Introduction
Prostate cancer (PCa) is a frequently occurring
malignancy of the male genitourinary system (1). Cancerous cells manifest an elevated
requirement for iron in comparison to ordinary non-cancerous cells
in order to stimulate proliferation (2). This reliance on iron renders
cancerous cells more susceptible to iron-catalyzed necrosis,
commonly termed ferroptosis (3).
This suggests the promise of targeting ferroptosis as a therapeutic
intervention. Ferroptosis has been implicated in several human
diseases, including PCa (4).
Increasing evidence indicates that ferroptosis inhibits tumor
growth (5) and the employment of
ferroptosis inducers or targeting ferroptosis-related genes may
represent promising strategies for treating castration-resistant
PCa (CRPC) (6). Traditional
Chinese medicine has multiple targets and can regulate various
signaling pathways, including ADAMTS18(7), reactive oxygen species (ROS)
(8), nuclear factor erythroid
2-related factor 2 (Nrf2) (9) and
glutathione peroxidase 4 (GPX4) (10), thereby modulating ferroptosis.
Therefore, the use of Chinese medicine to induce ferroptosis in PCa
cells may represent a promising avenue for future
investigation.
A study has shown a correlation between the Nrf2
signaling pathway and the mechanisms underpinning ferroptosis
(11). Nrf2 signaling is involved
in ferroptosis through the regulation of glutathione (GSH)
homeostasis, mitochondrial function and lipid metabolism (6,12).
The p62-Kelch-like ECH-associated protein 1 (Keap1)-Nrf2 axis
mainly governs the transcription of downstream genes associated
with the metabolism of iron and ROS metabolism, thus regulating the
occurrence of ferroptosis (13-15).
Suppression of heme oxygenase-1 (HO-1) in hormone-refractory PCa
cells reduces intracellular ROS levels and inhibits a variety of
carcinogenic properties (16).
Further investigation is required to fully understand the
regulation of prostate cancer cells by the Nrf2/HO-1 signaling
pathway, despite indications that suggest ferroptosis inducers may
promote ferroptosis in cancer cells through said pathway (17,18).
Icariin (19) and
curcumol are both extracted from traditional Chinese herbs, which
are widely available and cost-effective. The former is the primary
active component of Epimedium (20), and is used in traditional Chinese
medicine (21). The latter, a
sesquiterpene found in Curcuma zedoaria, exhibits
neuroprotective, anti-inflammatory and anti-tumor properties
(22). Curcumol modulates the
PDK1/AKT/mTOR signaling pathway via miR-9, influencing the onset of
PCa (23). It can act in tandem
with a number of synthetic drugs as an antibiotic or anti-cancer
agent (24). The present authors'
initial study indicated that curcumol effectively inhibits the
proliferation, invasion and migration of PC3 and 22RV1 cells,
mitigating the progression of PCa via the miR-125a/STAT3 axis
(25). Icariin and curcumol act
synergistically to modulate the miR-7/mTOR/SREBP1 pathway, inducing
autophagy in PCa cells and affecting lipid metabolism (26). Our latest experiments suggested
that icariin and curcumol increase Fe2+ and
malondialdehyde (MDA) contents while decreasing GSH levels, whereas
treatment with Fer-1, an iron death inhibitor, reversed these
indexes (26). However, it remains
to be seen whether icariin-curcumol interferes with PCa progression
by promoting ferroptosis of PCa cells.
Although ferroptosis has gradually become a research
hotspot in the field of cancer, the mechanism through which
traditional Chinese medicine regulates the ferroptosis of PCa cells
remains to be explored. The present study hypothesized that icariin
and curcumol regulate the ferroptosis of CRPC cells through the
Nrf2/HO-1 signaling axis, ultimately disrupting the progression of
CRPC.
Materials and methods
Cell culture and screening
Human prostate normal cells RWPE-2 (cat. no.
AW-CNH470; Abiowell) and human PCa cells PC-3 (cat. no. AW-CCH111;
Abiowell), VCAP (cat. no. AW-CCH367; Abiowell), DU145 (cat. no.
AW-CCH043; Abiowell) were cultured in a special medium with 10%
fetal bovine serum (FBS; cat. no. 10099141; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (cat. no. SV30010;
Cytiva). These cells were cultured in an incubator (cat. no.
DH-160I; SANTN Instrument Co., Ltd.) with saturated humidity at
37˚C and 5% CO2. Icariin (cat. no. N1705; APeXBIO
Technology LLC) and curcumol (cat. no. N1743, APeXBIO Technology
LLC) were dissolved in the solvent (PEG400:ethanol:normal saline
volume ratio of 57.1:14.3:28.6) and the drug concentrations were 30
µg/ml and 50 µg/ml (27),
respectively. Logarithmically grown cells were treated with icariin
and curcumol at different concentrations and the cells were
categorized into four distinct factions: Control, 10 mM [4.4 ml (30
µg) icariin and 21 µl (50 µg) curcumol], 20 mM [2.2 ml (30 µg)
icariin and 10.5 µl (50 µg) curcumol] and 40 mM [1.1 ml (30 µg)
icariin and 5.25 µl (50 µg) curcumol]. These cells were cultivated
in the presence of icariin-curcumol at concentrations ranging from
0-40 mM for intervals of 0, 24 and 48 h.
Cell grouping and treatment
The DU145 cells were selected and treated with 0 mM,
10, 20 and 40 mM icariin-curcumol for 24 h for subsequent studies.
Then, the DU145 cells were further divided into three groups:
Control, small interfering negative control (si-NC;
5'-TTCTCCGAACGTGTCACGT-3') and si-Nrf2 (5'-GTATGTCAATCAAATCCAT-3').
In the control group, DU145 was cultured in DMEM medium in a 37˚C
with 5% CO2 content and proper ventilation to keep the
intracellular environment moist. Lipofectamine 2000 (cat. no.
11668019, Thermo Fisher Scientific, Inc.) was used for transfecting
experiments. Cells in the si-NC group were transfected with 5 µl of
100 nM si-NC and cultured at 37˚C for 48 h according to the
manufacturer's protocols of Lipofectamine 2000, while the control
group was added a reagent of the same volume without siRNA.
Similarly, cells in the si-Nrf2 group were transfected with the
si-Nrf2 and cultured at 37˚C for 48 h. Follow-up experiments were
performed after 48 h.
Then, DU145 cells were divided into four groups:
Control, icariin + curcumol, icariin + curcumol + overexpression
(oe)-NC and icariin + curcumol + oe-Nrf2. In the control group,
DU145 was cultured in DMEM medium in a 37˚C incubator with 5%
CO2 content and proper ventilation to keep the
intracellular environment moist. In the icariin + curcumol group,
DU145 cells were treated with 40 mM icariin-curcumol for 24 h at
37˚C, while the control group received solvent of the same volume
without icariin and curcumol. In the icariin + curcumol +oe-NC and
icariin + curcumol+oe-Nrf2 groups, before being treated with 40 mM
icariin-curcumol for 24 h at 37˚C, the DU145 cells were transfected
with the NC plasmid and Nrf2 overexpression plasmid at 37˚C for 48
h, respectively. Plasmid transfection was performed by mixing the
plasmid with Lipofectamine 2000 Reagent to form a transfection
complex. The transfection complex was incubated on DU145 cells at
37˚C for 6 h. Subsequently, it was transferred to the culture
medium and continued to be incubated at 37˚C for 48 h. The control
and icariin + curcumol were added transfection reagents of the same
volume without plasmids. The siRNAs and plasmids were obtained from
Abiowell. Following treatment, the cells were collected for other
detection after 48 h.
Cell Counting Kit-8 (CCK-8)
The cells were seeded into 96-well plates at a
density of 5x103 cells/well according to the
instructions for CCK-8 (cat. no. AWC0114a; Abiowell). CCK8 solution
with the complete medium configuration was added to each well and
the cells were incubated for 4 h at 37˚C with 5% CO2.
The OD values at 450 nm were then analyzed with a multipurpose
microplate analyzer (cat. no. MB-530; HEALES, China).
Reverse transcription-quantitative
(RT-q) PCR
1x103 cells were collected with 1 ml of
TRIzol reagent (cat. no. 15596-026; Thermo Fisher Scientific, Inc.)
per group, following the manufacturer's instructions. The mRNA was
subsequently reverse transcribed into cDNA, using the mRNA reverse
transcription kit (cat. no. CW2569; CWBio) according to the
manufacturer's instructions. qPCR was performed in a fluorescence
quantitative RCP instrument (QuantStudio1, Thermo, USA). The
reaction conditions were denaturation at 95˚C for 10 min, then a
total of 40 cycles, including denaturation at 94˚C for 15 s,
annealing and extension at 60˚C for 30 s. The primers were designed
using Primer Premier 5 software (Premier Biosoft, USA) and are in
Table I. For normalization
purposes, β-actin was utilized as a reference gene. The experiments
were performed using a fluorescence quantitative PCR apparatus
(cat. no. PIKOREAL96; Thermo Fisher Scientific, Inc.). The
2-△△Ct method) was used to calculate the relative
transcription level of the target gene (28). The experiment was repeated three
times.
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Gene name | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| p53 |
ACATTCTCCACTTCTTGTTCCCC |
CTCCCCACAACAAAACACCAGT |
| SLC7A11 |
CTCCAGGTTATTCTATGTTGCGTCT |
CAAAGGGTGCAAAACAATAACAGC |
| GPX4 |
CGCCTTTGCCGCCTACTGAAGC |
AACCATGTGCCCGTCGATGTCC |
| Nrf2 |
CAACTACTCCCAGGTTGCCC |
AGTGACTGAAACGTAGCCGAA |
| HO-1 |
AAACTTCAGAGGGGGCGAAG |
GACAGCTGCCACATTAGGGT |
| β-actin |
ACCCTGAAGTACCCCATCGAG |
AGCACAGCCTGGATAGCAAC |
Western blotting
Total protein was extracted from cells in each group
using the RIPA lysate (cat. no. AWB0136; Abiowell), following the
guidelines provided by the manufacturer. A BCA concentration assay
kit (ab102536; Abcam) was employed to quantify protein
concentrations. Prepared protein samples were added to the loading
buffer with a volume of 200 µl of protein per lane. Electrophoresis
was performed with 10% gel to segregate proteins which were further
transferred to nitrocellulose membranes. The membranes were blocked
in a blocking buffer containing 5% skimmed milk at 25˚C for 1 h.
Membrane were incubated overnight at 4˚C with primary antibodies
p53 (cat. no. 10442-1-AP; Proteintech Group, Inc.), solute carrier
family 7 member 11 (SLC7A11; cat. no. ab175186; Abcam), GPX4
(23KDa, 67763-1-Ig; Proteintech Group, Inc.), Nrf2 (110KDa, cat.
no. 16396-1-AP; Proteintech Group, Inc.), HO-1 (33KDa, cat. no.
10701-1-AP; Proteintech Group, Inc.) and β-actin (42KDa, cat. no.
66009-1-Ig; Proteintech Group, Inc.). The membranes were then
incubated with the diluted secondary antibodies HRP goat anti-mouse
IgG (cat. no. SA00001-1; Proteintech Group, Inc.) and HRP goat
anti-rabbit IgG (cat. no. SA00001-2; Proteintech Group, Inc.) in
0.05% PBST (PBS with 0.05% Tween 20) for 90 min at room
temperature. Following incubation, the membranes were treated with
ECL chemiluminescence solution (cat. no. AWB0005; Abiowell) for 1
min. Finally, the membranes were analyzed in a chemiluminescence
imaging system (Chemiscope6100; Clinx Science Instruments Co.,
Ltd.). β-actin was used as an internal reference protein. The
optical density values of the protein bands were determined using
ImageJ software (Version 1.48v, NIH, USA).
The detection of Fe2+, GSH
and MDA content
The evaluation of intracellular Fe2+
level, GSH and MDA content was performed using the iron
colorimetric assay kit (cat. no. E-BC-K881-M; Elabscience
Biotechnology, Inc.), GSH (cat. no. A006-2-1, Nanjing Jiancheng
Bioengineering Institute) and MDA (cat. no. A003-1, Nanjing
Jiancheng Bioengineering Institute) detection kits were used
following the instructions provided by the manufacturer. The
membranes were immersed in Superecl plus (k-12045-d50, advansta,
USA) for luminescence development. β-actin was used as the internal
reference.
Flow cytometry
Levels of ROS within cells were assessed using a ROS
kit (cat. no. S0033S; Beyotime Institute of Biotechnology). First,
cells were digested to obtain a cell suspension. Then, the cell
suspension was incubated in a medium without serum, ultimately
reaching a concentration of 40 mM for 20 min at 37˚C. The staining
lasted for 1 h at 15˚C. A flow cytometer (CytoFLEX A00-1-1102;
Beckman Coulter, Inc.) and its corresponding software CytExpert
(Version 2.4; Beckman Coulter, Inc.) were used to detect the ROS
fluorescence intensity.
Statistical analysis
All measurement data were expressed as mean ±
standard deviation. Each test was repeated independently three
times. All data were analyzed by using SPSS 26.0 software (IBM
Corp.). Kolmogorov-Smirnov test and exploratory descriptive
statistics test were used to analyze whether the data conformed to
a normal distribution and homogeneity of variance. The measurement
data obeyed the normal distribution and homogeneity of variance.
One-way ANOVA and Tukey's post-hoc test were used to compare data.
Dunnett's test was used for comparison of multiple time points and
Bonferroni was used for post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
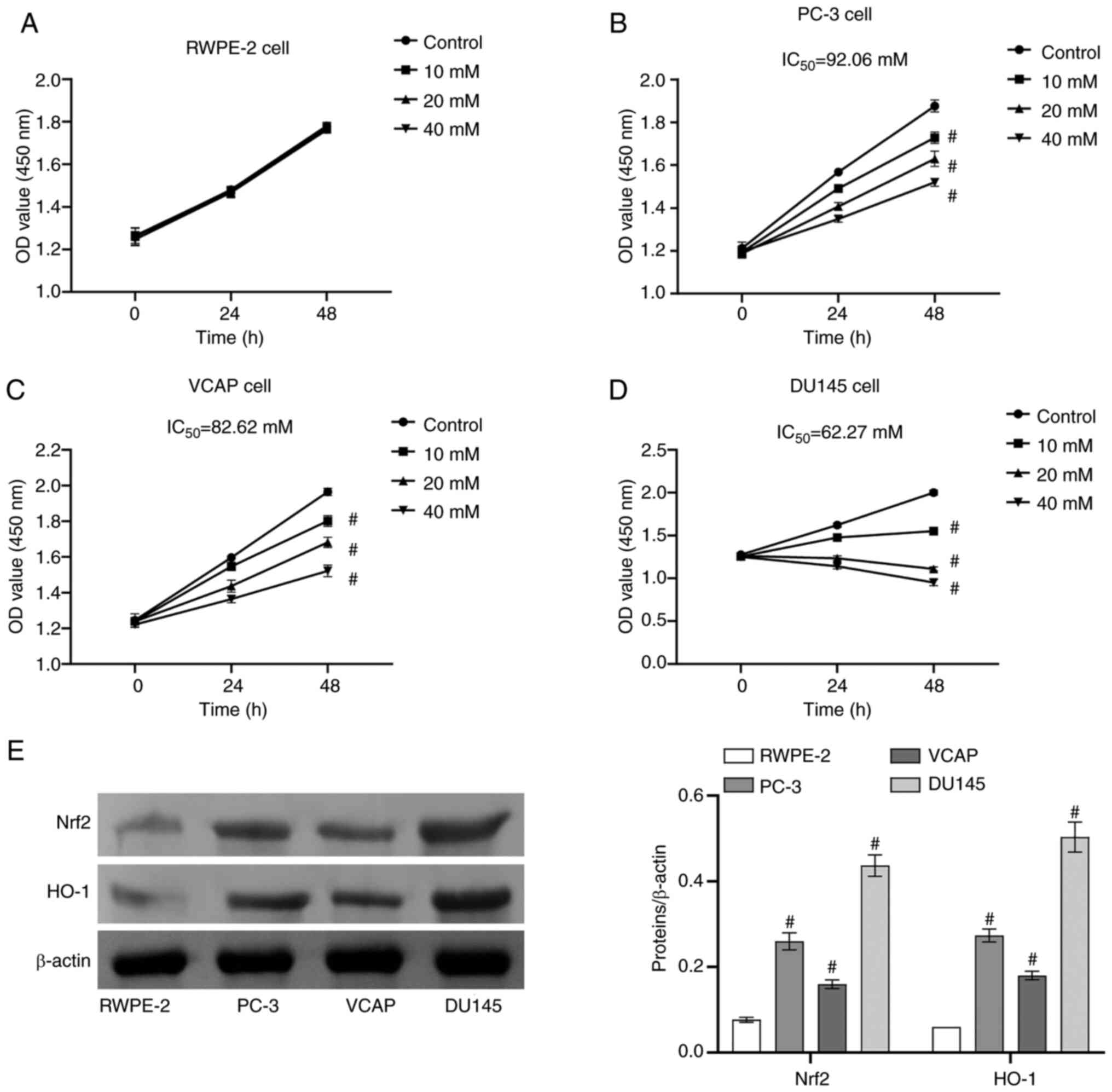
Selection of prostate cells and
icariin-curcumol concentration
Human PCa cell lines (PC-3, VCAP, DU145) and a human
normal prostate cell line (RWPE-2) were selected as subjects.
Icariin-curcumol concentrations were set at 0, 10, 20 and 40 mM.
With the increase of icariin-curcumol concentration, proliferation
of RWPE-2 cells showed no significant change (Fig. 1A), while that of PC-3 cells
(Fig. 1B), VCAP cells (Fig. 1C) and DU145 cells (Fig. 1D) was noticeably decreased.
According to IC50 data, DU145 cells (62.27) were the
most sensitive to icariin-curcumol concentration compared with PC-3
(92.06) and VCAP (82.26; Fig. 1).
The findings revealed that icariin-curcumol decreased the
proliferation of PCa cells, although it exerted no effect on the
normal prostate cells. Finally, it was found that Nrf2 and HO-1
protein levels were highest in DU145 cells compared with other cell
lines RWPE-2, PC-3 and VCAP (Fig.
1E). Therefore, DU145 cells were chosen for subsequent
studies.
Icariin-curcumol promoted ferroptosis
in DU145 cells
Next, DU145 cells were stimulated with different
concentrations of icariin-curcumol to elucidate the effect of
icariin-curcumol on the ferroptosis process in PCa cells. With the
increase of icariin-curcumol concentration, the p53 level was
gradually increased, while the protein and mRNA levels of SLC7A11
and GPX4 were gradually decreased (Fig. 2A and B). With the increase of icariin-curcumol
concentration, Fe2+ content (Fig. 2C) and ROS fluorescence intensity
(Fig. 2D and E) were gradually increased. Notably, GSH
content was decreased gradually with the increase of
icariin-curcumol concentration (Fig.
2F). In addition, the MDA level was also increased gradually
with the increase of icariin-curcumol concentration (Fig. 2F). With the increase of
icariin-curcumol concentration, the protein levels and mRNA levels
of Nrf2 and HO-1 gradually decreased (Fig. 2H). These results indicated that
icariin-curcumol promoted ferroptosis in PCa cells.
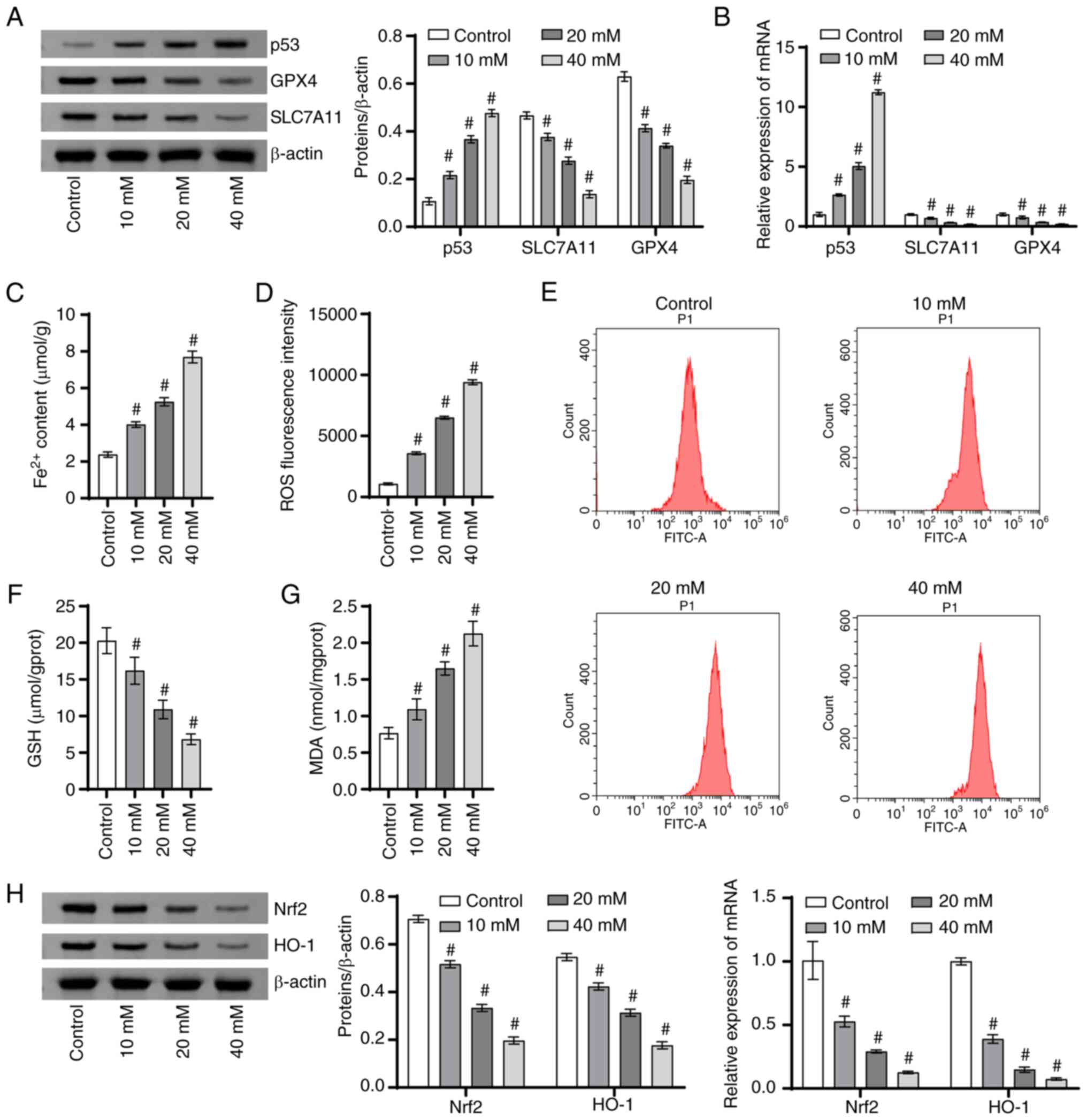 | Figure 2Icariin-curcumol promotes ferroptosis
in DU145 cells. (A) The p53, SLC7A11 and GPX4 (ferroptosis-related
proteins) levels were analyzed using a western blotting. (B) p53,
SLC7A11 and GPX4 levels were detected using RT-qPCR. (C)
Intracellular Fe2+ levels were assessed with an iron
colorimetric assay kit. (D) The fluorescence intensity of ROS. (E)
Flow cytometry of intracellular ROS production. (F) A GSH detection
kit was utilized to detect intracellular GSH content. (G) The MDA
level was assessed using an MDA detection kit. (H) The Nrf2 and
HO-1 levels in cells were analyzed using RT-qPCR and western
blotting. #P<0.05 vs. control; n=3. SLC7A11, solute
carrier family 7 member 11; GPX4, glutathione peroxidase 4;
RT-qPCR, reverse transcription-quantitative PCR; ROS, reactive
oxygen species; GSH, glutathione; MDA, malondialdehyde; Nrf2,
nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1. |
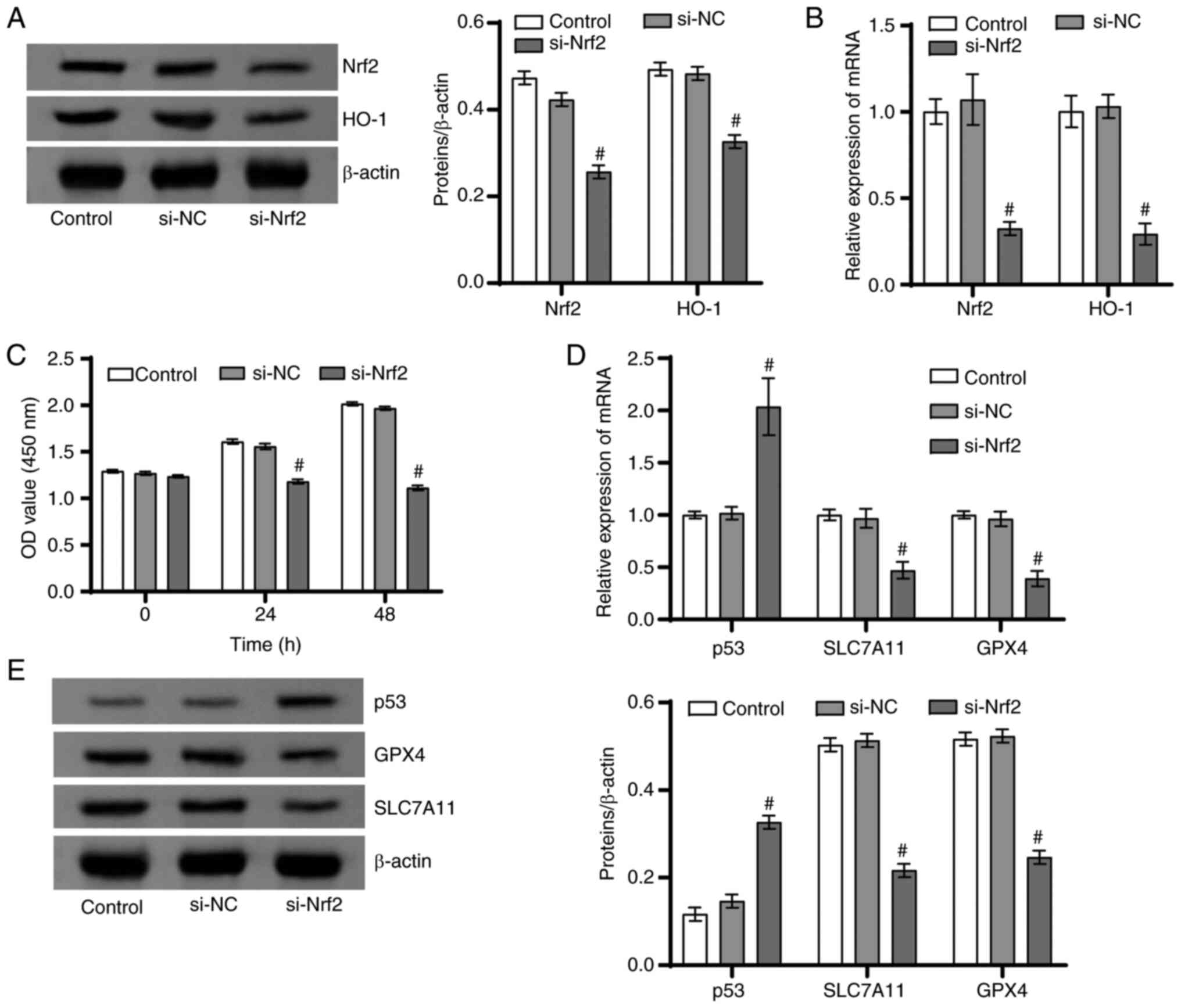
Silencing of Nrf2/HO-1 signaling axis
promoted ferroptosis in DU145 cells
The present study continued to use DU145 cells as
research objects to explore the function of the Nrf2/HO-1 signaling
axis on the ferroptosis of PCa cells. The protein and mRNA levels
of Nrf2 and HO-1 in the si-Nrf2 group were markedly lower than the
si-NC group (Fig. 3A and B). Moreover, DU145 cell proliferation
ability was signally lower in the si-Nrf2 group than in the si-NC
group (Fig. 3C). Furthermore, in
contrast to the si-NC group, the proteins and mRNA levels of
SLC7A11 and GPX4 in the si-Nrf2 group notably declined, while the
p53 level was markedly enhanced (Fig.
3D and E). These results
together suggested that Nrf2/HO-1 signaling axis inhibited
ferroptosis in PCa cells.
Icariin-curcumol regulated ferroptosis
of DU145 cells through Nrf2/HO-1 signaling axis
The present study further investigated whether
icariin-curcumol regulated ferroptosis in PCa cells through the
Nrf2/HO-1 signaling axis. In contrast to the control group, the
protein and mRNA levels of Nrf2 and HO-1 in the icariin + curcumol
group were markedly reduced. The protein and mRNA levels of Nrf2
and HO-1 in the icariin + curcumol + oe-Nrf2 group were markedly
enhanced when compared with the icariin + curcumol + oe-NC group
(Fig. 4A and B). Cell proliferation ability was
conspicuously reduced in the icariin + curcumol group in comparison
to the control group. Following transfection with overexpression of
Nrf2, the cell proliferation ability was signally enhanced in
contrast to the icariin + curcumol + oe-NC group (Fig. 4C). Furthermore, the protein and
mRNA levels of SLC7A11 and GPX4 levels in the icariin + curcumol
group were clearly higher, while the p53 levels were markedly lower
than the control group. The protein and mRNA levels of SLC7A11 and
GPX4 levels in the icariin + curcumol + oe-Nrf2 were evidently
increased, while the p53 levels were markedly decreased in
comparison to the icariin + curcumol + oe-NC group (Fig. 4D and E). It was worth noting that
Fe2+ content in the icariin + curcumol group was
markedly increased in contrast to the control group. After
overexpression of Nrf2 transfected, the Fe2+ content was
markedly decreased in contrast to the icariin + curcumol + oe-NC
group (Fig. 4F). The mean
fluorescence intensity of ROS in the icariin + curcumol group was
markedly increased in comparison with the control group. After
overexpression of Nrf2, ROS mean fluorescence intensity was
markedly decreased compared with the icariin + curcumol + oe-NC
group (Fig. 4G). Notably, in
contrast to the control group, GSH content in the icariin +
curcumol group was decreased, while MDA content was notably
increased. Overexpression of Nrf2 reversed the associated
regulation induced by icariin + curcumol. (Fig. 4H-J). These results indicated that
icariin-curcumol regulates ferroptosis in prostate cancer cells
through Nrf2/HO-1 signaling axis.
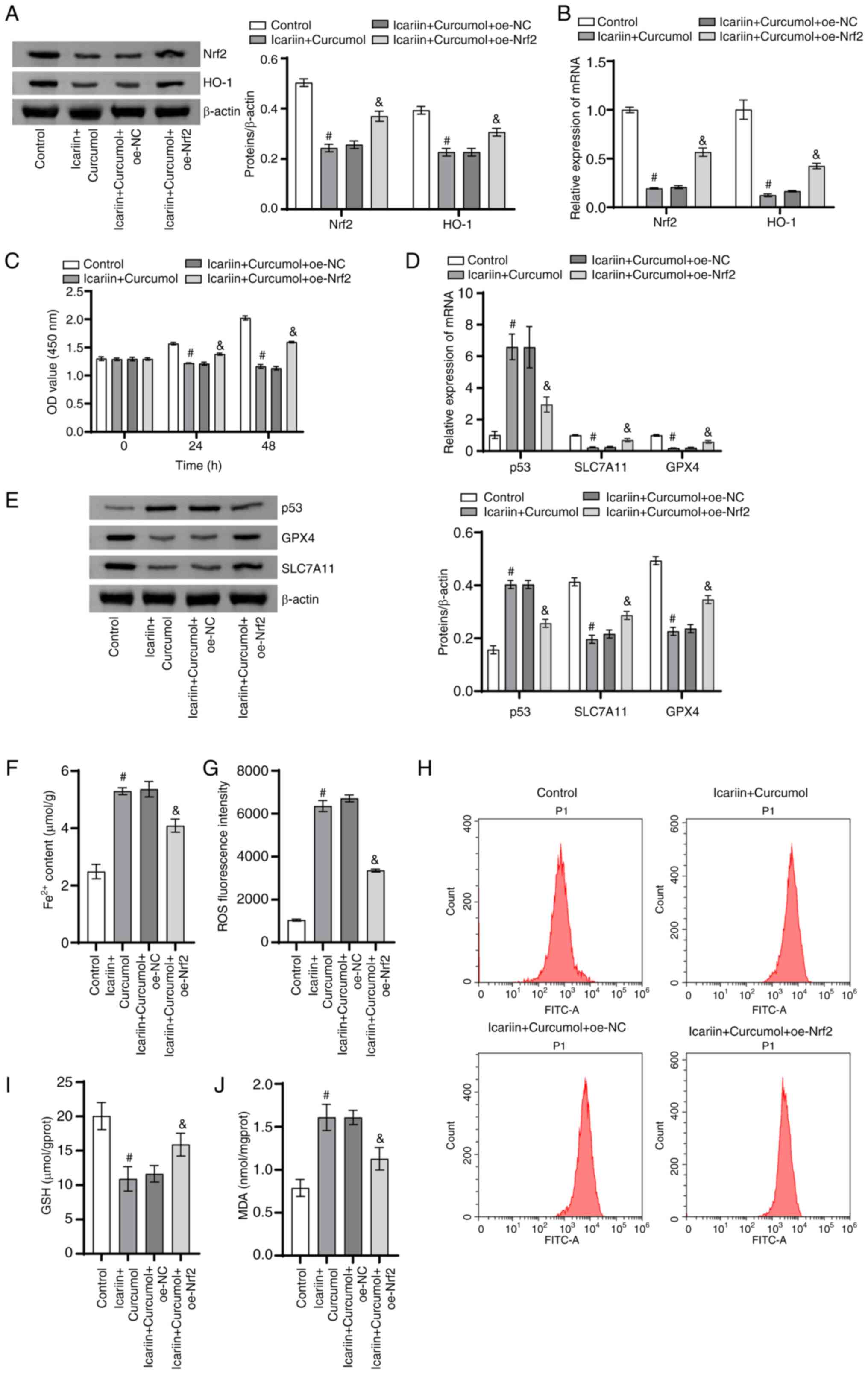 | Figure 4Icariin-curcumol promotes ferroptosis
in DU145 cells through Nrf2/HO-1 signaling axis. (A) The Nrf2 and
HO-1 levels in DU145 cells were analyzed using western blotting.
(B) The Nrf2 and HO-1 levels in DU145 cells were assessed using
RT-qPCR. (C) The proliferation ability of DU145 cells was examined
using CCK8. (D) The p53, SLC7A11 and GPX4 levels in DU145 cells
were tested using RT-qPCR. (E) Western blotting was used to detect
the p53, SLC7A11 and GPX4 levels in DU145 cells. (F) Intracellular
Fe2+ levels were assessed with an iron colorimetric
assay kit. (G) The fluorescence intensity of ROS. (H) Flow
cytometry of intracellular ROS production. (I) A GSH detection kit
was used to detect intracellular GSH content. (J) The MDA level in
DU145 cells was tested using an MDA detection kit. n=3.
#P<0.05 vs. control; &P<0.05 vs.
icariin + curcumol + oe-NC. Nrf2, nuclear factor erythroid
2-related factor 2; HO-1, heme oxygenase-1; SLC7A11, solute carrier
family 7 member 11; GPX4, glutathione peroxidase 4; si small
interfering; NC, negative control; oe, overexpression; GSH,
glutathione; MDA, malondialdehyde. |
Discussion
Currently, natural botanical extracts are considered
a significant source of anti-tumor medication. This is not only due
to their diverse chemical structures and biological activities, but
also because of their lower toxicity compared with drugs
synthesized chemically (29).
Therefore, identifying natural plant extracts and their functional
mechanisms has become a major area of research (30). The present study highlighted the
anti-tumor effects of icariin-curcumol on PCa cells. It found that
icariin-curcumol promoted ferroptosis in PCa cells through the
Nrf2/HO-1 signaling pathway.
Icariin is extracted from Yinyanghuo (Epimedium
brevicornu) and curcumol from Ezhu (Curcuma zedoaria),
which are both traditional Chinese herbal medicines with wide
geographic distribution and affordable prices (31,32).
Studies have shown that both icariin and curcumol have therapeutic
effects on PCa (23,33).
Icariin can promote ferroptosis of tumor cells by
interfering with ferroptosis metabolism pathways (26). Specifically, it can inhibit uptake
of ferroptosis in tumor cells and disrupt the balance of
ferroptosis storage and utilization, leading to ferroptosis
overload in tumor cells. This ferroptosis overload can trigger
excessive ROS production and induce apoptosis and ferroptosis in
cells (34). Curcumol is widely
used in anti-tumor therapy (35,36).
Study has shown that curcumol can induce ferroptosis death by
interfering with ferroptosis metabolism pathways in tumor cells
(37). Specifically, curcumol can
inhibit the endogenous production of ferroptosis in tumor cells and
increase the concentration of free ferroptosis in the cytoplasm.
These free ferroptosis can participate in the production of
excessive ROS, thereby triggering autophagy and apoptosis in cells
and inducing ferroptosis in tumor cells (38). Icariin and curcumol both have the
ability to promote apoptosis in cells (27). Apoptosis is a normal, orderly and
programmed cell death mechanism in the body. In the case of tumor
cells, the process of apoptosis is inhibited, leading to tumor
growth and spread (39). icariin
and curcumol can inhibit tumor growth and spread by promoting
apoptosis in PCa cells. They also have inhibitory effects on cell
proliferation. One of the characteristics of tumor cells is
abnormal cell proliferation, which leads to tumor growth and
spread. icariin and curcumol can inhibit tumor growth by
suppressing the proliferation of tumor cells. Additionally, both
icariin and curcumol have anti-inflammatory effects (26). Inflammation is an important factor
in the occurrence and development of tumors and by inhibiting the
occurrence of inflammatory reactions, tumor growth and spread can
be suppressed (27).
Icariin and curcumol both have pharmacological
activities and by mixing them together, they may produce a
synergistic effect, enhancing the therapeutic effect (27). Previous experiments have
demonstrated that Icariin and curcumin synergistically regulate the
miR-7/mTOR/SREBP1 pathway, inducing autophagy and ferroptosis in
PCa cells and affecting lipid metabolism (26). DU145 cells may have differences in
the regulation of the Nrf2/HO-1 signaling pathway compared with
other PCa cells such as RWPE-2, PC-3 and VCAP. The Nrf2/HO-1
pathway is an important cellular response mechanism to oxidative
stress and its overactivation can lead to increased antioxidant and
cell protective abilities (40).
Nrf2 is a transcription factor that regulates the antioxidant
stress pathway in cells (41).
HO-1 is a target gene downstream of the Nrf2 pathway and has
antioxidant and anti-inflammatory effects (42). Nrf2/HO-1 signaling pathway serves
an important role in development and progression of PCa (1). PCa cell lines with high expression of
Nrf2/HO-1 may have lower sensitivity to chemotherapy drugs. This is
because the high expression of Nrf2/HO-1 can enhance the
antioxidant stress capacity of cells and promote cell survival and
drug resistance (43). On the
other hand, some studies have found that inhibiting the Nrf2/HO-1
pathway may increase the sensitivity of PCa cells to chemotherapy
drugs (44,45). In the present study, the data
results showed that icariin combined with curcumol effectively
inhibited the proliferation of prostate cancer cells, particularly
DU145, which was the most sensitive. Nrf2 and HO-1 were expressed
more highly in DU145 cells, compared with other prostate cancer
cell lines. Icariin combined with curcumol effectively suppressed
the expression of Nrf2 and HO-1, thereby affecting the process of
ferroptosis in DU145 cells.
Ferroptosis, a type of programmed cell death that
depends on iron, is closely associated with the emergence and
advancement of tumors (46). In
particular, the mRNA molecule SLC7A11 is linked to ferroptosis
(47). Researchers have
demonstrated that regulating the SLC7A11/GSH/GPX4 axis induces
ferroptosis in vascular smooth muscle cells (48). Lethal depletion of GSH and GPX4
caused by iron-dependent ROS accumulation constitutes a crucial
step in the initiation of ferroptosis within cancerous cells
(49). The present study found,
consistent with these findings, that icariin-curcumol reduced the
SLC7A11 and GPX4 levels and GSH content. As intracellular iron and
ROS accumulate (12), ferroptosis
is triggered, playing a pivotal role in the development and
progression of cancer (50).
Cancer cells evade cell death by overexpressing SLC7A11. The
specific process was as follows: SLC7A11 facilitates GSH production
and decreases ROS-mediated cellular stress by exchanging cysteine
for intracellular glutamate (51).
The present study also recorded a reduction in ROS-mediated
cellular stress as a result of icariin-curcumol. These results
indicated that icariin-curcumol-activated ingredients regulated
ferroptosis to inhibit PCa.
Targeting the Nrf2/HO-1 axis has been introduced as
a revolutionary cancer therapy (52). Nrf2 is a fundamental transcription
factor that governs endogenous antioxidants, including
HO-1(53). The HO-1 gene is
accountable for the balance maintenance within cells and assumes a
critical part in controlling oxidative stress and the advancement
and progression of PCa (54). The
Keap1/Nrf2/ARE axis has been found to enhance the proliferation and
inhibit apoptosis and invasion of PCa cells (55). Prior exposure to puerarin resulted
in reduced protein expression levels of both Nrf2 and HO-1 in DU145
and PC3 cells (56). As the
present study demonstrated, Nrf2 and HO-1 showed a gradual decrease
with an increase in icariin-curcumol concentration. The tumor
suppressor protein p53 serves various roles while responding to
different stress signals (57).
Furthermore, it was found that the p53 level, Fe2+
content and MDA level were increased proportionally with an
increase in icariin-curcumol concentration. The focus of the
present study was on exploring the influence of the Nrf2/HO-1
signaling pathway on ferroptosis in PCa cells. It made a notable
discovery that the inhibition of Nrf2 expression resulted in a
significant reduction in ferroptosis-related protein levels. In
conditions of stress, p53 exerts stringent control over cellular
proliferation by facilitating apoptosis (58). The tumor-inhibitory action of p53
has been traditionally linked to its capacity to elicit cell cycle
arrest, senescence and apoptosis in cells (59). Upon comparing with the si-NC group,
inhibiting Nrf2 expression demonstrated a marked decrease of cancer
cell replication capability and a significant increase in the p53
level. The results indicated that inhibiting the activation of the
Nrf2/HO-1 pathway instead activated the P53-dependent ferroptosis
pathway, leading to the accumulation of iron and ultimately leading
to ferroptosis. It could be hypothesized that the Nrf2/HO-1
signaling pathway regulated ferroptosis in PCa cells. However,
whether the Nrf2/HO-1 pathway regulates the ferroptosis mechanism
in prostate cancer at the clinical level was not investigated and
therefore future studies will collect clinical samples for more
in-depth studies.
The present study unveiled a fresh potential
mechanism through which icariin-curcumol may induce ferroptosis in
PCa cells, most likely by operating through the Nrf2/HO-1 signaling
axis. Notably, ferroptosis can be stimulated in cancer cells by
Tagitinin C, a sesquiterpene lactone obtained from Tithonia
diversifolia, resulting in increased levels of ROS and MDA,
paired with reduced GSH levels (60). The present study observed that
icariin-curcumol also led to ferroptosis in conjunction with
depleted GSH levels, elevated oxidative stress and MDA expression.
Moreover, this phenomenon was accompanied by a substantial
reduction in the levels of Nrf2, HO-1, SLC7A11 and GPX4 in addition
to reducing cell proliferation ability and causing a marked
increase in p53 level and Fe2+ content. Finally,
overexpressing Nrf2 reversed the regulatory effects of
icariin-curcumol on the aforementioned factors.
To sum up, the present study demonstrated that
icariin-curcumol enhanced ferroptosis in PCa cells through
modulation of the Nrf2/HO-1 signaling pathway. Its findings offered
potential targets for PCa therapy and novel treatment strategies
for drug development.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Natural Science
Foundation of Hunan Province (grant no. 2023JJ40511), Excellent
Youth Project of Scientific Research Program of Hunan Education
Department (grant no. 22B0370), Project of Traditional Chinese
Medicine Administration of Hunan Province (grant no. B2023034),
Changsha Natural Science Foundation (grant no. kq2208204),
Excellent Youth Project of Hunan University of Chinese Medicine
(grant no. Z2023XJYQ05), and Health Commission of Hunan Province
Health research projects (grant no. W20243165).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WS conceived the present study. WS, BL, TS and CZ
collected and analyzed the data. YL administrated the project and
WX designed the methodology. WS wrote the original draft of the
manuscript which was then edited by YL and WX and reviewed by all
authors. WS, YL and WX confirm the authenticity of all the raw data
and agree to be accountable for all aspects of the present study in
ensuring that questions related to the accuracy or integrity of any
part of the present study are appropriately investigated and
resolved. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Liao D, Yang G, Yang Y, Tang X, Huang H,
Shao J and Pan Q: Identification of pannexin 2 as a novel marker
correlating with ferroptosis and malignant phenotypes of prostate
cancer cells. Onco Targets Ther. 13:4411–4421. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hentze MW, Muckenthaler MU and Andrews NC:
Balancing acts: Molecular control of mammalian iron metabolism.
Cell. 117:285–297. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Maccarinelli F, Coltrini D, Mussi S,
Bugatti M, Turati M, Chiodelli P, Giacomini A, De Cillis F, Cattane
N, Cattaneo A, et al: Iron supplementation enhances RSL3-induced
ferroptosis to treat naïve and prevent castration-resistant
prostate cancer. Cell Death Discov. 9(81)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ghoochani A, Hsu EC, Aslan M, Rice MA,
Nguyen HM, Brooks JD, Corey E, Paulmurugan R and Stoyanova T:
Ferroptosis inducers are a novel therapeutic approach for advanced
prostate cancer. Cancer Res. 81:1583–1594. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu B, Zhu WJ, Peng YJ and Cheng SD:
Curcumin reverses the sunitinib resistance in clear cell renal cell
carcinoma (ccRCC) through the induction of ferroptosis via the
ADAMTS18 gene. Transl Cancer Res. 10:3158–3167. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R,
Cui X, Yang H, Yang Y, Birnbaumer L, et al: Quercetin alleviates
acute kidney injury by inhibiting ferroptosis. J Adv Res.
28:231–243. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie R, Zhao W, Lowe S, Bentley R, Hu G,
Mei H, Jiang X, Sun C, Wu Y and Yueying Liu: Quercetin alleviates
kainic acid-induced seizure by inhibiting the Nrf2-mediated
ferroptosis pathway. Free Radic Biol Med. 191:212–226.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang X, Jiang L, Chen H, Wei S, Yao K,
Sun X, Yang G, Jiang L, Zhang C, Wang N, et al: Resveratrol
protected acrolein-induced ferroptosis and insulin secretion
dysfunction via ER-stress-related PERK pathway in MIN6 cells.
Toxicology. 465(153048)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suzuki T, Motohashi H and Yamamoto M:
Toward clinical application of the Keap1-Nrf2 pathway. Trends
Pharmacol Sci. 34:340–346. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
La Rosa P, Petrillo S, Turchi R,
Berardinelli F, Schirinzi T, Vasco G, Lettieri-Barbato D, Fiorenza
MT, Bertini ES, Aquilano K and Piemonte F: The Nrf2 induction
prevents ferroptosis in Friedreich's Ataxia. Redox Biol.
38(101791)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liang J, Liao Y, Wang P, Yang K, Wang Y,
Wang K, Zhong B, Zhou D, Cao Q, Li J, et al: Ferroptosis landscape
in prostate cancer from molecular and metabolic perspective. Cell
Death Discov. 9(128)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang S, Wei W, Ma N, Qu Y and Liu Q:
Molecular mechanisms of ferroptosis and its role in prostate cancer
therapy. Crit Rev Oncol Hematol. 176(103732)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Choi BH, Kim JM and Kwak MK: The
multifaceted role of NRF2 in cancer progression and cancer stem
cells maintenance. Arch Pharm Res. 44:263–280. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J
and Yang M: Melatonin suppresses ferroptosis induced by high
glucose via activation of the Nrf2/HO-1 signaling pathway in type 2
diabetic osteoporosis. Oxid Med Cell Longev.
2020(9067610)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen J, Zhou S, Zhang X and Zhao H:
S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane, a novel ferroptosis
inducer, promotes NSCLC cell death through inhibiting Nrf2/HO-1
signaling pathway. Front Pharmacol. 13(973611)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuk SS, Lim EM, Lee JY, Lee YJ, Kim YS,
Lee TH, Park SK, Bae H, Kim HM, Ko SG, et al: Antiinflammatory
effects of Epimedium brevicornum water extract on
lipopolysaccharide-activated RAW264.7 macrophages. Phytother Res.
24:1781–1787. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Jin J, Wang H, Hua X, Chen D, Huang C and
Chen Z: An outline for the pharmacological effect of icariin in the
nervous system. Eur J Pharmacol. 842:20–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gao JQ, Zhuang SX, Wang Y, Cao FX, Chen L,
Bao YH and Wei Y: Evaluation of Epimedium brevicornum Maxim extract
for anti-osteoporosis activity in rats. Trop J Pharm Res.
16:2185–2190. 2017.
|
|
22
|
Lo JY, Kamarudin MN, Hamdi OA, Awang K and
Kadir HA: Curcumenol isolated from Curcuma zedoaria suppresses
Akt-mediated NF-κB activation and p38 MAPK signaling pathway in
LPS-stimulated BV-2 microglial cells. Food Funct. 6:3550–3559.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sheng W, Xu W, Ding J, Li L, You X, Wu Y
and He Q: Curcumol inhibits the malignant progression of prostate
cancer and regulates the PDK1/AKT/mTOR pathway by targeting miR-9.
Oncol Rep. 46(246)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun DX, Fang ZZ, Zhang YY, Cao YF, Yang L
and Yin J: Inhibitory effects of curcumenol on human liver
cytochrome P450 enzymes. Phytother Res. 24:1213–1216.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Sheng W, Ding J, Liu L, Wang N, Lu B, You
X, He Q and Zhou Q: Curcumol inhibits the development of prostate
cancer by miR-125a/STAT3 Axis. Evid Based Complement Alternat Med.
2022(9317402)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu W, Ding J, Li B, Sun T, You X, He Q and
Sheng W: Effects of icariin and curcumol on autophagy, ferroptosis,
and lipid metabolism based on miR-7/m-TOR/SREBP1 pathway on
prostate cancer. Biofactors. 49:438–456. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu W, Ding J, Kuang S, Li B, Sun T, Zhu C,
Liu J, Zhu L, Li Y and Sheng W: Icariin-Curcumol promotes docetaxel
sensitivity in prostate cancer through modulation of the PI3K-Akt
signaling pathway and the Warburg effect. Cancer Cell Int.
23(190)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nie F, Yan J, Ling Y, Liu Z, Fu C, Li X
and Qin Y: Effect of Shuangdan Mingmu capsule, a Chinese herbal
formula, on oxidative stress-induced apoptosis of pericytes through
PARP/GAPDH pathway. BMC Complement Med Ther. 21(118)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the nearly four decades from 01/1981
to 09/2019. J Nat Prod. 83:770–803. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuan M, Zhang G, Bai W, Han X, Li C and
Bian S: The role of bioactive compounds in natural products
extracted from plants in cancer treatment and their mechanisms
related to anticancer effects. Oxid Med Cell Longev.
2022(1429869)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang S, Ma J, Zeng Y, Zhou G, Wang Y, Zhou
W, Sun X and Wu M: Icariin, an Up-and-Coming bioactive compound
against neurological diseases: Network pharmacology-based study and
literature review. Drug Des Devel Ther. 15:3619–3641.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei W, Rasul A, Sadiqa A, Sarfraz I,
Hussain G, Nageen B, Liu X, Watanabe N, Selamoglu Z, Ali M, et al:
Curcumol: From plant roots to cancer roots. Int J Biol Sci.
15:1600–1609. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee KS, Lee HJ, Ahn KS and Kim SH, Nam D,
Kim DK, Choi DY, Ahn KS, Lu J and Kim SH:
Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II
induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett.
280:93–100. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT
and Dong F: Icariin inhibits hypoxia/reoxygenation-induced
ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1
signaling pathway. FEBS Open Bio. 11:2966–2976. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei ZL, Juan W, Tong D, Juan LX, Sa LY,
Jie HFM, Xiao G, Xiang LG, Jie HM and Xu C: Curcumol inhibits
breast cancer growth via NCL/ERα36 and the PI3K/AKT pathway. Food
Funct. 14:874–885. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huang X, Qian J, Li L, Zhang X, Wei G, Lv
J, Qin F, Yu J, Xiao Y, Gong Z and Huo J: Curcumol improves
cisplatin sensitivity of human gastric cancer cells through
inhibiting PI3K/AKT pathway. Drug Dev Res. 81:1019–1025.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zheng Y, Zhao T and Wang J, Jiang R, Huang
J, Li W and Wang J: Curcumol alleviates liver fibrosis through
inducing autophagy and ferroptosis in hepatic stellate cells. FASEB
J. 36(e22665)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang X, Zhang M, Mao C, Zhang C, Ma W,
Tang J, Xiang D and Qi X: Icariin alleviates ferroptosis-related
atherosclerosis by promoting autophagy in xo-LDL-induced vascular
endothelial cell injury and atherosclerotic mice. Phytother Res.
37:3951–3963. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kashyap D, Garg VK and Goel N: Intrinsic
and extrinsic pathways of apoptosis: Role in cancer development and
prognosis. Adv Protein Chem Struct Biol. 125:73–120.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang Q, Liu J, Duan H, Li R, Peng W and
Wu C: Activation of Nrf2/HO-1 signaling: An important molecular
mechanism of herbal medicine in the treatment of atherosclerosis
via the protection of vascular endothelial cells from oxidative
stress. J Adv Res. 34:43–63. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tonelli C, Chio IIC and Tuveson DA:
Transcriptional Regulation by Nrf2. Antioxid Redox Signal.
29:1727–1745. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang Y, Yang C, Elsheikh NAH, Li C, Yang
F, Wang G and Li L: HO-1 reduces heat stress-induced apoptosis in
bovine granulosa cells by suppressing oxidative stress. Aging
(Albany NY). 11:5535–5547. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Furfaro AL, Piras S, Domenicotti C,
Fenoglio D, De Luigi A, Salmona M, Moretta L, Marinari UM, Pronzato
MA, Traverso N and Nitti M: Role of Nrf2, HO-1 and GSH in
neuroblastoma cell resistance to bortezomib. PLoS One.
11(e0152465)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X,
Jiang L and Ye L: Cetuximab promotes RSL3-induced ferroptosis by
suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant
colorectal cancer. Cell Death Dis. 12(1079)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Abdel-Wahab BA, Walbi IA, Albarqi HA, Ali
FEM and Hassanein EHM: Roflumilast protects from cisplatin-induced
testicular toxicity in male rats and enhances its cytotoxicity in
prostate cancer cell line. Role of NF-κB-p65, cAMP/PKA and
Nrf2/HO-1, NQO1 signaling. Food Chem Toxicol.
151(112133)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liang Y, Ye F, Xu C, Zou L, Hu Y, Hu J and
Jiang H: A novel survival model based on a Ferroptosis-related gene
signature for predicting overall survival in bladder cancer. BMC
Cancer. 21(943)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen ZA, Tian H, Yao DM, Zhang Y, Feng ZJ
and Yang CJ: Identification of a ferroptosis-related signature
model including mRNAs and lncRNAs for predicting prognosis and
immune activity in hepatocellular carcinoma. Front Oncol.
11(738477)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ye Y, Chen A, Li L, Liang Q, Wang S, Dong
Q, Fu M, Lan Z, Li Y, Liu X, et al: Repression of the antiporter
SLC7A11/glutathione/glutathione peroxidase 4 axis drives
ferroptosis of vascular smooth muscle cells to facilitate vascular
calcification. Kidney Int. 102:1259–1275. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jing S, Lu Y, Zhang J, Ren Y, Mo Y, Liu D,
Duan L, Yuan Z, Wang C and Wang Q: Levistilide a induces
ferroptosis by activating the Nrf2/HO-1 signaling pathway in breast
cancer cells. Drug Des Devel Ther. 16:2981–2993. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tang B, Yan R, Zhu J, Cheng S, Kong C,
Chen W, Fang S, Wang Y, Yang Y, Qiu R, et al: Integrative analysis
of the molecular mechanisms, immunological features and
immunotherapy response of ferroptosis regulators across 33 cancer
types. Int J Biol Sci. 18:180–198. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yadav P, Sharma P, Sundaram S, Venkatraman
G, Bera AK and Karunagaran D: SLC7A11/xCT is a target of miR-5096
and its restoration partially rescues miR-5096-mediated ferroptosis
and anti-tumor effects in human breast cancer cells. Cancer Lett.
522:211–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ghareghomi S, Moosavi-Movahedi F, Saso L,
Habibi-Rezaei M, Khatibi A, Hong J and Moosavi-Movahedi AA:
Modulation of Nrf2/HO-1 by natural compounds in lung cancer.
Antioxidants (Basel). 12(735)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang L, Guo J, Zhang Q, Zhou W, Li J, Yin
J, Cui L, Zhang T, Zhao J, Carmichael PL, et al: Flutamide induces
hepatic cell death and mitochondrial dysfunction via inhibition of
Nrf2-Mediated heme oxygenase-1. Oxid Med Cell Longev.
2018(8017073)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Labanca E, De Luca P, Gueron G, Paez A,
Moiola CP, Massillo C, Porretti J, Giudice J, Zalazar F, Navone N,
et al: Association of HO-1 and BRCA1 is critical for the
maintenance of cellular homeostasis in prostate cancer. Mol Cancer
Res. 13:1455–1464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jiang G, Liang X, Huang Y, Lan Z, Zhang Z,
Su Z, Fang Z, Lai Y, Yao W, Liu T, et al: p62 promotes
proliferation, apoptosis-resistance and invasion of prostate cancer
cells through the Keap1/Nrf2/ARE axis. Oncol Rep. 43:1547–1557.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li J, Xiong C, Xu P, Luo Q and Zhang R:
Puerarin induces apoptosis in prostate cancer cells via
inactivation of the Keap1/Nrf2/ARE signaling pathway.
Bioengineered. 12:402–413. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kwon SK, Saindane M and Baek KH: p53
stability is regulated by diverse deubiquitinating enzymes. Biochim
Biophys Acta Rev Cancer. 1868:404–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kanapathipillai M: Treating p53 mutant
aggregation-associated cancer. Cancers (Basel).
10(154)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Liu J, Zhang C, Hu W and Feng Z: Tumor
suppressor p53 and metabolism. J Mol Cell Biol. 11:284–292.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wei R, Zhao Y, Wang J, Yang X, Li S, Wang
Y, Yang X, Fei J, Hao X, Zhao Y, et al: Tagitinin C induces
ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal
cancer cells. Int J Biol Sci. 17:2703–2717. 2021.PubMed/NCBI View Article : Google Scholar
|