Introduction
Respiratory diseases are one of the most common
causes of death worldwide (1) and
pose a significant burden on any healthcare system and society. The
spread of COVID-19 has further complicated the respiratory disease
network and exacerbated the challenges of social healthcare
resources (2). Accurate diagnosis
and effective treatment of respiratory diseases require an in-depth
understanding of their pathogenesis and the pathophysiology of
airway epithelial cells. However, the existing airway epithelial
models cannot meet the growing scientific needs, mainly due to the
lack of models and the poor efficiency of existing model
production, making the study of the basis and application of
pathogenic processes of respiratory diseases a difficult and
tedious task. Considering the structural and physiological
differences between animals and humans, using human airway
epithelial cells (hAECs) cultured in vitro rather than the animal
as an airway epithelial model seems to be an ideal choice.
Therefore, how to obtain and expand human airway epithelial cells
is bound to become a crucial scientific mission in the future.
Basal cell (BC) is considered the central and the
key to airway regeneration. As one of the airway progenitor cells
responsible for regeneration, BC can regenerate itself,
differentiate into other types of airway epithelial cells to
maintain airway homeostasis, and participate in emergency repair in
situations such as airway injury (3). Thus, the successful amplification of
BC is significant to the amplification of hAECs.
Researchers cultured BC in vitro by the
technique named conditional reprogramming (CR) (4,5). CR
involves the mouse fibroblast cell line 3T3-J2 as feeder layer
cells and culture medium containing fetal bovine serum (FBS). The
feeder cells 3T3-J2 and FBS affect the clinical use of hAECs
because of containing animal components foreign to humans. Even if
patients receive autologous cell therapy, media supplemented with
FBS will bring risks of triggering immune rejection reactions,
making cell therapy much more uncertain (6). Besides, the preparation of feeder
cells is a high workload (7), and
FBS contains a number of poorly defined components (e.g., growth
factors, antibodies, and other immunologically active substances)
with different component concentrations from different production
batches (8). Therefore, hAECs
should be cultured and expanded in SFM without feeder
co-culture.
In the present study, we tested a novel developed
SFM for the culture of hAECs. The novel SFM is designed to be able
to monoculture hAECs, without FBS and the help of feeder cells. We
used PneumaCult™-Ex medium (STEMCELL, Canada) and DMEM + FBS as the
control groups and evaluated the cell morphology, cell markers,
proliferating, and differentiating accordingly. We hypothesized
that hAECs could expand in the novel SFM without altering
phenotype, proliferation, and differentiation.
Materials and methods
Media and reagents
To prepare the serum-free medium, the following
culture media and reagents were used: MCDB 153 medium, 40 µg/ml
L-Histidine, 100 µg/ml L-Isoleucine, 30 µg/ml L-Methionine, 60
µg/ml L-Phenylalanine, 16 µg/ml L-Tryptophan, 100 µg/ml L-Tyrosine,
500 µg/ml Db-cAMP (N, N-Dibutyladenosine 3',5'-phosphoric acid), 10
ng/ml rFGF1 (Recombinant fibroblast growth factor 1), 1 ng/ml rEGF
(Recombinant epidermal growth factor), 20 ng/ml rIGF1 (Recombinant
insulin growth factor 1), 0.1 µg/ml Hydrocortisone (STEMCELL,
Canada), 4 µg/ml Nicotinamide, 5 µmol/l Blebbistatin (Nuwacell,
Anhui, China). If not specifically noted, all agents above were
purchased from Sigma-Aldrich, USA.
Dulbecco's modified eagle medium (DMEM) and fetal
bovine serum (FBS) were from Gibco, USA. PneumaCult-Ex and
PneumaCult-ALI medium were from STEMCELL, Canada.
Cell culture
hAECs were collected through bronchoscopic brushing
samples. All cell samples were obtained from Shanghai Jiao Tong
University Affiliated Sixth People's Hospital (Shanghai, China),
with patient informed consent. Table
SI includes basic patient information and pulmonary function
test results to illustrate the physiological and pathological
background of samples. Patient 1 is a 60 years old female diagnosed
with bronchiectasis and hypertension, who was sampled on
2022/07/22. Patient 2 is a 56 years old female diagnosed with
interstitial pneumonia and no comorbidities, who was sampled on
2022/07/25. Both patients had restrictive hypoventilation. Patient
2 was milder but had diffusion dysfunction and small airway
dysfunction. Both patients were sampled at the basal segment of the
lower lobe of the right lobe.
Samples were washed in pre-chilled wash buffer
(Ham's F12, 5% fetal bovine serum, 100 µg/ml pen strep, 100 µg/ml
Gentamycin, and 0.25 µg/ml Fungizone) and were centrifuged at 1,000
rpm, 4̊C for 10 min and the supernatant was discarded. The
centrifugal process was repeated three times. The resulting cells
were plated into a 12-well plate (Corning, NY, USA) in the
serum-free medium containing 100 µg/ml pen strep and 1 ug/ml
Blebbistatin (Nuwacell, Anhui, China). The plates were designated
as passage 0 (P0) and were transferred into a 37̊C, 5%
CO2 saturated humidity incubator for further culturing.
The medium was changed every two days, and the cells were passaged
at a 1:1 to 1:2 ratio when they reached 80-90% confluency.
Brushing samples carry various cells, including
blood cells. Due to the selectivity of the medium, the unwanted
cell groups will fade by themselves during the culturing (usually
P0 to P2). Only basal cells are suitable to survive and thrive in
the medium. In order to avoid the unwanted cell groups affecting
experiment results, the subsequent experiments do not apply cells
before P3 unless necessary.
CCK-8 cell proliferation assay
hAECs obtained from P3 were seeded on 96-well plates
(Corning, NY, USA) at 6x103 cells/cm2 and
incubated at 37̊C in 5% CO2. At 24, 48, 72, 120, 168,
and 216 h after cell seeding, hAECs proliferation was measured by
CCK-8 assay. The old supernatant was replaced by WST-8 and fresh
medium at a 1:10 ratio. WST-8 refers to
2-(4-indophenyl)-3-(4-nitrophenyl)-5-(2,4-
disulpho-phenyl)-2H-tetrazolium monosodium salt (Beyotime
Biotechnology, Beijing, China). The processed plate was incubated
at 37̊C in 5% CO2 for 1.5 h. Staining intensity in the
medium was measured by determining the absorbance at 450 nm, and
the data were expressed as ratios of the control value. The data
are collected from three independent experiments performed in
duplicate. The proliferation curve was depicted by Graphpad Prism
8.
Immunohistochemical and
immunofluorescent analysis
Cells from P1, P3, P5, and P7 were fixed in 4% (w/v)
paraformaldehyde for 8 min, then washed with phosphate-buffered
saline (PBS). Cells were proceeded to permeabilization by 0.2%
Triton-X for 15 min, washed with PBS. The processed cells were
blocked with PBST (0.05% Tween-20, 5% FBS in PBS) for 1 h and then
incubated with primary antibodies diluted in PBS overnight at 4̊C.
Following incubation with primary antibodies, cells were rinsed
three times in PBS and incubated with a solution of secondary
antibodies diluted at 1:400 in PBS for 1.5 h in the dark. After
three further washes in PBS, 4',6-Diamidino-2-phenylindole (DAPI,
MilliporeSigma) diluted at 1:500 was added for 5 min to stain cell
nuclei. Afterward, the cells underwent confocal microscopy using a
laser scanning confocal microscope by Olympus FV1200. Images were
analyzed and processed by FV10-ASW 4.2 Viewer and Adobe Photoshop
19.0.
The following antibodies and dilutions were used:
mouse anti-P63 (ab735; Abcam, UK) at 1:200; mouse uteroglobin
monoclonal antibody (MA5-17170; Thermo Fisher Scientific, USA) at
1:200; rabbit anti-KRT5 (MA5-14473; Thermo Fisher Scientific, USA)
at 1:200; rabbit anti-KI67 (ab16667; Abcam, UK) at 1:250; mouse
anti-CDKN2A/P16 (sc-1661; Santa Cruz, USA); rabbit anti-P21
Waf1/Cip1 (#2947, Cell signaling Technology, USA); goat anti-mouse
Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 555 (Thermo Fisher
Scientific) at 1:400.
As for the immunofluorescence of air-liquid
interface (ALI) culture mentioned later, the following antibodies
and dilutions were used: mouse anti-acetylated tubulin (T7451;
Sigma, USA) at 1:200; mouse uteroglobin monoclonal antibody
(MA5-17170; Thermo Fisher Scientific, USA) at 1:200; rabbit
anti-MUC5AC (ab198294; Abcam, UK) at 1:200; goat anti-mouse Alexa
Fluor 488, goat anti-rabbit Alexa Fluor 555, and goat anti-mouse
Alexa Fluor 555 (Thermo Fisher Scientific) at 1:400.
Air-liquid interface (ALI)
culture
Cells from P3 were seeded at high density
(5*105/cm2) on Costar 6.5 mm Transwell, 0.4
µm Pore Polyester Membrane Inserts. After the seeding cells reached
full confluency, the proliferative medium was switched to the
differentiation medium (PneumaCult-ALI Medium, STEMCELL, Canada) to
induce mucociliary differentiation. Subsequently, the apical medium
was removed, and the cells received nutrients only from the
basolateral side (air-liquid interface condition) for 3 weeks. When
the differentiation was fully performed, pick up the Transwell from
the culturing plate. Cut a circle carefully at the bottom membrane
with a sterile surgical blade and then separate the membrane. The
harvested layers were fixed in 4% (w/v) paraformaldehyde and were
paraffin-sectioned to make the monolayer slices of ALI tissue. The
slices will show the sagittal plane or coronal plane of the
membranes. All cells are incubated under the circumstances of 37̊C
and 5% CO2. The cultured ALI tissues were further
analyzed by immunohistochemical and immunofluorescent analysis.
Statistical analysis
All experiments were repeated at least three times.
The data are shown as the average ± standard deviation. Comparisons
between groups were analyzed using a t-test. Statistical analyses
were performed using SPSS 20.0 and Graphpad Prism 8. P-values
<0.05 were considered to indicate statistically significant
differences.
Cell counting and nuclei mean area measuring were
performed via ImageJ. Transform the DAPI image into the 8-bit
format and set the threshold by the ‘Auto’ option without any other
adjustments. Use the ‘Fill holes’ tool to fill the gaps in
incomplete cells. Then use the tool of ‘Watershed’ to split
overlapping cells. At last, use the tool ‘Analyze Particles.’ The
number of cells and the nuclei size is calculated automatically by
ImageJ.
Results
hAECs expands similarly in the novel
SFM and PneumaCult-Ex
Cell samples from bronchoscopic brushing were seeded
in the novel SFM and STEMCELL PneumaCult-Ex medium, which was taken
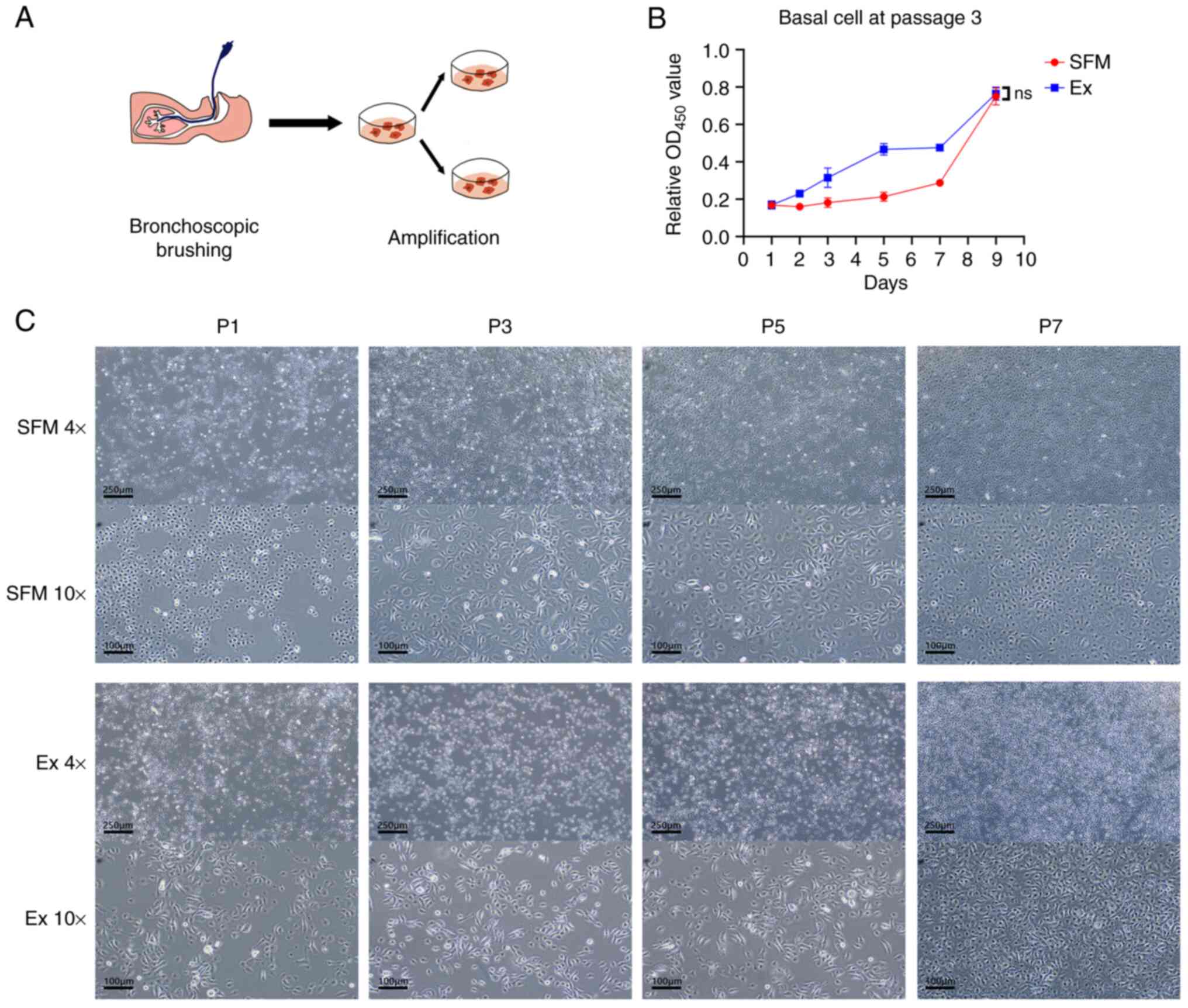
as a control group (Fig. 1A).
hAECs exhibited similar, consistent morphology at every passage,
generally showing the shape of cobblestones (Fig. 1C). Newly seeded cells appeared
round initially and began to sprawl into cobblestones-shape. Part
of the cells showed a more pointed form, which was like a spindle.
A proportion of cells in the novel SFM appeared in a larger form
with abundant cytoplasm and large nuclei (Table SII). At late passage, white
vesicles appeared in the cytoplasm of cells in Ex medium.
Previous research (9) shows basal cells cultured by CRC
retained colony-forming efficiency at P3. Also, during the early
culturing phase (P0-P2), unrelated cell groups may interfere with
the CCK-8 assay result. When the culturing progressed to P3, there
were ample cell materials for the assay. Thus, we applied hAECs
from P3 to depict the proliferation curve of cells in the novel SFM
and Ex medium (Fig. 1B). From day
1 to day 7, hAEC in the novel SFM grows relatively slowly compared
to Ex medium. However, on day 9, the result showed no significant
difference in proliferation capacity between hAECs culturing in the
novel SFM and Ex medium (P=0.7822).
To clearly illustrate the novel SFM's proliferating
ability, we seeded cells from P3 in the novel SFM and DMEM + FBS
(Fig. S1). The novel SFM expanded
hAECs successfully, whereas DMEM + FBS group can hardly perpetuate
the cell lineage (Fig. S1C). The
CCK-8 assay result validated the culture result. The difference in
the number of cells between the two groups on day 5 was
statistically significant (P=0.0024) (Fig. S1B).
As shown in Table
I, hAECs in novel SFM proliferated at a relatively constant
rate during P0 to P7. hAECs in Ex medium had a slow starter but
thrived from P1 to P4. Cells in Ex medium had a shorter duplication
time than the novel SFM during P1 to P4. However, the novel SFM had
no significant difference with Ex medium in proliferating speed
after P4. The proliferation decelerated in the experimental and
control groups at late passage.
 | Table IDuplication time between passages. |
Table I
Duplication time between passages.
| | SFM | Ex |
|---|
| Passage | Patient 1 | Patient 2 | Patient 1 | Patient 2 |
|---|
| P0 | 7 days | 7 days | 10 days | 7 days |
| P1 | 6 days | 7 days | 4 days | 3 days |
| P2 | 6 days | 8 days | 3 days | 4 days |
| P3 | 7 days | 7 days | 3 days | 3 days |
| P4 | 9 days | 6 days | 4 days | 5 days |
| P5 | 7 days | 7 days | 6 days | 8 days |
| P6 | 6 days | / | 6 days | |
| P7 | 7 days | / | 6 days | |
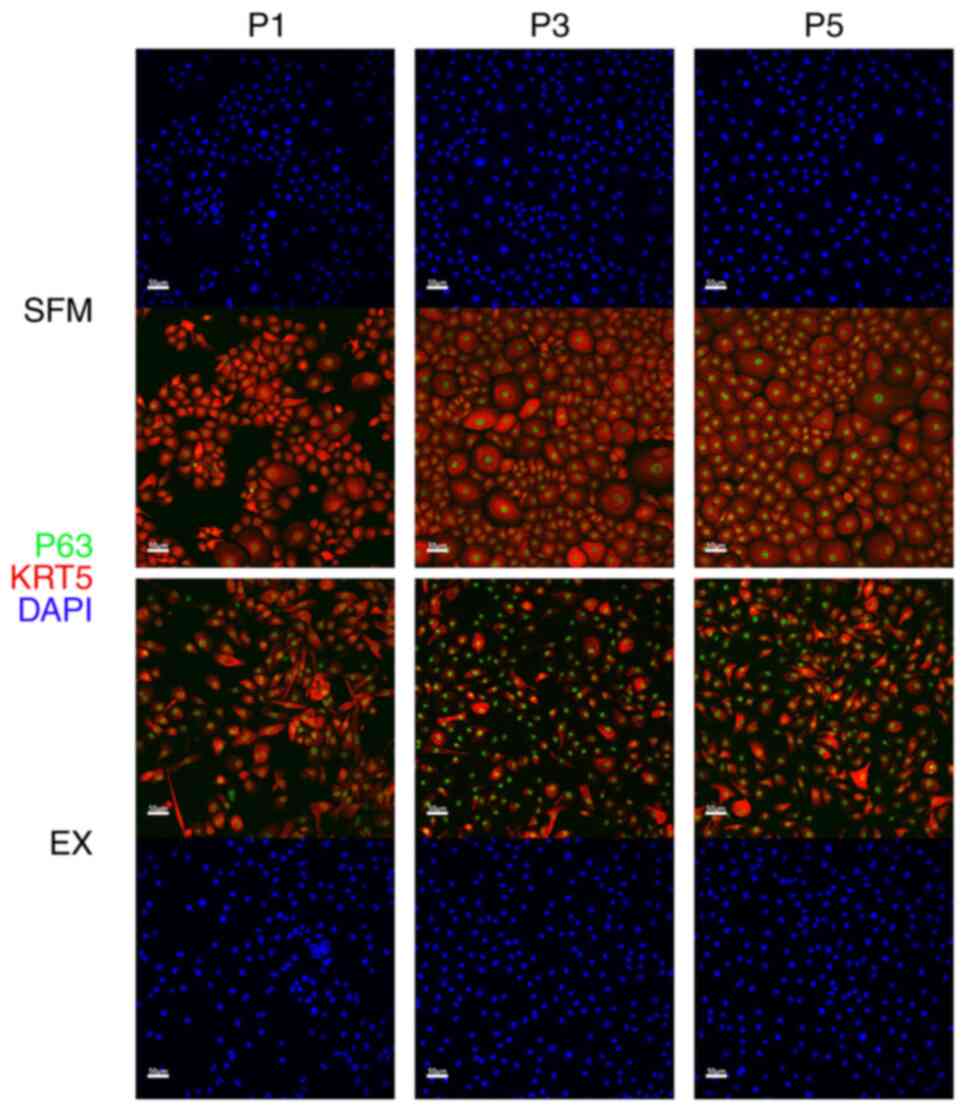
Immunofluorescent results show basal
cell markers strongly positive
In passages 1, 3, and 5, we used hAECs cultured from
the monoculture system to conduct immunohistochemical and
immunofluorescent analysis to identify whether the novel SFM can
maintain the characteristics of hAECs, especially basal cells. As
mentioned above in the Introduction, the success of proliferating
BCs is of great significance to the amplification of hAECs.
Staining for BC marker (P63+KRT5+) was
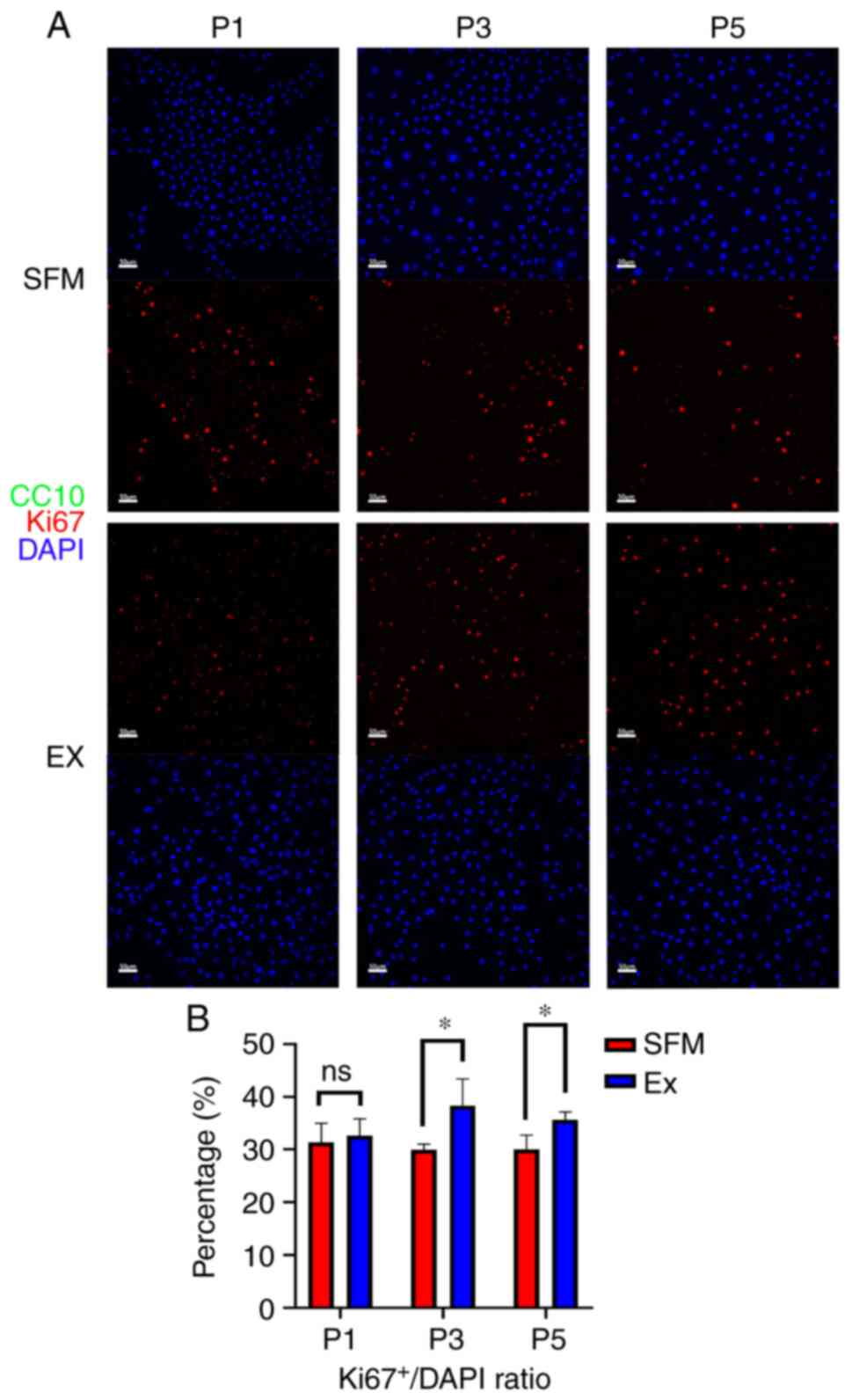
strongly positive in both novel SFM or Ex medium (Fig. 2). The proliferating marker Ki67 was
also positive in both groups (Fig.
3A). Compared with 32, 38, and 36% in the Ex medium, 31, 30,
and 30% of P63+KRT5+ BCs showed Ki67 positive
in the novel SFM in passages 1, 3, and 5. (Fig. 3B). CC10, a marker representing club
cells, was utterly negative in both groups.
hAECs differentiated into an
airway-like structure on the air-liquid interface (ALI)
We asked further whether hAECs from the novel SFM
can retain the differentiate ability. We chose a three-dimensional
platform air-liquid interface (ALI) to investigate possible
differences between the novel SFM and Ex medium. Cultured on ALI
successfully, the tissues were sent to subsequent
immunofluorescence. The result showed that both hAECs from the
novel SFM and Ex medium could differentiate into an airway-like
structure, which consists of ciliated cells (Acetylated
tubulin+), goblet cells (MUC5AC+), and club
cells (CC10+) (Fig.
4A).
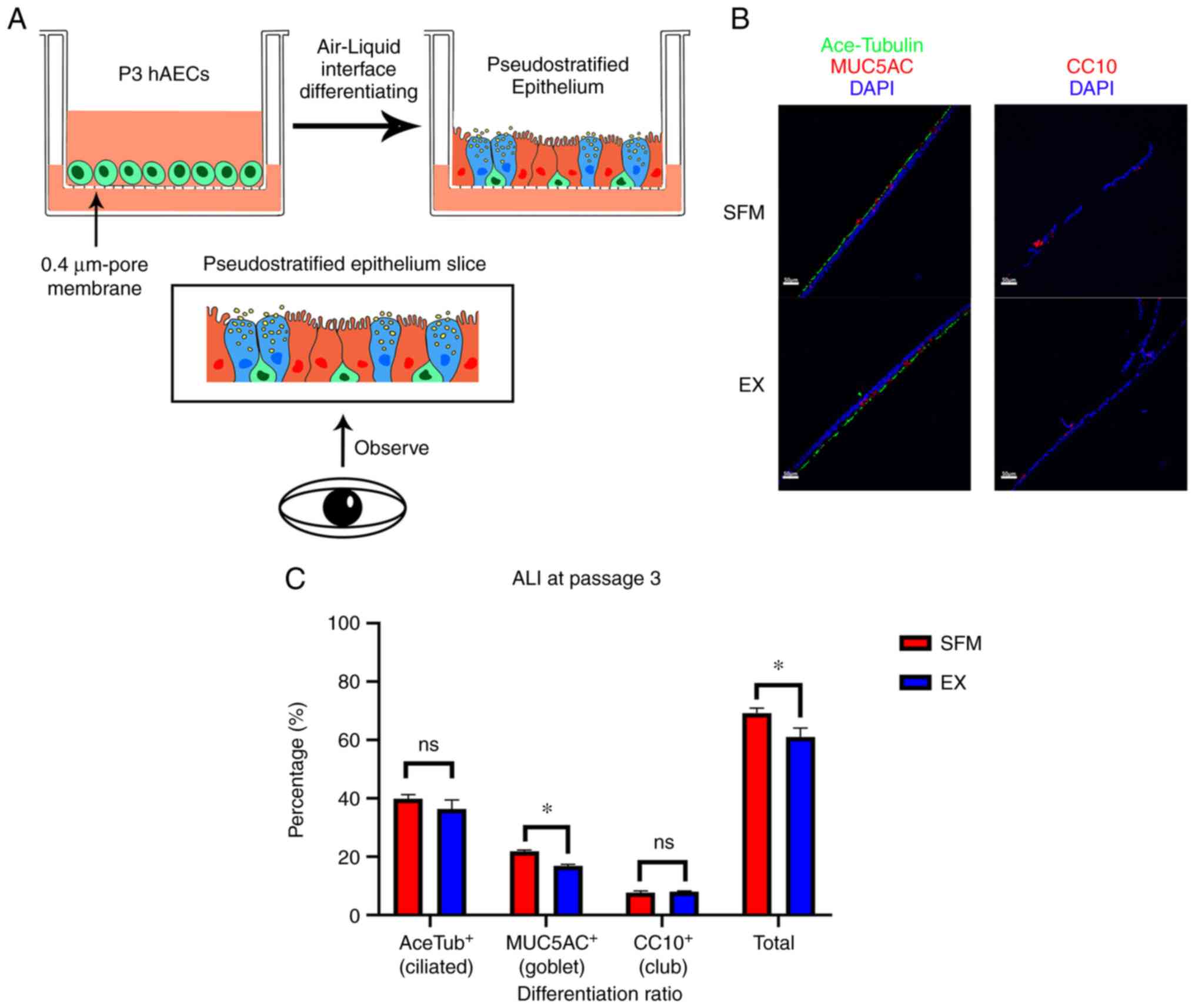 | Figure 4Differentiating results and
immunofluorescent results of ALI. (A) General procedures of ALI
culturing. hAECs from P3 were seeded in Transwell assay with 0.4-µm
pore polyester membrane. When the ALI culturing was fully
performed, the differentiated pseudostratified epithelium tissue
(basal cell, green; ciliated cell, red; goblet cell, blue) was
processed to be sliced. The slices show the sagittal plane or
coronal plane of the membranes. (B) Fluorescence micrographs of the
differentiation conditions of ALI tissue at day 21. Cell sources of
ALI tissues were basal cells at passage 3 cultured from the novel
SFM and Ex medium, respectively. Acetylated tubulin, green; MUC5AC,
red left; CC10, red right; DAPI, blue. Scale bar, 50 µm. (C)
Differentiation ratio of ALI tissue. *P<0.05. Ns, no
statistical significance; ALI, air-liquid interface; hAECs, human
airway epithelium cells; SFM, serum-free medium; Ex, PneumaCult-Ex
medium. |
The ciliated cells (green) formed a line on the top
layer, with goblet cells (red, left) forming niches. Sporadic club
cells (red, right) lay on the bottom side of the tissue.
Differentiated tissues presented similar cell composition: 35~40%
ciliated cells (39.8% SFM vs. 36.3% Ex), 15~22% goblet cells (21.8%
SFM vs. 16.8% Ex), and 8% club cells (7.7% SFM vs. 8.1% Ex)
(Fig. 4B). The composition is
comparable with the typical conducting airway differentiation cell
ratio.
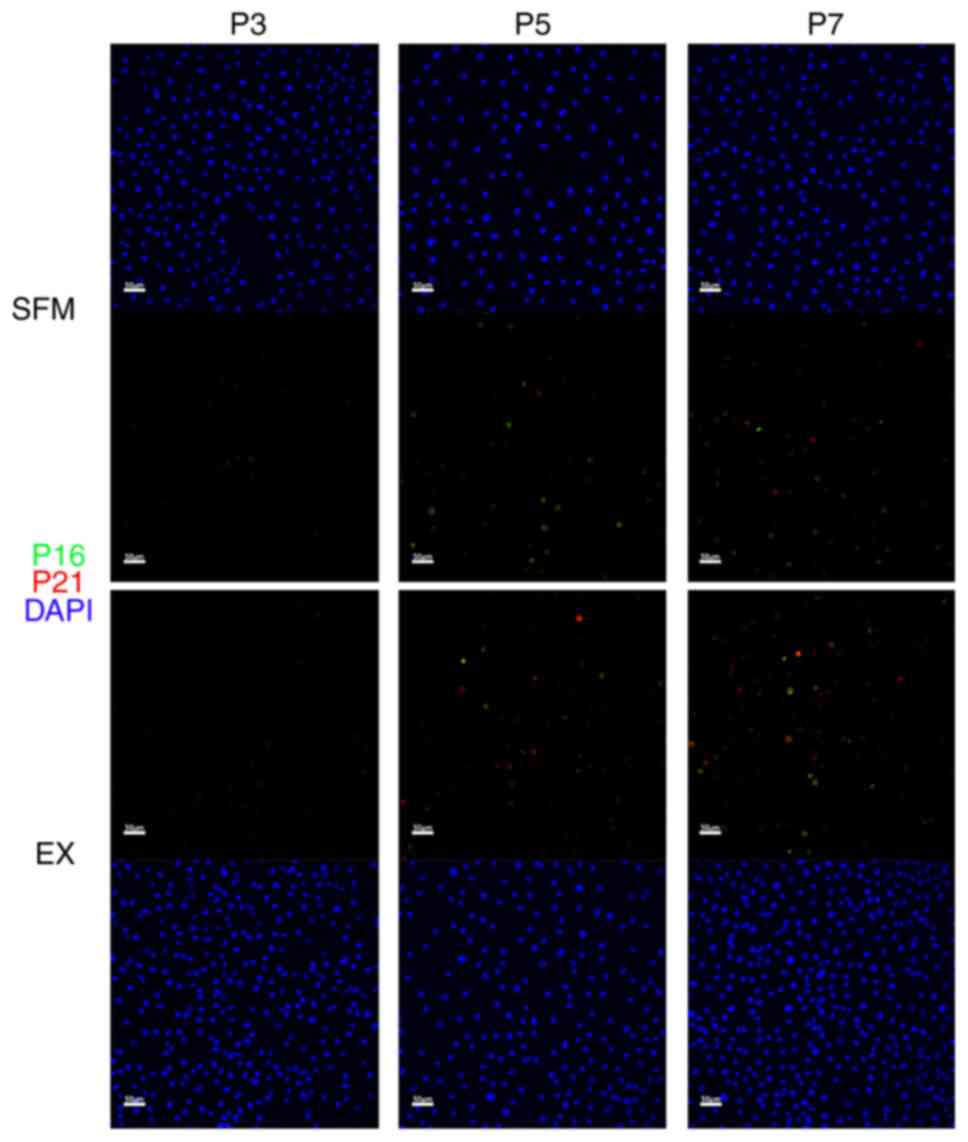
Immunofluorescent results show cell
senescence may initiate after passage 3
To investigate whether cell senescence will appear
in hAECs and when it will appear, we stained P16 and P21 markers
(both are senescence-indicating markers) in P3, P5, and P7 using
cells in the experimental and the Ex control group (Fig. 5). The immunofluorescent results
show barely any P16 or P21 positive cells in passage 3. P16 or P21
positive cells were found in passages 5 and 7, and there were more
P16 or P21 positive cells in passage 7 than in passage 5.
Discussion
The novel serum-free medium provides a new
alternative to amplify human airway epithelium cells. Compared with
other media used for hAECs amplification, the novel SFM has several
characteristics: 1) clear and well-defined components; 2) low cost
for ingredients; 3) simple and easy culturing procedure, no need to
coating or prepare feeder cells; 4) able to maintain proliferating
and differentiating ability 5) can retain linage marker consistency
(4,5).
Other culturing protocols for hAECs usually include
the application of FBS and feeder cells. On the one hand, FBS
contains a number of poorly defined components (e.g., growth
factors, antibodies, and other immunologically active substances).
Different production batches of FBS contain different
concentrations of components (8).
Moreover, FBS contains animal-origin components which are foreign
to humans. Even if patients receive autologous cell therapy, cells
cultured in a medium supplemented with FBS still carry the risk of
triggering immune rejection reactions, making cell therapy much
more uncertain (6). On the other
hand, feeder co-culture gives a much higher workload compared to
monoculture because of the necessity of preparing feeder cells
(7). Before co-culturing is
launched, feeder cells need to be expanded and adjusted for cell
status. The irradiation or mitomycin treatment is also required to
stop feeder cell proliferation. Feeder cells need to be removed by
manual or culture media change to prevent contamination of
animal-origin components. To diminish the workload of preparing
feeder cells, a conditioned medium after co-incubation with
irradiated feeder cells has been tested, but the result turned out
not to be as effective as direct co-culturing with feeder cells
(10). Although most feeder cells
can be manually removed during the culture process, any slight of
very few feeder cells can still render cell agents potentially
heterogeneous (11). Therefore,
for hAECs, monoculture using a serum-free medium is recommended for
high safety and stability.
Due to the reasons above, we have tested various
combinations and developed the novel SFM. Based on the basic MCDB
153 medium, we supplemented high amino acid supplement and Db-cAMP,
rFGF1, rEGF, rIGF1, hydrocortisone, and nicotinamide. The high
amino acid supplement includes histidine, isoleucine, methionine,
phenylalanine, tryptophan, and tyrosine, which are all essential
amino acids. Cell proliferation needs the support of essential
amino acids. Significantly when cells thrive, the consumption of
essential amino acids will increase. Isoleucine may play a more
important role because when isoleucine is deficient, the cell cycle
will be blocked at G or G/M phases, leading to proliferation
impairment (12).
Db-cAMP is involved in activating the second
messenger (cAMP) signal transduction pathway, whose activating can
lead to cell growth (13). Any
reagent which can activate the cAMP signal transduction pathway to
increase intracellular cAMP levels to activate cAMP-dependent
protein kinases is optional for components, including but not
limited to 8-Bromo-cAMP, 6-Bnz-cAMP, cAMPs-Sp, 8-CPT-Cyclic AMP,
Forskolin or IBMX. According to the previous research (14,15),
the increase of cAMP stimulates the growth via the activation of
protein kinase A (PKA), activation of the cAMP-responsive element
binding protein (CREB), and activation of the extracellular
signal-regulated kinases 1/2 (ERK1/2). The CREB family proteins are
well-characterized PKA substrates. This family is a group of the
basic leucine zipper (bZIP) superfamily transcription factors,
including CREB, cAMP-responsive element regulatory protein (CREM),
and transcriptional activator 1 (ATF1). Activated ATF1 and CREB can
form homodimer or heterodimer, bind to the cAMP response element
(CRE) in the promoter region of target genes and initiate gene
transcription, thereby regulating cell differentiation and
proliferation. cAMP can also activate Epac (Exchange protein
directly activated by cAMP). Epac proteins exert their diverse
biological functions either alone and/or in concert with PKA
(16). Epac was reported to have
effects on cell division and differentiating (17). However, The role of Epac in cell
division signaling by cAMP cannot be determined. Epac exerts
opposing effects even on the same cellular process (16). Whether Epac affects hAECs in
concert with/without PKA in proliferating and differentiating
positively or negatively still remains elusive.
FGF1 and EGF are potent growth factors that can
stimulate epithelial cell proliferation. They are both involved in
the receptor complex kinase and RTK-Ras protein signaling pathways
(18). IGF1 belongs to the insulin
factor family and is structurally and functionally similar to
insulin but has higher growth-promoting activity than insulin
(19). Hydrocortisone and
nicotinamide are common supplements for epithelial culture. They
can increase cell viability and maintain cell morphology (20,21).
Noticeably, there are no specific restrictions on the specific type
of components. Any component with similar effects is optional. The
supplemented ingredients above are all clear and well-defined. In
addition, because of the absence of feeder cells, the culturing
procedure using the novel SFM is simple and easy. Preparing feeder
cells or pre-coating is of no necessity for the novel SFM.
To investigate the culture potential of the novel
SFM, we used cell cultures and took the STEMCELL Ex medium and DMEM
+ FBS as control groups. The results show that serum-free medium
can expand hAECs successfully without involving FBS (Seen Fig. S1). Due to the poor practicability
of the DMEM + FBS combination, which can hardly keep basal cells
expanding, we chose STEMCELL PneumaCult-Ex medium as the control
group in the following research phases. The general morphology of
hAECs was consistent from the beginning to the end of the culture
process. However, white vesicles appeared in the cytoplasm of cells
at late passage (passage 3-5) in Ex medium (Fig. 1). We considered the phenomenon
might be the symbol of senescence and apoptosis, which is
consistent with the result that the percentage of Ki67+
basal cells dropped as the generation counts increased at late
culturing phase (Fig. 3).
For primary hAECs, it is natural to find the cell
expansion ability declines, representing as cell division slows
down and differentiation potential loss (22). Previous research (9) suggests basal cells cultured by CRC
methods retained colony-forming efficiency at least to passage 3.
To investigate whether cell senescence will appear in hAECs, we
stained P16 and P21 (Fig. 5). The
results suggested cell senescence may initiate after passage 3. We
supplement the novel SFM ROCK-inhibitor (Rho-associated coiled coil
protein kinase inhibitor, ROCKi) Blebbistatin to solve the problem.
According to previous studies (23-25),
ROCK-inhibitor can accelerate the cell cycle and inhibit apoptosis
and cell differentiation.
For the next step, we want to confirm whether cells
cultured in the different media have differences. We have conducted
cell marker immunofluorescence and found no significant difference,
suggesting that though the cells isolated from the samples have
morphological diversity in different media, they were all basal
cells
(P63+KRT5+KI67+CC10-).
The different medium formulas can be responsible for the
morphological diversity, but both medium managed to culture primary
hAECs into BCs. According to earlier research (26), KRT5 and P63 are markers showing
cell stemness. Differentiated cells or cells ready to differentiate
will lose these stemness markers. Approximately 30% of cells in the
Ex medium did not express the KRT5 marker, whereas 100% of cells in
the novel SFM were KRT5+. In other words, the novel SFM
can retain the basal cell marker better and thus has better
stemness retaining ability.
To ask further whether hAECs from the novel SFM can
retain the differentiating ability, we used a three-dimensional
differentiate platform air-liquid interface (ALI). Obviously, the
immunofluorescent result showed similar composition between tissues
cultured by the novel SFM or Ex medium. Notably, the composition of
ALI tissue is comparable with the cell proportion of conducting
airway (27). However,
differentiated cells from Ex medium account for only 60% (ideally,
it should be 70~80%) in ALI tissue. There were lesser goblet cells
in the Ex control group. A possible explanation is that the ALI
tissue from the Ex medium was not fully differentiated, or the
induction characteristics of the ALI medium should be considered.
In other words, hAECs cultured by the novel SFM or Ex medium can be
induced to differentiate into airway-like structure tissue,
implying adequate differentiating potential and the
de-differentiation effect of ROCKi.
There are still limitations and a few questions need
to be answered in this study. The reason for the relatively slow
growth speed in the early stage of the novel SFM remains unclear.
The main task in the future is to focus on increasing proliferating
cell ratio in the cell groups. Moreover, how to postpone cell
senescence and apoptosis and prolong the available duration are to
be addressed. It is also unknown how the cell physiology changes in
subsequent generations (such as P9) since we have terminated the
culture at P7.We should explore the effect of medium components and
specific mechanisms of hAECs in order to promote formula
improvements in future studies.
In Conclusion, the novel SFM is capable of culturing
hAECs. The hAECs cultured by the novel SFM can proliferate and
differentiate in vivo. The novel SFM does not change the
morphologic characteristics or biomarkers of hAECs. The novel SFM
has the potential for the amplification of hAECs for scientific
research and clinical application.
Supplementary Material
Cell culture results in the experiment
and the DMEM+FBS control group. (A) General design of the
experiment. Cell samples from bronchoscopic brushing were seeded in
the medium and subsequently amplificated. (B) Proliferation curve
of basal cells in the novel SFM and the DMFM + FBS group. (C)
Brightfield microscopy images of cell colonies in the novel SFM and
DMFM + FBS. Top row scale bar, 250 μm; bottom row scale bar,
100 μm. **P<0.01. DMEM, Dulbecco's modified
eagle medium; FBS, fetal bovine serum; SFM, serum-free medium.
Basic patient information.
Mean area of nuclei.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by China National Science
Foundation (grant nos. 81930001 and 81870005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD, QZ, ZL, SS and TR contributed to the study
concept and design. HD, QZ, ZL, SS, YL, JZ, CZ, YX and TR acquired,
analyzed and interpreted the data. HD, QZ, ZL and TR drafted the
manuscript. HD and QZ confirm the authenticity of all the raw data.
HD and QZ performed the statistical analysis. TR obtained funding.
HD, QZ and ZL provided administrative, technical and material
support. TR supervised the study. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Shanghai Jiao Tong University Affiliated Sixth People's
Hospital (approval no. 2020-152).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2017 Causes of Death Collaborators.
Global, regional, and national age-sex-specific mortality for 282
causes of death in 195 countries and territories, 1980-2017: A
systematic analysis for the global burden of disease study 2017.
Lancet. 392:1736–1788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Onyeaka H, Anumudu CK, Al-Sharify ZT,
Egele-Godswill E and Mbaegbu P: COVID-19 pandemic: A review of the
global lockdown and its far-reaching effects. Sci Prog.
104(368504211019854)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Parekh KR, Nawroth J, Pai A, Busch SM,
Senger CN and Ryan AL: Stem cells and lung regeneration. Am J
Physiol Cell Physiol. 319:C675–C693. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bukowy-Bieryllo Z: Long-term
differentiating primary human airway epithelial cell cultures: How
far are we? Cell Commun Signal. 19(63)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Butler CR, Hynds RE, Gowers KH, Lee Ddo H,
Brown JM, Crowley C, Teixeira VH, Smith CM, Urbani L, Hamilton NJ,
et al: Rapid expansion of human epithelial stem cells suitable for
airway tissue engineering. Am J Respir Crit Care Med. 194:156–168.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Naskou MC, Sumner SM, Chocallo A,
Kemelmakher H, Thoresen M, Copland I, Galipeau J and Peroni JF:
Platelet lysate as a novel serum-free media supplement for the
culture of equine bone marrow-derived mesenchymal stem cells. Stem
Cell Res Ther. 9(75)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hynds RE, Butler CR, Janes SM and
Giangreco A: Expansion of human airway basal stem cells and their
differentiation as 3D tracheospheres. Methods Mol Biol. 1576:43–53.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Savelli S, Trombi L, D'Alessandro D,
Moscato S, Pacini S, Giannotti S, Lapi S, Scatena F and Petrini M:
Pooled human serum: A new culture supplement for bioreactor-based
cell therapies. Preliminary results. Cytotherapy. 20:556–563.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee RE, Miller SM, Mascenik TM, Lewis CA,
Dang H, Boggs ZH, Tarran R and Randell SH: Assessing human airway
epithelial progenitor cells for cystic fibrosis cell therapy. Am J
Respir Cell Mol Biol. 63:374–385. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wolf S, Perez GF, Mukharesh L, Isaza N,
Preciado D, Freishtat RJ, Pillai D, Rose MC and Nino G: Conditional
reprogramming of pediatric airway epithelial cells: A new human
model to investigate early-life respiratory disorders. Pediatr
Allergy Immunol. 28:810–817. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Villa-Diaz LG, Ross AM, Lahann J and
Krebsbach PH: Concise review: The evolution of human pluripotent
stem cell culture: From feeder cells to synthetic coatings. Stem
Cells. 31:1–7. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McLean KJ and Jacobs-Lorena M: The
response of plasmodium falciparum to isoleucine withdrawal is
dependent on the stage of progression through the intraerythrocytic
cell cycle. Malar J. 19(147)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hannila SS and Filbin MT: The role of
cyclic AMP signaling in promoting axonal regeneration after spinal
cord injury. Exp Neurol. 209:321–332. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Al-Wadei HA, Takahashi T and Schuller HM:
Growth stimulation of human pulmonary adenocarcinoma cells and
small airway epithelial cells by beta-carotene via activation of
cAMP, PKA, CREB and ERK1/2. Int J Cancer. 118:1370–1380.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang H, Kong Q, Wang J, Jiang Y and Hua
H: Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol
Oncol. 9(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Grandoch M, Roscioni SS and Schmidt M: The
role of Epac proteins, novel cAMP mediators, in the regulation of
immune, lung and neuronal function. Br J Pharmacol. 159:265–284.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Borland G, Smith BO and Yarwood SJ: EPAC
proteins transduce diverse cellular actions of cAMP. Br J
Pharmacol. 158:70–86. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rabata A, Fedr R, Soucek K, Hampl A and
Koledova Z: 3D cell culture models demonstrate a role for FGF and
WNT signaling in regulation of lung epithelial cell fate and
morphogenesis. Front Cell Dev Biol. 8(574)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goshi N, Morgan RK, Lein PJ and Seker E: A
primary neural cell culture model to study neuron, astrocyte, and
microglia interactions in neuroinflammation. J Neuroinflammation.
17(155)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Darcy KM, Shoemaker SF, Lee PP, Ganis BA
and Ip MM: Hydrocortisone and progesterone regulation of the
proliferation, morphogenesis, and functional differentiation of
normal rat mammary epithelial cells in three dimensional primary
culture. J Cell Physiol. 163:365–379. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pu Q, Guo XX, Hu JJ, Li AL, Li GG and Li
XY: Nicotinamide mononucleotide increases cell viability and
restores tight junctions in high-glucose-treated human corneal
epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed
Pharmacother. 147(112659)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Martinovich KM, Iosifidis T, Buckley AG,
Looi K, Ling KM, Sutanto EN, Kicic-Starcevich E, Garratt LW, Shaw
NC, Montgomery S, et al: Conditionally reprogrammed primary airway
epithelial cells maintain morphology, lineage and disease specific
functional characteristics. Sci Rep. 7(17971)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu X, Krawczyk E, Suprynowicz FA,
Palechor-Ceron N, Yuan H, Dakic A, Simic V, Zheng YL, Sripadhan P,
Chen C, et al: Conditional reprogramming and long-term expansion of
normal and tumor cells from human biospecimens. Nat Protoc.
12:439–451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ligaba SB, Khurana A, Graham G, Krawczyk
E, Jablonski S, Petricoin EF, Glazer RI and Upadhyay G:
Multifactorial analysis of conditional reprogramming of human
keratinocytes. PLoS One. 10(e0116755)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu X, Wang S, Li M, Li J, Shen J, Zhao Y,
Pang J, Wen Q, Chen M, Wei B, et al: Conditional reprogramming:
Next generation cell culture. Acta Pharm Sin B. 10:1360–1381.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Girardet L, Cyr DG and Belleannée C:
Arl13b controls basal cell stemness properties and Hedgehog
signaling in the mouse epididymis. Cell Mol Life Sci.
79(556)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Basil MC, Katzen J, Engler AE, Guo M,
Herriges MJ, Kathiriya JJ, Windmueller R, Ysasi AB, Zacharias WJ,
Chapman HA, et al: The cellular and physiological basis for lung
repair and regeneration: Past, present, and future. Cell Stem Cell.
26:482–502. 2020.PubMed/NCBI View Article : Google Scholar
|