red-brown,
purulent | 147 |
Staphylococcus species | Streptococcus
dysgalactiae |
| 8 | Drainage | | Light yellow
transparent | 112 | Negative | Staphylococcus
lugdunensis; Acinetobacter johnsonii |
| 9 | Drainage | | Light yellow
transparent | 113 | Negative | Enterococcus
saccharolyticus; Acinetobacter johnsonii |
| 10 | Drainage | | Yellow
purulent | 120 | Escherichia
coli | Escherichia
coli |
| 11 | Drainage | | Light yellow
transparent | 103 | Streptococcus
agalactiae | Streptococcus
dysgalactiae; Acinetobacter johnsonii |
| 12 | Drainage | | Transparent | 128 | Streptococcus
equinus | Streptococcus
equinus; Streptococcus bovis group (sample mixed from both
cysts) |
| 13 | Drainage | Lt | Light yellow
transparent | 34.7 | Negative |
Pseudomonas |
| | | Rt | Light yellow
transparent | 39.4 | Negative |
pseudoalcaligenes; Acinetobacter
johnsonii; Pseudomonas fluorescens; Bradyrhizobium genosp.O;
Pseudomonas fluorescens; Novosphingobium aquaticum Glaeser
et al. 2013; Sphingomonas sp.; Pseudomonas
pseudoalcaligenes; Acinetobacter johnsonii; Propionispora
sp. |
| 14 | None | | | | | |
| 15 | None | | | | | |
| 16 | None | Lt | | | | |
| | | Rt | | | | |
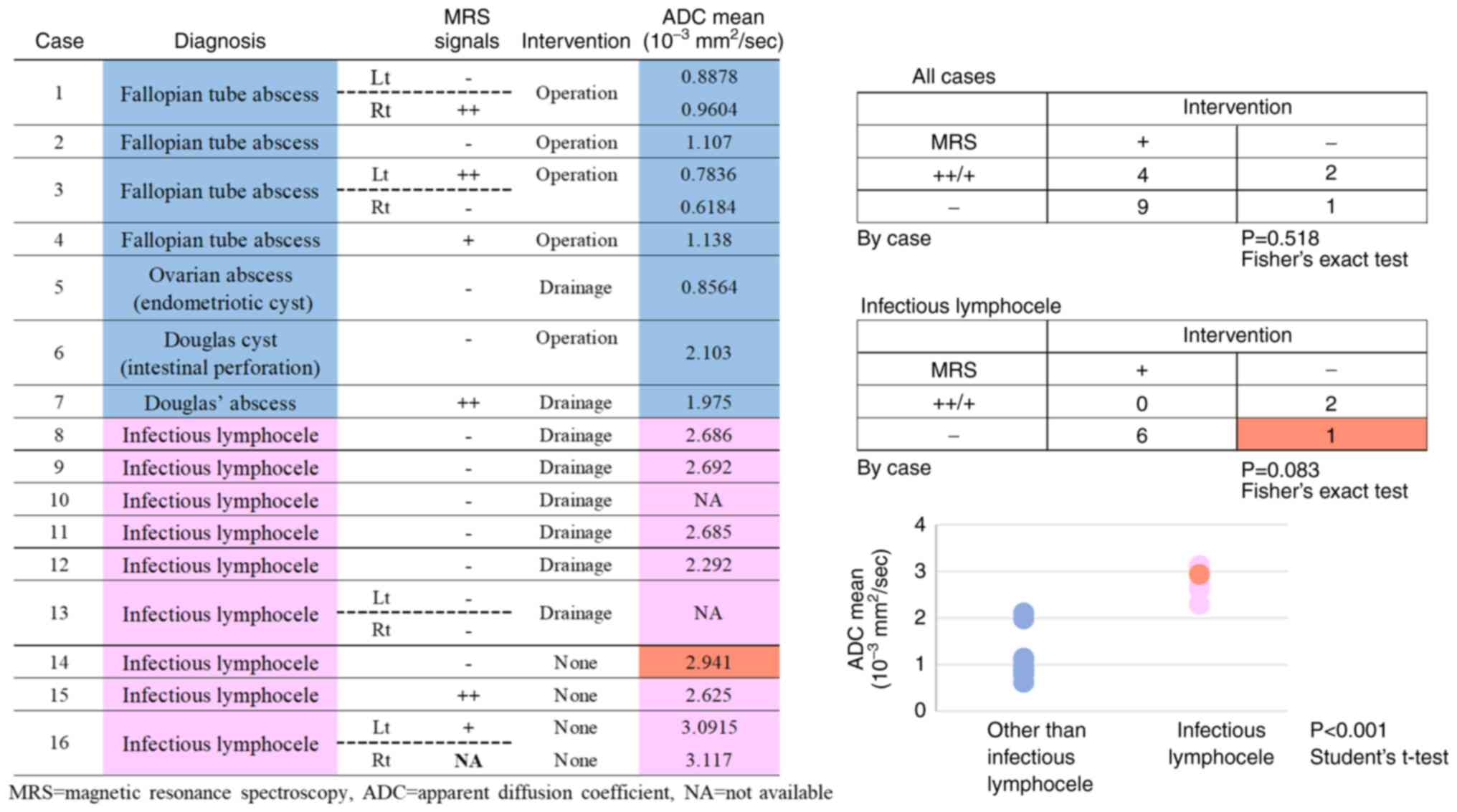
MRS signals and clinical course
MRS signals and the necessity for surgery and
drainage were examined in patients with infectious lymphocele. A
total of 7 cases were MRS (-) and 6 of these subjects required
drainage. Two (+) or (++) cases were improved without drainage
(P=0.083, Fig. 5). These results
suggest that MRS signals can predict the requirement for drainage
in patients with infectious lymphocele, although the number of
cases was small.
ADC values, contents of abscess
samples and clinical course
There was significant difference in ADC values
between lymphocele infection case and non-lymphocele infection
cases (P<0.001, Fig. 5).
Although we found no association between ADC values and the
causative organisms, all non-lymphocele infection cases required
surgery or drainage, and low ADC appears to be associated with
severity.
Compared only among the lymphocele infection cases,
those that required drainage tended to have lower ADC values. MRS
(-) tended to correlate with the requirement for drainage, but MRS
(-) case that did not require drainage had particularly high ADC
values (red point in Fig. 5).
Simultaneous determination of MRS signals and ADC values is likely
to provide a more accurate estimation of the clinical course.
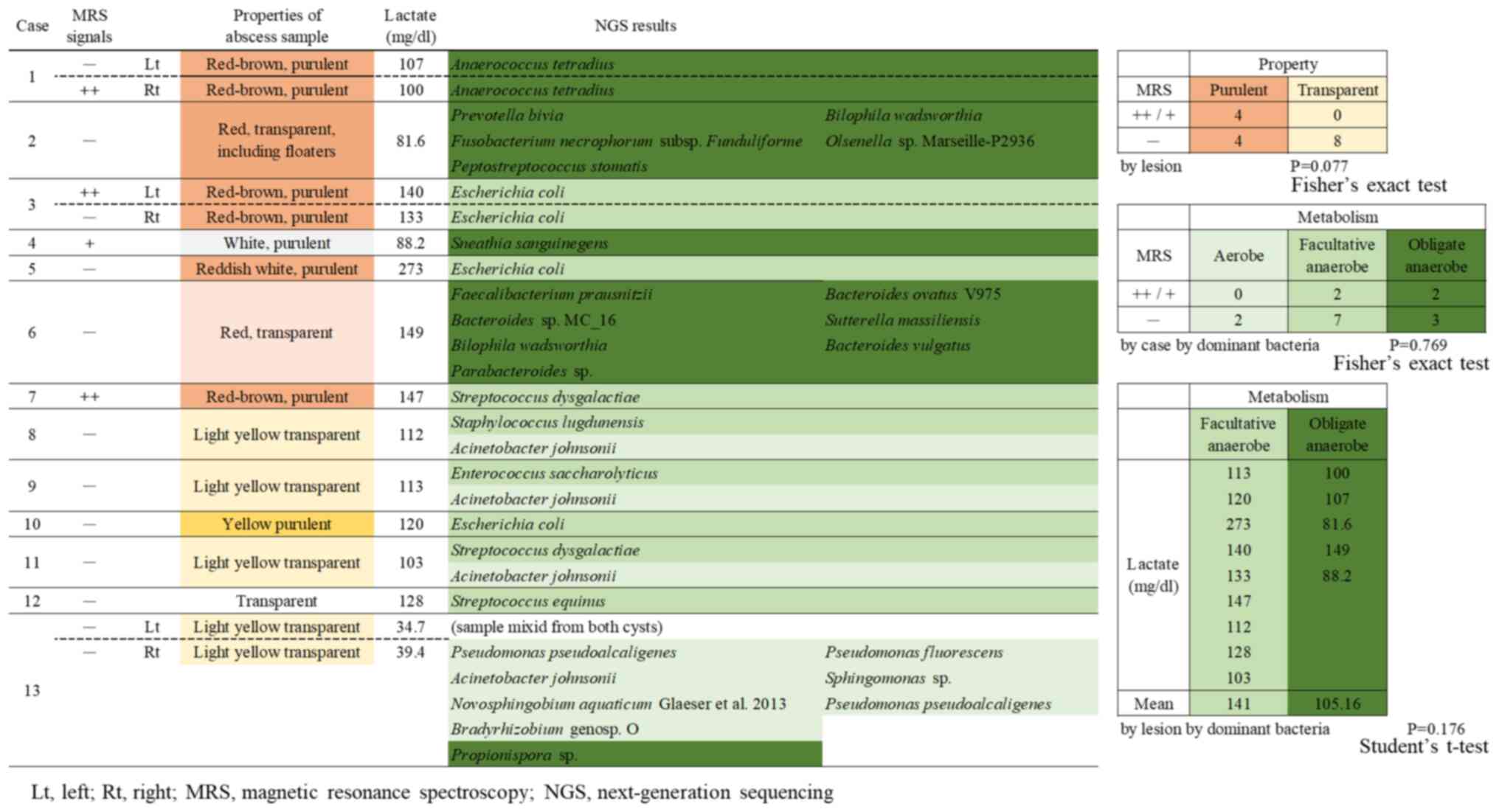
The contents abscess samples were purulent or
transparent yellow or red. We found no association between the
properties of the contents of abscess samples and the causative
bacteria. All MRS (+) lesion had the purulent contents, and
properties of contents of abscess samples tended to correlate with
MRS singles (Fig. 6).
Relationships among MRS signals,
lactic acid in abscess fluid, and bacterial strain analysis
MRS signals, lactic acid in abscess fluid, and
results from bacterial strain analysis were compared. In Fig. 6, aerobes identified by NGS are
shown in light green, and facultative and obligate anaerobes in
green and dark green, respectively. Correlation between MRS signals
and the causative bacteria identified by NGS, the most promising
result of this study, was documented in only Fig. 6. There was no correlation between
metabolism and MRS signals (P=0.521, Fig. 6). No significant differences in
lactic acid levels were found among bacterial strains, but lactic
acid tended to be high for facultative anaerobes and low for
obligate anaerobes (P=0.176, Fig.
6).
Discussion
One of the problems of abscess treatment is that it
is started without performance of culture tests, which are
important for choosing antibiotics, because sampling is invasive.
Drainage is performed for patients who do not respond to treatment,
but the true pathogenic bacteria often remain unclear because of
modifications by the initial antibiotics. Therefore, a test that
would allow the appropriate choice of antibiotics at the start of
treatment would be useful, and for this reason we examined MRS as a
basis for detection of bacteria in this study. To our knowledge,
there are few reports of evaluation of gynecological pelvic abscess
using MRS. In this study, we examined whether the pathogenic
bacteria and clinical course could be predicted by identifying
metabolites in abscess fluid. From previous reports, we predicted
that the MR peaks are due to metabolites such as lactic acid
(10,11). However, almost all detected peaks
other than that for H2O indicate the presence of lipids,
which suggests a limitation of scanning protocols and
postprocessing of MRS data in the current study.
The heights of the peaks reflect the concentration
of the metabolites, but are also affected by acquisition noises.
Okada's classification was used to analyze the peaks for
categorizing the peak heights, which are a quantitative and
continuous values, into qualitative values. Quantitative indicators
are better suited for developing specific numerical criteria using
large numbers of cases, but qualitative indicators are better
suited for seeking new findings and trends in a small number of
cases. Okada's criteria were not the result of a study for
abscesses, but they were for pelvic lesions as in the present
study.
A peak matching lactic acid was found in one
patient; however, we could not confirm the actual lactic acid level
in the content of the abscess in that case due to improvement
without drainage. If the true pathogenic bacteria identified by NGS
were facultative anaerobes that readily produce lactic acid in
abscesses, lactic acid should have a tendency to increase. A
correlation between bacterial metabolic activity and metabolites in
the abscess was found, which suggests that detection using MRS may
not always be accurate.
Identification of pathogenic bacteria has been
reported based on MRS signals in patients with brain abscess and in
case reports of pelvic abscess in women (11,12).
These studies were based on the presence of acetate and succinate,
as metabolites of facultative anaerobes similar to lactic acid. In
bacterial metabolism, facultative anaerobes ferment lactic acid
under anaerobic conditions and obtain energy, whereas bacterial
activities depend on environments and obligate anaerobes that
metabolize lactic acid, leading to consumption (13,14).
In clinical practice for infection, anaerobes are generally
obligate anaerobes and antibiotics are also classified by activity
against these anaerobes, including Bacteroides.
Identification of obligate anaerobes contributes to the choice of
antibiotics; however, optimal antibiotics for Gram-positive cocci
including Streptococcus sp. differ from those for
Gram-negative bacillus including Escherichia coli, although
both are facultative anaerobes. Therefore, it is difficult to
choose antibiotics based only on such information. These findings
indicate that MRS cannot be used definitively for treatment
decision-making, even if detection of metabolites in abscesses is
possible.
Difficulty with detecting signals inside an abscess
was also suggested by our results. Detection of true signals from
abscess contents requires establishment of ROIs inside the abscess
only. Because of the small respiratory variability in the pelvis,
we decided to set a cube with 20 mm long sides, which was the
largest possible size for a homogeneous area, due to obtaining the
signal strength and minimalizing sampling error. However, we still
could not deny the possibility that respiratory variability might
have affected the results. For lymphoceles, respiratory variability
were virtually absent because they were in the retroperitoneum and
were anchored in the vascular territory, whereas fallopian tubes
are inside the pelvis and may be affected by respiration.
Consequently, errors may be caused by variance during detection of
signals by MRS. Furthermore, as shown in ADC, abscesses were not
uniform among the subjects. The ROI was established at the center
to cover all layers, but setting of the ROI may also have led to
errors at certain sites.
Despite the difficulties with use of MRS, our
results suggest that MRS signals at the start of treatment may
allow prediction of the need for drainage due to lymphocele
infection. Patients with a signal other than H2O in MRS
at the start of treatment are likely to respond to conservative
therapy, whereas those without this signal are unlikely to be
improved and will need drainage. In addition, patients with an
abscess with low ADC levels and high viscous contents were likely
to require surgical intervention. For these patients, early
drainage may shorten the treatment period. Abscess size has been
suggested to be a predictor of the need for lymphocele drainage
(15), but this tendency was not
found in the current study. However, this is a small-scale study
and specimens were not collected from patients who responded to
treatment, which might explain this result. Further studies are
needed to examine this issue.
As for other diseases in terms of the use of MRS for
pelvic lesions, there are reports in the field of malignancies.
There were reports that in ovarian tumors, high peaks of choline
and lactate (more than twice the noise) were findings suggestive of
malignancy (16), and that in
locally advanced cervical cancer, high peak of lipid was predictive
marker of a poor response to neo-adjuvant chemotherapy (17). The indication for pelvic lesions
seems feasible, and future studies are expected on abscesses as
well.
Initial administration of antibiotics is involved in
identifying pathogenic bacteria causing the abscess, as described
above. In fact, the results of culture tests in this study showed
many cases that were negative for bacteria. If based on only
culture results, the analysis might be poorer than those on NGS
results. In NGS analysis of flora, pathogenic bacteria were
identified in all cases, which shows the value of this method for
abscess examination. The current study was performed
retrospectively, but NGS has been shown to be useful during
treatment in clinical practice (5)
and is expected to decrease costs and time.
The greatest limitation of this study was the small
number of cases. The disease of pelvic abscesses included a wide
variety of conditions, and focusing on each of them would lead to a
dispersion of cases. However, we found promising results in
lymphocele infections, which involved the largest number of cases.
In infectious lymphocele, existence of peaks of metabolites other
than H2O (lipid or lactate) in MRS might predict
improving without drainage. This was a finding that had never been
studied or reported before. An exploratory study of MRS for
treatment of gynecological pelvic abscess was conducted. In
conclusion, currently, we cannot show marked utility of MRS for
identification of pathogenic bacteria and prediction of the
clinical course. However, MRS may be useful for predicting the need
for drainage in patients with infectious lymphocele.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by JSPS KAKENHI (Tokyo, Japan;
grant no. 18K16779).
Availability of data and materials
The raw sequencing data generated and/or analyzed
during the current study are available in the DDBJ Sequence Read
Archive (DRA) under accession number DRA015101 (https://ddbj.nig.ac.jp/resource/sra-submission/DRA015101).
The other datasets used and/or analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
YN collected data and wrote the manuscript. KB
analyzed data, managed the project and contributed to critical
revision of the manuscript. YN and KB confirm the authenticity of
all the raw data. YK collected data, managed the patients and
advised on experimental design. ET collected data, managed the
patients and advised on experimental design. SO collected data,
analyzed the MRS signals, and advised on interpretation of MRI and
MRS results. DA supervised the project and advised on experimental
design. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients gave written informed consent after
receiving an oral explanation of the study for participating and
publication. The study was approved by the institutional research
ethics committee (approval no. 20140406) and is registered in the
UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctr/index-j.htm; registration
no. UMIN000016705 on March 11, 2015).
Patient consent for publication
All patients gave written informed consent after
receiving an oral explanation of the study for participating and
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weinberger V, Cibula D and Zikan M:
Lymphocele: Prevalence and management in gynecological
malignancies. Exp Rev Anticancer Ther. 14:307–317. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang H, Ma L, Wang Q, Zheng X, Wu C and
Xu BN: Role of magnetic resonance spectroscopy for the
differentiation of recurrent glioma from radiation necrosis: A
systematic review and meta-analysis. Eur J Radiol. 83:2181–2189.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Taylor JS, Langston JW, Reddick WE,
Kingsley PB, Ogg RJ, Pui MH, Kun LE, Jenkins JJ III, Chen G, Ochs
JJ, et al: Clinical value of proton magnetic resonance spectroscopy
for differentiating recurrent or residual brain tumor from delayed
cerebral necrosis. Int J Rad Oncol Biol Phys. 36:1251–1261.
1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anbarloui MR, Ghodsi SM, Khoshnevisan A,
Khadivi M, Abdollahzadeh S, Aoude A, Naderi S, Najafi Z and
Faghih-Jouibari M: Accuracy of magnetic resonance spectroscopy in
distinction between radiation necrosis and recurrence of brain
tumors. Iran J Neurol. 14:29–34. 2015.PubMed/NCBI
|
|
5
|
Guo LY, Feng WY, Guo X, Liu B, Liu G and
Dong J: The advantages of next-generation sequencing technology in
the detection of different sources of abscess. J Infect. 78:75–86.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song YG, Shim SG, Kim KM, Lee DH, Kim DS,
Choi SH, Song JY, Kang HL, Baik SC, Lee WK, et al: Profiling of the
bacteria responsible for pyogenic liver abscess by 16S rRNA gene
pyrosequencing. J Microbiol. 52:504–509. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Okada T, Harada M, Matsuzaki K, Nishitani
H and Aono T: Evaluation of female intrapelvic tumors by clinical
proton MR spectroscopy. J Magn Reson Imaging. 13:912–917.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nadatani Y, Watanabe T, Suda W, Nakata A,
Matsumoto Y, Kosaka S, Higashimori A, Otani K, Hosomi S, Tanaka F,
et al: Gastric acid inhibitor aggravates indomethacin-induced small
intestinal injury via reducing Lactobacillus johnsonii. Sci Rep.
9(17490)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kakiuchi T, Yamamoto K, Imamura I,
Hashiguchi K, Kawakubo H, Yamaguchi D, Fujioka Y and Okuda M: Gut
microbiota changes related to Helicobacter pylori
eradication with vonoprazan containing triple therapy among
adolescents: A prospective multicenter study. Sci Rep.
11(755)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Burn PR, Haider MA, Alfuhaid T, Brown MP
and Roberts TP: Proton magnetic resonance spectroscopy as a
potential tool for differentiating between abdominal fluid
collections. J Magn Reson Imaging. 18:740–744. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takeuchi M, Matsuzaki K and Harada M: In
vivo proton MR spectroscopy in uterine abscesses. J Magn Reson
Imaging. 38:955–957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pal D, Bhattacharyya A, Husain M, Prasad
KN, Pandey CM and Gupta RK: In vivo proton MR spectroscopy
evaluation of pyogenic brain abscesses: A report of 194 cases. AJNR
Am J Neuroradiol. 31:360–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Macfarlane S and Macfarlane GT: Regulation
of short-chain fatty acid production. Proc Nutr Soc. 62:67–72.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Watabe J: Carbohydrate fermentation in the
colon. J Intestinal Microbiol. 19:169–177. 2005.
|
|
15
|
Tanaka K, Tominaga E, Nogami Y, Nakamura
M, Nishio H, Yamagami W, et al: Therapeutic procedures for
postoperative infectious lymphocele. Tokyo J Obstet Gynecol.
64:571–575. 2015.
|
|
16
|
Mansour SM, Gomma MMM and Shafik PN:
Proton MR spectroscopy and the detection of malignancy in ovarian
masses. Br J Radiol. 92(20190134)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dolciami M, Canese R, Testa C, Pernazza A,
Santangelo G, Palaia I, Rocca CD, Catalano C and Manganaro L: The
contribution of the 1H-MRS lipid signal to cervical
cancer prognosis: A preliminary study. Eur Radiol Exp.
6(47)2022.PubMed/NCBI View Article : Google Scholar
|