The first oral vaccine was introduced in the 1960s,
and the oral polio vaccine (OPV) was the first oral vaccine proven
to be effective. The OPV remains widely used today in numerous
countries to prevent polio infections (1). Oral vaccines are administered via the
gastrointestinal mucosa for the delivery of antigens, and this
pathway generates a similar immune response to that of traditional
injectable immunizations (2).
However, the capacity of the gastrointestinal mucosa to induce
immunity through antigen presentation is limited (3). Furthermore, the high quantity of
antigen required for oral immunization poses a challenge to its
widespread use. Nevertheless, following advancements in medicine
and molecular biology, significant improvements in enhancing the
mucosal antigen presentation of oral vaccines have been made,
thereby improving immune responses and immune tolerance to the
antigens (4).
The aim of oral vaccines is to stimulate immune
responses in the mucosal tissues lining the gastrointestinal tract.
To achieve this, the antigens in oral vaccines undergo a series of
processes that involve their destruction and subsequent
presentation to the immune system (2). Upon ingestion, oral vaccines
encounter a number of challenges within the digestive system,
including exposure to low pH levels in the stomach and the presence
of various proteolytic enzymes in the gastrointestinal tract
(5). These hostile conditions pose
the risk of antigens being fully degraded before they reach the
target immune cells. To overcome these challenges, a number of
strategies have been devised to protect the antigens while ensuring
their destruction in a controlled manner. One common approach
involves encapsulating the antigen within specialized vehicles or
carriers, providing protection during transit through the digestive
system (6). These carriers are
typically composed of materials such as liposomes, microspheres or
protein-based nanoparticles. Additionally, oral vaccine antigens
have been engineered to be more resistant to degradation by
proteolytic enzymes. By modifying the structure or incorporating
stabilizing agents, vaccine antigens can withstand enzymatic
breakdown, to a certain extent, allowing them to reach the desired
sites of immune stimulation (7).
Once the antigen-carrier complex reaches the mucosal surfaces of
the intestine, it encounters specialized immune cells such as
dendritic cells. These cells possess mechanisms to capture, process
and present antigens to immune cells, effectively initiating an
immune response (8). The
differences between classical injectable vaccination and oral
vaccination are presented in Table
I.
Despite the number of challenges faced by oral
immunization, the existence of multiple licensed formulations
indicates that oral immunization is feasible. In the United States,
vaccines that target enteric pathogens such as rotavirus,
enterotoxigenic Escherichia coli, Vibrio cholerae and
Shigella (S. flexneri, S. sonnei, S. boydii
and S. dysenteriae) have been approved (9). These vaccines are also effective
against pathogens that enter the body through the intestinal
mucosa, leading to systemic diseases such as Salmonella enterica
serovar typhi and poliovirus (2). Rapid advancements in technology,
especially in relation to the global impact of COVID-19, have led
to significant developments in the field of vaccines. This paper
will provide a more up-to-date overview of the latest advancements
in vaccine development.
The immune response to oral immunization depends on
the dose of vaccine, the frequency of administration, the form of
spacer antigen and the metabolism of the individual. The degree of
immune response or immune tolerance differs in local and/or
systemic mucosa (10).
The immunogenicity of antigenic proteins
administered via oral mucosal inoculation alone is weak and is
typically enhanced by the addition of specific adjuvants or vectors
to achieve the desired effect. Different adjuvants, and even the
same adjuvant with modified subunits, elicit different immune
responses (11). Numerous
adjuvants and vectors possess molecular structures that are
recognized and targeted by innate immunity, such as
lipopolysaccharides, the acidic components of membranes, basic
peptidoglycan structures and non-methylated CpG structures
(12). These molecular structures
are recognized by gastrointestinal macrophages, dendritic cells and
Toll-like receptors, which facilitate antigen recognition,
presentation and subsequent T cell activation and differentiation
through a series of signal transductions and cytokine secretion
(13). The dendritic cell subtype
and the corresponding cytokines play a crucial role in determining
the proportion of subsequently activated CD4+ T cells
(14). When an antigen is
presented to the cell, T helper (Th)1 cells differentiate and
release cytokines such as interferon (IFN)-γ and tumor necrosis
factor (TNF)-α, to mediate cellular immunity and induce production
of the neutralizing antibody, IgG2a, by B cells (15). Antigens induce Th2 cell
differentiation and increase the secretion of IL-4, 5, 10 and 13,
which assist the production of neutralizing antibodies, IgE, IgA
and IgG (16). Liposomes, immune
stimulatory complexes, biodegradable microparticles and naked DNA
can present exogenous antigens via endogenous pathways to stimulate
the CD8+ T cell response and mediate cytotoxic immune
responses (17). The most
characteristic aspect of the immune response produced by oral
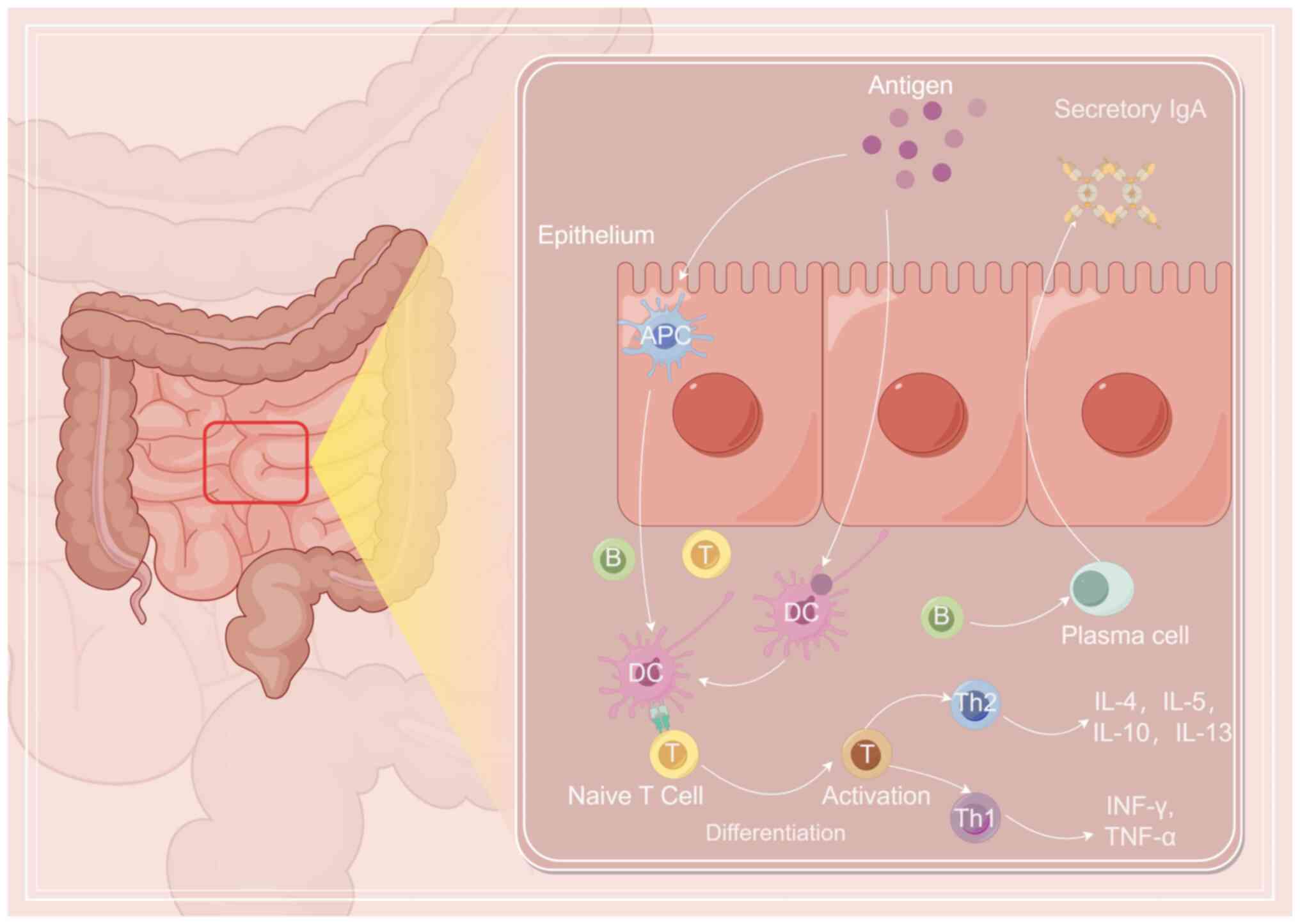
mucosal immunity is the production of secreted IgA (sIgA) (18). Following oral inoculation, the
antigen is taken up by M cells to activate dendritic cells and T
cell subsets, which then release a large number of cytokines and
chemokines (19). Expression of
major histocompatibility complex (MHC) class I and II antigens
eventually leads to the activation of B cells, specific integrin
expression and phenotypic conversion, especially to IgA (20). In addition to the local mucosa
where the antigen makes contact, the corresponding sIgA can also be
detected in the mucosal tissues of distant effector organs. The
migration of IgA-producing cells is associated with the
simultaneous expression of specific adhesion molecules in the
endothelium of these tissues. As aforementioned, both Th1 and Th2
cells, along with their cytokines, are involved in B cell
activation and sIgA production (21). However, studies suggest that
transcriptional growth factor (TGF)-β promotes the generation of
surface IgA+ B cells (22,23).
Fig. 1 shows the mechanism of oral
immunization (Fig. 1).
Oral immune tolerance is a process within the
mucosal immune response aimed at managing immunized antigens, which
operates through two primary mechanisms, namely clone inactivation
and active inhibition (24). A
previous study revealed that a solitary high dose of antigen can
trigger apoptosis of antigen-specific CD4+ T cells in
vivo (25). This apoptosis is
considered to be mediated by the p55 TNF receptor and is closely
associated with C-C motif chemokine ligand 2 (CCL2) and its
corresponding receptor, CCR2. By contrast, active inhibition arises
from repetitive stimulation by low-dose antigens. This induces the
activation of Th3 cells, which secrete TGF-β to initiate bystander
inhibition. The bystander suppression caused by TGF-β-secreting Th3
cells can broadly inhibit both cell-mediated and humoral immune
responses (26). However, it is
worth noting that TGF-β also promotes the generation of
IgA+ B cells, thereby reducing the production of other
antibodies while augmenting the synthesis and secretion of IgA
(27).
Not all oral tolerance processes exhibit elevated
levels of inhibitory factors. Several animal studies have
discovered that peripheral tolerance (such as the suppression of
delayed-type hypersensitivity) is accompanied by a notable increase
in IFN-γ levels, without any alteration in the inhibitory factor
(28,29). It is hypothesized that oral
antigens induce the expression of α4β7 and its interaction with
mucosal addressin cell adhesion molecule-1, which is expressed in
the intestinal epithelium, thus inducing the secretion of IFN-γ and
enhancing the local cellular immune response with the synergistic
effects of intestinal lumen bacteria (30). The interaction with bacteria in the
intestinal lumen synergistically enhances the immune response.
Simultaneously, the downregulation of α4β1 and p-selectin ligand
reduces the migration of memory T cells to peripheral tissues and
suppresses the peripheral immune response (31). Additionally, T lymphocyte
activation relies on antigen-presenting cells (APCs), and when
presenting antigens to T cells, APCs must express appropriate
co-stimulators. Quiescent APCs do not express the corresponding
co-stimulator or instead exhibit a very low expression level during
antigen presentation, thereby causing a loss of tolerance effect
and the auxiliary function of T cells (32). Regulatory and non-reactive cells
can directly mediate inhibition by producing inhibitory cytokines,
as well as indirectly through competition for growth factors,
MHC-peptide complexes or co-stimulatory molecules on APCs (33).
Mucosal tolerance is linked to the active function
of T cell subsets expressing γδ T cell receptor (TCR) in Peyer's
patch (PP) nodes and the epithelium of the small intestine. Mucosal
tolerance serves as a fundamental regulator of mucosal immune
tolerance and IgA production, which is primarily mediated by the
immune modulation of IL-4 and IL-10(34).
Immune tolerance can be induced by small doses of
antigen. T cells expressing γδTCR can inhibit the specific response
of traditional antigen-specific αβT cells, leading to an ‘immune
non-response’ to antigen stimulation. The immune tolerance induced
by ovalbumin (OVA) can be blocked by the anti-γδTCR monoclonal
antibody (35). Furthermore,
deficiency in γδT cells also results in downregulation of the
synthesis and activation of IgA+ B cells. However,
immune tolerance to OVA can still be induced in γδTCR-knockout
mice, suggesting that multiple factors are involved in the
development of oral immune tolerance (36).
The PP junction plays a crucial role in the immune
tolerance induced by oral proteins, while hapten tolerance is
primarily induced by the small intestinal epithelial barrier
(37). Additionally, the
generation of oral immune tolerance is closely associated with the
normal flora of the gastrointestinal tract (38). It is generally considered that the
failure of intestinal bacteria to induce oral tolerance is due to a
significant decrease in associated T lymphocytes in the PP
node.
COVID-19, caused by severe acute respiratory
syndrome coronavirus 2 (SARS-COV-2) (39), is a disease that has infected
nearly 780 million individuals and resulted in nearly 7 million
mortalities to date (2023) worldwide according to the World Health
Organization. Given the lack of effective medications, the
development and utilization of COVID-19 vaccines have become
crucial in controlling the COVID-19 disease outbreak (40). SARS-COV-2 is an enveloped, positive
sense, single-stranded RNA virus (41). The spike (S) protein (42) and the receptor binding domain
(43) of the S protein are the
primary targets for currently available COVID-19 vaccines. The
first two vaccines approved for clinical use were an inactivated
vaccine from China (Sinovac) and an mRNA-based vaccine from the USA
(Pfizer-BioNTech). As of December 2021, there were six different
vaccine platforms, including an inactivated virus vaccine, DNA and
mRNA vaccines, an adenovirus vector vaccine, a subunit vaccine, a
virus-like vaccine and a lentivirus vaccine. Moreover, >194
candidate vaccines have been approved for clinical trials worldwide
(44). However, due to the various
development platforms of COVID-19 vaccines, concerns regarding
safety, effectiveness and stability during transportation and
storage have arisen (45).
Although the safety of COVID-19 vaccines in phase III clinical
trials has been reported to be excellent, vaccine safety remains a
significant concern (46). For
example, live attenuated vaccines require replication within the
body and therefore entail the potential risk of virulent atavism
(47) or viral transmission
(48). DNA vaccines carry the risk
of oncogene activation (49) and
chromosome instability (50) due
to the integration of foreign DNA into the host genome. The
synthetic components and encapsulating materials utilized in mRNA
vaccine synthesis may exhibit toxicity and provoke apoptosis of
surrounding host cells (51).
According to the World Health Organization, a minimum protective
efficacy of 50% is required for the introduction of COVID-19
vaccines (52). Inactivated,
adenovirus vector and mRNA vaccines currently possess protective
efficacies of 79.34% (53), 62-90%
(54) and <90% (55), respectively, all meeting the
aforementioned requirements. Nonetheless, evaluating or comparing
the clinical efficacy of different vaccines for COVID-19 has proven
challenging due to variations in their clinical schemes.
Consequently, the effectiveness of vaccine protection still
necessitates extensive verification through subsequent large-scale
phase IV clinical trials.
The different types of injectable vaccines possess
their own merits and faults and, to the best of our knowledge,
different vaccines may have differences in effectiveness, safety
and suitability for different populations, so there is no one
vaccine that is considered to be universally optimal for all
situations.
Inactivated virus vaccines employ either heat or
chemical methods to render the virus obtained from culture inactive
(56). As such, inactivated
viruses lose their pathogenic virulence while retaining the primary
antigenic properties of the viral shell, thus stimulating a
specific immune response within the human body (57). The development process for an
inactivated virus vaccine is straightforward and does not require
any conceptual design or validation as it simply necessitates
finding the appropriate means to inactivate the virus, which
significantly enhances vaccine preparation time (58). However, inactivated vaccines can
lead to severe adverse reactions (59). For example, an inactivated vaccine
for respiratory syncytial virus was tested in clinical trials
during the mid-1960s, and it instead exacerbated disease
progression (60). Therefore,
despite achieving certain successes in clinical trials related to
the SARS virus, caution should still be exercised in the use of
inactivated virus vaccines for COVID-19.
In the design of live attenuated vaccines, a less
virulent strain is selected from the offspring and the process is
repeated until the pathogenicity of the strain is eliminated
(61). Live attenuated vaccines
provide stronger immunity and have a longer duration of action
compared with inactivated virus vaccines (62). However, there are certain
disadvantages to live attenuated vaccines. The screening process in
the early stages of development is time-consuming (63), making it challenging to develop
early products within a short timeframe.
Recombinant protein vaccines involve transferring
the gene sequence capable of expressing the viral surface antigen
into prokaryotes via genetic engineering (64). This method allows for large-scale
expression of the antigen protein. The recombinantly expressed
antigen protein is then extracted and purified for inoculation into
individuals. Recombinant protein vaccines have been extensively
utilized in clinical practice (65). For example, hepatitis B surface
antigen (HBsAg) is used in a commonly administered recombinant
protein vaccine for hepatitis B (66). One significant benefit of this
vaccine type is that the enriched or modified recombinant antigen
protein exhibits a high level of immunogenicity, and the production
process has reached a relatively advanced stage of development.
However, the development of recombinant protein vaccines is
hindered by factors such as the induction of non-specific immune
responses in the body (67).
Viral vector, DNA and mRNA vaccines share a similar
biological mechanism, as they all involve encoding the gene
sequence of the antigen protein in the human body (68). The utilization of host cells for
the production of viral antigen stimulates a specific immune
response. Both DNA and mRNA vaccines are primarily delivered
through non-biological methods, such as nanomaterial delivery
(69). However, the development of
viral vector vaccines is a complex process that involves not only
the screening of suitable antigens but also the selection of
appropriate vector viruses. Some studies have indicated that DNA
remains unmetabolized in the human body for up to 2 years (39,70).
The presence of foreign genetic information in the nucleus poses a
risk of integration into the host genome, which can result in
mutations and potentially cancer (71).
By contrast, mRNA is easily degraded, thus avoiding
issues related to gene recombination. However, certain patients in
mRNA vaccine clinical trials have experienced varying degrees of
adverse reactions, which may relate to a proinflammatory action of
the lipid nanoparticles used or the delivered mRNA (i.e., the
vaccine formulation), as well as to the unique nature, expression
pattern, binding profile and proinflammatory effects of the
produced antigens, S protein and/or its subunits/peptide fragments,
in human tissues or organs (72),
which hinder the widespread use of mRNA vaccines. In addition, when
considering the storage and transportation of COVID-19 vaccines,
mRNA vaccines are unstable and prone to degradation, necessitating
strict storage conditions (73).
The mRNA vaccine (Pfizer-BioNTech COVID-19 mRNA vaccine), jointly
developed by scientists in the USA and Germany, requires storage at
-70˚C, and once thawed the vaccine vials can only be stored at
2-8˚C for a maximum of 5 days. Additionally, there is another mRNA
vaccine (Moderna COVID-19 mRNA vaccine) that remains stable at
temperatures of 2-8˚C for a duration of 30 days. However, this
particular vaccine must be stored at -20˚C (73).
Oral vaccines provide a more feasible approach for
preventing contracting COVID-19. The mechanism of mucosal
absorption of oral vaccines has been comprehensively described in
previous studies (74-81).
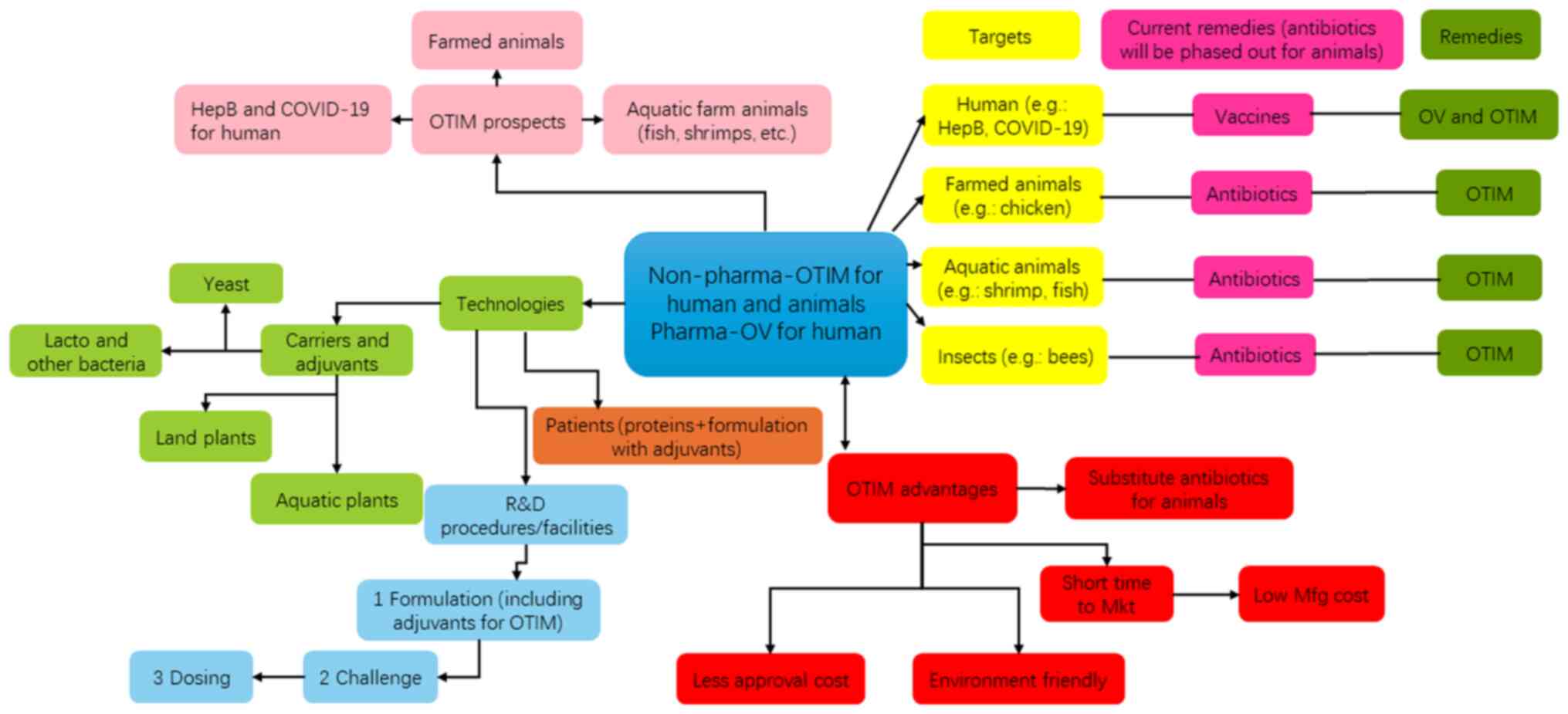
Fig. 2 shows the oral vaccine and
oral targeted immunomodulator platform. Oral vaccines have gained
significant attention in vaccine research and development in recent
years, as they offer several advantages over traditional systemic
vaccines. Oral vaccines primarily target gut-associated lymphoid
tissue (GALT) upon delivery into the gut. This strategy capitalizes
on the inherent immune properties of GALT, which play a crucial
role in the development and regulation of intestinal immune
responses (82). GALT is capable
of inducing strong and specific mucosal immune responses, including
sIgA, antibody-secreting cells and B and T cell memory cells
(83). These responses provide
protection at the mucosal surface and prevent the spread of
infectious material. In addition to targeting specific immune
responses, oral vaccines offer several advantages for individuals
with compromised immune systems. These benefits may include reduced
risk of infection, no need for medical personnel, non-invasive
administration and thermal stability (84). Unlike systemic vaccines, oral
vaccines do not affect the blood vessels or the circulatory system,
reducing the risk of adverse events (85).
The efficacy of oral vaccines is influenced by the
absorption of the vaccine by the gastrointestinal mucosa and the
efficiency of local mucosal APCs in presenting the antigen
(86). The immunogenicity of pure
antigenic proteins is significantly low. The key to a successful
oral vaccine lies in enhancing oral antigen presentation in the
gastrointestinal mucosa and inducing effective mucosal and systemic
immune responses. A well-designed adjuvant and antigen carrier
system can reduce the antigen dose required for inducing an optimal
immune response and immune tolerance (11). It is also important to note that,
in the development of oral vaccines, it is crucial to consider
their ability to withstand the various pH levels encountered
throughout the gastrointestinal tract and the presence of
proteolytic enzymes that can degrade antigen proteins (2). These challenges require a number of
strategies, such as the use of stabilizing agents and protective
encapsulation systems, to ensure the preservation of vaccine
integrity and its effective delivery to the immune system (87). Addressing these factors is
essential for the successful development of oral vaccines to elicit
robust immune responses (88).
The term ‘recombinant vaccine’ refers to the
purified antigenic protein or subunit that is produced in
vitro using recombinant technology. This process involves
removing the original virulence and infectivity of the pathogen
while retaining its immunogenicity (89). However, when these recombinant
antigens are administered alone, there are challenges involving
uptake via the mucosal route (90). Therefore, these antigens are often
combined with various adjuvants such as chitosan/aluminum, glucan
and squalene-based adjuvants or carriers (91). Recombinant vaccines against HBsAg
(92), tetanus toxin (93), diphtheria toxin (94) and pertussis toxin (95) have been developed and can be
produced on a large scale. Furthermore, research into the
development of oral forms of these recombinant vaccines is being
conducted.
The production of oral attenuated live vaccines
involves eradicating the virulence of pathogenic microorganisms,
while also exhibiting self-replication ability and natural adjuvant
activity (63). The effectiveness
of their adjuvant activity in preventing reinfection, generating
serum and mucosal immune responses and establishing long-lasting
immune memory is noteworthy (96).
However, further understanding and control of the toxicity of oral
attenuated live vaccines is imperative, as numerous studies are
currently only focused on animal experimentation (97,98).
Oral DNA vaccines are effective, but they require an
appropriate delivery system as naked DNA vaccines administered
orally are inefficient (2). To
enhance the efficacy of an oral DNA vaccine, recombinant herpes
simplex virus DNA can be used as a vector for Salmonella
typhimurium, allowing localization to both the mucosal and
systemic regions such as the spleen, ileal lymph nodes and PP node
(99). Oral vaccines are less
effective in eliciting humoral immune responses compared with
intramuscular injections, but they are more effective in inducing
local cell-mediated immune responses (100).
Microspheres are a type of biodegradable,
micron-structured material with a uniform particle size, and
include poly lactide-glycolide microspheres, polylactic acid
microspheres, polypropylene microspheres, starch microspheres and
alginate microspheres (106).
Most microspheres are natural and non-toxic, and have an adhesive
effect during the transportation of antigens. This effect helps
antigens pass through the intestinal mucosa epithelial cell layer.
When using microspheres as a carrier system, immune tolerance or
the immune response can be selectively induced through a single low
dose of antigen administered orally. Due to their small diameter,
microspheres can carry antigens and selectively deliver them to PP
nodes and the systemic lymphatic system (107). Microspheres also release antigens
slowly and in controlled amounts, resulting in a significant
reduction in the required antigen dose (108). The immune response triggered by
microspheres is closely related to their diameter, and as delivery
systems, they hold the promise of being comparatively safe
(109).
Significant advancements have also been made in the
study of viral agents as vectors. Particularly noteworthy
advancements include mucosal vaccines that utilize poliovirus and
adenovirus as live vectors. Research has demonstrated that a
poliovirus vector carrying an antigen can activate CD4+
T cells, thereby regulating the activity of IgA-associated B cells
and generating specific cytotoxic T lymphocytes (121). An oral vaccine for human
immunodeficiency virus (HIV) employs poliovirus as a carrier. The
poliovirus envelope gene is replaced with the pol and gag genes
from HIV, and the resulting recombinant virus expresses the P1
virus sheath protein (122). An
oral vaccine for measles utilizes a recombinant defective
adenovirus as a vector. This vector contains a mutated form of the
cytomegalovirus promoter that is missing a portion of the E1 region
and successfully induces a T cell immune response in mice through
expression of the measles virus H protein (123).
Oral vaccines present challenges in clinical trials.
Unlike injectable vaccines, which are typically administered in one
or multiple doses depending on the vaccine type, individual age,
weight and immune system, oral vaccines require additional testing
to study their tolerance to the acidic environment of the stomach
and their ability to remain intact and activate an immune response
during the digestive process (124).
The concept of producing oral vaccines using edible
plants was initially proposed by Dominic Lam and subsequently
implemented in the early 1990s (125). Plant-based oral vaccines refer to
vaccines that are generated from genetically modified plants; the
immunity provided by plant-based oral vaccines in human trials is
achieved by ingesting plant tissue containing the vaccine.
Plant-based vaccines have gained significant attention in the field
of biotechnology and notable advancements have been made in this
area. Thus far, the antigen genes expressed in transgenic plants
include hepatitis B virus surface antigen (74,5),
tuberculosis virus secretory protein, MPT64(126) and measles virus hemagglutinin
glycoprotein (79) gene. The
plants used include tobacco (127), potato (128), Arabidopsis (129), soybean (130), peanut (131), lettuce (132), carrot (133), tomato (134), white clover (135), alfalfa (136), corn (137), kelp (138) and lupine (139). Plant-based oral vaccines do not
require processing, purification or cryopreservation, making them
easy to use and promote.
Transgenic plants offer a novel platform for
developing recombinant proteins, with a number of advantages.
Through genetic modification, plants can be engineered to produce
proteins for pharmaceutical, industrial or agricultural use,
offering benefits such as low production costs, scalability and
increased safety (140).
Transgenic plants have been shown to be the most effective form of
oral vaccine due to their ability to facilitate easy
administration, reduce production and storage costs, and improve
accessibility, especially in areas with limited healthcare
infrastructure (141). As a
result, in addition to bacteria and viruses, plants have also been
successfully utilized to express and present vaccine antigens
(142). Transgenic tobacco,
potato, tomato and other plants have been found to be capable of
expressing various human pathogen antigens, including heat-labile
enterotoxin subunit B (LTB) subunit, hepatitis B surface antigen,
rotavirus and virus-like particles (143,144). These expressed antigens can
stimulate a specific immune response without the need for adjuvants
(11). Some transgenic plants can
also induce protective immune responses against certain allergens,
such as bacterial outer membrane vesicles from Pseudomonas
syringae and P. fluorescens activate plant immune
responses that protect against bacterial and oomycete pathogens
(145). However, the expression
level of antigens in transgenic plants is relatively low.
Furthermore, although they can enhance the immunogenicity of
presented antigens, transgenic plants also pose the risk of
compromising the body's tolerance to food such as inducing new
allergic reactions to foods that were previously non-allergenic, or
exacerbating existing allergies by increasing the immune system's
sensitivity to certain antigens found in foods. Therefore, further
research is needed to explore the development of plant-based oral
vaccines (146).
Due to their taste, lack of toxic ingredients and
high nutrient content, most vegetables are considered suitable for
use as receptors for plant-based oral vaccines. Among these
vegetables, potatoes have emerged as the primary plant model for
developing plant-based oral vaccines (147). However, since potatoes are not
edible in their raw state, they must be cooked before consumption,
which limits the applicability of this receptor. Tomatoes have also
emerged as a promising expression system and have successfully been
utilized for the transfer of various genes such as the hepatitis B
virus surface antigen gene, HIV gag and gp genes (148) and the rabies virus coat protein
(149). In addition, significant
advancements have been achieved in the tissue culture and genetic
transformation of carrots, making them another ideal candidate for
studying plant-based oral vaccines (150). For example, carrots have been
used to express the measles virus hemagglutinin, which exhibits
both antigenicity and immunogenicity. This engineered protein is
capable of stimulating a Th2 immune response (151), indicating its ability to activate
both humoral and cellular immunity. Additionally, the structural
protein, VP1, of the foot-and-mouth disease virus has been
effectively expressed in carrot leaves (152). Subsequent ELISA results indicate
that the expressed antigen exhibits specific and active binding to
the corresponding antibody (153). Therefore, the utilization of
genetically modified vegetables in the production of
orally-administered vaccines, particularly for COVID-19, holds
potential. Nevertheless, certain challenges were addressed in a
study (154). One such obstacle
is that most vegetables do not possess a high protein content,
which could impede the expression of antigens (112).
Research is currently being conducted to explore
fruits as a viable option for edible oral vaccines. For example,
vaccines synthesized in bananas using fruit-specific promoters
could be utilized for disease prevention through their consumption
(155). Papaya, a widely
available tropical and subtropical fruit, can be consumed in its
raw form (156). Thus, the papaya
transformation and regeneration system has been well-established,
and is currently regarded as an optimal candidate for oral vaccine
production (157). Transformation
of the Mycobacterium tuberculosis secreted protein, early
secreted antigenic target 6 kDa, in papaya is still undergoing
follow-up experiments (158). For
the production of oral vaccines, seed plants that contain
substantial amounts of soluble protein and that can maintain their
quality under storage conditions are typically regarded as more
appropriate candidates.. Cereals, such as corn and rice, are also
particularly well suited due to the abundance of soluble proteins
in the endosperm, which can be separated from the rest of the seed,
thereby increasing antigen concentration and reducing the required
dosage (159). Currently, antigen
gene expression has been successfully achieved in corn (160) and rice (161). In addition, with the
establishment of industrial algae production, research into the use
of transgenic algae as bioreactors for the production of exogenous
proteins has begun (162). At
present, the genetic transformation of algae has been successful in
Cyanobacteria (163) and
Arthrospira platensis (Spirulina) (164). Furthermore, hepatitis B virus
surface antigen has been successfully expressed in cyanobacteria
(165). Using algae as a
bioreactor to produce oral vaccines may solve a number of problems
such as high production costs, risk of contamination with human
pathogens, complex purification processes, and cold chain storage
and distribution requirements that are difficult to overcome with
other organisms.
In the development of future vaccines, promising
candidates for eliciting the necessary immune response and enabling
oral administration include Bacillus subtilis, yeast and
nanoparticles (166). These
innovative vaccine vectors offer unique advantages, such as their
inherent immunogenicity and ability to traverse the
gastrointestinal tract unharmed (53). B. subtilis, a versatile
bacterium, can deliver antigens effectively and stimulate both the
mucosal and systemic immune responses (167). Yeast-based vaccine platforms have
also demonstrated their potential in inducing strong humoral and
cellular immune responses (168).
Mucosal surfaces are the first line of defense against most
infectious diseases, and oral immunization can stimulate cellular
and humoral immune responses at both systemic and mucosal levels,
thereby inducing broad-spectrum and long-lasting immunity (169). However, successful oral vaccines
need to overcome the harsh gastrointestinal environment, including
extremely low pH, proteolytic enzymes, bile salts, low
permeability, and low immunogenicity (170).
Over recent years, innovative delivery systems
utilizing nanoparticles and microparticles have been meticulously
engineered to enhance the administration and efficacy of oral
vaccines. The incorporation of these particles into vaccine
formulations has been demonstrated to bolster antigen stability,
increase antigen availability and augment adjuvanticity.
Furthermore, they possess an enhanced capacity to stimulate the
immune system, ensure targeted delivery and facilitate controlled
release of the vaccine components (170). The use of these vaccine vectors
holds great promise for the future of oral vaccine development,
offering new avenues for achieving the desired immune response and
improving vaccine accessibility. Recombinant vaccines against
hepatitis B virus surface antigen (171), tetanus toxin (172), diphtheria toxin (173) and pertussis toxin (174) have been developed and can be
mass-produced. Live attenuated oral vaccines not only eliminate
pathogen toxicity as the carrier but also possess self-replication
ability and natural adjuvant activity. As such, they are highly
effective in preventing reinfection, establishing an immune
response between serum and the mucous membrane, and maintaining
lasting immune memory. Therefore, live attenuated vaccines hold
significant application value as oral vaccines. However, the
control of their toxicity requires further improvement, and a
number of studies are still in the animal experimentation stage
(175,176).
Research on oral vaccines in the past 20 years
suggests that this approach could be highly beneficial for mass
administration of vaccinations worldwide. The safety, efficacy,
convenience and cost-effectiveness of oral vaccines make them an
excellent option for disease prevention. We plan to further explore
this approach by developing oral vaccines for other human
infectious diseases such as hepatitis B, as well as for infectious
diseases in animals such as shrimp and chickens (Fig. 2).
Not applicable.
Funding: This study was provided financial support from the
following projects: Jie Bang Gua Shuai Project of Science &
Technology Department of Liaoning Province (grant no.
2022JH1/10800070), Postgraduate Education Teaching Research and
Reform Project of Jinzhou Medical University (grant no. YJ2023-018)
and Liaoning Province Science and Technology Program Joint Program
Fund Project (grant no. 2023-MSLH-059).
Not applicable.
YL, DL and ML were responsible for writing the
manuscript and investigation of the subject, such as conducting
literature reviews to understand the current state of research. WZ
and HA edited, conceptualized and supervised the study. All authors
have read and approved the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Plotkin S: History of vaccination. Proc
Natl Acad Sci USA. 111:12283–12287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vela Ramirez JE, Sharpe LA and Peppas NA:
Current state and challenges in developing oral vaccines. Adv Drug
Deliv Rev. 114:116–131. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pasetti MF, Simon JK, Sztein MB and Levine
MM: Immunology of gut mucosal vaccines. Immunol Rev. 239:125–148.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brisse M, Vrba SM, Kirk N, Liang Y and Ly
H: Emerging concepts and technologies in vaccine development. Front
Immunol. 11(583077)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Coffey JW, Gaiha GD and Traverso G: Oral
biologic delivery: advances toward oral subunit, DNA, and mRNA
vaccines and the potential for mass vaccination during pandemics.
Annu Rev Pharmacol Toxicol. 61:517–540. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang EY, Sarmadi M, Ying B, Jaklenec A and
Langer R: Recent advances in nano- and micro-scale carrier systems
for controlled delivery of vaccines. Biomaterials.
303(122345)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Serradell MC, Rupil LL, Martino RA, Prucca
CG, Carranza PG, Saura A, Fernández EA, Gargantini PR, Tenaglia AH,
Petiti JP, et al: Efficient oral vaccination by bioengineering
virus-like particles with protozoan surface proteins. Nat Commun.
10(361)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mann ER and Li X: Intestinal
antigen-presenting cells in mucosal immune homeostasis: Crosstalk
between dendritic cells, macrophages and B-cells. World J
Gastroenterol. 20:9653–9664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Seo H, Duan Q and Zhang W: Vaccines
against gastroenteritis, current progress and challenges. Gut
Microbes. 11:1486–1517. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zimmermann P and Curtis N: Factors that
influence the immune response to vaccination. Clin Microbiol Rev.
32(e00084)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Díaz-Dinamarca DA, Salazar ML, Castillo
BN, Manubens A, Vasquez AE, Salazar F and Becker MI: Protein-Based
adjuvants for vaccines as immunomodulators of the innate and
adaptive immune response: Current knowledge, challenges, and future
opportunities. Pharmaceutics. 14(1671)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y
and Tian X: Vaccine adjuvants: Mechanisms and platforms. Signal
Transduct Target Ther. 8(283)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ryan EJ, Daly LM and Mills KH:
Immunomodulators and delivery systems for vaccination by mucosal
routes. Trends Biotechnol. 19:293–304. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mayer L and Shao L: Therapeutic potential
of oral tolerance. Nat Rev Immunol. 4:407–419. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pishesha N, Harmand TJ and Ploegh HL: A
guide to antigen processing and presentation. Nat Rev Immunol.
22:751–764. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu J: T helper 2 (Th2) cell
differentiation, type 2 innate lymphoid cell (ILC2) development and
regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine.
75:14–24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mukherjee A, Bisht B, Dutta S and Paul MK:
Current advances in the use of exosomes, liposomes, and
bioengineered hybrid nanovesicles in cancer detection and therapy.
Acta Pharmacol Sin. 43:2759–2776. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Y, Jin L and Chen T: The effects of
secretory IgA in the mucosal immune system. Biomed Res Int.
2020(2032057)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hilligan KL and Ronchese F: Antigen
presentation by dendritic cells and their instruction of CD4+ T
helper cell responses. Cell Mol Immunol. 17:587–599.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen K and Cerutti A: Vaccination
strategies to promote mucosal antibody responses. Immunity.
33:479–491. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liew FY: TH1 and TH2 cells: A historical
perspective. Nat Rev Immunol. 2:55–60. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Spender LC, O'Brien DI, Simpson D, Dutt D,
Gregory CD, Allday MJ, Clark LJ and Inman GJ: TGF-beta induces
apoptosis in human B cells by transcriptional regulation of BIK and
BCL-XL. Cell Death Differ. 16:593–602. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Izikson L, Liu L and Weiner HL:
Activation of CD25(+)CD4(+) regulatory T cells by oral antigen
administration. J Immunol. 167:4245–4253. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pelaez-Prestel HF, Sanchez-Trincado JL,
Lafuente EM and Reche PA: Immune tolerance in the oral mucosa. Int
J Mol Sci. 22(12149)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Painter MM, Mathew D, Goel RR, Apostolidis
SA, Pattekar A, Kuthuru O, Baxter AE, Herati RS, Oldridge DA, Gouma
S, et al: Rapid induction of antigen-specific CD4(+) T cells is
associated with coordinated humoral and cellular immunity to
SARS-CoV-2 mRNA vaccination. Immunity. 54:2133–2142.e3.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weiner HL: Oral tolerance: Immune
mechanisms and the generation of Th3-type TGF-beta-secreting
regulatory cells. Microbes Infect. 3:947–954. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huai G, Markmann JF, Deng S and Rickert
CG: TGF-β-secreting regulatory B cells: Unsung players in immune
regulation. Clin Transl Immunology. 10(e1270)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang M, Zhai X, Li J, Guan J, Xu S, Li Y
and Zhu H: The role of cytokines in predicting the response and
adverse events related to immune checkpoint inhibitors. Front
Immunol. 12(670391)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Noh J, Noh G, Lee SJ, Lee JH, Kim A, Kim
HS and Choi WS: Tolerogenic effects of interferon-gamma with
induction of allergen-specific interleukin-10-producing regulatory
B cell (Br1) changes in non-IgE-mediated food allergy. Cell
Immunol. 273:140–149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
MacDonald TT and Monteleone G: IL-12 and
Th1 immune responses in human Peyer's patches. Trends Immunol.
22:244–247. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yoo JY, Groer M, Dutra SVO, Sarkar A and
McSkimming DI: Gut microbiota and immune system interactions.
Microorganisms. 8(1587)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ngo MC, Ando J, Leen AM, Ennamuri S,
Lapteva N, Vera JF, Min-Venditti A, Mims MP, Heslop HE, Bollard CM,
et al: Complementation of antigen-presenting cells to generate T
lymphocytes with broad target specificity. J Immunother.
37:193–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kenison JE, Stevens NA and Quintana FJ:
Therapeutic induction of antigen-specific immune tolerance. Nat Rev
Immunol: Dec 12, 2023 (Epub ahead of print).
|
|
36
|
Park JH and Lee HK: Function of γδ T cells
in tumor immunology and their application to cancer therapy. Exp
Mol Med. 53:318–327. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Odenwald MA and Turner JR: The intestinal
epithelial barrier: A therapeutic target? Nat Rev Gastroenterol
Hepatol. 14:9–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Maeda Y, Noda S, Tanaka K, Sawamura S,
Aiba Y, Ishikawa H, Hasegawa H, Kawabe N, Miyasaka M and Koga Y:
The failure of oral tolerance induction is functionally coupled to
the absence of T cells in Peyer's patches under germfree
conditions. Immunobiology. 204:442–457. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Deng S, Liang H, Chen P, Li Y, Li Z, Fan
S, Wu K, Li X, Chen W, Qin Y, et al: Viral vector vaccine
development and application during the COVID-19 Pandemic.
Microorganisms. 10(1450)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jeyanathan M, Afkhami S, Smaill F, Miller
MS, Lichty BD and Xing Z: Immunological considerations for COVID-19
vaccine strategies. Nat Rev Immunol. 20:615–632. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Alexandersen S, Chamings A and Bhatta TR:
SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are
not an indicator of active replication. Nat Commun.
11(6059)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sewell HF, Agius RM, Kendrick D and
Stewart M: Covid-19 vaccines: Delivering protective immunity. BMJ.
371(m4838)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu SC: Progress and Concept for COVID-19
vaccine development. Biotechnol J. 15(e2000147)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kudlay D and Svistunov A: COVID-19
vaccines: An overview of different platforms. Bioengineering
(Basel). 9(72)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Al-Jighefee HT, Najjar H, Ahmed MN, Qush
A, Awwad S and Kamareddine L: COVID-19 vaccine platforms:
Challenges and safety contemplations. Vaccines (Basel).
9(1196)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Su S, Du L and Jiang S: Learning from the
past: Development of safe and effective COVID-19 vaccines. Nat Rev
Microbiol. 19:211–219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhou F, Zhou J, Ma L, Song S, Zhang X, Li
W, Jiang S, Wang Y and Liao G: High-yield production of a stable
Vero cell-based vaccine candidate against the highly pathogenic
avian influenza virus H5N1. Biochem Biophys Res Commun.
421:850–854. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kamboj M and Sepkowitz KA: Risk of
transmission associated with live attenuated vaccines given to
healthy persons caring for or residing with an immunocompromised
patient. Infect Control Hosp Epidemiol. 28:702–707. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Smahel M, Síma P, Ludvíková V and Vonka V:
Modified HPV16 E7 Genes as DNA Vaccine against E7-Containing
oncogenic cells. Virology. 281:231–238. 2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Williams JA: Vector design for improved
DNA vaccine efficacy, safety and production. Vaccines (Basel).
1:225–249. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pardi N, Hogan MJ, Porter FW and Weissman
D: mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov.
17:261–279. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hodgson SH, Mansatta K, Mallett G, Harris
V, Emary KRW and Pollard AJ: What defines an efficacious COVID-19
vaccine? A review of the challenges assessing the clinical efficacy
of vaccines against SARS-CoV-2. Lancet Infect Dis. 21:e26–e35.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
He Q, Mao Q, Zhang J, Bian L, Gao F, Wang
J, Xu M and Liang Z: COVID-19 Vaccines: Current understanding on
immunogenicity, safety, and further considerations. Front Immunol.
12(669339)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hwang JK, Zhang T, Wang AZ and Li Z:
COVID-19 vaccines for patients with cancer: Benefits likely
outweigh risks. J Hematol Oncol. 14(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bernal JL, Andrews N, Gower C, Stowe J,
Robertson C, Tessier E, Simmons R, Cottrel S, Robertson R,
O'Doherty M, et al: Early effectiveness of COVID-19 vaccination
with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on
symptomatic disease, hospitalisations and mortality in older adults
in England. medRxiv: 2021.2003.2001.21252652, 2021.
|
|
56
|
Patterson EI, Prince T, Anderson ER,
Casas-Sanchez A, Smith SL, Cansado-Utrilla C, Solomon T, Griffiths
MJ, Acosta-Serrano Á, Turtle L and Hughes GL: Methods of
inactivation of SARS-CoV-2 for downstream biological assays. J
Infect Dis. 222:1462–1467. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Burrell CJ: Pathogenesis of Virus
Infections. Fenner and White's Medical Virology. 2017:77-104, 2017.
doi: 10.1016/B978-0-12-375156-0.00007-2. (Epub 2016 Nov 11).
|
|
58
|
Pavel STI, Yetiskin H, Uygut MA, Aslan AF,
Aydın G, İnan Ö, Kaplan B and Ozdarendeli A: Development of an
inactivated vaccine against SARS CoV-2. Vaccines (Basel).
9(1266)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kouhpayeh H and Ansari H: Adverse events
following COVID-19 vaccination: A systematic review and
meta-analysis. Int Immunopharmacol. 109(108906)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wright PF, Gruber WC, Peters M, Reed G,
Zhu Y, Robinson F, Coleman-Dockery S and Graham BS: Illness
severity, viral shedding, and antibody responses in infants
hospitalized with bronchiolitis caused by respiratory syncytial
virus. J Infect Dis. 185:1011–1018. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
61
|
Minor PD: Live attenuated vaccines:
Historical successes and current challenges. Virology.
479-480:379–392. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bournazos S and Ravetch JV: Attenuated
vaccines for augmented immunity. Cell Host Microbe. 21:314–315.
2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lauring AS, Jones JO and Andino R:
Rationalizing the development of live attenuated virus vaccines.
Nat Biotechnol. 28:573–579. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
De Berardinis P and Haigwood NL: New
recombinant vaccines based on the use of prokaryotic
antigen-display systems. Expert Rev Vaccines. 3:673–679.
2004.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pollet J, Chen WH and Strych U:
Recombinant protein vaccines, a proven approach against coronavirus
pandemics. Adv Drug Deliv Rev. 170:71–82. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Clark JR, Bartley K, Jepson CD, Craik V
and March JB: Comparison of a bacteriophage-delivered DNA vaccine
and a commercially available recombinant protein vaccine against
hepatitis B. FEMS Immunol Med Microbiol. 61:197–204.
2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Mosaddeghi P, Shahabinezhad F, Dorvash M,
Goodarzi M and Negahdaripour M: Harnessing the non-specific
immunogenic effects of available vaccines to combat COVID-19. Hum
Vaccin Immunother. 17:1650–1661. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Schlake T, Thess A, Fotin-Mleczek M and
Kallen KJ: Developing mRNA-vaccine technologies. RNA Biol.
9:1319–1330. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nanomedicine and the COVID-19 vaccines.
Nat Nanotechnol. 15(963)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Travieso T, Li J, Mahesh S, Mello JDFRE
and Blasi M: The use of viral vectors in vaccine development. NPJ
Vaccines. 7(75)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Becker PD, Noerder M and Guzmán CA:
Genetic immunization: Bacteria as DNA vaccine delivery vehicles.
Hum Vaccin. 4:189–202. 2008.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Trougakos IP, Terpos E, Alexopoulos H,
Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E and
Dimopoulos MA: Adverse effects of COVID-19 mRNA vaccines: The spike
hypothesis. Trends Mol Med. 28:542–554. 2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Uddin MN and Roni MA: Challenges of
storage and stability of mRNA-Based COVID-19 Vaccines. Vaccines
(Basel). 9(1033)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Mason HS, Lam DM and Arntzen CJ:
Expression of hepatitis B surface antigen in transgenic plants.
Proc Natl Acad Sci USA. 89:11745–11749. 1992.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lou XM, Yao QH, Zhang Z, Peng RH, Xiong AS
and Wang HK: Expression of the human hepatitis B virus large
surface antigen gene in transgenic tomato plants. Clin Vaccine
Immunol. 14:464–469. 2007.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Lei H, Xu Y, Chen J, Wei X and Lam DM-K:
Immunoprotection against influenza H5N1 virus by oral
administration of enteric-coated recombinant Lactococcus lactis
mini-capsules. Virology. 407:319–324. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Monreal-Escalante E, Ramos-Vega A, Angulo
C and Bañuelos-Hernández B: Plant-Based vaccines: Antigen design,
diversity, and strategies for high level production. Vaccines
(Basel). 10(100)2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kurup VM and Thomas J: Edible vaccines:
Promises and challenges. Mol Biotechnol. 62:79–90. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Lam J, Lam FW, Lam YO and Lam DM: Oral
immunization and edible vaccines: a viable option or mirage?
Biotechnology in Hong Kong. II:201–213. 2015.
|
|
80
|
De Smet R, Allais L and Cuvelier CA:
Recent advances in oral vaccine development: Yeast-derived β-glucan
particles. Hum Vaccin Immunother. 10:1309–1318. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Sung JC, Liu Y, Wu KC, Choi MC, Ma CH, Lin
J, He EIC, Leung DY, Sze ET, Hamied YK, et al: Expression of
SARS-CoV-2 spike protein receptor binding domain on recombinant B.
subtilis on spore surface: A potential COVID-19 oral vaccine
candidate. Vaccines (Basel). 10(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kunisawa J, Kurashima Y and Kiyono H:
Gut-associated lymphoid tissues for the development of oral
vaccines. Adv Drug Deliv Rev. 64:523–530. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Mörbe UM, Jørgensen PB, Fenton TM, von
Burg N, Riis LB, Spencer J and Agace WW: Human gut-associated
lymphoid tissues (GALT); diversity, structure, and function.
Mucosal Immunol. 14:793–802. 2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Van der Weken H, Cox E and Devriendt B:
Advances in oral subunit vaccine design. Vaccines (Basel).
9(1)2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kim Y, Kang J, Lee SG and Kim GT: COVID-19
vaccination-related small vessel vasculitis with multiorgan
involvement. Z Rheumatol. 81:509–512. 2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Huang M, Zhang M, Zhu H, Du X and Wang J:
Mucosal vaccine delivery: A focus on the breakthrough of specific
barriers. Acta Pharm Sin B. 12:3456–3474. 2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wen H, Jung H and Li X: Drug delivery
approaches in addressing clinical pharmacology-related issues:
Opportunities and challenges. AAPS J. 17:1327–1340. 2015.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Bachmann MF and Jennings GT: Vaccine
delivery: A matter of size, geometry, kinetics and molecular
patterns. Nat Rev Immunol. 10:787–796. 2010.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Nascimento IP and Leite LC: Recombinant
vaccines and the development of new vaccine strategies. Braz J Med
Biol Res. 45:1102–1111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
90
|
de Oliveira NR, Santos FDS, Dos Santos
VAC, Maia MAC, Oliveira TL and Dellagostin OA: Challenges and
strategies for developing recombinant vaccines against
leptospirosis: Role of expression platforms and adjuvants in
achieving protective efficacy. Pathogens. 12(787)2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Gong X, Gao Y, Shu J, Zhang C and Zhao K:
Chitosan-Based nanomaterial as immune adjuvant and delivery carrier
for vaccines. Vaccines (Basel). 10(1906)2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhao H, Zhou X and Zhou YH: Hepatitis B
vaccine development and implementation. Hum Vaccin Immunother.
16:1533–1544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Möller J, Kraner ME and Burkovski A: More
than a Toxin: Protein inventory of clostridium tetani toxoid
vaccines. Proteomes. 7(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Abulmagd S, Khattab AEA and Zedan H:
Expression of full and fragment-B of diphtheria toxin genes in
Escherichia coli for generating of recombinant diphtheria vaccines.
Clin Exp Vaccine Res. 11:12–29. 2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Chokephaibulkit K, Puthanakit T, Bhat N,
Mansouri S, Tang Y, Lapphra K, Rungmaitree S, Anugulruengkitt S,
Jantarabenjakul W, Andi-Lolo I, et al: A phase 2 randomized
controlled dose-ranging trial of recombinant pertussis booster
vaccines containing genetically inactivated pertussis toxin in
women of childbearing age. Vaccine. 40:2352–2361. 2022.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Pulendran B, S Arunachalam P and O'Hagan
DT: Emerging concepts in the science of vaccine adjuvants. Nat Rev
Drug Discov. 20:454–475. 2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Head JR, Vos A, Blanton J, Müller T,
Chipman R, Pieracci EG, Cleaton J and Wallace R: Environmental
distribution of certain modified live-virus vaccines with a high
safety profile presents a low-risk, high-reward to control zoonotic
diseases. Sci Rep. 9(6783)2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Radhakrishnan A, Vaseeharan B, Ramasamy P
and Jeyachandran S: Oral vaccination for sustainable disease
prevention in aquaculture-an encapsulation approach. Aquac Int.
31:867–891. 2023.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Karem KL, Bowen J, Kuklin N and Rouse BT:
Protective immunity against herpes simplex virus (HSV) type 1
following oral administration of recombinant Salmonella typhimurium
vaccine strains expressing HSV antigens. J Gen Virol. 78:427–434.
1997.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Mouro V and Fischer A: Dealing with a
mucosal viral pandemic: Lessons from COVID-19 vaccines. Mucosal
Immunol. 15:584–594. 2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Freytag LC and Clements JD: Mucosal
adjuvants. Vaccine. 23:1804–1813. 2005.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Verma SK, Mahajan P, Singh NK, Gupta A,
Aggarwal R, Rappuoli R and Johri AK: New-age vaccine adjuvants,
their development, and future perspective. Front Immunol.
14(1043109)2023.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Clements JD and Norton EB: The Mucosal
Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere. 3:e00215–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Kawamura YI, Kawashima R, Shirai Y, Kato
R, Hamabata T, Yamamoto M, Furukawa K, Fujihashi K, McGhee JR,
Hayashi H and Dohi T: Cholera toxin activates dendritic cells
through dependence on GM1-ganglioside which is mediated by
NF-kappaB translocation. Eur J Immunol. 33:3205–3212.
2003.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Heim JB, Hodnik V, Heggelund JE, Anderluh
G and Krengel U: Crystal structures of cholera toxin in complex
with fucosylated receptors point to importance of secondary binding
site. Sci Rep. 9(12243)2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Delafresnaye L, Feist F, Hooker JP and
Barner-Kowollik C: Microspheres from light-a sustainable materials
platform. Nat Commun. 13(5132)2022.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Welling MM, Duszenko N, van Meerbeek MP,
Molenaar TJM, Buckle T, van Leeuwen FWB and Rietbergen DDD:
Microspheres as a carrier system for therapeutic embolization
procedures: Achievements and advances. J Clin Med.
12(918)2023.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Hanes J, Cleland JL and Langer R: New
advances in microsphere-based single-dose vaccines. Adv Drug Deliv
Rev. 28:97–119. 1997.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Matsunaga Y, Wakatsuki Y, Tabata Y,
Kawasaki H, Usui T, Yoshida M, Itoh T, Habu S and Kita T: Oral
immunization with size-purified microsphere beads as a vehicle
selectively induces systemic tolerance and sensitization. Vaccine.
19:579–588. 2000.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Saleh S, Van Puyvelde S, Staes A,
Timmerman E, Barbé B, Jacobs J, Gevaert K and Deborggraeve S:
Salmonella Typhi, Paratyphi A, Enteritidis and Typhimurium core
proteomes reveal differentially expressed proteins linked to the
cell surface and pathogenicity. PLoS Negl Trop Dis.
13(e0007416)2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Galen JE, Pasetti MF, Tennant S,
Ruiz-Olvera P, Sztein MB and Levine MM: Salmonella enterica serovar
Typhi live vector vaccines finally come of age. Immunol Cell Biol.
87:400–412. 2009.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Rogers AWL, Tsolis RM and Bäumler AJ:
Salmonella versus the Microbiome. Microbiol Mol Biol Rev.
85(e00027)2021.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Sirard JC, Niedergang F and Kraehenbuhl
JP: Live attenuated Salmonella: A paradigm of mucosal vaccines.
Immunol Rev. 171:5–26. 1999.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Howlader DR, Koley H, Sinha R, Maiti S,
Bhaumik U, Mukherjee P and Dutta S: Development of a novel S. Typhi
and Paratyphi A outer membrane vesicles based bivalent vaccine
against enteric fever. PLoS One. 13(e0203631)2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Dempsey E and Corr SC: Lactobacillus spp.
for gastrointestinal health: Current and future perspectives. Front
Immunol. 13(840245)2022.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Li F, Wang X, Ma R, Wu W, Teng F, Cheng X,
Jiang Y, Zhou H, Wang L, Tang L, et al: Oral immunization with
lactobacillus casei expressing the porcine circovirus type 2 Cap
and LTB induces mucosal and systemic antibody responses in mice.
Viruses. 13(1302)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Shaw DM, Gaerthé B, Leer RJ, Van Der Stap
JG, Smittenaar C, Heijne Den Bak-Glashouwer M, Thole JE, Tielen FJ,
Pouwels PH and Havenith CE: Engineering the microflora to vaccinate
the mucosa: Serum immunoglobulin G responses and activated draining
cervical lymph nodes following mucosal application of tetanus toxin
fragment C-expressing lactobacilli. Immunology. 100:510–518.
2000.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Betancor M, Moreno-Martínez L, López-Pérez
Ó, Otero A, Hernaiz A, Barrio T, Badiola JJ, Osta R, Bolea R and
Martín-Burriel I: Therapeutic Assay with the Non-toxic C-Terminal
fragment of tetanus toxin (TTC) in transgenic murine models of
prion disease. Mol Neurobiol. 58:5312–5326. 2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Mathiesen G, Øverland L, Kuczkowska K and
Eijsink VGH: Anchoring of heterologous proteins in multiple
Lactobacillus species using anchors derived from Lactobacillus
plantarum. Sci Rep. 10(9640)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Ding C, Ma J, Dong Q and Liu Q: Live
bacterial vaccine vector and delivery strategies of heterologous
antigen: A review. Immunol Lett. 197:70–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Yun SO, Shin HY, Kang CY and Kang HJ:
Generation of antigen-specific cytotoxic T lymphocytes with
activated B cells. Cytotherapy. 19:119–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Porter DC, Ansardi DC and Morrow CD:
Encapsidation of poliovirus replicons encoding the complete human
immunodeficiency virus type 1 gag gene by using a complementation
system which provides the P1 capsid protein in trans. J Virol.
69:1548–1555. 1995.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Sharpe S, Fooks A, Lee J, Hayes K, Clegg C
and Cranage M: Single oral immunization with replication deficient
recombinant adenovirus elicits long-lived transgene-specific
cellular and humoral immune responses. Virology. 293:210–216.
2002.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Kwong KW, Xin Y, Lai NC, Sung JC, Wu KC,
Hamied YK, Sze ET and Lam DM: Oral vaccines: A better future of
immunization. Vaccines. 11(1232)2023.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Langridge WH: Edible Vaccines. Sci Am.
283:66–71. 2000.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Zhang Y, Chen S, Li J, Liu Y, Hu Y and Cai
H: Oral immunogenicity of potato-derived antigens to Mycobacterium
tuberculosis in mice. Acta Biochim Biophys Sin (Shanghai).
44:823–830. 2012.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Wen SX, Teel LD, Judge NA and O'Brien AD:
A plant-based oral vaccine to protect against systemic intoxication
by Shiga toxin type 2. Proc Natl Acad Sci USA. 103:7082–7087.
2006.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Arakawa T, Chong DK and Langridge WH:
Efficacy of a food plant-based oral cholera toxin B subunit
vaccine. Nat Biotechnol. 16:292–297. 1998.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Greco R, Michel M, Guetard D,
Cervantes-Gonzalez M, Pelucchi N, Wain-Hobson S, Sala F and Sala M:
Production of recombinant HIV-1/HBV virus-like particles in
Nicotiana tabacum and Arabidopsis thaliana plants for a bivalent
plant-based vaccine. Vaccine. 25:8228–8240. 2007.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Mehrizadeh V, Dorani E, Mohammadi SA and
Ghareyazie B: Expression of recombinant human IFN-γ protein in
soybean (Glycine max L.). Plant Cell Tiss Organ Cult. 146:127–136.
2021.
|
|
131
|
Ren C, Zhang Q, Wang G, Ai C, Hu M, Liu X,
Tian F, Zhao J, Chen Y, Wang M, et al: Modulation of peanut-induced
allergic immune responses by oral lactic acid bacteria-based
vaccines in mice. Appl Microbiol Biotechnol. 98:6353–6364.
2014.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Joh LD, Wroblewski T, Ewing NN and
VanderGheynst JS: High-level transient expression of recombinant
protein in lettuce. Biotechnol Bioeng. 91:861–871. 2005.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Luchakivskaya Y, Kishchenko O, Gerasymenko
I, Olevinskaya Z, Simonenko Y, Spivak M and Kuchuk M: High-level
expression of human interferon alpha-2b in transgenic carrot
(Daucus carota L.) plants. Plant Cell Rep. 30:407–415.
2011.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Beihaghi M, Marashi H, Bagheri A and
Sankian M: Transient expression of CCL21as recombinant protein in
tomato. Biotechnol Rep (Amst). 17:10–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Lee RW, Strommer J, Hodgins D, Shewen PE,
Niu Y and Lo RY: Towards development of an edible vaccine against
bovine pneumonic pasteurellosis using transgenic white clover
expressing a Mannheimia haemolytica A1 leukotoxin 50 fusion
protein. Infect Immun. 69:5786–5793. 2001.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Peréz Aguirreburualde MS, Gómez MC,
Ostachuk A, Wolman F, Albanesi G, Pecora A, Odeon A, Ardila F,
Escribano JM, Dus Santos MJ and Wigdorovitz A: Efficacy of a BVDV
subunit vaccine produced in alfalfa transgenic plants. Vet Immunol
Immunopathol. 151:315–324. 2013.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Streatfield SJ, Lane JR, Brooks CA, Barker
DK, Poage ML, Mayor JM, Lamphear BJ, Drees CF, Jilka JM, Hood EE
and Howard JA: Corn as a production system for human and animal
vaccines. Vaccine. 21:812–815. 2003.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Wee S and Gombotz WR: Protein release from
alginate matrices. Adv Drug Deliv Rev. 31:267–285. 1998.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Ma Y, Lin SQ, Gao Y, Li M, Luo WX, Zhang J
and Xia NS: Expression of ORF2 partial gene of hepatitis E virus in
tomatoes and immunoactivity of expression products. World J
Gastroenterol. 9:2211–2215. 2003.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Eidenberger L, Kogelmann B and
Steinkellner H: Plant-based biopharmaceutical engineering. Nat Rev
Bioeng. 1:426–439. 2023.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Ortega-Berlanga B and Pniewski T:
Plant-Based vaccines in combat against coronavirus diseases.
Vaccines (Basel). 10(138)2022.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Smart V, Foster PS, Rothenberg ME, Higgins
TJ and Hogan SP: A plant-based allergy vaccine suppresses
experimental asthma via an IFN-gamma and CD4+CD45RBlow T
cell-dependent mechanism. Immunol. 171:2116–2126. 2003.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Guan ZJ, Guo B, Huo YL, Guan ZP and Wei
YH: Overview of expression of hepatitis B surface antigen in
transgenic plants. Vaccine. 28:7351–7362. 2010.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Jin S, Wang T, Zhao Y, Liu X, Wang Y,
Jiang L and Zhang Q: The heat-labile toxin B subunit of E. coli
fused with VP6 from GCRV (Grass carp reovirus) was expressed and
folded into an active protein in rice calli. Protein Expr Purif.
197(106099)2022.PubMed/NCBI View Article : Google Scholar
|
|
145
|
McMillan HM, Zebell SG, Ristaino JB, Dong
X and Kuehn MJ: Protective plant immune responses are elicited by
bacterial outer membrane vesicles. Cell Rep.
34(108645)2021.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Lee J, Woodruff MC, Kim EH and Nam JH:
Knife's edge: Balancing immunogenicity and reactogenicity in mRNA
vaccines. Exp Mol Med. 55:1305–1313. 2023.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Jan N, Shafi F, Hameed Ob, Muzaffar K, Dar
SM, Majid I and Na N: An Overview on Edible Vaccines and
Immunization. Austin J Nutri Food Sci. 4(1078)2016.
|
|
148
|
Zhang X, Buehner NA, Hutson AM, Estes MK
and Mason HS: Tomato is a highly effective vehicle for expression
and oral immunization with Norwalk virus capsid protein. Plant
Biotechnol J. 4:419–432. 2006.PubMed/NCBI View Article : Google Scholar
|
|
149
|
McGarvey PB, Hammond J, Dienelt MM, Hooper
DC, Fu ZF, Dietzschold B, Koprowski H and Michaels FH: Expression
of the rabies virus glycoprotein in transgenic tomatoes.
Biotechnology (N Y). 13:1484–1487. 1995.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Jain A, Saini V and Kohli DV: Edible
transgenic plant vaccines for different diseases. Curr Pharm
Biotechnol. 14:594–614. 2013.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Chan HT and Daniell H: Plant-made oral
vaccines against human infectious diseases-Are we there yet? Plant
Biotechnol J. 13:1056–1070. 2015.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Rao JP, Agrawal P, Mohammad R, Rao SK,
Reddy GR, Dechamma HJ and S Suryanarayana VV: Expression of VP1
protein of serotype A and O of foot-and-mouth disease virus in
transgenic sunnhemp plants and its immunogenicity for guinea pigs.
Acta Virol. 56:91–99. 2012.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Han L, An C, Liu D, Wang Z, Bian L, He Q,
Liu J, Wang Q, Liu M, Mao Q, et al: Development of an ELISA Assay
for the Determination of SARS-CoV-2 protein subunit vaccine antigen
content. Viruses. 15(62)2022.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Khalid F, Tahir R, Ellahi M, Amir N, Rizvi
SFA and Hasnain A: Emerging trends of edible vaccine therapy for
combating human diseases especially COVID-19: Pros, cons, and
future challenges. Phytother Res. 36:2746–2766. 2022.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Sharma M and Sood B: A banana or a
syringe: Journey to edible vaccines. World J Microbiol Biotechnol.
27:471–477. 2011.
|
|
156
|
Surridge C: Oral vaccines: Papaya salad.
Nat Plants. 3(17034)2017.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Azad MA, Rabbani MG, Amin L and Sidik NM:
Development of transgenic papaya through agrobacterium-mediated
transformation. Int J Genomics. 2013(235487)2013.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Thach PN and Hoi NT: Result of
homogenization of sputum with papaya for faster detection of
Mycobacterium tuberculosis. Probl Tuberk. 37(85)1959.PubMed/NCBI(In Russian).
|
|
159
|
Stöger E, Vaquero C, Torres E, Sack M,
Nicholson L, Drossard J, Williams S, Keen D, Perrin Y, Christou P
and Fischer R: Cereal crops as viable production and storage
systems for pharmaceutical scFv antibodies. Plant Mol Biol.
42:583–590. 2000.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Rosales-Mendoza S, Sández-Robledo C,
Bañuelos-Hernández B and Angulo C: Corn-based vaccines: Current
status and prospects. Planta. 245:875–888. 2017.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Nochi T, Takagi H, Yuki Y, Yang L,
Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T,
et al: Rice-based mucosal vaccine as a global strategy for
cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA.
104:10986–10991. 2007.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Specht EA and Mayfield SP: Algae-based
oral recombinant vaccines. Front Microbiol. 5(60)2014.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Sami N, Ahmad R and Fatma T: Exploring
algae and cyanobacteria as a promising natural source of antiviral
drug against SARS-CoV-2. Biomed J. 44:54–62. 2021.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Satyaraj E, Reynolds A, Engler R, Labuda J
and Sun P: Supplementation of diets with spirulina influences
immune and gut function in dogs. Front Nutr.
8(667072)2021.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Genetically Engineered Plants as a Source
of Vaccines Against Wide Spread Diseases: An Integrated View.
Springer, New York, NY, 2014.
|
|
166
|
Gebre MS, Brito LA, Tostanoski LH, Edwards
DK, Carfi A and Barouch DH: Novel approaches for vaccine
development. Cell. 184:1589–1603. 2021.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Chan BC, Li P, Tsang MS, Sung JC, Kwong
KW, Zheng T, Hon SS, Lau CP, Cheng W, Chen F, et al: Creating a
vaccine-like supplement against respiratory infection using
recombinant bacillus subtilis spores expressing SARS-CoV-2 spike
protein with natural products. Molecules. 28(4996)2023.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Soutter F, Werling D, Nolan M, Küster T,
Attree E, Marugán-Hernández V, Kim S, Tomley FM and Blake DP: A
novel whole yeast-based subunit oral vaccine against eimeria
tenella in chickens. Front Immunol. 13(809711)2022.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Li M, Wang Y, Sun Y, Cui H, Zhu SJ and Qiu
HJ: Mucosal vaccines: Strategies and challenges. Immunol Lett.
217:116–125. 2020.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Jazayeri SD, Lim HX, Shameli K, Yeap SK
and Poh CL: Nano and microparticles as potential oral vaccine
carriers and adjuvants against infectious diseases. Front
Pharmacol. 12(682286)2021.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Kong Q, Richter L, Yang YF, Arntzen CJ,
Mason HS and Thanavala Y: Oral immunization with hepatitis B
surface antigen expressed in transgenic plants. Proc Natl Acad Sci
USA. 98:11539–11544. 2001.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Figueiredo D, Turcotte C, Frankel G, Li Y,
Dolly O, Wilkin G, Marriott D, Fairweather N and Dougan G:
Characterization of recombinant tetanus toxin derivatives suitable
for vaccine development. Infect Immun. 63:3218–3221.
1995.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Lee CW, Lee SF and Halperin SA: Expression
and immunogenicity of a recombinant diphtheria toxin fragment A in
Streptococcus gordonii. Appl Environ Microbiol. 70:4569–4574.
2004.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Barry EM, Gomez-Duarte O, Chatfield S,
Rappuoli R, Pizza M, Losonsky G, Galen J and Levine MM: Expression
and immunogenicity of pertussis toxin S1 subunit-tetanus toxin
fragment C fusions in Salmonella typhi vaccine strain CVD 908.
Infect Immun. 64:4172–4181. 1996.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Kenner JR, Coster TS, Taylor DN, Trofa AF,
Barrera-Oro M, Hyman T, Adams JM, Beattie DT, Killeen KP, Spriggs
DR, et al: Peru-15, an improved live attenuated oral vaccine
candidate for Vibrio cholerae O1. J Infect Dis. 172:1126–1129.
1995.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Banda R, Yambayamba V, Lalusha BD, Sinkala
E, Kapulu MC and Kelly P: Safety of live, attenuated oral vaccines
in HIV-infected Zambian adults: Oral vaccines in HIV. Vaccine.
30:5656–5660. 2012.PubMed/NCBI View Article : Google Scholar
|