Introduction
Intervertebral disc degeneration (IDD) is a
universal chronic disease that is frequently characterized by back
pain (1). Complicated pathogenic
mechanisms increase the therapeutic difficulties of IDD (2,3). The
inflammatory response and apoptosis in nucleus pulposus cells
(NPCs) promote the degradation of NP tissues (4-7).
Therefore, identifying the regulators of inflammation and apoptosis
in NPCs should improve the prevention and treatment of IDD.
Chemokine families are regularly of significant
activity in research due to their important roles in various
disease processes. CX3 chemokine ligand 1 (CX3CL1) belongs to the
CX3 chemokine family and exerts its biological effects by binding
to the specific receptor CX3C motif chemokine receptor 1 (CX3CR1)
(8-10).
The anti-inflammatory and anti-apoptotic roles of CX3CL1 have been
discovered in different diseases (11,12).
Although the mRNA expression of CX3CL1 and the CX3CR1 axis has been
detected in the nucleus pulpous tissues (NPs) of cervical and
lumbar vertebrae, the effect of the CX3CL1/CX3CR1 axis on IDD
progression remains to be elucidated (13).
Macrophages play crucial roles in multiple
pathophysiological processes, including IDD (14-20).
Several studies have reported that p38 in NPCs regulates macrophage
polarization and polarized macrophages are involved in IDD
progression (14,20,21).
Regulation of the phenotypic transition of infiltrated macrophages
(M1 proinflammatory and M2 anti-inflammatory phenotypes) is
important for controlling tissue inflammation (22,23).
Nakazawa et al (24)
detected M2 macrophage accumulation in degenerative intervertebral
discs and positive staining significantly correlated with
degenerative grades. In addition, Li et al (25) reported that M2
macrophage-conditioned medium alleviated IDD. Therefore, it is
unclear how monocyte migration and macrophage polarization occur in
NPs during IDD (26,27). Meanwhile, factors released from
polarized macrophages have been shown to regulate apoptosis and
further investigation of whether and how infiltrated macrophages
affect apoptosis in HNPCs will be valuable (28,29).
The CX3CL1/CX3CR1 axis affects macrophage chemotaxis and it would
be useful to explore the association of the CX3CL1/CX3CR1 axis with
macrophages and IDD.
The present study evaluated the role of macrophages
in IDD and further assessed the involvement of macrophages in the
molecular mechanism of the IDD process. In addition, the present
study assessed the contribution of the CX3CL1/CX3CR1 axis to the
development of IDD and the underlying communication of human
nucleus pulposus cells (HNPCs) with macrophages during IDD. The
studies were intended to provide a new way for IDD therapy in the
future.
Materials and methods
Patient samples
The degenerative nucleus pulposus (NP) tissues were
obtained from IDD patients [n=5 (female, 2; and male, 3); age range
29-74 years, median age 58 years] undergoing posterior lumbar
interbody fusion (PLIF) due to degenerative lumbar disc disease in
The Affiliated Nanhua Hospital of University of South China. The
control NP tissues were obtained from lumbar vertebrae fracture
patients without IDD [n=5 (female, 2; and male, 3); age range 14-65
years; median age 54 years] in The Affiliated Nanhua Hospital of
University of South China. Voluteers were recruited between
February and December 2021. The present study protocol conformed to
the globally accepted regulations on clinical studies involving
human data, and approval was conferred by the ethics committee of
University of South China (approval no. 2021-ky-41). Written
informed consent was obtained from all of the participants or
donors' families before using the samples.
Immunofluorescence staining
Human NP tissue sections were incubated with UV
block (Pierce; Thermo Fisher Scientific, Inc.) containing 10% goat
serum (Abcam) for 30 min at room temperature and then with mouse
cluster of differentiation (CD)68 (1:200, cat. no. ab283654, Abcam)
overnight in a humid chamber at 4˚C. After, sections were incubated
with a secondary antibody (1:500, cat. no. ab150077, Abcam) for 2 h
at room temperature. Nuclei were counterstained with DAPI. After
washing with PBS for 15 min, images were captured at x200
magnification on an Olympus FV1000 (Olympus Corporation).
Cell culture and transfection
HNPCs and THP-1-derived monocytes were purchased
from Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and cultured in DMEM or 1640 supplemented with 0.1% nonessential
amino acids, penicillin (100 U/ml), streptomycin (100 mg/ml) and
10% FBS at 37˚C for 24 h. 100 ng/ml TNF-α (MedChemExpress) or 200
nmol/l PMA (MilliporeSigma) were used for inducing apoptosis of
HNPCs and the differentiation of THP-1 derived monocytes into
macrophages, respectively. Control vector and CX3CL1 and CCL17
overexpression vectors were packaged and purchased from Hanbio
Biotechnology Co., Ltd. The 293T cell line from ATCC was used for
generating viral particles. The mass of used vectors included 10 µg
pHBLV, 10 µg psPAX2 and 5 µg pMD2G. HNPCs and THP-1-derived
macrophages were cultured in 6-well plate at 37˚C with 5%
CO2. When cells reached 40-50% confluence, 1 ml of
serum-free medium was replaced with serum-containing medium and
2x108 TU/ml viral particles [HNPC (MOI: 30) and
THP-1-derived macrophage (MOI: 10)]. After 12 h, 1 ml of
serum-containing medium was added to wells and the medium changed
to fresh serum-containing medium at 24 h. After 48 h, puromycin was
used for screening of stably transfected cells. The stably
expressed cells were screened using 4 µg/ml puromycin for 7 days
followed by use for different experiments. The generation used of
transfected cells were controlled at third generation. Cells were
incubated with CX3CR1 inhibitor JMS-17-2 (MedChemExpress) or
JAK2/STAT3 inhibitor SD1029 (MedChemExpress).
Reverse transcription-quantitative
(RT-q) PCR
Human nucleus pulpous cells and THP-1-derived
macrophages were cultured in 6-well plates at 37˚C and were used
for RNA extraction when the confluent reached ~95%. The RNA
extraction, complementary DNA (cDNA) synthesis, and qPCR were
performed according to the manufacturer's protocols. Human nucleus
pulpous cells, THP-1-derived macrophages and NPs were treated and
lysed for RNA extraction using the TRIzol® (Thermo
Fisher Scientific, Inc.) extraction method. Total RNA was reversed
transcribed into cDNA with HiScript III RT SuperMix (R323-01,
Vazyme). qPCR amplifications were performed according to
manufacture manual from ChamQ SYBR qPCR Master Mix (cat. no.
RQ311-02; Vazyme Biotech Co., Ltd.), which was 95˚C for 30 sec,
95˚C 5 sec, 60˚C 20 sec for additional 40 cycles. Using 18s as a
control, the 2-ΔΔCq method was applied to calculate the
relative mRNA expression of target genes. The sequences of the
primers were: Human CX3CL1, 5'-GCCACAGGCGAAAGCAGTA-3' and
5'-GGAGGCACTCGGAAAAGCTC-3'; Human CX3CR1,
5'-AGTGTCACCGACATTTACCTCC-3' and 5'-AAGGCGGTAGTGAATTTGCAC-3'; Human
CD86: 5'-AGTGGAATAGCCTCCCTGTAACTCC-3' and
5'-CCCATAAGTGTGCTCTGAAGTGAAA-3', Human CD206:
5'-TCCGGGTGCTGTTCTCCTA-3', 5'-TCCGGGTGCTGTTCTCCTA-3'; Human IL-1β:
5'-ATGATGGCTTATTACAGTGGCAA-3' and 5'-GTCGGAGATTCGTAGCTGGA-3', Human
IL-6: 5'-ACTCACCTCTTCAGAACGAATTG-3' and
5'-CCATCTTTGGAAGGTTCAGGTTG-3', Human IL-4:
5'-CGGCAACTTTGTCCACGGA-3' and 5'-TCTGTTACGGTCAACTCGGTG-3', Human
IL-10: 5'-GACTTTAAGGGTTACCTGGGTTG-3' and
5'-TCACATGCGCCTTGATGTCTG-3' and 18s: 5'-TTGACGGAAGGGCACCACCAG-3'
and 5'-GCACCACCACCCACGGAATCG-3'. Analysis of mRNA levels was
performed using ABI PRISM7900 sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Monocyte migration
THP-1-derived monocytes were added to the upper
chamber and culture supernatant to the lower chamber of 5.0 µm
Transwell inserts (Corning, Inc.). The number of migrated cells was
assessed after 24 h by incubating with calcein at 37˚C and positive
cells in low chambers counted. Fluorescence microscopy images were
captured at x200 magnification on an Olympus BX-53-LV2000 (Olympus
Corporation).
TUNEL assay
TUNEL staining was used to evaluate cell apoptosis.
The cells were fixed in 4% paraformaldehyde for 1 h at room
temperature and then treated with 0.5% TritonX-100 for 10 min.
After washing with PBS, the cells were incubated with the cell
death detection kit (Elabscience Biotechnology, Inc.) according to
the manufacturer's instructions. Nuclei were counterstained with
DAPI (Beyotime Institute of Biotechnology). Fluorescence microscopy
images were captured at x200 magnification on an Olympus
BX-53-LV2000 (Olympus Corporation).
Western blot analysis
Total protein were extracted with RIPA cell lysis
buffer (Beyotime Institute of Biotechnology) and Protease and
Phosphatase Inhibitor Cocktail (MedChemExpress) on ice for 30 min
and centrifuged at 13,400 x g for 10 min at 4˚C. Total protein were
quantified by a BCA kit (Beyotime Institute of Biotechnology).
Primary antibodies included anti-CX3CL1 (Proteintech Group, Inc.;
cat. no. 10108-2-AP; 1:1,000), anti-CX3CR1 (Proteintech Group,
Inc.; cat. no. 13885-1-AP; 1:1,000), BCL-2 (Proteintech Group,
Inc.; cat. no. 68103-1-Ig; 1:1,000), Bax (Proteintech Group, Inc.;
cat. no. 50599-2-Ig; 1:1,000), anti-STAT3 (Abcam; cat. no. ab68153;
1:200), anti-phosphorylated (p-)STAT3 (Abcam; cat. no. ab76315;
1:1,000), anti-JAK2 (Abcam; cat. no. ab108596; 1:1,000),
anti-p-JAK2 (Abcam; cat. no. ab32101; 1:1,000), anti-GAPDH (Abcam;
cat. no. ab8245; 1:2,000) and anti-β-actin (Abcam; cat. no. ab8226;
1:2,000). Secondary antibodies were HRP-labelled Goat anti-Mouse or
anti-Rabbit IgG (H + L) (Abcam; cat. no. ab6789 or ab6721;
1:5,000). The amount of GTP bound RHOA in cell-free lysates was
determined using the RHO Activation Assay kit (MilliporeSigma).
Protein (30 µg) of each sample were electrophoresed via 12% SDS gel
and transferred to polyvinylidene fluoride membrane
(MilliporeSigma). The membranes were blocked with 5% fat-free milk
for 1 h at room temperature, incubated with indicated primary
antibodies overnight at 4˚C and incubated with HRP-conjugated
secondary antibodies at room temperature for 2 h. Antibody binding
was visualized with Tanon 5500 (Tanon Science and Technology Co.,
Ltd.) and BeyoECL Plus (Beyotime Institute of Biotechnology), and
ImageJ software (version 1.8.0.112; National Institutes of Health)
was used for analyzing the blots.
ELISA assay
Supernatant from treated HNPCs and THP-1-derived
macrophages were collected and centrifuged at 2,000 x g for 10 min.
The supernatants then were used to ELISA according to the
instruction of kits (cat. nos. CX3CL1, ab192145; IL-4, ab215089;
IL-10, ab185986; CCL17, ab183366; Abcam).
Statistical analysis
All data were presented as means ± SD. Statistical
significance was evaluated by either one-way ANOVA or Student's
t-test. Student's t-test was used for assessing the differences
between two groups, while one-way ANOVA with Scheffe's test was
used for analyzing the difference among multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
CX3CL1 is downregulated in human
degenerative NPs
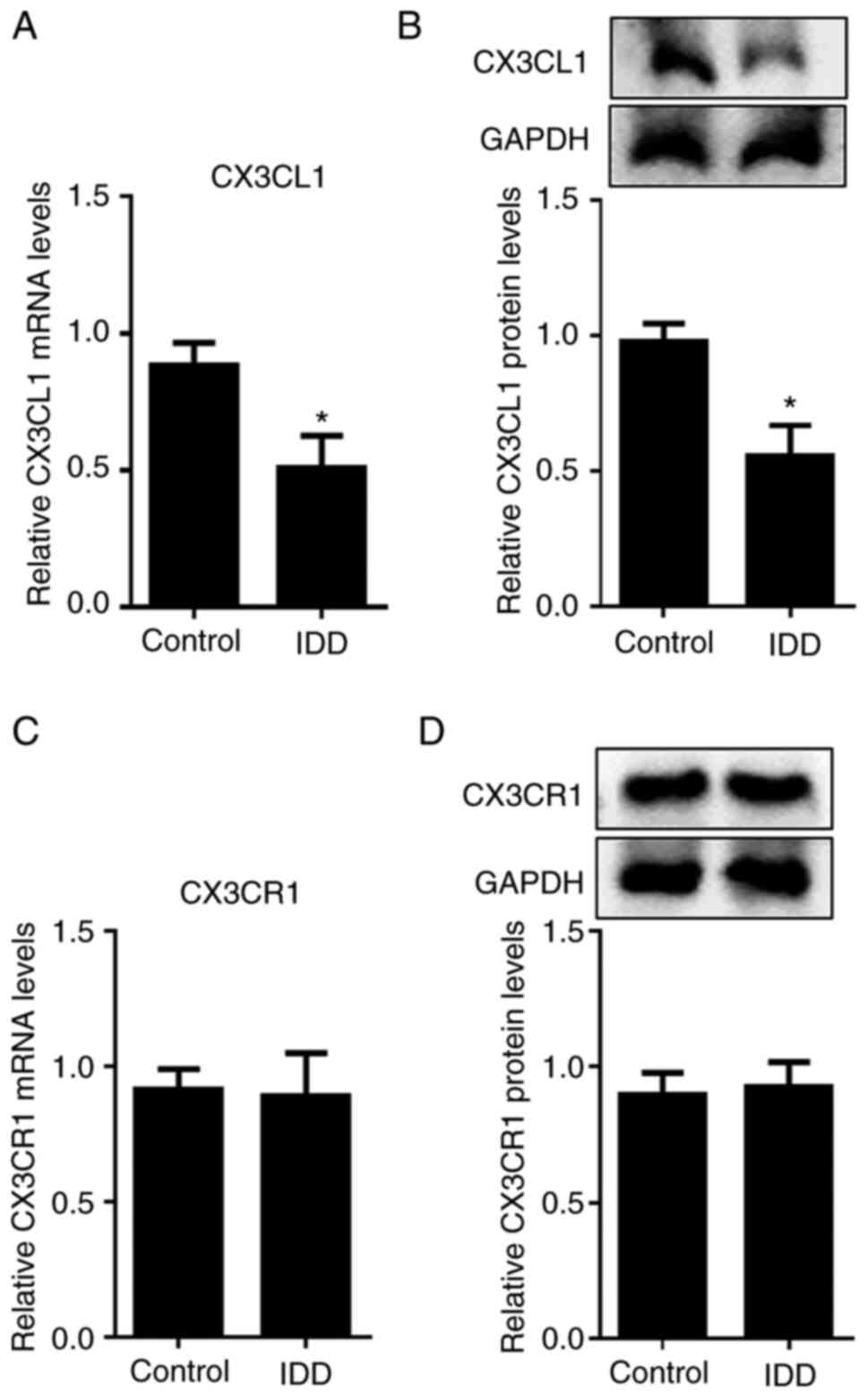
To identify whether CX3CL1 contributed to IDD, the
levels of CX3CL1 were detected in NPs of IDD patients. As shown in
Fig. 1A and B, the mRNA and protein levels of CX3CL1
were significantly decreased in degenerative NP tissues. However,
CX3CR1 expression in degenerative NPs were unchanged (Fig. 1C and D). This indicated that CX3CL1 might
possess a potential regulatory effect on IDD progression.
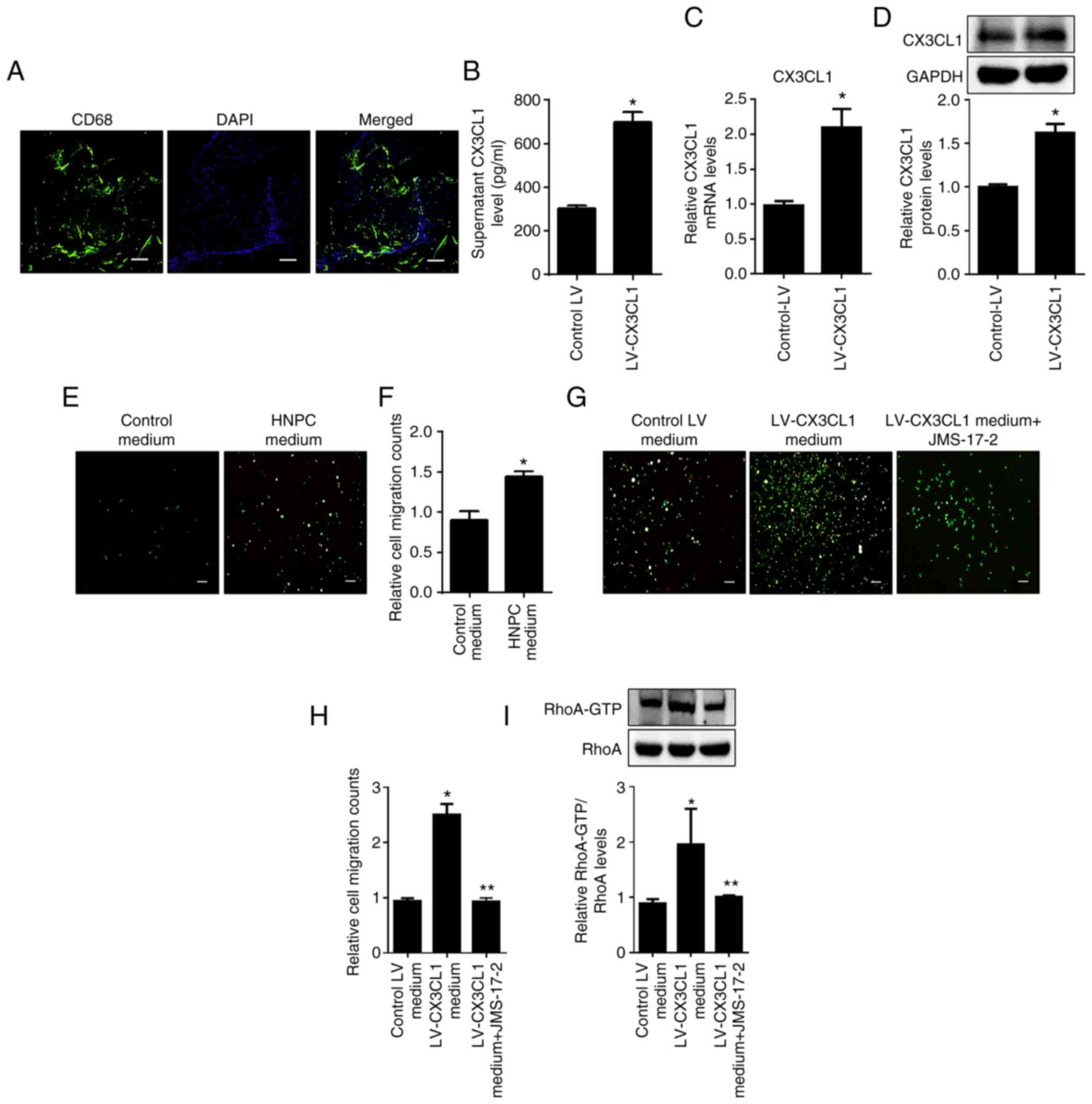
HNPC-derived CX3CL1 promotes monocyte
migration via CX3CR1
Macrophages have been reported to be involved in
inflammatory response during IDD progression (30,31).
As shown in Fig. 2A, abundant CD68
positive fluorescence staining was detected in NPs of IDD patients.
Given that the tissue macrophages are mainly derived from
circulating monocytes, the present study aimed to identify whether
CX3CL1 was involved the regulation of monocyte migration. As shown
in Fig. 2B-E, HNPC supernatant
significantly promoted THP-1-derived monocytes migration and
overexpression of CX3CL1 markedly promoted this effect.
Furthermore, CX3CR1 antagonist JMS-17-2 significantly blocked
CX3CL1 overexpression and supernatant-induced THP-1-derived
monocytes migration (Fig. 2D-E).
Inhibition of CX3CR1 also impaired RhoA signaling in THP-1-derived
monocytes (Fig. 2F and G). These data suggested that HNPC-derived
CX3CL1 bound to monocyte CX3CR1 followed by the promotion of
monocyte migration via RhoA signaling.
CX3CL1/CX3CR1 axis induces M2-like
macrophage via JAK2/STAT3 signaling and reduces inflammation of
HNPCs
Macrophages act as an important regulator during
tissue inflammation via the conversion between pro- and
anti-inflammatory phenotypes. The mRNA expression level of M2
marker CD206 significantly decreased (Fig. 3B) whereas M1 marker significantly
increased in degenerative NPs (Fig.
3A). The present study next aimed to investigate the role of
HNPC-derived CX3CL1 in macrophage polarization and reveal its
molecular detail. As shown in Fig.
3C and D, the supernatant from
CX3CL1 overexpression HNPCs significantly promoted macrophage
polarization into the M2 phenotype while reducing M1-like
macrophages, and JMS-17-2 incubation almost eliminated these
effects. Moreover, the conditional medium from CX3CL1/CX3CR1
axis-induced M2-like macrophages significantly increased
anti-inflammatory cytokines release from HNPCs (Fig. 3E). The activation of classical
JAK2/STAT3 signaling is known to occur during M2 polarization
(32). The present study suggested
that CX3CL1 elevated the phosphorylated levels of JAK2 and STAT3 in
THP-1-derived macrophage, while incubation with JMS-17-2 almost
reversed these (Fig. 3F-G).
Furthermore, JAK2/STAT3 signaling inhibitor SD1029 effectively
prevented CX3CL1 overexpression supernatant-induced M2-like
macrophages and inhibited M2-like macrophage-promotion of
anti-inflammatory cytokines secretion from HNPCs (Fig. 3H-J). In addition, the present study
detected increased pro-inflammatory cytokines IL-6 levels and
decreased anti-inflammatory cytokines IL-4 and IL-10 levels in NPs
of IDD patients (Fig. 3K).
Meanwhile, increased pro-inflammatory factors and M1 marker CD86
levels was found in NPs of IDD patients with low CX3CL1 expression
group (Fig. 3L and M). These data revealed that the
polarization of macrophage in NPs may be dependent on CX3CL1/CX3CR1
axis.
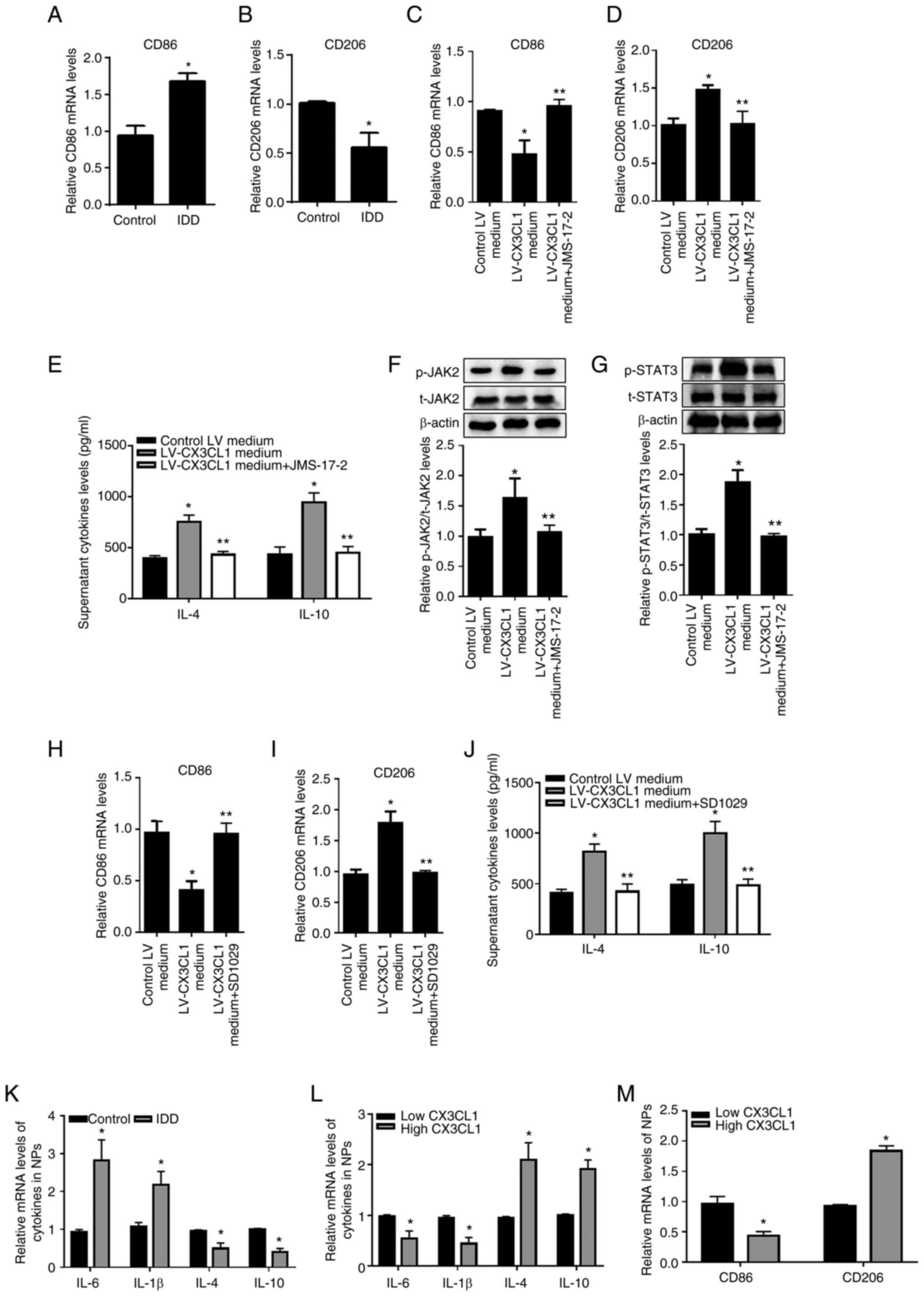 | Figure 3CX3CL1/CX3CR1 axis accelerates M2
macrophage polarization via JAK2/STAT3 signaling and inhibited the
inflammatory response in HNPCs. Analysis of (A) M1 (CD86) and (B)
M2 (CD206) markers in NPs via RT-qPCR. (C-D) Analysis of M1 (CD86)
and M2 (CD206) markers of THP-1-derived macrophages via RT-qPCR.
(E) Measurement of media IL-4 and IL-10 level of HNPCs using ELISA.
(F and G) Analysis the protein levels of t-JAK2, p-JAK2, t-STAT3
and p-STAT3 via western blotting. Analysis of (H) M1 (CD86) and (I)
M2 (CD206) markers via RT-qPCR. (J) Measurement of media IL-4 and
IL-10 level of HNPCs using ELISA. (K) Analysis of pro-inflammatory
cytokines (IL-1β and IL-6) and anti-inflammatory cytokines (IL-4
and IL-10) markers in NPs via RT-qPCR. Analysis of (L)
pro-inflammatory cytokines (IL-1β and IL-6), anti-inflammatory
cytokines (IL-4 and IL-10) and (M) M1 (CD86) and M2 (CD206) markers
in NPs of low or high CX3CL1 expression via RT-qPCR. All data are
mean ± SD from three independent experiment with each performed in
triplicate. *P<0.05 vs. Control or Control LV medium
or Low CX3L1 groups; **P<0.05 vs. LV-CX3CL1 medium
group. CX3CL1, CX3C chemokine ligand 1; CX3CR1, CX3C motif
chemokine receptor 1; HNPCs, human nucleus pulposus cells; CD,
cluster of differentiation; NPs, nucleus pulposus tissues; RT-qPCR,
reverse transcription-quantitative PCR; t-, total; p-,
phosphorylated; LV, lentivirus. |
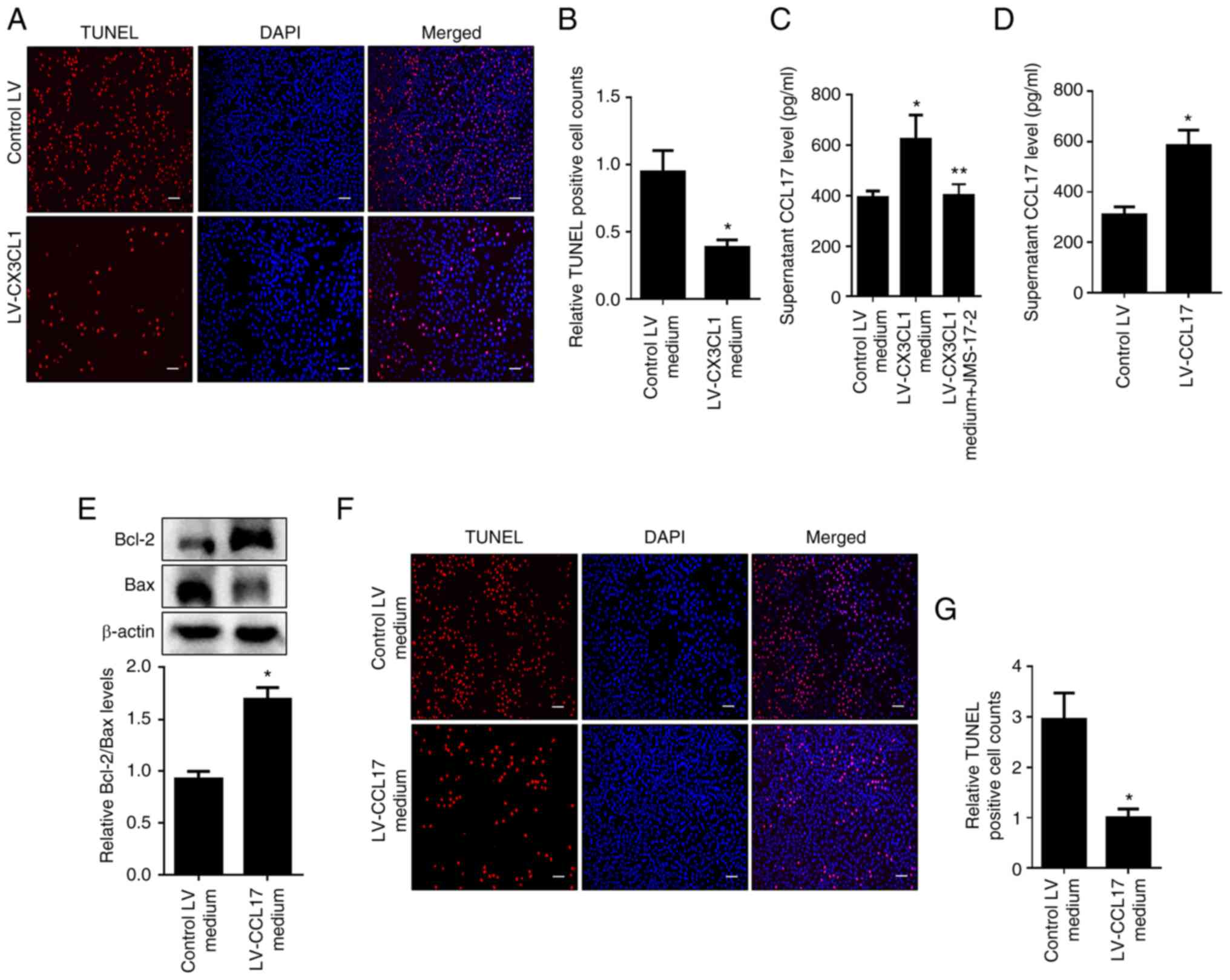
CCL17-mediated the anti-apoptotic
effect of M2 macrophage on HNPCs
As macrophages have been reported to be involve in
the regulation of cell death (33), the present study aimed to reveal
whether an anti-inflammatory type M2-like macrophage serves a role
in HNPC apoptosis. As shown in Fig.
4A and B, the data from TUNEL
assays indicated that the medium from CX3CL1 overexpression
supernatant-incubated macrophages evidently inhibited apoptosis of
HNPCs. To further identify the trigger during this process, it was
found that JMS-17-2 clearly blocked the increase of CCL17 level in
the medium of CX3CL1-overexpressed supernatant-cultured macrophages
(Fig. 4C). In addition, the
supernatant from CCL17 overexpressed macrophages significantly
increased the ratio of Bcl-2 to Bax and reduced apoptosis in HNPCs
(Fig. 4D-G). These results
indicated that increased CCL17 secretion from M2 macrophages
exerted an anti-apoptotic effect on HNPCs.
Discussion
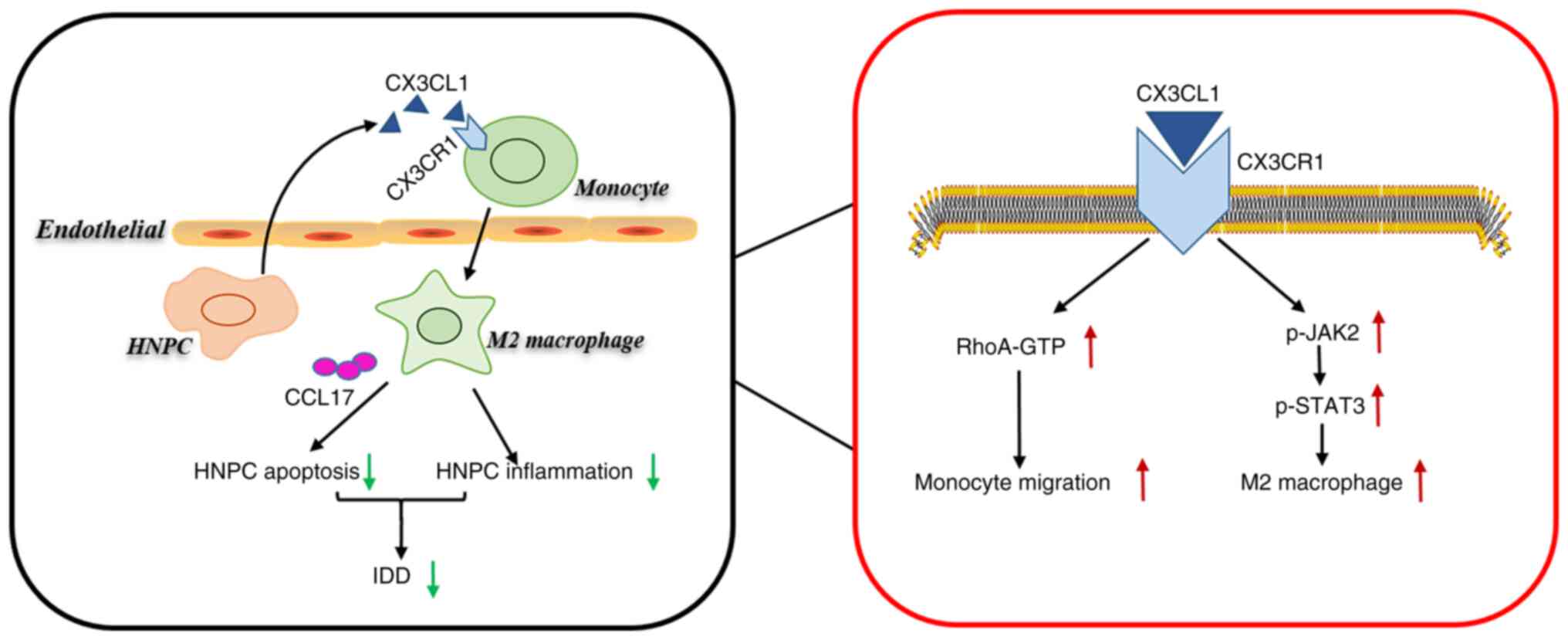
The present study first uncovered a decrease in
CX3CL1 expression in the NPs of IDD patients. It provided the first
evidence demonstrating that CX3CL1 reduced inflammation and
apoptosis in HNPCs (Fig. 5).
Mechanistically, the binding of CX3CL1 to monocyte CX3CR1
accelerated monocyte migration via RhoA signaling. In addition, the
CX3CL1/CX3CR1 axis activated JAK2/STAT3 signaling to promote
M2-like macrophage polarization. The medium from M2 macrophages
increased anti-inflammatory cytokine secretion by HNPCs and
CX3CL1-induced M2 macrophages reduced HNPC apoptosis by releasing
CCL17.
Pro-inflammatory M1 macrophage accumulation promotes
the degenerative phenotype of intervertebral discs (18,34,35).
Consistent with previous studies, the present study observed
macrophage infiltration in degenerative NPs (24). The present study found increased
pro-inflammatory cytokine and M1 marker CD86 levels and decreased
anti-inflammatory cytokine and M2 marker CD206 levels in
degenerative NPs. The important roles of chemokines in different
diseases, including IDD (36-38)],
by regulating cell chemotaxis are well known. CX3CL1-regulated
immune cell recruitment has been revealed, but there is still a
lack of evidence about its role and regulatory mechanism in IDD
(39,40). The present study detected
significantly elevated levels of the M1 macrophage marker CD86 and
pro-inflammatory cytokines (IL-1β and IL-6) in IDD patients with
low CX3CL1 expression in NPs, indicating that the protective effect
of CX3CL1 on intervertebral discs may involve controlling
macrophage polarization and alleviating inflammation. The data from
the present study suggested that HNPC-derived CX3CL1 induced
monocyte migration by increasing cytoskeletal remodeling via
CX3CR1/RhoA signaling. Furthermore, the binding of CX3CL1 to
macrophage CX3CR1 activated JAK2/STAT3 signaling to induce M2
macrophages. In addition, it was verified that the medium from
CX3CL1-induced M2 macrophages increased anti-inflammatory cytokine
levels in HNPCs. Although the data provided evidence to support the
protective role of CX3CL1 in IDD, it did not verify this in
vivo. To confirm the clinical value of CX3CL1, the effect of
CX3CL1 on IDD of mice will be tested in future work.
In addition to being regulators of the inflammatory
response, macrophages also serve a role in apoptosis (26,27).
Although abundant macrophages have been examined in degenerative
NPs, whether they participate in apoptosis in NPCs was unknown
(20,21). Huang et al (12) found that CX3CL1 effectively
prevented neuronal apoptosis. The present study verified that
conditioned medium from CX3CL1-induced M2 macrophages reduced
apoptosis in HNPCs. Previous studies verify that M2 macrophages
release a large amount of CCL17 and the anti-apoptotic effect of
CCL17 has also been confirmed in the nervous system (41,42). The present study found increased
secretion of CCL17 by CX3CL1-induced M2 macrophages. In addition,
overexpression of CCL17 reduced apoptosis in HNPCs. Revealing the
mechanism by which the CX3CL1/CX3CRL1 axis promotes CCL17 secretion
is helpful for further understanding the protective effect of
CX3CL1 on NPCs.
In short, the present study showed that the
CX3CL1/CX3CR1 axis protected against the progression of IDD.
Further analysis verified that CX3CL1 bound to CX3CR1 and triggered
monocyte migration and M2 macrophage polarization via RhoA and
JAK2/STAT3 signaling, respectively. In addition, M2 macrophages not
only mitigate inflammation but also prevent apoptosis in HNPCs.
Therefore, targeting the CX3CL1/CX3CR1 axis is a new approach for
alleviating IDD.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Technology
Innovation Guidance Plan of Hunan Province (grant no.
2018SK51709).
Availability of data and materials
The datasets analyzed and/or generated during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XG was responsible for conceptualization,
validation, investigation, methodology and manuscript writing. HH
was responsible for data curation, software based data analysis,
validation, investigation and visualization. MX was responsible for
data validation, methodology and project administration. CT gave
guidance on experimental technologies and was responsible for data
curation and validation. JO was responsible for data curation,
validation, investigation, visualization, project administration,
manuscript revision and funding support. ZL was responsible for
data curation, validation, investigation, visualization, project
administration, manuscript revision and funding support. JO and ZL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol conformed to the globally
accepted regulations on clinical studies involving human data, and
approval was conferred by the ethics committee of University of
South China (approval no. 2021-ky-41). Written informed consent was
obtained from all of the participants or donors' families before
using the samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X
and Yang Y: Possible pathogenesis of painful intervertebral disc
degeneration. Spine. 31:560–566. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adams MA, Freeman BJ, Morrison HP, Nelson
IW and Dolan P: Mechanical initiation of intervertebral disc
degeneration. Spine (Phila Pa 1976). 25:1625–1636. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Freemont AJ, Watkins A, Le Maitre C,
Jeziorska M and Hoyland JA: Current understanding of cellular and
molecular events in intervertebral disc degeneration: Implications
for therapy. J Pathol. 196:374–379. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jia J, Nie L and Liu Y: Butyrate
alleviates inflammatory response and NF-κB activation in human
degenerated intervertebral disc tissues. Int Immunopharmacol.
78(106004)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu H, Liu Y, Xie W, Xie Q, Liu Q and Cheng
L: IL-38 alleviates the inflammatory response and the degeneration
of nucleus pulposus cells via inhibition of the NF-κB signaling
pathway in vitro. Int Immunopharmacol. 85(106592)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Risbud MV, Fertala J, Vresilovic EJ,
Albert TJ and Shapiro IM: Nucleus pulposus cells upregulate
PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions
and resist apoptosis induced by serum withdrawal. Spine (Phila Pa
1976). 30:882–889. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Erwin WM, Islam D, Inman RD, Fehlings MG
and Tsui FW: Notochordal cells protect nucleus pulposus cells from
degradation and apoptosis: Implications for the mechanisms of
intervertebral disc degeneration. Arthritis Res Ther.
13(R215)2011.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Apostolakis S and Spandidos D: Chemokines
and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta
Pharmacol Sin. 34:1251–1256. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Limatola C and Ransohoff RM: Modulating
neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci.
8(229)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wojdasiewicz P, Poniatowski LA, Kotela A,
Deszczyński J, Kotela I and Szukiewicz D: The chemokine CX3CL1
(fractalkine) and its receptor CX3CR1: Occurrence and potential
role in osteoarthritis. Arch Immunol Ther Exp (Warsz). 62:395–403.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aoyama T, Inokuchi S, Brenner DA and Seki
E: CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced
liver inflammation and fibrosis in mice. Hepatology. 52:1390–1400.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ,
Zhang WW, Li YY and Xin WJ: CX3CL1-mediated macrophage activation
contributed to paclitaxel-induced DRG neuronal apoptosis and
painful peripheral neuropathy. Brain Behav Immun. 40:155–165.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oh IS, Suh DW and Ha KY: Hypertrophy of
the ligament flavum in degenerative lumbar stenosis associated with
the increased expression of fractalkine (CX3CL1)/CX3CR1. chemokine.
54:380–385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang C, Cao P, Gao Y, Wu M, Lin Y, Tian Y
and Yuan W: Differential expression of p38 MAPK α, β, γ, δ isoforms
in nucleus pulposus modulates macrophage polarization in
intervertebral disc degeneration. Sci Rep. 6(22182)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu ZH, Sun Z, Wang HQ, Ge J, Jiang TS,
Chen YF, Ma Y, Wang C, Hu S, Samartzis D and Luo ZJ: FasL
expression on human nucleus pulposus cells contributes to the
immune privilege of intervertebral disc by interacting with
immunocytes. Int J Med Sci. 10:1053–1060. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim JH, Moon HJ, Lee JH, Kim JH, Kwon TH
and Park YK: Rabbit notochordal cells modulate the expression of
inflammatory mediators by human annulus fibrosus cells cocultured
with activated macrophage-like THP-1 cells. Spine (Phila Pa 1976).
37:1856–1864. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Park JJ, Moon HJ, Park JH, Kwon TH, Park
YK and Kim JH: Induction of proinflammatory cytokine production in
intervertebral disc cells by macrophage-like THP-1 cells requires
mitogen-activated protein kinase activity. J Neurosurg Spine.
24:167–175. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ni L, Zheng Y, Gong T, Xiu C, Li K,
Saijilafu Li B, Yang H and Chen J: Proinflammatory macrophages
promote degenerative phenotypes in rat nucleus pulpous cells partly
through ERK and JNK signaling. J Cell Physiol. 234:5362–5371.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ling Z, Liu Y, Wang Z, Zhang Z, Chen B,
Yang J, Zeng B, Gao Y, Jiang C, Huang Y, et al: Single-cell RNA-Seq
analysis reveals macrophage involved in the progression of human
intervertebral disc degeneration. Front Cell Dev Biol.
9(833420)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamagishi A, Nakajima H, Kokubo Y,
Yamamoto Y and Matsumine A: Polarization of infiltrating
macrophages in the outer annulus fibrosus layer associated with the
process of intervertebral disc degeneration and neural ingrowth in
the human cervical spine. Spine J. 22:877–886. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamamoto Y, Kokubo Y, Nakajima H, Honjoh
K, Watanabe S and Matsumine A: Distribution and polarization of
hematogenous macrophages associated with the progression of
intervertebral disc degeneration. Spine (Phila Pa 1976).
47:E149–E158. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li C, Xu MM, Wang K, Adler AJ, Vella AT
and Zhou B: Macrophage polarization and meta-inflammation. Transl
Res. 191:29–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bashir S, Sharma Y, Elahi A and Khan F:
Macrophage polarization: The link between inflammation and related
diseases. Inflamm Res. 65:1–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nakazawa KR, Walter BA, Laudier DM,
Krishnamoorthy D, Mosley GE, Spiller KL and Iatridis JC:
Accumulation and localization of macrophage phenotypes with human
intervertebral disc degeneration. Spine J. 18:343–356.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li XC, Luo SJ, Fan W, Zhou TL, Huang CM
and Wang MS: M2 macrophage-conditioned medium inhibits
intervertebral disc degeneration in a tumor necrosis factor-α-rich
environment. J Orthop Res. 40:2488–2501. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mondragon AA, Betts-Obregon BS, Moritz RJ,
Parvathaneni K, Navarro MM, Kim HS, Lee CF, LeBaron RG, Asmis R and
Tsin AT: BIGH3 protein and macrophages in retinal endothelial cell
apoptosis. Apoptosis. 20:29–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ing DJ, Zang J, Dzau VJ, Webster KA and
Bishopric NH: Modulation of cytokine-induced cardiac myocyte
apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res. 84:21–33.
1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Truman LA, Ford CA, Pasikowska M, Pound
JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA,
Nibbs R, et al: CX3CL1/fractalkine is released from apoptotic
lymphocytes to stimulate macrophage chemotaxis. Blood.
112:5026–5036. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gevrey JC, Isaac BM and Cox D: Syk is
required for monocyte/macrophage chemotaxis to CX3CL1
(Fractalkine). J Immunol. 175:3737–3745. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li XC, Luo SJ, Fan W, Zhou TL, Tan DQ, Tan
RX, Xian QZ, Li J, Huang CM and Wang MS: Macrophage polarization
regulates intervertebral disc degeneration by modulating cell
proliferation, inflammation mediator secretion, and extracellular
matrix metabolism. Front Immunol. 13(922173)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu B, Jiang Q, Chen R, Gao S, Xia Q, Zhu
J, Zhang F, Shao C, Liu X, Li X, et al: Tacrolimus ameliorates
bleomycin-induced pulmonary fibrosis by inhibiting M2 macrophage
polarization via JAK2/STAT3 signaling. Int Immunopharmacol.
113(109424)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li X, Luo J, Li Y, Jia L, Li Y, Ye S, Liu
L, Yu Y, Lu Y and Luan Y: Macrophage-derived exosomes in
TLR9-/- mice ameliorate sepsis-induced mitochondrial
oxidative stress and apoptosis in cardiomyocytes. Oxid Med Cell
Longev. 2022(5719974)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang S, Wang P, Hu B, Liu W, Lv X, Chen S
and Shao Z: HSP90 inhibitor 17-AAG attenuates nucleus pulposus
inflammation and catabolism induced by M1-polarized macrophages.
Front Cell Dev Biol. 9(796974)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao F, Guo Z, Hou F, Fan W, Wu B and Qian
Z: Magnoflorine alleviates ‘M1’ polarized macrophage-induced
intervertebral disc degeneration through repressing the
HMGB1/Myd88/NF-κB pathway and NLRP3 inflammasome. Front Pharmacol.
12(701087)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Phillips KL, Cullen K, Chiverton N,
Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK
and Le Maitre CL: Potential roles of cytokines and chemokines in
human intervertebral disc degeneration: Interleukin-1 is a master
regulator of catabolic processes. Osteoarthritis Cartilage.
23:1165–1177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stich S, Möller A, Cabraja M, Krüger JP,
Hondke S, Endres M, Ringe J and Sittinger M: Chemokine CCL25
induces migration and extracellular matrix production of anulus
fibrosus-derived cells. Int J Mol Sci. 19(2207)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ying JW, Wen TY, Pei SS, Su LH and Ruan
DK: Stromal cell-derived factor-1α promotes recruitment and
differentiation of nucleus pulposus-derived stem cells. World J
Stem Cells. 11:196–211. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Klapproth E, Witt A, Klose P, Wiedemann J,
Vavilthota N, Künzel SR, Kämmerer S, Günscht M, Sprott D, Lesche M,
et al: Targeting cardiomyocyte ADAM10 ectodomain shedding promotes
survival early after myocardial infarction. Nat Commun.
13(7648)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Komissarov A, Potashnikova D, Freeman ML,
Gontarenko V, Maytesyan D, Lederman MM, Vasilieva E and Margolis L:
Driving T cells to human atherosclerotic plaques: CCL3/CCR5 and
CX3CL1/CX3CR1 migration axes. Eur J Immunol. 51:1857–1859.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hsu AT, Lupancu TJ, Lee MC, Fleetwood AJ,
Cook AD, Hamilton JA and Achuthan A: Epigenetic and transcriptional
regulation of IL4-induced CCL17 production in human monocytes and
murine macrophages. J Biol Chem. 293:11415–11423. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang A, Liu Y, Xu H, Zhang Z, Wang X,
Yuan L, Lenahan C, Zhang C, Jiang J, Fang C, et al: CCL17 exerts
neuroprotection through activation of CCR4/mTORC2 axis in microglia
after subarachnoid haemorrhage in rats. Stroke Vasc Neurol. 8:4–16.
2022.PubMed/NCBI View Article : Google Scholar
|