Introduction
Most osteoarthritis (OA) resulting from degenerative
diseases of joints, traumas and inflammation will unavoidably
progress to osteochondral defects (1). The particular nature of hyaline
articular cartilage, including the avascular nature and the low
number of chondrocytes and stem cells in the surrounding cartilage
lesions, results in limited potential to reconstruct osteochondral
defects through a self-healing process (1,2).
Moreover, to produce acceptable structural and functional repair,
all three kinds of tissues involved in osteochondral lesions,
including subchondral bone, the osteochondral interface and
articular cartilage, need to be reconstructed simultaneously
(3). Although microfractures,
arthroscopic debridement, and cell-based or cell-free approaches
have been introduced for osteochondral reconstruction, the clinical
application and repair effects remain barely satisfactory owing to
donor morbidity, possible contamination and potential problems in
cell transportation (4,5). Tissue engineering and function
reconstruction through host remodeling and autologous cell
recruitment effectively was shown to overcome the aforementioned
limitations and represent a fundamental shift from cell-based
approaches (6-8).
To provide appropriate channels for the migration of
recruited cells into lesion regions, marrow-stimulation techniques
were applied and achieved encouraging repair results (9). Bioactive agents, including cytokines
and growth factors such as stromal cell-derived factor-1,
platelet-derived growth factor, VEGF, and others, were shown to
promote cell recruitment and have a helpful effect on the repair of
articular cartilage injuries (8,10,11).
Some of the aforementioned growth factors are released from
activated platelets. Consequently, different types of platelet
concentrates (PCs) should also be regarded as alternative sources
of autologous growth factors for cartilage regeneration (11). Platelet-rich fibrin (PRF), commonly
known as a second-generation PC, was shown to have a high capacity
to improve wound healing and tissue repair owing to the gradual
release of growth factors during its slow degradation along with
its intrinsic fibrin scaffolding, which offers a unique
three-dimensional (3-D) microstructure for promoting proliferation
and differentiation of recruited cells (12,13).
In previous studies performed by the present authors, PRF was
proven to markedly promote the proliferative and osteogenic
capability of bone marrow mesenchymal stem cells (BMSCs) and
significantly improve the repair effect of osteogenic BMSC sheets
in vitro and in vivo (14,15).
Furthermore, PRF has been generally applied in oral implants,
severe periodontitis, soft tissue, bone and cartilage defect repair
(15). Nonetheless, fresh PRF
(F-PRF) is currently difficult to apply immediately following
preparation and is impossible to store for a long period and
therefore impossible to commercialize (16). Several approaches for freeze-drying
platelet-rich plasma (PRP) have been studied to determine storage
issues and broaden its clinical application (17). Freeze-drying is a common strategy
that improves the long-term preservation of these proteins and
maintains their stability for tissue engineering. Lyophilized
proteins in PRF not only maintain their original biological
activity but also exhibit excellent stability and storage potential
(18,19). The present authors previously
investigated the preparation of lyophilized PRF (L-PRF) and
discussed the combination of L-PRF and osteogenic BMSC sheets for
bone tissue engineering in nude mice, but the repair effect of
L-PRF in vivo remains unclear (16).
Consequently, the present study aimed to assess the
potential of L-PRF in the reconstruction of osteochondral defects
in rabbits using micro-CT and macroscopic and histological
observations.
Materials and methods
Ethics approval
The present study was reviewed and approved by the
Institutional Animal Care and Use Committee of the General Hospital
of Southern Theater of PLA (Guangzhou, China; approval no.
2019010402).
Preparation of L-PRF
PRF was first prepared according to a previously
published approach, with minor modifications (14). In brief, blood samples (20 ml each)
were collected from the central auricular artery of rabbits and
centrifuged promptly for 10 min at 1,200 x g at 26˚C in a
laboratory centrifuge. These centrifuged samples were composed of
three layers, with the middle layer being considered fresh PRF
clot. The PRF clot was harvested with tweezers and gently pressed
into a flexible, compact and elastic fibrin membrane between two
sterile pieces of gauze for 10 sec to keep the membrane wet. The
fresh PRF clot was referred to as F-PRF and half of the fresh clots
were processed through lyophilization (freeze-drying), according to
a previously published method with minor modifications (19). After freeze-drying, lyophilized
platelet-rich fibrin (L-PRF) samples were obtained and stored at
room temperature for 2 weeks. F-PRF should be used immediately
after preparation and cannot be stored for a long period.
Therefore, after L-PRF was prepared for further experiments, F-PRF
was immediately prepared and used for in vitro and in
vivo experiments. The prepared L-PRF and F-PRF fragments were
processed for histological observation and inspection under
scanning electron microscopy (SEM; S-4800, Hitachi, Ltd.). Finally,
the harvested F-PRF and L-PRF samples were preserved in sterile
Petri dishes for subsequent experiments.
To acquire BMSCs, a total of 3 male 4-week-old New
Zealand rabbits were euthanized by intraperitoneal injection of an
overdose of barbital sodium (3%; 150 mg/kg). Briefly, limb long
bone and iliac bone were dissected from the rabbits and rinsed with
PBS. Subsequently, the bone marrow in the limb long and iliac bones
was flushed out with DMEM-F12; (HyClone; Cytiva) supplemented with
10% FBS (HyClone; Cytiva), 200 U/ml penicillin, 100 µg/ml
streptomycin and 272 µg/ml L-glutamine (all Amresco, LLC). The
obtained clumps of bone marrow cells were repeatedly pipetted and
dispersed to obtain a homogeneous cell suspension. Subsequently,
the isolated cell suspension was plated in a 10-cm culture dish at
a concentration of 5x105 cells/cm2. After the
third, fifth and seventh days of seeding, floating cells were
removed and fresh medium was added to the remaining adherent cells,
which were considered BMSCs. The medium was changed every 3 days
until the cells reached 90-95% confluence; then were subcultured at
a ratio of 1:3. Second-passage BMSCs were used for experiments.
Surface marker expression of BMSCs was investigated using flow
cytometry (FACSCalibur™; BD Biosciences). Cells were labeled with
monoclonal antibodies against CD29 (cat. no. ab95623) and CD31
(cat. no. ab24590) (all from Abcam). Differentiation into
osteogenic and adipogenic lineages was also evaluated by Alizarin
red staining and Oil-Red-O staining in vitro. Second-passage
cells were obtained, cultured in osteogenic induction medium
(containing 10% FBS, 10 mmol/l β-sodium glycerophosphate,
10-7 mol/l dexamethasone, 50 mg/l L-ascorbic acid) (all
Amresco, LLC) and in adipogenic induction medium [containing 10%
FBS, 1 µmol/l dexamethasone, 10 µmol/l insulin, 200 µmol/l
indomethacin and 0.5 mmol/l isobutylmethylxanthine (IBMX)],
respectively. Then, alizarin red staining for 8 min at room
temperature and Oil-Red-O staining for 10 min at room temperature,
observed and photographed under an inverted microscope (X100 and
X200 of magnification, OlympasIX71).
Animal surgery
In the present study, fifteen male mature New
Zealand white rabbits (age, 3-month-old; weight, 2.0-2.5 kg; Huadu
Xinhua experimental animal farm, Guangzhou, China, were used.
General anesthesia was induced before surgery through intravenous
injection of ketamine hydrochloride (60 mg/kg) and xylazine (6
mg/kg) (7). Subsequently, the knee
joint capsule was exposed sufficiently after the lateral
parapatellar skin was incised. A steel trephine bar (5 mm in
diameter and 6 mm in height (Taobao), which is currently applied in
oral dental implant surgery, was used to produce a full-thickness
osteochondral defect 5 mm in diameter and 4 mm in depth in the
center of the trochlear grove. To minimize any possible variance in
the operation, the same well-trained surgeon completed all the
surgeries. The 24 osteochondral defects produced in 12 rabbits were
randomly divided into the following three groups: i) Defects
without treatment, i.e. untreated group used as a blank control;
ii) defects filled with F-PRF fragments, i.e. F-PRF group; and iii)
defects filled with L-PRF fragments, i.e. L-PRF group. Finally,
eight osteochondral samples in each group were subject to
histological analysis.
The PRFs were cut into small pieces (~1
mm3) and implanted into the osteochondral defects in
rabbits, according to a previously published method with minor
modifications (20). A total of
one PRF membrane obtained from a 10 ml blood sample was used to
fill one osteochondral defect. Finally, the remaining three rabbits
were also surgically treated, but no osteochondral defect was
created, to be used as positive control. Postoperatively, all of
the rabbits were returned to their housing when they could lie on
their stomachs and were conscious. Thereafter, rabbits were
maintained in separate cages and allowed to move freely immediately
after surgery. The vital signs of the animal were observed for the
whole animal experiments process that lasted for 16 weeks; vital
signs included the healing of the wound, the mental state of the
rabbit, feeding and drinking conditions, the body temperature and
movement. The rabbits were monitored by the present authors every
day for 2 weeks after surgery and at least once every two days
after the surgical area healed. Moreover, the staff of the
Laboratory Animal Center monitored the rabbits every day.
Furthermore, no rabbits showed treatment-specific effusions or any
overt evidence of infection and all wounds healed well. After 16
weeks, all 15 rabbits were euthanized by intravenous injection of
an overdose of barbital sodium (3%; 150 mg/kg) and osteochondral
specimens were harvested for macroscopic assessment, micro-CT
scanning and histological observations.
Gross morphology
To evaluate the effect of PRF fragments on the
repair of osteochondral defects in vivo, the macroscopic
evaluation scoring method for cartilage repair established by the
International Cartilage Repair Society (ICRS), which divided four
grades: Grade I: normal(12);
Grade II: nearly normal (11-8); Grade III: abnormal(7-4) and Grade
IV: severely abnormal (3-1), was used to examine and score
specimens. At the same time, blinded scoring was calculated three
times to reduce the bias as much as possible (21).
Micro-CT scanning
Micro-CT scanning and examinations (Siemens AG; 80
kV, 500 mA, 1200 msec integration time) were used to analyze the
grade of repair of the articular cartilage and subchondral bone in
the PRF transplanted defect areas. Briefly, the specimens were
collected and fixed in 4% formaldehyde at 26˚C for 24 h and then
subjected to micro-CT scanning. In addition, all of the scanning
data were reconstructed in 3-D based on Cobra software (Siemens
reconstruction software; version VC40) to display the newly formed
bone and cartilage tissues. Furthermore, the newly regenerated bone
tissue in defects was also examined and reported as the bone volume
(BV)/total volume (TV) ratio to quantitatively present newly formed
bone tissue in the three experimental groups. Moreover, the
specimens from the three positive control rabbits were examined and
reconstructed to provide reference data.
Histological and immunohistochemical
examination
After macroscopic assessment and micro-CT scanning
observations, 10% EDTA was used to decalcify all the collected
specimens for 14 days, followed by dehydration with graded ethanol.
Next, the processed specimens were embedded in paraffin, sectioned
into slices of 5 µm thickness and stained with safranin-O for 3 min
at room temperature to study the glycosaminoglycan distribution,
stained with H&E and Masson's trichrome (MTC) for 15 min at
room temperature for general histological observations, and stained
with Sirius Red for 8 min at room temperature to analyze kinds of
collagen in newly formed tissue. Moreover, blinded scoring based on
the ICRS scale for histological evaluation was calculated three
times to reduce the bias (22).
Furthermore, the expression of collagen type I
(COL-I) and type II (COL-II) was investigated through
immunohistochemical staining to analyze the collagen distribution
in the reconstructed tissue. Briefly, sections (thickness 5 µm were
blocked with 1% bovine serum albumin Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C for 40 min, followed by incubation with
primary anti-COL-I (1:100, bs-10423R, Bioss) and anti-COL-II
antibodies (1:100, bs-10589R, Bioss, Beijing, China) at 4˚C
overnight. Subsequently, the secondary antibody goat anti-rabbit
IgG (H+L) HRP (1:200, Cat# S0001, Affinity Biosciences, Beijing,
China) was added and cultured at 37˚C for 60 min after rinsing with
PBS three times. Finally, peroxidase-antiperoxidase method (PAP)
and hematoxylin (for 50 sec at room temperature) was used to stain
these sections for further observations. In addition, the
percentage of the positive surface area occupied by COL-I and
COL-II relative to the total target area (mean density=IOD Sum/Area
Sum) was quantitatively calculated based on Image-Pro Plus 6.0
software (Media Cybernetics, Inc.). The signal density of tissue
areas from five randomly selected fields was counted blindly and
subject to statistical analysis.
Statistical analysis
SPSS version 21.0 (IBM Corp.) was used to perform
the statistical analysis. Data are presented as the mean ± standard
deviation. One-way ANOVA was used for multiple group comparisons,
followed by Tukey's honestly significant difference tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of L-PRF
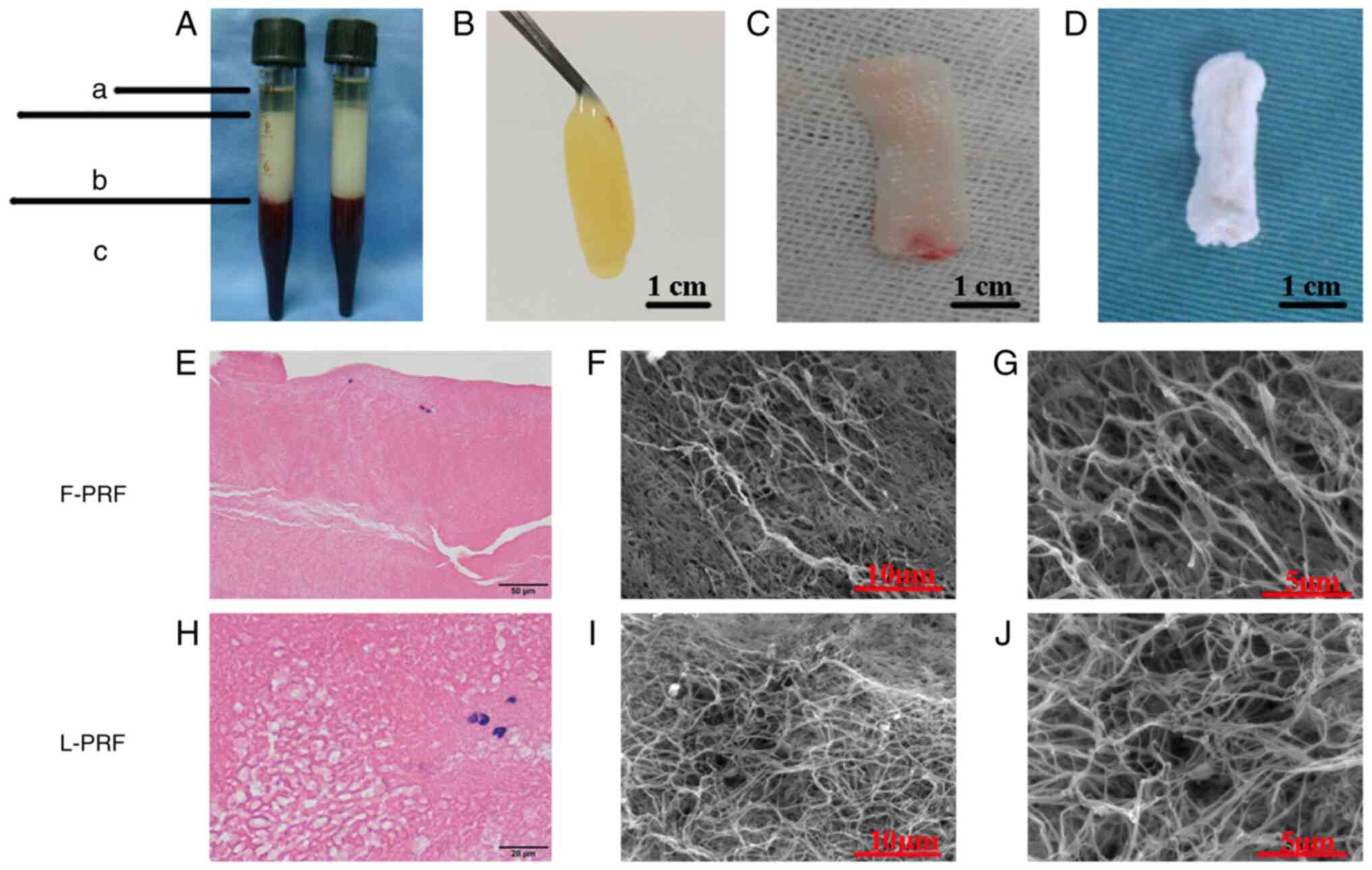
Blood samples were separated into three layers by
centrifugation with acellular plasma at the faint yellow liquid top
layer (Fig. 1A-a), fibrin clots at
the pale yellow gel middle layer (Fig.
1A-b) and red blood cells at the loose red jelly bottom layer
(Fig. 1A-c). After the acellular
plasma was removed, PRF clots were obtained easily with sterile
tweezers and the red blood cells were discarded (Fig. 1B). When the fluids within the PRF
matrix were gently squeezed out, a flexible, compact and highly
elastic fibrin membrane was obtained, which was regarded as F-PRF
(Fig. 1C). L-PRF with a loose
structure and rough surface was harvested after F-PRF was
freeze-dried (Fig. 1D).
H&E staining results showed that F-PRF was
composed of a mass of red-stained and closely arranged fiber
bundles, in which blue-stained mononuclear leukocytes were observed
(Fig. 1E). In comparison, the
fibrous fibrin mesh of L-PRF was not compactly aligned with
abundant pores of unequal size and blue-stained mononuclear
leukocytes were also observed (Fig.
1H). Microstructural examination through SEM revealed that the
fibrous fibrin network was arranged more regularly and compactly in
the F-PRF clots (Fig. 1F) than in
the L-PRF (Fig. 1I). However, a
large number of fibrin fibers assembled into a porous 3-D fibrin
network of trimolecular branch junctions could be seen throughout
both the L-PRF (Fig. 1J) and F-PRF
(Fig. 1G). These histological and
microstructural results indicated that freeze-drying had little
effect on the 3-D microstructure of PRF.
BMSCs presented a fibroblast-like morphology and
closely spaced growth (Fig. S1A)
and exhibited the typical characteristics of stem cells, including
rapid proliferation and self-renewal (Fig. S1B). Alizarin red (Fig. S1C) and Oil red O (Fig. S1D) confirmed their osteogenesis
and adipogenesis. Moreover, the cells were highly CD29-positive
(Fig. S1E) and CD31 negative
(Fig. S1F), which confirmed the
identification of mesenchymal stem cells.
Scheme of the knee joint osteochondral
defect model in vivo
As shown in Fig.
2A, 24 defects obtained from 12 rabbits were randomly divided
into three groups. In addition, the remaining three rabbits, which
were only surgically skin-incised without undergoing any procedure
to create osteochondral defects, were used as a normal control. The
specific grouping diagram is displayed in Fig. 2B-E. After the patella was medially
dislocated, the articular joint was exposed (Fig. 2F) and a graduated stainless steel
trephine bar (red arrow; Fig. 2G)
was used to create an osteochondral defect, which measured 5 mm in
diameter and 4 mm in depth (Fig.
2H). Fresh blood promptly occupied the defect site. Fig. 2I and J show the implantation of F-PRF fragments
(black arrow) and L-PRF fragments (yellow arrow) into the defects,
respectively. After the PRF fragments were placed into the defect
site, they were promptly mixed with blood. Subsequently, the
transplanted PRF scaffolds were pressed softly for 2 min and PRF
scaffolds immersed in blood from the bone marrow cavity filled the
defects (yellow arrow; Fig. 2I and
J).
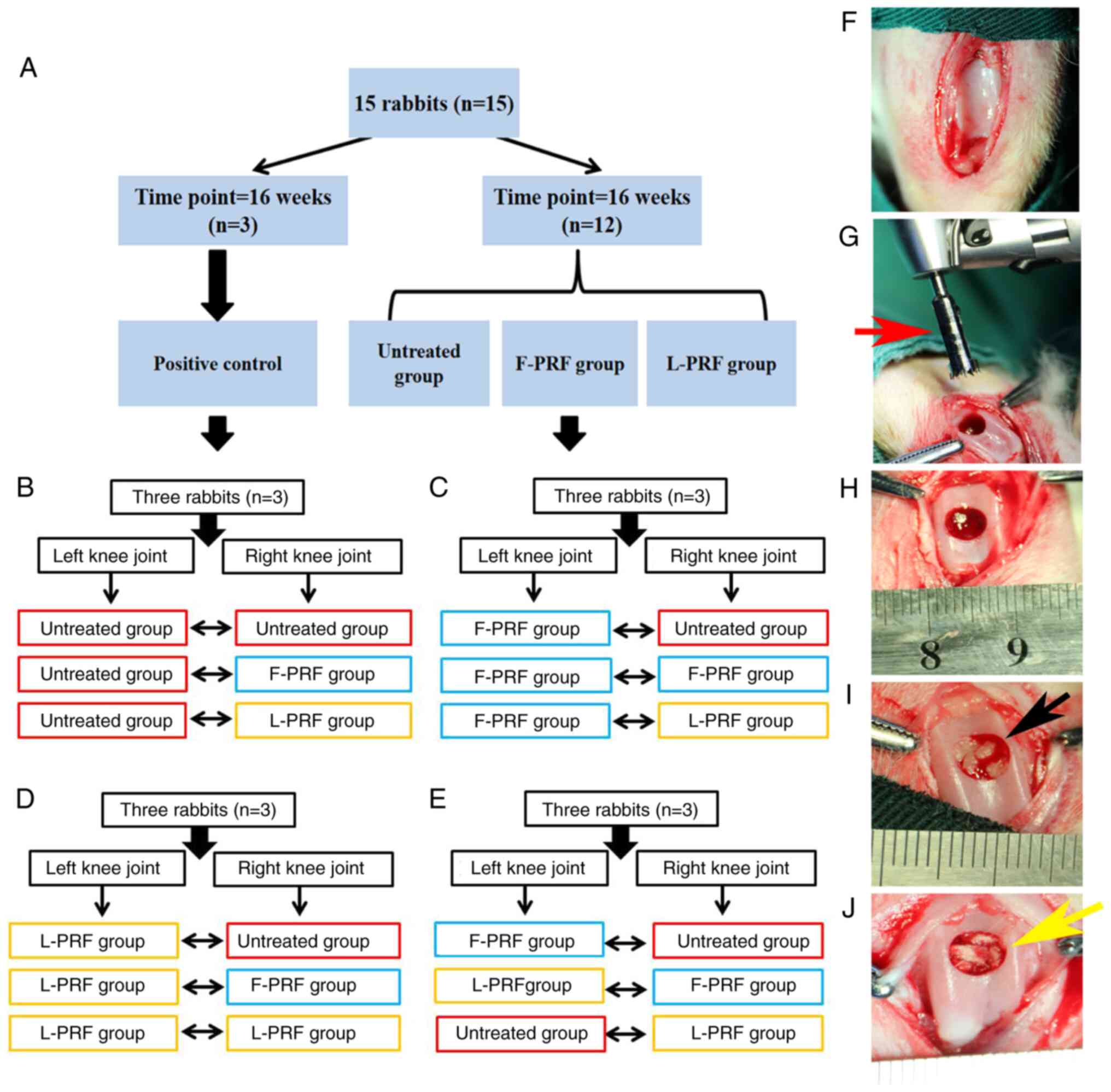 | Figure 2Animal surgery of the knee joint of
rabbits. (A) Grouping scheme. (B) Left side was untreated, whereas
the contralateral osteochondral defect received untreated, F-PRF,
and L-PRF, respectively. (C) Left side was F-PRF, whereas the
contralateral osteochondral defect received untreated, F-PRF, and
L-PRF, respectively. (D) Left side was L-PRF, whereas the
contralateral osteochondral defect received untreated, F-PRF, and
L-PRF, respectively. (E) Left side was F-PRF, and L-PRF, and
untreated, whereas the contralateral osteochondral defect received
the untreated, F-PRF, and L-PRF, respectively. (F) Representative
photography of an exposed knee joint. (G) Graduated stainless steel
trephine bar used in creating defects (red arrow). (H)
Representative photography of surgically induced cylindrical
osteochondral defect with a diameter of 5 mm and a depth of 4 mm.
Blood from the subchondral bone marrow cavity occupied the defect
sites. (I) The complex of the F-PRF scaffold (black arrow) and
blood clots adequately occupied the defect after coagulation. (J)
Complex of the L-PRF (yellow arrow) scaffold and blood clots
adequately occupied the defect after coagulation. PRF,
platelet-rich fibrin; L-PRF, lyophilized PRF; F-PRF, free PRF. |
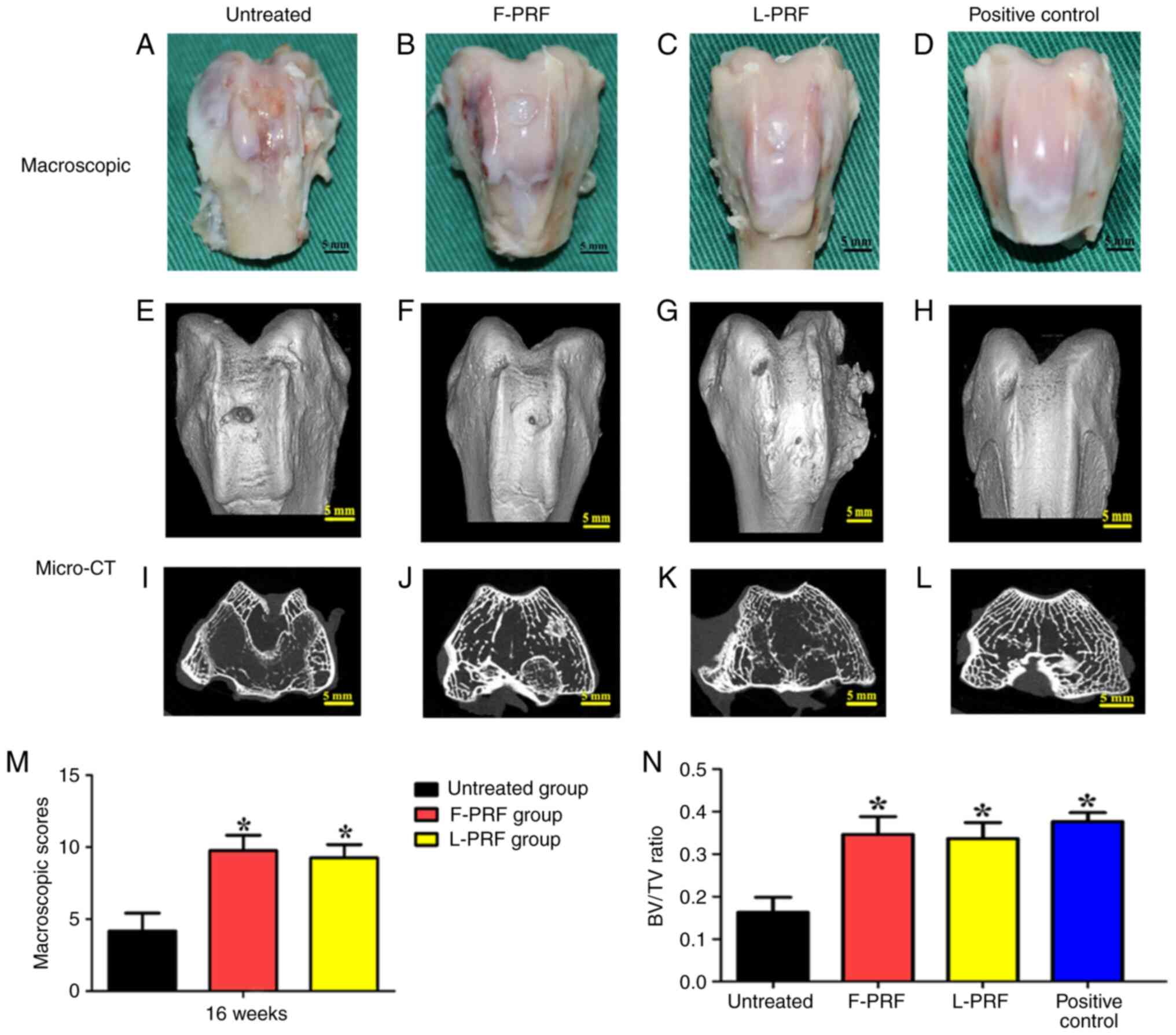
Macroscopic evaluations
At 16 weeks after surgery, macroscopic evaluations
of the osteochondral defects showed that the newly formed tissues
had a rough surface and manifested as fibrous tissues covered with
a spot of cartilage-like tissue (Fig.
3A). The newly formed tissues in the L-PRF and F-PRF groups
appeared fully connected with the circumambient native articular
cartilage and smooth hyaline cartilage similar to that of the
positive control was located on the surface (Fig. 3B-D). Furthermore, Fig. 3M showed that the macroscopic
examination based on the ICRS scoring system provided the following
mean scores for the three experimental groups: F-PRF group (10±1)
showed no significant difference compared with that of the L-PRF
group (9±1), which suggested that similar repair results were
observed in both PRF-treated groups. The score of the untreated
group (4±1) was the lowest among the three groups (P<0.05),
reflecting the worst results compared with those of the PRF-treated
groups.
BV/TV ratio was evaluated based on micro-CT data to
analyze the newly regenerated bone tissue in the transplanted sites
(Fig. 3E-L). Fig. 3E-G display the different quantities
of newly regenerated bone and bone-like tissues in the different
groups. The knee joints of the PRF-treated groups (Fig. 3E and G) were similar to the knee joints of the
positive control (Fig. 3H).
Furthermore, the newly formed bone tissues in each group showed
large differences in the cross-sectional images of defect sites
(Fig. 3I-L). The quantity and
quality of bone tissue (BV/TV ratio) in the untreated group was
significantly worse than that in the PRF-treated and normal control
groups (Fig. 3N; P<0.05).
Moreover, there was no obvious discrepancy between the PRF groups
and the normal control group (P>0.05).
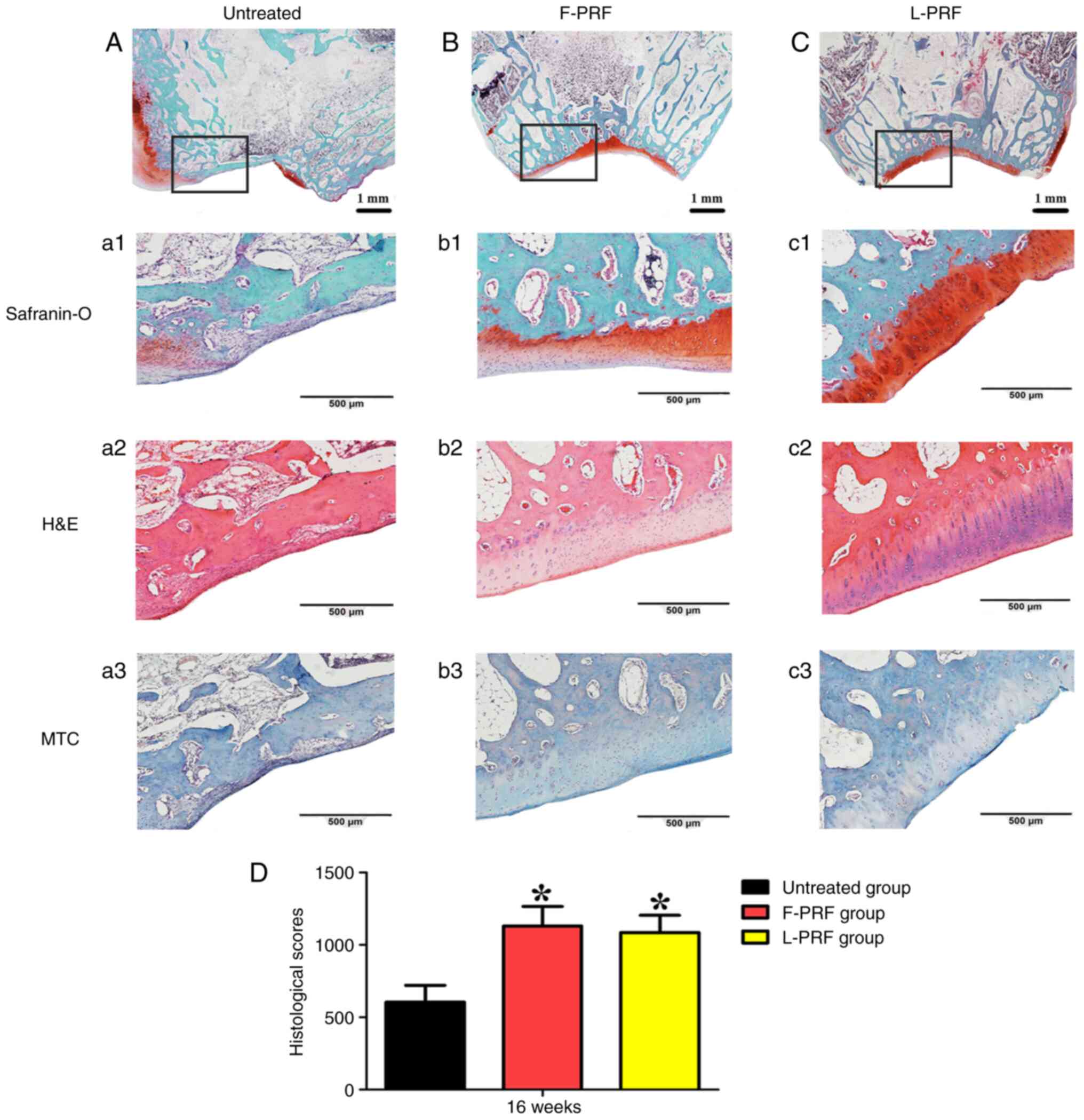
Histological examinations
Histological analysis through safranin-O, H&E,
MTC and Sirius red stain and immunostaining (COL-I and COL-II)
showed differences in the tissues occupying the defects in all
groups at 4 months after surgery (Figs. 4 and 5). Almost no newly formed cartilage or
cartilage-like tissues were observed, where the defects were filled
with fibrous tissues and a small amount of cartilage tissue, and
almost no hyaline cartilage formed in the untreated group (Fig. 4A). In contrast, the merged images
of safranin-O staining showed that well-regenerated cartilage and
bone tissues filled the defects in the PRF-treated groups (Fig. 4B and C) and completely reconstructed the
consistency of the trochlear grove surfaces of joints. In detail,
in the untreated group, fibrous tissues with poor vascularization
adhered to the surrounding normal tissues (Fig. 4a1-a3). By contrast, the regenerated
cartilage layer in the PRF-treated groups conformed to the
surrounding normal cartilage layer. Reassuringly, the
characteristic hierarchical zonal cell alignments in cartilage
appeared in the PRF-treated groups (Fig. 4b2 and b2), which was in accordance with the
structure of normal cartilage tissue (Fig. S2C). In addition, histological
staining, such as H&E and MTC, indicated well-vascularized
reconstructed bone combined with newly formed cartilage (Fig. 4b2, c2, b3
and c3). The ICRS scoring method
was applied to quantitatively analyze histological observations.
The scores were 605±115 in the untreated group, 1,130±135 in the
F-PRF group, and 1,085±118 in the L-PRF group at 16 weeks after
surgery (Fig. 4D). The untreated
group had lower scores than the PRF-treated groups (P<0.05).
However, the scores in the F-PRF and L-PRF groups were similar
(P>0.05).
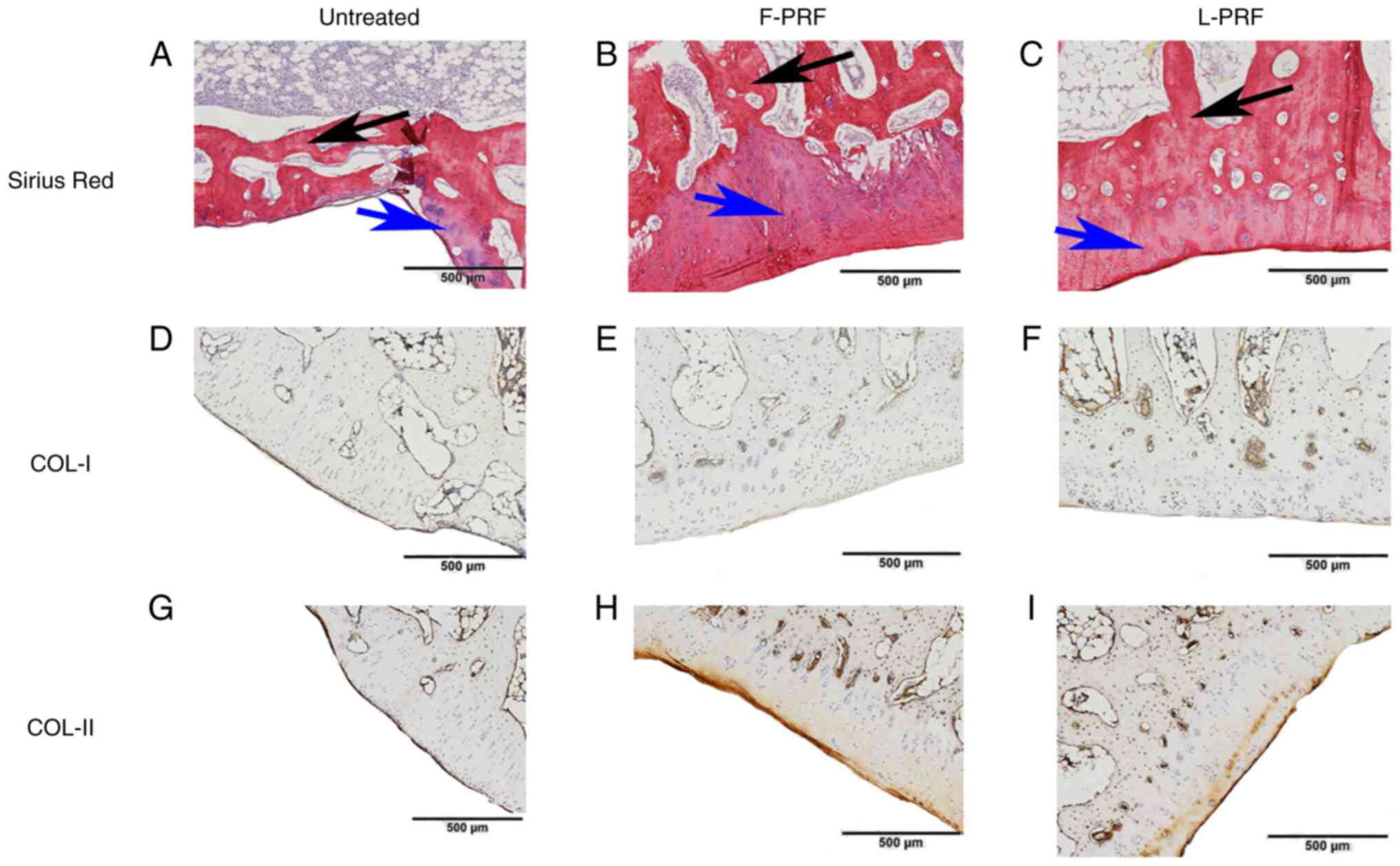
Furthermore, the different kinds of newly formed
collagen in all three groups were displayed more clearly using
Sirius red staining. A large mass of regenerated cartilage matrix
(blue arrow) and bone matrix (black arrow) was observed in the
PRF-treated groups (Fig. 5B and
C), which was very similar to the
normal cartilage in the positive control (Fig. S2D). However, in the untreated
group, only a small amount of newly regenerated cartilage matrix
was observed (blue arrow; Fig.
5A). Immunohistochemical COL-I and COL-II staining also
indicated the phenotypic stability of the newly formed cartilage
tissues in PRF-treated groups. In detail, COL-I, as a
characteristic bone constituent, was weakly stained (Fig. 5E and F), whereas COL-II, as a characteristic
cartilage component, was highly positively stained in the newly
formed cartilage tissues in the PRF-treated groups (Fig. 5H and I). Moreover, COL-II staining was weak in
the untreated group because the amount of newly formed cartilage
was minimal (Fig. 5G).
Furthermore, the mean density of COL-I and COL-II in the
PRF-treated groups was also similar to that in the positive
control. Finally, the quantitative analysis of the
immunohistochemical COL-I and COL-II staining also provided similar
results; that is, there was no significant difference between the
F-PRF and L-PRF groups, but both of them had significantly higher
values than those in the untreated group (Fig. S3).
Discussion
The present study demonstrated that full-thickness
osteochondral defects 5 mm in diameter and 4 mm in depth could be
successfully reconstructed by implanting F-PRF or L-PRF scaffolds,
without the need for additional cell seeding, and the simultaneous
reconstruction of avascular articular cartilage and
well-regenerated subchondral bone was proven through macroscopic
observations, micro-CT scanning and histological examinations.
Compared with several commonly reported approaches used for the
repair and regeneration of osteochondral defects, the present
method possesses the following advantages: First, F-PRF and L-PRF
are derived wholly from autologous blood, they are convenient and
versatile, have a high ratio of quality to price. For example, for
bone defects after dental implant and apical cyst curettage in the
dental clinic, if GBR is performed at the same time, the cost of
bone powder, collagen membrane and membrane nail will be a large
amount of cost. However, if PRF is used, only a small amount of the
cost of autologous blood centrifugation is required. Relatively
speaking, it reduces economic burden for patients.) and lack the
drawback of donor morbidity (14).
Second, autologous F-PRF and L-PRF, as rich sources of growth
factors and bioactive scaffolds, provide good biocompatibility and
no risk of immunological rejection. Third, this method does not
require additional cell transplantation or an exogenous scaffold
for cell transportation; therefore, it can avoid the shortcomings
of implanting such scaffolds or cells (7). Finally, transplanted F-PRF or L-PRF
scaffolds retained the blood effused from subchondral bone marrow,
which contained a mass of BMSCs, within the osteochondral defects,
thus initiating the process of wound healing and improving the
remodeling of newly formed tissues (23).
Due to the gradual release of large amounts of
growth factors that can accelerate tissue regeneration and
remodeling during the biodegradation of PRF, this has been broadly
applied in tissue engineering and regenerative medicine (24-27).
However, one limitation of the PRF application is that it has to be
used immediately, commonly within 10 min after preparation. In a
previous study performed by the present authors, L-PRF was
processed and prepared by vacuum freeze-drying F-PRF and the effect
of these two PRFs on the osteogenicity of BMSCs was compared in
vitro and in vivo (16). The results of the present study
indicated that the lyophilization of F-PRF had a slight effect on
the microstructure of the PRF, which was verified through H&E
staining and SEM examination. L-PRF was composed of abundant
compactly arranged fibers and could release growth factors. SEM
observations showed that BMSCs possessed good attachment on the
surface of both F-PRF and L-PRF (Fig.
S4). In addition, L-PRF markedly increased the osteogenicity
and proliferation of BMSCs in a dose-dependent manner in
vitro and stimulated the osteogenicity of osteoblastic BMSC
sheets in vivo (Fig. S5)
(16). The numerous 3-D network
microstructures of L-PRF could stimulate cell migration and tissue
bonding (28). Furthermore,
because the contact surface area of L-PRF was increased,
lyophilization not only facilitated cell proliferation and
mineralization by increasing the pore size of L-PRF but also
increased the degradation rate of L-PRF, which was verified to be
faster than that of F-PRF (29,30).
In addition, F-PRF and L-PRF could recruit stem cells to targeted
sites and then initiate the tissue repair process by stimulating
the rapid proliferation and differentiation of BMSCs (11,26).
These results suggest that L-PRF can be considered an emerging
biomaterial that adequately supplies the persistent discharge of
cytokines and growth factors that are crucial for tissue repair and
regeneration (31,32).
Successful wound repair in various kinds of adult
tissues relies strongly on the process of repair mediated by a
stable blood clot that can release a mass of cues attracted by
hemostasis and trigger the recruitment and migration of cells
(9,33). Therefore, an essential and stable
blood clot in defects of the articular knee joint could stimulate
cartilage repair and subchondral bone reconstruction by offering a
stable microenvironment for surrounding stem cell recruitment and
migration and tissue regeneration (23,34).
On the other hand, osteochondral defects on the femoral condyle
joint provided an opening and suitable microenvironment for
recruiting cells from the surrounding joint cavity, synovium, and,
most importantly, from the subchondral bone marrow (7,35).
During surgery, PRF fragments placed in the defect site promptly
transformed into soft matrices after absorbing bone marrow blood.
In addition, because of the 3-D fibrous network and bioactive
cytokines or growth factors that appeared in the L-PRF and F-PRF
scaffolds, PRF scaffolds can recruit progenitor or stem cells from
the subchondral bone marrow or surrounding synovium and stimulate
them to transfer through channels among PRF fragments, which also
stimulates the recruited and migrated progenitor or stem cells to
undergo proliferation and differentiation (36,37).
The upper part of the defect in the joint surface was enclosed by
surrounding articular cartilage and the recruited cells were
influenced by the biological cues from the cartilage and joint
fluid, which formed a chondrogenic environment (6,7,38).
By contrast, the lower part of the osteochondral defects was
directly connected with the bone marrow cavity and enclosed by the
surrounding subchondral bone, which has been proven to be strongly
osteogenic. BMSCs undergo endochondral ossification with the
induction of osteogenic medium and chondrogenesis occurs during
this process and before ossification (39). Therefore, environmental factors
play a dominant role in the reconstruction of osteochondral
defects, including the engineering of cartilage and subchondral
bone (40). Furthermore,
histological results showed that a decreased inflammation occurred,
which confirmed the excellent biocompatibility of autologous F-PRF
and L-PRF scaffolds. Concerning implantation, blood clots are mixed
with transplanted PRF fragments, which provide an opening
microenvironment. Such a microenvironment enables migrated cells to
experience a low oxygen level, which is considered to promote
chondrogenesis in the upper part of osteochondral defects (41,42).
Moreover, the natural structure of the load-bearing femoral condyle
poses the risk of PRF scaffold displacement and remodeling. The
purpose of the present study was to discuss the effect of L-PRF
scaffolds on the reconstruction of osteochondral defects. Hence,
the trochlear groove was selected as the experimental model of
defects in rabbits.
Mechanical testing is more frequently used for
examining the mechanical properties of the articular cartilage
(7). However, F-PRF or L-PRF
fragments used in this experiment are small in thickness, which
could not meet the requirements for compressive tests. In addition,
the trochlear groove was chosen as the defect model as the present
study is a preliminary attempt to test the osteochondral
reconstruction using L-PRF fragments. Load-bearing femoral condyle
model inevitably increases the risk of scaffold collapse and
displacement, mainly owing to their weak mechanical strength. The
present study aimed to investigate the cell-recruiting and
cartilage-repairing effects of F-PRF or L-PRF fragments. Although
only one treatment displayed good satisfactory effect in the
present rabbit model, multiple treatments may be required on large
animals like dogs or pigs, which needs further study.
The newly formed cartilage tissue failed to achieve
complete regeneration because the zonal hierarchical structure of
the native osteochondral tissue could not be completely
reconstructed (23). Therefore,
the present results indicated that the PRF scaffolds elicited
tissue regeneration, which could be regarded as cartilage
replacement. Because an osteochondral defect 5 mm in diameter and 4
mm in depth was produced in knee joints in vivo, the
regenerated tissue was damaged when the newly reconstructed
cartilage and subchondral bone were harvested; therefore, the
compressive strength assay was not performed. In the future, a
larger defect in larger model animals will be created (such as
miniature pigs and goats) to test the compressive strength of the
reconstructed cartilage tissue.
The present study demonstrated that autologous L-PRF
prepared by freeze-drying F-PRF significantly improved the
reconstruction of osteochondral defects in the knee joint of
rabbits, thus offering a novel bioscaffold for the simultaneous
regeneration of subchondral bone and articular cartilage without
cell transplantation.
Supplementary Material
Characterization of BMSCs. (A) BMSCs
presented a fibroblast-like morphology and closely spaced growth.
(B) MTT assay showed BMSCs self-renewal and rapid proliferation.
BMSCs potency was characterized based on their (C) Adipogenic
(alizarin red stain) and (D) Osteogenic (oil red O)
differentiation. BMSCs were identified through flow cytometric
analysis which showed that the cells were (E) CD29 positive and (F)
CD31 negative. BMSC, bone marrow mesenchymal stem cell.
Histological analysis of normal
femoral condyles joint used as a positive control. (A) Merged views
of safranin-O staining. Higher-magnification views of (B)
Safranin-O staining, (C) H&E staining, (D) Sirius red staining,
(E) COL-I immu-nostaining and (F) COL-II immunostaining. The number
for COL-I and COL-II in the positive was 6.51 and 6.23,
respectively. COL-I, collagen type I; COL-II, collagen type
II.
Comparison of the mean density of
COL-I and COL-II immunostaining. There was no statistically
significant difference in mean COL-I or COL-II immunostaining
density between the F-PRF and L-PRF groups, but the values in both
were markedly higher than those in the untreated group.
*P<0.05 vs. untreated group. COL-I, collagen type I;
COL-II, collagen type II; PRF, platelet-rich fibrin; L-PRF,
lyophilized PRF; F-PRF, free PRF.
SEM imaging displayed the effects of
PRF on the attachment of BMSCs. BMSCs showed good attachment on the
surface of PRFs. BMSCs attached on the surface of (A and B) F-PRF
and (C and D) L-PRF. SEM, scanning electron microscopy; BMSC, bone
marrow mesenchymal stem cell; PRF, platelet-rich fibrin; L-PRF,
lyophilized PRF; F-PRF, free PRF.
Effects of PRF on the proliferation of
BMSCs. The proliferation of BMSCs was markedly increased by adding
L-PRF or F-PRF at concentrations ranging from 0.2X PRF to 1X PRF to
the culture medium. *P<0.05. BMSC, bone marrow
mesenchymal stem cell; OD, optical density; PRF, platelet-rich
fibrin; L-PRF, lyophilized PRF; F-PRF, free PRF.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Military Medical
Technology Youth Cultivation Project of PLA (grant no. 20QNPY081),
the National Natural Science Foundation of China (grant no.
81700943), the Natural Science Foundation of Guangdong Province
(grant no. 2017A030310671) and the Science and Technology Planning
Project of Guangzhou city, China (grant no. 202102021269).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWS, LH and ZFW conceived and designed the study.
JWS, LH and CDL carried out in vitro and in vivo
studies, participated in the sequence alignment and drafted the
manuscript. JLM, XL and SHS carried out the in vitro and
in vivo studies. SHS and ZFW confirm the authenticity of all
the raw data and performed the statistical analysis. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were reviewed and approved by
the Institutional Animal Care and Use Committee of the General
Hospital of Southern Theater of PLA (Guangzhou, China; approval no.
2019010402).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng C, Chang J and Wu C: Bioactive
scaffolds for osteochondral regeneration. J Orthop Translat.
17:15–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xue J, Feng B, Zheng R, Lu Y, Zhou G, Liu
W, Cao Y, Zhang Y and Zhang WJ: Engineering ear-shaped cartilage
using electrospun fibrous membranes of gelatin/polycaprolactone.
Biomaterials. 34:2624–2631. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huey DJ, Hu JC and Athanasiou KA: Unlike
bone, cartilage regeneration remains elusive. Science. 338:917–921.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brittberg M, Lindahl A, Nilsson A, Ohlsson
C, Isaksson O and Peterson L: Treatment of deep cartilage defects
in the knee with autologous chondrocyte transplantation. N Engl J
Med. 331:889–895. 1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nooeaid P, Salih V, Beier JP and
Boccaccini AR: Osteochondral tissue engineering: Scaffolds, stem
cells and applications. J Cell Mol Med. 16:2247–2270.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mendelson A, Frank E, Allred C, Jones E,
Chen M, Zhao W and Mao JJ: Chondrogenesis by chemotactic homing of
synovium, bone marrow, and adipose stem cells in vitro. FASEB J.
25:3496–3504. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Z, Li Z, Li Z, Wu B, Liu Y and Wu W:
Cartilaginous extracellular matrix derived from decellularized
chondrocyte sheets for the reconstruction of osteochondral defects
in rabbits. Acta Biomater. 81:129–145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee CH, Cook JL, Mendelson A, Moioli EK,
Yao H and Mao JJ: Regeneration of the articular surface of the
rabbit synovial joint by cell homing: A proof of concept study.
Lancet. 376:440–448. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zlotnick HM, Locke RC, Stoeckl BD, Patel
JM, Gupta S, Browne KD, Koh J, Carey JL and Mauck RL: Marked
differences in local bone remodelling in response to different
marrow stimulation techniques in a large animal. Eur Cell Mater.
41:546–557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park MS, Kim YH, Jung Y, Kim SH, Park JC,
Yoon DS, Kim SH and Lee JW: In situ recruitment of human bone
marrow-derived mesenchymal stem cells using chemokines for
articular cartilage regeneration. Cell Transplant. 24:1067–1083.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kazemi D, Fakhrjou A, Dizaji VM and
Alishahi MK: Effect of autologous platelet rich fibrin on the
healing of experimental articular cartilage defects of the knee in
an animal model. Biomed Res Int. 2014(486436)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part I: Technological
concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e37–e44. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Serra CI, Soler C, Carrillo JM, Sopena JJ,
Redondo JI and Cugat R: Effect of autologous platelet-rich plasma
on the repair of full-thickness articular defects in rabbits. Knee
Surg Sports Traumatol Arthrosc. 21:1730–1736. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Z, Weng Y, Lu S, Zong C, Qiu J, Liu Y
and Liu B: Osteoblastic mesenchymal stem cell sheet combined with
Choukroun platelet-rich fibrin induces bone formation at an ectopic
site. J Biomed Mater Res B Appl Biomater. 103:1204–1216.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Z, Hu H, Li Z, Weng Y, Dai T, Zong C,
Liu Y and Liu B: Sheet of osteoblastic cells combined with
platelet-rich fibrin improves the formation of bone in
critical-size calvarial defects in rabbits. Br J Oral Maxillofac
Surg. 54:316–321. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Z, Han L, Sun T, Wang W, Li X and Wu
B: Preparation and effect of lyophilized platelet-rich fibrin on
the osteogenic potential of bone marrow mesenchymal stem cells in
vitro and in vivo. Heliyon. 5(e02739)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lei X, Yang Y, Shan G, Pan Y and Cheng B:
Preparation of ADM/PRP freeze-dried dressing and effect of mice
full-thickness skin defect model. Biomed Mater.
14(035004)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chumroenphat T, Somboonwatthanakul I,
Saensouk S and Siriamornpun S: Changes in curcuminoids and chemical
components of turmeric (Curcuma longa L.) under freeze-drying and
low-temperature drying methods. Food Chem.
339(128121)2021.PubMed/NCBI View Article : Google Scholar : Haugh MG, Murphy
CM and O'Brien FJ: Novel freeze-drying methods to produce a range
of collagen-glycosaminoglycan scaffolds with tailored mean pore
sizes. Tissue Eng Part C Methods 16: 887-894, 2010.
|
|
19
|
Shi L, Li R, Wei S, Zhou M, Li L, Lin F,
Li Y, Guo Z, Zhang W, Chen M and Shan G: Effects of a protective
agent on freeze-dried platelet-rich plasma. Blood Coagul
Fibrinolysis. 30:58–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ba R, Wei J, Li M, Cheng X, Zhao Y and Wu
W: Cell-bricks based injectable niche guided persistent ectopic
chondrogenesis of bone marrow-derived mesenchymal stem cells and
enabled nasal augmentation. Stem Cell Res Ther.
6(16)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
van den Borne MP, Raijmakers NJ, Vanlauwe
J, Victor J, de Jong SN, Bellemans J and Saris DB: International
Cartilage Repair Society. International cartilage repair society
(ICRS) and oswestry macroscopic cartilage evaluation scores
validated for use in autologous chondrocyte implantation (ACI) and
microfracture. Osteoarthritis Cartilage. 15:1397–1402.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mainil-Varlet P, Van Damme B, Nesic D,
Knutsen G, Kandel R and Roberts S: A new histology scoring system
for the assessment of the quality of human cartilage repair: ICRS
II. Am J Sports Med. 38:880–890. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Z, Han L, Sun T, Ma J, Sun S, Ma L
and Wu B: Extracellular matrix derived from allogenic
decellularized bone marrow mesenchymal stem cell sheets for the
reconstruction of osteochondral defects in rabbits. Acta Biomater.
118:54–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Temmerman A, Cleeren GJ, Castro AB,
Teughels W and Quirynen M: L-PRF for increasing the width of
keratinized mucosa around implants: A split-mouth, randomized,
controlled pilot clinical trial. J Periodontal Res. 53:793–800.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sameera S, Nagasri M, Aravind Kumar P,
Indeevar P, Raviraj K and Musalaiah SVVS: Comparison of two
surgical techniques in the treatment of multiple gingival
recessions sandwiched with a combination of A-PRF and L-PRF. Saudi
Dent J. 30:183–189. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Beitzel K, McCarthy MB, Cote MP, Russell
RP, Apostolakos J, Ramos DM, Kumbar SG, Imhoff AB, Arciero RA and
Mazzocca AD: Properties of biologic scaffolds and their response to
mesenchymal stem cells. Arthroscopy. 30:289–298. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part III: Leucocyte
activation: a new feature for platelet concentrates? Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 101:e51–e55. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma L, Wang X, Zhao N, Zhu Y, Qiu Z, Li Q,
Zhou Y, Lin Z, Li X, Zeng X, et al: Integrating 3D printing and
biomimetic mineralization for personalized enhanced osteogenesis,
angiogenesis, and osteointegration. ACS Appl Mater Interfaces.
10:42146–42154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Isobe K, Watanebe T, Kawabata H, Kitamura
Y, Okudera T, Okudera H, Uematsu K, Okuda K, Nakata K, Tanaka T and
Kawase T: Mechanical and degradation properties of advanced
platelet-rich fibrin (A-PRF), concentrated growth factors (CGF),
and platelet-poor plasma-derived fibrin (PPTF). Int J Implant Dent.
3(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pichotano EC, de Molon RS, de Souza RV,
Austin RS, Marcantonio E and Zandim-Barcelos DL: Evaluation of
L-PRF combined with deproteinized bovine bone mineral for early
implant placement after maxillary sinus augmentation: A randomized
clinical trial. Clin Implant Dent Relat Res. 21:253–262.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lundquist R, Dziegiel MH and Agren MS:
Bioactivity and stability of endogenous fibrogenic factors in
platelet-rich fibrin. Wound Repair Regen. 16:356–363.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dohan Ehrenfest DM, Del Corso M, Diss A,
Mouhyi J and Charrier JB: Three-dimensional architecture and cell
composition of a Choukroun's platelet-rich fibrin clot and
membrane. J Periodontol. 81:546–555. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
White JG: Platelet secretion during clot
retraction. Platelets. 11:331–343. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mao Z, Bi X, Wu C, Zheng Y, Shu X, Wu S,
Guan J and Ritchie RO: A cell-free silk fibroin biomaterial
strategy promotes in situ cartilage regeneration via programmed
releases of bioactive molecules. Adv Healthc Mater.
12(e2201588)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ravindran S, Kotecha M, Huang CC, Ye A,
Pothirajan P, Yin Z, Magin R and George A: Biological and MRI
characterization of biomimetic ECM scaffolds for cartilage tissue
regeneration. Biomaterials. 71:58–70. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Crapo PM, Gilbert TW and Badylak SF: An
overview of tissue and whole organ decellularization processes.
Biomaterials. 32:3233–3243. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cortiella J, Niles J, Cantu A, Brettler A,
Pham A, Vargas G, Winston S, Wang J, Walls S and Nichols JE:
Influence of acellular natural lung matrix on murine embryonic stem
cell differentiation and tissue formation. Tissue Eng Part A.
16:2565–2580. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Z, Ba R, Wang Z, Wei J, Zhao Y and Wu
W: Angiogenic potential of human bone marrow-derived mesenchymal
stem cells in chondrocyte brick-enriched constructs promoted stable
regeneration of craniofacial cartilage. Stem Cells Transl Med.
6:601–612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li J, Kang F, Gong X, Bai Y, Dai J, Zhao
C, Dou C, Cao Z, Liang M, Dong R, et al: Ceria nanoparticles
enhance endochondral ossification-based critical-sized bone defect
regeneration by promoting the hypertrophic differentiation of BMSCs
via DHX15 activation. FASEB J. 33:6378–6389. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gaut C and Sugaya K: Critical review on
the physical and mechanical factors involved in tissue engineering
of cartilage. Regen Med. 10:665–679. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nukavarapu SP and Dorcemus DL:
Osteochondral tissue engineering: current strategies and
challenges. Biotechnol Adv. 31:706–721. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brown BN and Badylak SF: Extracellular
matrix as an inductive scaffold for functional tissue
reconstruction. Transl Res. 163:268–285. 2014.PubMed/NCBI View Article : Google Scholar
|