Introduction
Adenomyosis (AD) is defined as invasion of
endometrial glands and stroma into the myometrium (1). Following a limited or diffuse growth
pattern, AD develops into endometrial glands in the myometrium
(2) and can coexist with
endometriosis (3). Manifesting as
pelvic pain and abnormal uterine bleeding, AD has a close
association with female infertility, dysmenorrhea and dyspareunia
(2,4,5). AD
is defined as a benign gynecological disease, however, according to
some reports, patients with AD may have a risk of malignant
transformation (6,7), during which the endometrium invaded
by AD can progress into endometrioid adenocarcinoma followed by
serous and clear cell carcinoma and poorly differentiated
adenocarcinoma, severely threatening health (8). Therefore, early diagnosis and timely
treatment are key for suppressing the progression and the malignant
transformation of AD. Understanding of the AD condition remains
poor despite the upgrade in diagnostic tools (6) and current management of AD still
lacks international guidelines (9).
It may be a novel approach to develop the treatment
of AD from the perspective of genetics, as long non-coding RNAs
(lncRNAs) are aberrantly expressed in both eutopic and ectopic
endometria of patients with AD (10,11).
Classified as a subgroup of ncRNAs, lncRNAs are >200 nucleotides
in length and possess regulatory effects on AD progression
(12-14).
Placenta-enriched lncRNA MIR503 host gene (MIR503HG), located on
chromosome Xq26.3 where genes associated with human reproduction
are enriched (15), is highly
expressed and associated with unfavorable outcomes in endometrial
cancer (16) and preeclampsia
(17), but is lowly expressed and
suppresses tumor growth by inhibiting cell migration and invasion
in triple-negative breast cancer (18). However, the specific expression and
mechanism of MIR503HG in AD remain unclear.
In addition, the conventional view is that AD
results from the abnormal down-growth and invagination of the
endometrium into the myometrium (19). Studies have reported that
lncRNA-mediated inhibition of proliferation and enhancement of
apoptosis in endometrial stromal cells (ESCs) ameliorates AD
(12-14).
Meanwhile, at only ~22 nucleotides in length and also classified as
ncRNAs, microRNAs (miRNAs or miRs) have been discovered to act as a
downstream mechanism of lncRNA-mediated changes in processes of
ESCs during AD progression (13).
Among them, miR-191 is notably dysregulated in the endometrium
tissues of patients with AD (20).
Therefore, identifying downstream miRNAs of lncRNAs that regulate
AD is key.
In the present study, the expression of MIR503HG in
AD was characterized and a potential mechanism by which MIR503HG
regulates AD by modulating its downstream miRNAs was investigated.
Meanwhile, since MIR503HG has been reported to target miR-191 to
promote tumor inhibition in cervical cancer (21), further experiments were performed
to determine their roles in AD.
Materials and methods
Ethics approval
The study was ratified by the Ethics Committee of
People's Hospital of Deyang City (approval no. GD202000524; Deyang,
China) and written informed consent from all participants was
obtained for experimental work involving tissues obtained from
humans.
Clinical samples
AD tissue (n=30) was collected from the endometrium
of patients with AD (mean age, 42.75±5.62 years; range, 31-58
years) who had been diagnosed clinically and pathologically at
People's Hospital of Deyang City in July 2020 to November 2020.
None of the patients had received preoperative chemotherapy,
radiotherapy or hormone therapy or had a history of chronic disease
such as coronary heart disease and hypertension. During the same
period, specimens taken from the endometrium (n=30) of patients
with cervical lesions or uterine fibroids after hysterectomy but
without AD (mean age, 40.24±3.73 years; range, 32-49 years) were
used as the control group. In the control group, the patients had
no history of hormone therapy or chronic diseases. All tissues were
immediately snap-frozen in liquid nitrogen at -80˚C for 40 min for
further use.
Isolation, identification and culture
of ESCs
ESCs were isolated from the endometrium of patients
with AD (n=3) as follows: The endometrium was cut into 0.5-1.0
mm3 sections and digested with 0.25% trypsin (cat. no.
9002-07-7; Sigma-Aldrich; Merck KGaA) that was diluted to 1.25 mg/l
with Dulbecco's Modified Eagle Medium (DMEM)/F-12 (cat. no.
21041025; Thermo Fisher Scientific, Inc.). The sections were
incubated at 37˚C with 5% CO2 for 80 min, then a cell
suspension was obtained and filtered twice through a nylon mesh
(140- and 37-µm mesh in sequence). Subsequently, the filtered
suspension was centrifugated at 1,000 x g for 5 min at 4˚C and ESCs
were obtained as previously described (22). When the confluence of ESCs was 90%
under an optical microscope (BX50; Olympus Corporation;
magnification, x200), ESCs were maintained in DMEM/F12 (50 ml),
supplemented with 10% fetal bovine serum (FBS; cat. no. F2442), 2
mmol glutamine (cat. no. 1294808) and 1% penicillin-streptomycin
(cat. no. P4333; all Sigma-Aldrich; Merck KGaA) at 37˚C with 5%
CO2.
Immunocytochemistry assay
After being fixed in 4% paraformaldehyde (cat. no.
P6148; Sigma-Aldrich; Merck KGaA) for 15 min at room temperature,
ESCs (2x104 cells/ml) were washed with PBS (cat. no.
P5493, Sigma-Aldrich; Merck KGaA) three times and incubated with
0.1% Triton X-100 (cat. no. X100; Sigma-Aldrich; Merck KGaA) for
improvement of permeability. The cells were washed with PBS three
times and blocked in 5% bovine serum albumin (cat. no. A7030;
Sigma-Aldrich; Merck KGaA) for 20 min at room temperature.
Subsequently, anti-vimentin (cat. no. PA5-27231; 1:1,000; Thermo
Fisher Scientific, Inc.) and anti-cytokeratin antibody (cat. no.
PA5-32465; 1:100; Thermo Fisher Scientific, Inc.) were used to
incubate the cells at 4˚C overnight. After being washed with PBS,
cells were incubated with goat anti-Rabbit IgG HRP (cat. no. 31466;
1:1,000; Thermo Fisher Scientific, Inc.) at 4˚C for 60 min in the
dark. Following washing with PBS, cells were color-developed using
diaminobenzidine (cat. no. D8001; Sigma-Aldrich; Merck KGaA) for 5
min at room temperature and counterstained with hematoxylin (cat.
no. H3136, Sigma-Aldrich; Merck KGaA) for 1 min at room
temperature. ESCs were observed by a light microscope (IX71;
Olympus Corporation; magnification, x200).
Cell transfection
MIR503HG overexpression plasmids were constructed
with pcDNA3.1 vector (cat. no. V79520; Thermo Fisher Scientific,
Inc.). Short hairpin (sh)-MIR503HG (5'-CATCCAGCATCTCCAGTTA-3') was
constructed using MISSION pLKO.1-puro Empty Vectors (cat. no.
SHC001; Sigma-Aldrich; Merck KGaA). Empty vector and scrambled
sequence (5'-TTCTCCGAACGTGTCACGT-3') were used as negative controls
(NCs). miR-191 inhibitor/inhibitor control (IC; miR20000440-1-5,
5'-CAGCUGCUUUUGGGAUUCCGUUG-3'; miR2N0000001-1-5,
5'-UCUACUCUUUCUAGGAGGUUGUGA-3') and mimic/mimic control
(miR10000440-1-5, 5'-CAACGGAAUCCCAAAAGCAGCUG-3'; miR1N0000001-1-5,
5'-UUCUCCGAACGUGUCACGU-3') were purchased from Guangzhou RiboBio
Co., Ltd. ESCs were transfected with MIR503HG overexpression
plasmids, sh-MIR503HG, miR-191 inhibitor/mimic or a combination of
sh-MIR503HG and miR-191 inhibitor using Lipofectamine
3000® (cat. no. L3000015; Thermo Fisher Scientific,
Inc.). Briefly, ESCs were plated in 96-well plates at a density of
1x104 cells/well and cultured to 80% confluence.
Opti-MEM (10 µl; cat. no. 31985062; Thermo Fisher Scientific, Inc.)
and P3000 reagents (0.4 µl; Thermo Fisher Scientific, Inc.) were
used in combination to dilute the plasmids (0.2 µg) and
Lipofectamine 3000 (0.15 µl), and the diluted Lipofectamine 3000
reagent and diluted plasmid was mixed, followed by incubation at
37˚C for 10 min. Finally, RNA-lipid complex was added to 96-well
plates to incubate the cells at 37˚C for 24 or 48 h before further
use.
Dual-luciferase reporter assay
Starbase v.2.0 (https://starbase.sysu.edu.cn/) was used to predict the
targeting association between MIR503HG and miR-191. Wild-type (WT;
5'-ATGCTGCTTTT-GATTTCCGTTA-3') and mutant (MUT;
5'-ATGCTGACTTT-GATTTCCGTTA-3') MIR503HG 3'-untranslated region were
cloned into pmirGLO vectors (cat. no. E1330, Promega Corporation)
to synthesize pMirGLO-MIR503HG-WT and pMirGLO-MIR503HG-MUT,
respectively. ESCs were plated into 12-well plates at a density of
1x107 cells/well and cultured to 70% confluence at 37˚C.
The cells were co-transfected with miR-191 mimic (100 ng) and
pmirGLO vectors (100 ng) inserted with fragments of MIR503HG WT or
MUT sequence using Lipofectamine 3000® (Thermo Fisher
Scientific, Inc.) at 37˚C for 48 h. Dual-luciferase reporter assay
was immediately performed with Dual-Luciferase Reporter Assay
System (cat. no. E1980, Promega Corporation). Firefly luciferase
activity, normalized to Renilla luciferase activity and
measured using a luminometer (GloMax® 20/20; Promega
Corporation), was used to express the binding specificity.
RNA immunoprecipitation (RIP)
assay
RIP assay was performed using a RIP kit (cat. no.
RIP-12RXN; Sigma-Aldrich; Merck KGaA). ESCs were digested with
trypsin (Sigma-Aldrich; Merck KGaA) and collected the cells, and
then cells were ransferred into a mixed solution containing PBS,
nuclear separation buffer and double-distilled H2O. The
solution was stirred on ice and centrifuged at 2,500 x g for 15 min
at 4˚C. The precipitated nucleus of ESCs was resuspended in RIP
buffer. The solution was uniformly divided into IgG and the Ago2
group. The supernatant in the IgG group was added to 10 µg anti-IgG
antibody (cat. no. ab171870; 1:1,000; Abcam) while that in the Ago2
group was added to 10 µg Ago2 antibody (cat. no. ab186733; 1:30;
Abcam), gently shaken and then incubated at 4˚C overnight. The
supernatant was added to 40 µl protein A/G magnetic beads (cat. no.
M2400; Beijing Solarbio Science & Technology Co., Ltd.) and
cultured at 4˚C for 1 h. Unconjugated protein was removed by RIP
buffer washing. The untreated cell lysate was used as an input
group. The RNAs in the input group, IgG and Ago2 group were
extracted and reverse-transcribed into cDNA. Subsequently, the
expression of miR-191 and MIR503HG were measured via quantitative
polymerase chain reaction (qPCR). Expression in the input group was
considered to be positive control.
Cell Counting Kit (CCK)-8 assay
Following transfection, ESCs were plated into
96-well plates at a density of 5x103 cells/well. CCK-8
reagent (cat. no. C0037; Beyotime Institute of Biotechnology) was
added into the cells at a ratio of 1:10, after which cell
incubation was performed at 37˚C for 1 h. Cell viability was
measured based on the optical density using a microplate reader
(Synergy Neo2; BioTek Instruments, Inc.; Agilent Technologies,
Inc.) at a wavelength of 450 nm.
Transwell assay
Cell migration and invasion of transfected ESCs were
evaluated by Transwell chambers (cat. no. 428; Corning, Inc.). For
invasion assays, Transwell upper chambers were precoated with
Matrigel (cat. no. 356234; Corning, Inc.) diluted at a ratio of 1:3
and incubated at 37˚C for 2 h. Following transfection, ESCs were
suspended in serum-free DMEM/F12 to a concentration of
2x105 cells/ml and cell suspension (100 µl) was poured
into the upper chamber. The lower chamber was filled with DMEM/F12
(600 µl) supplemented with 10% FBS (cat. no. F2442; Sigma-Aldrich;
Merck KGaA). The whole Transwell chamber was incubated at 37˚C for
24 h, after which non-migratory or non-invading cells in the upper
chamber were removed. The remaining cells were washed twice with
PBS, fixed in 4% paraformaldehyde (cat. no. P6148; Sigma-Aldrich;
Merck KGaA) at room temperature for 20 min and stained with Giemsa
(800 µl; cat. no. 10092013; Thermo Fisher Scientific, Inc.) at room
temperature for 15 min. Finally, stained cells were observed under
an inverted light microscope (IX71; Olympus Corporation) and
counted with ImageJ v.1.47 (National Institutes of Health,
Bethesda, MD, USA) from eight randomly selected fields. The cell
migration or invasion rate was set to 100% in control groups, while
that in other groups was calculated by comparing the mean cell
number with that in the control group.
Flow cytometry
The apoptosis of transfected ESCs was measured using
Annexin V-FITC/PI apoptosis detection kit (cat. no. 40302ES20;
Shanghai Yeasen Biotechnology Co., Ltd.). After transfection, ESCs
were digested in EDTA-free trypsin (cat. no. T2600000;
Sigma-Aldrich; Merck KGaA) at room temperature for 2 min and
centrifugated at 3,000 x g for 5 min at 4˚C, followed by washing
with PBS. Subsequently, the cells were resuspended with 1X Binding
Buffer to reach the concentration of 1x106 cells/ml.
Annexin V-FITC solution (5 µl) and PI solution (10 µl) were used to
incubate the cells in the dark for 10 min at room temperature.
Apoptotic cells (early and late apoptosis) were examined using a
flow cytometer (CytoFLEX; Beckman Coulter, Inc.) and analyzed with
CytExpert software (Version 2.2.0.97; Beckman Coulter, Inc.).
Reverse transcription-(RT)qPCR
AD tissues were homogenized using a UH-05
homogenizer (Union-Biotech). Total RNA and miRNA were extracted
from ESCs, with or without transfection and AD and control tissues
using TRIzol® (cat. no. 15596026; Thermo Fisher Scientific, Inc.)
and PureLink miRNA Isolation kit (cat. no. K157001; Thermo Fisher
Scientific, Inc.), respectively. The extracted total RNA and miRNA
were reverse-transcribed to cDNA using SuperScript IV reverse
transcriptase (cat. no. 18090010; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. qPCR was performed on a
CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.) using PowerUp SYBR Green Master Mix (cat. no. A25742; Thermo
Fisher Scientific, Inc.). The primer pairs used for qPCR are listed
in Table I. The thermocycling
conditions were as follows: Initial denaturation at 95˚C for 10
min; 40 cycles of annealing at 95˚C for 15 sec and elongation at
60˚C for 60 sec. Quantification was performed using the
2-ΔΔCq method (23) and
the expression levels of MIR503HG were normalized to GAPDH while
miR-191 was normalized to U6.
 | Table IHuman primers used for reverse
transcription-quantitative PCR. |
Table I
Human primers used for reverse
transcription-quantitative PCR.
| Gene | Forward, 5'→3' | Reverse, 5'→3' |
|---|
| MIR503HG |
CTTGAAGGCATCCAGCATCTC |
TTGGGACACTTGGGTGGTTTT |
| microRNA-191 |
CGGAATCCCAAAAGCAGCTG |
TGTCGTGGAGTCGGCAATTG |
| GAPDH |
GAGAAGGCTGGGGCTCATTT |
AGTGATGGCATGGACTGTGG |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Western blotting
Total protein from transfected ESCs was extracted
using RIPA Buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.)
and quantified using a BCA assay (cat. no. A53227; Thermo Fisher
Scientific, Inc.). The extracted protein (40 µg/lane) and marker (4
µl; cat. no. PR1910; Beijing Solarbio Science & Technology Co.,
Ltd.) were loaded and separated by SDS-PAGE on a 10 or 12% gel
(cat. nos. P0670 and P0672, Beyotime Institute of Biotechnology)
and then transferred onto PVDF membranes (cat. no. P2438;
Sigma-Aldrich; Merck KGaA). The membranes were blocked with 5%
non-fat milk in TBS with 1% Tween-20 (TBST; cat. no. T9039,
Sigma-Aldrich; Merck KGaA) for 1 h at 37˚C. Subsequently, membranes
were incubated with primary antibodies against E-cadherin (cat. no.
ab40772; 97 kDa; 1:10,000; Abcam), N-cadherin (cat. no. ab18203;
130 kDa; 1:1,000; Abcam), β-catenin (cat. no. 9562; 92 kDa,
1:1,000; Cell Signaling Technology, Inc.), cleaved caspase-3 (cat.
no. ab32042; 17 kDa; 1:500; Abcam) and GAPDH (cat. no. 5174; 37
kDa; 1:1,000; Cell Signaling Technology, Inc.) at 4˚C overnight.
Following washing with TBST, membranes were incubated with a
secondary Goat anti-Rabbit IgG Alexa Fluor™ Plus 488 (cat. no.
A32731; 1:10,000; Thermo Fisher Scientific, Inc.) at 37˚C for 1 h.
Immunoreactive bands were visualized using enhanced
chemiluminescence reagent kit (cat. no. WP20005; Thermo Fisher
Scientific, Inc.) on an imaging device (iBright CL750; Thermo
Fisher Scientific, Inc.) and analyzed using ImageJ Software (1.52s
version; National Institutes of Health).
Statistical analysis
All statistical analyses were conducted with
GraphPad Prism (version 8.0; GraphPad Software Inc.). Data were
obtained from independent experiments performed in triplicate and
are presented as mean ± standard deviation. Differences between two
groups were analyzed using the unpaired Student's t test, while
those between multiple groups were analyzed using one-way analysis
of variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MIR503HG is lowly expressed in AD
tissue and regulates viability, migration, invasion and apoptosis
in ESCs derived from the endometrium of patients with AD
RT-qPCR demonstrated that the MIR503HG expression in
AD tissues was much lower than that in tissues collected from
patients with cervical lesions or uterine fibroids (P<0.001;
Fig. 1A). The physiological and
pathological characteristics of ESCs isolated from the endometrium
of patients with AD were investigated. Morphological examination
via an optical microscope of isolated ESCs revealed that ESCs
derived from patients with AD were polygons with an elliptical
nucleus that was primarily located at the center of the cell and
had noticeable nucleoli (Fig. 1B).
In addition, immunocytochemistry assay illustrated that the
cytoplasm of the ESCs was positive for vimentin with brownish
yellow, but was negative for cytokeratin with purplish red nuclei
(Fig. 1C). To investigate the role
of MIR503HG in AD, isolated ESCs were subjected to transfection
with MIR503HG overexpression plasmids or sh-MIR503HG; MIR503HG was
successfully overexpressed and knocked down (P<0.001; Fig. 1D), respectively. CCK-8 and
Transwell assays were performed to detect the viability, migration
and invasion rates of ESCs derived from patients with AD; these
were confirmed to be significantly decreased by MIR503HG
overexpression (P<0.01 for viability and P<0.001 for both
migration and invasion vs. NC; Fig.
1E-I) and increased by MIR503HG knockdown (P<0.05, P<0.01
and P<0.001 vs. NC, respectively; Fig. 1F-I). Moreover, flow cytometry
analysis showed that apoptosis of ESCs was promoted by MIR503HG
overexpression but inhibited by MIR503HG knockdown (P<0.001;
Fig. 1J and K).
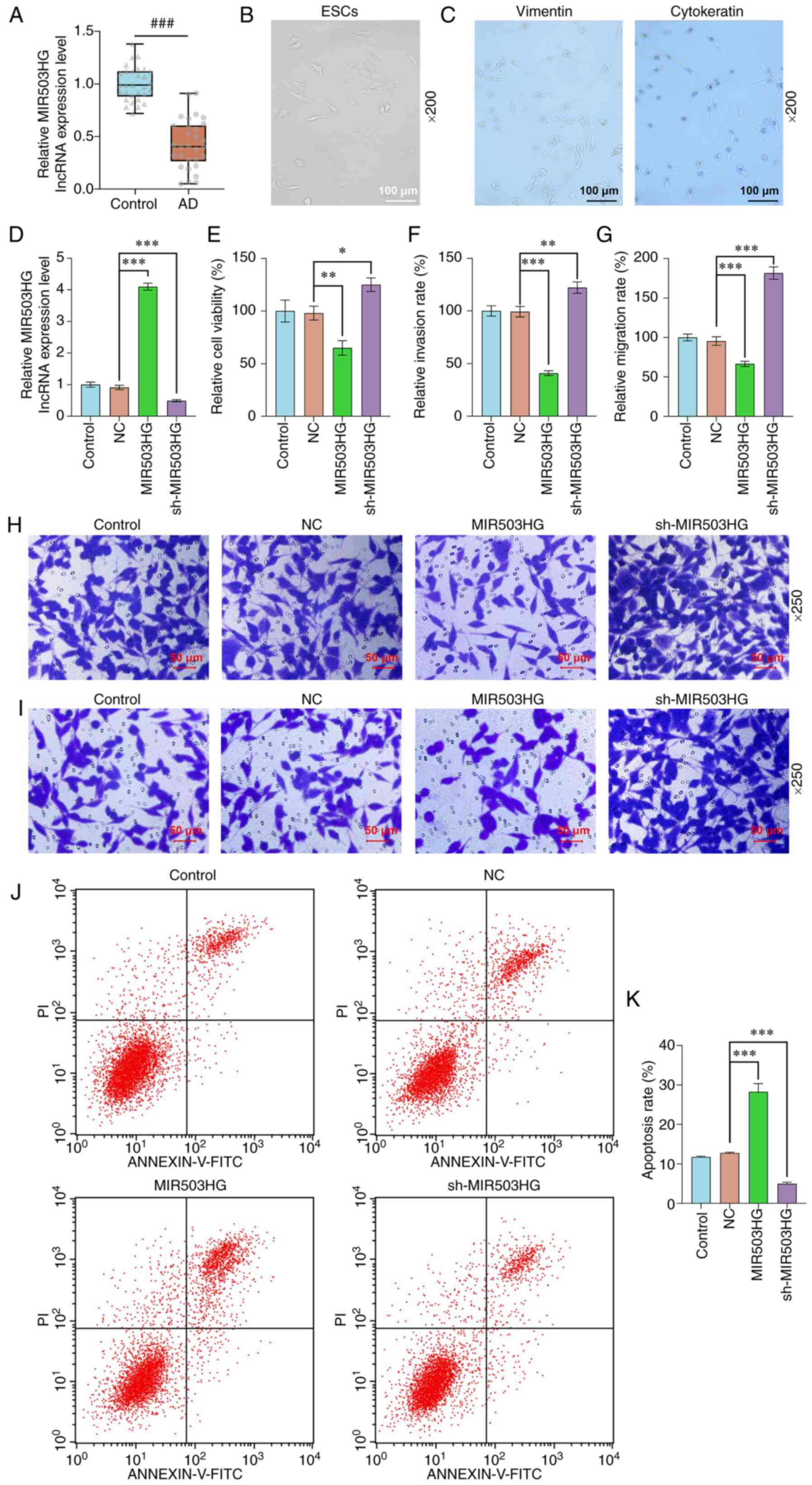 | Figure 1MIR503HG is lowly expressed in AD
tissue and regulates viability, migration, invasion and apoptosis
in ESCs derived from patients with AD. (A) Expression of MIR503HG
in the endometrium of patients with AD or cervical lesions/uterine
fibroids was analyzed using RT-qPCR. (B) ESCs isolated from AD
tissue were observed under an optical microscope (scale bar, 100
µm). (C) Cytokeratin and vimentin expression in ESCs was assessed
via immunocytochemistry (scale bar, 100 µm). (D) Expression of
MIR503HG in ESCs derived from patients with AD transfected with
MIR503HG overexpression plasmids/sh-MIR503HG was analyzed using
RT-qPCR. (E) Viability of ESCs derived from patients with AD
transfected with MIR503HG overexpression plasmids/sh-MIR503HG was
measured via Cell Counting Kit-8 assay. (F) Invasion and (G)
migration rate of ESCs derived from patients with AD transfected
with MIR503HG overexpression plasmids/sh-MIR503HG were evaluated.
(H) Invasion and (I) migration of ESCs transfected with MIR503HG
overexpression plasmids/sh-MIR503HG was detected via Transwell
assay (scale bar, 50 µm). (J) Apoptosis of ESCs derived from
patients with AD transfected with MIR503HG overexpression
plasmid/sh-MIR503HG was measured via flow cytometry. (K) Apotosis
rate of ESCs was quantified. *P<0.05,
**P<0.01 and ***P<0.001 vs. NC.
###P<0.001 vs. control. AD, adenomyosis; ESCs,
endometrial stromal cells; RT-q, reverse
transcription-quantitative; NC, negative control; sh, short
hairpin; lnc, long non-coding. |
MIR503HG is a sponge of miR-191 and
inhibits miR-191 expression in ESCs derived from patients with AD
while miR-191 is highly expressed in AD tissues
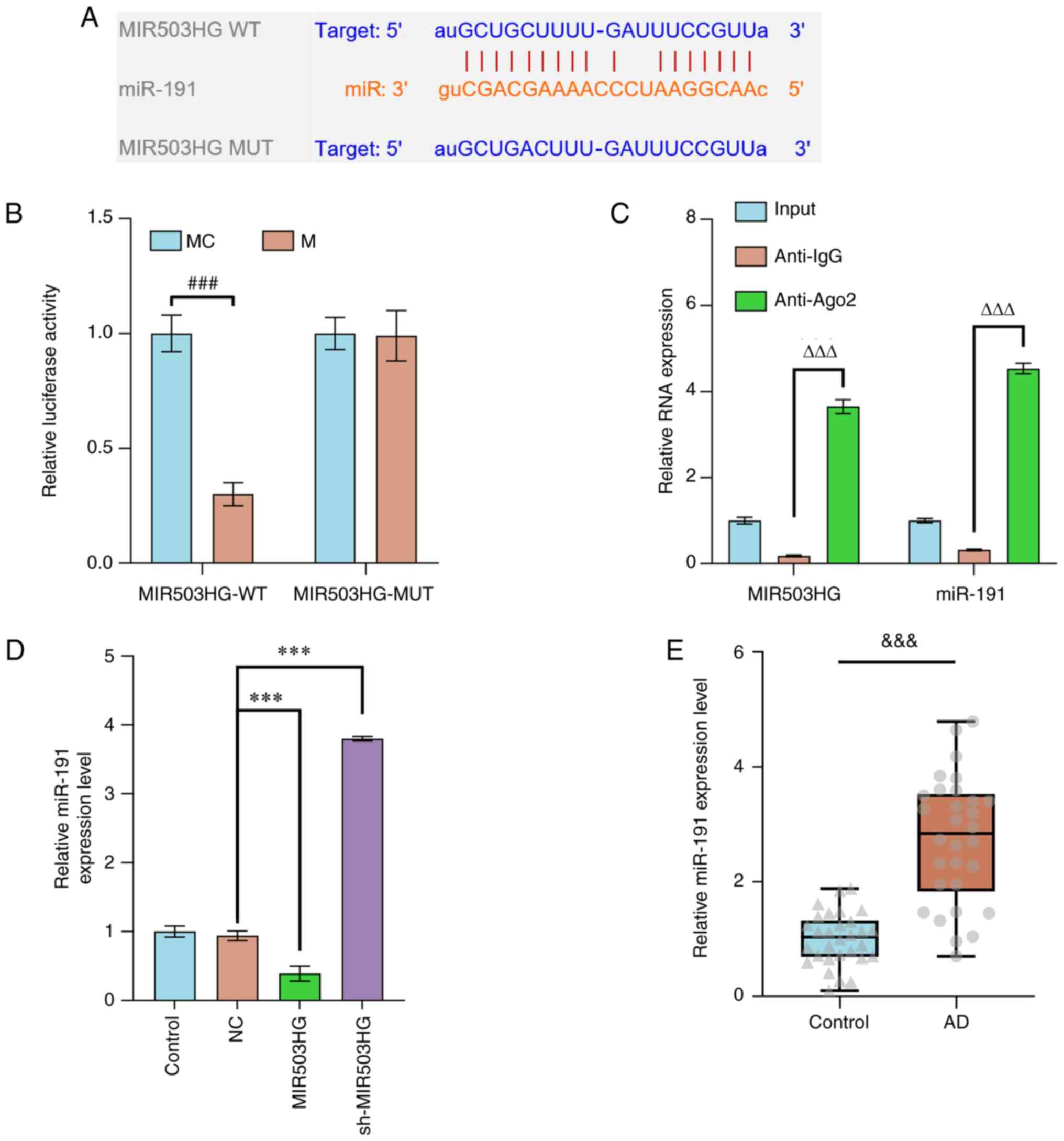
Starbase bioinformatics analysis demonstrated that
miR-191 had binding sites with MIR503HG (Fig. 2A). Dual-luciferase reporter assay
was performed, the result of which showed that with transfection of
miR-191 mimic, luciferase activity of ESCs derived from patients
with AD and transfected with pMirGLO-MIR503HG-WT was suppressed
(P<0.001; Fig. 2B), while that
of cells transfected with pMirGLO-MIR503HG-MUT remained unchanged
(Fig. 2B). RIP assay demonstrated
that the expression levels of both miR-191 and MIR503HG were
increased in the anti-Ago2 group, indicating binding of miR-191 and
MIR503HG (P<0.001 vs. IgG; Fig.
2C). RT-qPCR showed that miR-191 expression was downregulated
by MIR503HG overexpression but upregulated by MIR503HG knockdown in
ESCs derived from patients with AD (P<0.001; Fig. 2D), while high expression of miR-191
was observed in AD tissue (P<0.001; Fig. 2E).
miR-191 inhibition partially reversed
the effect of MIR503HG knockdown on the AD-derived ESC viability
and migration/invasion and apoptosis
miR-191 inhibitor was introduced to determine the
role of miR-191 in the MIR503HG-associated effects on ESCs derived
from patients with AD. According to RT-qPCR results, expression of
miR-191 was significantly decreased after ESCs were transfected
with miR-191 inhibitor (P<0.001; Fig. 3A) and upregulation of miR-191 was
observed following MIR503HG knockdown (P<0.001; Fig. 3B), and miR-191 inhibitor reversed
MIR503HG knockdown-induced upregulation of miR-191 (P<0.001;
Fig. 3B). CCK-8, Transwell and
flow cytometry assay results demonstrated that miR-191 inhibition
decreased the viability and the migration and invasion rates of
ESCs derived from patients with AD and augmented the apoptosis rate
(P<0.001 vs. NC + IC; Fig.
3C-I). MIR503HG knockdown promoted the viability and the
migration and invasion of ESCs derived from patients with AD and
inhibited the apoptosis (P<0.01, P<0.001 vs. NC + IC;
Fig. 3C-I). In addition, miR-191
inhibition attenuated the effect of MIR503HG knockdown on viability
and migration, invasion and apoptosis of ESCs derived from patients
with AD (P<0.001; Fig. 3C-3I).
Moreover, cleaved caspase-3 was used as the indicator of apoptosis
and its expression was decreased by MIR503HG knockdown (P<0.001;
Fig. 3J and K) but increased by miR-191 inhibitor in
ESCs (P<0.001; Fig. 3J and
K). Moreover, miR-191 inhibitor
attenuated the effect of MIR503HG knockdown on the expression of
cleaved caspase-3 (P<0.001; Fig.
3J and K).
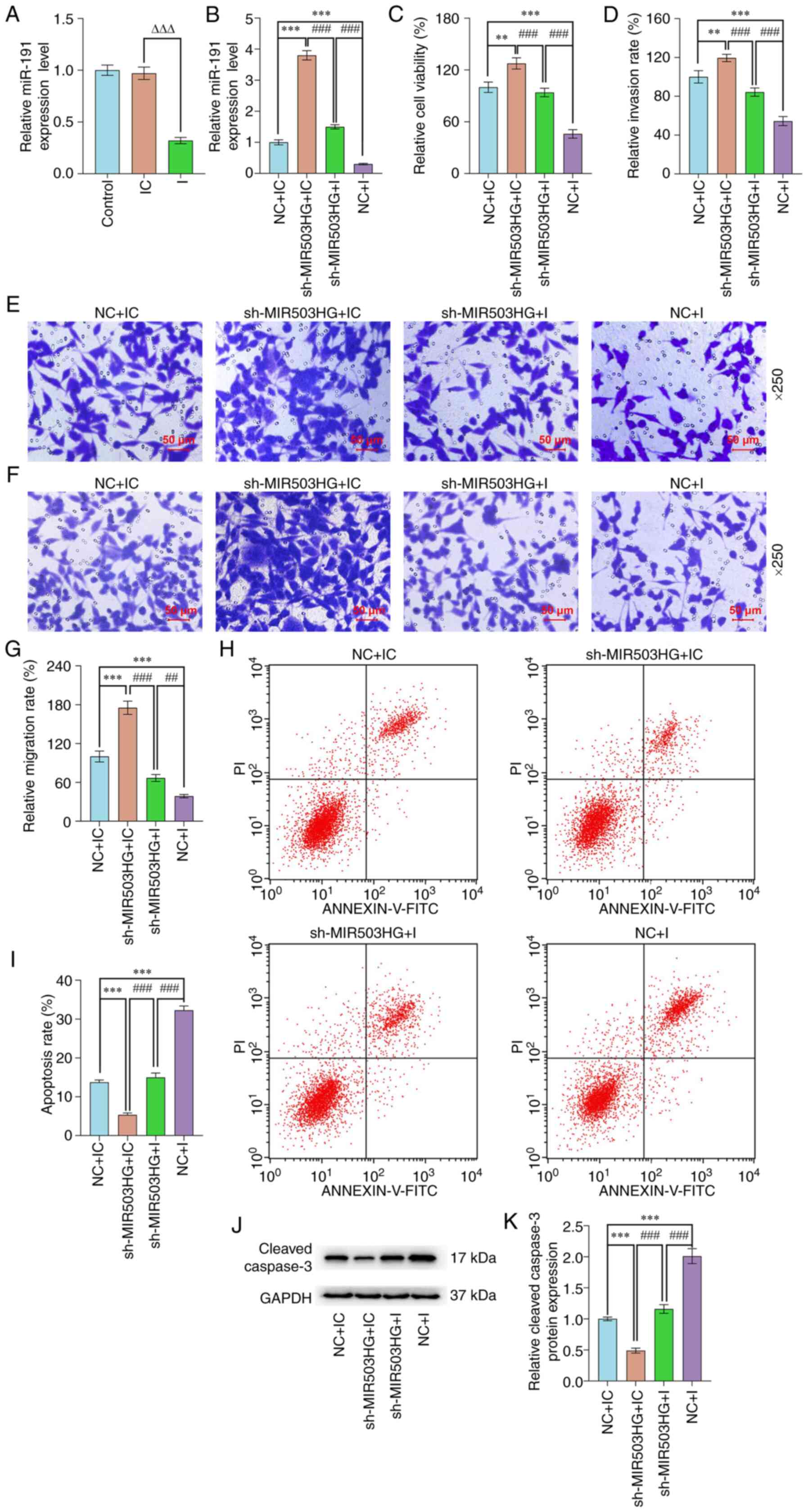 | Figure 3miR-191 inhibition partially reversed
the effect of MIR503HG knockdown on the viability and
migration/invasion and apoptosis in ESCs derived from patients with
AD potentially. (A) Expression of miR-191 in ESCs derived from
patients with AD that were transfected with I was analyzed via
RT-qPCR. (B) Expression of miR-191 in ESCs derived from patients
with AD transfected with sh-MIR503HG or I alone or in combination
was analyzed via RT-qPCR. (C) Viability of ESCs derived from
patients with AD transfected with sh-MIR503HG or I alone or in
combination was measured via Cell Counting Kit-8 assay. (D)
Invasion rate of ESCs derived from patients with AD transfected
with sh-MIR503HG or I alone or in combination was quantified. (E)
Invasion of ESCs derived from patients with AD transfected with
sh-MIR503HG or I alone or in combination were evaluated via
Transwell assay (scale bar, 50 µm). (F) Migration of ESCs derived
from patients with AD transfected with sh-MIR503HG or I alone or in
combination were evaluated via Transwell assay (scale bar, 50 µm).
(G) Migration rate of ESCs derived from patients with AD
transfected with sh-MIR503HG or I alone or in combination was
quantified. (H) Apoptosis of ESCs derived from patients with AD
transfected with sh-MIR503HG or I alone or in combination was
measured via flow cytometry. (I) Apotosis rate of ESCs was
quantified. (J) Expression of cleaved caspase-3 in ESCs derived
from patients with AD transfected with sh-MIR503HG or I alone or in
combination was measured by western blotting with GAPDH as
reference gene. (K) Protein expression of cleaved caspase-3 in ESCs
was quantified. ΔΔΔP<0.001 vs. IC.
**P<0.01 and ***P<0.001 vs. NC + IC.
##P<0.01 ###P<0.001 vs. sh-MIR503HG +
I. AD, adenomyosis; ESC, endometrial stromal cell; RT-q, reverse
transcription-quantitative; I, miR-191 inhibitor; IC, inhibitor
control; NC, negative control; miR, microRNA; sh, short
hairpin. |
MIR503HG knockdown facilitates
epithelial-mesenchymal transition (EMT) and activates the
Wnt/β-catenin pathway by regulating miR-191 expression in ESCs
derived from patients with AD
A previous study demonstrated that EMT is a cellular
process key for miR-191 upregulation-promoted cancer progression
(24). In addition, the
Wnt/β-catenin pathway is one of the underlying mechanisms of EMT in
AD (25). Western blotting showed
that MIR503HG knockdown significantly downregulated the levels of
E-Cadherin and upregulated those of N-cadherin and β-catenin
(P<0.01, P<0.001 and P<0.01 vs. NC + IC, respectively;
Fig. 4A-D), while miR-191
inhibition significantly upregulated the levels of E-Cadherin and
downregulated those of N-cadherin and β-catenin (P<0.001,
P<0.05 and P<0.001 vs. NC + IC, respectively; Fig. 4A-D). Moreover, miR-191 inhibition
attenuated the effect of MIR503HG knockdown on levels of
E-cadherin, N-cadherin and β-catenin (P<0.05, P<0.01 and
P<0.001, vs. sh-MIR503HG + IC, respectively; Fig. 4A-D).
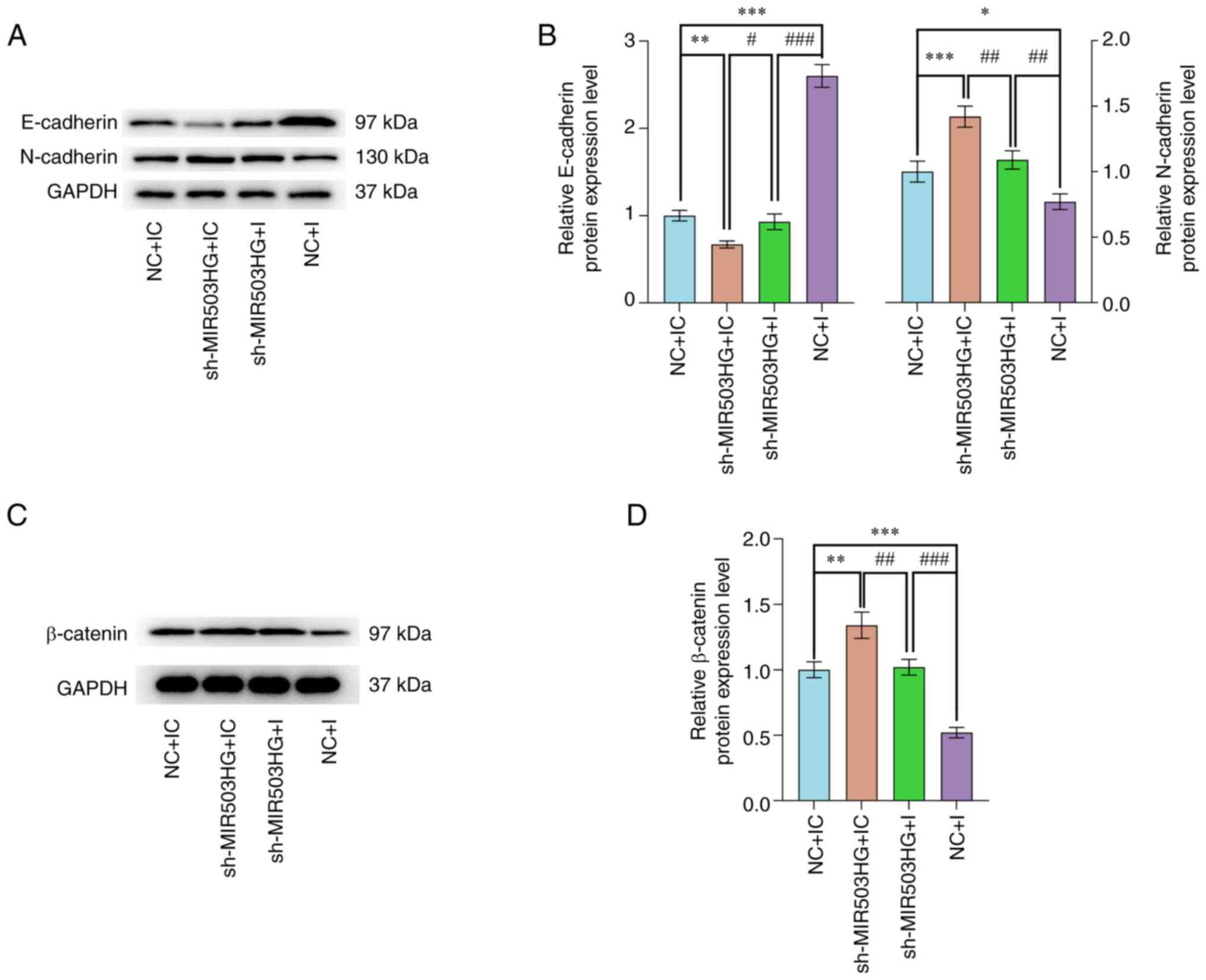 | Figure 4MIR503HG knockdown promotes
epithelial-mesenchymal transition and activates the Wnt/β-catenin
pathway by reversing miR-191 inhibition in ESCs derived from
patients with AD. (A) Expression of E-cadherin and N-cadherin in
ESCs derived from patients with AD transfected with sh-MIR503HG or
I alone or in combination was detected by western blotting. (B)
Protein expression of E-cadherin and N-cadherin in ESCs was
quantified. (C) Expression of β-catenin in ESCs derived from
patients with AD transfected with sh-MIR503HG or I alone or in
combination were analyzed by western blotting with GAPDH as the
reference gene. (D) Protein expression of β-catenin in ESCs was
quantified. *P<0.01, **P<0.05 and
***P<0.001 vs. NC + IC. #P<0.05,
##P<0.01 and ###P<0.001 vs. sh-MIR503HG
+ I. AD, adenomyosis; ESCs, endometrial stromal cells; RT-q,
reverse transcription-quantitative; I, miR-191 inhibitor; IC,
inhibitor control; NC, negative control; sh, short hairpin; miR,
microRNA. |
Discussion
Although no direct evidence has indicated that AD
triggers infertility, AD interferes with the success of in
vitro fertilization (26).
Prolonged treatment time of AD can result not only in placental
dislocation, preeclampsia and premature delivery, but also cause
endometrial adenocarcinoma (7,27,28).
Currently, AD, as a common benign pathology, is asymptomatic in
one-third of cases (29) and
diagnostic indices for AD are still lacking (6). There is no early classification of
the extent of the disease (30)
and biomarkers need to be discovered for the management of AD.
Aberrantly expressed lncRNAs, which are been
proposed to serve as diagnostic and therapeutic markers in assorted
diseases, such as cancer, cardiovascular disease and
osteoarthritis, have been detected in patients with AD (10,11).
MIR503HG has been previously identified as key in sustaining
endothelial cell biology and angiogenic processes (31) and is highly expressed in the
placenta and other reproductive tissues (32). As AD refers to invasion of the
endometrium into the myometrium, it is reasonable to hypothesize
that MIR503HG is lowly expressed in patients with AD; this was
confirmed in AD tissue in the present study.
ESCs are closely associated with the pathogenesis of
AD, and inhibited ESC proliferation contributes to AD progression
(13). Previous studies have
demonstrated that transition of ESCs to epithelial cells suppresses
AD progression (33) and
resistance to the apoptosis of ESCs may account for the
pathogenesis of AD (22).
Moreover, inhibition of proliferation, migration and enhancement of
apoptosis are considered a radically and efficiently conservative
method for the treatment of AD (22). Overexpression of MIR503HG in
preeclampsia-derived trophoblast cells inhibits cell proliferation,
migration and invasion, which underly the pathogenesis of
preeclampsia (17). MIR503HG
overexpression leads to enhanced apoptosis of high glucose-cultured
human proximal tubular (HK-2) cells (34). Contrary to the role of MIR503HG in
preeclampsia but consistent with that in high glucose-induced HK-2
cells, the present study demonstrated that overexpression of
MIR503HG impeded the viability, migration and invasion and enhanced
the apoptosis of ESCs isolated successfully from the endometrial
tissue of patients with AD. In addition, the pathogenesis of AD is
affected by EMT (33). EMT is
characterized by epithelial cells obtaining mesenchymal phenotypes
through undergoing loss of cell polarity, remodeling of the
cytoskeleton, acquired invasiveness and resistance to apoptosis
(35,36). During this process, E-cadherin (an
epithelial marker) expression is downregulated and N-cadherin (a
mesenchymal marker) expression is upregulated (37). The present study demonstrated that
the knockdown of MIR503HG led to downregulated E-cadherin and
upregulated N-cadherin, indicating that MIR503HG overexpression may
reverse EMT during AD progression. Overall, the present findings
demonstrated that restoration of MIR503HG expression may help
suppress AD progression.
Eutopic endometrium of patients with AD exhibits
reciprocally dysregulated miRNAs such as upregulated miR-191, the
analysis of which may be a promising low-invasive method for
efficient diagnosis of AD (20).
In addition, miRNAs participate in lncRNA-mediated AD progression
by serving as the downstream targets of lncRNAs (13). The present study identified that
miR-191 was directly targeted by MIR503HG and enriched in AD
tissue, which is in line with its expression in adipose-derived
mesenchymal stem cells that have stromal features (38). In addition, inhibition of miR-191
reversed the MIR503HG knockdown-induced the viability, migration,
invasion increased and apoptosis decreased in ESCs derived from
patients with AD to suppress AD progression, which signified that
inhibition of AD progression induced by restoration of MIR503HG
expression was miR-191 inhibition-dependent.
Additionally, a previous study discovered that the
activation of the Wnt/β-catenin signaling pathway contributes to
the pathogenesis of AD via EMT (39). In the nucleus of epithelial cells,
functioning as the main effector of the canonical Wnt signaling
which initiates EMT, β-catenin is key for tissue differentiation
during embryonic development (39)
and elevated β-catenin levels indicate activation of Wnt/β-catenin
signaling (25). By analyzing
β-catenin expression, the present study demonstrated that the
Wnt/β-catenin pathway was activated by MIR503HG knockdown but
suppressed by miR-191 inhibition, which also reversed MIR503HG
knockdown-induced Wnt/β-catenin activation, denoting that the
MIR503HG/miR-191 axis-mediated amelioration of AD resulted from
inhibited Wnt/β-catenin signaling.
In conclusion, the present findings demonstrated
that MIR503HG was lowly expressed in AD, and MIR503HG exerted a
suppressive impact on AD progression by targeting miR-191, which
was enriched in ESCs derived from patients with AD, to decrease
viability, migration, invasion and EMT while enhancing apoptosis.
This suppression may involve inhibition of the Wnt/β-catenin
pathway. The present study provided new insights into the
therapeutic strategies for AD. However, examinations in a clinical
situation or peripheral blood samples were not performed to
elucidate the role of MIR503HG or miR-191 in survival or prognosis
of patients with AD. Also, the size of samples was small. In
addition, the analysis of Wnt/β-catenin signaling pathway in future
studies requires MIR503HG overexpression and rescue
experiments.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Deyang Science
and Technology Bureau (grant no. 2020SZZ078).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and BC provided substantial contributions to
conception and design and wrote and revised the manuscript. YL, RL
and JL performed data acquisition, analysis and interpretation. All
authors have read and approved the final manuscript. XX and BC
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of People's Hospital of Deyang City (approval no.
GD202000524) and written informed consent from all participants was
obtained for experiments involving tissue.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferenczy A: Pathophysiology of
adenomyosis. Hum Reprod Update. 4:312–322. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lacheta J: Uterine adenomyosis:
Pathogenesis, diagnostics, symptomatology and treatment. Ceska
Gynekol. 84:240–246. 2019.PubMed/NCBI
|
|
3
|
Leyendecker G, Bilgicyildirim A, Inacker
M, Stalf T, Huppert P, Mall G, Böttcher B and Wildt L: Adenomyosis
and endometriosis. Re-visiting their association and further
insights into the mechanisms of auto-traumatisation. An MRI study.
Arch Gynecol Obstet. 291:917–932. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harada T, Khine YM, Kaponis A, Nikellis T,
Decavalas G and Taniguchi F: The impact of adenomyosis on women's
fertility. Obstet Gynecol Surv. 71:557–568. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Peric H and Fraser IS: The symptomatology
of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 20:547–555.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vannuccini S and Petraglia F: Recent
advances in understanding and managing adenomyosis. F1000Rese.
8(F1000)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khalifa MA, Atri M, Klein ME, Ghatak S and
Murugan P: Adenomyosis as a confounder to accurate endometrial
cancer staging. Semin Ultrasound CT MR. 40:358–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yuan H and Zhang S: Malignant
transformation of adenomyosis: Literature review and meta-analysis.
Arch Gynecol Obstet. 299:47–53. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vannuccini S, Luisi S, Tosti C, Sorbi F
and Petraglia F: Role of medical therapy in the management of
uterine adenomyosis. Fertil Steril. 109:398–405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang JF, Sun AJ, Xue W, Deng Y and Wang
YF: Aberrantly expressed long noncoding RNAs in the eutopic
endometria of patients with uterine adenomyosis. Eur J Obstet
Gynecol Reprod Biol. 199:32–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou C, Zhang T, Liu F, Zhou J, Ni X, Huo
R and Shi Z: The differential expression of mRNAs and long
noncoding RNAs between ectopic and eutopic endometria provides new
insights into adenomyosis. Mol Biosyst. 12:362–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liang N, Zhang W, Wang H, Shi W, Wang L
and Ma L: Levonorgestrel ameliorates adenomyosis via lncRNA
H19/miR-17/TLR4 pathway. Drug Des Devel Ther. 14:3449–3460.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu XY, Zhang J, Qi YH, Kong M, Liu SA and
Hu JJ: Linc-ROR promotes endometrial cell proliferation by
activating the PI3K-Akt pathway. Eur Rev Med Pharmacol Sci.
22:2218–2225. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fiedler J, Baker AH, Dimmeler S, Heymans
S, Mayr M and Thum T: Non-coding RNAs in vascular disease-from
basic science to clinical applications: scientific update from the
working group of myocardial function of the european society of
cardiology. Cardiovasc Res. 114:1281–1286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang H, Wu Z, Zhang Y, Xia T, Liu D, Cai J
and Ye Q: Identification and function analysis of a five-long
noncoding RNA prognostic signature for endometrial cancer patients.
DNA Cell Biol. 38:1480–1498. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng D, Jiang S, Chen J, Li J, Ao L and
Zhang Y: The increased lncRNA MIR503HG in preeclampsia modulated
trophoblast cell proliferation, invasion, and migration via
regulating matrix metalloproteinases and NF-κB signaling. Dis
Markers. 2019(4976845)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fu J, Dong G, Shi H, Zhang J, Ning Z, Bao
X, Liu C, Hu J, Liu M and Xiong B: LncRNA MIR503HG inhibits cell
migration and invasion via miR-103/OLFM4 axis in triple negative
breast cancer. J Cell Mol Med. 23:4738–4745. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Parrott E, Butterworth M, Green A, White
IN and Greaves P: Adenomyosis-a result of disordered stromal
differentiation. Am J Pathol. 159:623–630. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Borisov E, Knyazeva M, Novak V, Zabegina
L, Prisyazhnaya T, Karizkiy A, Berlev I and Malek A: Analysis of
reciprocally dysregulated miRNAs in eutopic endometrium is a
promising approach for low invasive diagnostics of adenomyosis.
Diagnostics (Basel). 10(782)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu YL, Zhang YX, Liu N, Liu H and Yuan YC:
LncRNA MIR503HG regulated cell viability, metastasis and apoptosis
of cervical cancer via miR-191/CEBPB axis. Eur Rev Med Pharmacol
Sci. 25:3200–3210. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Yanyan M, Mu L, Chen X and Zheng W:
The expression of Bcl-2 in adenomyosis and its effect on
proliferation, migration, and apoptosis of endometrial stromal
cells. Pathol Res Pract. 215(152477)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, Lu
X, Zhao Y, Luo F and Wang B: MicroRNA-191, by promoting the EMT and
increasing CSC-like properties, is involved in neoplastic and
metastatic properties of transformed human bronchial epithelial
cells. Mol Carcinog. 54 (Suppl 1):E148–E161. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Feng T, Wei S, Wang Y, Fu X, Shi L, Qu L
and Fan X: Rhein ameliorates adenomyosis by inhibiting NF-κB and
β-Catenin signaling pathway. Biomed Pharmacother. 94:231–237.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vercellini P, Bonfanti I and Berlanda N:
Adenomyosis and infertility: Is there a causal link? Expert Rev
Endocrinol Metab. 14:365–367. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hashimoto A, Iriyama T, Sayama S, Nakayama
T, Komatsu A, Miyauchi A, Nishii O, Nagamatsu T, Osuga Y and Fujii
T: Adenomyosis and adverse perinatal outcomes: Increased risk of
second trimester miscarriage, preeclampsia, and placental
malposition. J Matern Fetal Neonatal Med. 31:364–369.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shin YJ, Kwak DW, Chung JH, Kim MY, Lee SW
and Han YJ: The risk of preterm births among pregnant women with
adenomyosis. J Ultrasound Med. 37:1937–1943. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aleksandrovych V, Basta P and Gil K:
Current facts constituting an understanding of the nature of
adenomyosis. Adv Clin Exp Med. 28:839–846. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dueholm M: Uterine adenomyosis and
infertility, review of reproductive outcome after in vitro
fertilization and surgery. Acta Obstet Gynecol Scand. 96:715–726.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fiedler J, Breckwoldt K, Remmele CW,
Hartmann D, Dittrich M, Pfanne A, Just A, Xiao K, Kunz M, Müller T,
et al: Development of long noncoding RNA-based strategies to
modulate tissue vascularization. J Am Coll Cardiol. 66:2005–2015.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Muys BR, Lorenzi JC, Zanette DL, Lima e
Bueno Rde B, de Araújo LF, Dinarte-Santos AR, Alves CP, Ramão A, de
Molfetta GA, Vidal DO and Silva WA Jr: Placenta-enriched LincRNAs
MIR503HG and LINC00629 decrease migration and invasion potential of
JEG-3 cell line. PLoS One. 11(e0151560)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, Zhang Q, Liu F, Zhang Z, Zou Y, Yang
B, Luo Y, Wang L and Huang O: Inhibition of formin like 2 promotes
the transition of ectopic endometrial stromal cells to epithelial
cells in adenomyosis through a MET-like process. Gene. 710:186–192.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cao X and Fan QL: LncRNA MIR503HG promotes
high-glucose-induced proximal tubular cell apoptosis by targeting
miR-503-5p/Bcl-2 pathway. Diabetes Metab Syndr Obes. 13:4507–4517.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zheng D, Duan H, Wang S, Xu Q, Gan L, Li J
and Dong Q: FAK regulates epithelial-mesenchymal transition in
adenomyosis. Mol Med Rep. 18:5461–5472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang C, Wang P, Mohammed A, Zhou Z, Zhang
S, Ni S and Tang Z: Function of adipose-derived mesenchymal stem
cells in monocrotaline-induced pulmonary arterial hypertension
through miR-191 via regulation of BMPR2. Biomed Res Int.
2019(2858750)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY,
Ahn JY, Broaddus RR, Taketo MM, Lydon JP, Leach RE, et al:
β-Catenin activation contributes to the pathogenesis of adenomyosis
through epithelial-mesenchymal transition. J Pathol. 231:210–222.
2013.PubMed/NCBI View Article : Google Scholar
|