Introduction
In Traditional Chinese Medicine (TCM) it is believed
that blood deficiency can be defined as suffering from Qi and blood
loss, deficiency in the stomach and spleen, and insufficient
hematogenesis. Common symptoms are pale or pale-yellow complexion,
pale lips, dizziness, blurred vision, hand and foot numbness, and
low menstrual volume in women (1).
These symptoms are in line with the symptoms of anemia in modern
medicine (2). Patients with
malignant tumors are treated with chemotherapy drugs, which usually
cause myelosuppression and immunosuppression. The reduction of
erythrocytopenia and thrombocytopenia in chemotherapy patients may
impede the chemotherapy process, which can affect both the
therapeutic outcome and quality of life of patients (3). Therefore, compounds that can treat
and/or prevent the side effects of anemia in patients with
malignant tumors are of great interest.
Panax notoginseng (Burk.) F.H. Chen (PN) is a
well-known traditional herb in China and has been used in TCM for
>2,000 years. PN can promote blood circulation, hemostasis,
detumescence and relieve pain. Saponins are a general term for a
type of glycoside composed of triterpenes or spirostanes. The
saponins first found in ginseng that were named as ginsenosides
Rb1, Rg1, Rd, etc. and in PN were named as notoginsenosides R1,
Ft1, and etc. The main active components of PN are saponins,
including notoginsenoside R1 (nR1), and ginsenosides Rb1, Rg1 and
Rd (4). These saponins are used as
anti-inflammatory and antitumor treatments and are also considered
to support the immune system, provide cardiovascular protection and
promote blood circulation (5,6).
Traditionally, PN has two different forms, raw and processed. The
processed PN, known as steamed PN, is the key component in
generating blood (7), which
increases the production of blood cells and is involved in the
activation of immune cells through the JAK-STAT signaling pathway,
finally promoting hematopoiesis in anemia (8). The process of steaming PN increases
the amount and activities of certain ginsenosides. For example, the
ginsenoside Rb1 is converted into the more bioactive ginsenoside
compound K (CK) and protopanaxadiol (PPD) (9). Although PN is typically processed by
steaming, it can also be processed by fermentation. The enzymes
produced by microorganisms in the fermentation process hydrolyze
the carbohydrate side chains at C-3, C-6 and C-20, which changes
the composition and contents of saponins (10). Previous studies (9,10)
have reported that the processed PN generates a large number of
effective ginsenosides, which differ from the ones found in raw PN.
Ginsenosides Rk3, Rh4, Rk1, Rg5, F4, 20(S/R)-Rg3, CK and
20(S/R)-Rh1 are unique saponins that only exist in processed PN but
not in raw PN (11). The microbial
transformation method is stable in the production of PN or NS
(12,13) and can also improve drug efficacy
and reduce toxicity. Several studies have reported that the
β-glucosidase enzyme produced by Lactobacillus plantarum,
Lactobacillus delbrueckii, Lactobacillus fermentum,
Bifidobacterium longum or Leuconostoc mesenteroides
could transform the ginsenosides into rare ginsenosides, which are
the deglycosylated secondary metabolic derivatives of major
ginsenosides and function as active substances (14,15).
In the present study, the chemical composition and content of the
total saponins from PN before and after fermentation with
Lactobacillus plantarum were compared.
In anemia, functional recovery of hematopoietic
organs is a key process. Hematopoiesis is the differentiation of a
small pool of self-renewing pluripotent hematopoietic stem cells
(HSCs) to produce blood cells, including white blood cells (WBCs),
red blood cells (RBCs), platelets (PLTs) and reticulocytes (Rets)
(16). HSCs can produce a new
blood cell count to resist the reduction caused by blood
deficiency. Hematopoietic cytokines, such as thrombopoietin (TPO),
erythropoietin (EPO) and granulocyte-macrophage colony-stimulating
factor (GM-CSF), perform vital roles in the progress of hemopoiesis
(17). Cytokines are crucial in
the inflammatory response to anemia and are necessary for
re-establishing flow to the afflicted organs (18). Decreased levels of cytokines
suggest organ pathology, which inhibits the development of T cells
and other immune cells (19). The
effect of saponins on hematopoiesis and immunity should be
evaluated for use in treating anemia. However, to the best of our
knowledge, no studies have previously reported the therapeutic
effect of notoginseng saponins (NS) and fermented NS (FNS) on blood
deficiency.
In the present study, the total saponins from PN
were fermented with Lactobacillus plantarum, and the changes
in saponin content were evaluated. Blood deficiency was induced in
rats using acetylphenylhydrazine (APH) and cyclophosphamide (CP),
and the effects of FNS and NS on blood deficiency parameters were
assessed. The present study aimed to provide a useful theoretical
basis for the future clinical treatment of blood deficiency.
Materials and methods
Materials
The saponins [notoginsenoside R1 (nR1), ginsenosides
Rg1, Rb1, Re, Rd, Rh2, CK, PPD and protopanaxatriol (PPT)] and NS
were purchased from Shanghai Yuanye Biotechnology Co., Ltd. The
saponins were supplied with a purity of >99.0%. APH and CP were
purchased from MilliporeSigma. The ELISA kits for
interleukin(IL)-4, IL-6, IL-10, IL-12, IL-13, transforming growth
factor-β (TGF-β), interferon-γ (IFN-γ), tumor necrosis factor-α
(TNF-α), thrombopoietin (TPO), erythropoietin (EPO),
granulocyte-macrophage colony-stimulating factor (GM-CSF), alkaline
phosphatase (ALP), alanine aminotransferase (ALT), aspartate
aminotransferase (AST), direct bilirubin (DBIL), lactate
dehydrogenase (LDH), and transferrin (TRF) were purchased from
Nanjing Jiancheng Bioengineering Institute. Cell cycle and
apoptosis kits were supplied by BD Biosciences. A blood routine
reagent kit was purchased from IDEXX Laboratories Inc.
Preparation of FNS
NS was inoculated with Lactobacillus
plantarum culture (10% MRS broth (Beijing Solarbio Science
& Technology Co., Ltd).; 3.1% of inoculation amount; pH 7.0)
and fermented for 3.2 days at 37.6˚C. After fermentation, the
culture was freeze-dried and smashed. The FNS and the raw NS
powders were stored at -20˚C for later use.
HPLC analysis
A mixed standard solution containing nR1,
ginsenosides Rg1, Rb1, PPD, Re, Rd, PPT, CK and Rh2 at 1.5 mg/ml
was dissolved in methanol and filtered through a 0.22-µm filter
membrane. Sample solutions of FNS and NS (2 mg/ml) were dissolved
in methanol and filtered. The saponin contents of FNS and NS were
analyzed using the LC-2030 HPLC system (Shimadzu Corporation) and a
C18 column (Agilent, 150x4.6 mm; 5 µm). The mobile phase comprised
acetonitrile (A) and water (B). The elution gradient was as
follows: 0-10 min, 18-23% A; 10-30 min, 23-44% A; 30-38 min, 44-68%
A; 38-45 min, 68% A; 45-55 min, 68-100% A; 55-60 min, 100% A; and
60-65 min, 100-18% A. The flow rate was 1.0 ml/min, the detection
wavelength was 203 nm, the column oven was maintained at 25˚C and
injection volume was 10 µl.
Animal model
A total of 32 male Wistar rats (weight, 200.0±20.0
g; age, 8 weeks) were supplied by Changchun Yisi Experimental
Animal Technology Co., Ltd. [animal license no. SCXK
(Ji)-2021-0003]. The rats had ad libitum access to food and
water and were kept in an environment with controlled light (12 h
light/dark cycle), temperature (25±1˚C) and relative humidity
(60±5%). This experiment was authorized by the Bioethics Committee
of Changchun University of Chinese Medicine and the Institutional
Animal Care (approval no. 2022156; Changchun, China), and was
performed based on the NIH guide for the care and use of laboratory
animals (20). After a 3-day
period of acclimation, 32 rats were divided into the control,
model, FNS and NS groups (n=8). To induce the blood deficiency
model, the rats (model, FNS and NS groups) were subcutaneously
injected in the neck with 2% APH normal saline (20 and 10 mg/kg) on
days 1 and 4, respectively. At 2 h after injection on day 4, the
rats were intraperitoneally injected with CP normal saline (20
mg/kg), this was repeated on days 5, 6 and 7(21). After APH and CP treatment for 24 h,
which started on day 8, the rats were intragastrically administered
FNS (250 mg/kg) and NS (250 mg/kg), and the rats in the control and
model groups were intragastrically administered 0.9% normal saline
(1 ml/100 g body weight), once a day for 21 consecutive days. The
safe clinical NS dosage was 2.5-10 mg/kg per day (22), which could be transformed into
15.75-63 mg/kg daily for rats (obversion coefficient=6.3). A dose
of 250 mg/kg is four times the amount of the maximum clinical dose
(22). The body weight of each rat
was measured daily. After 21 days of drug treatment, all rats were
euthanized by cervical dislocation under anesthesia with 30 mg/kg
pentobarbital sodium via intraperitoneal injection after fasting
for 24 h.
Routine blood tests
After rats were anesthetized as aforementioned,
blood from the abdominal aorta was collected in a tube with
K2-EDTA. The WBC, RBC, hemoglobin (HGB), PLT and Ret
parameters of rats were detected using the XT-2000i automated
hematology analyzer (Sysmex Corporation) (n=6). In the remaining
rats, the blood was used in other tests. HCT parameter was
calculated as follows: HGB (%)=RBC (1012/l) x MCV
(fl).
Cytokine and biochemical parameter
assays in serum
Blood prepared with K2-EDTA as
aforementioned was centrifuged at 4˚C at 5,760 x g for 5 min to
collect serum. Hematopoiesis-related cytokines EPO (cat. no. H051),
TPO (cat. no. H482-1) and GM-CSF (cat. no. H060), and inflammatory
cytokines IL-4 (cat. no. H005-1-2), IL-6 (cat. no. H007-1-2), IL-10
(cat. no. H009-1-2), IL-12 (cat. no. H010-1-2), IL-13 (cat. no.
H011), TGF (cat. no. H034-1-2), IFN-γ (cat. no. H025-1-2) and TNF-α
(cat. no. H052-1-2) were analyzed using ELISA kits.
The whole blood of rats was collected from the
abdominal aorta in a tube with an additive clot activator and then
centrifuged at 4˚C at 5,760 x g for 5 min to collect the serum. The
biochemical parameters, such as alkaline phosphatase (ALP; cat. no.
A059-2-2), alanine aminotransferase (ALT; cat. no. C009-2-1),
aspartate aminotransferase (AST; cat. no. C010-2-1), direct
bilirubin (DBIL; cat. no. C019-2-1), lactate dehydrogenase (LDH;
cat. no. A020-2-2) and transferrin (TRF; cat. no. H130-1-2), were
detected using ELISA kits.
Cell cycle and apoptosis analysis of
bone marrow (BM) cells
BM from the left femur was flushed with sterile PBS.
A single cell suspension (1x106 cells/ml) in PBS was
centrifuged at 300 x g for 5 min at room temperature. The BM cells
were fixed with 70% ethanol at 4˚C overnight. After washing twice
with PBS, the cells were resuspended with 1 ml PI/Triton X-100
staining solution with RNase A (Beyotime Institute of
Biotechnology) and incubated for 30 min at room temperature (n=6).
The cells were quantified using a DxFLEX flow cytometer (Beckman
Coulter, Inc.). The cell cycle distribution of the BM cells was
analyzed using ModFit LT 5.0 software (Verity Software House,
Inc.).
The apoptosis rate of the BM cells (1x106
cells/ml) prepared as aforementioned was measured using an Annexin
V-FITC/PI apoptosis kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions and a DxFLEX flow
cytometer with CytExpert software 5.0 (Beckman Coulter, Inc.)
(n=6). In the remaining rats, the tissue was used in other
tests.
Western blotting
Single suspension cells from rats for each group
were collected from the left femur, total protein was obtained by
using RIPA lysis buffer containing protease/phosphatase inhibitor
cocktail (Beyotime Institute of Biotechnology). The total protein
concentration was determined using a BCA Protein Assay Kit
(Beyotime Institute of Biotechnology) and 30 µg protein per lane
was separated by 10% SDS-PAGE and transferred to PVDF membranes.
The membranes were blocked with 5% (w/v) nonfat dried milk for 2 h
at room temperature and incubated with specific primary antibodies
at 4˚C overnight. The primary antibodies used were as follows:
Cyclin A polyclonal antibody (1:500; cat. no. BS1083; Bioworld
Technology, Inc.), cyclin D1 polyclonal antibody (1:500; cat. no.
BS1741; Bioworld Technology, Inc.), Bcl-2 (1:500; cat. no. BS80057;
Bioworld Technology, Inc.), Bax (1:500; cat. no. BS79682; Bioworld
Technology, Inc.) and β-actin monoclonal antibody (1:5,000; cat.
no. BS6007M; Bioworld Technology, Inc.). The membranes were
subsequently washed in TBS with 0.1% Tween-20 (TBST) and incubated
with secondary antibodies, HRP-labelled goat anti-mouse IgG (H+L)
(1:5,000; cat. no. ZJ2020-M; Bioworld Technology, Inc.) or
HRP-labelled goat anti-rabbit IgG (H+L) (1:5,000; cat. no.
ZJ2020-R; Bioworld Technology, Inc.), at room temperature for 1.5
h. Following washing with TBST (0.1% Tween-20), protein bands were
visualized using a BeyoECL Plus Kit (Beyotime Institute of
Biotechnology). Immunoreactive protein bands were quantified by
using a ChemiDocTM MP imaging system (Bio-Rad Laboratories,
Inc.).
Splenic T-lymphocyte (LYMPH)
subpopulation assay
Splenocytes (1x106 cells/ml) from rat
spleen were prepared using sterile PBS according to the
aforementioned method used for BM cells. Splenocytes were labeled
with FITC-conjugated anti-rat CD4 (2.5 µg/ml final concentration;
cat. no. 201505; BioLegend, Inc.) and phycoerythrin-conjugated
anti-rat CD25 antibodies (2.5 µg/ml final concentration; cat. no.
202105; BioLegend, Inc.). The labeled splenocytes were washed twice
with PBS and resuspended in the PBS buffer, and the expression of
CD4 and CD25 by splenocytes was detected using a DxFLEX flow
cytometer with CytExpert software 5.0 (Beckman Coulter, Inc.).
Thymus and spleen indexes
After the rats were sacrificed, the spleen and
thymus were collected and weighed. Thymus and spleen indexes were
calculated as follows: Organ index=organ weight (g)/body weight
(g).
H&E staining
Spleen and thymus specimens were fixed in 10%
formalin solution for 48 h at room temperature, paraffin embedded
and cut into 4 µm sections. The paraffin slices underwent H&E
staining, with hematoxylin for 3 min and eosin for 30 sec at room
temperature, for routine morphological analysis. Images were
captured using a fluorescence microscope (Nikon Corporation) at a
magnification of x400.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the rat spleens with
TRIzol® reagent (Thermo Fisher Scientific, Inc.). Total
RNA was reverse transcribed into cDNA using the FastKing RT Kit
(Tiangen Biotech Co., Ltd.) according to the manufacturer's
protocol. qPCR was performed using the CFX Connect Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.) with SuperReal PreMix
Plus SYBR Green reagent (Tiangen Biotech Co., Ltd.). The primer
sequences used for qPCR were as follows: β-actin forward (F),
5'-CTGTCCCTGTATGCCTCTG-3' and reverse (R),
5'-ATGTCACGCACGATTTCC-3'; EPO F, 5'-GGGGGTGCCCGAACG-3' and R,
5'-GGCCCCCAGAATATCACTGC-3'; TPO F, 5'-GAACCCAGCTTCCTCCACAG-3' and
R, 5'-CCTTTCCCCGAAGCAGTTGT-3'; GM-CSF F, 5'-TCCTAAATGACATGCGTGCT-3'
and R, GCCATTGAGTTTGGTGAGGT; IL-4 F, 5'-CTTGCTGTCACCCTGTTC-3' and
R, 5'-CATGGAAGTGCAGGACTGC-3'; IL-6 F, 5'-GAGTTCCGTTTCTACCTG-3' and
R, 5'-CTCTGGCTTTGTCTTTCT-3'; IFN-γ F, 5'-CGTCTTGGTTTTGCAGCTC-3' and
R, 5'-ACTCCTTTTCCGCTTCCTT-3'; GATA binding protein 3 (GATA-3) F,
5'-CTGGCTGGATGGCGGCAAAG-3' and R, 5'-TGGGCGGGAAGGTGAAGAG-3'; and
T-bet F, 5'-AACCAGTATCCTGTTCCCAGC-3' and R,
5'-TGTCGCCACTGGAAGGATA-3'. The thermocycling conditions were as
follows: 95˚C for 15 min, followed by 40 cycles of 95˚C for 10 sec,
55˚C for 20 sec and 72˚C for 30 sec. The transcript levels were
quantified and normalized to the internal reference gene β-actin
using the 2-IICq method (23).
Statistical analysis
All experiment data are presented as the mean ± SD.
The significance of differences was analyzed using one-way ANOVA
with Tukey's post hoc test using GraphPad Prism 8.0 software
(Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
HPLC analysis of FNS and NS
Saponins were the main active ingredients in FNS and
NS with nine saponins detected using HPLC. As shown in Fig. 1 and Table I, the levels of the ginsenosides
PPD, Rd, PPT, CK and Rh2 were increased in FNS, and nR1, Rg1, Rb1
and Re levels were decreased in FNS compared with NS. In summary,
there was a marked difference in saponin content between FNS and
NS.
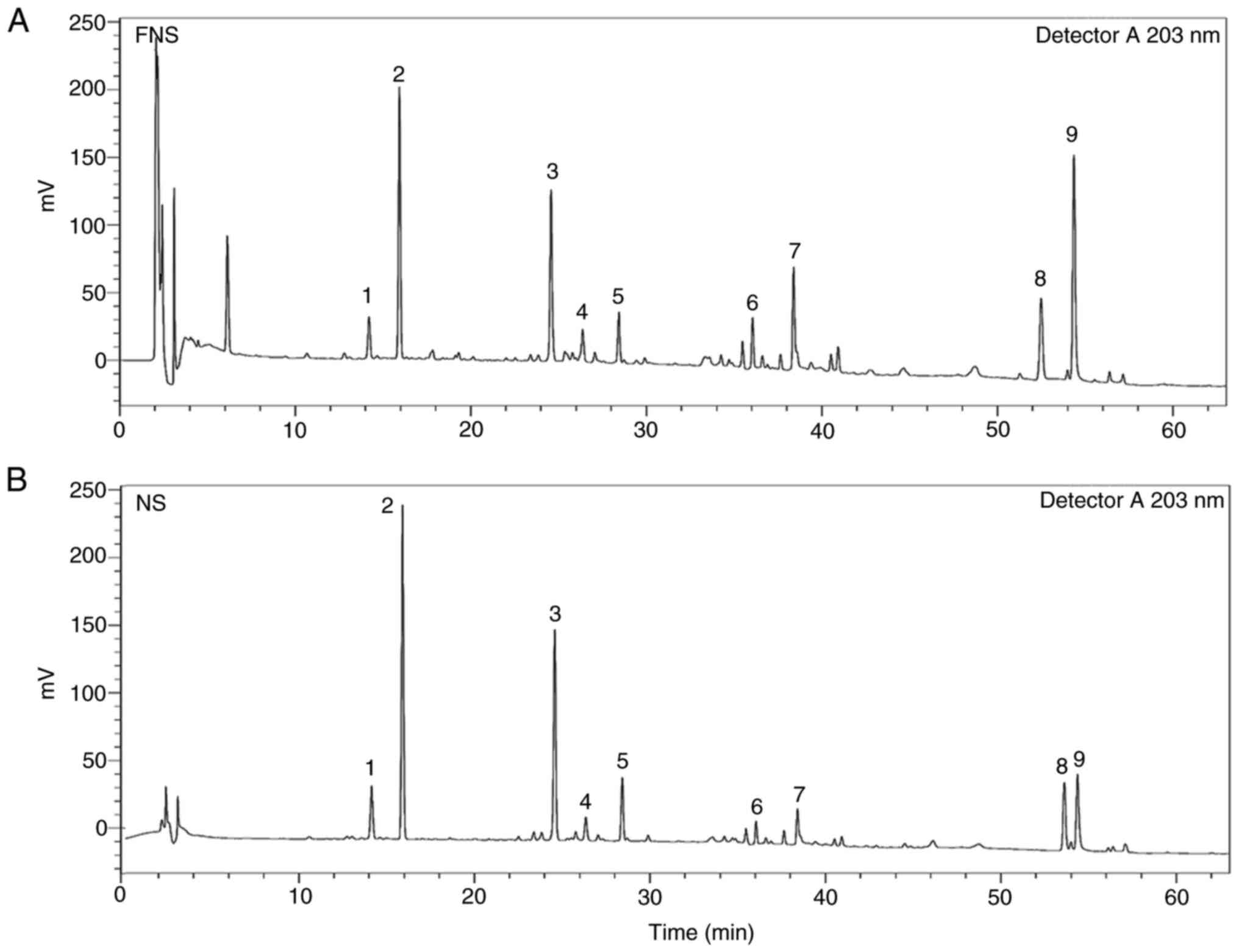 | Figure 1Chromatograms of (A) FNS and (B) NS
from high-performance liquid chromatography. 1, notoginsenoside R1;
2, Rg1; 3, Rb1; 4, protopanaxadiol; 5, Re; 6, Rd; 7,
protopanaxatriol; 8, compound K; and 9, Rh2. FNS, fermented
notoginseng saponins; NS, notoginseng saponins. |
 | Table ISaponin contents of FNS and NS. |
Table I
Saponin contents of FNS and NS.
| Peak no. | Ginsenosides | Type of
saponin | FNS, g/100 g | NS, g/100 g |
|---|
| 1 | nR1 | PPT | 5.36±0.68 | 7.58±0.75 |
| 2 | Rg1 | PPT | 12.42±0.93 | 19.84±1.31 |
| 3 | Rb1 | PPD | 16.25±2.35 | 23.34±2.22 |
| 4 | PPD | PPD | 1.37±0.21 | 0.87±0.19 |
| 5 | Re | PPT | 2.64±0.37 | 3.15±0.32 |
| 6 | Rd | PPD | 1.93±0.29 | 0.57±0.08 |
| 7 | PPT | PPT | 5.50±0.67 | 2.27±0.38 |
| 8 | CK | PPD | 14.08±1.97 | 10.80±1.21 |
| 9 | Rh2 | PPD | 11.23±1.33 | 5.65±0.83 |
Effect of FNS and NS on blood cell
parameters of blood deficiency rats
APH and CP treatment in the model rats significantly
reduced the WBC, RBC, HGB, PLT and Ret levels compared with those
in the control group. After FNS and NS treatment for 21 days, most
blood cell parameters were significantly increased compared with
the model group (Fig. 2).
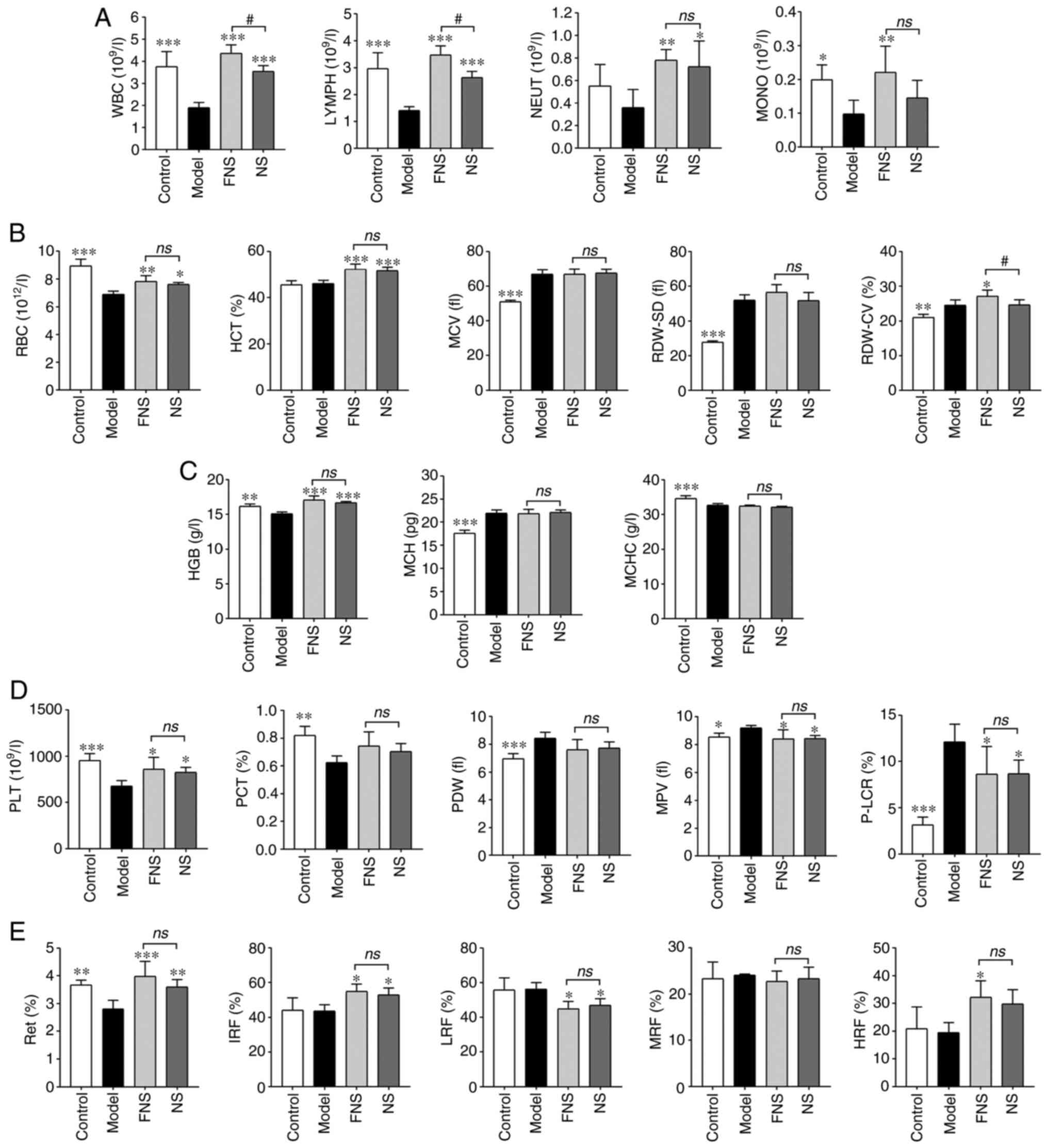 | Figure 2Effect of FNS and NS on (A) WBC, (B)
RBC, (C) HGB, (D) PLT and (E) Ret parameters in blood deficiency
rats. The data are presented as the mean ± SD (n=6).
*P<0.05, **P<0.01 and
***P<0.001 compared with the model group; and
#P<0.05 compared with the NS group. NS, notoginseng
saponins; FNS, fermented notoginseng saponins; WBC, white blood
cell; LYMPH, lymphocyte in the plasma; NEUT, neutrophil; MONO,
monocyte; RBC, red blood cell; HCT, hematocrit; MCV, mean
corpuscular volume; RDW-SD, red cell distribution width-standard
deviation; RDW-CV, red cell distribution width-coefficient of
variation; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC,
mean corpuscular hemoglobin concentration; PLT, platelet; PCT,
plateletcrit; PDW, platelet distribution width; MPV, mean platelet
volume; P-LCR, platelet larger cell ratio; Ret, reticulocyte; IRF,
immature reticulocyte fraction; LRF, low fluorescence ratio; MRF,
middle fluorescence ratio; HRF, high fluorescence ratio; ns, not
statistically significant. |
WBC parameters. For the WBC parameters, the
model group exhibited significantly decreased WBC, LYMPH and
monocyte (MONO) levels compared with the control group. A decrease
in neutrophil (NEUT) levels was observed compared with the model
group; however, this was not statistically significant. FNS
treatment significantly increased WBC, LYMPH, NEUT and MONO levels,
and NS significantly increased WBC, LYMPH and NEUT levels compared
with the model group. MONO levels were also increased in the NS
group; however, this was not statistically significant compared
with the model group. Furthermore, the WBC and LYMPH levels of rats
treated with FNS were significantly higher than those of rats in
the NS group (Fig. 2A).
RBC parameters. In terms of the RBC
parameters, the RBC levels of rats in the model group were
significantly reduced, and the mean corpuscular volume (MCV), red
cell distribution width-standard deviation (RDW-SD) and red cell
distribution width-coefficient of variation (RDW-CV) levels were
significantly increased compared with the control group. No
significant decrease in MCV and RDW-SD was seen in the FNS and NS
treatment groups compared with the model group. However, a
significant increase in RDW-CV levels was seen in FNS-treated rats
compared with the model group, and these were also significantly
higher than the levels in the NS-treated group. The hematocrit
(HCT) was calculated according to the RBCs and MCV. According to
the results, the HCT of the model rats demonstrated no significant
difference compared with the control group. Both FNS and NS
significantly increased the RBC and HCT levels compared with the
model group. (Fig. 2B).
HGB parameters. The HGB and mean corpuscular
hemoglobin concentration (MCHC) levels of rats were significantly
decreased and mean corpuscular hemoglobin (MCH) levels were
significantly increased in the model group compared with the
control group. Both FNS and NS significantly elevated the HGB level
compared with the model group; however, there was no significant
difference in MCH and MCHC levels compared with the model rats
(Fig. 2C).
PLT parameters. PLT and plateletcrit (PCT)
levels of model rats were significantly decreased, and the platelet
distribution width (PDW), mean platelet volume (MPV) and platelet
larger cell ratio (P-LCR) were significantly increased in the model
group compared with the control group. FNS and NS treatment
significantly increased PLT levels, and significantly reduced MPV
and P-LCR levels compared with the model group. However, there was
no statistically significant difference in PCT and PDW levels
compared with the model group (Fig.
2D).
Ret parameters. The Ret level of model rats
was significantly reduced compared with the control group, and the
model group exhibited no significant change in immature Ret
fraction (IRF), low fluorescence ratio (LRF), middle fluorescence
ratio (MRF) and high fluorescence ratio (HRF) compared with the
control group. FNS significantly increased the Ret, IRF and HRF
levels of rats, and NS significantly elevated the Ret and IRF
levels compared with the model group. NS and FNS also significantly
decreased the LRF level of rats compared with the model group
(Fig. 2E).
Effect of FNS and NS on
hematopoiesis-related cytokines and biochemical parameters of blood
deficiency rats
In model rats, the GM-CSF, EPO and TPO levels were
significantly reduced compared with the control group. Treatment
with FNS and NS significantly elevated the GM-CSF, EPO and TPO
levels of rats compared with the model group. The GM-CSF, EPO and
TPO levels of rats treated with FNS were significantly higher than
those of rats treated with NS (Fig.
3A).
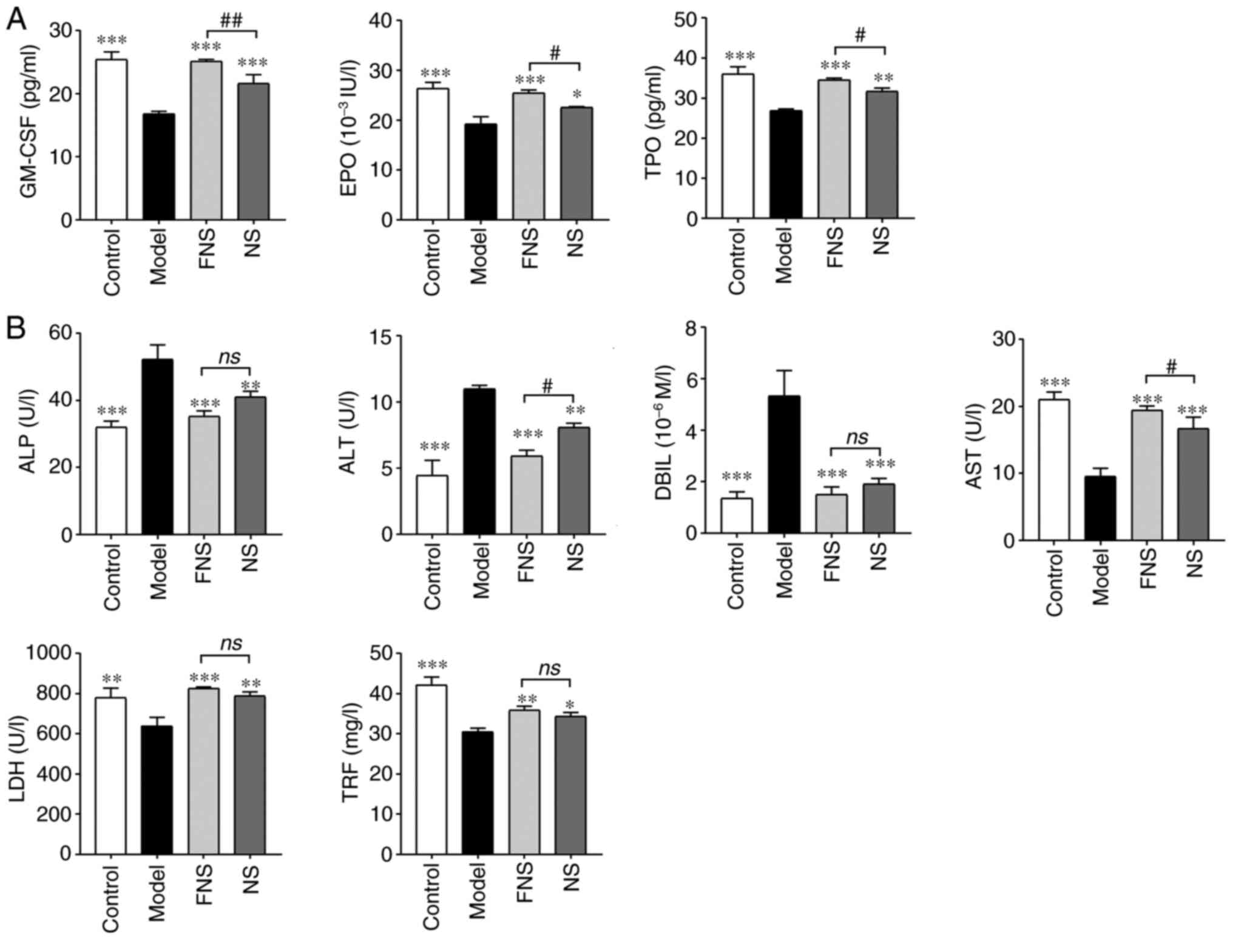 | Figure 3Effect of FNS and NS on (A)
hematopoiesis-related cytokines and (B) biochemical parameters of
blood deficiency rats. The data are presented as the mean ± SD
(n=8). *P<0.05, **P<0.01 and
***P<0.001 compared with the model group; and
#P<0.05 and ##P<0.01 compared with the
NS group. GM-CSF, granulocyte-macrophage colony-stimulating factor;
EPO, erythropoietin; TPO, thrombopoietin; ALP, alkaline
phosphatase; ALT, alanine aminotransferase; DBIL, direct bilirubin;
AST, aspartate aminotransferase; LDH, lactate dehydrogenase; TRF,
transferrin; NS, notoginseng saponins; FNS, fermented notoginseng
saponins; ns, not statistically significant. |
CP is metabolized in the liver and can cause liver
damage (24) as seen in the model
rats where the ALP, ALT and DBIL levels were significantly
increased and AST, LDH and TRF levels were significantly decreased
compared with the control group. FNS and NS significantly reduced
ALP, ALT and DBIL and significantly increased AST, LDH and TRF
levels compared with the model group. ALT levels in FNS-treated
rats were significantly reduced and AST levels were significantly
increased compared with those in the NS-treated rats (Fig. 3B).
Effect of FNS and NS on inflammatory
cytokines of blood deficiency rats
APH and CP can reduce the efficiency of the immune
system and cause an imbalance between anti-inflammatory and
pro-inflammatory cytokines (25,26).
The levels of anti-inflammatory cytokines (IL-4,
IL-10, IL-12, IL-13 and TGF-β) and pro-inflammatory cytokines
(IL-6, IFN-γ and TNF-α) in the model rats were significantly
reduced compared with those in the control group (27,28).
Both FNS and NS significantly increased the levels of these
indicators compared with those in the model rats. There was a
significant increase in IL-10, IL-12, IL-13 and TNF-α levels in
FNS-treated rats compared with the NS-treated group (Fig. 4).
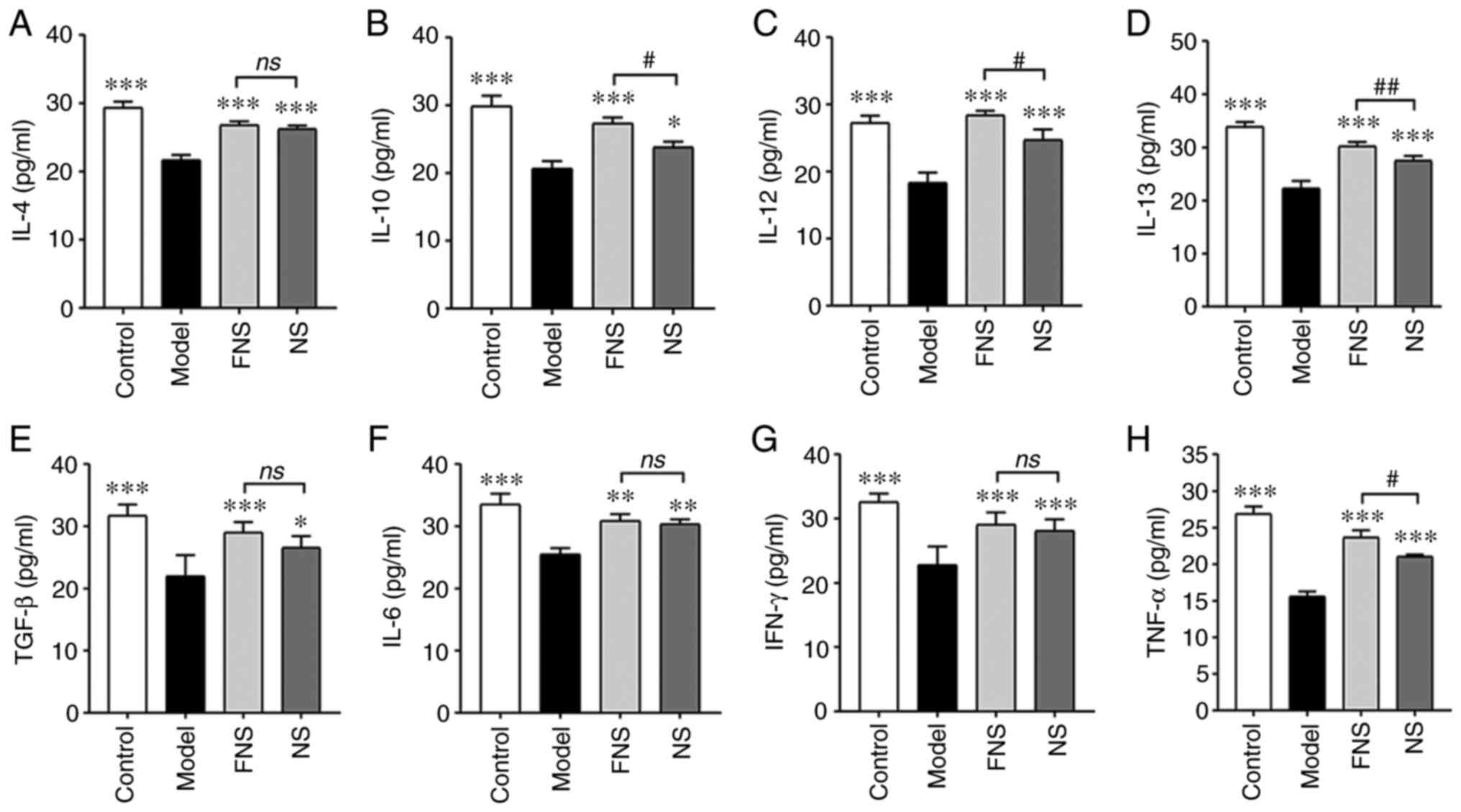 | Figure 4Effect of FNS and NS on inflammatory
cytokines of blood deficiency rats. Levels of (A) IL-4, (B) IL-10,
(C) IL-12, (D) IL-13, (E) TGF-β, (F) IL-6, (G) IFN-γ and (H) TNF-α
were detected using an ELISA. The data are presented as the mean ±
SD (n=8). *P<0.05, **P<0.01 and
***P<0.001 compared with the model group; and
#P<0.05 and ##P<0.01 compared with the
NS group. NS, notoginseng saponins; FNS, fermented notoginseng
saponins; ns, not statistically significant. |
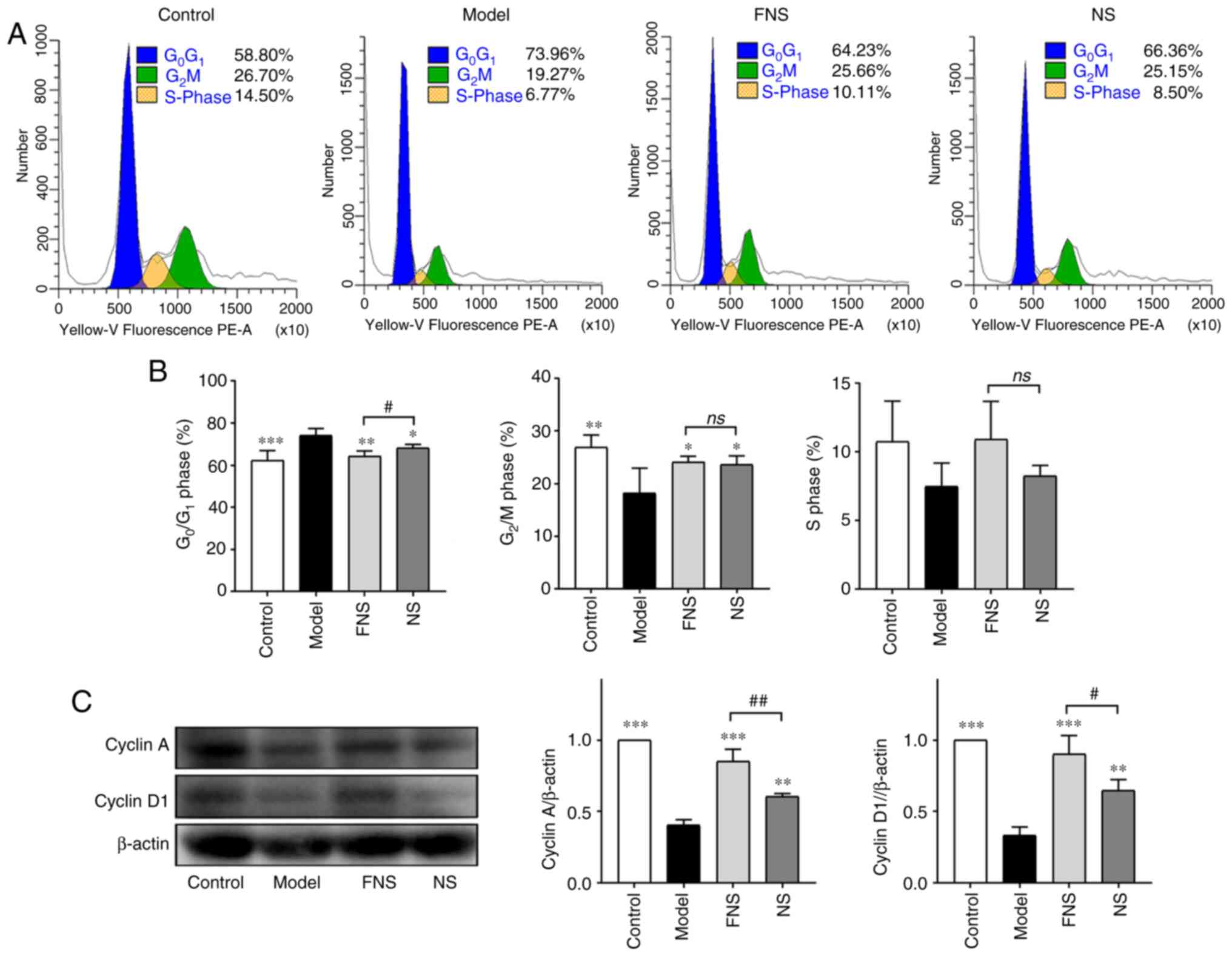
Effect of FNS and NS on the cell cycle
and apoptosis of BM cells from blood deficiency rats
Chemotherapy causes myelosuppression, damages the
DNA, affects normal hematopoiesis and changes the proportions of
cells in different cell cycle phases (29).
The percentage of BM cells in the
G0/G1 phase was significantly increased and
the percentage of BM cells in the G2/M phase was
significantly decreased in model rats compared with the control
group (Fig. 5A and B). No significant difference in the
percentage of cells in the S phase was seen compared with the
control. After treatment with FNS and NS, the percentage of BM
cells in the G0/G1 phase was significantly
decreased and the percentage of BM cells in G2/M was
significantly increased compared with the model group. An increase
in the proportion of cells in S phase was also seen after treatment
with FNS and NS; however, the difference was not statistically
significant compared with the model group (Fig. 5B). These results demonstrated that
FNS and NS effectively improved the recovery of hemopoiesis in
blood deficiency rats by increasing the progression of BM cells
from G0/G1 phase arrest into G2/M
and S phases.
The protein expression levels of cyclin A and cyclin
D1 in BM cells were significantly decreased in blood deficiency
model rats compared with the control group. FNS and NS treatment
significantly increased the protein expression levels of cyclin A
and cyclin D1 compared with the model group. Furthermore, a
significant increase in cyclin A and cyclin D1 protein expression
levels was seen in FNS-treated rats compared with NS-treated rats
(Fig. 5C).
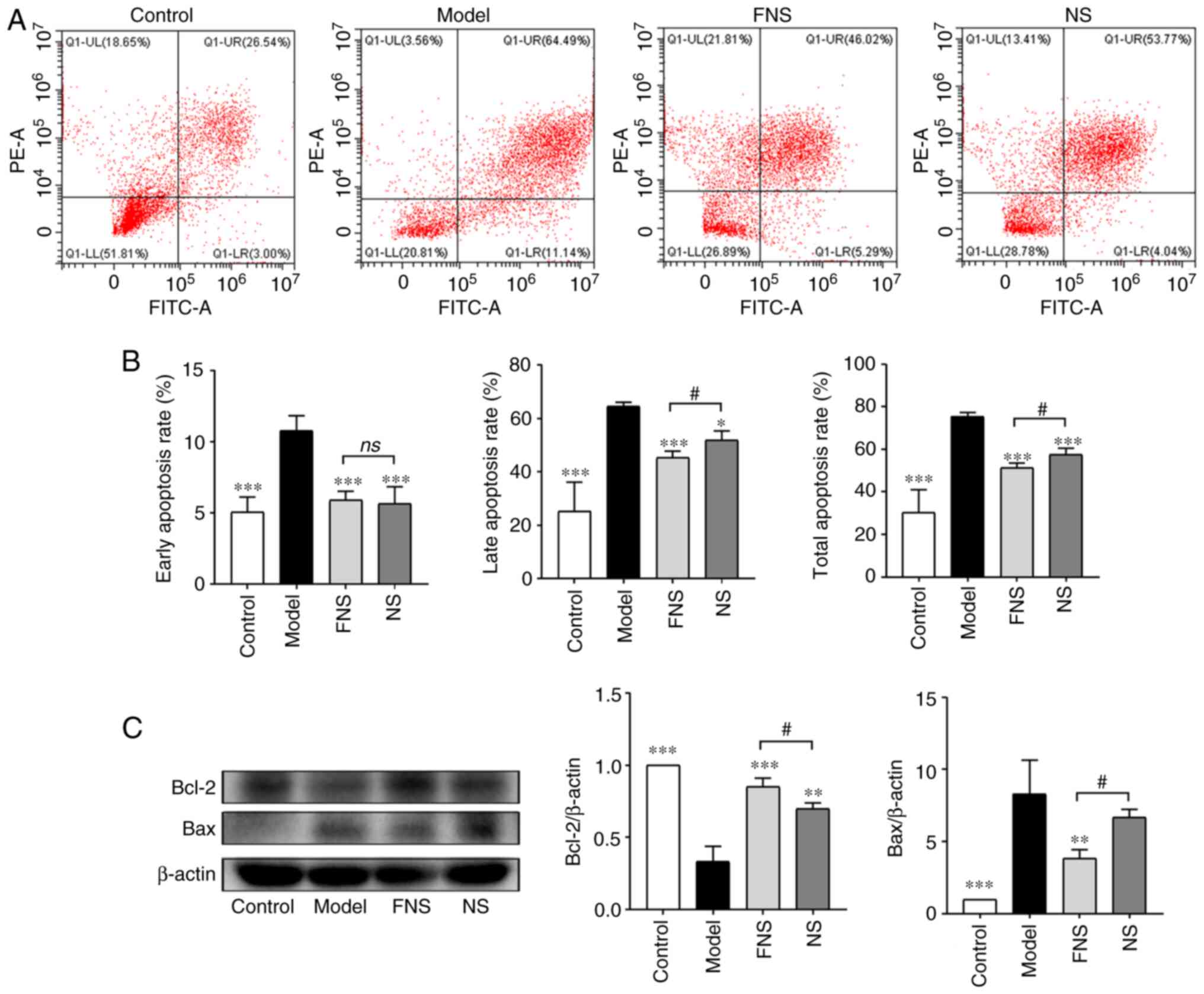
Apoptosis and proliferation of BM cells are coupled
and are responsible for the maintenance of hematopoiesis in the
hematopoietic system (30). The
apoptosis rates of BM cells in the model group, including the
early, late and total apoptosis rates, were significantly increased
compared with the control group. The early, late and total
apoptosis rates of BM cells were significantly decreased after FNS
and NS administration compared with the model group. Furthermore,
the late and total apoptosis rates of BM cells isolated from rats
treated with FNS were significantly decreased compared with the NS
group (Fig. 6A and B).
Furthermore, the expression levels of anti-apoptotic
protein Bcl-2 and pro-apoptotic protein Bax were detected. These
proteins can prevent or promote cell apoptosis and prolong or
shorten cell lifespan, respectively (31,32).
In model rats, the relative expression levels of Bcl-2 were
significantly decreased and the relative expression levels of Bax
were significantly increased compared with the control group. FNS
and NS significantly increased Bcl-2 protein expression and FNS
significantly reduced Bax protein expression compared with the
model group. The reduction of Bax protein expression in the NS
group was not statistically significant compared with the model
group. Furthermore, the relative protein expression levels of Bcl-2
were significantly increased in FNS-treated rats and the relative
protein expression levels of Bax were significantly reduced
compared with the NS-treated group (Fig. 6C). These results suggested that APH
and CP could accelerate the apoptosis of BM cells and that the
anti-apoptotic effect of FNS was superior to NS in the relative
expression of Bcl-2 and Bax.
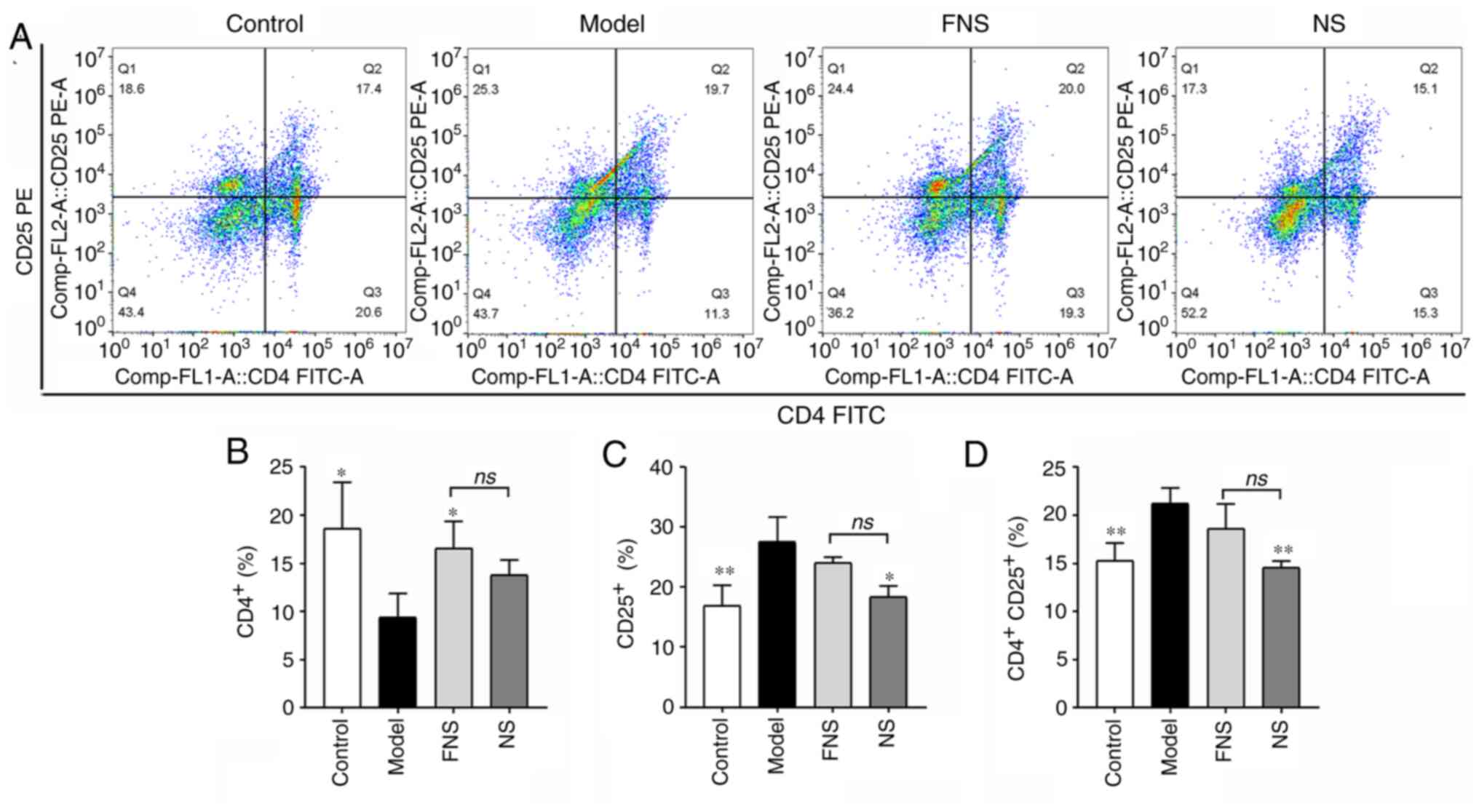
Effect of FNS and NS on T cells of
blood deficiency rats
APH and CP can damage immunological self-tolerance
and homeostasis, and significantly influence the function of T
cells (33). The levels of
CD4+, CD25+ and
CD4+CD25+ T cells from the spleen were
measured using flow cytometry. In the model group, CD4+
T cell levels were significantly decreased and the levels of
CD25+ and CD4+CD25+ T cells were
significantly increased compared with those in the control group.
FNS treatment significantly increased the percentage of
CD4+ T cells compared with the model group. There was a
significant decrease in the percentages of CD25+ and
CD4+CD25+ T cells in the NS-treated group
compared with the model group. There was no significant difference
in the percentage of CD4+, CD25+ and
CD4+CD25+ T cells between the FNS and NS
groups (Fig. 7).
Effect of FNS and NS on body weight,
organ indexes, and the morphology of the spleen and thymus of blood
deficiency rats
APH and CP seriously affect immune organs, such as
the spleen and thymus (33,34).
In the model group, there was a significant decrease in the body
weight and thymus index, and a significant increase in the spleen
index compared with the control group. FNS and NS treatment
significantly increased the body weight and thymus index and
significantly decreased the spleen index compared with the model
group. However, there was no statistically significant difference
in the body weight, spleen index or thymus index between the FNS
and NS groups (Fig. 8A-C).
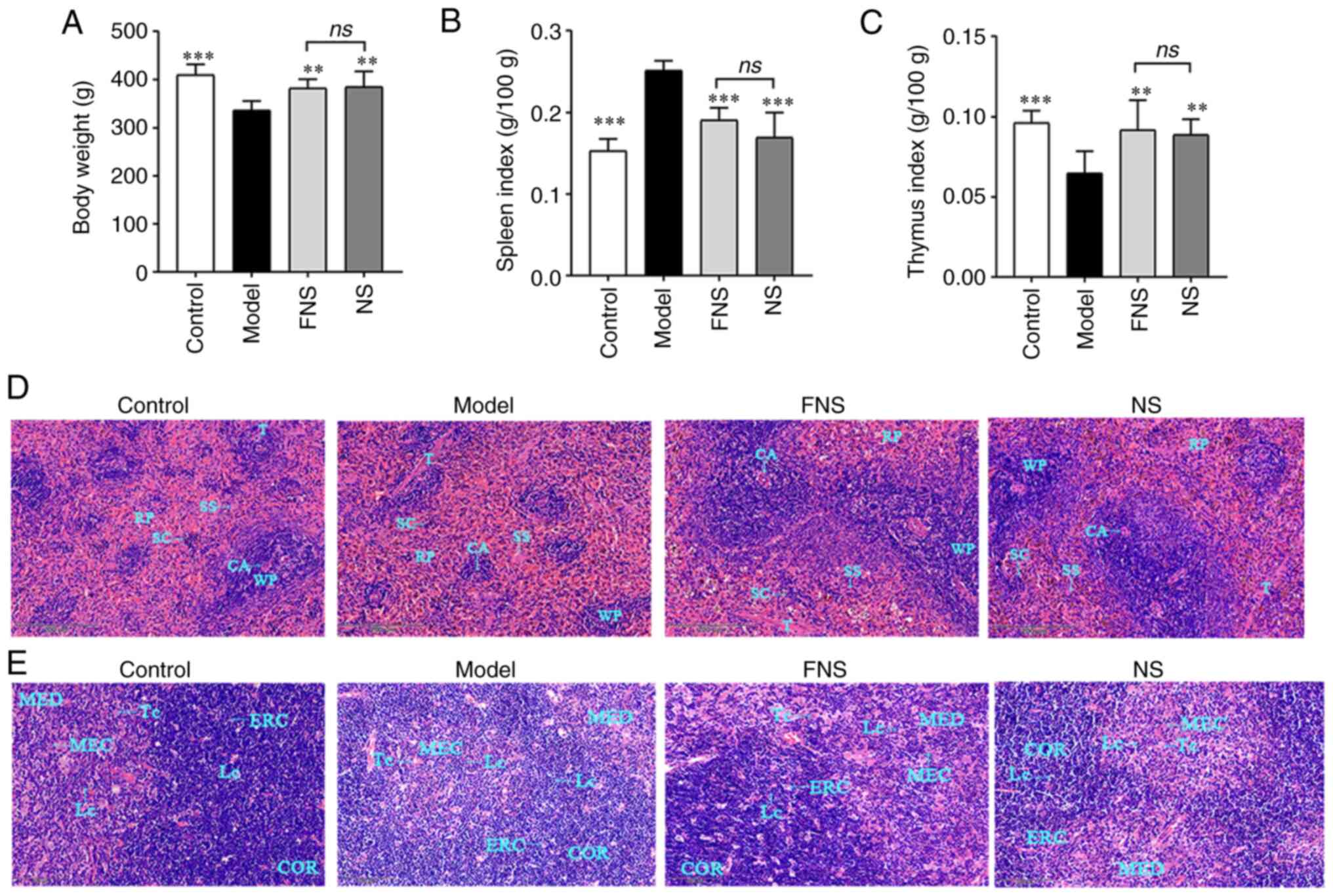 | Figure 8Effect of FNS and NS on (A) body
weight, (B) spleen index and (C) thymus index, and
hematoxylin-eosin staining of histological structure of blood
deficiency rat (D) spleen and (E) thymus at x400 magnification. The
data are presented as the mean ± SD (n=8). **P<0.01
and ***P<0.001 compared with the model group. WP,
white pulp; RP, red pulp; CA, central artery; SS, splenic sinus;
SC, splenic cord; T, trabecula; MED, medulla; MEC, medullary
epithelial cell; Lc, lymphocyte in tissues; TC, thymic corpuscle;
ERC, epithelial reticular cell; COR, cortex; NS, notoginseng
saponins; FNS, fermented notoginseng saponins; ns, not
statistically significant. |
APH and CP damage the histological structure changes
of the rat spleen and thymus, including induction of
disorganization in splenic structures, thymic apoptosis,
hypocellularity and atrophy (35,36).
H&E staining showed that the splenic cord, splenic sinus and
trabecula in the red pulp (RP) were displayed clearly in the
control group. However, in the model group, RP expansion, white
pulp (WP) and central artery shrinking were visible, which
indicated neutrophil accumulation and a decreasing level of LYMPHs,
respectively. The marginal zone was ambiguous between RP and WP.
These results showed a decreasing level of LYMPHs and an increasing
level of macrophages. FNS and NS improved the histological
structure of the rat spleens compared with the model group. The WP
was darker and had extensive distribution which related to an
increasing level of macrophages, and the marginal zone was clear in
the FNS and NS groups (Fig.
8D).
The cortex (COR) and medulla (MED) of the model rat
thymus, which related to the change of thymus morphology and
atrophy, became fused in some areas with no apparent marginal zones
between them. In the model group, the number of Lcs and epithelial
reticular cells (ERCs) was reduced compared with the control, and
ERCs provided a scaffold in the COR. The medullary epithelial cells
(MECs) and thymic corpuscle (TC) are important to T cell
development, and became expanded and irregular in the MED. In the
FNS and NS groups, the number of Lcs, ERCs and MECs increased, the
COR had deep staining, and round or oval TCs were seen in the MED.
Both FNS and NS appeared to reduce tissue damage induced by APH and
CP in the model group (Fig.
8E).
Effect of FNS and NS on mRNA
expression levels in blood deficiency rats
APH and CP break the balance of body immunity,
affect immune organs and the release of immunity cytokines and
transcription factors and prevent stem cells from differentiating
into hematopoietic cell lineages by affecting the release of
cytokines and transcription factors (37,38).
In the model group, the mRNA expression levels of hematopoietic
cytokines (GM-CSF, EPO and TPO), inflammatory cytokines (IL-4, IL-6
and IFN-γ) and transcription factors (GATA-3 and T-bet) were
significantly reduced compared with those in the control group.
With NS and FNS treatment, the mRNA expression levels of GM-CSF,
EPO, TPO, IL-4, IL-6, IFN-γ, GATA-3 and T-bet were significantly
increased compared with the model group. FNS treatment
significantly increased GM-CSF, TPO, IL-4, IL-6 and T-bet mRNA
expression compared with NS treatment (Fig. 9).
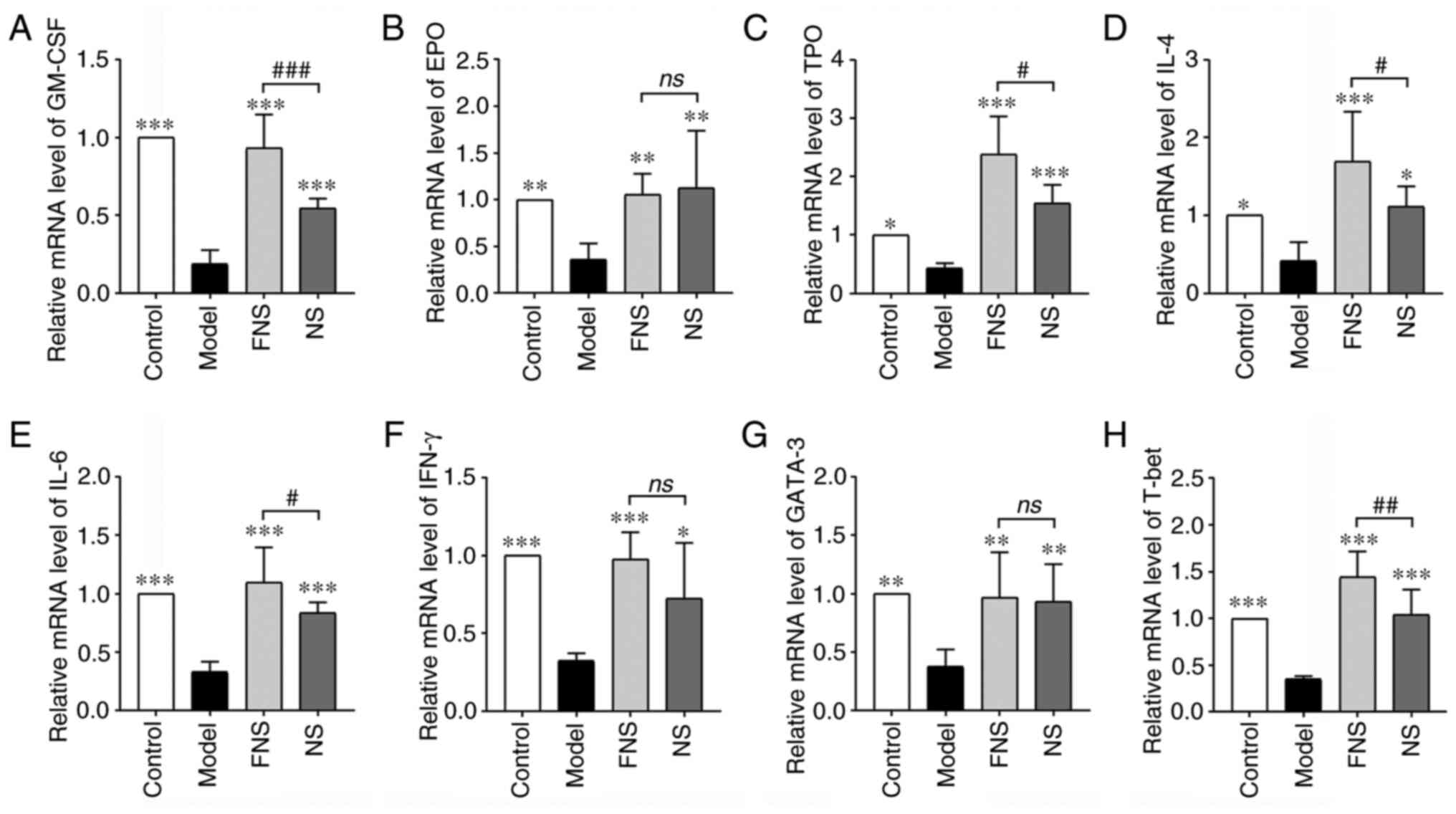 | Figure 9Effect of FNS and NS on mRNA
expression levels in the spleens of blood deficiency rats. mRNA
expression levels of (A) GM-CSF, (B) EPO, (C) TPO, (D) IL-4, (E)
IL-6, (F) IFN-γ, (G) GATA-3 and (H) T-bet. The data are presented
as the mean ± SD (n=8). *P<0.05,
**P<0.01 and ***P<0.001 compared with
the model group; and #P<0.05, ##P<0.01
and ###P<0.001 compared with the NS group. GM-CSF,
granulocyte-macrophage colony-stimulating factor; EPO,
erythropoietin; TPO, thrombopoietin; GATA-3, GATA binding protein
3; T-bet, T-box expressed in T cell; NS, notoginseng saponins; FNS,
fermented notoginseng saponins; ns, not statistically
significant. |
Discussion
According to the theory of TCM, deficiency of
viscera and insufficiency of Qi and blood are attributed to blood
deficiency. The effect of PN in promoting hemostasis is known as
‘the raw materials eliminate and the steamed ones tonify’ and
steamed PN is considered to possess the function of warming and
toning viscera, benefiting Qi and nourishing blood in TCM (39). In TCM it is believed that there are
marked differences in composition, activities and efficacy between
raw PN and the steamed PN (8). For
example, in TCM raw PN is considered to primarily stop bleeding,
promote apokatastasis, strengthen the heart and provide pain
relief. Whereas steamed PN is used in TCM to nourish blood,
regulate circulation and improve immune function (40).
Previous research has shown that fermentation of PN
extracts produces a similar ginsenoside profile to steamed PN
(41). L. plantarum
metabolizes ginsenosides mainly through deglycosylation and
dehydration (42). NS contains
Rb1, Rg1, Re and nR1 as the major active compounds, which are
metabolized by β-glycosidases produced by the gut microbiota
(43). The C-20 glucosides of
Rb1/Rb2/Rc are deglycosylated to form Rd, which further be
converted to Rg3. The C-20 of Rg3 is dehydrated to form Rk1 or
Rg5(44). The C-3 glucoside of Rg3
is deglycosylated to form Rh2 which is then transformed into PPD
(45). In addition, the C-3
glucoside of Rb1 is deglycosylated to convert X VII to LXXV/F2, CK
and PPD (46,47).
Previous studies have reported on the metabolic
pathway for the elimination of the C-20 sugar moieties in Re and
Rg1 produce 20(S)-Rg2 and 20(S)-Rh1, respectively
(48,49). It has been suggested that the C-6
rhamnose of Rg2 is eliminated to generate Rh1 (50,51).
The elimination of the C-6 glucoside of Re produces Rg1. Rg1 is
transformed into Rh1, which further changed into PPT by L.
plantarum fermentation. Furthermore, the C-20 glucoside of Re
is deglycosylated to produce Rg2, which is further dehydrated to
Rh4, F4 or Rg6. The C-6 glucoside of Rg6 and nR1 can also be
deglycosylated to produce Rk3 and Rg1, respectively (51). In the present study, according to
HPLC analysis, L. plantarum fermentation transformed nR1,
Rg1, Rb1 and Re into their corresponding metabolites, and increased
the PPD, Rd, PPT, CK and Rh2 content in FNS compared with
unfermented NS.
Hypodermic injection of APH and intraperitoneal
injection of CP were used to establish a blood deficiency rat model
(52). Hua et al (53) and Li et al (54) reported that Sprague Dawley rats
were hypodermically injected with 2% APH saline solution on days 1
and 4 at a dose of 20 and 40 mg/kg, respectively; 2 h after the
hypodermic injection with 2% APH saline solution on day 4, the rats
were intraperitoneally injected with CP saline solution on days 4,
5, 6 and 7 at a dose of 20 mg/kg. A similar modeling method has
been previously reported in mice; however, the dose of CP was 40
mg/kg on days 4, 5, 6 and 7(55).
Then, the blood deficiency model was created. APH and CP decreased
WBCs, RBCs, HGB, PLTs and Rets, and model rats exhibited mental
sluggishness, movement retardation, peripheral blood cell count
reduction and weight loss (25,53).
Liu et al (56) reported
that PN extract treated with a microwave processing method
increased the WBCs and HGB of blood deficiency model mice induced
by APH and CP compared with raw PN. In an additional study, steamed
PN was demonstrated to elevate the levels of WBCs, RBCs, HGB and
PLTs in mice with blood deficiency induced by APH and CP. The PN
contents were examined and the main saponins included the
notoginsenoside R1, Rg1, Re, Rh1, Rb1, Rd, Rk3, Rh4 and
Rg3(7). The results of the present
study demonstrated that FNS and NS significantly improved the blood
cell parameters. Notably, the levels of WBCs and LYMPHs of rats
treated with FNS were increased compared with those of rats treated
with NS.
BM is a key site of hematopoiesis and is responsible
for producing new blood cells (57). A series of hematopoiesis-related
cytokines, including GM-CSF, EPO and TPO are required for blood
cell formation (58). TPO has been
reported to improve thrombocytopenia and markedly augment
megakaryopoiesis (59). EPO and
GM-CSF have been suggested to promote erythropoiesis and the
generation of myeloid cell subsets, respectively (60). CP damage the BM and cause cell
apoptosis by increasing the expression of the pro-apoptotic protein
Bax and decreasing the expression of anti-apoptotic proteins in the
model mice, such as Bcl-2(61). CP
induce G0/G1 phase arrest of BM (62) and inhibit the protein expressions
of Cyclin D1(63). NS has
previously been reported to decrease the apoptosis rate, Bax
expression and caspase-3 activity of BM stromal cells induced by
hydrogen peroxide (64,65). Ginsenoside CK could control
apoptosis and promote cells to enter the normal cell cycle via the
Bcl-2/Bax and MEK/ERK signaling pathways in myelosuppression mice
induced by CP (66). Ginsenoside
Rg1 increased the number of hematopoietic stem and progenitor cells
and restored the function of BM in CP-treated myelosuppressed
mice.
The results of the present study demonstrated that
both FNS and NS reduced the cell apoptosis rate, recovered the
normal pattern of the cell cycle of BM cells and increased the
levels of GM-CSF, EPO and TPO. Furthermore, treatment with FNS
further increased the levels of WBCs, LYMPHs, GM-CSF, EPO and TPO,
and the protein expression levels of cyclin A and D1 compared with
NS treatment. FNS treatment further decreased the total apoptosis
rate of BM cells compared with NS treatment.
The liver stores blood and regulates the quantity of
blood in circulation. CP is converted to phosphoramide mustard and
acrolein by the liver cytochrome P450, which can result in liver
damage (24). ALT and AST indicate
the degree of liver damage. A high level of ALT suggests liver
damage (67). DBIL represents the
liver metabolic capacity, acting as an indicator of liver damage,
and the level of DBIL is increased in patients with hepatitis and
cirrhosis (68). ALP is released
from the liver and bones, and its levels are increased in certain
liver diseases and bone disorders (69). TRF is responsible for transporting
iron from the digestive tract and degrading RBCs that enter the BM
as a complex of TRF-Fe3+ (70). Due to the barrier of iron
utilization by RBCs, the TRF is reduced during anemia (71). NS can improve hepatic function in
non-alcoholic fatty liver disease and acute ethanol-induced liver
injury (72,73). Zhong et al (74) reported that NS promoted liver
regeneration through activation of the PI3K/AKT/mTOR cell
proliferation pathway and upregulation of the AKT/Bad cell survival
pathway in mice. The results of the present study indicated that
FNS and NS protected the liver and maintained normal biochemical
parameters in blood deficiency rats by reducing the ALP, ALT and
DBIL levels and increasing the AST, LDH and TRF levels. The effect
on ALT and AST levels in FNS rats was greater than that in NS
treated rats.
APH and CP can damage the spleen and thymus, immune
cells, such as T cells, B-LYMPHs and granulocytes, and cause a
reduction in levels of inflammatory cytokines, such as IL-2, IL-4
and IL-6 (27,75). Lcs are produced in the BM and
mature in the thymus gland or BM (76), and are the key cells involved in
the regulation of immune function throughout the body (77). CD4+ T cells are
activated by antigen-presenting cells and regulate immune responses
via the production of cytokines and helper T (Th) cells, such as
Th1, Th2, Th17 and regulatory T cells (78). T-bet can induce Th1 cells to
produce IL-2, IFN-γ and TNF-α, which are pro-inflammatory
cytokines. These cytokines enhance antigen presentation and
facilitate phagocytic function by macrophages (79). GATA-3 can induce Th2 cells to
secrete IL-4, IL-10 and IL-13, which are anti-inflammatory
cytokines involved in humoral immunity (80). Th1 and Th2 serve essential roles in
the coordination and intercellular communication of lymphoid,
inflammatory and hematopoietic cells in the immune system (81). CP inhibits the expression of TNF-α,
IFN-γ, IL-4 and IL-10, and decreases the Th1/Th2 cytokine secretion
ratio (17,82). Both APH and CP decrease the levels
of TNF-α and IL-6(38). In
radiation-induced aplastic anemia mice, NS regulates Th1 and Th2
immune responses by downregulating the production of Th1 cytokines
and T-bet protein expression, and upregulating the production of
Th2 cytokines and expression of GATA-3(83). Furthermore, Rd can promote the Th1
and Th2 immune responses by increasing IL-2, IFN-γ, IL-4 and IL-10
mRNA expression in mice splenocytes (84).
The results of the present study demonstrated that
FNS and NS increased the protein expression levels of IL-4, IL-10,
IL-12, IL-13, TGF-β, IL-6, IFN-γ and TNF-α, and regulated Th1 and
Th2 immune responses by increasing the protein expression levels of
GATA-3 and T-bet. Furthermore, FNS treatment significantly
increased the levels of immune cytokines (IL-10, IL-12, IL-13 and
TNF-α) and transcription factor T-bet compared with NS treatment.
This difference may be due to the increase of certain ginsenosides
during fermentation.
Most ginsenosides in NS have low oral
bioavailability (85). In L.
plantarum fermentation, the hydrophilic ginsenosides (nR1, Rb1,
Rg1, Rc, Re and R1) are deglycosylated and converted into
hydrophobic ginsenosides (Rd, Rh2, CK, PPT and PPD). This increased
hydrophobicity allows passage through cell membranes and increases
bioavailability (86-88).
In the present study, the total contents of nR1, Rg1, Rb1 and Re,
as the main bioactive components in NS (89,90),
were reduced from 53.91 g/100 g to 36.67 g/100 g, and the total
content of Rd, Rh2, CK, PPT and PPD was increased from 20.16 g/100
g to 34.11 g/100 g, during the NS Lactobacillus fermentation
process. Zhu et al (91)
reported that the levels of Rb1, Rd, Rk1, Rg5, Rk3, Rh4 and
20(S)-PPD increased in steamed PN and could be used as
pharmacokinetic markers of the steamed PN based on their elevated
levels in the plasma. The ginsenosides in PN are classified as
oleanane type, protopanaxadiol (PPD) and protopanaxatriol (PPT)
types, according to the chemical structure. The PPD-type
ginsenosides showed improved absorption compared with PPT-types in
rat gastrointestinal systems. The peak concentration
(Cmax) and area under the concentration-time
curve (AUC) of PPD-type ginsenosides Fa, Rb1, Rd, Rk1 Rg5 and PPD
were higher than PPT-type ginsenosides R1, Re, Rg1, Rg2, F4, Rh1
and PPT; the peak time (Tmax) of the PPT-types
ginsenosides Rg1 (0.83 h), R1 (1.17 h), Re (1.33 h), Rg2 (1.00 h),
Rh1 (0.63 h) and 20(R)-Rh1 (0.79 h) was shorter than that of the
PPD-types ginsenosides Fa (8.00 h), Rb1 (8.00 h), Rb2 (8.00 h), Rd
(9.33 h), CK (12.00 h), Rk1 (3.67 h), Rg5 (3.67 h) and PPD (12.00
h) (91).
The present study demonstrated that the content of
PPD-type ginsenosides Rd, CK and PPD in FNS was increased during
the fermentation process, with results suggesting increased blood
concentration, prolonged the drug duration and increased activity,
and so served an important role in the treatment of blood
deficiency rats.
CK is a secondary ginsenoside, which is more
bioavailable and soluble than its parent ginsenoside (92). The Cmax of CK is
double that of ginsenoside Rb1, and the Tmax of
CK is higher than that of Rb1(93). Fukami et al (94) reported that the
Tmax, Cmax and AUC were different
between Lactobacillus paracasei A221 fermented ginseng (FG)
and non-FG (NFG). The Tmax of CK was 2.2 and 16 h
and the Cmax was 41.5 and 1.16 ng/ml in the FG
and NFG group, respectively. The AUC0-12 h and
AUC0-24 h of healthy adults treated with FG were 58.3
and 17.5-fold higher than those in the NFG group (94). Choi et al (95) reported that the AUC0-24
h and Cmax of CK from FG were 6.3-fold and
6.0-fold higher than those from NFG in rats. The
Tmax of CK in humans and rats was 2.54 and 3.33 h
for FG and 9.11 and 6.75 h for NFG, respectively. The results of
the present study demonstrated that the content of CK in FNS was
higher than that in NS. These results suggested that administration
of FNS resulted in a higher and faster absorption of CK in blood
deficiency rats compared with NS.
In conclusion, both FNS and NS treatment appeared
to reduce the changes in the blood deficiency parameters induced by
APH and CP. For certain parameters, FNS exhibited a greater impact
compared with NS, improving the function of the BM, spleen, thymus
and liver.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of China (grant no. U19A2013), the National Key Research
and Development Program of China (grant no. 2021YFD1600903-02),
Science and Technology Projects of the Education Department of
Jilin Province (grant no. JJKH20230990KJ), and the Science and
Technology Development Plan Project of Jilin Province (grant nos.
202002053JC, 20210304001YY, 20210304002YY and
212558JC010387462).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WS, ZL, DP, LZ, YZ, TY, XG, HS and HZ contributed
to the study conception and design. WS also contributed to project
development and data collection; WS, ZL and DP contributed to
protocol development and manuscript writing. LZ, YZ and TY
contributed to data collection and analysis. XG contributed to data
analysis. HS and HZ contributed to data analysis and manuscript
editing. WS and HZ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures in the present study were approved
by the Bioethics Committee of Changchun University of Chinese
Medicine and the Institutional Animal Care (approval no. 2022156;
Changchun, China), and the study was conducted based on the
guidelines for the care and use of laboratory animals (20).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi XQ, Yue SJ, Tang YP, Chen YY, Zhou GS,
Zhang J, Zhu ZH, Liu P and Duan JA: A network pharmacology approach
to investigate the blood enriching mechanism of Danggui Buxue
decoction. J Ethnopharmacol. 235:227–242. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li PL, Sun HG, Hua YL, Ji P, Zhang L, Li
JX and Wei Y: Metabolomics study of hematopoietic function of
Angelica sinensis on blood deficiency mice model. J Ethnopharmacol.
166:261–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang Q, Feng L, Li H, Zheng L, Qi X, Wang
Y, Feng Q, Liu Z, Liu X and Lu L: Jian-Pi-Bu-Xue-formula alleviates
cyclophosphamide-induced myelosuppression via up-regulating
NRF2/HO1/NQO1 signaling. Front Pharmacol. 11(1302)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Uzayisenga R, Ayeka PA and Wang Y:
Anti-diabetic potential of Panax notoginseng saponins (PNS):
A review. Phytother Res. 28:510–516. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li YH, Li YY, Fan GW, Yu JH, Duan ZZ, Wang
LY and Yu B: Cardioprotection of ginsenoside Rb1 against
ischemia/reperfusion injury is associated with mitochondrial
permeability transition pore opening inhibitiz. Chin J Integr Med:
Jan 6, 2016 (Epub ahead of print).
|
|
6
|
Li L, Wang Y, Qi B, Yuan D, Dong S, Guo D,
Zhang C and Yu M: Suppression of PMA-induced tumor cell invasion
and migration by ginsenoside Rg1 via the inhibition of
NF-κB-dependent MMP-9 expression. Oncol Rep. 32:1779–1786.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Z, Zhang Y, Gao M, Cui X, Yang Y,
van Duijn B, Wang M, Hu Y, Wang C and Xiong Y: Steamed Panax
notoginseng attenuates anemia in mice with blood deficiency
syndrome via regulating hematopoietic factors and JAK-STAT pathway.
Front Pharmacol. 10(1578)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xiong Y, Chen L, Man J, Hu Y and Cui X:
Chemical and bioactive comparison of Panax notoginseng root
and rhizome in raw and steamed forms. J Ginseng Res. 43:385–393.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Quan LH, Piao JY, Min JW, Kim HB, Kim SR,
Yang DU and Yang DC: Biotransformation of ginsenoside Rb1 to
prosapogenins, gypenoside XVII, ginsenoside Rd, ginsenoside F2, and
compound K by Leuconostoc mesenteroides DC102. J Ginseng
Res. 35:344–351. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li J, Huang Q, Yao Y, Ji P, Mingyao E,
Chen J, Zhang Z, Qi H, Liu J, Chen Z, et al: Biotransformation,
pharmacokinetics, and pharmacological activities of ginsenoside Rd
against multiple diseases. Front Pharmacol.
13(909363)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang D, Liao PY, Zhu HT, Chen KK, Xu M,
Zhang YJ and Yang CR: The processing of Panax notoginseng
and the transformation of its saponin components. Food Chem.
132:1808–1813. 2012.
|
|
12
|
Zheng F, Zhang MY, Wu YX, Wang YZ, Li FT,
Han MX, Dai YL and Yue H: Biotransformation of Ginsenosides
(Rb1, Rb2, Rb3, Rc) in human
intestinal bacteria and its effect on intestinal flora. Chem
Biodivers. 18(e2100296)2021.
|
|
13
|
Liu Z, Li JX, Wang CZ, Zhang DL, Wen X,
Ruan CC, Li Y and Yuan CS: Microbial conversion of
protopanaxadiol-type ginsenosides by the edible and medicinal
mushroom schizophyllum commune: A green biotransformation strategy.
ACS Omega. 4:13114–13123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim BG, Shin KS, Yoon TJ, Yu KW, Ra KS,
Kim JM, Kim SY and Suh HJ: Fermentation of Korean red ginseng by
Lactobacillus plantarum M-2 and its immunological
activities. Appl Biochem Biotechnol. 165:1107–1119. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park SE, Na CS, Yoo SA, Seo SH and Son HS:
Biotransformation of major ginsenosides in ginsenoside model
culture by lactic acid bacteria. J Ginseng Res. 41:36–42.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Barreda D, Hanington P and Belosevic M:
Regulation of myeloid development and function by colony
stimulating factors. Dev Comp Immunol. 28:509–554. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang H, Sun Y, Fan M, Zhang Y, Liang Z,
Zhang L, Gao X, He X, Li X, Zhao D, et al: Prevention effect of
total ginsenosides and ginseng extract from Panax ginseng on
cyclophosphamide-induced immunosuppression in mice. Phytother Res.
37:3583–3601. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Barone FC and Feuerstein GZ: Inflammatory
mediators and stroke: new opportunities for novel therapeutics. J
Cereb Blood Flow Metab. 19:819–834. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sharir R, Semo J, Shaish A, Landa-Rouben
N, Entin-Meer M, Keren G and George J: Regulatory T cells influence
blood flow recovery in experimental hindlimb ischaemia in an
IL-10-dependent manner. Cardiovasc Res. 103:585–596.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Research Council Committee for
the Update of the Guide for the C and Use of Laboratory A: The
National Academies Collection: Reports funded by National
Institutes of Health. In: Guide for the Care and Use of Laboratory
Animals. National Academies Press (US), Copyright ©. 2011, National
Academy of Sciences, Washington (DC), 2011.
|
|
21
|
Li W, Tang Y, Guo J, Shang E, Qian Y, Wang
L, Zhang L, Liu P, Su S, Qian D and Duan JA: Comparative
metabolomics analysis on hematopoietic functions of herb pair
Gui-Xiong by ultra-high-performance liquid chromatography coupled
to quadrupole time-of-flight mass spectrometry and pattern
recognition approach. J Chromatogr A. 1346:49–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao J, Liu J, Yao M, Zhang W, Yang B and
Wang G: Panax notoginseng saponins stimulates neurogenesis
and neurological restoration after microsphere-induced cerebral
embolism in rats partially via mTOR signaling. Front Pharmacol.
13(889404)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Steinbrecht S, Kiebist J, König R,
Thiessen M, Schmidtke KU, Kammerer S, Küpper JH and Scheibner K:
Synthesis of cyclophosphamide metabolites by a peroxygenase from
Marasmius rotula for toxicological studies on human cancer cells.
AMB Express. 10(128)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang J, Wang F, Yuan L, Ruan H, Zhu Z, Fan
X, Zhu L and Peng X: Blood-enriching effects and immune-regulation
mechanism of steam-processed polygonatum sibiricum polysaccharide
in blood deficiency syndrome mice. Front Immunol.
13(813676)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu B, Yang Z, Bo L, Zhao Z, Zhou Q and
Sun C: Cytotoxic effects, inflammatory response and apoptosis
induction of cyclophosphamide in the peripheral blood leukocyte of
blunt snout bream (Megalobrama amblycephala). Fish Shellfish
Immunol. 93:174–182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng Y, Liu L, Mo S, Gao J, Zhang H,
Zhang H, Zhang C, Song X, Li L and Geng Z: The immunomodulatory
effects of phellodendri cortex polysaccharides on
cyclophosphamide-induced immunosuppression in mice. Evid Based
Complement Alternat Med. 2021(3027708)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang S, Huang S, Ye Q, Zeng X, Yu H, Qi D
and Qiao S: Prevention of cyclophosphamide-induced
immunosuppression in mice with the antimicrobial peptide sublancin.
J Immunol Res. 2018(4353580)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Economopoulou C, Pappa V, Papageorgiou S,
Kontsioti F, Economopoulou P, Charitidou E, Girkas K, Kapsimali V,
Papasteriadi C, Tsirigotis P, et al: Cell cycle and apoptosis
regulatory gene expression in the bone marrow of patients with de
novo myelodysplastic syndromes (MDS). Ann Hematol. 89:349–358.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vermeulen K, Berneman ZN and Van
Bockstaele DR: Cell cycle and apoptosis. Cell Prolif. 36:165–175.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Czabotar PE and Garcia-Saez AJ: Mechanisms
of BCL-2 family proteins in mitochondrial apoptosis. Nat Rev Mol
Cell Bio. 24:732–748. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Westphal D, Dewson G, Czabotar PE and
Kluck RM: Molecular biology of Bax and Bak activation and action.
Biochim Biophys Acta. 1813:521–531. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Raj S and Gothandam K: Immunomodulatory
activity of methanolic extract of Amorphophallus commutatus var.
Wayanadensis under normal and cyclophosphamide induced
immunosuppressive conditions in mice models. Food Chem Toxicol.
81:151–159. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hou F, Yang H, Yu T and Chen W: The
immunosuppressive effects of 10 mg/kg cyclophosphamide in Wistar
rats. Environ Toxicol Pharmacol. 24:30–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shrief AI, Hamed WHE, Mazroa SA and
Moustafa AM: Histological study of the role of CD34+ stem cells and
mast cells in cyclophosphamide-induced thymic injury in rats and
the possible attenuating role of melatonin. Histochem Cell Biol.
159:501–512. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Khazaei F, Ghanbari E and Khazaei M:
Protective effect of royal jelly against cyclophosphamide-induced
thrombocytopenia and spleen and bone marrow damages in rats. Cell
J. 22:302–309. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen H, Luo Z, Shen H, Ren C, Li Z, Tang
J, Wang J and Wu T: Research on the roles of transcription factors
T-bet and GATA-3 in aplastic anemia. Clin Lab. 60:291–295.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang H, Wang H, Liu Y, Huang L, Wang Z
and Li Y: The haematopoietic effect of Panax japonicus on blood
deficiency model mice. J Ethnopharmacol. 154:818–824.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang Y, Fei QQ, Wang J, Zhu FX, Chen Y,
Tang DQ and Chen B: Study on blood enrichment mechanism of steamed
notoginseng based on metabolomics method. Zhongguo Zhong Yao Za
Zhi. 44:2139–2148. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
40
|
Zhang Z, Chen L, Cui X, Zhang Y, Hu Y,
Wang C and Xiong Y: Identification of anti-inflammatory components
of raw and steamed Panax notoginseng root by analyses of
spectrum-effect relationship. RSC Adv. 9:17950–17958.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shin NR, Bose S, Choi Y, Kim YM, Chin YW,
Song EJ, Nam YD and Kim H: Anti-obesity effect of fermented
Panax notoginseng is mediated via modulation of appetite and
gut microbial population. Front Pharmacol.
12(665881)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bai Y and Gänzle MG: Conversion of
ginsenosides by Lactobacillus plantarum studied by liquid
chromatography coupled to quadrupole trap mass spectrometry. Food
Res Int. 76:709–718. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen MY, Shao L, Zhang W, Wang CZ, Zhou
HH, Huang WH and Yuan CS: Metabolic analysis of Panax
notoginseng saponins with gut microbiota-mediated
biotransformation by HPLC-DAD-Q-TOF-MS/MS. J Pharmaceut Biomed.
150:199–207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang L, Yang X, Yu X, Yao Y and Ren G:
Evaluation of antibacterial and anti-inflammatory activities of
less polar ginsenosides produced from polar ginsenosides by
heat-transformation. J Agric Food Chem. 61:12274–12282.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bae Ea, Han MJ, Kim EJ and Kim DH:
Transformation of ginseng saponins to ginsenoside Rh2 by acids and
human intestinal bacteria and biological activities of their
transformants. Arch Pharm Res. 27:61–67. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Akao T, Kanaoka M and Kobashi K:
Appearance of compound K, a major metabolite of ginsenoside Rb1 by
intestinal bacteria, in rat plasma after oral
administration-measurement of compound K by enzyme immunoassay.
Biol Pharm Bull. 21:245–249. 1998.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Choi HS, Kim SY, Park Y, Jung EY and Suh
HJ: Enzymatic transformation of ginsenosides in Korean Red Ginseng
(Panax ginseng Meyer) extract prepared by Spezyme and Optidex. J
Ginseng Res. 38:264–269. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lee SY, Jeong JJ, Eun SH and Kim DH:
Anti-inflammatory effects of ginsenoside Rg1 and its metabolites
ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with
TNBS-induced colitis. Eur J Pharmacol. 762:333–343. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu Z, Wen X, Wang CZ, Li W, Huang WH, Xia
J, Ruan CC and Yuan CS: Remarkable impact of amino acids on
ginsenoside transformation from fresh ginseng to red ginseng. J
Ginseng Res. 44:424–434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang HY, Hua HY, Liu XY, Liu JH and Yu BY:
In vitro biotransformation of red ginseng extract by human
intestinal microflora: Metabolites identification and metabolic
profile elucidation using LC-Q-TOF/MS. J Pharm Biomed Anal.
98:296–306. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee BH, You HJ, Park MS, Kwon B and Ji GE:
Transformation of the glycosides from food materials by probiotics
and food microorganisms. J Microbiol Biotechn. 16:497–504.
2006.
|
|
52
|
Li W, Tang Y, Guo J, Huang M, Li W, Qian D
and Duan J: Enriching blood effect comparison in three kinds of
blood deficiency model after oral administration of drug pair of
Angelicae Sinensis Radix and Chuanxiong Rhizoma and each single
herb. Zhongguo Zhong Yao Za Zhi. 36:1808–1814. 2011.PubMed/NCBI(In Chinese).
|
|
53
|
Hua YL, Ma Q, Yuan ZW, Zhang XS, Yao WL,
Ji P, Hu JJ and Wei YM: A novel approach based on metabolomics
coupled with network pharmacology to explain the effect mechanisms
of Danggui Buxue Tang in anaemia. Chin J Nat Med. 17:275–290.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Li S, Lin H, Qu C, Tang Y, Shen J, Li W,
Yue S, Kai J, Shang G, Zhu Z, et al: Urine and plasma metabonomics
coupled with UHPLC-QTOF/MS and multivariate data analysis on
potential biomarkers in anemia and hematinic effects of herb pair
Gui-Hong. J Ethnopharmacol. 170:175–183. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hua Y, Yao W, Ji P and Wei Y: Integrated
metabonomic-proteomic studies on blood enrichment effects of
Angelica sinensis on a blood deficiency mice model. Pharm Biol.
55:853–863. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu H, Pan J, Yang Y, Cui X and Qu Y:
Production of minor ginenosides from Panax notoginseng by
microwave processing method and evaluation of their blood-enriching
and hemostatic activity. Molecules. 23(1243)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu
Y, Wang G and Zou W: Bone marrow and the control of immunity. Cell
Mol Immunol. 9:11–19. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sieff C: Hematopoietic growth factors. J
Clin Invest. 79:1549–1557. 1987.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hokom MM, Lacey D, Kinstler OB, Choi E,
Kaufman S, Faust J, Rowan C, Dwyer E, Nichol JL, Grasel T, et al:
Pegylated megakaryocyte growth and development factor abrogates the
lethal thrombocytopenia associated with carboplatin and irradiation
in mice. Blood. 86:4486–4492. 1995.PubMed/NCBI
|
|
60
|
Kumar A, Taghi Khani A, Sanchez Ortiz A
and Swaminathan S: GM-CSF: A double-edged sword in cancer
immunotherapy. Front Immunol. 13(901277)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu H, Yan Y, Zhang F and Wu Q: The
immuno-enhancement effects of tubiechong (eupolyphaga sinensis)
lyophilized powder in cyclophosphamide-induced immunosuppressed
mice. Immunol Invest. 48:844–859. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen QQ, Han X, Wang WM, Zhao L and Chen
A: Danggui sini decoction ameliorates myelosuppression in animal
model by upregulating thrombopoietin expression. Cell Biochem
Biophys. 71:945–950. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang LF, Xu ZY, Jin CJ, Sha HF, Wang ZQ,
Zhou WD, Zhang M, Wu J and Bai B: Dual regulation of cell cycles by
Shuanghuang Shengbai Granule in Lewis-bearing mice with
chemotherapy-induced myelosuppression and its mechanism. Zhong Xi
Yi Jie He Xue Bao. 7:453–457. 2009.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
64
|
Qiang H, Wang KZ, Shi ZB and Fan LH:
Panax notoginseng saponins protect rabbit bone marrow
stromal cells from hydrogen peroxide-induced apoptosis. Zhong Xi Yi
Jie He Xue Bao. 8:131–137. 2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Qiang H, Zhang C, Shi ZB, Yang HQ and Wang
KZ: Protective effects and mechanism of Panax notoginseng
saponins on oxidative stress-induced damage and apoptosis of rabbit
bone marrow stromal cells. Chin J Integr Med. 16:525–530.
2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Han J, Wang Y, Cai E, Zhang L, Zhao Y, Sun
N, Zheng X and Wang S: Study of the effects and mechanisms of
ginsenoside compound K on myelosuppression. J Agric Food Chem.
67:1402–1408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Senior J: Alanine aminotransferase: A
clinical and regulatory tool for detecting liver injury-past,
present, and future. Clin Pharmacol Ther. 92:332–339.
2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lee CH and Kim IH: Direct
hyperbilirubinemia as a predictor of mortality in patients with
liver cirrhosis. Gut Liver. 15:490–491. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Vimalraj S: Alkaline phosphatase:
Structure, expression and its function in bone mineralization.
Gene. 754(144855)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Gkouvatsos K, Papanikolaou G and
Pantopoulos K: Regulation of iron transport and the role of
transferrin. Biochim Biophys Acta. 1820:188–202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Boshuizen M, van der Ploeg K, von
Bonsdorff L, Biemond BJ, Zeerleder SS, van Bruggen R and Juffermans
NP: Therapeutic use of transferrin to modulate anemia and
conditions of iron toxicity. Blood Rev. 31:400–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ding RB, Tian K, Cao YW, Bao JL, Wang M,
He C, Hu Y, Su H and Wan JB: Protective effect of Panax
notoginseng saponins on acute ethanol-induced liver injury is
associated with ameliorating hepatic lipid accumulation and
reducing ethanol-mediated oxidative stress. J Agric Food Chem.
63:2413–2422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Xu Y, Wang N, Tan HY, Li S, Zhang C and
Feng Y: Gut-liver axis modulation of Panax notoginseng
saponins in nonalcoholic fatty liver disease. Hepatol Int.
15:350–365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Zhong H, Wu H, Bai H, Wang M, Wen J, Gong
J, Miao M and Yuan F: Panax notoginseng saponins promote
liver regeneration through activation of the PI3K/AKT/mTOR cell
proliferation pathway and upregulation of the AKT/Bad cell survival
pathway in mice. BMC Complem Altern Med. 19(122)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ahlmann M and Hempel G: The effect of
cyclophosphamide on the immune system: Implications for clinical
cancer therapy. Cancer Chemother Pharmacol. 78:661–671.
2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Travlos GS: Normal structure, function,
and histology of the bone marrow. Toxicol Pathol. 34:548–565.
2006.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kar UK and Joosten LAB: Training the
trainable cells of the immune system and beyond. Nat Immunol.
21:115–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Dong C: Cytokine regulation and function
in T cells. Annu Rev Immunol. 39:51–76. 2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Oestreich KJ and Weinmann AS: T-bet
employs diverse regulatory mechanisms to repress transcription.
Trends Immunol. 33:78–83. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Elenkov IJ and Chrousos GP: Stress
hormones, proinflammatory and antiinflammatory cytokines, and
autoimmunity. Ann N Y Acad Sci. 966:290–303. 2002.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Mei YX, Chen HX, Zhang J, Zhang XD and
Liang YX: Protective effect of chitooligosaccharides against
cyclophosphamide-induced immunosuppression in mice. Int J Biol
Macromol. 62:330–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhu G, Luo J, Du H, Jiang Y, Tu Y, Yao Y
and Xu M: Ovotransferrin enhances intestinal immune response in
cyclophosphamide-induced immunosuppressed mice. Int J Biol
Macromol. 120:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhao Y, Sun X, Yu X, Gao R and Yin L:
Saponins from Panax notoginseng leaves improve the symptoms
of aplastic anemia and aberrant immunity in mice. Biomed
Pharmacother. 102:959–965. 2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Yang Z, Chen A, Sun H, Ye Y and Fang W:
Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in
mice. Vaccine. 25:161–169. 2007.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Won HJ, Kim HI, Park T, Kim H, Jo K, Jeon
H, Ha SJ, Hyun JM, Jeong A, Kim JS, et al: Non-clinical
pharmacokinetic behavior of ginsenosides. J Ginseng Res.
43:354–360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Han M and Fang XL: Difference in oral
absorption of ginsenoside Rg1 between in vitro and in vivo models.
Acta Pharmacol Sin. 27:499–505. 2006.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Paek IB, Moon Y, Kim J, Ji HY, Kim SA,
Sohn DH, Kim JB and Lee HS: Pharmacokinetics of a ginseng saponin
metabolite compound K in rats. Biopharm Drug Dispos. 27:39–45.
2006.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Xu QF, Fang XL and Chen DF:
Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1
from Panax notoginseng in rats. J Ethnopharmacol.
84:187–192. 2003.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Peng Y, Wu Z, Huo Y, Chen Y, Lu F, Peng Q
and Liang Y: Simultaneous determination of ginsenosides Rg1, Re,
and Rb1 and notoginsenoside R1 by solid phase extraction followed
by UHPLC-MS/MS and investigation of their concentrations in various
kinds of cosmetics. Anal Methods. 9:5441–5448. 2017.
|
|
90
|
Li W, Wu Y, Wan M, Chu Y, Wang X, Li S,
Liu Z, Chen X, Polachi N, Zhou S and Sun H: Simultaneous
determination of three saponins in human plasma after oral
administration of compound danshen dripping pills by LC-MS/MS and
its application in a pharmacokinetic study. J Pharm Biomed Anal.
169:254–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhu D, Zhou Q, Li H, Li S, Dong Z, Li D
and Zhang W: Pharmacokinetic characteristics of steamed notoginseng
by an efficient LC-MS/MS method for simultaneously quantifying
twenty-three triterpenoids. J Agric Food Chem. 66:8187–8198.
2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Sharma A and Lee HJ: Ginsenoside compound
K: Insights into recent studies on pharmacokinetics and
health-promoting activities. Biomolecules. 10(1028)2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kim HK: Pharmacokinetics of ginsenoside
Rb1 and its metabolite compound K after oral administration of
Korean Red Ginseng extract. J Ginseng Res. 37:451–456.
2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Fukami H, Ueda T and Matsuoka N:
Pharmacokinetic study of compound K in Japanese subjects after
ingestion of Panax ginseng fermented by Lactobacillus
paracasei A221 reveals significant increase of absorption into
blood. J Med Food. 22:257–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Choi ID, Ryu JH, Lee DE, Lee MH, Shim JJ,
Ahn YT, Sim JH, Huh CS, Shim WS, Yim SV, et al: Enhanced absorption
study of ginsenoside compound K
(20-O-β-(D-Glucopyranosyl)-20(S)-protopanaxadiol) after oral
administration of fermented red ginseng extract (HYFRG™)
in healthy Korean volunteers and rats. Evid Based Complement
Alternat Med. 2016(3908142)2016.PubMed/NCBI View Article : Google Scholar
|