Introduction
Parkinsonism is a neurological syndrome and is
divided into primary and secondary parkinsonism. Primary
parkinsonism is caused by neurodegenerative disease and secondary
parkinsonism can be caused by a variety of factors, such as drugs,
vascular disease, toxicity, infection, and autoimmune, neoplastic,
metabolic, and functional diseases (1). The clinical symptoms are motor and
nonmotor. It is characterized by motor symptoms, including tremors,
rigidity, bradykinesia, gait disorders, and akinesia, and nonmotor
symptoms, such as cognitive decline, depression, anxiety, sleep
disturbance, and dysautonomia (2,3).
Accurate diagnosis of Parkinson's disease (PD) and parkinsonism
remains challenging. Clinical symptoms and levodopa challenge tests
are important for differentiation. PD typically responds better to
levodopa than parkinsonism (4).
Among adults, meningiomas occur most frequently in
individuals aged 65 and above. Overall, these tumors are observed
2.3 times more often in women than in men. Meningiomas are usually
benign and develop slowly. They are the most prevalent type of
extraparenchymal brain tumors, accounting for approximately 40% of
all brain tumors (5). The initial
symptoms of meningiomas typically include headaches, focal
symptoms, and cranial nerve symptoms. Some patients are diagnosed
without clinical symptoms during routine medical examinations. The
occurrence of meningiomas becomes more frequent as people get older
(5). Although meningiomas can
exhibit a range of clinical symptoms, parkinsonism is an unusual
initial symptom. Tumoral parkinsonism is rare and is defined as
parkinsonism that develops as a direct or indirect result of
tumors, such as infiltration or compression (6).
In this report, we present an unusual case of
meningioma in which parkinsonism manifested as the primary clinical
symptom. We describe the patient's clinical course of treatment,
explore the underlying mechanism responsible for this occurrence,
and predict symptom improvement based on preoperative imaging
findings.
Case report
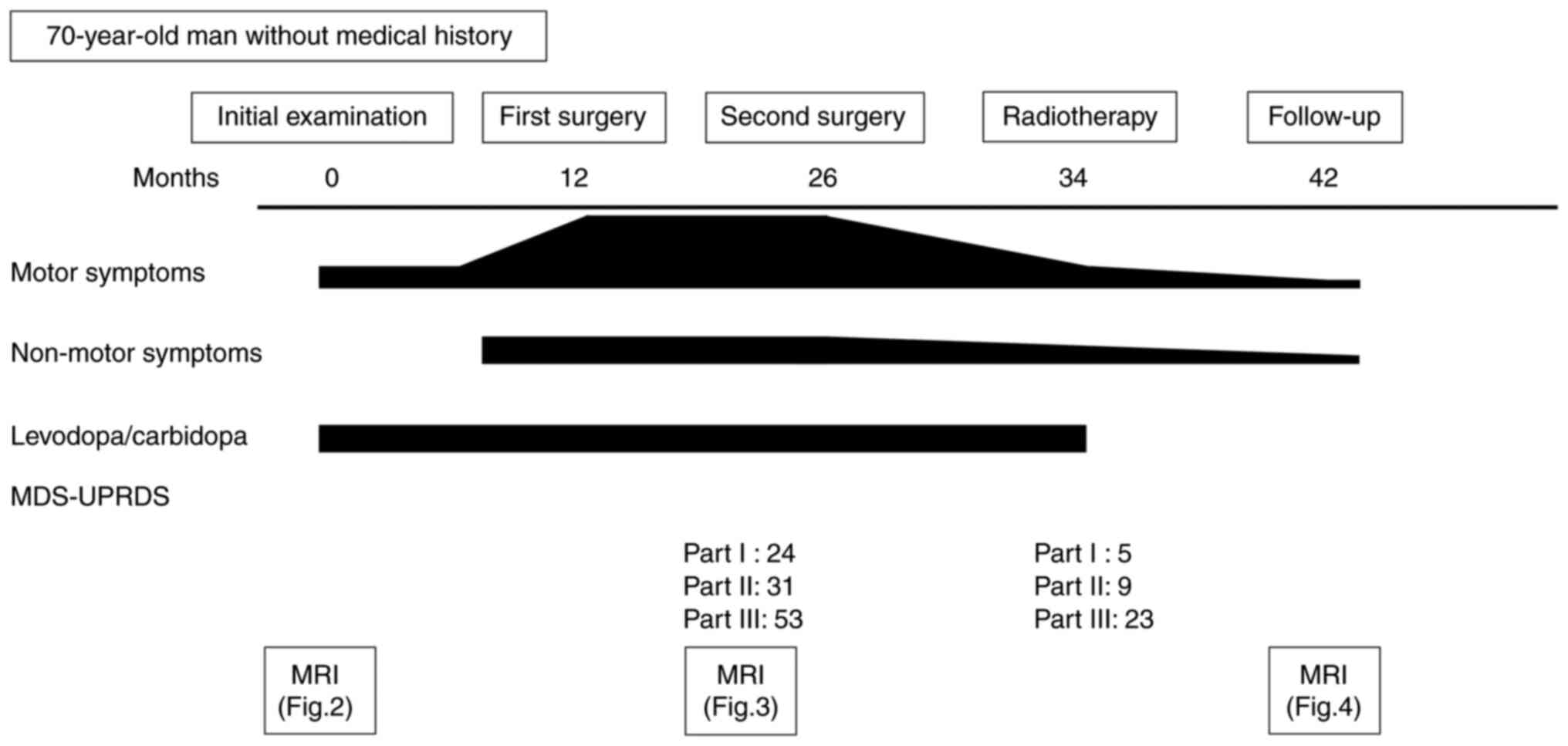
A 70-year-old man without a family history was
referred in November 2020 to the University of Occupational and
Environmental Health Hospital in Kitakyushu, Japan because of
involuntary movement 3 years prior to presentation. Examination
revealed resting tremors, pill-rolling tremors, and muscle rigidity
on the left side. Asymmetrical bradykinesia (left > right) and
mild postural instability were also observed. His eyeballs showed
no saccadic slowing or eye-movement limitations. Deep tendon
reflexes of the upper and lower extremities were normal. He did not
experienced paralysis and non-motor symptoms such as sensory
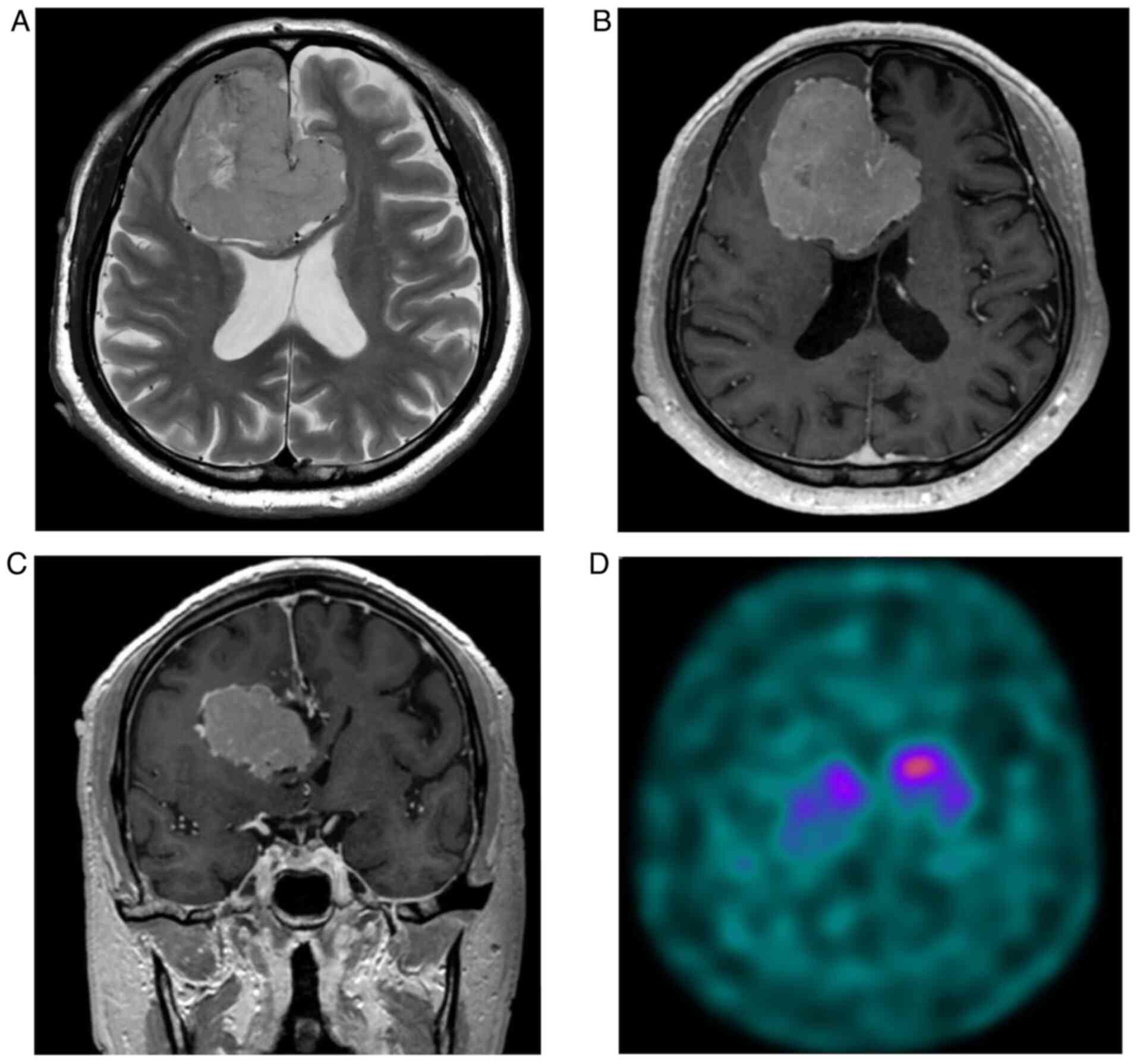
disorder, urinary incontinence, or cognitive decline (Fig. 1). Magnetic resonance imaging (MRI)
with T2-weighted images revealed dilated tortuous veins (Fig. 2A), and contrast-enhanced
T1-weighted imaging (CE-T1WI) revealed homogeneous enhancement of a
frontal lobe extra-axial giant lesion suggestive of a falx
meningioma (Fig. 2B and C). Dopamine transporter single-photon
emission computed tomography (DAT-SPECT) revealed decreased
123I-ioflupane uptake in the right striatum (Fig. 2D). Considering the diagnosis of PD
based on the DAT-SPECT findings, he was started on oral
levodopa/carbidopa. The patient refused surgery and was discharged
for frequent follow-up visits.
One year later, the patient continued to take
medication; however, his symptoms did not improve. Additionally,
his condition deteriorated, and he presented with urinary
incontinence, cognitive decline, and focal behavioral arrest
seizures. MRI with CE-T1WI revealed a growing tumor. The patient
subsequently underwent surgery. The tumor was removed using an
interhemispheric approach; however, it persisted due to
intraoperative blood loss. Pathological examination revealed the
proliferation of spindle or oval meningothelial cells, including
components arranged in fascicles or whorls, corresponding to a
transitional meningioma. The patient's symptoms did not improve
because the residual tumor compressed the right basal ganglia and
caused venous congestion. After 14 months, the residual tumor
enlarged. The Movement Disorder Society-Unified Parkinson's Disease
Rating Scale (MDS-UPRDS) Part I score was 24. In brief, the patient
experienced non-motor symptoms of PD such as cognitive decline,
urinary incontinence, depression, and constipation. Parts II and
III had Hoehn and Yahr (H&Y) stage 5 scores of 31 and 53,
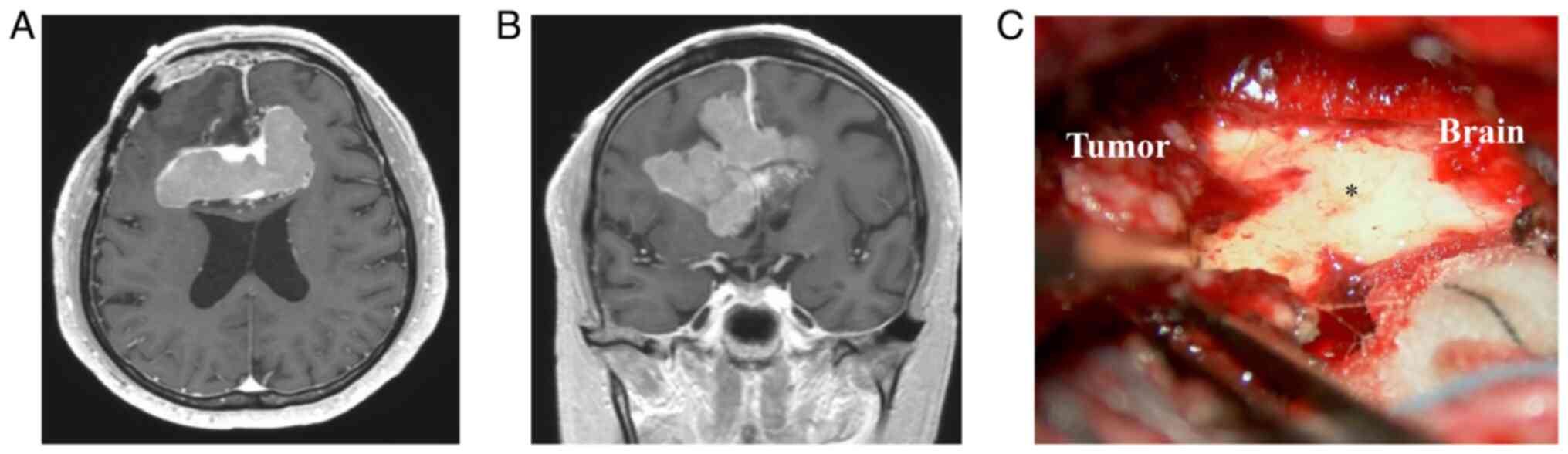
respectively. MRI with CE-T1WI revealed a homogeneously enhanced
tumor (Fig. 3A and B). The patient then underwent surgery
again. The tumor was resected via an interhemispheric approach. The
interhemispheric fissure was identified by opening the dura mater
and the tumor, which was covered with a hard capsule. Although the
tumor was attached to the surrounding brain, the arachnoid membrane
was preserved in some areas (Fig.
3C). The pathology was similar to that observed after the
initial surgery, and the intraoperative findings showed no tumor
invasion of the brain. The patient did not develop any
complications. Eight months later, the residual tumor had grown
slightly, and the patient underwent radiation therapy. The
MDS-UPRDS Part I score improved from 24 to 5. Overall, the
non-motor symptoms showed improvement, with the exception of minor
urinary incontinence and constipation. Parts II and III scored 9
and 23, respectively, as functions of H&Y stage 1. The patient
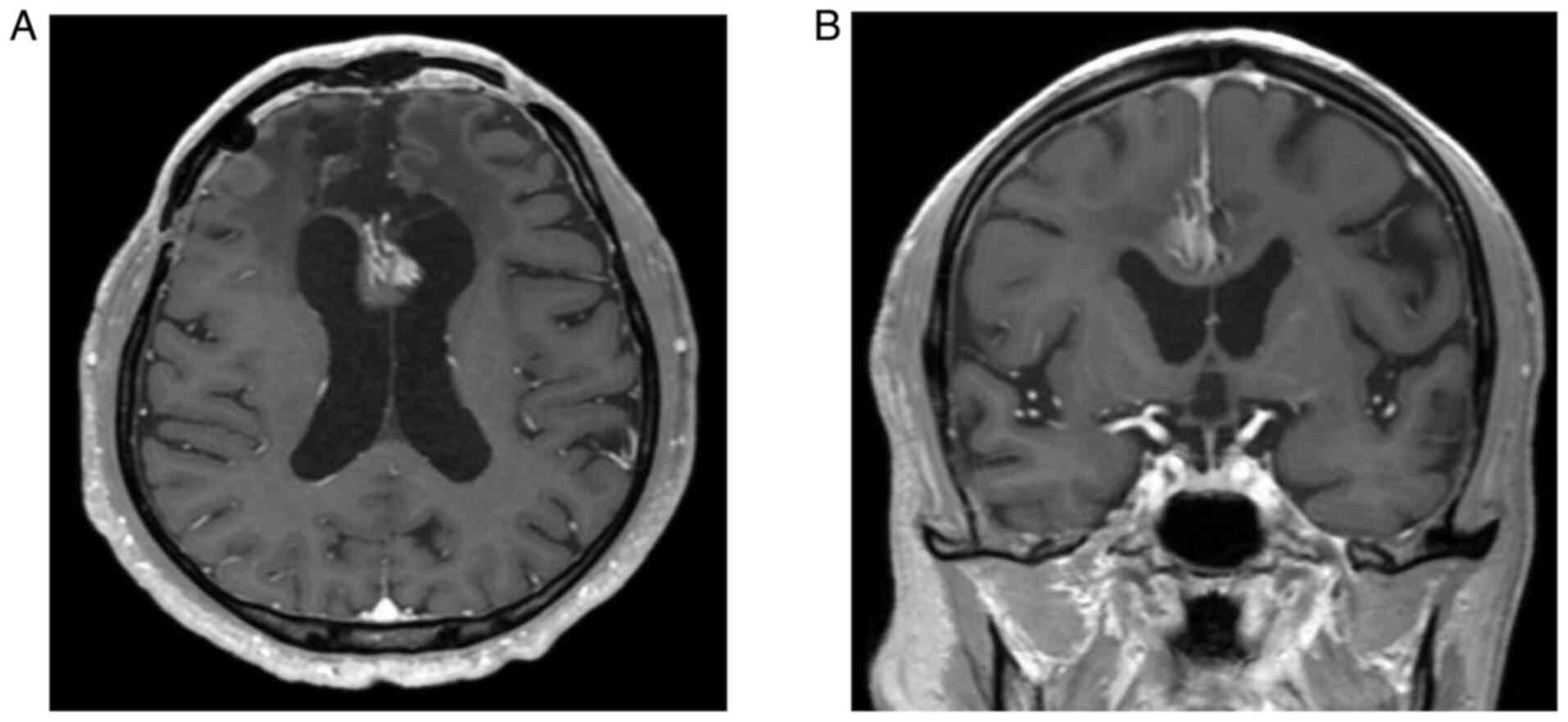
discontinued oral levodopa/carbidopa. Eight months later, an MRI
revealed a residual tumor; however, the basal ganglia were no
longer compressed (Fig. 4A and
B). The tumor was stable, and the
patient's symptoms did not change.
Discussion
Focal brain lesions can induce involuntary movement
disorders, such as hemichorea, hemiballism, dystonia, tremor,
myoclonus, parkinsonism, and asterixis (7). Cerebrovascular disease and stroke are
the major causes of this condition; however, other factors include
tumors, trauma, anoxia, and multiple sclerosis (7,8).
Parkinsonism has been observed in approximately 0.3% of patients
with supratentorial tumors, particularly those located in the
sphenoidal ridge or the frontal or parietal lobes (9). Brain tumors in the basal ganglia,
corpus callosum, periventricular white matter, midbrain, and
hypothalamus cause parkinsonism. Intraparenchymal tumors, such as
primary central nervous system lymphomas and gliomas, are
associated with parkinsonism (10,11).
Previous reports have described basal ganglia lymphoma-induced
parkinsonism, suggesting that tumor cell infiltration and damage to
neuronal membranes contribute to its development (11). Conversely, tumors with
extraparenchymal locations, such as meningiomas, may disrupt
neuronal circuits, including presynaptic dopaminergic neuronal
axons and the output pathway from the postsynaptic cells of the
basal ganglia circuit to the cortex. This disruption can result
from the mass effect of the tumor, leading to parkinsonism
(1). In tumor-associated
parkinsonism, DAT-SPECT may show decreased uptake due to tumor
invasion or compression (12,13).
In the present case, DAT SPECT revealed decreased
123I-ioflupane uptake in the right striatum.
Levodopa/carbidopa was administered for 1 year; however, symptoms
did not improve. Therefore, the patient was diagnosed with
meningioma-associated parkinsonism.
We hypothesized that involuntary movements,
including parkinsonism, are associated with the following two
parallel pathways: the cortico-cerebellar-cortical pathway and the
dentato-rubro-olivary pathway (Guillain-Mollaret triangle)
(8). The
cortico-cerebellar-cortical pathway comprises major afferent and
efferent fibers. Afferent fibers extend from the frontal lobe to
the cerebellar cortex via the pons. In contrast, efferent fibers
extend from the dentate nucleus to the motor cortex via the red
nucleus and thalamus. The dentato-rubro-olivary pathway comprises
the inferior olivary nucleus (ION). Efferent fibers from the
dentate nucleus to the contralateral red nucleus, red nucleus to
the ipsilateral ION, and ION to the contralateral cerebellum form a
triangular circuit that governs motor activity. Both basal ganglia
and cerebellar circuits function as subcortical loops that receive
and return cortical information. Brain tumors can influence the
output pathway of the basal ganglia circuit from the postsynaptic
cells to the cortex. These pathways may be infiltrated and
compressed by tumors (8). It is
often difficult to alleviate symptoms when tumor infiltration is
involved; however, meningiomas are more prone to mechanical
compression than infiltration into the basal ganglia circuit and
show improvement after surgery. Involuntary movements in meningioma
are rare. Since 2010, to our knowledge, involuntary movements in
seven meningioma cases improved after surgery (Table I) (1,14-19).
The mean age at diagnosis was 54.9 years (range, 41-67 years), and
all patients were female. Several clinical symptoms have been
reported previously. Six patients presented with tremors, followed
by parkinsonism in five patients. Two of the five patients received
levodopa but responded poorly. All patients demonstrated laterality
of clinical symptoms as opposed to tumor location; however, only
one patient had a tumor located in the bifrontal region. Four
tumors were located in the sphenoid ridge, followed by the frontal
lobe and midbrain. The meningiomas were removed in all patients,
and the symptoms included involuntary movements due to compression,
which improved postoperatively. Only seven cases of involuntary
movement in meningiomas have been reported in the literature.
Involuntary movements in patients with tumors may mimic these
symptoms, leading to misdiagnosis. Clinical features are not well
known; however, for tremors and parkinsonism, especially when there
are laterality symptoms and opposite to tumor location, clinicians
should suspect the risk of tumor-associated involuntary
movements.
 | Table ISummary of involuntary movements in
patients treated for meningioma since 2010. |
Table I
Summary of involuntary movements in
patients treated for meningioma since 2010.
| First author,
year | Case | Age, years/sex | Clinical
symptoms | Tumor location | Laterality | Treatment | Prognosis | (Refs.) |
|---|
| Diyora, 2014 | 1 | 50/F | Headache, dystonic
head tremor, resting tremor | Lt. sphenoid
ridge | Right | Surgery | Improved | (14) |
| Kim, 2014 | 2 | 58/F | Resting tremor,
bradykinesia, gait disorder | Lt. sphenoid
ridge | Right | Surgery | Improved | (1) |
| Kleib, 2016 | 3 | 41/F | Resting tremor,
bradykinesia, rigidity, paralysis | Lt. sphenoid
ridge | Right | Surgery | Improved | (15) |
| Fong, 2016 | 4 | 58/F | Hypomimic face,
hypophonic speech, gait disorder, resting pill-rolling tremor,
rigidity, bradykinesia | Lt. frontal
tumor | Right | Surgery | Improved | (16) |
| Labate, 2018 | 5 | 67/F | Ataxia, hypotension,
urinary incontinence, hyperreflexia, gait disorder, bradykinesia,
rigidity, postural and resting tremor | Lt. midbrain | Both | Surgery | Improved | (17) |
| Al-Janabi, 2019 | 6 | 65/F | Resting tremor,
bradykinesia, rigidity | Bil. anterior cranial
fossa | Right | Surgery | Improved | (18) |
| Inoue, 2021 | 7 | 45/F | Hemichorea | Rt. sphenoid
ridge | Left | Surgery | Improved | (19) |
| Present study | 8 | 70/M | Tremor, pill-rolling
tremor, rigidity, bradykinesia, postural instability | Bil. anterior cranial
fossa | Left | Surgery | Improved | - |
In our cases, the patient showed laterality of
parkinsonism. The first surgery slightly improved the mechanical
compression but did not improve the parkinsonism because the venous
congestion did not improve; however, the second surgery improved
symptoms by relieving the compression of the basal ganglia and
venous congestion. Previous reports have focused on mechanical
compression caused by meningiomas, and the fact that the symptoms
did not improve after the first surgery, even though the mechanical
compression was relieved, may be due to cortical damage from venous
congestion. Considering tumors associated with parkinsonism is
important when clinicians suspect the laterality of parkinsonism.
Tumor removal decompresses the basal ganglia, resulting in the
improvement of parkinsonism, especially in meningiomas.
In conclusion, various pathogeneses, including
trauma, drug-induced cerebrovascular disorders, and brain tumors
can cause parkinsonism. This report presents a rare case of
meningioma presenting with parkinsonism as an initial manifestation
in an older adult. Parkinsonism is related to the
cortico-cerebellar-cortical pathway and the Guillain-Mollaret
triangle. Therefore, parkinsonism is rare in both intraparenchymal
and extraparenchymal tumors, however, parkinsonism caused by tumor
compression or venous congestion is more easily ameliorated than
that caused by tumor cell infiltration. Parkinsonism in patients
with brain tumors, particularly meningiomas, can be reversed with
surgical treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ST and SN drafted the manuscript and wrote the final
draft. KS, KF and JY revised the manuscript and provided
constructive feedback. SN and JY performed the surgeries. ST, KF,
KS and SN analyzed all the images. SN, KF and JY confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case details and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim J-I, Choi JK, Lee J-W and Hong JY:
Intracranial meningioma-induced parkinsonism. J Lifestyle Med.
4:101–103. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hayes MT: Parkinson's disease and
parkinsonism. Am J Med. 132:802–807. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tolosa E, Garrido A, Scholz SW and Poewe
W: Challenges in the diagnosis of Parkinson's disease. Lancet
Neurol. 20:385–397. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sveinbjornsdottir S: The clinical symptoms
of Parkinson's disease. J Neurochem. 139 (Suppl):318–324.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2015-2019. Neuro Oncol. 24
(Suppl):v1–v95. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cedergren Weber G, Timpka J, Rydelius A,
Bengzon J and Odin P: Tumoral parkinsonism-Parkinsonism secondary
to brain tumors, paraneoplastic syndromes, intracranial
malformations, or oncological intervention, and the effect of
dopaminergic treatment. Brain Behav. 13(e3151)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Defebvre L and Krystkowiak P: Movement
disorders and stroke. Rev Neurol (Paris). 172:483–487.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi S-M: Movement disorders following
cerebrovascular lesions in cerebellar circuits. J Mov Disord.
9:80–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Krauss JK, Paduch T, Mundinger F and
Seeger W: Parkinsonism and rest tremor secondary to supratentorial
tumours sparing the basal ganglia. Acta Neurochir. 133:22–29.
1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi K-H, Choi S-M, Nam T-S and Lee M-C:
Astrocytoma in the third ventricle and hypothalamus presenting with
parkinsonism. J Korean Neurosurg Soc. 51:144–146. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Merrill S, Mauler DJ, Richter KR,
Raghunathan A, Leis JF and Mrugala MM: Parkinsonism as a late
presentation of lymphomatosis cerebri following high-dose
chemotherapy with autologous stem cell transplantation for primary
central nervous system lymphoma. J Neurol. 267:2239–2244.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Okano R, Suzuki K, Nakano Y and Yamamoto
J: Primary central nervous system lymphoma presenting with
Parkinsonism as an initial manifestation: A case report and
literature review. Mol Clin Oncol. 14(95)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rodriguez W, Fedorova M and Chand P:
Levodopa-responsive parkinsonian syndrome secondary to a
compressive craniopharyngioma: A case report. Cureus.
15(e35621)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Diyora B, Kukreja S and Sharma A: Cerebral
meningioma presenting as dystonic head tremor. Mov Disord.
29(40)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kleib AS, Sid'Ahmed E, Salihy SM,
Boukhrissi N, Diagana M and Soumaré O: Hemiparkinsonism secondary
to sphenoid wing meningioma. Neurochirurgie. 62:281–283.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fong M, Ghahreman A, Masters L and Huynh
W: Large intracranial meningioma masquerading as Parkinson's
disease. J Neurol Neurosurg Psychiatry. 87(1251)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Labate A, Nisticò R, Cherubini A and
Quattrone A: Midbrain meningioma causing subacute parkinsonism.
Neurol Clin Pract. 8:166–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Al-Janabi WSA, Zaman I and Memon AB:
Secondary parkinsonism due to a large anterior cranial fossa
meningioma. Eur J Case Rep Intern Med. 6(001055)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Inoue H, Yamamura R, Yamada K, Hamasaki T,
Inoue N and Mukasa A: Hemichorea induced by a sphenoid ridge
meningioma. Surg Neurol Int. 12(201)2021.PubMed/NCBI View Article : Google Scholar
|