Introduction
The most common causes of malignant biliary
stricture (MBS) are the primary pancreaticobiliary tumors and other
local tumors (such as gallbladder cancer and liver metastatic
cancer) that compress the bile duct (1). Patients with MBS often have no
obvious symptoms or signs in the early stage of the disease and
typically have unexplained jaundice or manifestations of
cholangitis such as abdominal pain and fever in the advanced stage
(2). When MBS is diagnosed,
several patients are in the advanced stage and may have lost the
opportunity for surgery. Certain patients that need surgical
treatment may also be inoperable due to old age and/or poor
conditions. As a result, the 5-year survival rate of MBS patients
is <5% (3). At present,
endoscopic placement of bile duct stents is the first choice for
palliative treatment of unresectable MBS and is also recommended to
relieve biliary obstruction for patients who plan to undergo
surgery but have cholangitis prior to surgery (4,5).
Currently, available bile duct stents include
plastic stents (PSs) and self-expandable metal stents (SEMSs). The
latter can be subdivided into uncovered self-expandable metal
stents (USEMSs) and covered self-expandable metal stents (CSEMSs).
PSs are composed of polyethylene, polyurethane, or Teflon, whereas
SEMSs are made of various metal alloys that are constructed to
achieve adequate radial expandable force without sacrificing
flexibility and conformability to the duct (6). To better counteract tumor in growth
in USEMSs, CSEMSs were developed by placing a thin nonporous
membrane on the inside of the metal mesh (5,7).
Studies have shown that SEMSs have the advantages of longer stent
patency and lower stent obstruction rates over PSs in the
palliative treatment of patients with MBS that cannot be surgically
resected (7). However, the
efficacy and safety of CSEMSs vs. USEMSs in the treatment of MBS
have not been clarified. There is still controversy in the
selection of stents, and most choices are made based on the
preference and experiences of endoscopists. Furthermore, to the
best of our knowledge, no similar studies have been reported in the
Chinese population. In the present study, the efficacy and safety
of USEMSs and CSEMSs in the palliative treatment of malignant
common bile duct strictures were compared as a 5-year retrospective
study from the Chinese population in order to provide a reference
for endoscopic physicians to choose the appropriate stent.
Patients and methods
Patient selection
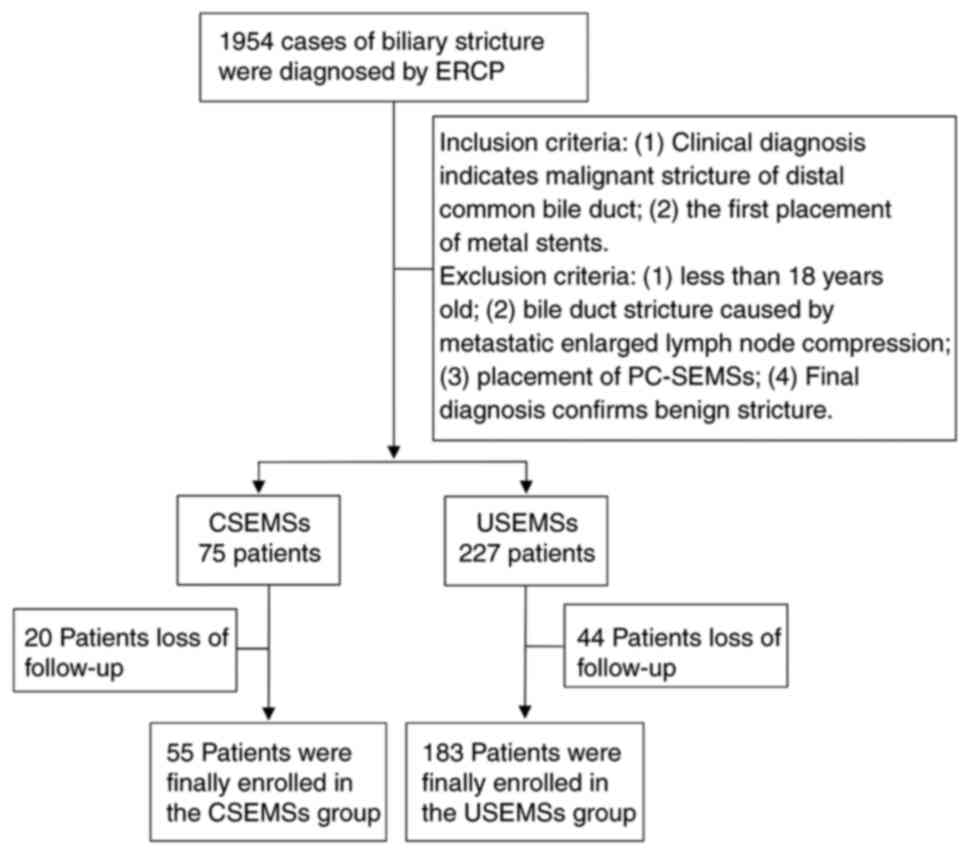
The study was designed as a single-center
retrospective study that collected data from all patients with a
clinical diagnosis of distal MBS who underwent SEM placement for
the first time at The First Affiliated Hospital of Nanchang
University (Nanchang, China) between November 2014 and March 2019.
All the patients with distal MBS involved in this research did not
undergo surgery after stent placement due to the advanced nature of
the tumor, old age, or poor conditions. Exclusion criteria
included: i) <18 years old; ii) metastatic enlarged lymph node
compression in the bile duct; iii) placement of partially covered
SEMs (PC-SEMSs) in the bile duct; and iv) benign stricture
confirmed by a final diagnosis (Fig.
1). The indications for CSEMS placement included distal biliary
strictures and the patient intention for stent removal or
replacement if the old one was obstructed. The contraindications
for CSEMS placement were hilar biliary strictures. The indications
for USEMS placement included malignant biliary strictures and
patient intention to not remove the stent. The contraindications
for USEMS placement were benign biliary strictures. All patients
involved in this study provided informed consent for future
research when they underwent endoscopic retrograde
cholangiopancreatography (ERCP). The study was approved by the
Ethics Committee of the First Affiliated Hospital of Nanchang
University (approval number, IIT2019036) and was performed in
accordance with the ethical standards described in the 1964
Declaration of Helsinki and its later amendments (8). All included cases were recorded in
the Human Genetic Resources Center of the First Affiliated Hospital
of Nanchang University.
Patient characteristics
General information on the patients was collected,
including sex, age, tumor type, tumor staging, and laboratory test
results (routine blood tests and liver function tests) within 1
week prior to and following stent placement. There were 33 males
(60.0%) and 22 females (40.0%) in the CSEMS group, and 94 males
(51.4%) and 89 females (48.6%) in the USEMS group, with no
significant difference in sex between the two groups (P=0.260). The
age of all patients ranged from 25 to 93 years old. The age (mean ±
standard deviation) of the patients in the CSEMS and USEMS groups
was 71.47±12.22 and 70.10±10.92 years old, respectively, with no
significant difference in age between the two groups (P=0.192). The
presence of the gallbladder, and whether antibiotics were used
before stent placement was also recorded. Procedure-related data
included pre-cut before stent placement, endoscopic sphincterotomy,
pancreatic duct stent placement and biliary stent specifications.
Post-ERCP surgical operation and radiotherapy or chemotherapy after
stent placement were also recorded. Adverse events were recorded
including biliary infection, post-ERCP pancreatitis (PEP),
hyperamylasemia, bleeding, and perforation, as well as
procedure-related mortality.
Outcome variables and end events
The primary outcomes included the average stent
patency time, stent patency rate, and incidence of adverse events.
The secondary outcomes included average patient survival time,
survival rate, and liver function. The end point of this study was
stent dysfunction or patient death during follow-up.
Stent placement
All ERCP procedures were performed by experienced
endoscopic physicians (each performing >200 ERCP procedures per
year). All patients underwent ERCP after anesthesia with propofol.
The diameter of SEMSs was 10 mm, and SEMSs with different lengths
(50, 60, 70 or 80 mm) were selected according to the location and
length of the biliary stricture. The proximal end of the stent was
placed at least 10 mm beyond the stricture, and the distal end was
placed at least 10 mm outside the duodenal papilla. Both CSEMSs and
USEMSs were WallFlex™ biliary self-expandable metal
stents produced by Boston Scientific Corporation.
Event definition
Distal biliary stricture was defined as a stricture
of the distal half of the extrahepatic bile duct (9). The diagnostic criteria for MBS were
malignant signs confirmed by cytological examination, endoscopic
biopsy, surgical specimens, or other pathological examinations. For
patients who could not be diagnosed by the above-mentioned
pathological examinations, or patients who refused or could not
complete the examinations, the stricture was regarded as MBS if the
patients demonstrated malignant progression after 1 year of
follow-up (10,11). Stent patency was assessed as the
period from stent insertion to stent dysfunction or patient death.
Survival time was defined as the overall survival time, from stent
insertion to death. Survival rate was defined as the percentage of
patients alive as a product of the starting number of patients.
Stent dysfunction was diagnosed when the patient developed signs of
cholangitis (fever, tenderness on the right upper quadrant, and/or
≥2-fold elevation of the total serum bilirubin above the baseline
level following stent placement) (12). Technical success was defined as the
successful placement of the stent across the stricture according to
appropriate radiographic positioning with bile or contrast outflow
(13).
Statistical analysis
All analyses were performed using SPSS version 25.0
(IBM Corp.). A Student's t-test was used for the comparison of
continuous variables. A χ2 test or Fisher's exact test
was used for comparison of categorical variables. Mixed ANOVA
followed by Bonferroni/Sidak's test was used for multiple
comparisons. The cumulative stent patency rate and patient survival
rate were analyzed using Kaplan-Meier curves. If the Kaplan-Meier
curves of the two groups did not cross each other, a log-rank test
was performed. Otherwise, the two-stage procedure was performed. If
the log-rank test gave a significant result, then the entire
two-stage procedure was halted and it was concluded that the
Kaplan-Meier curves of the two groups were significantly different.
Otherwise, the stage-II test was performed, which was designed
specifically for detecting the crossing difference between the two
hazard rate functions and has the property that its test statistic
was independent of the log-rank test statistic. P<0.05 was
considered to indicate a statistically significant difference.
Results
General patient information
A total of 238 patients who underwent SEMS placement
with ERCP were included in the study (55 in the CSEMSs group and
183 in the USEMSs group). The primary reason for the large
difference in the numbers between the two groups was hospital
procurement. USEMSs were introduced into The First Affiliated
Hospital of Nanchang University in 2014, whereas CSEMSs were not
introduced until 2016, thus USEMSs were the only choice of SEMSs
for patients prior to 2016. All the patients that accepted SEMSs
were included in this retrospective study, such that the number of
USEMS patients was ~3x larger than that of the CSEMSs patients.
Elderly patients >70 years old were predominant in both groups
and the age distribution between the two groups did not differ
significantly (P=0.192). Regarding the causes of MBS, there were 27
cases (49.1%) of pancreatic cancer, 15 (27.3%) of
cholangiocarcinoma, 1 (1.8%) of gallbladder cancer, 8 (14.5%) of
duodenal papillary cancer, and 4 (7.3%) of others in the CSEMSs
group and 76 (41.6%), 74 (40.4%), 9 (4.9%), 13 (7.1%) and 11
(6.0%), respectively, in the USEMSs group. There was no significant
difference in the cause distribution of MBS between the two groups
(P>0.05), no difference in the tumor staging between the two
groups (P>0.05), and the technical success rate of stent
placement in both groups was 100%. The number of patients who
underwent cholecystectomy prior to stent placement was 6 (10.9%) in
the CSEMSs group and 14 (7.7%) in the USEMSs group, with no
significant difference (P=0.418). Antibiotics use prior to stenting
was more frequently used in the CSEMSs group (n=13, 23.6%) than in
the USEMSs group (n=20, 10.9%) (P=0.017). In terms of ERCP-related
procedures, no significant difference was seen in the pre-cut,
endoscopic sphincterotomy, and pancreatic duct stent placement
between the two groups (P>0.05). In terms of stent length,
biliary stents 60 mm in length were more commonly used in the
CSEMSs group (n=41, 74.5%) than in the USEMSs group (n=76, 41.5%)
(P<0.001). No patients underwent surgical operations after stent
placement in either group and there was no significant difference
with regard to radiotherapy or chemotherapy after stent placement
between the two groups (P=0.458) (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristics | CSEMSs | USEMSs | Ρ-value |
|---|
| Number of patients,
n | 55 | 183 | |
| Sex, n (%) | | | 0.260 |
|
Male | 33 (60.0) | 94 (51.4) | |
|
Female | 22 (40.0) | 89 (48.6) | |
| Age, years | | | |
|
Mean ±
SD | 71.47±12.22 | 70.10±10.92 | 0.192 |
|
<50 | 4 (7.3) | 9 (4.9) | 0.504 |
|
50-60 | 7 (12.7) | 25 (13.7) | 0.859 |
|
61-70 | 12 (21.8) | 50 (27.3) | 0.415 |
|
>70 | 32 (58.2) | 99 (54.1) | 0.593 |
| Causes of
strictures, n (%) | | | |
|
Pancreatic
cancer | 27 (49.1) | 76 (41.6) | 0.321 |
|
Cholangiocarcinoma | 15 (27.3) | 74 (40.4) | 0.077 |
|
Gallbladder
cancer | 1 (1.8) | 9 (4.9) | 0.461 |
|
Duodenal
papillary carcinoma | 8 (14.5) | 13 (7.1) | 0.104 |
|
Others | 4 (7.3) | 11 (6.0) | 0.754 |
| Tumor staging, n
(%) | | | |
|
I | 23 (41.8) | 79 (43.2) | 0.859 |
|
II | 5 (9.1) | 20 (10.9) | 0.697 |
|
III | 6 (10.9) | 21 (11.5) | 0.908 |
|
IV | 21 (38.2) | 63 (34.4) | 0.609 |
| Technical success,
n (%) | 55(100) | 183(100) | 1.000 |
| Cholecystectomy
before ERCP, n (%) | 6 (10.9) | 14 (7.7) | 0.418 |
| Antibiotics use
before stent placement, n (%) | 13 (23.6) | 20 (10.9) | 0.017a |
| Pre-cut before
stent placement, n (%) | 10 (18.2) | 24 (13.1) | 0.346 |
| Endoscopic
sphincterotomy, n (%) | 20 (36.4) | 54 (29.5) | 0.335 |
| Pancreatic duct
stent placement, n (%) | 16 (29.1) | 39 (21.3) | 0.230 |
| Size of SEMSs in
mmc | | | |
|
10x50 | Unavailable | 6 (3.3) | - |
|
10x60 | 41 (74.5) | 76 (41.5) |
<0.001b |
|
10x70 | Unavailable | 33 (18.0) | - |
|
10x80 | 14 (25.5) | 68 (37.2) | 0.109 |
| Post-ERCP surgical
operation, n (%) | 0 | 0 | - |
| Radiotherapy or
chemotherapy after stent placement, n (%) | 7 (12.7) | 17 (9.3) | 0.458 |
Laboratory results
There was no significant difference in liver
function between the CSEMSs and USEMSs groups both prior to and
following the placement of SEMSs (P>0.05). The levels of serum
bilirubin, aminotransferase, γ-glutamyl transferase (γ-GT), and
other parameters. after SEMS placement were significantly lower
than those prior to SEMSs placement in both groups (P<0.05). The
amylase levels after ERCP were significantly higher than that
before ERCP in both groups (P<0.05) (Table II).
 | Table IILiver function parameters (mean ± SD)
prior to and following stent placement. |
Table II
Liver function parameters (mean ± SD)
prior to and following stent placement.
| Parameter | Time | CSEMSs | USEMSs | P1a | P2b | P3c |
|---|
| TBIL, µmol/l | Pre-ERCP | 212.6±139.1 | 226.9±127.2 |
<0.001a | 0.209 | 0.431 |
| | Post-ERCP | 172.9±119.8 | 196.4±106.1 | | | |
| |
P4d | 0.021e |
<0.001f | | | |
| DBIL, µmol/l | Pre-ERCP | 164.1±103.6 | 173.9±97.5 |
<0.001e | 0.545 | 0.241 |
| | Post-ERCP | 127.7±87.6 | 146.0±78.2 | | | |
| |
P4d | 0.040e |
<0.001f | | | |
| ALT, U/l | Pre-ERCP | 109.4±83.0 | 127.9±92.6 |
<0.001a | 0.597 | 0.181 |
| | Post-ERCP | 80.1±68.4 | 86.6±52.3 | | | |
| |
P4d | 0.044e |
<0.001f | | | |
| AST, U/l | Pre-ERCP | 105.1±68.8 | 121.1±77.3 |
<0.001a | 0.341 | 0.200 |
| | Post-ERCP | 77.8±85.3 | 87.5±67.2 | | | |
| |
P4d | 0.030e |
<0.001f | | | |
| ALP, U/l | Pre-ERCP | 525.7±314.2 | 574.7±341.9 | 0.071 | 0.890 | 0.211 |
| | Post-ERCP | 474.9±305.0 | 515.5±259.9 | | | |
| |
P4d | 0.356 | 0.061 | | | |
| γ-GT, U/l | Pre-ERCP | 569.2±483.8 | 510.1±309.8 | 0.001e | 0.493 | 0.329 |
| | Post-ERCP | 440.5±358.5 | 425.9±261.2 | | | |
| |
P4d | 0.032e | 0.005g | | | |
| Amylase, U/l | Pre-ERCP | 79.8±80.8 | 121.3±417.9 |
<0.001a | 0.183 | 0.800 |
| | Post-ERCP | 296.1±427.2 | 232.5±381.5 | | | |
| |
P4d | 0.001f | 0.005g | | | |
Stent patency and patient
survival
The overall stent dysfunction (caused by stent
obstruction or stent migration) rates in the CSEMSs group and the
USEMSs group within the follow-up period were 20.0 and 18.6%
respectively, with no significant differences between the two
groups. The stent patency time of the CSEMSs group (262.8±195.3
days) was significantly longer than that of the USEMSs group
(169.5±155.7 days) (P=0.002). The stent patency rates at 1, 3, 6
and 12 months after stent placement were 90.9, 74.5, 56.4 and
29.1%, respectively, in the CSEMSs group, and 89.6, 62.3, 33.9 and
12.0%, respectively, in the USEMSs group. The stent patency rates
of the two groups did not differ significantly 1 and 3 months after
stent placement (P>0.05), but the stent patency rates of the
CSEMSs group at 6 and 12 months were significantly higher than
those of the USEMSs group (P<0.05). The patient survival time of
the CSEMSs group (273.9±197.6 days) was significantly longer than
that of the USEMSs group (184.9±167.6 days) (P=0.003). Patient
survival rates at 1, 3, 6 and 12 months were 94.5, 76.4, 58.2 and
34.5%, respectively, in the CSEMSs group and 90.7, 65.6, 37.2 and
13.7%, respectively, in the USEMSs group. No significant difference
in survival rates was observed between the two groups at 1 and 3
months after stent placement (P>0.05), but the survival rates of
the CSEMSs group at 6 and 12 months were significantly higher than
those of the USEMSs group (P<0.05) (Table III). It was noted that 5 patients
with stage I tumors from the USEMSs group lived for a longer period
of time (>600 days after stent placement) than all the patients
from the CSEMSs group although there was no significant difference
between the two groups in the ratios of all the stages of tumors.
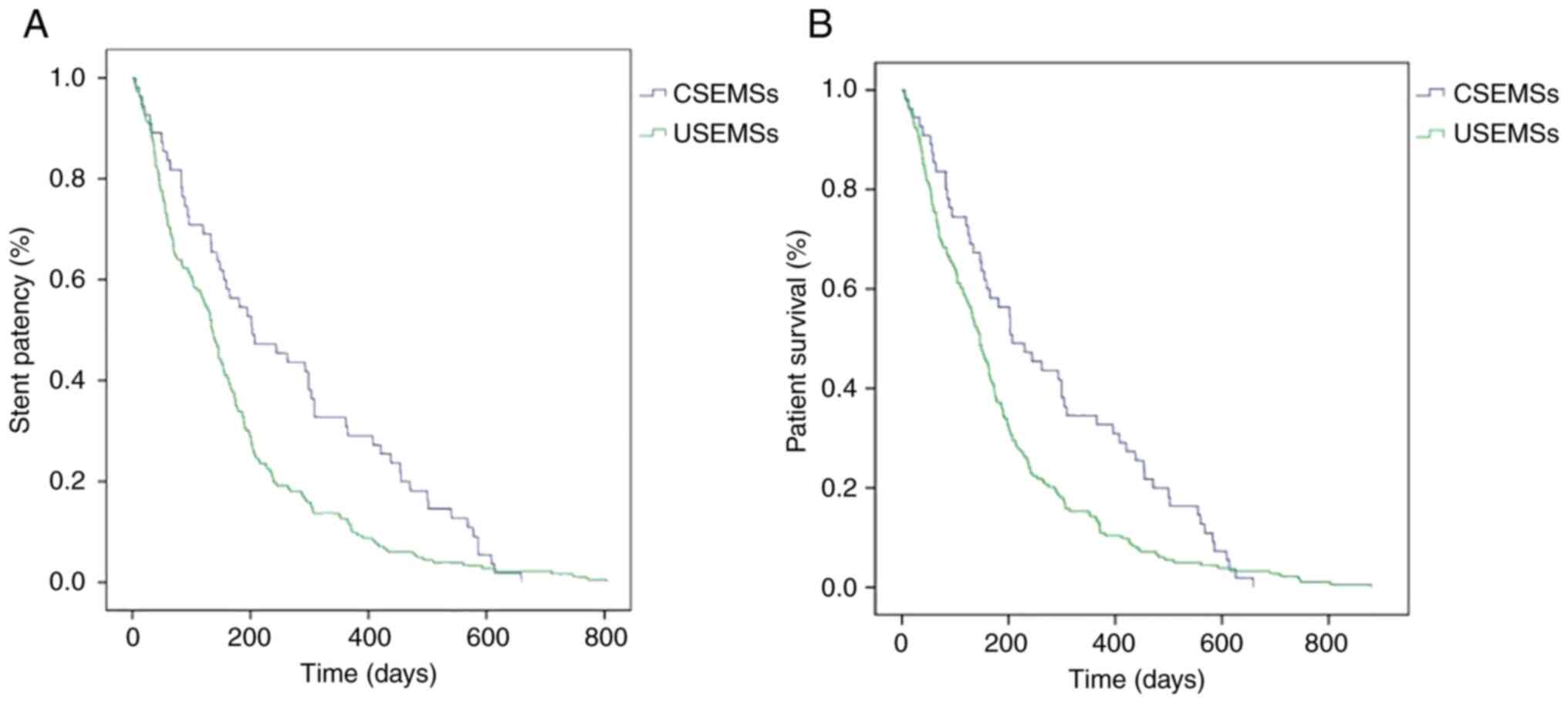
The Kaplan-Meier curve showed that the cumulative stent patency
rate of the CSEMSs group was higher than that of the USEMSs group
(P=0.003) (Fig. 2A) and the
cumulative patient survival rate of the CSEMSs group was
significantly higher than that of the USEMSs group (P=0.009)
(Fig. 2B).
 | Table IIIComparison of stent patency time
(rate) and patient survival time (rate). |
Table III
Comparison of stent patency time
(rate) and patient survival time (rate).
| Parameter | CSEMSs | USEMSs | P-value |
|---|
| Stent dysfunction,
n (%) | 11 (20.0) | 34 (18.6) | 0.813 |
|
Stent
obstruction | 10 (90.9) | 34(100) | 0.548 |
|
Stent
migration | 1 (9.1) | 0 (0.0) | 0.548 |
| Stent patency time,
daysa | 262.8±195.3 | 169.5±155.7 | 0.002b |
| Stent patency rate,
% | | | |
|
1 month | 90.9 | 89.6 | 0.780 |
|
3
months | 74.5 | 62.3 | 0.095 |
|
6
months | 56.4 | 33.9 | 0.003b |
|
12
months | 29.1 | 12.0 | 0.002b |
| Patient survival
time, daysa | 273.9±197.6 | 184.9±167.6 | 0.003b |
| Patient survival
rate, % | | | |
|
1 month | 94.5 | 90.7 | 0.579 |
|
3
months | 76.4 | 65.6 | 0.132 |
|
6
months | 58.2 | 37.2 | 0.006b |
|
12
months | 34.5 | 13.7 |
<0.001c |
Adverse events
The total incidence of postoperative adverse events
in the CSEMSs group and the USEMSs group was 25.5 and 19.7%,
respectively, with no significant difference (P=0.356). The
incidence of PEP in the CSEMSs group (18.1%) was higher than that
in the USEMSs group (8.8%) (P=0.049). Some of the patients in both
groups had hyperamylasemia or pancreatitis after ERCP, but not
before ERCP. Thus, amylase levels after ERCP in these patients were
significantly higher than those before ERCP, which further raised
the average amylase levels of all the patients following ERCP in
both groups. Therefore, both post-ERCP pancreatitis and post-ERCP
hyperamylasemia were the cause of the increase in amylase levels
after ERCP. There was no significant difference in the incidence of
biliary infection, hyperamylasemia, bleeding, or perforation
between the two groups (P>0.05). There was no procedure-related
death in the CSEMSs group; however, 3 patients died in the USEMSs
group, of which 1 patient died of bleeding after ERCP, and 2 died
of severe biliary infection. There was no significant difference in
procedure-related mortality between the two groups (P=1.000)
(Table IV).
 | Table IVAdverse events following stent
placement. |
Table IV
Adverse events following stent
placement.
| Adverse events | CSEMSs, n (%) | USEMSs, n (%) | P-value |
|---|
| Total | 14 (25.5) | 36 (19.7) | 0.356 |
| Biliary
infection | 2 (3.7) | 7 (3.8) | >0.999 |
| Post-ERCP
pancreatitis | 10 (18.1) | 16 (8.8) | 0.049a |
|
Hyperamylasemia | 2 (3.7) | 12 (6.6) | 0.531 |
| Bleeding | 0 (0.0) | 1 (0.5) | >0.999 |
| Perforation | 0 (0.0) | 0 (0.0) | - |
| Procedure-related
mortality | 0 (0) | 3 (1.6) | >0.999 |
Discussion
Endoscopic placement of stents such as SEMSs and PSs
can effectively relieve symptoms such as fever, jaundice,
itchiness, and dyspepsia, amongst others, in patients with MBS and
improve their quality of life (6).
Endoscopic stent placement has become the first choice for the
palliative treatment of patients with MBS in which the tumor cannot
be resected by surgery, and it is also the primary method of
preoperative biliary drainage for patients with cholangitis
(14). The disadvantage of PSs is
that re-obstruction occurs earlier. PSs usually need to be replaced
in ~3 months. Otherwise, fatal cholangitis may occur (15). It has been reported that SEMSs have
a longer patency time, lower stent occlusion rate, fewer adverse
events, and requires fewer interventions than PSs (15). As a result, SEMSs exhibit improved
cost-effectiveness and are currently widely used, especially for
patients whose expected survival time is >3 months (9).
In recent years, the endoscopic stent placement
technique has improved significantly. Recently, studies have
demonstrated that the technical success rate of endoscopic stenting
is close to 100% (15-17).
The results of the present study showed that the technical success
rates in both the CSEMSs group and the USEMSs group were 100%. The
demographic characteristics of the patients indicated that there
seemed to be more male patients in both the CSEMSs group and the
USMESs group than the female patients, but there was no significant
difference in sex distribution between the two groups. The majority
of patients in both groups were elderly patients, most of whom were
>70 years old, but there was no significant difference in the
age distribution between the two groups. Etiological analysis of
MBS indicated that pancreatic cancer and cholangiocarcinoma were
predominant, but there was no significant difference in the
etiology composition and tumor staging between the two groups. In
addition, there was no significant difference in cholecystectomy
before ERCP between the two groups. In terms of ERCP-related
techniques, there was no significant difference in pre-cut,
endoscopic sphincterotomy, and pancreatic duct stent placement.
Interestingly, it was noticed that antibiotics were more frequently
used prior to ERCP in the CSEMSs group than in the USEMSs group,
likely due to the possibility that endoscopic physicians tended to
use CSEMSs for patients with severe infections based on their
experience, and clinicians were more likely to use antibiotics for
patients with severe infections. It was also found that biliary
stents 60 mm in length were more often used than 80 mm in the
CSEMSs group but not in the USEMSs group and biliary stents 60mm in
length were more often used in the CSEMS group than in the USEMS
group. We hypothesize that the possible reason for this might be
that all the cases included in the present study were distal bile
duct strictures and when CSEMSs were used, endoscopists preferred
shorter stents for relieving bile duct obstructions in the CSEMSs
group to minimize the blockade of the cystic duct as longer CSEMSs
for distal biliary strictures have a higher risk of blocking the
cystic duct by the covering membrane of CSEMSs. Liver function
tests indicated that there was a significant decrease in serum
bilirubin, transaminase, and γ-GT in both the CSEMSs group and the
USEMSs group after the placement of SEMSs, indicating that both
stents could significantly improve the liver function of patients
in the short term. In addition, there was no significant difference
between the two groups in the liver function tests before or after
ERCP, indicating that CSEMSs and USEMSs played an equivalent role
in improving liver function in the short term. It was also noted
that post-ERCP serum amylase in both groups was significantly
higher than that before ERCP, possibly because certain patients in
both groups had PEP or post-ERCP hyperamylasemia. Hence, amylase
levels after ERCP in these cases were significantly higher than
those before ERCP, which further raised the average amylase level
after ERCP in both groups.
It is still contested whether to use CSEMSs or
USEMSs in the palliative treatment of patients with MBS. The
comparison of the efficacy and safety of CSEMSs and USEMSs remains
uncertain, and the results of several studies differed. Certain
studies showed that the patency time of the stents in the CSEMSs
group was significantly longer than that in the USEMSs group
(18,19), and the patency rate of the stents
in the CSEMSs group was also significantly higher (20). However, several studies have also
shown that there is no difference in the stent patency time and
stent patency rate between the two groups for the treatment of MBS
(13,21-25).
In fact, a recent randomized multicenter study by Conio et
al (26) showed the opposite
result to the aforementioned studies. Conio et al (26) found that the median stent patency
time of the USEMSs group was significantly longer than that of the
CSEMSs group (541 days vs. 240 days), but there was no significant
difference in the stent patency rate. The reason for the above
inconsistent results may lie in differences in study design,
patient selection criteria, stent materials and structures, and the
experience of endoscopists. The present study showed that although
there was no significant difference in the overall stent
dysfunction rate between the two groups within the follow-up
period, the average stent patency time of the CSEMSs group was
significantly longer than that of the USEMSs group (262.8±195.3
days vs. 169.5±155.7 days). In terms of the stent patency rate,
there was no significant difference between the two groups at 1 and
3 months after stent placement, but the stent patency rate of the
CSEMSs group at 6 and 12 months was significantly higher than that
of the USEMSs group. This indicates that CSEMSs are superior to
USEMSs in terms of stent patency time and long-term patency
rate.
Regarding the prognosis of the patients, most of the
previous studies revealed that there was no significant difference
in survival time between the CSEMSs group and the USEMSs group
(13,18,20).
However, a meta-analysis showed that patients in the CSEMSs group
had longer survival times than those in the USEMSs group (19). The results of the present study
showed that the survival time and the cumulative 1-year survival
rate of patients in the CSEMSs group were better than those in the
USEMSs group. Further analysis in the present study showed that
there was no significant difference in the survival rate between
the two groups at 1 and 3 months after stent placement, but the
survival rate of the CSEMSs group was significantly higher than
that of the USEMSs group at 6 and 12 months, which demonstrated
that the long-term survival rate of patients in the CSEMSs group
was higher than that in the USEMSs group. The survival time and
survival rate for inoperable patients may be affected by tumor
staging and chemotherapy after stent placement. In this study, it
was demonstrated that there was no significant difference in tumor
staging or chemotherapy between the two groups (Table I). Therefore, it was considered
that the reason why the CSEMS group had a longer survival time and
higher survival rate may be due to the longer stent patency time
and higher patency rate. This is because the covering membrane in
CSEMSs prevents tumor growth across the stents so CSEMSs may be
obstructed slower and obstructive cholangitis may occur later than
USEMSs. A recent randomized control trial (RCT) conducted by Seo
et al (27) showed that
during the new adjuvant therapy for patients with bile duct
obstruction caused by pancreatic cancer, the cumulative 1-year
survival rates of patients in the CSEMSs group and the USEMSs group
were 60.2 and 56.8%, respectively, with no significant difference.
However, in the present study, the cumulative 1-year survival rates
of both groups were notably lower than that reported by Seo et
al. A possible reason for this difference may lie in the fact
that fewer patients in the present study accepted radiotherapy or
chemotherapy after stent placement. It was noted that overall
survival time/rate was used in the present study rather than
disease-free survival time/rate as no patients in the present study
were actually disease-free prior to death. Furthermore, there were
two reasons stent-dysfunction-free survival was not used either:
Firstly, the stent-dysfunction-free survival time had the same
period as stent patency time according to the definition of stent
patency in the present study design, thus there was no need to show
dysfunction-free survival; secondly, overall survival was used to
demonstrate that the possible reason why the CSEMS group had a
longer survival time and higher survival rate may be the longer
stent patency time and higher patency rate as the patient overall
survival time and survival rate for inoperable patients may have
been affected by stent patency as well as tumor staging and
chemotherapy; however, there were no significant differences in
tumor staging and chemotherapy between the two groups.
A retrospective study showed that the incidence of
total post-ERCP adverse events in the CSEMSs group and the USEMSs
group was 22.8 and 15.9%, respectively (17). Similarly, our study showed that the
incidence of post-ERCP adverse events in the CSEMSs group and the
USEMSs group was 25.5 and 19.7%, respectively, with no significant
difference. However, in the present study, it was found that the
incidence of PEP in the CSEMSs group was higher than that in the
USEMSs group (18.1% vs. 8.8%). The cause of higher PEP in the
CSEMSs group may be the obstruction of pancreatic duct drainage by
the covering membrane of CSEMSs. In addition, the present study
showed no significant difference in post-ERCP biliary duct
infection and hyperamylasemia between the two groups. There were no
cases of bleeding in the CSEMSs group and 1 case in the USEMSs
group, with no significant difference in bleeding between the two
groups. In the present study, there were no perforation
complications in either group, which may be due to the experience
of the endoscopic physicians and the small sample size in this
study. In addition, all the patients in the present study exhibited
jaundice and impaired liver function, and certain patients even had
poor blood coagulation function, so only a few patients underwent
endoscopic sphincterotomy during ERCP, which might be another
reason for the lack of perforation adverse events.
Partially covered self-expandable metal stents
(PC-SEMSs) were not included in the present study according to the
study design. However, previous studies demonstrated the
application value of PC-SEMSs, although these have also been
contested. A study by Kim et al (13) showed that compared to uncovered
SEMSs, PC-SEMSs did not prolong stent patency in unresectable
malignant distal biliary obstruction. Stent migration was more
frequent with PC-SEMSs; however, tumor ingrowth was less frequent
with PC-SEMSs compared to uncovered SEMSs. Conversely, a study by
Yokota et al (17) showed
that PC-SEMSs with a proximal uncovered flared end had a longer
patency than uncovered and fully covered SEMSs by preventing tumor
ingrowth and stent migration.
Previous studies determined the diagnosis of
malignant bile duct stricture by pathology or by patients' clinical
manifestations, laboratory data, and abdominal imaging such as CT
and MRCP (12,13,15-38).
However, benign and malignant biliary strictures often have similar
clinical manifestations and imaging characteristics at the early
stage, so it is difficult to distinguish them only by clinical and
imaging data. Furthermore, the differential diagnostic yield by
ERCP is limited even by means of the SpyGlass choledochoscope
(10). Finally, some patients with
indeterminate bile duct strictures were inoperable or reluctant to
accept surgery due to old age and/or poor conditions. As a result,
whether the indeterminate biliary stricture is benign or malignant
might not be clarified during hospitalization. In this situation,
the follow-up was extremely important for the differential
diagnosis. It is recommended that the benign bile duct stricture be
considered if no malignant progression is observed by imaging or
ERCP during follow-up for at least 6 months. Otherwise, a malignant
bile duct stricture should be diagnosed (9). One year of follow-up in the present
study was performed for patients with indeterminate biliary
stricture unless they died, to maximize the reliability of the
diagnosis of malignant bile duct strictures. To sum up, the
patients with malignant bile duct strictures were enrolled based on
pathology or by follow-up according to the international consensus
to ensure the reliability of diagnosis, and this inclusion
criterion was not mentioned by previous studies. This is one
innovation and advantage of the present study compared with
previous studies.
However, the present study has some limitations. It
was a single-center and retrospective study, so there were
inevitable shortcomings. Firstly, the choice of stent type was
based on the preference of endoscopists, and there may be a
selection bias. Secondly, the prognosis of patients with advanced
stage or tumor metastasis was poor, such that died soon after stent
placement. As a result, their follow-up time was short, which may
have affected the long-term evaluation of stent function and
stent-related adverse events. Thirdly, patients receiving PC-SEMSs
were excluded, thus, it was not possible to compare all the
different types of SEMSs as palliative treatments of malignant bile
duct strictures. Additional multicenter RCTs are required for
further confirmation of the findings of the present study. Finally,
the number of patients who received USEMSs was ~3x larger than that
of the CSEMSs patients in the present study given the hospital
procurement criteria, which may have biased the results.
In conclusion, the present study showed that CSEMSs
were better than USEMSs for malignant distal biliary strictures in
terms of stent patency time and patient survival time as well as
stent patency rate and patient survival rate in the long term
(>6 months). Adverse events in the two groups occurred at a
similar rate, although the incidence of PEP was higher in the
CSEMSs group.
Acknowledgements
The authors would like to thank Professor Quqin Lu
(Medical College, Nanchang University, Nanchang, China) for
providing advice on the statistical analysis.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant no. 82160694), the project of
the Jiangxi Department of Science and Technology (grant no.
20202BBGL73109), the project of the Health and Family Planning
Commission of Jiangxi Province (grant no. 20195082), the project of
Jiangxi Clinical Research Center for Gastroenterology (grant no.
20201ZDG02007) and the Jiangxi Postgraduate Innovation Special Fund
Project (grant no. YC2021-S192).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ collected and analyzed the data, and drafted the
manuscript. ZW, ZH, XY, ZY and RC participated in the follow-up of
the patients, and the collection and analysis of the data. YC
designed the study, supervised the project and reviewed the final
manuscript. LZ, ZW, ZH, XY, ZY, RC and YC confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Nanchang University
(approval number, IIT2019036) and was performed in accordance with
the ethical standards described in the 1964 Declaration of Helsinki
and its later amendments. All included cases were recorded in the
Human Genetic Resources Center of the First Affiliated Hospital of
Nanchang University. Informed consent was obtained from all
subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gerges C, Beyna T, Tang RSY, Bahin F, Lau
JYW, van Geenen E, Neuhaus H, Nageshwar Reddy D and Ramchandani M:
Digital single-operator peroral cholangioscopy-guided biopsy
sampling versus ERCP-guided brushing for indeterminate biliary
strictures: A prospective, randomized, multicenter trial (with
video). Gastrointest Endosc. 91:1105–1113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dorrell R, Pawa S and Pawa R: Endoscopic
management of malignant biliary stricture. Diagnostics (Basel).
10(390)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aadam AA and Liu K: Endoscopic palliation
of biliary obstruction. J Surg Oncol. 120:57–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang CC, Yang TW, Sung WW and Tsai MC:
Current endoscopic management of malignant biliary stricture.
Medicina (Kaunas). 56(114)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sawas T, Al Halabi S, Parsi MA and Vargo
JJ: Self-expandable metal stents versus plastic stents for
malignant biliary obstruction: A meta-analysis. Gastrointest
Endosc. 82:256–267.e7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Almadi MA, Barkun A and Martel M: Plastic
vs. self-expandable metal stents for palliation in malignant
biliary obstruction: A series of meta-analyses. Am J Gastroenterol.
112:260–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
World Medical Association General
Assembly. World medical association declaration of Helsinki:
Ethical principles for medical research involving human subjects. J
Int Bioethique. 15:124–129. 2004.PubMed/NCBI
|
|
9
|
Nakai Y, Isayama H, Wang HP, Rerknimitr R,
Khor C, Yasuda I, Kogure H, Moon JH, Lau J, Lakhtakia S, et al:
International consensus statements for endoscopic management of
distal biliary stricture. J Gastroenterol Hepatol. 35:967–979.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fernandez Y, Viesca M and Arvanitakis M:
Early diagnosis and management of malignant distal biliary
obstruction: A review on current recommendations and guidelines.
Clin Exp Gastroenterol. 12:415–432. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singhi AD, Nikiforova MN, Chennat J,
Papachristou GI, Khalid A, Rabinovitz M, Das R, Sarkaria S, Ayasso
MS, Wald AI, et al: Integrating next-generation sequencing to
endoscopic retrograde cholangiopancreatography (ERCP)-obtained
biliary specimens improves the detection and management of patients
with malignant bile duct strictures. Gut. 69:52–61. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoon WJ, Lee JK, Lee KH, Lee WJ, Ryu JK,
Kim YT and Yoon YB: A comparison of covered and uncovered
Wallstents for the management of distal malignant biliary
obstruction. Gastrointest Endosc. 63:996–1000. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JY, Ko GB, Lee TH, Park SH, Lee YN,
Cho YS, Jung Y, Chung IK, Choi HJ, Cha SW, et al: Partially covered
metal stents may not prolong stent patency compared to uncovered
stents in unresectable malignant distal biliary obstruction. Gut
Liver. 11:440–446. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Smith AC, Dowsett JF, Russell RC, Hatfield
AR and Cotton PB: Randomised trial of endoscopic stenting versus
surgical bypass in malignant low bileduct obstruction. Lancet.
344:1655–1660. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaassis M, Boyer J, Dumas R, Ponchon T,
Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF and Burtin
P: Plastic or metal stents for malignant stricture of the common
bile duct? Results of a randomized prospective study. Gastrointest
Endosc. 57:178–182. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song TJ, Lee JH, Lee SS, Jang JW, Kim JW,
Ok TJ, Oh DW, Park DH, Seo DW, Lee SK, et al: Metal versus plastic
stents for drainage of malignant biliary obstruction before primary
surgical resection. Gastrointest Endosc. 84:814–821.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yokota Y, Fukasawa M, Takano S, Kadokura
M, Shindo H, Takahashi E, Hirose S, Kawakami S, Fukasawa Y, Sato T
and Enomoto N: Partially covered metal stents have longer patency
than uncovered and fully covered metal stents in the management of
distal malignant biliary obstruction: A retrospective study. BMC
Gastroenterol. 17(105)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kitano M, Yamashita Y, Tanaka K, Konishi
H, Yazumi S, Nakai Y, Nishiyama O, Uehara H, Mitoro A, Sanuki T, et
al: Covered self-expandable metal stents with an anti-migration
system improve patency duration without increased complications
compared with uncovered stents for distal biliary obstruction
caused by pancreatic carcinoma: a randomized multicenter trial. Am
J Gastroenterol. 108:1713–1722. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Saleem A, Leggett CL, Murad MH and Baron
TH: Meta-analysis of randomized trials comparing the patency of
covered and uncovered self-expandable metal stents for palliation
of distal malignant bile duct obstruction. Gastrointest Endosc.
74:321–327. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Isayama H, Komatsu Y, Tsujino T, Sasahira
N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H, et al:
A prospective randomised study of ‘covered’ versus ‘uncovered’
diamond stents for the management of distal malignant biliary
obstruction. Gut. 53:729–734. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kullman E, Frozanpor F, Söderlund C,
Linder S, Sandström P, Lindhoff-Larsson A, Toth E, Lindell G, Jonas
E, Freedman J, et al: Covered versus uncovered self-expandable
nitinol stents in the palliative treatment of malignant distal
biliary obstruction: Results from a randomized, multicenter study.
Gastrointest Endosc. 72:915–923. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee JH, Krishna SG, Singh A, Ladha HS,
Slack RS, Ramireddy S, Raju GS, Davila M and Ross WA: Comparison of
the utility of covered metal stents versus uncovered metal stents
in the management of malignant biliary strictures in 749 patients.
Gastrointest Endosc. 78:312–324. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li J, Li T, Sun P, Yu Q, Wang K, Chang W,
Song Z and Zheng Q: Covered versus uncovered self-expandable metal
stents for managing malignant distal biliary obstruction: A
meta-analysis. PLoS One. 11(e0149066)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen MY, Lin JW, Zhu HP, Zhang B, Jiang
GY, Yan PJ and Cai XJ: Covered stents versus uncovered Stents for
unresectable malignant biliary strictures: A meta-analysis. Biomed
Res Int. 2016(6408067)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park DH, Kim MH, Choi JS, Lee SS, Seo DW,
Kim JH, Han J, Kim JC, Choi EK and Lee SK: Covered versus uncovered
wallstent for malignant extrahepatic biliary obstruction: A cohort
comparative analysis. Clin Gastroenterol Hepatol. 4:790–796.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Conio M, Mangiavillano B, Caruso A,
Filiberti RA, Baron TH, De Luca L, Signorelli S, Crespi M, Marini
M, Ravelli P, et al: Covered versus uncovered self-expandable metal
stent for palliation of primary malignant extrahepatic biliary
strictures: A randomized multicenter study. Gastrointest Endosc.
88:283–291. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Seo DW, Sherman S, Dua KS, Slivka A, Roy
A, Costamagna G, Deviere J, Peetermans J, Rousseau M, Nakai Y, et
al: Covered and uncovered biliary metal stents provide similar
relief of biliary obstruction during neoadjuvant therapy in
pancreatic cancer: A randomized trial. Gastrointest Endosc.
90:602–612. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Flores Carmona DY, Alonso Lárraga JO,
Hernández Guerrero A and Ramírez Solís ME: Comparison of covered
and uncovered self-expandable stents in the treatment of malignant
biliary obstruction. Rev Esp Enferm Dig. 108:246–249.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tringali A, Hassan C, Rota M, Rossi M,
Mutignani M and Aabakken L: Covered vs. uncovered self-expandable
metal stents for malignant distal biliary strictures: A systematic
review and meta-analysis. Endoscopy. 50:631–641. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Almadi MA, Barkun AN and Martel M: No
benefit of covered vs uncovered self-expandable metal stents in
patients with malignant distal biliary obstruction: A
meta-analysis. Clin Gastroenterol Hepatol. 11:27–37.e1.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang MJ, Kim JH, Yoo BM, Hwang JC, Yoo JH,
Lee KS, Kang JK, Kim SS, Lim SG, Shin SJ, et al: Partially covered
versus uncovered self-expandable nitinol stents with anti-migration
properties for the palliation of malignant distal biliary
obstruction: A randomized controlled trial. Scand J Gastroenterol.
50:1490–1499. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Siddiqui AA, Mehendiratta V, Loren D, Hong
SK and Kowalski T: Fully covered self-expandable metal stents are
effective and safe to treat distal malignant biliary strictures,
irrespective of surgical resectability status. J Clin
Gastroenterol. 45:824–827. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Moole H, Bechtold ML, Cashman M, Volmar
FH, Dhillon S, Forcione D, Taneja D and Puli SR: Covered versus
uncovered self-expandable metal stents for malignant biliary
strictures: A meta-analysis and systematic review. Indian J
Gastroenterol. 35:323–330. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mangiavillano B, Montale A, Frazzoni L,
Bianchetti M, Sethi A, Repici A and Fuccio L: Endoscopic biliary
self-expandable metallic stent in malignant biliary obstruction
with or without sphincterotomy: Systematic review and
meta-analysis. Endosc Int Open. 7:E26–E35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yamashita Y, Tachikawa A, Shimokawa T,
Yamazaki H, Itonaga M, Sakai Y, Sugiyama H, Nakai Y, Tanaka K,
Isayama H and Kitano M: Covered versus uncovered metal stent for
endoscopic drainage of a malignant distal biliary obstruction:
Meta-analysis. Dig Endosc. 34:938–951. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Isayama H, Komatsu Y, Tsujino T, Yoshida
H, Tada M, Shiratori Y, Kawabe T and Omata M: Polyurethane-covered
metal stent for management of distal malignant biliary obstruction.
Gastrointest Endosc. 55:366–370. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee BS, Ryu JK, Jang DK, Chung KH, Yoon
WJ, Kim J, Woo SM, Lee SH, Lee WJ and Kim YT: Reintervention for
occluded metal stent in malignant bile duct obstruction: A
prospective randomized trial comparing covered and uncovered metal
stent. J Gastroenterol Hepatol. 31:1901–1907. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Telford JJ, Carr-Locke DL, Baron TH,
Poneros JM, Bounds BC, Kelsey PB, Schapiro RH, Huang CS,
Lichtenstein DR, Jacobson BC, et al: A randomized trial comparing
uncovered and partially covered self-expandable metal stents in the
palliation of distal malignant biliary obstruction. Gastrointest
Endosc. 72:907–914. 2010.PubMed/NCBI View Article : Google Scholar
|