Introduction
Psoriasis is a common, chronic, immune-mediated
systemic disease characterized by skin lesions, including red
plaques, scaly patches, and papules. This serious global disease
caused a heavy psychosocial burden on patients and continues to
increase in incidence (1).
Clinically, evidence has shown that a number of psoriasis patients
usually suffer from obesity, insulin resistance, metabolic
syndrome, cardiovascular disease and other immune-mediated gut
chronic inflammation (2). As
observed, psoriasis is refractory and easily relapses and it can be
triggered by lifestyle factors (3), such as intake of stimulating food
(SF). According to the Traditional Chinese Medicine (TCM), SF is
usually defined as foods with high nutrition and energy,
particularly animal proteins and fat (red meat such as beef and
mutton, fried food and cream), with pungent properties (Chinese
liquor, and plants such as pepper, fennel, Chinese ginger,
cinnamon, verum, amomum tsao-ko and cardamon) and with high sugar
content (dessert and sweetmeats). Long-term high consumption of SF
can invoke the onset and/or aggravate the progression of diverse
skin diseases, especially psoriasis, eczema and allergic dermatitis
(4). It appears that certain
dietary patterns, such as SF, can induce gut disorder in
susceptible individuals and trigger systemic inflammation via
unknown signaling and subsequent production of pro-inflammatory
cytokines further exacerbate unstable skin immunity and caused
recurrence of psoriasis (5). Such
phenomena have been noticed in clinical practice and aroused
patients' concern. The present study highlighted patients with
inflammatory bowel condition who are usually more susceptible to
SF.
Report on psoriasis and diet first appeared in the
1950s and researchers tried nutrient therapy in psoriasis and
designed clinical trials to verify the observational results
(6). During the past 60 years,
various dietary regimens have been proposed, such as a
low-tryptophan diet (7), a
low-protein diet (8), a low-fat
diet (9), a fasting diet (10), a gluten-free diet (11) and even dietary habits from
geographical regions, such as the turkey diet (12) and a Mediterranean diet (13). Additionally, supplementing elements
in the diet has also been evaluated, mainly on polyunsaturated
fatty acids from marine fish oil (omega-3 and -6; ethyl ester
lipids-angiosan) (14), vitamins
(A, D, E, B1, B2, B6, niacin, biotin and C) (15,16),
micronutrients (selenium, zinc, iron and copper) (17) and other additives (taurine, fumaric
acid, arachidonic acid, bile acids and bioflavonoids) (18-20).
However, a Western diet (WD) plays a crucial diet pattern in modern
societies and growing researches had confirmed that WD intake leads
to psoriasis exacerbation and is even related to its comorbidities
(21,22).
Gradually, it became noticed that some metabolites
of food were higher in the blood plasma of psoriasis, especially
increased cholesterol, triglyceride (fatty acids), and glucose,
which are closely associated with food intake and metabolic
condition (23). Unsurprisingly,
the management of metabolic disturbance by ‘lifestyle intervention’
and weight loss has been proposed (24).
However, the pathophysiologic mechanism behind
dietetics and skin lesions remain to be elucidated. There is
emerging evidence that diet-driven changes in gut microbiota
composition and metabolome, which directly or indirectly affect
human health, are particularly obvious in people with in
inflammatory bowel diseases (IBD) or metabolic syndrome (25,26).
Additionally, a WD induced adipose inflammation characterized by
increases in the M1:M2 macrophage ratio and proinflammatory
cytokine expression contributes to IBD (27). Unexpectedly, such macrophages also
exist in the skin as antigen-presenting cells (APCs) and play key
role in defending against cutaneous pathogens (28). In light of evidence that psoriasis
is a systemic inflammatory disorder linked to the gut, the present
study hypothesized that long-term intake of SF induces unbalance of
gut immune, which results in the releasing of inflammatory
cytokines in circulation which then interact with unstable immune
cells residing in the skin lesions of psoriasis. The present study
constructed psoriasis-like mice fed with SF, then gut histology and
immune condition, serum cytokines and skin lesions were compared
with normal fed mice, thus providing valuable information for the
prevention and treatment of psoriasis.
Materials and methods
Preparation of SF
According to the Chinese cooking culture, SF usually
consists of several basic ingredients, such as high in fat and
energy (butter, yolk, dried whole power and soybean oil added in SF
formulations; and beef and mutton providing protein content in
subsequent research), sugar (honey water), pungent tastes (Chinese
liquor and soybean oil infused with plant spices) and less abundant
in fibrin. Based on normal diet ingredients (Table I), SF was produced by Xietong
Biotech, except for additives in soybean oil (obtained from TCM
pharmacies). In theory, long-term intake of SF (Table II) could induce gut inflammation
in mice.
 | Table INormal dietary ingredient composition
(% by weight) of mice. |
Table I
Normal dietary ingredient composition
(% by weight) of mice.
| Ingredient | g/kg diet |
|---|
| Casein, 30
Mesh | 200 |
| L-Cystine | 3 |
| Corn starch | 397 |
| Maltodextrin
10 | 132 |
| Sucrose | 100 |
| Cellulose | 50 |
| Soybean oil (no
additives) | 70 |
|
t-butylhydroquinone | 0.014 |
| Mineral Mix
S10022M | 35 |
| Vitamin Mix
V10037 | 10 |
| Choline
Bitartrate | 2.5 |
| Total | 1,000 |
 | Table IIThe proportion of constituents of
‘stimulating food’ |
Table II
The proportion of constituents of
‘stimulating food’
| Supplement
regimen | Proportion |
|---|
| Normal diet of
mice | 60% |
| Soybean Oil (with
additivesa) | 10% |
| Yolk | 15% |
| Butter | 5% |
| Dried whole milk
powder | 7% |
| Sugar | 3% |
| Drinking
waterb | ad
libitum |
Psoriasis-like animal model and
experimental design
Male BALB/c mice (n=18, aged 5-6 weeks, body weight
16.5±0.4 g) were obtained from Xipuer-BiKai (Shanghai, China),
housed at the Zhejiang Chinese Medical University Laboratory Animal
Research Center and randomly divided into three groups (normal
control, psoriasis, psoriasis + SF). Mice in the psoriasis + SF
group were fed SF and the other groups had free access to standard
chow and water. At the third week, their back skin hair was removed
by shaver and depilatory cream. Mice were topically administered
62.5 mg of 5% imiquimod cream (Aldara; 3M Health Care Ltd.) three
times every other day and the control mice were treated with
Vaseline. If signs of abnormal coat condition and excessive
scratching, or weight loss was >10%, the mice were sacrificed
with 70% volume displacement per min of 100% carbon dioxide. The
indicator of mortality was loss of heartbeat. Mice were euthanized
when their psoriatic lesions were spontaneously alleviated 1 week
later. After sacrifice, skin lesions and alimentary tract tissue
samples and blood were collected for further analysis. All
experimental procedures were performed in compliance with the
guidelines set by the Zhejiang University of Traditional Chinese
Medicine Animal Care and Use Committee (approval no.
IACUC-20190408-12).
Scoring severity of skin lesion under
dermascopy
Mouse skin lesions were scored based on the modified
Psoriasis Area and Severity Index (mPASI) scores, which were
comprised of scale (scores 0-4), erythema (scores 0-4) and
infiltration (scores 0-4). From day 0 and days 1, 3, and 6 after
establishment of psoriasis-like dermatitis, images of lesions
captured by dermascopy (HEINE mini 3000 LED Dermatoscope; HEINE
Optotechnik GmbH & Co. KG) were evaluated for scoring on a
scale from 0-4: None, 0; slight, 1; moderate, 2; marked, 3; very
marked, 4. The level of erythema was scored using a scoring table
with red taints. The thickness of the skin lesion was measured by a
digital caliper. The cumulative score (erythema plus scaling plus
thickening) was marked as a measure of the severity of psoriatic
lesions (scale 0-12).
Tissue preparation and
immunohistochemistry (IHC)
Back skin lesion tissue and alimentary tract tissue
(stomach, jejunum, ileum and colon) of each mouse were fixed with
10% neutral buffered formalin separately at 4˚C for 1 h, and then
transferred to room temperature for 12 h. Briefly, the above
samples of each mouse were processed through a paraffin embedding
protocol, with dehydration through a series of alcohol-water
solutions beginning with 75% and up to absolute alcohol, clearing
(Histoclear), embedded in paraffin wax and sectioned at 5 µm using
a Leica RM2145 microtome (Leica Microsystems, Inc.). Slides were
stained with hematoxylin and eosin (H&E) Leica Autostainer XL
Protocol Sheet, each step in the protocol was accurately timed and
room temperature was used for all steps and solutions. The slides
were stained in hematoxylin solution for 3 min, and in working
eosin solution for 1 min. For IHC, slides of sections from the
above paraffin-embedded tissues were incubated with anti-rabbit
CD11b antibody (cat. no. ab133357; Abcam; 1:500) and MRC1 antibody
(cat. no. abs141228; Absin Bioscience Inc. 1:500) overnight at 4˚C,
followed by incubation with biotinylated secondary antibody Goat
anti-Rabbit IgG H&L (HRP; cat. no. ab205728; Abcam; 1:1,000)
for 1 h at room temperature and development with
3,3'-diaminobenzidine. Samples were scanned by NanoZoomer Slide
Scanner (Hamamatsu Photonics K.K.), five random high-power field
images (magnification, x20) of each slide were captured and CD11b-
and MRC1-positive parts were counted using ImageJ software version
2.0 (National Institutes of Health).
ELISA
Blood samples from each mouse were centrifuged at
1,000 x g at 4˚C to obtain serum. ELISA kits for mouse interleukin
(IL)-17 (cat. no. MM-0170M1), IL-35 (cat. no. MM-0904M1), IL-10
(cat. no. MM-0176M2) and TNF-α (cat. no. MM-0132M1) were purchased
from Jiangsu Meimian Industrial Co. The expression levels in the
blood serum of mice were measured according to the manufacturer's
instructions.
Protein extraction and western
blotting
Proteins of skin tissue were extracted with Enhanced
RIPA lysate (cat. no. AR0102; Wuhan Boster Biological Technology,
Ltd.) mixed with complete Protease Inhibitor Cocktail (Roche Life
Science). Total protein content in the tissue lysates were
determined by a bicinchoninic acid assay kit (cat. no. AR0146;
Wuhan Boster Biological Technology, Ltd.) and mixed with loading
buffer when subjected to SDS-PAGE on Bolt 10% Bis-Tris Plus Gels
(Invitrogen; Thermo Fisher Scientific, Inc.). Proteins were
transferred onto a PVDF membrane (MilliporeSigma), and then
incubated with primary antibodies specific for NF-κB p65 (cat. no.
6956), phospho-NF-κB p65 (cat. no. 3033), Toll-like receptor-2
(cat. no. 2229S), IKBα (cat. no. 9242), Jagged-1 (cat. no. 70109s),
Notch-1 (cat. no. 3608s; all CST; 1:1,000), Hes-5 (cat. no.
ab25374; Abcam; 1:1,000) and anti-β-actin (cat. no. BM0627; Wuhan
Boster Biological Technology, Ltd.; 1:2,000) overnight at 4˚C and
then followed by incubation with horseradish peroxidase-conjugated
secondary anti-mouse or anti-rabbit IgG (cat. nos. 7076 and 7074;
CST; 1:2,000) at room temperature for 2 h. The blots were
visualized by Beyo ECL Star (cat. no. P0018AS; Beyotime Institute
of Biotechnology) with Bio-Rad Gel Doc XR+ and quantified with
Image Lab software (Bio-Rad Laboratories, Inc.). The relative
expression of proteins in each band was quantified using ImageJ v
1.8.0 (National Institutes of Health) and normalized to β-actin as
an internal loading control.
Cell culture and immunofluorescence
(IF)
HaCaT cells (human immortal keratinocytes) were
obtained from the Chinese Academy of Sciences Kunming Cell Bank
(cat. no. KCB 200442YJ) with cell line authenticated by STR
profiling, cultured with DMEM containing 10% fetal bovine serum and
penicillin (100 U/ml)/streptomycin (100 mg/ml; Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C and 5% CO2. Prior to
performing IF, HaCaT cells were cultured with adding serum (filter
sterilization) from the aforementioned three groups of mice on
glass slides for 1, 3 and 6 h separately. At the end of culture,
cells were harvested and fixed with 4% paraformaldehyde for 15 min
at room temperature. Then, IF experiments were performed according
to the manufacturer's protocol. Briefly, cells were permeabilized
for 10 min, and then incubated with primary antibodies against
NF-κB p65 (cat. no. 6956; CST; 1:1,000) at 4˚C overnight. Then
cells were incubated with secondary anti-mouse IgG antibodies
conjugated to Alexa Fluor 594 (cat. no. 8890; CST; 1:1,000). Nuclei
were counterstained with DAPI obtained from Abcam.
Statistical analyses
Data analyses was performed using SPSS 20.0 (IBM
Corp.) and GraphPad Prism version 6.0 (Dotmatics). The data were
presented as the mean ± standard error of the mean and each
experiment was performed in triplicate independently. Comparisons
with the other two groups were analyzed by one-way analysis of
variance (ANOVA) followed by the Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
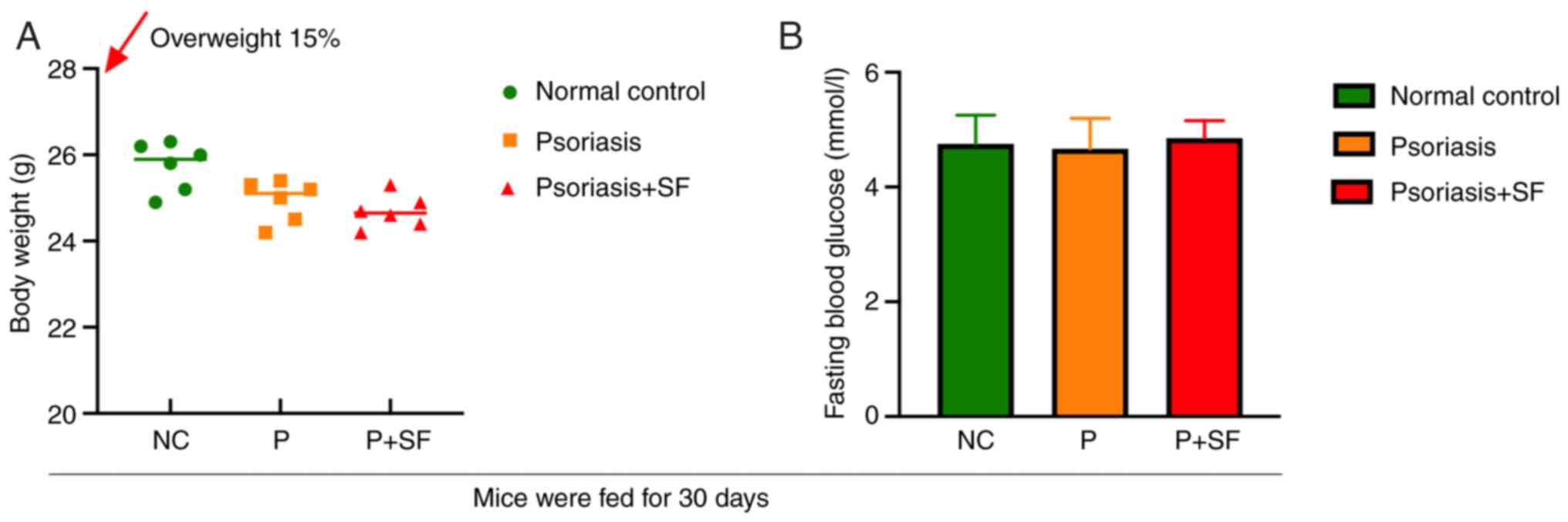
SF diet and normal diet did not induce
prediabetes in mice
Impaired fasting blood glucose was recognized as an
indicator of prediabetes in mice. After 30 days of regular diet and
SF feeding, mice in the SF-disposed group did not exhibit
overweight and blood glucose disorders or features of obesity or
even emaciation (Fig. 1). This
result suggested that an SF diet may induce harmful irritating
factors other than energy metabolic disorders during this phase and
that their gut perturbations were more similar to the inflammatory
‘stressed state’.
Long-term SF feeding exacerbates
imiquimod-induced psoriasis-like lesions
Compared with the normal control, the application of
Aldara induced psoriasis-like skin lesions in BALB/c mice in
dermascopic features and pathological manifestations. Such skin
lesions were estimated by mPASI scores (Fig. 2A). Psoriasis-like skin lesions on
the 3rd day after Aldara irritation were most typical and then
started to resolve spontaneously. Comparison of erythema, scaling,
thickening and mPASI scores were highly consistent and higher in
the psoriasis + SF group. Additionally, compared with skin lesion
pathology of normal control and psoriasis group, there was
significant thickening of stratum corneum and epidermis, along with
inflammatory cell infiltration and neoangiogenesis in dermis,
without distinctive Munro microabscess (neutrophil accumulation at
the stratum corneum) or Kogoj spongy microabscess (neutrophilic
aggregates in the epidermal stratum spinosum) (Fig. 2B), which represent distinctive
features for psoriasis although not often present, only being
observed in pustular psoriasis.
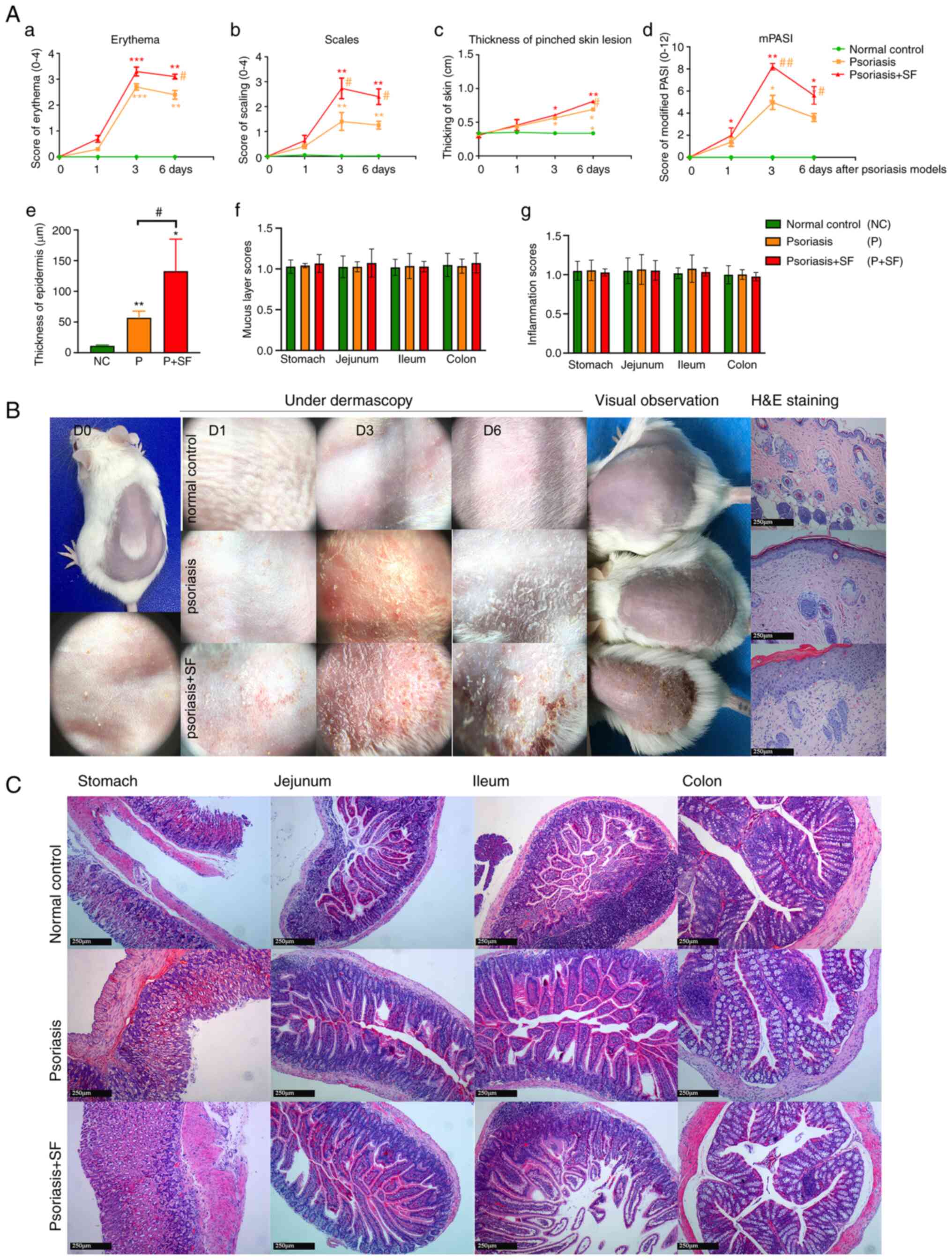 | Figure 2Score index of skin lesions under
dermascopy and H&E staining. (A) Scores of (a) erythema, (b)
scaling, (c) thickening, (d) mPASI, (e) thickness of epidermis and
histological sores of (f) gastrointestinal mucus layer and (g)
inflammation of the three groups. The results showed that the SF
group had more severe skin lesions, by either mPASI or H&E
staining. *P<0.05, **P<0.01,
***P<0.001 vs. the normal control;
#P<0.05, ##P<0.01 vs. the psoriasis
group. (B) Under skin dermatology, the progression of skin lesions
was classified into three stages: D1, Compared with normal control
skin (D0), the subcutaneous bleeding point in the psoriasis + SF
group was more than that in the psoriasis group; D3, Erythema
scaling of the psoriasis + SF group (erythematous covered with
thick scales, sporadic blood crust) was more obvious than that of
the normal control smooth skin group (erythema with thin silvery
scales); D6, Compared with normal control with hair growth skin,
skin lesion started to remission in other groups, erythema and
scale still obvious in SF disposed group, while only chaff scales
were observed in psoriasis group. (C) The effect of food on the
gastrointestinal tract. The pathology of the gut tract
(magnification, x10) demonstrated that mucosa, luminal contents and
even acute inflammatory cell infiltration were not changed during
the experimental process. H&E, hematoxylin and eosin; mPASI,
modified Psoriasis Area and Severity Index; SF, stimulating
food. |
The pathology of the gut (from the greater gastric
curvature of stomach, jejunum and ileum to colon) were analyzed.
The result (Fig. 2C) indicated
that there were no significant differences in the distribution and
construction of the gut mucosal features and inflammatory
infiltrations between SF-disposed group and other groups.
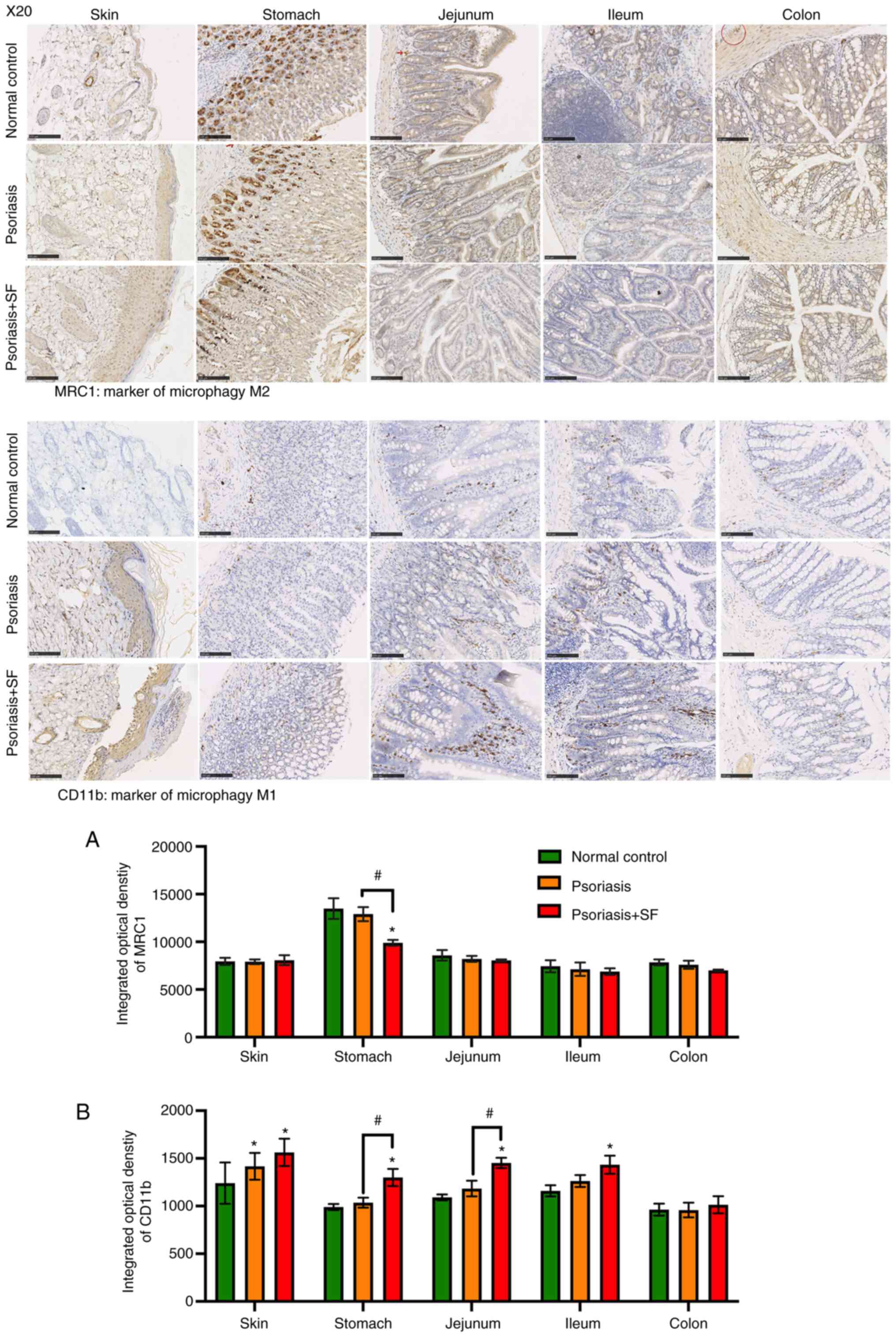
Correlation of macrophage polarization
in the gut tract and skin lesions
Skin and gut tract tissues from each mouse were
paraffin embedded and sectioned to confirm the correlation of gut
and skin. The quantification analysis of IHC staining (Fig. 3) showed that the macrophage
polarization (M1/M2) in the gut tract of the psoriasis + SF group
was different from that in the normal control and psoriasis groups
(both without SF). In the normal control, MRC1 + macrophages
(marker of M2 macrophage; anti-inflammatory cells) were enriched
throughout the whole gut tract and skin, being especially distinct
in the basal part of gastric glands and around the hair follicle,
while M1 macrophages marked by CD11b (pro-in inflammatory cells)
had generally a lower expression. Such condition also presented in
the psoriasis group, excepting an increased expression of CD11b in
the dermis. In the psoriasis + SF group, a decreased expression of
MRC1 was observed in the stomach, followed by an increased
expression levels of CD11b in the gut tract and dermis, excepting
only the colon part. The expression levels of CD11b in skin lesions
were positively associated with their expression in the gut tract,
especially the stomach, jejunum and ileum. It indicated that SF may
promote M1 macrophage activation. Notably, epidermis of several
mice in the SF disposed group showed a severe injury which almost
reach to a spongiotic dermatitis and necrotic foci.
Cytokines in blood serum of mice and
their effect on nuclear translocation of HaCaT cell
Cytokines released by tissue cells (mainly by immune
cells) are important for signal transduction. In the SF predisposed
group (Fig. 4A), the TNF-α
(higher; P=0.007)/IL-10 (lower; P=0.047) ratio was unbalanced,
which mediated a chronic inflammation condition. Additionally,
IL-35, which is usually an anti-inflammatory cytokine in the gut
(28), was also decreased mildly
(P=0.084) in the SF predisposed group. No significant result was
observed in serum level of IL-17 in psoriasis-like dermatitis
mice.
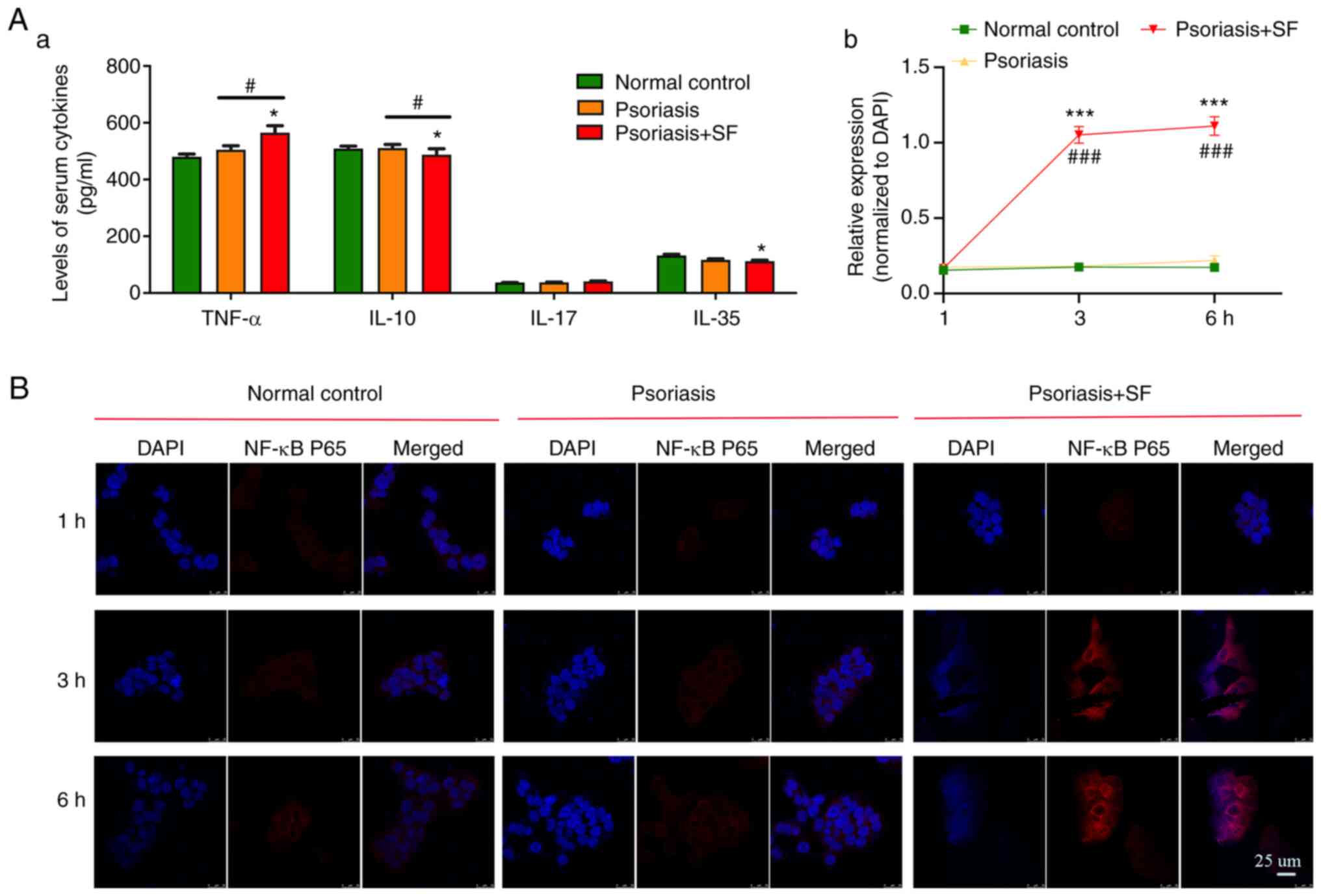 | Figure 4Serum levels of cytokines in the three
groups of mice and their effect on translocation of HaCaT cells. (A
and a) There was a significant difference in the expression of
TNF-α, IL-10, and IL-35 in the SF, while there was no significant
difference in the expression of IL-17, which was higher in the
SF-disposed group. (B and b) The serum of SF-disposed mice promotes
the proliferation of HaCaT cells by activating the transcription of
NF-κB p65. HaCaT cells were cultured with serum from different
groups of mice over time (1, 3 and 6 h), and whole mounts of HaCaT
cells stained for NF-κB p65 (protein, red) and DAPI (cell nuclei
distribution, blue) were observed by microscopy. The data are the
mean ± SD values (*P<0.05, ***P<0.001
vs. the normal control; #P<0.05,
###P<0.001 vs. the psoriasis group) and were
statistically analyzed with one-way ANOVA. |
To demonstrate the influence of such serum cytokines
on skin, HaCaT cells were stimulated by serum from aforementioned
three groups of mice. After culturing for 1 h, IF showed no
difference in NF-κB p65 translocation in cells. However, after 3
and 6 h, NF-κB p65 was significantly activated by serum from
SF-disposed mice, although there was no obvious activation in the
other groups (Fig. 4B). Thus, it
was hypothesized that cytokines (more TNF-α and less IL-10) from
the serum of SF-disposed mice mediated translocation of NF-κB p65
in HaCaT cells.
Expression of the TLR-2/NF-κB p65 and
Notch signaling pathways in skin lesions
TLR-2/NF-κB p65 is a classic inflammatory signaling
pathway that is highly activated in advanced stage of psoriasis
(29). Toll-like receptor-2
(TLR-2) is usually expressed on macrophages to recognize
structurally conserved molecules of microbes, also known as
pathogen-associated molecular patterns (PAMPs) and then activates
the immune cell response, such as activation of NF-κB p65 and
subsequent effector molecules to mediate psoriasis relapse.
Moreover, TLR activation can activate canonical and non-canonical
Notch signaling. In psoriatic skin lesions, Notch signaling
molecules have been reported to be highly expressed with diffused
mode (30). The results of western
blotting (Fig. 5) demonstrated
that TLR-2/NF-κB p65 signaling and Notch signaling were activated
in psoriasis lesions. Protein expressions of Jagged-1 (a ligand for
Notch receptor), Notch-1 and TLR-2, NF-κB p65 and its
phosphorylated NF-κB p65 were upregulated significantly in the
psoriasis group, while abnormally downregulated in the SF
group.
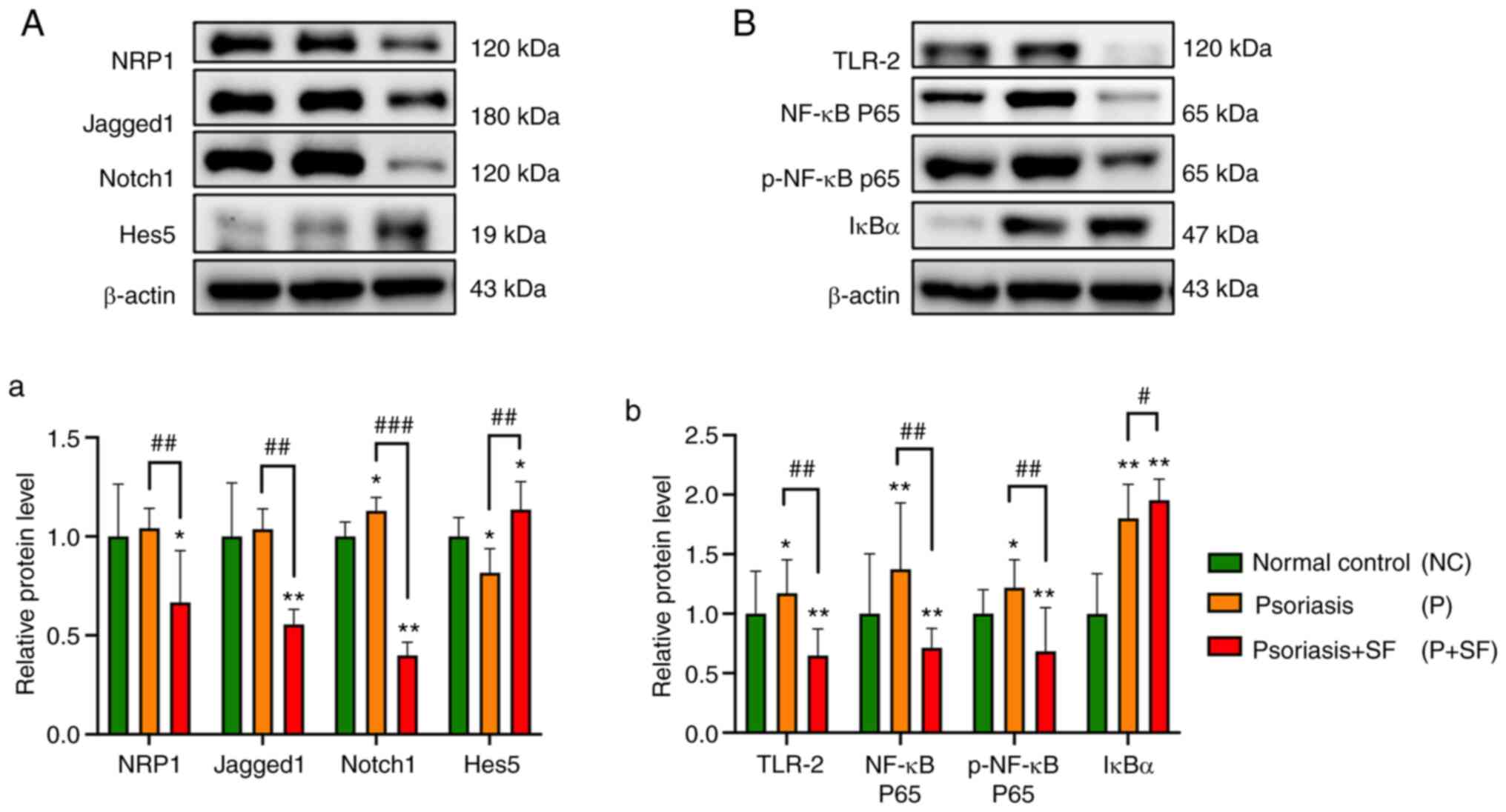 | Figure 5Protein expressions of
Jagged1/Notch1/HES5 and TLR-2/IκB-α/NF-κB p65 signaling pathway on
skin lesions of imiquimod-induced psoriasis model. (A and a)
Western blot analysis of Notch signaling and NRP1 in skin lesions.
The mammalian Notch signaling pathway consists of four Notch
receptors (Notch 1, 2, 3, and 4) and five Notch ligands (Jagged 1,
and 2, and Delta-like 1, 3 and 4). HES5 is an important downstream
target gene of Notch signaling pathway. NRP1 is involved in
keratinocyte hyperproliferation of psoriasis accompanied by
inflammation activated angiogenesis. (B and b) Western blot
analysis of TLR-2/IκB-α/NF-κB p65 signaling in skin lesions. Values
are expressed as mean ± SD (*P<0.05,
**P<0.01 vs. the normal control;
#P<0.05, ##P<0.01,
###P<0.001 vs. the psoriasis group) and were
statistically analyzed with one-way ANOVA. NC, normal control; P,
psoriasis; P + SF, psoriasis + SF; SF, stimulating food; p-,
phosphorylated. |
Discussion
The major finding of the current study is that SF
could aggravate the progression of psoriasis, which may occur by
inducing a chronic inflammatory gut tract with unbalanced
polarization of macrophages (M1/M2) and production of
proinflammatory cytokines. Once these cytokines are released into
blood circulation and transported to skin lesions, they easily
activate the primary abnormal immune cells of skin lesions and
cause an inflammatory response. Finally, the disease is exacerbated
after certain food intake. Future research should explore the role
of serum cytokines of psoriasis in crosstalk to resident immune
cells of skin lesions, such as Langerhans cells (31) and CD8+ tissue-resident memory T
cells (TRMs). Therefore, the potential efficacy of preventative
measures against gut inflammation should be assessed and could be
of great value.
According to the skin lesion of psoriasis and PASI
scores, guidelines of different countries have recommended detailed
therapeutic regimens. While alternative options for the treatment
of psoriasis with various comorbidities are still being sought. In
Asia, a 15-year nationwide population-based cohort study of Korea
showed that atherosclerotic cardiovascular risk increased in
psoriasis (32). In the US,
results from the National Health and Nutrition Examination Survey
also indicated that an increased risk of psoriatic mortality is
partially mediated by an increased prevalence of cardiovascular
disease, diabetes and cancer (33). In TCM, the body condition of all
individuals who suffer from psoriasis has been taken into
consideration more than mere skin lesions. Based on the
constitution of patients, suggestions as to what type of food in
certain nature and taste should be avoided have been given to
patients. As mentioned earlier, it amount to one thing, metabolic
disorder, which is closely related to lifestyle, particularly what
one eats. Data from real world concerning psoriasis gradually
underlined the importance of lifestyle changes. To clarify the
underlying mechanisms of SF on the progression of psoriasis,
preliminarily tests were performed.
In the present study, BALB/c mice were administered
an SF diet every day to construct ‘unhealthy gut’ type. After
psoriasis-like dermatitis modeling, skin lesions in the SF-disposed
group significantly worsened. During these processes, SF tended not
to increase the body weight and impact on defecation of the mouse
as was expected. Additionally, the ethology of mice showed that
SF-disposed mice were more irritable and like to bully one another.
Thus, it was hypothesized that SF-induced aggravated lesions might
mediated by unknown response in the gut.
In the present study, SF-induced pathology of the
gut tract and its corresponding skin lesions were evaluated. The
mucosal immune system of the gastrointestinal tract plays a unique
role in the balance of commensal microbes and resident immune
cells. Among them, accumulating evidence suggests that an
overwhelming load of nutrients and their metabolites disturbs the
polarization of macrophages, which are closely related to
inflammatory response (34). The
data of the present study showed an increased M1 macrophages in gut
tract and skin lesions of the SF group, which was marked by
elevated expression of CD11b. Subsequently, alterations in the
serum levels of cytokines (more TNF-α, and less IL-10, IL-35)
suggested a low gradation systemic inflammation due to activation
of M1 macrophages. However, as key cytokine in the progression of
psoriasis, IL-17 levels were not enhanced as expected. At present,
researchers have found IL-17 levels were higher both in serum and
lesions of psoriasis patients, such just reported in patients who
suffered from serious advanced psoriasis and with higher PASI score
(35,36). In vivo, skin lesions of
psoriasis-like dermatitis were different from patients both in
pathogenesis and PASI. Thus, such differences of serum cytokines in
mice were probably induced by food intake, which further confirmed
the influence of food on gut tissue.
Mechanistically, unbalanced macrophages caused gut
local immune response disorder and caused aforementioned cytokines
release, which induced mild systemic inflammation of SF diet mice.
Once these cytokines were translocated to skin and interacted with
local immune cells, progression of primary skin lesions of
psoriasis occurred, present as lesions worsening in mPASI scores
and skin histology. Therefore, NF-κB p65 nuclear transcription of
HaCaT cells was promoted significantly by serum from SF diet mice
and was highly associated with inflammation-induced proliferation
of epidermis.
The present study found that unstable resident
immune cells in primary skin lesions was the reason for interval or
sequential therapy of calcipotriol or Daivobet (37) even in the stable stage, which has
been highly recommended in guidelines of psoriasis (38). In the pathogenesis of psoriasis, a
signal transduction pathway that connects PAMPs recognition to
NF-κB activation is involved in the progression of psoriasis. TLRs,
especially TLR-2, build a bridge between innate immunity and
autoimmunity. The present study revealed that TLR-2/NF-κB p65 and
Notch signaling were activated in psoriasis-like dermatitis but
overinhibited by SF, which was inconsistent with aforementioned
findings. There are several possibilities to account for this. As
mentioned in the section on IHC, some skin lesions in SF-disposed
mice showed necrotic foci and spongiotic condition. Normal
expression of NF-κB p65 and Notch are indispensable for skin
development and homeostasis (39,40).
Taken together, it was hypothesized that such an unusual
downregulation of the aforementioned proteins could be attributed
to serious skin damage triggered by cytokines from gut or negative
feedback regulation of mice.
The national epidemiological survey of psoriasis in
1984 showed that psoriasis prevalence was higher in North China
than South China and more frequent in men compared with women
(41). Cases of psoriasis flare
following some risk factors, cold weather in mild-high latitudes
and more consumption of red meat (beef and mutton, often considered
as common kinds of SF) are more likely to be the demographic and
epidemiological drivers (42). As
is well-known, men have more social intercourse and unhealthful
dietary behaviors when exposed to urban food environment (43). It is recommended that patients
should avoid taking SF when suffering from an acute skin
inflammation. Although the present study offered new insights on
the role of SF in psoriasis relapse and preliminarily explored its
possible mechanism, effects of diet on female mice and small number
of experimental mice should be investigated. To further investigate
influence of the common kinds of SF on the progress of psoriasis,
key laboratory of meat processing and quality control on SF diets
with added beef and mutton and the aforementioned limits should be
employed.
At present, diet-induced intestinal homeostasis, gut
microbiota and their metabolites are still unknown in psoriasis.
Future studies in other fields of medicine, 16S rRNA amplicon
sequencing would be valuable in determining how microbes respond to
diet. Otherwise, microbiota metabolites in stool could be
identified by gas chromatographic mass spectrometry, including
l-aspartic acid, cholestan-3-ol (5β, 3α), campesterol, lactic acid,
and butyrate, which have been associated with lipogenesis and
inflammation. However, these effects on psoriasis remain to be
elucidated and should be further studied.
SF is one of the danger factors responsible for the
progression of psoriasis and can change the gut barrier and
metabolite of the patient. Then, damaging substances from the gut
are released into the blood circulation and cause low-level
systemic inflammatory response, thereby exacerbating local unstable
TRMs or APCs in primary skin lesions. Consequently, it induces the
progression of the advanced stage of psoriasis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Construction
Fund of Medical Key Disciplines of Hangzhou (grant no.
2020SJZDXK03), National Key Research and Development Program of
China (grant no. 2018YFC1705300) and Zhejiang Hospital of
Traditional Chinese Medicine, CaoYi TCM studio (grant no.
321028-2019-0001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization and visualization was by FX.
Methodology and investigation by WZ and YW. FX and XY confirm the
authenticity of all the raw data. FX and XY analyzed the data,
revised the draft and revised the figures and tables. Funding
acquisition and work modification was by YC. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
compliance with the guidelines set by the Zhejiang Chinese Medical
University Laboratory Animal Research Center (approval no.
IACUC-20190408-12).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Griffiths CEM, Armstrong AW, Gudjonsson JE
and Barker JNWN: Psoriasis. Lancet. 397:1301–1315. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Takeshita J, Grewal S, Langan SM, Mehta
NN, Ogdie A, Van Voorhees AS and Gelfand JM: Psoriasis and comorbid
diseases: Epidemiology. J Am Acad Dermatol. 76:377–390.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Madden SK, Flanagan KL and Jones G: How
lifestyle factors and their associated pathogenetic mechanisms
impact psoriasis. Clin Nutr. 39:1026–1040. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhu FY and Xie GQ: Discussion on Chinese
Medicine Diet taboo-fawu. Chinese Journal of Traditional Chinese
Medicine and Pharmacy. 7:3104–3106. 2018.
|
|
5
|
Yu S, Wu X, Zhou Y, Sheng L, Jena PK, Han
D, Wan YJY and Hwang ST: A Western diet, but not a high-fat and
low-sugar diet, predisposes mice to enhanced susceptibility to
imiquimod-induced psoriasiform dermatitis. J Invest Dermatol.
139:1404–1407. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boericke H: Critical considerations on a
new treatment for psoriasis. Dtsch Gesundheitsw. 7:919–922.
1952.PubMed/NCBI(In German).
|
|
7
|
Petrozzi JW and Rosenbloom J:
Low-tryptophan diet in treatment of psoriasis. JAMA. 205:345–346.
1968.PubMed/NCBI
|
|
8
|
Zackheim HS and Farber EM: Low-protein
diet and psoriasis. A hospital study. Arch Dermatol. 99:580–586.
1969.PubMed/NCBI
|
|
9
|
Kragballe K and Fogh K: A low-fat diet
supplemented with dietary fish oil (Max-EPA) results in improvement
of psoriasis and in formation of leukotriene B5. Acta Derm
Venereol. 69:23–28. 1989.PubMed/NCBI
|
|
10
|
Adawi M, Damiani G, Bragazzi NL,
Bridgewood C, Pacifico A, Conic RRZ, Morrone A, Malagoli P, Pigatto
PDM, Amital H, et al: The impact of intermittent fasting (Ramadan
Fasting) on psoriatic arthritis disease activity, enthesitis, and
dactylitis: A multicentre study. Nutrients. 11(601)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Passali M, Josefsen K, Frederiksen JL and
Antvorskov JC: Current evidence on the efficacy of gluten-free
diets in multiple sclerosis, psoriasis, type 1 diabetes and
autoimmune thyroid diseases. Nutrients. 12(2316)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Spiera H and Lefkovits AM: Turkey,
tryptophan, and psoriasis. Lancet. 2(1418)1967.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Phan C, Touvier M, Kesse-Guyot E, Adjibade
M, Hercberg S, Wolkenstein P, Chosidow O, Ezzedine K and Sbidian E:
Association between mediterranean anti-inflammatory dietary profile
and severity of psoriasis: Results from the NutriNet-Santé cohort.
JAMA Dermatol. 154:1017–1024. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Balić A, Vlašić D, Žužul K, Marinović B
and Mokos ZB: Omega-3 versus omega-6 polyunsaturated fatty acids in
the prevention and treatment of inflammatory skin diseases. Int J
Mol Sci. 21(741)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barrea L, Savanelli MC, Di Somma C,
Napolitano M, Megna M, Colao A and Savastano S: Vitamin D and its
role in psoriasis: An overview of the dermatologist and
nutritionist. Rev Endocr Metab Disord. 18:195–205. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Millsop JW, Bhatia BK, Debbaneh M, Koo J
and Liao W: Diet and psoriasis, part III: Role of nutritional
supplements. J Am Acad Dermatol. 71:561–569. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen W, Zhou X and Zhu W: Trace elements
homeostatic imbalance in psoriasis: A meta-analysis. Biol Trace
Elem Res. 191:313–322. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ely PH: Is psoriasis a bowel disease?
Successful treatment with bile acids and bioflavonoids suggests it
is. Clin Dermatol. 36:376–389. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zackheim HS: Taurine and diet in
psoriasis. Arch Dermatol. 118(961)1982.PubMed/NCBI
|
|
20
|
Reich K, Augustin M, Thaçi D, Pinter A,
Leutz A, Henneges C, Schneider E, Schacht A, Dossenbach M and
Mrowietz U: A 24-week multicentre, randomized, open-label,
parallel-group study comparing the efficacy and safety of
ixekizumab vs. fumaric acid esters and methotrexate in patients
with moderate-to-severe plaque psoriasis naive to systemic
treatment. Br J Dermatol. 182:869–879. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jena PK, Sheng L, McNeil K, Chau TQ, Yu S,
Kiuru M, Fung MA, Hwang ST and Wan YJY: Long-term Western diet
intake leads to dysregulated bile acid signaling and dermatitis
with Th2 and Th17 pathway features in mice. J Dermatol Sci.
95:13–20. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu S, Wu X, Shi Z, Huynh M, Jena PK, Sheng
L, Zhou Y, Han D, Wan YJY and Hwang ST: Diet-induced obesity
exacerbates imiquimod-mediated psoriasiform dermatitis in anti-PD-1
antibody-treated mice: Implications for patients being treated with
checkpoint inhibitors for cancer. J Dermatol Sci. 97:194–200.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fernández-Armenteros JM, Gómez-Arbonés X,
Buti-Soler M, Betriu-Bars A, Sanmartin-Novell V, Ortega-Bravo M,
Martínez-Alonso M, Garí E, Portero-Otín M, Santamaria-Babi L and
Casanova-Seuma JM: Psoriasis, metabolic syndrome and cardiovascular
risk factors. A population-based study. J Eur Acad Dermatol
Venereol. 33:128–135. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mahil SK, McSweeney SM, Kloczko E, McGowan
B, Barker JN and Smith CH: Does weight loss reduce the severity and
incidence of psoriasis or psoriatic arthritis? A critically
appraised topic. Br J Dermatol. 181:946–953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
De Angelis M, Garruti G, Minervini F,
Bonfrate L, Portincasa P and Gobbetti M: The food-gut human axis:
The effects of diet on gut microbiota and metabolome. Curr Med
Chem. 26:3567–3583. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gentile CL and Weir TL: The gut microbiota
at the intersection of diet and human health. Science. 362:776–780.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tran HQ, Bretin A, Adeshirlarijaney A,
Yeoh BS, Vijay-Kumar M, Zou J, Denning TL, Chassaing B and Gewirtz
AT: ‘Western Diet’-induced adipose inflammation requires a complex
gut microbiota. Cell Mol Gastroenterol Hepatol. 9:313–333.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ye C, Yano H, Workman CJ and Vignali DAA:
Interleukin-35: Structure, function and its impact on
immune-related diseases. J Interferon Cytokine Res. 41:391–406.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang BY, Cheng YG, Liu Y, Liu Y, Tan JY,
Guan W, Guo S and Kuang HX: Datura Metel L. Ameliorates
imiquimod-induced psoriasis-like dermatitis and inhibits
inflammatory cytokines production through TLR7/8-MyD88-NF-κB-NLRP3
inflammasome pathway. Molecules. 24(2157)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Marasca C, Scala E, Di Caprio R, Raimondo
A, Cacciapuoti S, Balato A and Fabbrocini G: Notch dysregulation
and hidradenitis suppurativa, psoriasis, atopic dermatitis and
lichen planus: Let's talk about Numb. Br J Dermatol. 180:950–951.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kashem SW, Haniffa M and Kaplan DH:
Antigen-presenting cells in the skin. Annu Rev Immunol. 35:469–499.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim ES, Han K, Kim MK, Park YM, Baek KH,
Moon SD, Han JH, Song KH and Kwon HS: Impact of metabolic status on
the incidence of psoriasis: A Korean nationwide cohort study. Sci
Rep. 7(1989)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Semenov YR, Herbosa CM, Rogers AT, Huang
A, Kwatra SG, Cohen B, Anadkat MJ and Silverberg JI: Psoriasis and
mortality in the United States: Data from the national health and
nutrition examination survey. J Am Acad Dermatol. 85:396–403.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mohammadi A, Blesso CN, Barreto GE, Banach
M, Majeed M and Sahebkar A: Macrophage plasticity, polarization and
function in response to curcumin, a diet-derived polyphenol, as an
immunomodulatory agent. J Nutr Biochem. 66:1–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Brembilla NC, Senra L and Boehncke WH: The
IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front
Immunol. 9(1682)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yilmaz SB, Cicek N, Coskun M, Yegin O and
Alpsoy E: Serum and tissue levels of IL-17 in different clinical
subtypes of psoriasis. Arch Dermatol Res. 304:465–469.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
De Simone C, Dapavo P, Malagoli P,
Martella A, Campanati A, Campione E, Errichetti E, Franchi C,
Gambardella A, Megna M, et al: Long-term proactive management of
psoriasis with calcipotriol and betamethasone dipropionate foam: An
Italian consensus through a combined nominal group technique and
Delphi approach. Int J Dermatol. 61:1543–1551. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Benhadou F, Mintoff D and Del Marmol V:
Psoriasis: Keratinocytes or immune cells-which is the trigger?
Dermatology. 235:91–100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Thatikonda S, Pooladanda V, Sigalapalli DK
and Godugu C: Piperlongumine regulates epigenetic modulation and
alleviates psoriasis-like skin inflammation via inhibition of
hyperproliferation and inflammation. Cell Death Dis.
11(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Siebel C and Lendahl U: Notch signaling in
development, tissue homeostasis, and disease. Physiol Rev.
97:1235–1294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
National Psoriasis Epidemic Survey Group.
Distribution of psoriasis in China: A nation-wide screening in
1984. J Dermatol Venereol. 11:66–78. 1989.
|
|
42
|
Qiao J, Jia QN, Li F, He CX, Wu C, Yu XL,
et al: Epidemic Analysis of Dietary Risk Factors for Psoriasis
Vulgaris. The Chinese Journal of Dermatovenereology,. 31:1301–1305.
2017.
|
|
43
|
Xu Y, Yang J and Yang L: Research on
social phenomenon of Chinese dining tables. Sci Technol
Information. 10:241–242. 2011.
|