Introduction
Kidney transplantation is a treatment option for
end-stage renal disease patients, which has several benefits for
the patients, such as a higher quality of life, lower costs, and
fewer dietary restrictions, amongst others (1-3).
However, this surgery carries the risks of post-transplant
complications, including delayed graft function, vascular
calcification, bone fractures, diabetes, and infection, amongst
other issues (4-8).
These complications threaten the success of a graft and may further
lead to the death of patients who received kidney transplantation
(9-11).
Therefore, exploring therapeutic approaches that reduce the
occurrence of these post-transplant complications is crucial to
improve the clinical outcomes of patients receiving kidney
transplantation.
Vitamin K is a hydrophobic vitamin that serves as a
cofactor of the enzyme γ-glutamyl carboxylase to activate several
vitamin K-dependent proteins, thereby improving vascular and bone
health (12). Recently, several
studies have explored the effect of vitamin K on improving clinical
outcomes (such as renal function, vascular calcification, and
all-cause mortality) in patients receiving kidney transplantation
(13-20);
however, these findings are contested. For example, one previous
study found that a higher vitamin K status was related to increased
renal function in patients receiving kidney transplantation
(20). Additionally, vitamin K
sufficiency is correlated with lower all-cause mortality in these
patients (15). However, another
study found that vitamin K supplementation did not reduce vascular
stiffness, vascular calcification, or renal function in patients
receiving kidney transplantation, which indicated that vitamin K
supplementation had no influence on improving the clinical outcomes
of these patients (19). As a
result, whether vitamin K improves clinical outcomes in patients
receiving kidney transplantation should be further explored.
Accordingly, this meta-analysis was performed to
explore the correlation of vitamin K status with all-cause
mortality, renal function, inflammation, as well as vascular and
bone health in patients receiving kidney transplantation.
Materials and methods
Search strategy
Electronic databases (EMBASE, PubMed, and Cochrane)
were used to screen the papers relating to the effects of vitamin K
status on clinical outcomes of patients who received kidney
transplantation from conception to December 2022. Key words and
medical subject headings were applied, including ‘vitamin K’, ‘VK’,
‘vitamin-K’, ‘V-K’, ‘kidney transplant’, ‘renal transplant’,
‘kidney transplantation’, ‘renal transplantation’, ‘kidney graft’,
and ‘renal graft’.
Eligibility criteria
Inclusion criteria for study screening were: i)
Patients >18 years old; ii) patients received a kidney
transplant; iii) studies assessed the impact of vitamin K status or
vitamin K supplementation on clinical outcomes after kidney
transplantation; iv) studies involved at least one clinical
outcomes of interest to the present study; and v) published in
English. The exclusion criteria were: i) Reviews, case reports, or
letters; or ii) had no available data for extraction.
The clinical outcomes of interest in the present
study were: i) All-cause mortality; ii) renal function indexes;
iii) and C-reactive protein (CRP).
Study selection
In the present meta-analysis, two reviewers
independently completed the study screening. In brief, the titles
and abstracts were assessed for preliminary screening. Then, the
full texts which met the inclusion criteria were downloaded and
assessed. The studies which met the exclusion criteria were
ineligible for inclusion, and the excluded cause was recorded.
Additionally, the relevant publications lists were also identified.
Any disagreements were resolved by conversation and reaching a
consensus. For studies with overlapping populations, those with a
larger population or a longer follow-up period were included.
Data collection and risk of bias
Two reviewers independently finished the data
collection and assessment of bias risk. The disagreements were
resolved by consensus. The extracted data included authors' names,
publication year, study design, demographic information of
patients, and outcomes. The Newcastle-Ottawa Scale criteria were
utilized to assess the risk of bias, involving 3 domains:
Selection, comparability, and outcome (21). The risk of bias in the included
studies was classified as low risk (score, >8), medium risk
(score, 5-7), or high risk (score, ≤4).
Statistical analysis
The analyses were completed per the Preferred
Reporting Item for Systematic Reviews and Meta-analyses (PRISMA)
using Stata (version 14.0, StataCorp LP). In the present
meta-analysis, normal vitamin K status or vitamin K supplementation
was considered as the experimental group; while vitamin K
deficiency or no vitamin K supplementation was considered as the
control group. Relative risk (RR) with 95% confidence intervals
(CIs) was used for dichotomous outcomes, and mean difference (MD)
with 95% CI was used for continuous outcomes. The heterogeneity was
determined using I2 statistics: If I2≤50.0%
and/or P≥0.05, the heterogeneity was considered insignificant, and
the fixed effects model was used; otherwise, the random effects
model was used (22). The
sensitivity analysis was performed by omitting each study and then
repeating the analysis. Egger's and Begg's tests were utilized to
evaluate publication bias and P<0.05 was considered to indicate
a statistically significant difference. R version 3.6 and R studio
version 4.2.2 were utilized for analyses (23,24).
Results
Study screening procedure
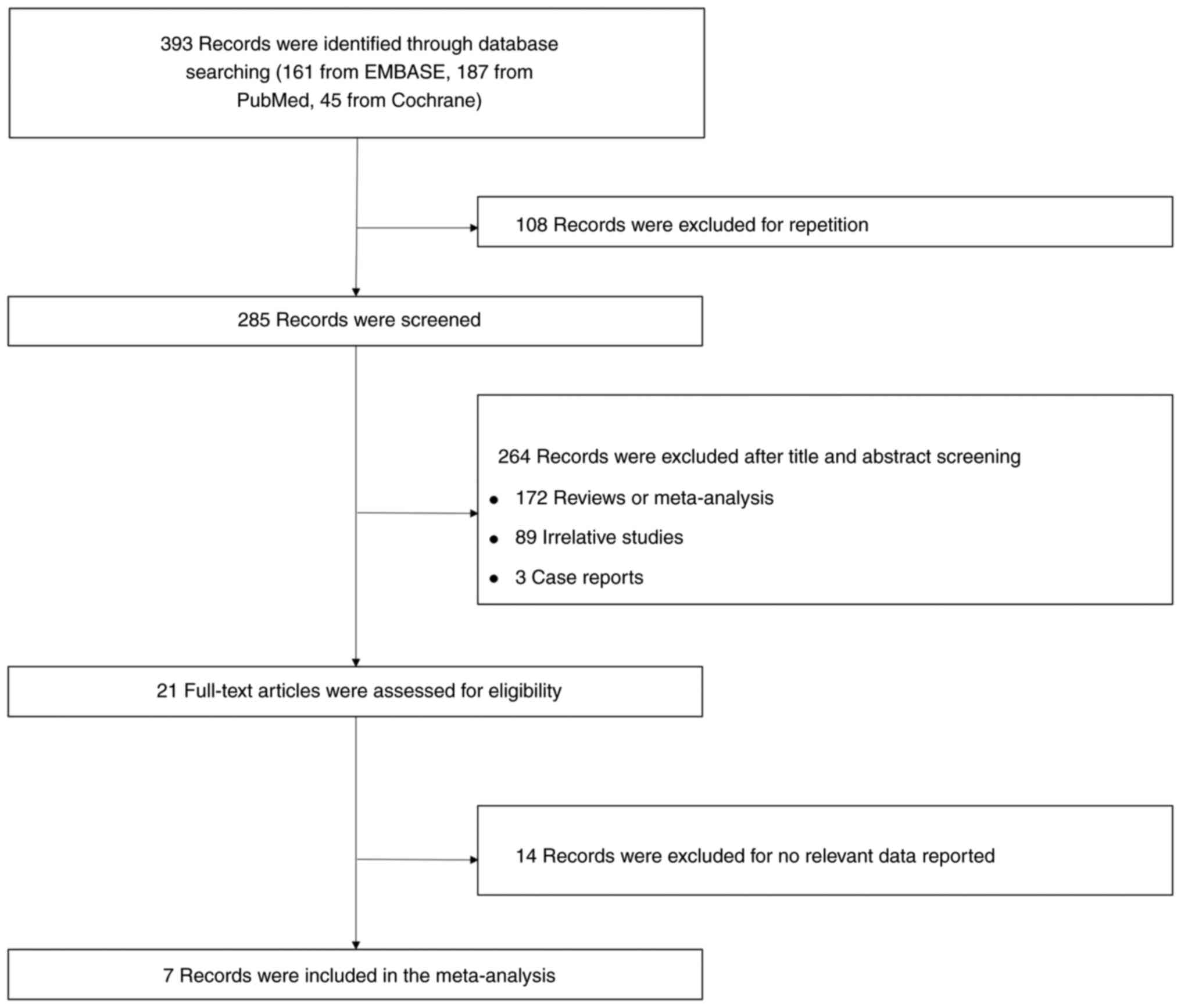
A total of 393 records were identified through
searching of databases, including 161 records from EMBASE, 187
records from PubMed, and 45 records from Cochrane, of which 108
records were excluded as duplicates, leaving 285 records to be
screened. A further 264 records were excluded after screening of
the titles and abstracts, including 172 reviews or meta-analyses,
89 irrelevant studies, and 3 case reports. Subsequently, 21
full-text records were assessed for eligibility, and 14 records
with no relevant data reported were further excluded. Ultimately, 7
records were included in the present meta-analysis (Fig. 1).
Characteristics of the included
studies
The included studies were published between 2012 and
2021 in various countries, including the Netherlands, Lebanon, the
Kingdom of Belgium, and the United Kingdom (14-20).
Regarding study design, there were 5 cohort studies (14,15,17,18,20),
1 subgroup analysis of a single-arm trial (16), and 1 randomized controlled trial
(19). Notably, 1,752 patients
receiving kidney transplantation were involved, including 1,101
patients in the experimental group and 651 patients in the control
group. The detailed information on the included studies is listed
in Table I.
 | Table IDetails of the included studies. |
Table I
Details of the included studies.
| First author,
year | Country | Study design | Sample size of
cohorts, n | Age in years, mean
± SD | Male, n (%) | Outcomes | (Ref.) |
|---|
| Boxma et al,
2012 | Netherlands | Cohort | Experimental, 30;
Control, 30 | Experimental,
55.0±NK; Control, 57.0±NK | Experimental: 15
(50.0) Control: 15 (50.0) | Creatinine, CRP,
systolic blood pressure | (14) |
| Keyzer et
al, 2015 | Netherlands | Cohort | Experimental, 130;
Control, 129 | Experimental,
48.1±12.6; Control, 54.6±10.8 | Experimental: 70
(54.0) Control: 70 (54.0) | All-cause
mortality, eGFR, albumin, CRP, triglycerides, hemoglobin,
calcium | (15) |
| Mansour et
al, 2017 | Lebanese | Subgroup analysis
of the single-arm trial | Experimental, 56;
Control, 56 | NK | NK | Creatinine,
albumin, systolic blood pressure, diastolic blood pressure,
hemoglobin, 25-hydroxyvitamin D | (16) |
| Evenepoel et
al, 2018 | The Kingdom of
Belgium | Cohort | Experimental, 155;
Control, 159 | Experimental,
51.6±14.4; Control, 57.6±11.0 | NK | Creatinine, CRP
triglycerides, calcium, 25-hydroxyvitamin D | (17) |
| van Ballegooijen
et al, 2020 | Netherlands | Cohort | Experimental, 107;
Control, 108 | Experimental,
50.3±12.1; Control, 51.6±11.2 | Experimental: 59
(55.0) Control: 68 (63.0) | All-cause
mortality, eGFR, albumin, CRP, systolic blood pressure, diastolic
blood pressure, triglycerides, hemoglobin | (18) |
| Lees et al,
2021 | United Kingdom | Randomized
controlled trial | Experimental, 45;
Control, 45 | Experimental, 5
6.3±11.1; Control, 58.9±7.8 | Experimental, 32
(71.7) Control: 31 (68.9) | All-cause
mortality, eGFR, creatinine, albumin, systolic blood pressure,
diastolic blood pressure, triglycerides, hemoglobin, calcium,
25-hydroxyvitamin D | (19) |
| Kremer et
al, 2021 | Netherlands | Cohort | Experimental, 578;
Control, 124 | Experimental,
56.0±13.0; Control, 53.0±14.0 | Experimental, 341
(59.0); Control, 79 (64.0) | Creatinine,
systolic blood pressure, hemoglobin, 2 5-hydroxyvitamin D | (20) |
Quality assessment
The included studies were assessed using the
Newcastle-Ottawa Scale criteria, which suggested that 1 study was
ranked as low risk of bias with a total score of 8(18). Additionally, 5 studies were ranked
as medium risk of bias with a range of total scores from 5 to 7
(14,15,17,19,20).
Notably, 1 study was ranked as a high risk of bias with a total
score of 4(16); in detail, the
scores of the selection bias, comparability bias, and outcome bias
were evaluated as 1, 2 and 1, respectively (Table II).
 | Table IIAssessment of the risk of bias using
the Newcastle-Ottawa Scale criteria. |
Table II
Assessment of the risk of bias using
the Newcastle-Ottawa Scale criteria.
| First author,
year | Selection | Comparability | Outcome | Total score | (Ref.) |
|---|
| Boxma et al,
2012 | 2 | 1 | 2 | 5 | (14) |
| Keyzer et
al, 2015 | 3 | 1 | 2 | 6 | (15) |
| Mansour et
al, 2017 | 1 | 2 | 1 | 4 | (16) |
| Evenepoel et
al, 2018 | 2 | 2 | 1 | 5 | (17) |
| van Ballegooijen
et al, 2020 | 4 | 1 | 3 | 8 | (18) |
| Lees et al,
2021 | 4 | 1 | 2 | 7 | (19) |
| Kremer et
al, 2021 | 3 | 1 | 3 | 7 | (20) |
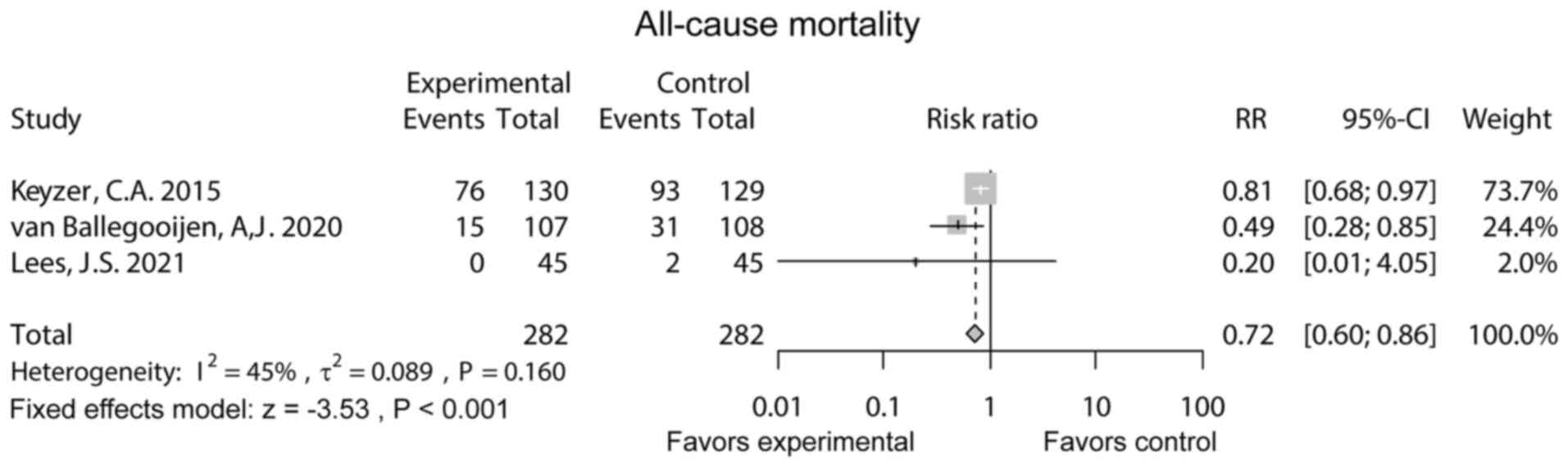
All-cause mortality
A total of 3 studies reported all-cause mortality.
The fixed effects model revealed that all-cause mortality was
reduced in the experimental group compared to the control group [RR
(95% CI): 0.72 (0.60-0.86), P<0.001]. Heterogeneity did not
exist among studies (I2=45%, P=0.160; Fig. 2).
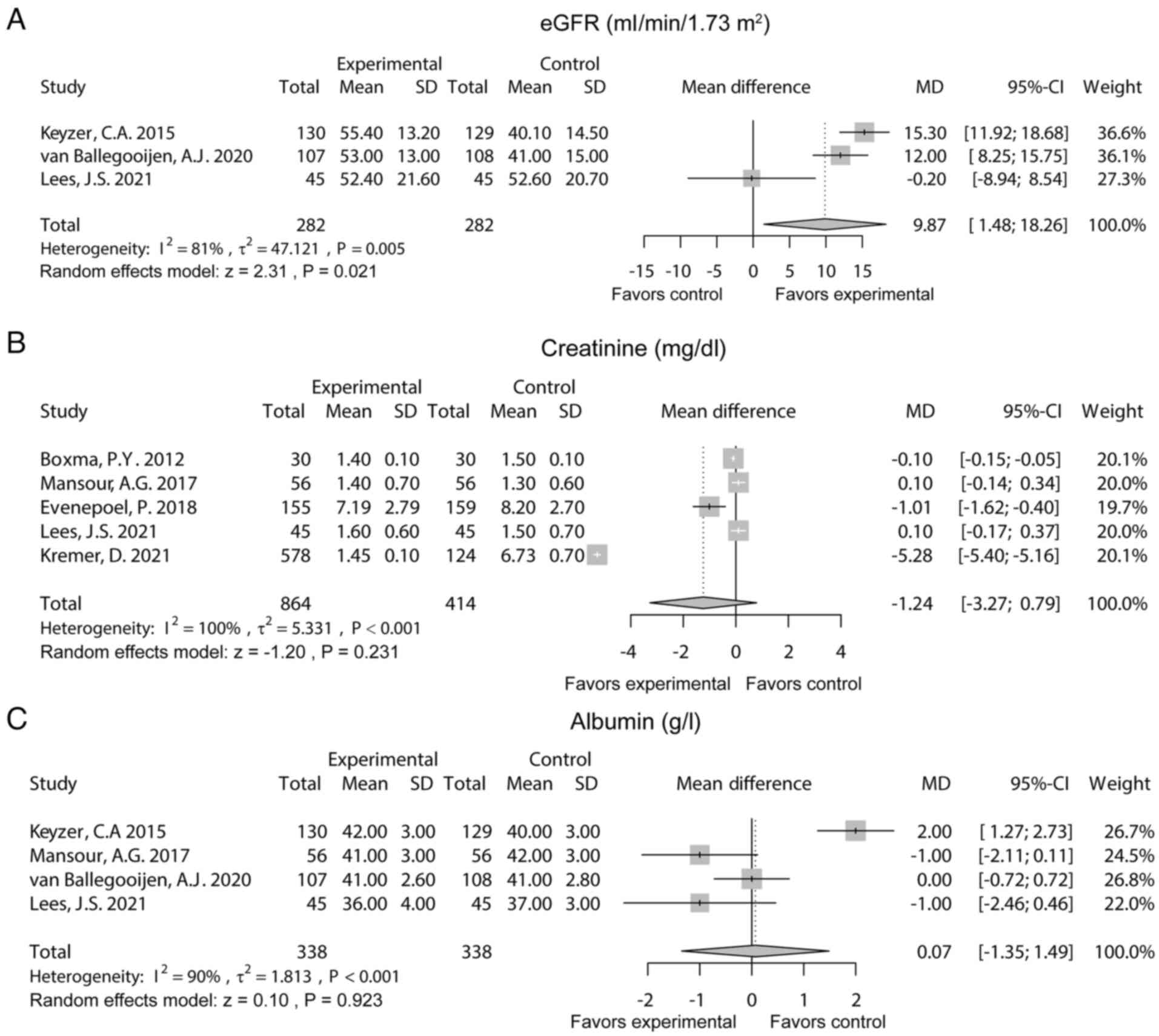
Renal function indexes
A total of 3 studies reported the estimated
glomerular filtration rate (eGFR). The random effects model
suggested that eGFR was increased in the experimental group
compared with the control group [MD (95% CI): 9.87 (1.48-18.26),
P=0.021). Heterogeneity existed among these studies
(I2=81%, P=0.005) (Fig.
3A). Additionally, five studies reported creatinine. After the
random effects model was applied, it was found that creatinine did
not differ between the two groups (MD (95% CI): -1.24 (-3.27-0.79),
P=0.231). Heterogeneity existed among these studies
(I2=100%, P<0.001; Fig.
3B). Moreover, five studies reported albumin. The random
effects model revealed that albumin was not different between the
two groups [MD (95% CI): 0.07 (-1.35-1.49), P=0.923]. Heterogeneity
existed among these studies (I2=90%, P<0.001;
Fig. 3C).
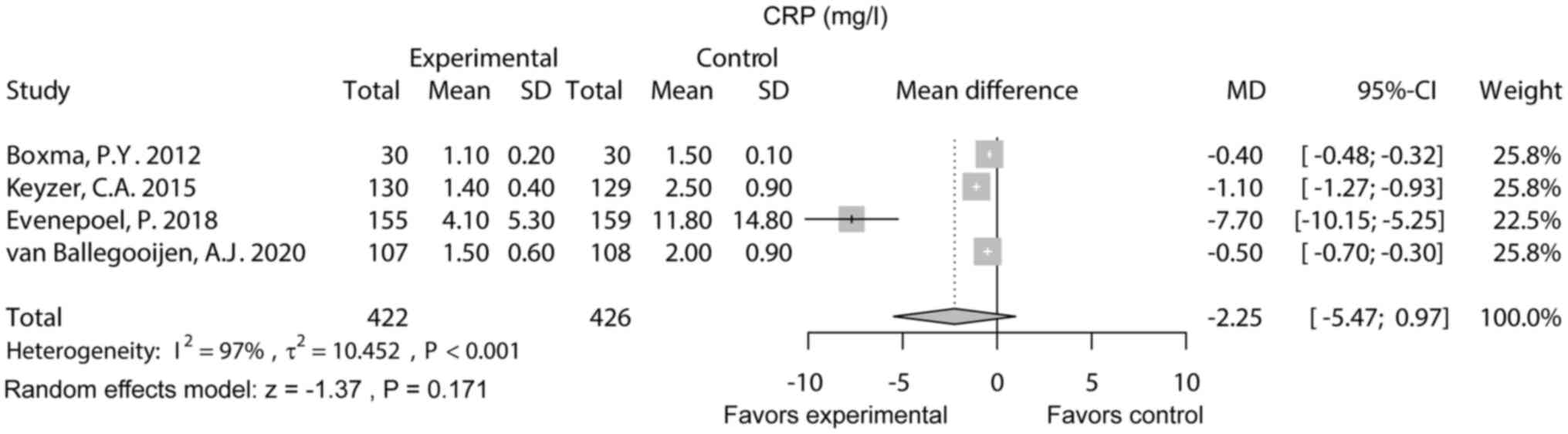
CRP
CRP was reported in four studies. Notably, the
random effects model showed that CRP did not differ between the
experimental group and the control group [MD (95% CI): -2.25
(-5.47-0.97), P=0.171]. Heterogeneity existed among these studies
(I2=97%, P<0.001; Fig.
4).
Cardiovascular and bone health
indexes
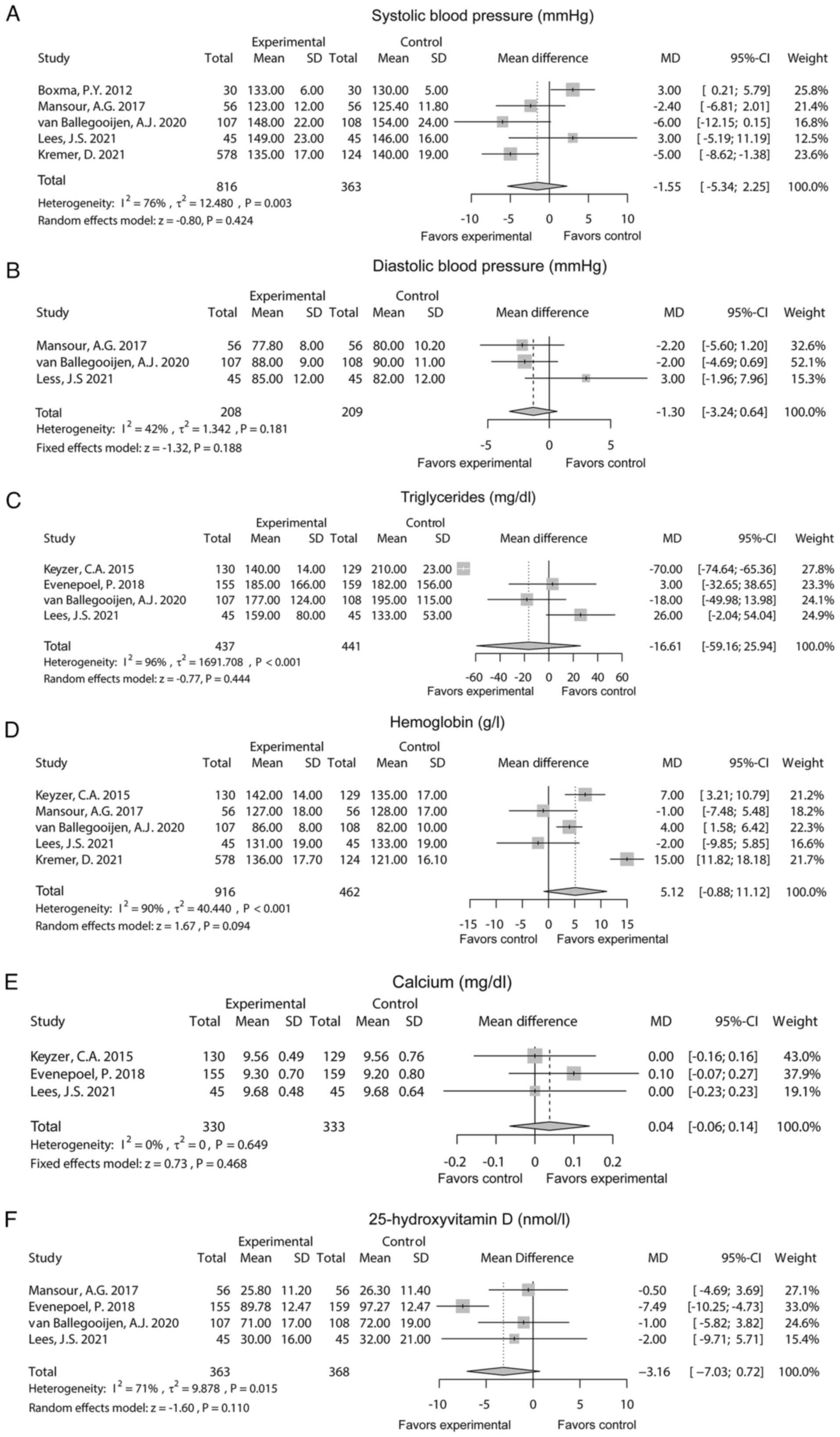
There were 5 studies that reported systolic blood
pressure. After the random effects model was applied, it was found
that systolic blood pressure did not differ between the
experimental group and the control group [MD (95% CI): -1.55
(-5.34-2.25), P=0.424]. Heterogeneity existed among these studies
(I2=76%, P=0.003; Fig.
5A). Additionally, 3 studies reported diastolic blood pressure.
The fixed effects model showed that diastolic blood pressure
remained unchanged between the two groups [MD (95% CI): -1.30
(-3.24-0.64), P=0.188]. Heterogeneity did not exist among these
studies (I2=42%, P=0.181; Fig. 5B). There were 4 studies that
reported triglycerides. The random effects model suggested that
triglycerides did not differ between the two groups [MD (95% CI):
-16.61 (-59.16-25.94), P=0.444] with heterogeneity among these
studies (I2=96%, P<0.001; Fig. 5C). Moreover, 5 studies reported
hemoglobin. The random effects model showed that no difference in
hemoglobin was found between the two groups [MD (95% CI): 5.12
(-0.88-11.12), P=0.094] with heterogeneity among these studies
(I2=90%, P<0.001; Fig.
5D).
A total of 3 studies reported calcium. The fixed
effects model found that calcium remained unchanged between the two
groups [MD (95% CI): 0.04 (-0.06-0.14), P=0.468) without
heterogeneity among these studies (I2=0%, P=0.649;
Fig. 5E). There were 4 studies
reported on 25-hydroxyvitamin D levels. The random effects model
suggested that 25-hydroxyvitamin D did not differ between the two
groups [MD (95% CI): -3.16 (-7.03-0.72); P=0.110] with
heterogeneity among these studies (I2=71%, P=0.015;
Fig. 5F).
Publication bias and sensitivity
analysis
Begg's test and Egger's test were performed to
estimate the potential publication bias, which indicated that no
publication bias existed for all-cause mortality, eGFR, creatinine,
albumin, CRP, systolic blood pressure, diastolic blood pressure,
triglycerides, hemoglobin, calcium, and 25-hydroxyvitamin D (all
P>0.05; Table III).
 | Table IIIPublication bias. |
Table III
Publication bias.
| Outcomes | Number of included
studies | P-value, Begg's
test | P-value, Egger's
test |
|---|
| All-cause
mortality | 3 | 0.602 | 0.310 |
| eGFR | 3 | 0.117 | 0.173 |
| Creatinine | 5 | 0.327 | 0.644 |
| Albumin | 4 | 0.497 | 0.351 |
| CRP | 4 | 0.497 | 0.192 |
| Systolic blood
pressure | 5 | 1.000 | 0.560 |
| Diastolic blood
pressure | 3 | 0.120 | 0.300 |
| Triglycerides | 4 | 0.500 | 0.060 |
| Hemoglobin | 5 | 1.000 | 0.592 |
| Calcium | 3 | 0.602 | 0.854 |
| 25-hydroxyvitamin
D | 4 | 1.000 | 0.265 |
The sensitivity analysis showed that omitting Keyzer
et al (15) or van
Ballegooijen et al (18)
resulted in eGFR remaining unchanged between the experimental group
and the control group. Meanwhile, omitting Evenepoel et al
(17) resulted in a decrease in
CRP in the experimental group compared to the control group.
Omitting Boxma et al (14)
may have contributed to systolic blood pressure reduction in the
experimental group vs. the control group. Additionally, hemoglobin
was increased in the experimental group compared to the control
group after omitting Lees et al (19). Apart from these, the RR of
all-cause mortality, as well as the MD of creatinine, albumin,
diastolic blood pressure, triglycerides, calcium, and
25-hydroxyvitamin D did not significantly change by omitting any
single study, which suggested the stability of this meta-analysis
(Table IV).
 | Table IVSensitivity analysis. |
Table IV
Sensitivity analysis.
| | 95% Confidence
interval |
|---|
| Omitted study | Estimate | Lower | Upper |
|---|
| All-cause
mortality, relative risk | | | |
|
Keyzer et
al, 2015 | 0.47 | 0.27 | 0.81 |
|
van
Ballegooijen et al, 2020 | 0.80 | 0.66 | 0.95 |
|
Lees et
al, 2021 | 0.73 | 0.61 | 0.88 |
|
Combined | 0.72 | 0.60 | 0.86 |
| eGFR, ml/min/1.73
m2, MD | | | |
|
Keyzer et
al, 2015 | 6.57 | -5.32 | 18.45 |
|
van
Ballegooijen et al, 2020 | 8.10 | -7.06 | 23.25 |
|
Lees et
al, 2021 | 13.75 | 10.53 | 16.98 |
|
Combined | 9.87 | 1.48 | 18.26 |
| Creatinine, mg/dl,
MD | | | |
|
Boxma et
al, 2012 | -1.53 | -4.04 | 0.99 |
|
Mansour
et al, 2017 | -1.58 | -4.05 | 0.90 |
|
Evenepoel
et al, 2018 | -1.30 | -3.90 | 1.31 |
|
Lees et
al, 2021 | -1.58 | -4.05 | 0.90 |
|
Kremer et
al, 2021 | -0.16 | -0.57 | 0.26 |
|
Combined | -1.24 | -3.27 | 0.79 |
| Albumin, g/l,
MD | | | |
|
Keyzer et
al, 2015 | -0.51 | -1.27 | 0.25 |
|
Mansour
et al, 2017 | 0.41 | -1.30 | 2.12 |
|
van
Ballegooijen et al, 2020 | 0.06 | -1.96 | 2.08 |
|
Lees et
al, 2021 | 0.37 | -1.35 | 2.09 |
|
Combined | 0.07 | -1.35 | 1.49 |
| CRP, mg/l, MD | | | |
|
Boxma et
al, 2012 | -2.95 | -7.31 | 1.42 |
|
Keyzer et
al, 2015 | -2.72 | -7.31 | 1.87 |
|
Evenepoel
et al, 2018 | -0.66 | -1.09 | -0.23 |
|
van
Ballegooijen et al, 2020 | -2.91 | -7.32 | 1.49 |
|
Combined | -2.25 | -5.47 | 0.97 |
| Systolic blood
pressure, mmHg, MD | | | |
|
Boxma et
al, 2012 | -3.66 | -6.09 | -1.23 |
|
Mansour
et al, 2017 | -1.33 | -6.23 | 3.56 |
|
van
Ballegooijen et al, 2020 | -0.65 | -4.80 | 3.50 |
|
Lees et
al, 2021 | -2.22 | -6.43 | 1.98 |
|
Kremer et
al, 2021 | -0.47 | -4.73 | 3.79 |
|
Combined | -1.55 | -5.34 | 2.25 |
| Diastolic blood
pressure, mmHg, MD | | | |
|
Mansour
et al, 2017 | -0.87 | -3.23 | 1.50 |
|
van
Ballegooijen et al, 2020 | -0.54 | -3.34 | 2.26 |
|
Lees et
al, 2021 | -2.08 | -4.18 | 0.03 |
|
Combined | -1.30 | -3.24 | 0.64 |
| Triglycerides,
mg/dl, MD | | | |
|
Keyzer et
al, 2015 | 4.63 | -21.71 | 30.97 |
|
Evenepoel
et al, 2018 | -22.10 | -77.78 | 33.58 |
|
van
Ballegooijen et al, 2020 | -15.38 | -74.05 | 43.29 |
|
Lees et
al, 2021 | -31.25 | -75.69 | 13.20 |
|
Combined | -16.61 | -59.16 | 25.94 |
| Hemoglobin, g/l,
MD | | | |
|
Keyzer et
al, 2015 | 4.47 | -3.30 | 12.24 |
|
Mansour
et al, 2017 | 6.48 | -0.15 | 13.12 |
|
van
Ballegooijen et al, 2020 | 5.27 | -2.60 | 13.14 |
|
Lees et
al, 2021 | 6.55 | 0.13 | 12.96 |
|
Kremer et
al, 2021 | 3.06 | -0.71 | 6.82 |
|
Combined | 5.12 | -0.88 | 11.12 |
| Calcium, mg/dl,
MD | | | |
|
Keyzer et
al, 2015 | 0.07 | -0.07 | 0.20 |
|
Evenepoel
et al, 2018 | 0.00 | -0.13 | 0.13 |
|
Lees et
al, 2021 | 0.05 | -0.07 | 0.16 |
|
Combined | 0.04 | -0.06 | 0.14 |
| 25-hydroxyvitamin
D, nmol/l, MD | | | |
|
Mansour
et al, 2017 | -4.12 | -8.76 | 0.53 |
|
Evenepoel
et al, 2018 | -0.90 | -3.82 | 2.02 |
|
van
Ballegooijen et al, 2020 | -3.78 | -8.65 | 1.09 |
|
Lees et
al, 2021 | -3.29 | -7.99 | 1.40 |
|
Combined | -3.16 | -7.03 | 0.72 |
Discussion
Vitamin K deficiency is very common in patients
receiving kidney transplantation, which may ultimately contribute
to an increase in all-cause mortality (15). Therefore, several studies have
explored the effect of vitamin K sufficiency on all-cause mortality
in patients receiving kidney transplantation (15,18,19).
A previous study found that higher vitamin K status is related to
reduced all-cause mortality in patients receiving kidney
transplantation (15).
Additionally, another study also showed that vitamin K sufficiency
estimates decreased premature mortality in patients receiving
kidney transplantation (18). The
present meta-analysis discovered that higher vitamin K status or
supplementation of vitamin K was related to decreased all-cause
mortality in patients receiving kidney transplantation. The
possible reasons may be: i) Vitamin K may inhibit the progression
of vascular calcification by increasing the activity of matrix Gla
protein (MGP) by accelerating γ-carboxylation (25,26);
ii) vitamin K may also improve bone health by regulating
osteocalcin (27,28). Notably, vascular calcification and
bone damage were two major causes of mortality in patients
receiving kidney transplantation (29), and vitamin K can improve these
situations as discussed above. As a result, vitamin K may reduce
all-cause mortality in these patients.
This meta-analysis also explored the effect of
vitamin K on improving renal function in patients receiving kidney
transplantation, and it was found that a higher vitamin K status or
supplementation of vitamin K was related to increased eGFR in
patients receiving kidney transplantation. A possible reason would
be that vitamin K may activate MGP through carboxylation to improve
renal function, which further led to the increase of eGFR (30). Thus, a positive correlation was
found between vitamin K status or supplementation and eGFR in
patients receiving kidney transplantation. Notably, heterogeneity
existed among the analyzed 3 studies; meanwhile, sensitivity
analysis displayed that omitting Keyzer et al (15) or van Ballegooijen et al
(18) affected the results of
eGFR, which indicated the notable weight these two articles had on
the outcomes. Thus, these findings still require additional studies
to verify these results. In addition, the present meta-analysis
also observed that higher vitamin K status or supplementation of
vitamin K was slightly associated with reduced CRP in patients
receiving kidney transplantation, but this was statistically
significant. A possible interpretation may be that vitamin K may
reduce inflammation by regulating the nuclear factor κB pathway, a
Gla-rich protein (31,32). However, heterogeneity existed among
the four analyzed studies. Omitting Evenepoel et al
(17) affected the results of CRP,
highlighting the notable weight of this study on the results. Thus,
this finding still requires further exploration.
The effect of vitamin K on improving vascular and
bone health is contested based on previous studies (15,16,18-20).
The present meta-analysis found that vitamin K status or
supplementation of vitamin K was not related to systolic blood
pressure, diastolic blood pressure, triglycerides, hemoglobin,
calcium, or 25-hydroxyvitamin D in patients receiving kidney
transplantation. A possible reason may be that the disease
conditions were complicated in patients after kidney
transplantation, and the change of a single factor (vitamin K) does
not greatly influence these vascular and bone health indexes; thus,
the benefits of vitamin K alone in improving vascular and bone
health would not be notable (27,33,34).
Heterogeneity of systolic blood pressure, triglycerides,
hemoglobin, calcium, and 25-hydroxyvitamin D existed among the
analyzed studies; meanwhile, sensitivity analysis found that
omitting Boxma et al (14)
and Lees et al (19)
affected the results of systolic blood pressure and hemoglobin,
respectively. this highlighted the notable weight of these two
studies on the corresponding results. Therefore, these findings
require further studies to confirm the results.
Although several interesting findings were
discovered in the present meta-analysis, some limitations should be
noted: i) Although robustness assessed by sensitivity analysis was
acceptable, omitting certain articles did affect the corresponding
results; thus, additional studies are required to further improve
the reliability of the results; ii) most of the included studies
were cohort studies; thus, the findings of this meta-analysis
should be further validated; and iii) one included study was ranked
as high risk of bias according to the Newcastle-Ottawa Scale
criteria, which may have interfered with the results.
In conclusion, vitamin K may improve all-cause
mortality and renal function in patients receiving kidney
transplantation. Clinically, the intake of vitamin K after kidney
transplantation may improve the clinical outcomes of these
patients. However, additional large-scale studies are required to
validate these findings.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Basic Research Program
of Guizhou Province (Guizhou Science and Technology Combination
Foundation) [grant no. ZK (2023) General 380].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and YN contributed to the research design, data
analysis, writing the paper, and critical review of the paper. KZ,
GL, and FY collected the data and wrote the paper. SC and LJ
contributed to the data analysis and critical review of the paper.
ZS and YN confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wouk N: End-stage renal disease: Medical
management. Am Fam Physician. 104:493–499. 2021.PubMed/NCBI
|
|
2
|
Wang Y, Hemmelder MH, Bos WJW, Snoep JD,
de Vries APJ, Dekker FW and Meuleman Y: Mapping health-related
quality of life after kidney transplantation by group comparisons:
A systematic review. Nephrol Dial Transplant. 36:2327–2339.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Axelrod DA, Schnitzler MA, Xiao H, Irish
W, Tuttle-Newhall E, Chang SH, Kasiske BL, Alhamad T and Lentine
KL: An economic assessment of contemporary kidney transplant
practice. Am J Transplant. 18:1168–1176. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Agrawal A, Ison MG and Danziger-Isakov L:
Long-term infectious complications of kidney transplantation. Clin
J Am Soc Nephrol. 17:286–295. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sotomayor CG, Benjamens S, Gomes-Neto AW,
Pol RA, Groothof D, Te Velde-Keyzer CA, Chong G, Glaudemans AWJM,
Berger SP, Bakker SJL and Slart RHJA: Bone mineral density and
aortic calcification: evidence for a bone-vascular axis after
kidney transplantation. Transplantation. 105:231–239.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ponticelli C, Favi E and Ferraresso M:
New-onset diabetes after kidney transplantation. Medicina (Kaunas).
57(250)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ponticelli C, Reggiani F and Moroni G:
Delayed graft function in kidney transplant: Risk factors,
Consequences and prevention strategies. J Pers Med.
12:2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bahl D, Haddad Z, Datoo A and Qazi YA:
Delayed graft function in kidney transplantation. Curr Opin Organ
Transplant. 24:82–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Haberal M, Boyvat F, Akdur A, Kirnap M,
Ozcelik U and Yarbug Karakayali F: Surgical complications after
kidney transplantation. Exp Clin Transplant. 14:587–595.
2016.PubMed/NCBI
|
|
10
|
Ammi M, Daligault M, Sayegh J, Abraham P,
Papon X, Enon B and Picquet J: Evaluation of the vascular surgical
complications of renal transplantation. Ann Vasc Surg. 33:23–30.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ghadiani MH, Peyrovi S, Mousavinasab SN
and Jalalzadeh M: Delayed graft function, allograft and patient
srvival in kidney transplantation. Arab J Nephrol Transplant.
5:19–24. 2012.PubMed/NCBI
|
|
12
|
Cozzolino M, Mangano M, Galassi A, Ciceri
P, Messa P and Nigwekar S: Vitamin K in chronic kidney disease.
Nutrients. 11(168)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eelderink C, Kremer D, Riphagen IJ, Knobbe
TJ, Schurgers LJ, Pasch A, Mulder DJ, Corpeleijn E, Navis G, Bakker
SJL, et al: Effect of vitamin K supplementation on serum
calcification propensity and arterial stiffness in vitamin
K-deficient kidney transplant recipients: A double-blind,
randomized, placebo-controlled clinical trial. Am J Transplant.
23:520–530. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Boxma PY, van den Berg E, Geleijnse JM,
Laverman GD, Schurgers LJ, Vermeer C, Kema IP, Muskiet FA, Navis G,
Bakker SJ and de Borst MH: Vitamin K intake and plasma
desphospho-uncarboxylated matrix Gla-protein levels in kidney
transplant recipients. PLoS One. 7(e47991)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Keyzer CA, Vermeer C, Joosten MM, Knapen
MH, Drummen NE, Navis G, Bakker SJ and de Borst MH: Vitamin K
status and mortality after kidney transplantation: A cohort study.
Am J Kidney Dis. 65:474–483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mansour AG, Hariri E, Daaboul Y, Korjian
S, El Alam A, Protogerou AD, Kilany H, Karam A, Stephan A and
Bahous SA: Vitamin K2 supplementation and arterial stiffness among
renal transplant recipients-a single-arm, single-center clinical
trial. J Am Soc Hypertens. 11:589–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Evenepoel P, Claes K, Meijers B, Laurent
M, Bammens B, Naesens M, Sprangers B, Pottel H, Cavalier E and
Kuypers D: Poor vitamin K status is associated with low bone
mineral density and increased fracture risk in end-stage renal
disease. J Bone Miner Res. 34:262–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Ballegooijen AJ, Beulens JWJ, Keyzer
CA, Navis GJ, Berger SP, de Borst MH, Vervloet MG and Bakker SJL:
Joint association of vitamins D and K status with long-term
outcomes in stable kidney transplant recipients. Nephrol Dial
Transplant. 35:706–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lees JS, Rankin AJ, Gillis KA, Zhu LY,
Mangion K, Rutherford E, Roditi GH, Witham MD, Chantler D,
Panarelli M, et al: The ViKTORIES trial: A randomized,
double-blind, placebo-controlled trial of vitamin K supplementation
to improve vascular health in kidney transplant recipients. Am J
Transplant. 21:3356–3368. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kremer D, Groothof D, Keyzer CA, Eelderink
C, Knobbe TJ, Post A, van Londen M, Eisenga MF, TransplantLines
Investigators, Schurgers LJ, et al: Kidney function-dependence of
vitamin K-status parameters: Results from the transplantlines
biobank and cohort studies. Nutrients. 13(3069)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wells GA, Shea B, O'Connell D, je
Peterson, Welch V, Losos M and Tugwell P: The newcastle-ottawa
scale (NOS) for assessing the quality of nonrandomized studies in
meta-analyses. Ottawa Hospital Research Institute, Ottawa, ON,
2015.
|
|
22
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
R Core Team: A language and environment
for statistical computing. R foundation for statistical computing,
Vienna, 2013. http://www.R-project.org/.
|
|
24
|
RStudio Team: RStudio: Integrated
development for R. RStudio, Inc., Boston MA. 2015. http://www.rstudio.com/.
|
|
25
|
Shioi A, Morioka T, Shoji T and Emoto M:
The inhibitory roles of vitamin K in progression of vascular
calcification. Nutrients. 12(583)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roumeliotis S, Duni A, Vaios V, Kitsos A,
Liakopoulos V and Dounousi E: Vitamin K supplementation for
prevention of vascular calcification in chronic kidney disease
patients: Are we there yet? Nutrients. 14(925)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alonso N, Meinitzer A, Fritz-Petrin E,
Enko D and Herrmann M: Role of vitamin K in bone and muscle
metabolism. Calcif Tissue Int. 112:178–196. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fusaro M, Cianciolo G, Brandi ML, Ferrari
S, Nickolas TL, Tripepi G, Plebani M, Zaninotto M, Iervasi G, La
Manna G, et al: Vitamin K and osteoporosis. Nutrients.
12(3625)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bellone F, Cinquegrani M, Nicotera R,
Carullo N, Casarella A, Presta P, Andreucci M, Squadrito G,
Mandraffino G, Prunestì M, et al: Role of vitamin K in chronic
kidney disease: A focus on bone and cardiovascular health. Int J
Mol Sci. 23(5282)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei FF, Drummen NE, Thijs L, Jacobs L,
Herfs M, Van't Hoofd C, Vermeer C and Staessen JA:
Vitamin-K-dependent protection of the renal microvasculature:
Histopathological studies in normal and diseased kidneys. Pulse
(Basel). 4:85–91. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ohsaki Y, Shirakawa H, Miura A, Giriwono
PE, Sato S, Ohashi A, Iribe M, Goto T and Komai M: Vitamin K
suppresses the lipopolysaccharide-induced expression of
inflammatory cytokines in cultured macrophage-like cells via the
inhibition of the activation of nuclear factor κB through the
repression of IKKα/β phosphorylation. J Nutr Biochem. 21:1120–1126.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bordoloi J, Dihingia A, Kalita J and Manna
P: Implication of a novel vitamin K dependent protein, GRP/Ucma in
the pathophysiological conditions associated with vascular and soft
tissue calcification, osteoarthritis, inflammation, and carcinoma.
Int J Biol Macromol. 113:309–316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vermeer C, Shearer MJ, Zittermann A,
Bolton-Smith C, Szulc P, Hodges S, Walter P, Rambeck W, Stöcklin E
and Weber P: Beyond deficiency: Potential benefits of increased
intakes of vitamin K for bone and vascular health. Eur J Nutr.
43:325–335. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kluch M, Bednarkiewicz P, Orzechowska M,
Grzelak P and Kurnatowska I: Vitamin K1 and K2 in the diet of
patients in the long term after kidney transplantation. Nutrients.
14(5070)2022.PubMed/NCBI View Article : Google Scholar
|