Introduction
Diabetic nephropathy (DN), a major contributor to
end-stage renal disease (ESRD), exhibited an increased morbidity
and mortality rate worldwide in the past few years (1,2). DN
accounts globally for 35-40% of all new clinical cases that need
dialysis therapy. DN features morphological and ultrastructural
changes in the kidney, such as glomerular hypertrophy, decreased
glomerular filtration and renal fibrosis, which make the
pathogenesis of DN complicated and poorly understood (3,4).
Podocytes, which are localized at the outer layer of the glomerular
basement membrane, are well-differentiated epithelial cells
constituting the glomerular filtration barrier (5). Notably, the aberrant structure and
function of podocytes are considered a predominant driver of DN
progression (6). Therefore,
focusing on investigating the molecular mechanisms involved in
podocyte injury is pivotal to finding novel therapies against
DN.
Diosgenin (DSG), a member of the spirostanol
steroidal compound family, is abundant in natural herbal medicinal
plants and exhibits various biological activities, including
cardiovascular protection (7),
anti-inflammatory (8) and
anticancer (9) activities. On this
basis, DSG has attracted widespread attention and has been widely
adopted for the management of several diseases, such as
cardiovascular diseases (10),
diabetes mellitus (11) and
osteoporosis (12). Furthermore,
DSG is implicated in the process of DN by protecting against
podocyte injury (13,14); therefore, preliminary studies on
its mechanism of action may confirm the possible application of DSG
in DN therapy.
As a primary sensor of cellular energy status,
AMP-activated protein kinase (AMPK) is highly conserved in all
eukaryotic species (15). In
addition, AMPK inhibits the expression of NF-κB by upregulating
sirtuin 1 (SIRT1) expression, thus minimizing the inflammatory
reaction (16). Moreover, the
AMPK/SIRT1/NF-κB signaling pathway has been indicated to be closely
associated with the progression of DN (17). Furthermore, a previous study
reported that DSG could activate AMPK, thus suppressing the
inflammatory response and ameliorating endothelial dysfunction
(18).
More importantly, hyperglycemia is involved in the
advancement and progression of DN (19); therefore, high glucose (HG) was
utilized in the present study to establish an in vitro DN
model in podocyte cells. The current study aimed to investigate the
efficacy of DSG in an in vitro DN podocyte model, as well as
explore the relationship between DSG and the AMPK/SIRT1/NF-κB
signaling pathway.
Materials and methods
Cell culture and treatment
The human podocyte CIHP-1 cell line (Ximbio) was
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Sigma-Aldrich;
Merck KGaA) at 37˚C with 5% CO2. Thereafter, cells were
treated with HG (30 mM D-glucose) for 48 h at 37˚C (20). Cells treated with normal glucose
(NG; 5 mM D-glucose) for 48 h at 37˚C are referred to the control
group. To further investigate the mechanism of DSG (cat. no.
ID0350; Beijing Solarbio Science & Technology Co., Ltd.), cells
were treated with compound C (CC; 10 µM; cat. no. IB0330; Beijing
Solarbio Science & Technology Co., Ltd.) for 2 h at 37˚C
(21). Mannitol (30 mmol/l; cat.
no. SM8120; Beijing Solarbio Science & Technology Co., Ltd.)
was used as osmotic control (20).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was measured using the CCK-8 assay
(cat. no. C0037; Beyotime Institute of Biotechnology). CIHP-1 cells
were seeded into 96-well plates (1x103 cells/well) and
incubated with different concentrations of DSG (0.1, 1.0 and 10.0
µM) for 24 h at 37˚C. Subsequently, the CCK-8 reagent was added to
the cells and incubated for 2 h. Finally, the optical density was
measured at 450 nm using a microplate reader (BioTek Instruments,
Inc.).
Lactate dehydrogenase (LDH) assay
LDH is a stable cytoplasmic enzyme that is found in
all cells, and measuring the activity of cytoplasmic enzymes
released by damaged cells is a common method for determining
cytotoxicity (22). CIHP-1 cells
were cultured into a 96-well plate up to 80-90% confluence.
Following the different treatments in the respective groups as
aforementioned, the plate was centrifuged at 400 x g for 5 min at
4˚C. LDH assay (cat. no. C0016; Beyotime Institute of
Biotechnology) was utilized for the determination of LDH activity
according to the manufacturer instructions. A total of 100 µl
supernatant was added to 100 µl of cytotoxicity assay reagent in a
96-well measurement plate. The LDH activity was determined using
the colorimetric detection of sodium pyruvate reduction. Absorbance
was measured at 490 nm using a microplate reader (BioTek
Instruments, Inc.).
ELISA
CIHP-1 cells were seeded into a 24-well plate
(5x104 cells/well) and treated as previously indicated.
Thereafter, cells were centrifuged at 1,000 x g for 5 min at 4˚C.
The supernatant was collected and used for ELISA. Tumor necrosis
factor-α (TNF-α; cat. no. PT518; Beyotime Institute of
Biotechnology), interleukin-1β (IL-1β; cat. no. PI305; Beyotime
Institute of Biotechnology) and interleukin-6 (IL-6; cat. no.
PI330; Beyotime Institute of Biotechnology) ELISA kits were used.
The levels of the inflammatory cytokines TNF-α, IL-1β and IL-6 were
determined by measuring the optical density at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
TUNEL assay
To investigate the effects of DSG on the apoptosis
of podocyte cells, a TUNEL assay (cat. no. C1082; Beyotime
Institute of Biotechnology) was employed. Briefly, CIHP-1 cells
(2x104 cells/well) seeded into a 24-well plate were
fixed with 4% paraformaldehyde for 15 min and permeabilized in
0.25% Triton X-100 for 20 min at room temperature. After rinsing in
phosphate-buffered saline (PBS; cat. no. C0221A; Beyotime Institute
of Biotechnology) three times, the cells were labeled with TUNEL
reagent for 60 min at 37˚C, according to the manufacturer's
instructions. DAPI staining solution (1 mg/ml; cat. no. C1002;
Beyotime Institute of Biotechnology) was utilized to counterstain
the cells at 37˚C for 30 min, which were then mounted in an
anti-fade reagent (Beijing Solarbio Science & Technology Co.,
Ltd.). Finally, the images of TUNEL-positive cells were observed in
five fields of view selected at random under a fluorescence
microscope.
Western blotting
Total protein was extracted from CIHP-1 cells using
RIPA lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) and then quantified using a bicinchoninic acid protein assay
kit (cat. no. P0010S; Beyotime Institute of Biotechnology). Total
protein (30 µg/lane) was separated on an 8% gel using SDS-PAGE and
then transferred onto a PVDF membrane. After blocking with 5%
skimmed milk for 2 h at room temperature, membranes were incubated
with primary antibodies against Bcl-2 (1:1,000; cat. no. ab32124;
Abcam), Bax (1:1,000; cat. no. ab32503), cleaved caspase-3 (1:500;
cat. no. ab32042), caspase-3 (1:5,000; cat. no. ab32351), cleaved
caspase-9 (1:1,000; cat. no. ab2324), caspase-9 (1:2,000; cat. no.
ab32068), nephrin (1:1,000; cat. no. ab216341), phosphorylated
(p)-AMPK (1:1,000; cat. no. ab92701), AMPK (1:1,000; cat. no.
ab32047), SIRT1 (1:1,000; cat. no. ab189494), NF-κB (1:1,000 cat.
no. ab16502) and GAPDH (1:2,500 cat. no. ab9485) (all from Abcam)
at 4˚C overnight. On the following day, membranes were incubated
with HRP-conjugated secondary antibody (1:2,000; cat. no. ab6721;
Abcam) at room temperature for 2 h. Protein bands were visualized
using the Immobilon Western Chemiluminescent HRP substrate (cat.
no. WBKLS0500; EMD Millipore) and quantified using Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc.).
Glucose uptake evaluation
CIHP-1 cells (5x105) were washed with PBS
and incubated in RPMI-1640 medium without serum for 2 h at 37˚C.
Subsequently, cells were rinsed in pre-warmed Krebs-Ringer
phosphate solution (Sigma-Aldrich; Merck KGaA) containing 2% FBS
for 30 min at 37˚C. After being treated with 500 nM insulin
(Sigma-Aldrich; Merck KGaA) for 20 min at 37˚C, cells were further
incubated with [3H]2-deoxy-D-glucose (2-DOG; 50 µmol/l;
Sigma-Aldrich: Merck KgaA) (23)
for 20 min at 37˚C. Subsequently, ice-cold PBS buffer containing
cytochalasin B (10 µmol/l; cat. no. C8080; Beijing Solarbio Science
& Technology Co., Ltd.) was added to block the non-specific
uptake for 5 min at 37˚C. Finally, the concentration of
2-deoxy-D-glucose-6-phosphate (2-DG6P) was measured at 450 nm using
a microplate reader (BioTek Instruments, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from CIHP-1 cells seeded into a 6-well
plate (6x104 cells/well) was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). Total
RNA was reverse transcribed into cDNA using the PrimerScript
reverse transcriptase (Takara Biotechnology Co., Ltd.) according to
the manufacturer's protocol. qPCR analysis was performed on a 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using SYBR green Premix Ex Taq II (Qiagen GmbH), according to
the manufacturer's protocol. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95˚C for 5 min;
followed by 40 cycles of denaturation at 95 ˚C for 45 sec,
annealing at 50˚C for 45 sec and elongation at 72˚C for 45 sec; and
a final extension step at 72˚C for 10 min. GAPDH was used as an
internal reference gene and the relative gene expression was
determined using the 2-ΔΔCq method (24). The following primer pairs were used
for qPCR: Nephrin forward, 5'-CTGCCTGAAAACCTGACGGT-3' and reverse,
5'-GACCTGGCACTCATACTCCG-3'; and GAPDH forward,
5'-TGTGGGCATCAATGGATTTGG-3' and reverse,
5'-ACACCATGTATTCCGGGTCAAT-3'.
Statistical analysis
All data were obtained from three independent
repeats and presented as the mean ± standard deviation. The data
analysis was performed using GraphPad Prism 8.0 software (GraphPad
Software; Dotmatics). Normal distribution and variance
heterogeneity were confirmed using the Shapiro-Wilk and
Brown-Forsythe tests, respectively. The comparisons among multiple
groups were performed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
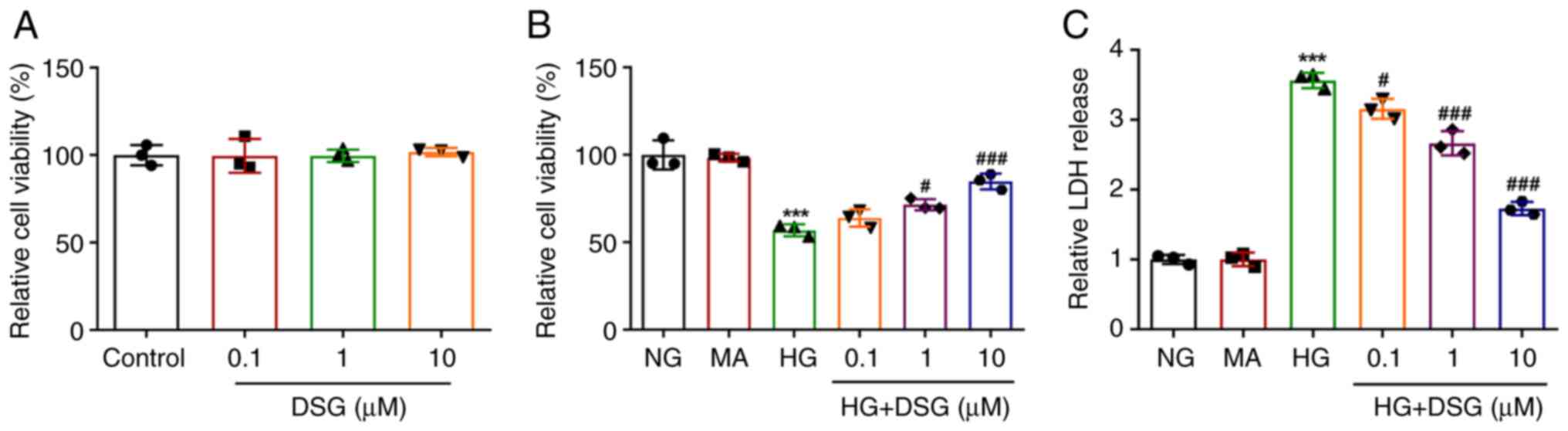
DSG enhances the viability of
HG-induced podocyte cells
CCK-8 assay was performed to investigate the impact
of DSG on the viability of HG-induced podocyte cells. Cell
viability of DSG-treated podocyte cells was not different compared
with that in the control group (Fig.
1A). Compared with that in the NG group, the viability of
podocyte cells was significantly decreased after HG induction;
however, the decreased viability in HG-induced podocyte cells was
counteracted by DSG treatment in a concentration-dependent manner
(Fig. 1B). HG induction increased
the release of LDH in comparison with that in the NG group
(Fig. 1C). In addition, DSG
decreased the release of LDH in HG-induced podocyte cells compared
with that in the NG group.
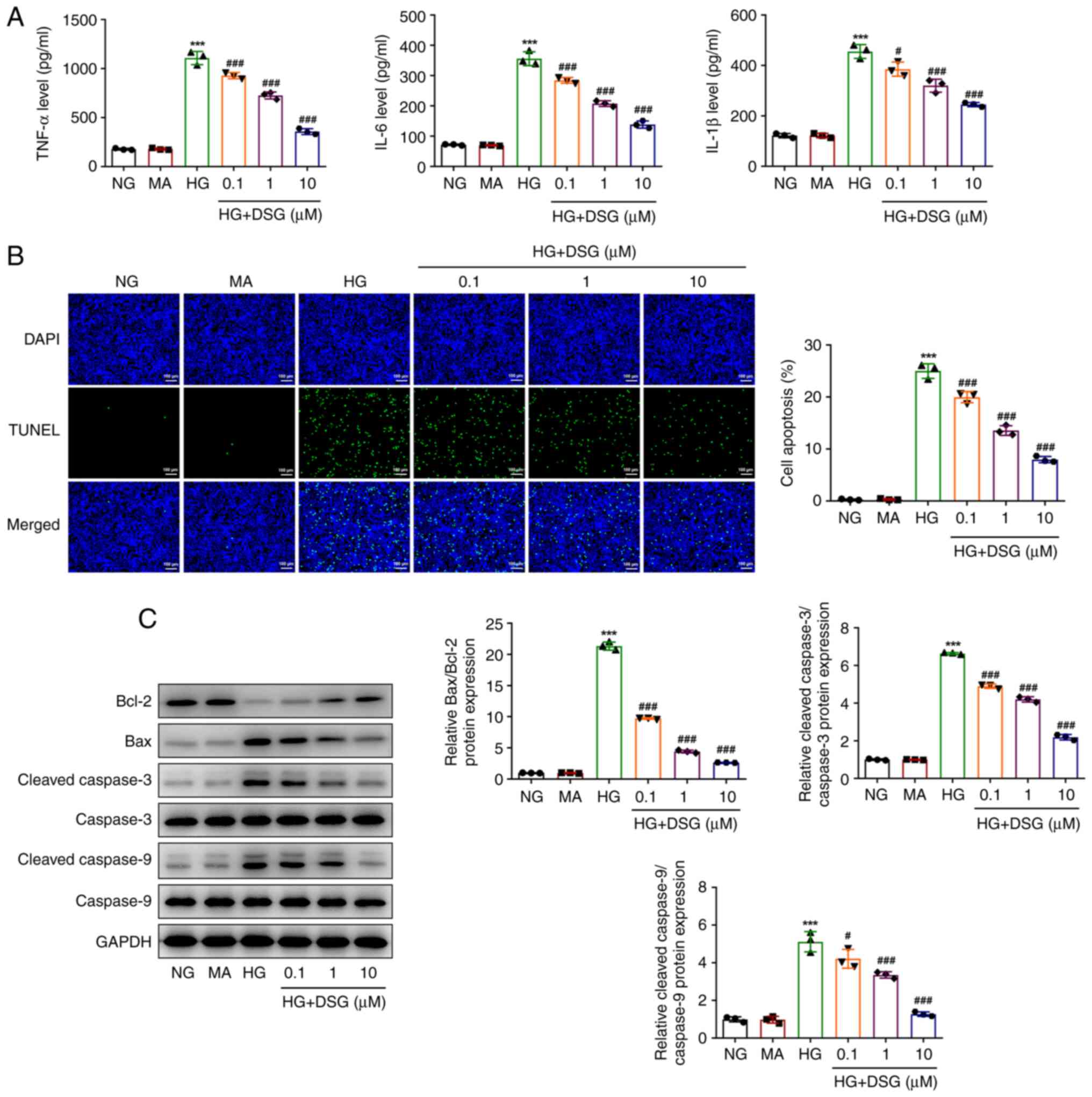
DSG inhibits the inflammatory damage
and apoptosis of HG-induced podocyte cells
To explore the effects of DSG on the inflammatory
response and apoptosis of HG-induced podocyte cells, ELISA and
TUNEL were used to evaluate the expression of inflammatory
cytokines, as well as the cell apoptosis levels. The levels of
TNF-α, IL-6 and IL-1β were significantly increased in HG-induced
podocyte cells compared with those in the NG group, while they were
reduced in HG-induced podocyte cells treated with different
concentrations of DSG compared with those in the HG group (Fig. 2A). DSG exhibited inhibitory effects
on inflammatory damage in a dose-dependent manner.
Similarly, the apoptosis of podocyte cells was
promoted by HG induction compared with that in the NG group
(Fig. 2B). However, the increase
in apoptosis of HG-induced podocyte cells was subsequently
counteracted by DSG treatment (Fig.
2B). In addition, HG induction downregulated Bcl-2 expression,
but upregulated the expression of Bax and the ratios of cleaved
caspase-3/caspase 3 and cleaved caspase-9/caspase-9 compared with
those in the NG group, while DSG treatment reversed the effect of
HG induction on the level of these proteins (Fig. 2C). The aforementioned results
suggested that DSG inhibited the inflammatory damage and apoptosis
of HG-induced renal podocyte cells.
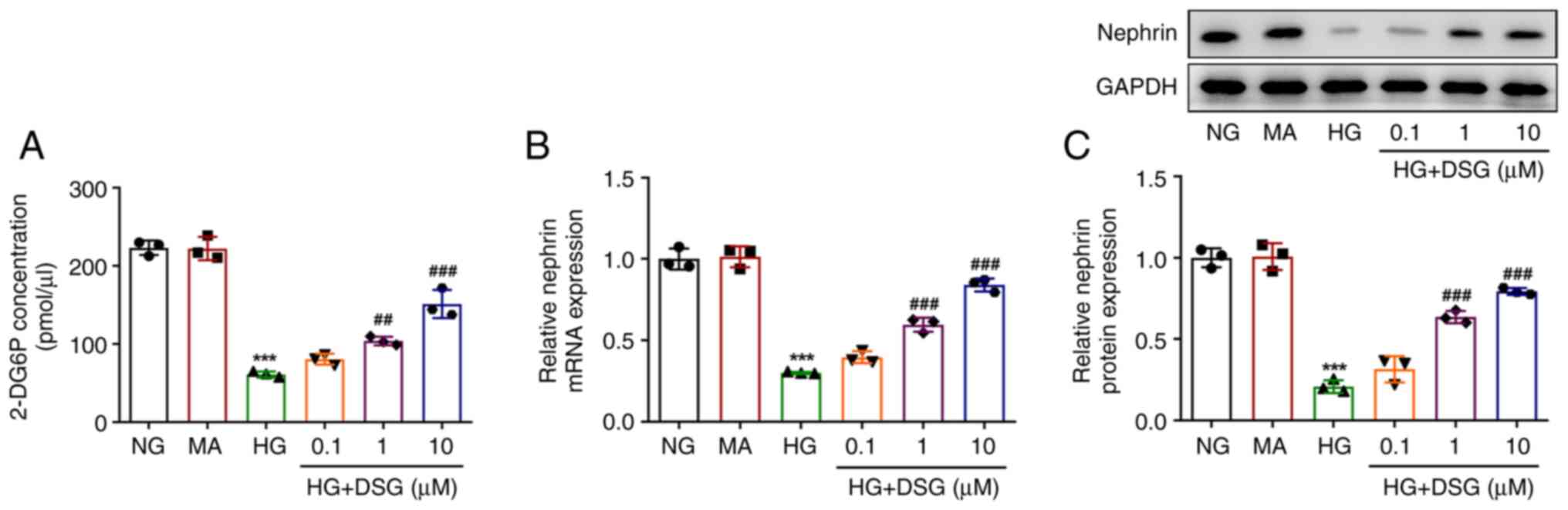
DSG attenuates the insulin resistance
(IR) of HG-induced podocyte cells
To clarify the impact of DSG on IR in HG-induced
podocyte cells, a 2-DOG assay was performed to assess
insulin-stimulated glucose uptake. The expression of nephrin was
also evaluated using RT-qPCR and western blotting. Compared with
that in the NC group, 2-DG6P levels were decreased in podocyte
cells after HG induction. Nevertheless, the decreased levels of
2-DG6P in HG-induced podocyte cells were gradually recovered by
treatment with DSG at several concentrations (HG + 1 µM DSG vs. HG,
P=0.005; HG + 10 µM DSG vs. HG, P=0.001) (Fig. 3A). Nephrin is a key regulator of
podocyte cell permeability, therefore, the mRNA and protein
expression of nephrin was measured via RT-qPCR and western
blotting. HG induction reduced the expression of nephrin at both
mRNA and protein levels compared with those in the NG group,
whereas DSG treatment partially counteracted the effect of HG on
the nephrin levels, as shown by the increased mRNA and protein
expression of nephrin in HG-induced podocyte cells with DSG
treatment compared with that in cells induced with HG (Fig. 3B and C).
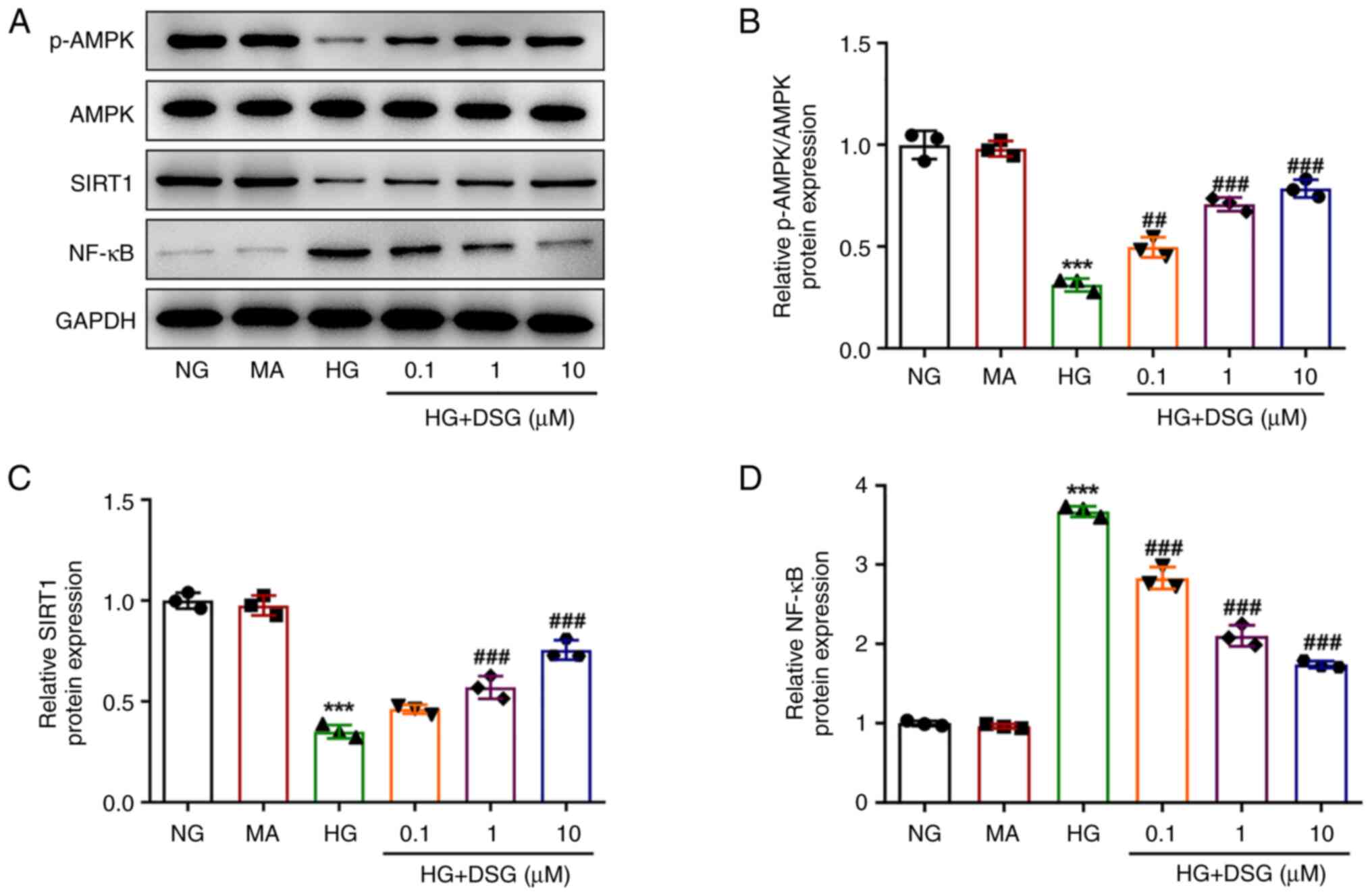
DSG participates in the regulation of
the AMPK/SIRT1/NF-κB signaling pathway
The p-AMPK/AMPK, SIRT1 and NF-κB protein levels were
measured to explore the relationship between DSG and the
AMPK/SIRT1/NF-κB signaling pathway. HG induction downregulated
p-AMPK and SIRT1 expression levels, but upregulated the expression
of NF-κB compared with that in the NG group. The effects of HG
induction on the expression levels of the aforementioned proteins
were reversed after treatment with DSG, suggesting that DSG was
involved in the regulation of the AMPK/SIRT1/NF-κB signaling
pathway (Fig. 4).
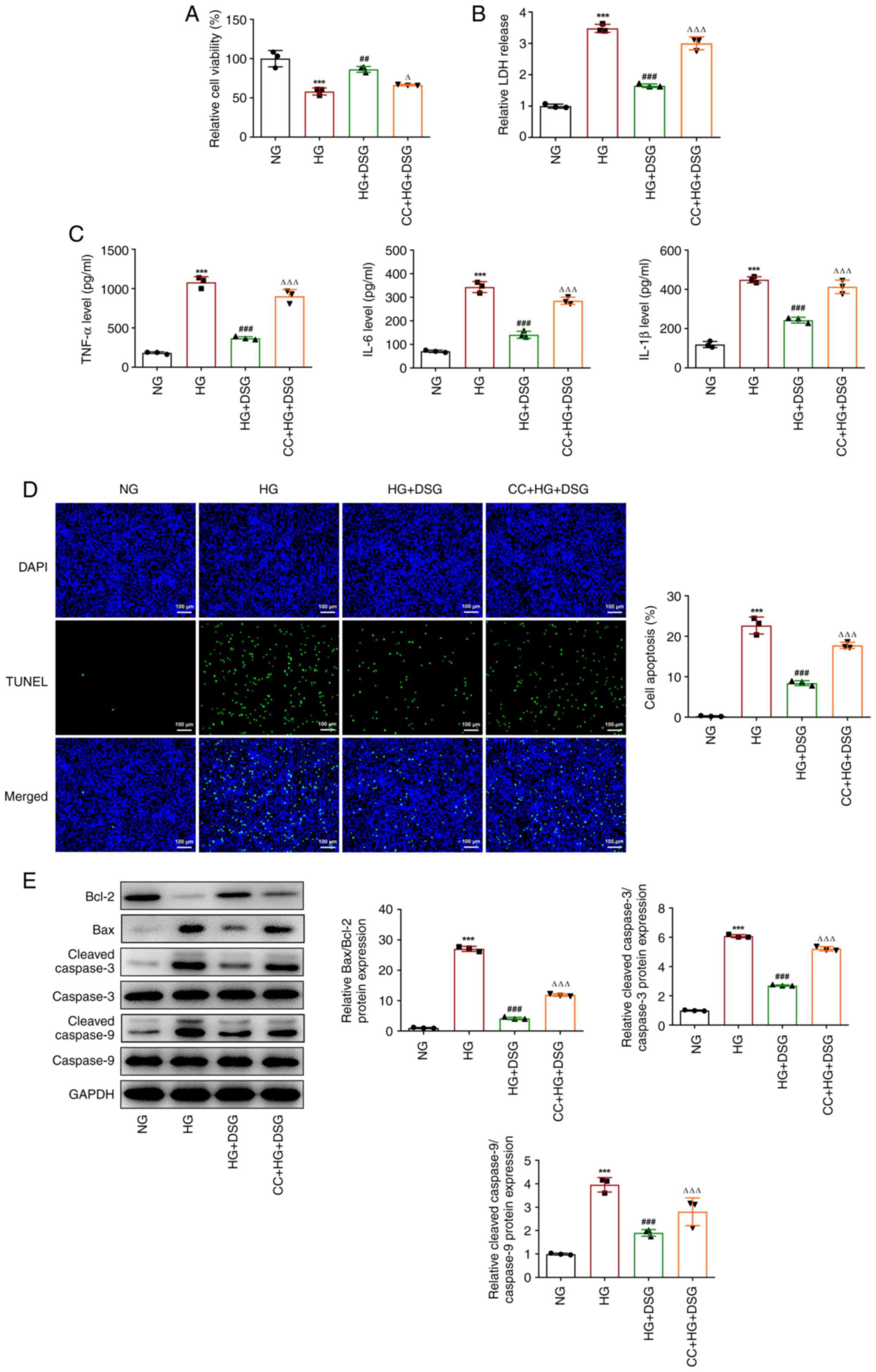
CC reverses the protective effects of
DSG on HG-induced podocyte cells
To further investigate the mechanism of DSG, CC, an
inhibitor of AMPK, was employed to treat podocyte cells, and
subsequently a series of functional experiments were performed. The
decreased viability of HG-induced podocyte cells was rescued by DSG
treatment; however, CC partially counteracted the effects of DSG,
as shown by the reduced viability found in the CC + HG + DSG group
compared with that in the HG+DSG group (HG+DSG vs. HG, P=0.002;
CC+HG+DSG vs. HG+DSG, P=0.015) (Fig.
5A). Similarly, DSG treatment decreased the LDH release in
HG-induced podocyte cells compared with that in the HG group, which
was then reversed by CC (Fig. 5B).
In addition, compared with that in the NG group, HG increased the
expression of inflammatory cytokines, which was then decreased by
DSG treatment (Fig. 5C). CC
promoted the inflammatory response, as shown by the increase in
TNF-α, IL-6 and IL-1β levels compared with those in the HG + DSG
group (Fig. 5C). In addition, the
inhibitory effects of DSG on the apoptosis of HG-induced podocyte
cells were also partially counterbalanced by CC treatment (Fig. 5D). Moreover, the upregulation of
Bcl-2, as well as the downregulation of Bax and the cleaved
caspase-3/caspase 3 and cleaved caspase-9/caspase-9 ratios caused
by DSG in HG-induced podocyte cells compared with the HG group,
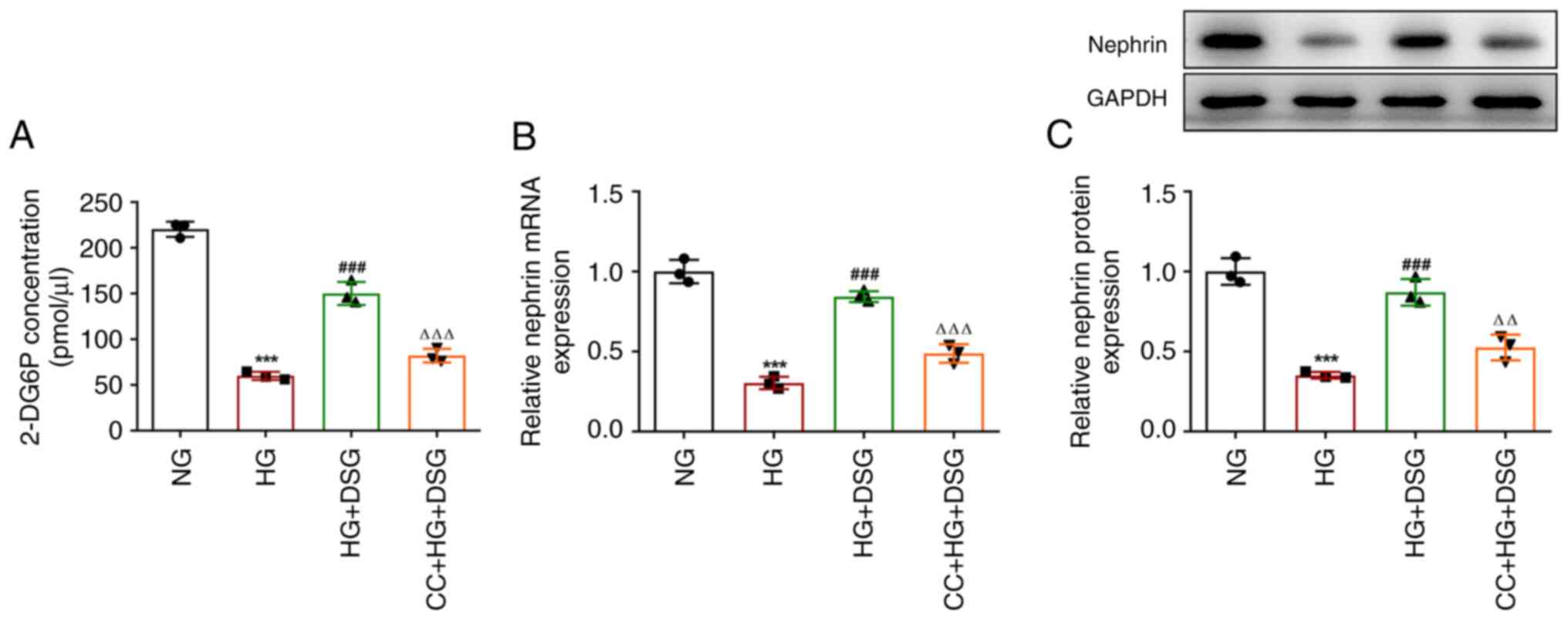
were attenuated after CC treatment (Fig. 5E). Compared with that in the HG +
DSG group, the levels of 2-DG6P were significantly decreased in the
CC + HG + DSG (Fig. 6A). In
addition, HG induction downregulated nephrin at both mRNA and
protein levels compared with the NG group, while DSG treatment
increased the nephrin mRNA and protein expression compared with
that in the HG group. Finally, the effects of DSG on nephrin were
inhibited by CC treatment (Fig. 6B
and C). The aforementioned results
indicated that CC partially counterbalanced the protective effects
of DSG on HG-induced podocyte cells.
Discussion
In the present study, HG was used to establish an
in vitro DN model in podocyte cells. The present results
showed that DSG increased cell viability while relieving the
inflammatory response, apoptosis and IR of HG-induced podocyte
cells in a concentration-dependent manner. In addition, DSG was
involved in the regulation of the AMPK/SIRT1/NF-κB signaling
pathway, and the AMPK inhibitor CC partially abolished the
protective effects of DSG.
As a natural steroidal saponin, DSG is extracted
from certain medicinal plants or vegetables, such as fenugreek and
yam, and has various pharmacological actions (25). Previous studies have highlighted
the beneficial effect of DSG on DN by ameliorating podocyte injury
(13,14). In the current study, different
concentrations of DSG did not affect the viability of podocyte
cells. On the other hand, DSG increased cell viability while
suppressing LDH release in HG-induced podocyte cells in a
dose-dependent manner. It has been reported that inflammation, as a
pathogenic mechanism, is involved in glomerular injury,
glomerulosclerosis and eventually ESRD (26). Moreover, DSG has been shown to
alleviate inflammatory responses in multiple human diseases. For
example, DSG diminished the infiltration of inflammatory cells and
the serum levels of inflammatory cytokines in a psoriasis mouse
model (27). In addition, Du et
al (28) suggested that DSG
could suppress the release of inflammatory factors in alveolar
macrophages, thus protecting against silicosis. Moreover, DSG
showed protective effects on testicular damage in
streptozotocin-induced diabetic rats by inhibiting the inflammatory
response (29). Accordingly, the
present study found that the increased levels of TNF-α, IL-6 and
IL-1β caused by HG induction were decreased after DSG treatment,
revealing the inhibitory effects of DSG on the inflammatory damage
of HG-induced podocyte cells. Additionally, the exacerbated
apoptosis and the decreased Bcl-2 and increased Bax, cleaved
caspase-3/caspase-3 and cleaved caspase-9/caspase-9 expression
caused by HG in podocytes were attenuated and reversed by DSG
treatment.
IR is a pathophysiological state, and results from a
progressive decline in insulin responsiveness in peripheral
tissues, including skeletal muscle, adipose tissue and liver
(30). Previous studies have shown
that DSG treatment could regulate IR. For example, DSG could
relieve IR in palmitate-treated human umbilical vein endothelial
cells (31). In addition, DSG was
also indicated to modulate IR by alleviating the metabolic
dysregulation of lipid profile in diabetic rats (32). In the present study, DSG attenuated
IR in HG-induced podocyte cells, as demonstrated by the increased
levels of 2-DG6P concentration and nephrin expression in HG-exposed
podocytes following DSG administration compared with HG treatment
alone. Therefore, the current findings suggested that DSG could
relieve IR in HG-induced podocyte cells.
It has been reported that the activation of
AMPK/SIRT could alleviate inflammation, oxidative stress and
apoptosis of podocyte cells (15).
Moreover, the activity of AMPKα could alleviate podocyte cells from
IR in a DN model (33). More
importantly, Ran et al (34) showed that DSG could regulate the
AMPK and NF-κB signaling pathways. In addition, DSG protected
against non-alcoholic fatty liver disease through the regulation of
the SIRT1/AMPK signaling pathway (35). In the present study, DSG
downregulated NF-κB expression, but upregulated the expression of
p-AMPK and SIRT1 in HG-induced podocyte cells, suggesting that DSG
participated in the regulation of the AMPK/SIRT1/NF-κB signaling
pathway. Furthermore, the protective effects of DSG against
decreased cell viability and increased inflammatory response,
apoptosis and IR in HG-induced podocyte cells were reversed by CC,
an inhibitor of AMPK.
In conclusion, DSG treatment protected HG-induced
podocyte cells against the increased inflammatory response and IR
by regulating the AMPK/SIRT1/NF-κB signaling pathway, indicating
that DSG may represent a novel therapy for the treatment of DN. The
present study may offer theoretical insights into the development
of DN and the pursuit of novel diagnostic and interventional
approaches, and lay a favorable foundation for further
investigation. However, there are some limitations in the current
study. Firstly, the toxicity of DSG was not investigated. Secondly,
the protective role and therapeutic value of DSG in DN need to be
further investigated in vivo.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY, HS and SL conceived and designed the study, and
acquired and interpreted the data. HY and HS performed the
experiments. HY and SL wrote the manuscript. HY, HS and SL confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heerspink HJL, Parving HH, Andress DL,
Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H,
McMurray JJV, et al: Atrasentan and renal events in patients with
type 2 diabetes and chronic kidney disease (SONAR): A double-blind,
randomised, placebo-controlled trial. Lancet. 393:1937–1947.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bell S, Fletcher EH, Brady I, Looker HC,
Levin D, Joss N, Traynor JP, Metcalfe W, Conway B, Livingstone S,
et al: End-stage renal disease and survival in people with
diabetes: A national database linkage study. QJM. 108:127–134.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Verzola D, Cappuccino L, D'Amato E,
Villaggio B, Gianiorio F, Mij M, Simonato A, Viazzi F, Salvidio G
and Garibotto F: Enhanced glomerular Toll-like receptor 4
expression and signaling in patients with type 2 diabetic
nephropathy and microalbuminuria. Kidney Int. 86:1229–1243.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rossing P and Persson F: What have we
learned so far from the use of sodium-glucose cotransporter 2
inhibitors in clinical practice? Adv Chronic Kidney Dis.
28:290–297. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu X, Tang L, Mao J, Hameed Y, Zhang J,
Li N, Wu D, Huang Y and Li C: Decoding the Mechanism behind the
pathogenesis of the focal segmental glomerulosclerosis. Comput Math
Methods Med. 2022(1941038)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tung CW, Hsu YC, Shih YH, Chang PJ and Lin
CL: Glomerular mesangial cell and podocyte injuries in diabetic
nephropathy. Nephrology (Carlton). 23 (Suppl 4):S32–S37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pi WX, Feng XP, Ye LH and Cai BC:
Combination of morroniside and diosgenin prevents high
glucose-induced cardiomyocytes apoptosis. Molecules.
22(163)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang WS, Moon SY, Lee MJ, Lee EK and Park
SK: Diosgenin, an activator of 1,25D3-MARRS receptor/ERp57,
attenuates the effects of TNF-α by causing ADAM10-dependent
ectodomain shedding of TNF receptor 1. Cell Physiol Biochem.
43:2434–2445. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Long C, Chen J, Zhou H, Jiang T, Fang X,
Hou D, Liu P and Duan H: Diosgenin exerts its tumor suppressive
function via inhibition of Cdc20 in osteosarcoma cells. Cell Cycle.
18:346–358. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kalailingam P, Kannaian B, Tamilmani E and
Kaliaperumal R: Efficacy of natural diosgenin on cardiovascular
risk, insulin secretion, and beta cells in streptozotocin
(STZ)-induced diabetic rats. Phytomedicine. 21:1154–1161.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hua S, Li Y, Su L and Liu X: Diosgenin
ameliorates gestational diabetes through inhibition of sterol
regulatory element-binding protein-1. Biomed Pharmacother.
84:1460–1465. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chiang SS, Chang SP and Pan TM:
Osteoprotective effect of Monascus-fermented dioscorea in
ovariectomized rat model of postmenopausal osteoporosis. J Agric
Food Chem. 59:9150–9157. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gan Q, Wang J, Hu J, Lou G, Xiong H, Peng
C, Zheng S and Huang Q: The role of diosgenin in diabetes and
diabetic complications. J Steroid Biochem Mol Biol.
198(105575)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Z, Wu Q, Wang H, Gao Y, Nie K, Tang
Y, Su H, Hu M, Gong J, Fang K and Dong H: Diosgenin protects
against podocyte injury in early phase of diabetic nephropathy
through regulating SIRT6. Phytomedicine. 104(154276)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xue W, Mao J, Chen Q, Ling W and Sun Y:
Mogroside IIIE alleviates high glucose-induced inflammation,
oxidative stress and apoptosis of podocytes by the activation of
AMPK/SIRT1 signaling pathway. Diabetes Metab Syndr Obes.
13:3821–3830. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen B, Li J and Zhu H: AMP-activated
protein kinase attenuates oxLDL uptake in macrophages through
PP2A/NF-κB/LOX-1 pathway. Vascul Pharmacol. 85:1–10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li F, Chen Y, Li Y, Huang M and Zhao W:
Geniposide alleviates diabetic nephropathy of mice through
AMPK/SIRT1/NF-kappaB pathway. Eur J Pharmacol.
886(173449)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen Y, Xu X, Zhang Y, Liu K, Huang F, Liu
B and Kou J: Diosgenin regulates adipokine expression in
perivascular adipose tissue and ameliorates endothelial dysfunction
via regulation of AMPK. J Steroid Biochem Mol Biol. 155:155–165.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021(1497449)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li F, Dai B and Ni X: Long non-coding RNA
cancer susceptibility candidate 2 (CASC2) alleviates the high
glucose-induced injury of CIHP-1 cells via regulating
miR-9-5p/PPARγ axis in diabetes nephropathy. Diabetol Metab Syndr.
12(68)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang F, Qin Y, Wang Y, Meng S, Xian H, Che
H, Lv J, Li Y, Yu Y, Bai Y and Wang L: Metformin Inhibits the NLRP3
inflammasome via AMPK/mTOR-dependent effects in diabetic
cardiomyopathy. Int J Biol Sci. 15:1010–1019. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the lactate dehydrogenase assay. Cold
Spring Harb Protoc 2018: doi: 10.1101/pdb.prot095497, 2018.
|
|
23
|
Zhan X, Yan C, Chen Y, Wei X, Xiao J, Deng
L, Yang Y, Qiu P and Chen Q: Celastrol antagonizes high
glucose-evoked podocyte injury, inflammation and insulin resistance
by restoring the HO-1-mediated autophagy pathway. Mol Immunol.
104:61–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Y, Li Y, Yang T and Wang M: Dioscin
attenuates oxLDL uptake and the inflammatory reaction of dendritic
cells under high glucose conditions by blocking p38 MAPK. Mol Med
Rep. 21:304–310. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li G, Huang D, Li N, Ritter JK and Li PL:
Regulation of TRPML1 channel activity and inflammatory exosome
release by endogenously produced reactive oxygen species in mouse
podocytes. Redox Biol. 43(102013)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu S, Zhao M, Sun Y, Xie M, Le K, Xu M and
Huang C: The potential of Diosgenin in treating psoriasis: Studies
from HaCaT keratinocytes and imiquimod-induced murine model. Life
Sci. 241(117115)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Du S, Li C, Lu Y, Lei X, Zhang Y, Li S,
Liu F, Chen Y, Weng D and Chen J: Dioscin alleviates crystalline
silica-induced pulmonary inflammation and fibrosis through
promoting alveolar macrophage autophagy. Theranostics. 9:1878–1892.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khosravi Z, Sedaghat R, Baluchnejadmojarad
T and Roghani M: Diosgenin ameliorates testicular damage in
streptozotocin-diabetic rats through attenuation of apoptosis,
oxidative stress, and inflammation. Int Immunopharmacol. 70:37–46.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Opazo-Rios L, Mas S, Marin-Royo G, Mezzano
S, Gómez-Guerrero C, Moreno JA and Egido J: Lipotoxicity and
diabetic nephropathy: Novel mechanistic insights and therapeutic
opportunities. Int J Mol Sci. 21(2632)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu K, Zhao W, Gao X, Huang F, Kou J and
Liu B: Diosgenin ameliorates palmitate-induced endothelial
dysfunction and insulin resistance via blocking IKKβ and IRS-1
pathways. Atherosclerosis. 223:350–358. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Naidu PB, Ponmurugan P, Begum MS, Mohan K,
Meriga B, RavindarNaik R and Saravanan G: Diosgenin reorganises
hyperglycaemia and distorted tissue lipid profile in high-fat
diet-streptozotocin-induced diabetic rats. J Sci Food Agric.
95:3177–3182. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lu J, Chen PP, Zhang JX, Li XQ, Wang GH,
Yuan BY, Huang SJ, Liu XQ, Jiang TT, Wang MY, et al: GPR43
deficiency protects against podocyte insulin resistance in diabetic
nephropathy through the restoration of AMPKalpha activity.
Theranostics. 11:4728–4742. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ran X, Yan Z, Yang Y, Hu G, Liu J, Hou S,
Guo W, Kan X and Fu S: Dioscin improves pyroptosis in LPS-induced
mice mastitis by activating AMPK/Nrf2 and inhibiting the NF-kappaB
signaling pathway. Oxid Med Cell Longev.
2020(8845521)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yao H, Tao X, Xu L, Qi Y, Yin L, Han X, Xu
Y, Zheng L and Peng J: Dioscin alleviates non-alcoholic fatty liver
disease through adjusting lipid metabolism via SIRT1/AMPK signaling
pathway. Pharmacol Res. 131:51–60. 2018.PubMed/NCBI View Article : Google Scholar
|