Introduction
Intracranial cavernous malformations (CMs) are
vascular lesions that have an annual bleeding rate of ~0.2 to 3%
per individual per year (1). In
the literature, there are three management options for addressing
CMs: Operative resection, radiosurgery (SRS) and conventional
treatment; however, the debate regarding the treatment options for
CMs has a long history and remains controversial (2).
The effects of SRS on cavernoma remain hypothetical
(3-5).
The outcome of CM management can be stated only as a decreased
bleeding rate for a large number of patients, which then
necessitates dependable data on the natural course. On an
individual level, the treatment result is extremely hypothetical,
as it is necessary to have knowledge of the natural history of the
condition to ensure that a good benefit is achieved (6,7).
Surgery is an alternative treatment option for CMs,
with a complete resection to reach a temporary morbidity rate
varying from 29 to 67% and a 1.9% combined post-operative
re-bleeding and surgery-related mortality rate (1,8). In
addition, 58% of incomplete resection cases re-bleed. Hence,
concerning surgical management, the proportion of no active
treatment cases, the direct morbidity and mortality rates, and the
risk of partial removal of CMs with the prospect of re-hemorrhage
have to be recalculated (1).
In addition, a number of CMs are considered
untreatable due to their placement in eloquent areas. Thus,
surgically approachable cavernomas consist of a detailed
assortment, whereas a number of untreatable cases can eventually be
managed with SRS. Hence, the effectiveness of surgery and SRS can
only be estimated based on an accurate designation of exclusion
criteria and the exact risks for complications and re-hemorrhage
for both treatment options.
The present systematic review and meta-analysis
compared with previous reports (9,10),
aimed to evaluate the safety of surgical or SRS treatment for the
management of CMs and also evaluate their potential outcomes
compared with conservative treatment.
Materials and methods
Literature research strategy
The present meta-analysis investigated the relative
articles involving intracranial cavernous malformation (CMs)
natural history vs. surgical or radiosurgical (SRS) treatment
option through electronic databases, counting the Cochrane Library,
PubMed (until June, 2023), Embase (until June, 2023), and MEDLINE
(until June, 2023). For the study protocol establishing and design,
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines were applied. ‘Cerebral Cavernous
Malformation’, ‘Cerebral Cavernus Malformation natural history vs.
surgical or radiosurgical treatment’, ‘Intracranial Cavernus
Malformation natural history vs. surgical or radiosurgical
treatment option’, and ‘Cerebral Cavernous Malformation natural
history vs. surgical treatment’, were used in the MeSH list as
keywords.
Selection of studies
In the present study, two authors (VEG and GF)
separately pulled out data from the contained articles, following
the guidelines for the epidemiology of meta-analysis. The
subsequent crucial information was attained: The main authors, year
of publication, sample size in the CM natural history vs. surgical
or SRS treatment option groups, study type, outcome indicator, etc.
The extracted data were contributed to a designed, standardized
table according to the Cochrane Handbook. The flow of the study
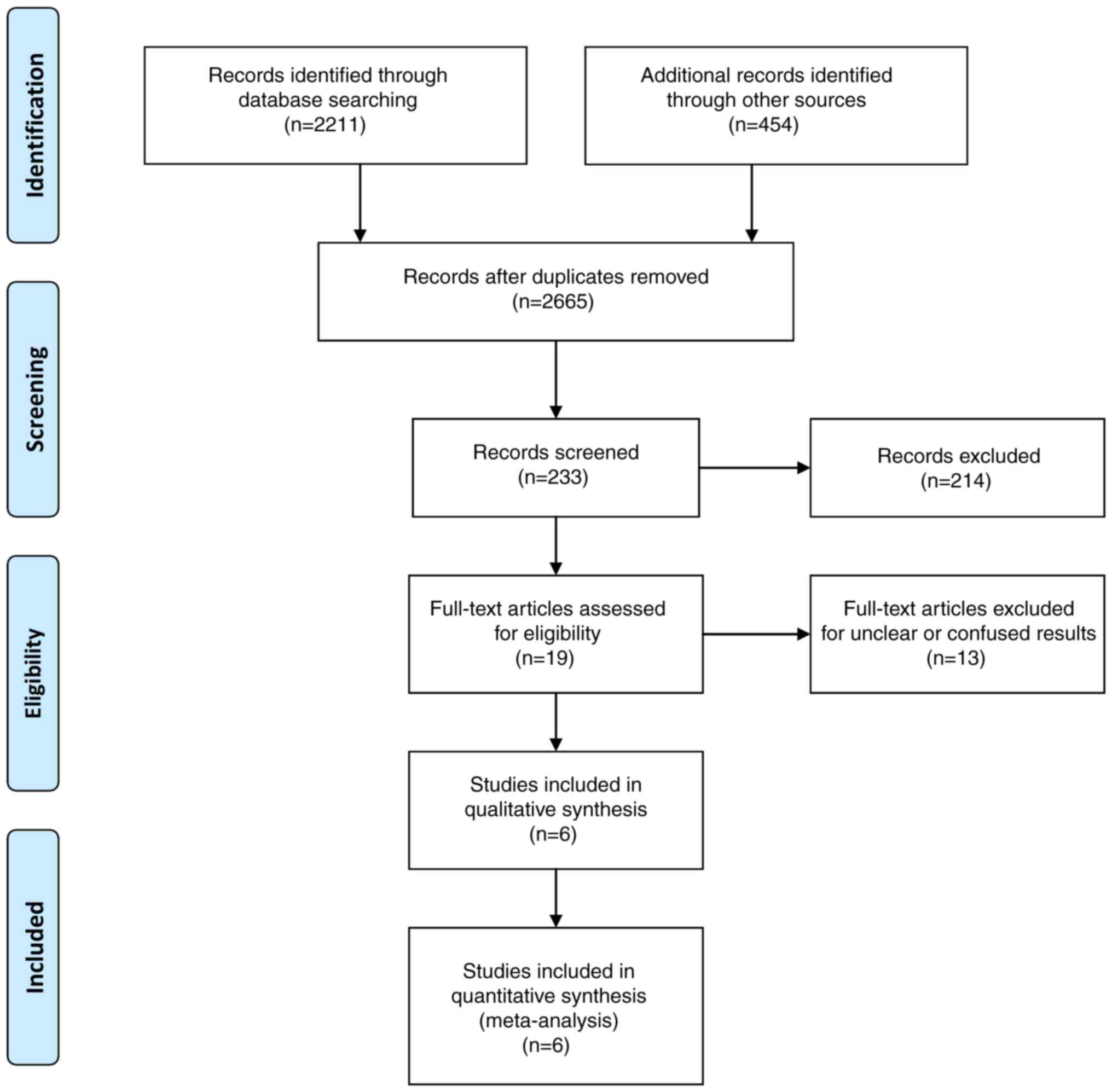
selection process is presented in Fig.
1.
Inclusion and exclusion criteria
If an article fulfilled the subsequent population,
intervention, comparison, outcomes and study (PICOS) design
criteria, it was eligible for inclusion in the present
meta-analysis: i) Population: Limited to patients with intracranial
CMs; ii) Intervention: Limited to patients with CMs natural history
vs. surgical or/and SRS treatment option; iii) Comparison: Studies
comparing the outcomes between the CM natural history (conservative
treatment; Cons) vs. the surgical or/and SRS (surg/SRS) treatment
option; iv) the comprehensive data of these articles are presented
in Table I. To avoid publication
bias, the final aim was to collect a homogeneous pool of
manuscripts, including articles that compare only two modalities:
Patients with intracranial CMs treated with the Cons vs. Surg/SRS
treatment option.
 | Table IDesign and baseline characteristics
of the trials included in the present meta-analysis. |
Table I
Design and baseline characteristics
of the trials included in the present meta-analysis.
| | | Location | Size |
| |
|---|
| | Sample size | Mean age
(years) | No. of males | Lobar | Deep | Brain stem | Cereb. | <2 cm | 2-6 cm | Hemos. in the
pre-MRI | Free of
seizures | Neurol.
deficit | Re-bleeding | OHS 2-6 | Mortality | |
|---|
| Authors, year of
publication | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | S/S | C | (Refs.) |
|---|
| Mathiesen et
al, 2003 | 34 | 34 | N | N | 33 | 33 | 0 | N | 23 | N | 6 | N | 5 | N | N | N | N | N | 33 | 34 | 20 | 9 | 6 | 11 | 21 | 14 | 26 | 11 | 4 | 0 | (15) |
| Tarnaris et
al, 2008 | 6 | 9 | 34.2 | 37.9 | 3 | 4 | 0 | N | 1 | N | 4 | N | 1 | N | N | N | N | N | 4 | 7 | 0 | 1 | 6 | 8 | 0 | 4 | 3 | 13 | 0 | 2 | (16) |
| Fernández et
al, 2012 | 26 | 17 | 44.8 | 50.2 | 16 | 10 | 24 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 8 | 15 | 8 | 20 | 10 | 14 | 16 | 2 | 0 | N | N | N | N | N | N | (1) |
| Moultrie et
al, 2014 | 25 | 109 | 34 | 43 | 10 | 45 | 19 | 71 | 1 | 8 | 1 | 16 | 4 | 14 | N | N | N | N | 8 | 10 | N | N | 8 | 17 | 5 | 1 | 9 | 40 | 17 | 30 | (17) |
| Dammann et
al, 2017 | 41 | 38 | 39 | 36 | 21 | 10 | 19 | 24 | 14 | 17 | 0 | 0 | 0 | 0 | N | N | N | N | 41 | 38 | 30 | 9 | 9 | 9 | 3 | 0 | 4 | 18 | N | N | (18) |
| Kang et al,
2018 | 25 | 35 | 44 | 56 | 13 | 17 | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | 5 | 2 | N | N | (19) |
All retrospective and prospective studies that
assessed these two modalities together were included, whereas
editorials, reviews, case reports and articles focusing on the
pediatric population, unrelated outcomes, co-morbidities,
experimental techniques, or one of the two modalities separately
from that article pool were excluded. Additionally, to decrease the
risk of bias in the included articles, a quality assessment tool
[the Newcastle-Ottawa Scale (NOS)] was used (Table II) (11).
 | Table IINewcastle-Ottawa Scale (NOS) quality
assessment of the final article pool. |
Table II
Newcastle-Ottawa Scale (NOS) quality
assessment of the final article pool.
| | Newcastle-Ottawa
Scale | |
|---|
| Author, year of
publication | Study design | Selection | Comparability | Exposure | Total scores | (Refs.) |
|---|
| Mathiesen et
al, 2003 | Prosp | 3 | 3 | 3 | 9 | (15) |
| Tarnaris et
al, 2008 | Prosp | 3 | 3 | 3 | 9 | (16) |
| Fernández et
al, 2012 | Retro | 3 | 2 | 2 | 7 | (1) |
| Moultrie et
al, 2014 | Prosp | 3 | 3 | 3 | 9 | (17) |
| Dammann et
al, 2017 | Retro | 3 | 3 | 2 | 8 | (18) |
| Kang et al,
2018 | Retro | 3 | 2 | 2 | 7 | (19) |
Outcomes' definition
The primary outcome was ‘poor outcome’, defined as
at least two successive ratings of the Oxford Handicap Scale (OHS)
(12) and OHS 2-6 (suggestive of
‘some restraints to lifestyle, but the patient can look after
themselves’, or worse). It was used only for OHS ratings after the
initial presentation to time progression to this event at the
midpoint between the last OHS score of 0-1 and the first of the
successive OHS 2-6 ratings for the conservatively managed group
(12).
Secondary outcomes were the frequency of seizures in
the surgical or + SRS and cons patients, neurological deficit,
re-bleeding and mortality. Information regarding age, sex,
localization (lobar, deep, brainstem, cerebellum), size (<2, 2-6
mm) and use of hemosiderin in the pre-surgical MRI is presented in
Table I. Re-bleeding was defined
as hemorrhage clearly demonstrated on imaging at the time of
admission.
Evaluation of the risk of bias
The Cochrane Collaboration tool was used to assess
the risk of bias and was used by two authors (GF and VEG) for each
study (13). The assessment
contained allocation concealment, random sequence generation, the
blinding of outcome evaluation, the blinding of participants and
assessors, unfinished outcome data, discriminating reports and
other biases. The evaluated results were classified into three
levels: Low risk, high risk and unclear risk. In the case of a
discrepancy, another author with authority gave the final
solution.
Statistical analysis and assessment of
heterogeneity
All analyses were carried out using Review Manager
Software (RevMan), version 5.4. Heterogeneity across trials was
identified using I2 statistics; considering
I2 >50% as high heterogeneity, a meta-analysis was
conducted using a random-effect model according to the Cochrane
Handbook for Systematic Reviews of Interventions (version 5.1.0)
(14). Otherwise, the fixed-effect
model was performed. The continuous outcomes were expressed as a
weighted mean difference with 95% confidence intervals (CIs). For
discontinuous variables, odds ratios (OR) with 95% CIs were applied
for the assessment. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
In total, six articles (1,15-19)
met the eligibility criteria. The total number of patients was 399
(157 in the Surg/SRS group and 242 in the Cons treatment group).
The study sample was based on six studies (Table I). Of these six studies, two
studies were retrospective and four studies were prospective.
Epidemiological and clinical
features
The mean age of the patients among the included
studies ranged from 34.9 to 56 years. The male-to-female ratio was
1.5 for the Surg/SRS group and 0.9 for the Cons treatment group
(95/62 and 119/123) (Table I).
Location
Lobar. Information regarding lobar brain
location was available in three articles (1,17,18).
No significant difference was found between the Surg/SRS and Cons
groups (OR, 1.03; 95% CI, 0.55 to 1.93; P=0.07), with heterogeneity
(P=0.92 and I2=62%) (Table III and Fig. S1).
 | Table IIIParameters for the results of the
present meta-analysis. |
Table III
Parameters for the results of the
present meta-analysis.
| | Groups | Overall effect | Heterogeneity | |
|---|
| Parameter | ‘Leave out one’
model; Authors/(Refs.), year of publication | Included trials,
n=6 | Surg/SRS | Cons | Effect
estimate | 95% CI | P-value | I2
(%) | P-value |
|---|
| Location | | | | | | | | | |
|
Lobar | - | 3 | 62 | 108 | 1.03 | (0.55-1.93) | 0.93 | 62 | 0.07 |
|
Deep | - | 3 | 15 | 25 | 0.62 | (0.27-1.43) | 0.26 | 0 | 0.87 |
|
Brainstem | - | 3 | 1 | 18 | 0.24 | (0.03-1.92) | 0.18 | - | 0.18 |
|
Cerebellum | - | 3 | 4 | 14 | 1.29 | (0.39-4.32) | 0.68 | - | 0.68 |
| Use of hemosiderin
in the pre-MRI | - | 5 | 106 | 99 | 2.56 | (1.20-5.47) | 0.22 | 32 | <0.05 |
| Free of
seizures | - | 4 | 64 | 35 | 3.49 | (1.79-6.83) | <0.05 | 82 | <0.05 |
| | Mathiesen et
al (15), 2003 | 3 | 44 | 26 | 3.17 | (1.31-7.71) | <0.05 | 88 | <0.05 |
| | Tarnaris et
al (16), 2008 | 3 | 64 | 34 | 3.81 | (1.92-7.55) | <0.05 | 87 | <0.05 |
| | Fernández et
al (1), 2012 | 3 | 50 | 19 | 5.27 | (2.60-10.68) | 0.18 | 41 | <0.05 |
| | Dammann et
al (18), 2017 | 3 | 34 | 26 | 1.72 | (0.71-4.20) | 0.23 | 82 | <0.05 |
| Neurological
deficit | - | 5 | 31 | 45 | 0.57 | (0.32-1.00) | 0.34 | 11 | 0.05 |
| Re-bleeding | - | 4 | 29 | 19 | 2.84 | (1.25-6.46) | <0.05 | 67 | <0.05 |
| | Mathiesen et
al (15), 2003 | 3 | 8 | 5 | 4.79 | (1.02-22.40) | 0.05 | 76 | <0.05 |
| | Tarnaris et
al (16), 2008 | 3 | 29 | 15 | 3.65 | (1.56-8.57) | <0.05 | 53 | 0.12 |
| | Moultrie et
al (17), 2014 | 3 | 24 | 18 | 1.97 | (0.81-4.78) | 0.13 | 55 | 0.11 |
| | Dammann et
al (18), 2017 | 3 | 26 | 19 | 2.64 | (1.12-6.20) | <0.05 | 77 | <0.05 |
| | Mathiesen et
al (15), 2003 and Tarnaris
et al (16), 2008 | 2 | 8 | 1 | 16.83 | (2.86-99.12) | 0.48 | 0 | <0.05 |
| | Mathiesen et
al (15), 2003 and Moultrie
et al (17), 2014 | 2 | 3 | 4 | 0.89 | (0.10-7.80) | 0.92 | 74 | 0.05 |
| | Mathiesen et
al (15), 2003 and Dammann
et al (18), 2017 | 2 | 5 | 5 | 4.17 | (0.69-25,25) | 0.12 | 88 | <0.05 |
| | Tarnaris et
al (16), 2008 and Moultrie
et al (17), 2014 | 2 | 24 | 14 | 2.56 | (1.02-6.47) | 0.49 | 0 | 0.05 |
| | Tarnaris et
al (16), 2008 and Dammann
et al (18), 2017 | 2 | 26 | 15 | 3.45 | (1.42-8.39) | <0.05 | 75 | <0.05 |
| | Moultrie et
al (17), 2014 and Dammann
et al (18), 2017 | 2 | 21 | 18 | 1.74 | (0.69-4.41) | 0.24 | 73 | 0.06 |
| OHS 2-6 | - | 5 | 51 | 79 | 1.44 | (0.83-2.49) | <0.05 | 86 | 0.19 |
| | Mathiesen et
al (15), 2003 | 4 | 25 | 68 | 0.83 | (0.44-1.57) | <0.05 | 84 | 0.57 |
| | Tarnaris et
al (16), 2008 | 4 | 48 | 71 | 1.61 | (0.92-2.82) | <0.05 | 88 | 0.10 |
| | Moultrie et
al (17), 2014 | 4 | 38 | 39 | 1.22 | (0.60-2.46) | <0.05 | 90 | 0.58 |
| | Dammann et
al (18), 2017 | 4 | 47 | 61 | 2.72 | (1.47-5.03) | <0.05 | 67 | <0.05 |
| | Kang et al
(19), 2018 | 4 | 46 | 77 | 1.28 | (0.72-2.28) | <0.05 | 89 | 0.40 |
| Mortality | - | 3 | 21 | 32 | 4.68 | (1.97-11.09) | 0.15 | 47 | <0.05 |
Deep. Information regarding deep brain
location was available in three articles (1,17,18).
No significant difference was found between the Surg/SRS and Cons
groups (OR, 0.62; 95% CI, 0.27 to 1.43; P=0.87), without
heterogeneity (P=0.26 and I2=0%) (Table III and Fig. S2).
Brainstem. As regards brainstem location,
information was available in three articles (1,17,18).
No significant difference was found between groups (OR, 0.24; 95%
CI, 0.03 to 1.92; P=0.18) (Table
III and Fig. S3).
Cerebellum. As regards cerebellum location,
information was available in three articles (1,17,18).
No significant difference was found between groups (OR, 1.29; 95%
CI, 0.39 to 4.32; P=0.68) (Table
III and Fig. S4).
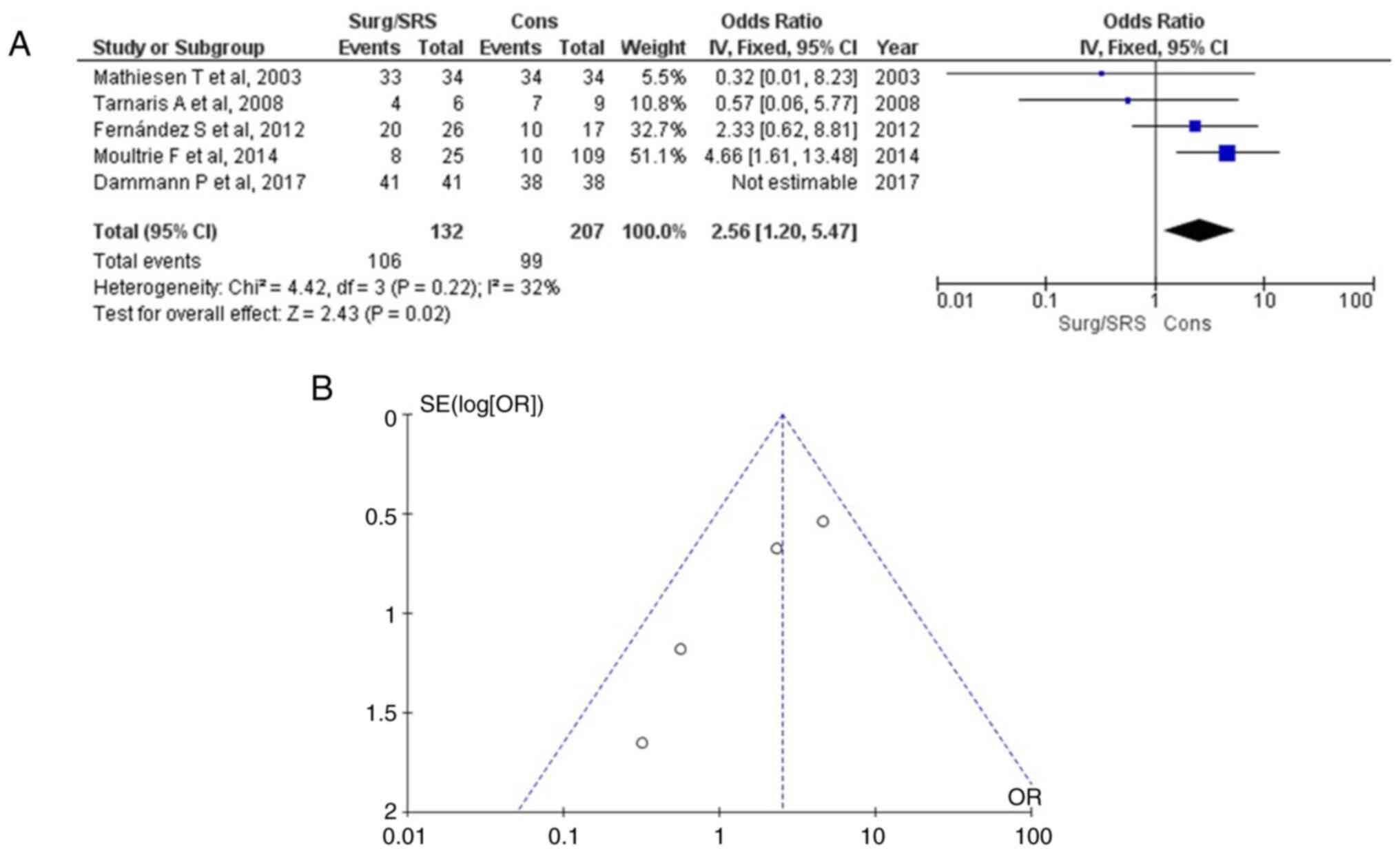
Hemosiderin in the pre-MRI
As regards the use of hemosiderin in the pre-MRI,
information was available in six articles (1,15-18),
and this demonstrated a statistically significant result (OR, 2.56;
95% CI, 1.20 to 5.47; P<0.05), with very low heterogeneity
(P=0.22; I2=32%) (Table
III and Fig. 2). The use of
hemosiderin in the pre-MRI was found in 106 of 132 (80.3%) patients
in the Surg/SRS group and in 99 of 207 (47.8%) patients in the Cons
group. When examining the funnel plot of the same parameter, no
publication bias was found.
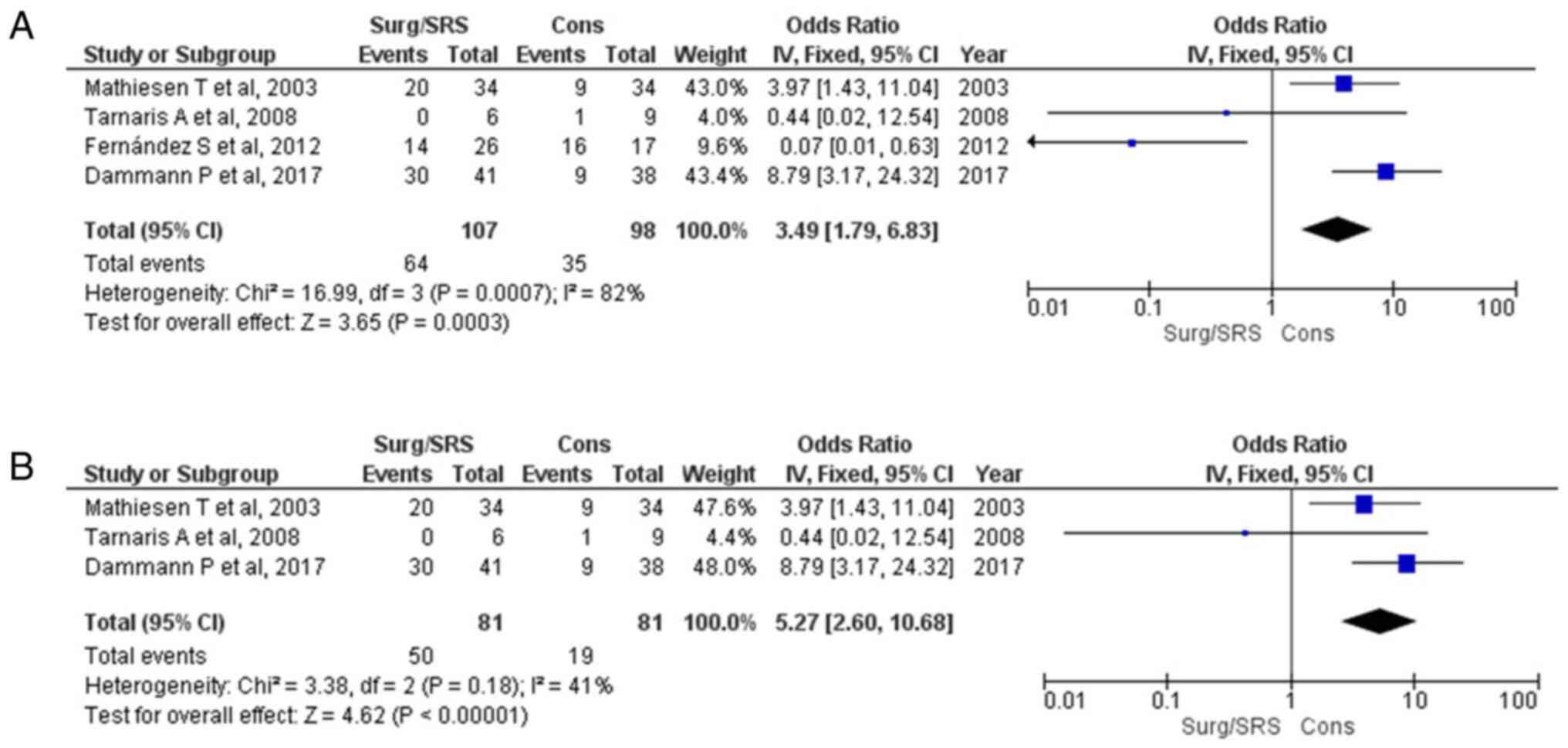
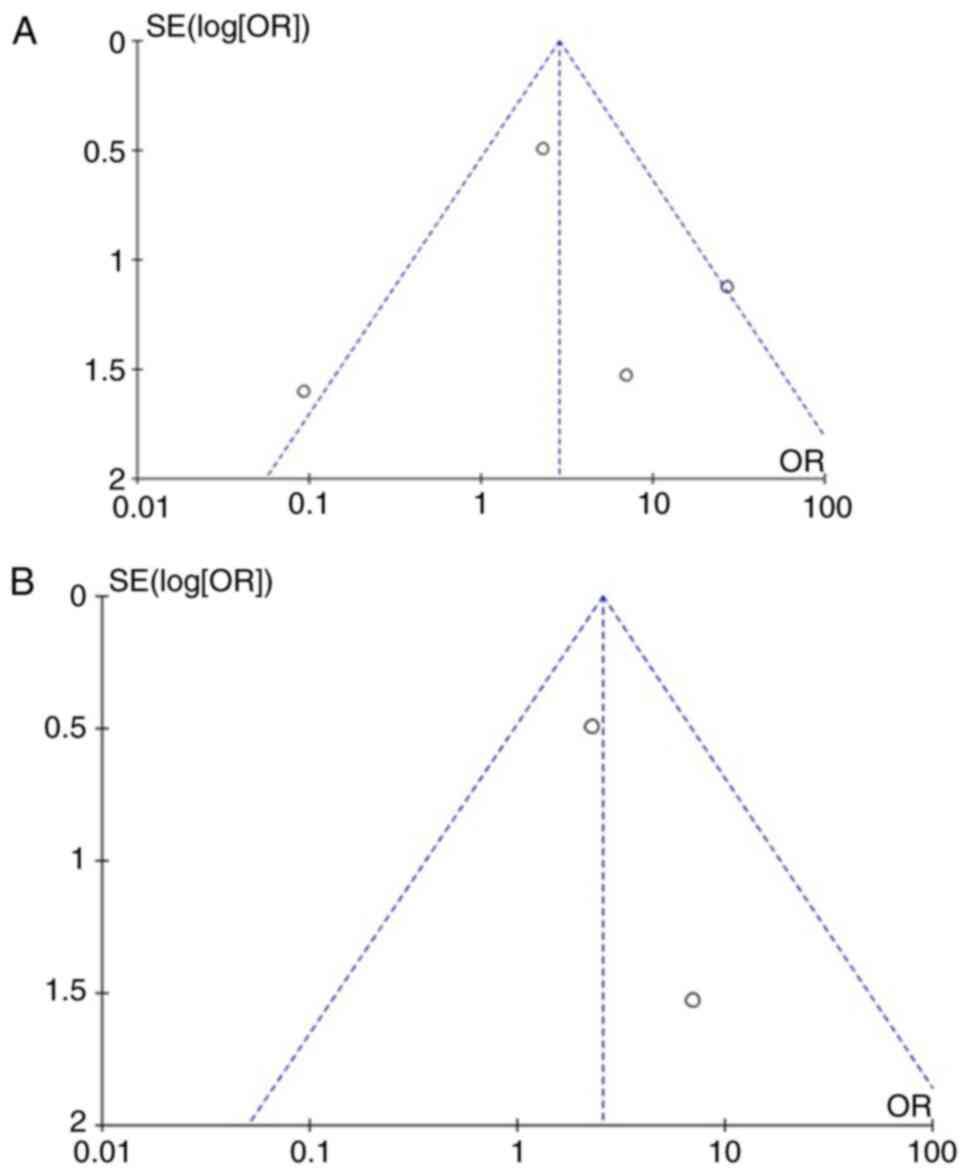
Free of seizures parameter
Information regarding the free of seizures parameter
was available in four articles (1,15,16,18)
and demonstrated a statistically significant result between the
patients in the Surg/SRS and Cons groups (OR, 3.49; 95% CI, 1.79 to
6.83; and P<0.05), but with heterogeneity (P<0.05 and
I2=82%) (Fig. 3A). For
testing the sensitivity, the ‘leave out one’ model was used, and
one study was removed at a time (Table III). Low heterogeneity (P=0.18
and I2=41%) was achieved only after removing the article
by Fernández et al (1);
again, a statistically significant difference was found (OR, 5.27;
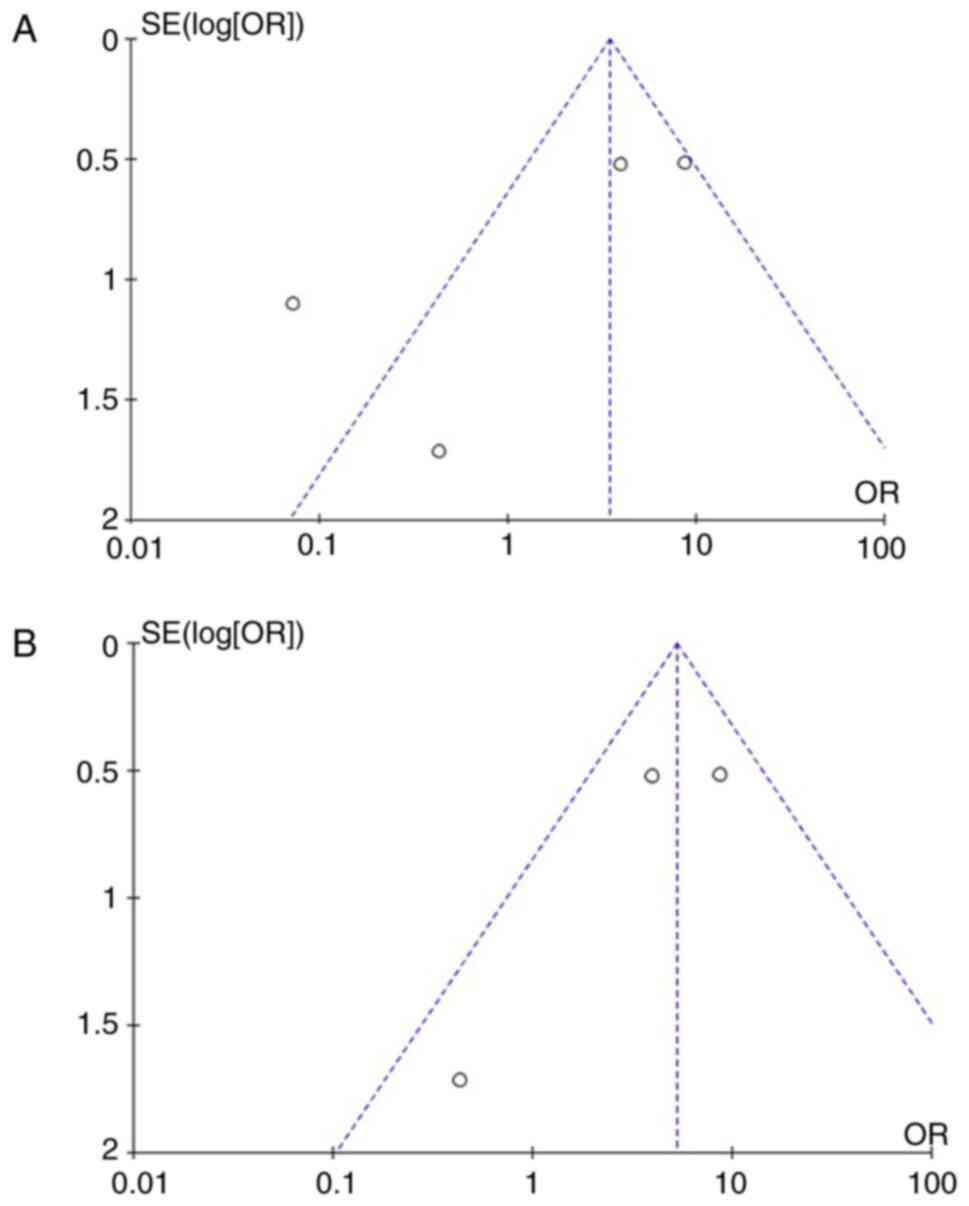
95% CI, 2.60 to 10.68; P<0.05) (Fig. 3B). When examining the funnel plot
of the same parameter, it was found that the study results without
the study by Fernández et al (1) displayed better dispersion with a low
publication bias (Fig. 4).
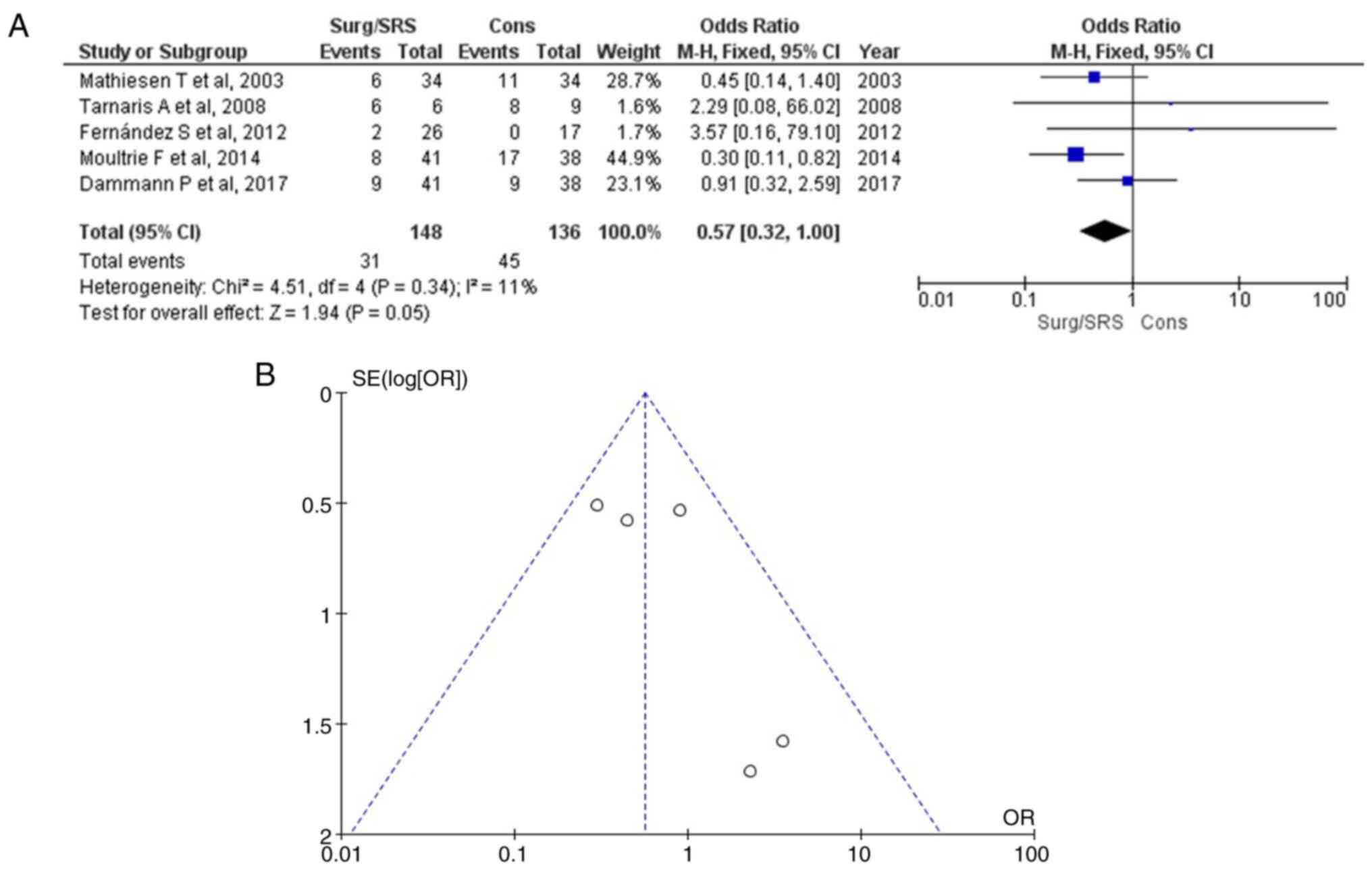
Neurological deficit parameter
As regards neurological deficit, information was
available in four articles (1,15-18),
and this demonstrated a statistically significant result (OR, 0.57;
95% CI, 0.32 to 1.00; P=0.05) with no heterogeneity (P=0.34;
I2=11%) (Table III
and Fig. 5). A neurological
deficit was found in 31 of 148 (20.9%) patients in the Surg/SRS
group and in 45 of 136 (33.0%) patients in the Cons group. When
examining the funnel plot of the same parameter, no publication
bias was found. Thus, the Surg/SRS (experimental) group exhibited
superiority over the Cons (control) group.
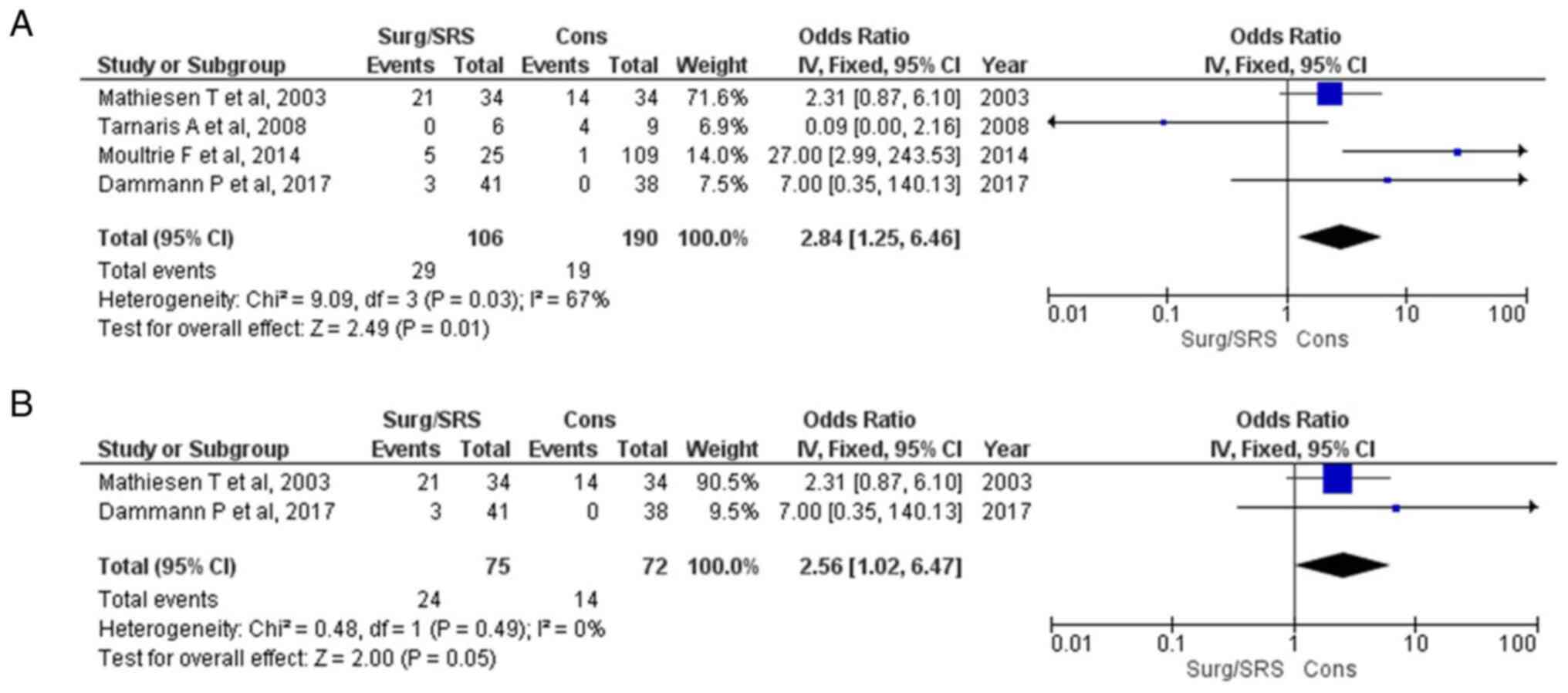
Re-bleeding
Information regarding the re-bleeding parameter was
available in four articles (15-18)
and demonstrated a statistically significant result between the
patients with Surg/SRS and the Cons groups (OR, 2.84; 95% CI, 1.25
to 6.46; and P<0.05), but with heterogeneity (P<0.05 and
I2=67%) (Fig. 6A). For
testing the sensitivity, the ‘leave out one’ model was used, and
one study was removed at a time (Table III). No heterogeneity (P=0.49 and
I2=0%) was achieved only after removing the articles by
Tarnaris et al (16) and
Moultrie et al (17);
again, a statistically significant difference was found (OR, 5.56;
95% CI, 1.02 to 6.67; P=0.05) (Fig.
6B). When examining the funnel plot of the same parameter, it
was found that the study results without the studies by Tarnaris
et al (16) and Moultrie
et al (17) displayed
better dispersion with a low publication bias (Fig. 7).
OHS 2-6
As regards OHS 2-6, information was available in
five articles (15-19).
No significant difference was found between groups (OR, 1.44; 95%
CI, 0.83 to 2.49; P=0.19) (Table
III and Fig. S5). In
addition, after applying the ‘leave out one’ model, no
statistically significant result was obtained (Table III).
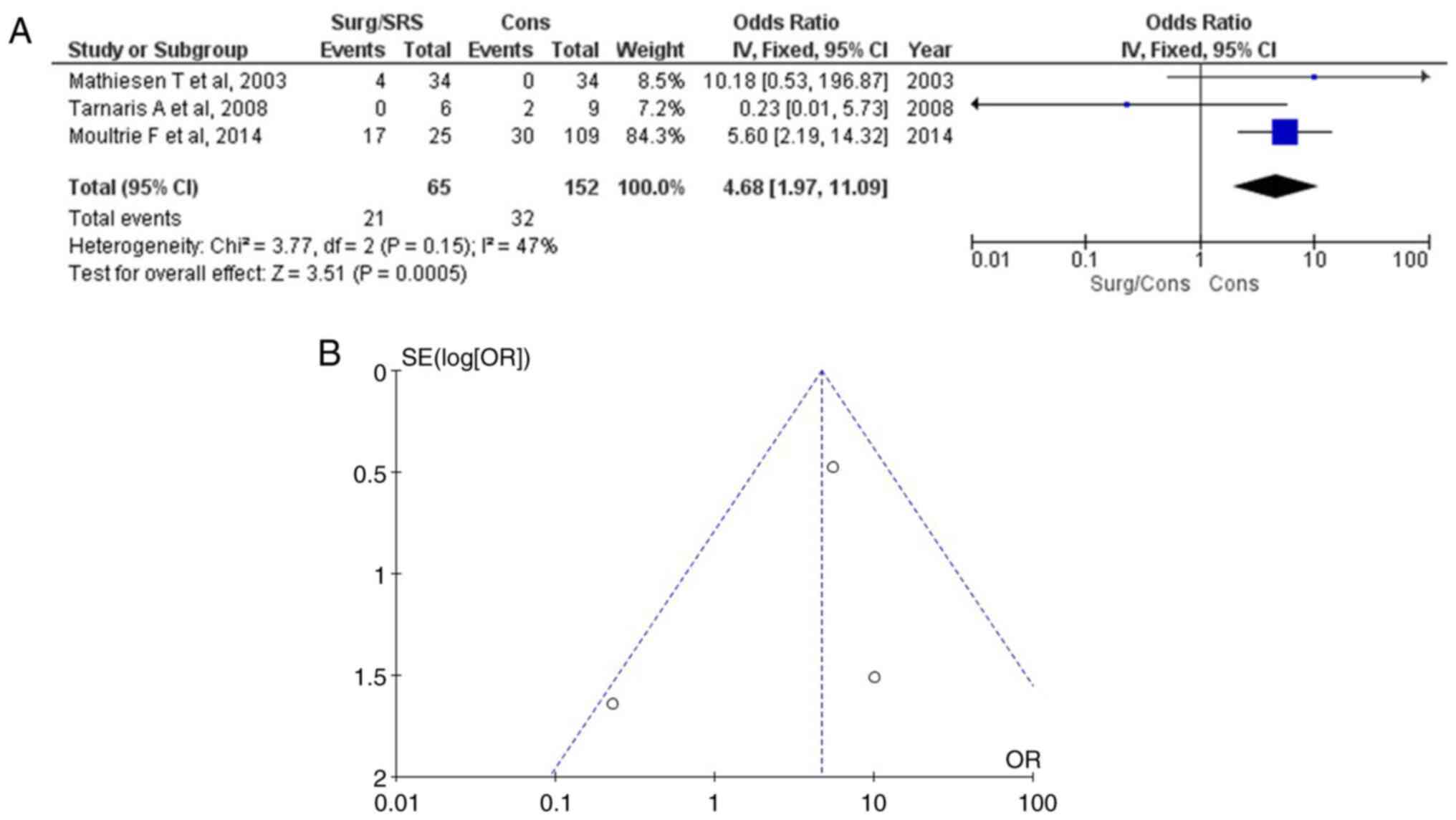
Mortality
Information regarding mortality was available in
three articles (15-19)
and demonstrated a statistically significant result between the
patients with Surg/SRS and the Cons groups (OR, 4.68; 95% CI, 1.97
to 11.09; and P<0.05) (Table
III and Fig. 8A). Mortality
was found in 21 of 65 (32.3%) patients in the Surg/SRS group and in
32 of 152 (21.1%) in the Cons group of patients. When examining the
funnel plot of the same parameter, it was found that the study
results had low heterogeneity (P=0.15 and I2=47%) and a
low publication bias (Fig. 8B).
Thus, the Cons (control) group exhibited superiority over the
Surg/SRS (experimental) group.
Discussion
The present study suggests that surgical or SRS
management may be a safe procedure as regards the outcomes of
patients with CMs compared with conservative treatment. More
precisely, neurological deficit was a statistically significant
parameter in these patients, exhibiting the superiority of surgery
and/or SRS over conservative management. Of note, the of
hemosiderin in the pre-MRI, the free of seizures parameter, and the
re-bleeding and neurological deficit parameters yielded
statistically significant results, predicting a better outcome in
the surgical or SRS group of patients. On the other hand, the
mortality rate was lower in the Cons group of patients compared
with the surgical or SRS treatment groups.
The treatment of CMs has been an ongoing topic of
debate due to the issues concerning their management. The major
difficulty with obtaining a clear perspective of their natural
history is determining when these injuries should be operated on.
In addition, published surgical series may ignore cases that never
hemorrhage and are only found on the follow-up for other reasons.
SRS is applied for the obliteration of the CMs, preventing any risk
of re-bleeding, and is an alternative to surgical treatment
(20,21). However, it appears that CMs
following SRS re-hemorrhage repeatedly and thus no benefit has been
observed (22,23). The present meta-analysis
demonstrated that the re-bleeding rate was a statistically
significant parameter in patients with CM who underwent surgical
or/and SRS management.
Based on the literature data, it appears evident
that the microsurgical management of CMs is the best option for
patients with epilepsy (1).
Nevertheless, there are no randomized studies evaluating
pharmaceutical and surgical treatment in patients with CMs and
epilepsy. In the present meta-analysis, the free of seizures
parameter yielded a statistically significant result, predicting a
better outcome in the surgical or SRS group of patients.
Researchers have asserted that patients with CMs
have an amplified risk of re-hemorrhage following an initial bleed
(21). Since a brain MRI is needed
to diagnose CMs without other pathological examinations, apart from
the use of hemosiderin in the MRI, intracranial hemorrhage in the
pre-MRI brain imaging may increase the risk of re-hemorrhage
(24). These risks may help to
determine whether to treat CMs with neurosurgical excision and/or
SRS or to opt for conservative management. In the present
meta-analysis, the use of hemosiderin in the pre-MRI was a
statistically significant parameter, predicting a better outcome in
the surgical or SRS group of patients than in patients in the Cons
group.
As regards outcomes due to surgical or/and
SRS-related morbidity or CM-related mortality, a poorer outcome in
surgically managed patients, such as that detailed in a previous
study (17), was also found in the
present meta-analysis. This may be explained by the complications
associated with CM excisions. In the present meta-analysis,
mortality was a statistically significant parameter, predicting a
better outcome in the Cons group of patients.
The present meta-analysis has certain limitations,
which should be mentioned. Half of the included studies were
retrospective, and a limited number of cases were presented,
particularly in the surgically treated group. In addition, there is
an argument suggesting that surgical outcome depends on the time of
the intervention, and some researchers have advocated for surgery
at 4 weeks after ictus (15,23).
Thus, the present meta-analysis did not include the time-dependent
intervention parameter.
In conclusion, the present meta-analysis proposes
that surgical or SRS management is a safe procedure as regards the
out outcomes of patients with CMs compared with conservative
treatment. More precisely, neurological deficit was statistically
significant parameter in these patients, exhibiting the superiority
of the surgical or/and SRS option over conservative management. Of
note, the use of hemosiderin in the pre-MRI, and the free of
seizures, re-bleeding and neurological deficit parameters yielded
statistically significant results, predicting a better outcome in
the surgical or SRS group of patients. On the other hand, the
mortality rate was lower in the Cons group of patients compared
with surgical or SRS treatment. Future studies are required to
examine more precisely the outcomes in the natural history cases
(conservative treatment), as the debate regarding the management of
CMs remains controversial.
Supplementary Material
(A) Forest plot for lobar (location).
The results demonstrated no statistically significant difference
between the surgical or/+ SRS and Cons (OR, 1.03; 95% CI, 0.55 to
1.93; P=0.07). (B) Funnel plot, testing the sensitivity of the
lobar (location); there was heterogeneity (P=0.92 and
I2=62%). In addition, after the applying ‘leave out one’
model, no statistically significant result was obtained (Table III). SRS, radiotherapy; Cons,
conservative management group; OR, odds ratio; I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval.
(A) Forest plot for deep (location).
The results demonstrated no statistically significant difference
between the surgical or/+ SRS and Cons (OR, 0.62; 95% CI, 0.27 to
1.43; P=0.87). (B) Funnel plot, testing the sensitivity of deep
(location); there was no heterogeneity (P=0.26 and I2=0
%). In addition, after the applying ‘leave out one’ model, no
statistically significant result was obtained (Table III). SRS, radiotherapy; Cons,
conservative management group; OR, odds ratio; I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval.
(A) Forest plot for brainstem
(location). The results demonstrated no statistically significant
difference between the surgical or/+ SRS and Cons (OR, 0.24; 95%
CI, 0.03 to 1.92; P=0.18). (B) Funnel plot, testing the sensitivity
of the brainstem (location): there was no heterogeneity. In
addition, after the applying ‘leave out one’ model, no
statistically significant result was obtained (Table III). SRS, radiotherapy; Cons,
that is due to heterogeneity rather than chance; CI, confidence
interval.
(A) Forest plot for cerebellum
(location). The results demonstrated no statistically significant
difference between the surgical or/+ SRS and Cons (OR, 1.29; 95%
CI, 0.39 to 4.32; P=0.68). (B) Funnel plot, testing the sensitivity
of the brainstem (location): there was no heterogeneity. In
addition, after the applying ‘leave out one’ model, no
statistically significant result was obtained (Table III). SRS, radiotherapy; Cons,
conservative management group; OR, odds ratio; I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval.
(A) Forest plot for OHS 2-6. The
results demonstrated no statistically significant difference
between the surgical or/+ SRS and Cons (OR, 1.44; 95% CI, 0.83 to
2.49; P=0.19). (B) Funnel plot, testing the sensitivity of the
brainstem (location): there was no heterogeneity. In addition,
after the applying ‘leave out one’ model, no statistically
significant result was obtained (Table III). OHS, Oxford Handicap Scale;
SRS, radiotherapy; Cons, conservative management group; OR, odds
ratio; I2, the percentage of total variation across
studies that is due to heterogeneity rather than chance; CI,
confidence interval.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF and VEG conceptualized the study. VEG, DAS, NT,
PS, PP, GF and KNF analyzed the data, and wrote and prepared the
draft of the manuscript. VEG and GF provided critical revisions.
All authors contributed to manuscript revision and have read and
approved the final version of the manuscript. GF and VEG confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Fernández S, Miró J, Falip M, Coello A,
Plans G, Castañer S and Acebes JJ: Surgical versus conservative
treatment in patients with cerebral cavernomas and non refractory
epilepsy. Seizure. 21:785–788. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Steiner L, Karlsson B, Yen CP, Torner JC,
Lindquist C and Schlesinger D: Radiosurgery in cavernous
malformations: Anatomy of a controversy. J Neurosurg. 113:16–22.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fotakopoulos G, Andrade-Barazarte H,
Kivelev J, Tjahjadi M, Goehre F and Hernesniemi J: Brainstem
cavernous malformations management: Microsurgery vs radiosurgery, a
meta-analysis. Front Surg. 8(630134)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fotakopoulos G, Kivelev J,
Andrade-Barazarte H, Tjahjadi M, Goehre F and Hernesniemi J:
Outcome in patients with spinal cavernomas presenting with symptoms
due to mass effect and/or hemorrhage: Conservative versus surgical
management: Meta-analysis of direct comparison of approach-related
complications. World Neurosurg. 152:6–18. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fotakopoulos G, Brotis AG and Fountas KN:
Dilemmas in managing coexisting arteriovenous and cavernous
malformations: Case report. Brain Circ. 8:45–49. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Robinson JR, Awad IA and Little JR:
Natural history of the cavernous angioma. J Neurosurg. 75:709–714.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sandalcioglu IE, Wiedemayer H, Secer S,
Asgari S and Stolke D: Surgical removal of brain stem cavernous
malformations: Surgical indications, technical considerations, and
results. J Neurol Neurosurg Psychiatry. 72:351–355. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gross BA, Batjer HH, Awad IA and Bendok
BR: Brainstem cavernous malformations. Neurosurgery. 64:E805–E818.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lippitz B: Treatment of cavernoma: An
evidence-based dilemma? Acta Neurochir Suppl. 116:99–101.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rinkel LA, Al-Shahi Salman R, Rinkel GJ
and Greving JP: Radiosurgical, neurosurgical, or no intervention
for cerebral cavernous malformations: A decision analysis. Int J
Stroke. 14:939–945. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos mM and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. Ottawa Hospital Research Institute, 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
|
|
12
|
Bamford JM, Sandercock PA, Warlow CP and
Slattery J: Interobserver agreement for the assessment of handicap
in stroke patients. Stroke. 20(828)1989.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Higgins JPT and Green S (eds): Cochrane
handbook for systematic reviews of interventions version 5.1.0. The
Cochrane Collaboration, 2011. www.cochrane-handbook.org. Updated March 2011.
|
|
15
|
Mathiesen T, Edner G and Kihlström L: Deep
and brainstem cavernomas: A consecutive 8-year series. J Neurosurg.
99:31–37. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tarnaris A, Fernandes RP and Kitchen ND:
Does conservative management for brain stem cavernomas have better
long-term outcome? Br J Neurosurg. 22:748–757. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moultrie F, Horne MA, Josephson CB, Hall
JM, Counsell CE, Bhattacharya JJ, Papanastassiou V, Sellar RJ,
Warlow CP, Murray GD and Al-Shahi Salman R: Scottish Audit of
Intracranial Vascular Malformations (SAIVMs) steering committee and
collaborators. Outcome after surgical or conservative management of
cerebral cavernous malformations. Neurology. 83:582–589.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dammann P, Wrede K, Jabbarli R, Neuschulte
S, Menzler K, Zhu Y, Özkan N, Müller O, Forsting M, Rosenow F and
Sure U: Outcome after conservative management or surgical treatment
for new-onset epilepsy in cerebral cavernous malformation. J
Neurosurg. 126:1303–1311. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang K, Ju Y, Wang D, Li H, Sun L, Ma K,
Zhao X and Lu J: Cerebral venous malformations in a chinese
population: Clinical manifestations, radiological characteristics,
and long-term prognosis. World Neurosurg. 120:e472–e479.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liscák R, Vladyka V, Simonová G, Vymazal J
and Novotny J Jr: Gamma knife surgery of brain cavernous
hemangiomas. J Neurosurg. 102 (Suppl):S207–S213. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kondziolka D, Lunsford LD, Flickinger JC
and Kestle JR: Reduction of hemorrhage risk after stereotactic
radiosurgery for cavernous malformations. J Neurosurg. 83:825–831.
1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Karlsson B, Kihlström L, Lindquist C,
Ericson K and Steiner L: Radiosurgery for cavernous malformations.
J Neurosurg. 88:293–297. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Steinberg GK, Chang SD, Gewirtz RJ and
Lopez JR: Microsurgical resection of brainstem, thalamic, and basal
ganglia angiographically occult vascular malformations.
Neurosurgery. 46:260–271. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Al-Shahi Salman R, Berg MJ, Morrison L and
Awad IA: Angioma Alliance Scientific Advisory Board. Hemorrhage
from cavernous malformations of the brain: Definition and reporting
standards. Angioma alliance scientific advisory board. Stroke.
39:3222–3230. 2008.PubMed/NCBI View Article : Google Scholar
|