Introduction
Bipolar disorder (BD) is associated with severe
mental disability and an increased risk of suicide, significantly
contributing to the societal burden (1,2).
Insomnia and anxiety, two common concomitant symptoms of BD, often
persist throughout the course of the illness. Approximately 45% of
individuals with BD will experience co-morbid anxiety disorders
over their lifetime (3). This
comorbidity further exacerbates the risk of substance dependence
and suicide (4), worsening social
functioning and quality of life in individuals with BD (5). Crucially, anxiety symptoms have
become a key target for early intervention in individuals at risk
of BD (6), anxiety in BD is
associated with an increased risk of subjective sleep disturbances
(7). Sleep, as a key regulator of
both metabolic homeostasis and oxidative stress, plays a
particularly significant role; when disrupted, it can contribute to
the onset and progression of BD. Given their role as
cross-diagnostic precursors, insomnia and anxiety represent
high-priority therapeutic targets for preventing BD episodes
(8,9).
The N-methyl-D-aspartate receptor subunit 2B
(GRIN2B) gene, which is located at 12p13.1, spans 419
kilobases and consists of 15 exons. N-methyl-D-aspartate receptors
(NMDARs), a class of ionotropic glutamate receptors, are involved
in regulating neuronal activity, synaptic plasticity and excitatory
transmission, with substantial implications for the pathophysiology
and treatment of affective disorders (10). The GRIN2B gene encodes the
NMDAR subunit 2B (NR2B), which is crucial for determining both the
structure and functional dynamics of NMDARs; overexpression of NR2B
leads to long-lasting enhancement and increased synaptic efficacy
(11). Light, which is the primary
driver of sleep rhythm regulation (12), triggers numerous intracellular
cascades through NMDARs in the suprachiasmatic nucleus (SCN),
ultimately affecting the expression of clock genes (13) studies have demonstrated that
circadian rhythm disturbances in mice correlate with reduced
expression of the NR2B subunit of NMDARs in the SCN (14,15).
Additionally, rats subjected to maternal separation showed a
significant increase in anxiety, accompanied by upregulated
expression of the GRIN2B gene (16), while ethanol-exposed mice displayed
anxiety-like behaviors with elevated levels of GRIN2B mRNA
expression in the cerebellum (17).
DNA methylation, one of the most stable epigenetic
mechanisms, is increasingly recognized as a potential biomarker for
numerous neuropsychiatric disorders (18) and various malignancies (19). It plays a significant role in the
pathophysiology of BD (20) and
may serve as a biomarker for variability in BD treatment response
(21). Numerous studies have
identified significant DNA methylation changes in patients with BD,
particularly in regions such as the prefrontal cortex and
peripheral leukocytes (22-24),
with potential links to suicidal behavior (25), cognitive impairment (26) and substance dependence (27). Emerging evidence from cancer
epigenetics demonstrates that methylation patterns can effectively
stratify disease subtypes and predict clinical outcomes (28,29),
suggesting similar precision medicine applications could be
explored in BD. However, few studies have specifically examined the
associations of DNA methylation with anxiety and insomnia in BD,
despite established methodological frameworks for epigenetic
analysis in other neuropsychiatric conditions, such as depression
(30) and schizophrenia (31).
Based on these findings, GRIN2B epigenetics
appears to play a significant role in the development of BD and
related anxiety and insomnia symptoms. However, the role of
GRIN2B in BD remains controversial; a study detected no
disease-associated variants in glutamatergic genes (including
GRIN2B) through targeted sequencing in patients with BD
(32), whereas another study
reported associations between GRIN2B genotypes and both
psychotic symptoms and disease relapse in BD (33). Research on GRIN2B gene
inheritance as it relates to anxiety and insomnia has been largely
restricted to animal models (34,35).
Therefore, to further clarify the relationship between
GRIN2B gene inheritance (particularly DNA methylation) and
BD, as well as its potential role in anxiety and insomnia among
patients with BD, DNA methylation levels were measured in the
GRIN2B gene's promoter region in peripheral blood leukocytes
from patients with BD and healthy controls via the MassARRAY method
and the possibility of an association was analyzed.
Patients and methods
Participants and procedure
A total of 31 patients diagnosed with BD (in the
depressive phase) were recruited from the inpatient units of the
Clinical Psychology Department at the People's Hospital of Xinjiang
Uygur Autonomous Region (Urumqi, China) between April and December
2023, while 32 healthy controls (HCs) were concurrently enrolled
from hospital staff and students at the affiliated Medical
University during the same study period. All participants were aged
18-55 years. The upper age limit was set to 55 years to minimize
age-related confounding factors. This threshold aligns with
evidence that epigenetic drift accelerates after age 55(36), age-associated methylation changes
increasingly interact with inflammatory/metabolic pathways beyond
age 50(37) and neurodegenerative
comorbidities in older populations may introduce spurious
associations (38). BD diagnosis
was confirmed using the Diagnostic and Statistical Manual of Mental
Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria
(39), based on structured
clinical interviews conducted by two senior psychiatrists. Eligible
patients met the following criteria: i) No medication,
psychotherapy or physical therapy for ≥6 months before recruitment;
ii) Hamilton Depression Rating Scale (HAMD)-24 [specifying the
24-item Hamilton Depression Rating Scale (HAMD-24); total score
range: 0-76] to distinguish clinical severity thresholds from the
17-item version (HAMD-17), total score >20(40); and iii) Young Mania Rating Scale
(YMRS) total score <7(41). The
exclusion criteria (applied to all participants) were as follows:
i) Comorbid psychiatric disorders (DSM-IV-TR axis I/II) (42); ii) history of organic brain disease
or severe systemic illness; iii) pregnancy or lactation (women);
and iv) substance abuse/dependence (drugs, alcohol or psychoactive
substances). This study was reviewed and approved by the Ethics
Committee of the People's Hospital of Xinjiang Uygur Autonomous
Region (Urumqi, China; approval no. KY2023020968). All subjects
volunteered to participate in the study and provided written
informed consent prior to the study.
Data collection
Demographic data, including age, gender, education
level and history of smoking and alcohol use, were collected from
all participants. Clinical data, such as age at onset, illness
duration, subtype, HAMD-24 score, depression severity, YMRS score,
14-item Hamilton Anxiety Scale (HAMA-14) (43) score and Pittsburgh Sleep Quality
Index (PSQI) (44) score, were
obtained from patients only.
DNA extraction and bisulfite
conversion
Peripheral fasting venous blood samples (~5 ml) were
collected from patients with BD using EDTA anticoagulant tubes (BD
Biosciences) within 24 h of hospital admission, prior to the
initiation of any new pharmacotherapy. Healthy control samples were
obtained the following morning after enrollment. Genomic DNA was
subsequently extracted using the solution-based Genenode DNA
extraction kit (Wuhan Genenode Biotech) according to the
manufacturer's protocol and stored at -80˚C. Quality control
assessments included spectrophotometric analysis for determining
DNA concentration and agarose gel electrophoresis to verify DNA
integrity. Following extraction, the isolated DNA underwent
modification and purification using the EZ DNA Methylation-Gold™
Kit (Zymo Research Corp.) according to the manufacturer's protocol.
During this process, cytosine residues that were non-methylated
were deaminated to uracil, while methylated cytosine residues
remained stable.
DNA methylation assay. Primer
design
The CpG island prediction website was utilized to
predict potential CpG islands in the GRIN2B promoter region
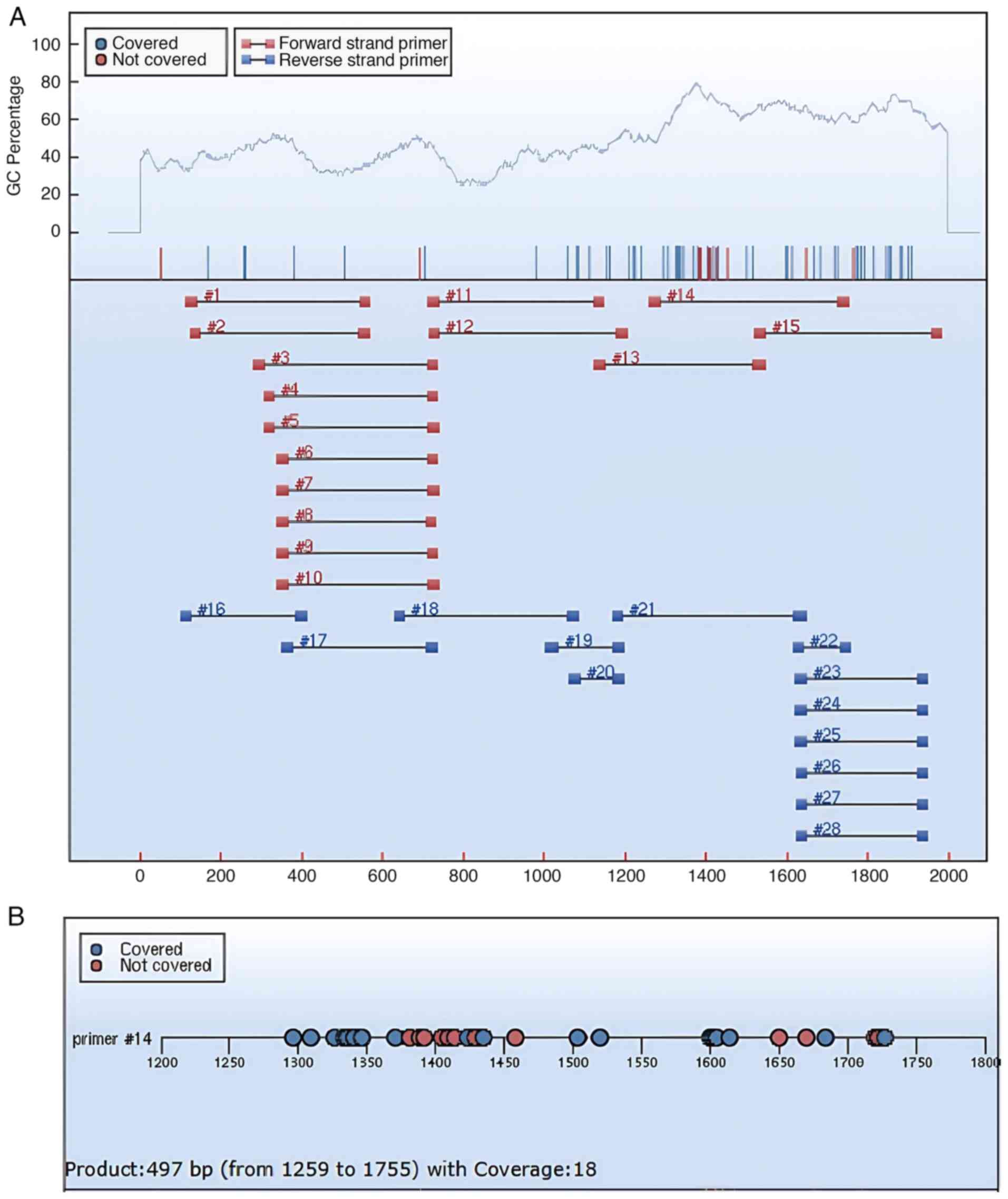
gene sequences, identifying two CpG islands (Fig. 1A). The Agena EpiDesigner program
(http://www.epidesigner.com) was used to
design primers for the target GRIN2B sequences (Fig. 1B), and a fragment with a high level
of CpG methylation (#14 in Fig.
2A) was selected as the primer sequence (Fig. 2A and B). Details of the primer sequences are
provided in Table I.
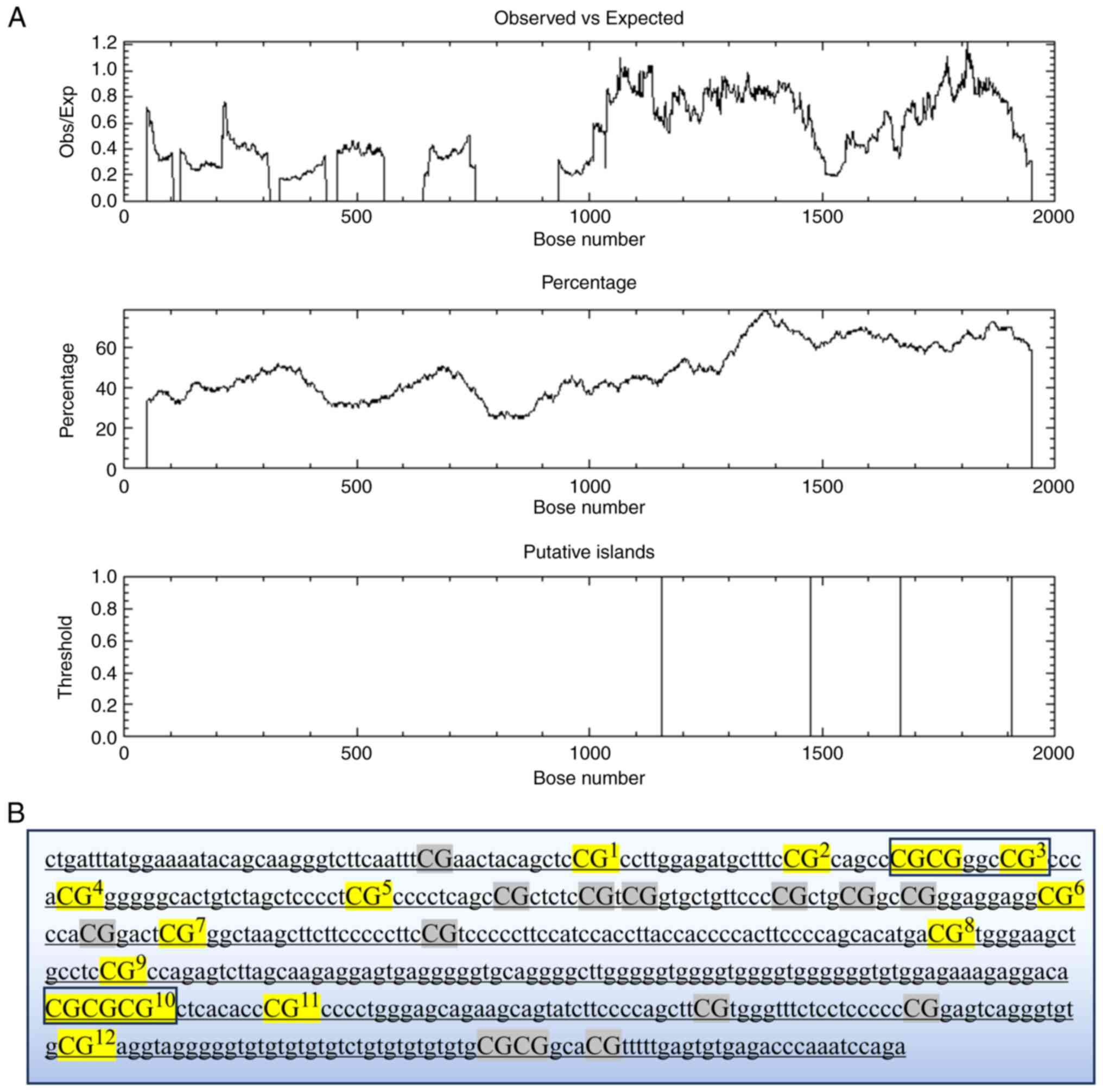 | Figure 1(A) CpG islands predicted by EMBOSS.
CpG island locations: Island 1 (1,157-1,475, 319 bp); Island 2
(1,670-1,908, 239 bp); Core criteria: Obs/Exp, >0.6; GC%,
>50; length, >200 bp. (B) The target GRIN2B sequences: The
black boxes highlight the contiguous CpG sites analyzed, with the
reported values representing the average methylation level across
these sites. Undetectable CpG sites are indicated in gray, while
the 12 successfully interrogated CpG sites are marked in yellow.
Obs, observed; Exp, expected. |
 | Table IPrimer information. |
Table I
Primer information.
| Primer | 5' end primer
sequence | 3' end primer
sequence |
|---|
| Start, bp | 1,259 | 1,755 |
| Size, bp | 28 | 27 |
| Tm, ˚C | 58.14 | 57.06 |
| GC% | 25 | 22 |
| Cs | 4 | 5 |
| Original
sequence |
CTGATTTATGGAAAATACAGCAAGGGTC |
TCTGGATTTGGGTCTCACACTCAAAAA |
| Methylation
sequence |
TTGATTTATGGAAAATATAGTAAGGGTT |
TCTAAATTTAAATCTCACACTCAAAAA |
| Insert
sequence | aggaagagag |
cagtaatacgactcactatagggagaaggct |
Methylation assay of GRIN2B. The
bisulfite-converted GRIN2B DNA was amplified by PCR using
the primers mentioned above. Optimal amplification conditions were
achieved through digestion with shrimp alkaline phosphatase,
transcriptional cleavage and resin-based purification. The purified
PCR product was then transferred to a 384-well
SpectroCHIP® bioarray (Axygen; Corning, Inc.) for
precise spot sampling using the Agena Nanodispenser RS1000 spotter
(Agena Bioscience). The spotted bioarray was analyzed by
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry with the MassARRAY Analyzer 4.0 (Axygen; Corning,
Inc.) to generate mass spectra. Methylation levels were then
quantified from these spectra using Epityper 1.2 software
(Sequenom).
Statistical analysis
All analyses were performed using SPSS 27.0 (IBM
Corp.) and OriginPro 8.5.0 (OriginLab Corp.). Continuous variables
were assessed for normality using Shapiro-Wilk tests. Normally
distributed data were expressed as the mean ± standard deviation
and compared using independent-samples t-tests. Non-normally
distributed data were reported as the median (interquartile range)
and analyzed with Mann-Whitney U-tests. Categorical variables were
presented as frequencies (percentages) and evaluated by χ² tests.
All group comparisons were adjusted using the Bonferroni method to
control for Type I error inflation. Binary logistic regression
models adjusted for age, sex and education duration were
constructed to examine the association between GRIN2B
promoter methylation (independent variable) and bipolar disorder
diagnosis (dependent variable). Partial correlation analyses (based
on the Pearson correlation coefficient) controlling for age, sex,
education level and HAMD scores were conducted to assess
relationships between GRIN2B methylation levels and clinical
symptom severity, specifically insomnia severity (PSQI) and anxiety
severity (HAMA-14). A two-sided α-level of 0.05 was set as the
statistical significance threshold for all analyses. All tests were
performed with 95% confidence intervals (95% CI).
Results
Sample description
In Table II, the
demographic and clinical profiles of patients with BD and healthy
controls were presented. The BD group included 31 participants [8
male (25.8%), 23 female (74.2%)] with a median age of 23 years
[interquartile range (IQR), 20-39], while the HC group comprised 32
individuals [11 male (34.4%), 21 female (65.6%)] with a median age
of 26.5 years (IQR, 22.0-37.5). No statistically significant
differences were observed between the groups in terms of age, years
of education, gender or smoking and drinking history
(P>0.05).
 | Table IIDemographic and clinical information
of BD and HC groups. |
Table II
Demographic and clinical information
of BD and HC groups.
| Item | BD (n=31) | HC (n=32) |
χ2/t/Z | P-value |
|---|
| Age, years | 23.0
(20.0-39.0) | 26.5
(22.0-37.5) | -1.343 | 0.179 |
| Education,
years | 16.0
(15.0-16.0) | 16.0
(16.0-16.0) | -0.748 | 0.455 |
| Sex,
male/female | (8,23) | (11,21) | 0.207 | 0.649 |
| Smoking,
yes/no | (6,25) | (8,24) | 0.290 | 0.590 |
| Drinking,
yes/no | (6,25) | (7,25) | 0.061 | 0.805 |
| Age of onset,
years | 20
(16.58-31.00) | - | - | - |
| Disease duration,
years | 4 (1.42-8.00) | - | - | - |
| HAMD-24 | 32 (23-36) | - | - | - |
| YMRS | 2 (2-3) | - | - | - |
| HAMA-14 | 19 (15-29) | - | - | - |
| PSQI | 14.32±4.05 | - | - | - |
| Subtype
(BDI/BDII) | (13,18) | - | - | - |
| Depression severity
(moderate/severe) | (18,13) | - | - | - |
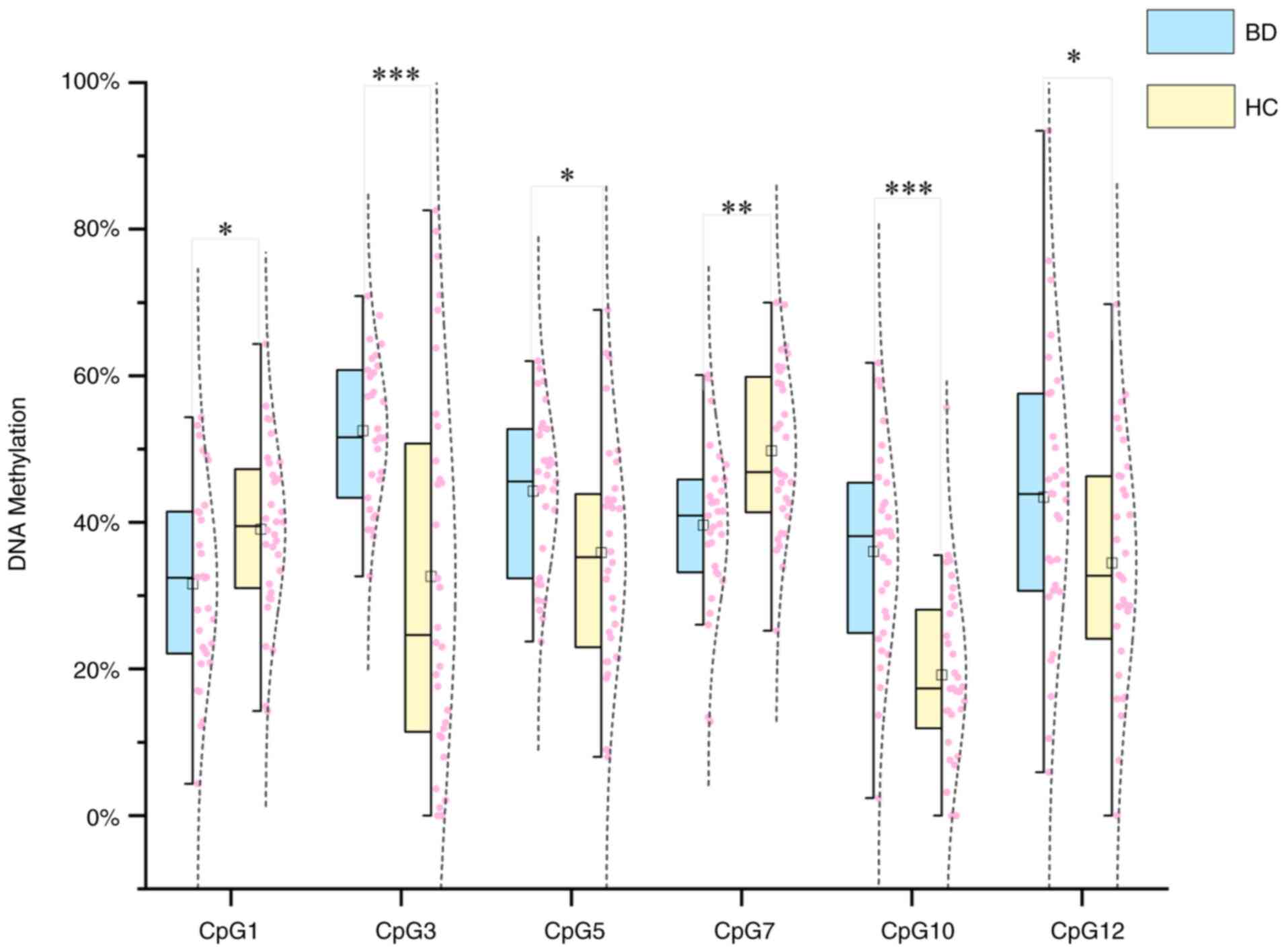
GRIN2B DNAm and BD. Altered GRIN2B
DNAm patterns in patients with BD
In the present study, the DNA methylation levels of
12 CpG sites in the GRIN2B promoter region were compared
between patients with BD and HCs. An independent-samples t-test
showed that 6 out of the 12 CpG sites (50%) had statistically
significant differences in DNA methylation levels between the
groups (Fig. 3). Specifically,
CpG3, CpG5, CpG10 and CpG12 showed hypermethylation, while CpG1 and
CpG7 showed hypomethylation in patients with BD compared to HCs.
Among these, CpG3 (BD: 52.52±10.00%; HC: 32.60±25.70%) and CpG1
(BD: 36.03±13.94%; HC: 19.15±12.33%) exhibited the most significant
differences in methylation levels between the two groups
(P<0.001).
Association of BD with GRIN2B methylation.
The predictive role of GRIN2B DNA methylation in the development of
BD was analyzed using a binary logistic regression model with
stepwise forward selection. Age, gender and years of education were
included as covariates and the final model chosen through stepwise
selection yielded a chi-square test value of χ²=44.49 (P<0.001),
indicating model significance. The Hosmer-Lemeshow test result was
χ²=3.896 (P=0.866), suggesting a good fit. The model identified
four variables associated with the likelihood of BD development,
specifically the hypermethylation of CpG3, CpG5, CpG10 and CpG12.
Among these, CpG3 [P=0.002, odds ratio (OR)=1.079, 95% CI:
1.029-1.132] and CpG10 (P=0.002, OR=1.113, 95% CI: 1.042-1.190)
demonstrated a significant predictive role (Table III).
 | Table IIIBinary logistic regression analysis
of the effect of GRIN2B DNA methylation on bipolar
disorder. |
Table III
Binary logistic regression analysis
of the effect of GRIN2B DNA methylation on bipolar
disorder.
| CpG methylation
site | B | SE | Wald | P-value | OR | 95%CI |
|---|
| CpG3 | 0.076 | 0.024 | 9.759 | 0.002 | 1.079 | 1.029-1.132 |
| CpG5 | 0.082 | 0.034 | 5.798 | 0.016 | 1.085 | 1.015-1.160 |
| CpG10 | 0.107 | 0.034 | 10.058 | 0.002 | 1.113 | 1.042-1.190 |
| CpG12 | 0.045 | 0.022 | 4.153 | 0.042 | 1.046 | 1.002-1.093 |
Association of GRIN2B DNA methylation
with anxiety and insomnia in BD
Partial correlation analysis (Table IV) revealed that the HAMA-14 score
was positively associated with methylation levels of CpG9 (r=0.408,
P=0.038) after controlling for age, gender and years of education
(r=0.419, P=0.033). Similarly, the PSQI score showed a positive
correlation with methylation levels of CpG8 at the GRIN2B
locus. No significant correlations were observed between other CpG
sites and either the HAMA or PSQI scores (P>0.05).
 | Table IVCorrelation analysis of GRIN2B
DNA methylation with anxiety and insomnia in bipolar disorder. |
Table IV
Correlation analysis of GRIN2B
DNA methylation with anxiety and insomnia in bipolar disorder.
| | HAMA score | PSQI score |
|---|
| Methylation % at
CpG site | r | P-value | r | P-value |
|---|
| CpG1 | -0.178 | 0.383 | -0.172 | 0.402 |
| CpG2 | 0.098 | 0.635 | -0.033 | 0.874 |
| CpG3 | -0.036 | 0.862 | -0.061 | 0.767 |
| CpG4 | 0.107 | 0.602 | 0.194 | 0.342 |
| CpG5 | -0.043 | 0.833 | -0.286 | 0.157 |
| CpG6 | 0.169 | 0.408 | -0.029 | 0.887 |
| CpG7 | -0.240 | 0.237 | -0.198 | 0.332 |
| CpG8 | 0.159 | 0.439 | 0.419 | 0.033 |
| CpG9 | 0.408 | 0.038 | 0.312 | 0.121 |
| CpG10 | 0.144 | 0.484 | 0.251 | 0.215 |
| CpG11 | -0.081 | 0.696 | 0.057 | 0.783 |
| CpG12 | -0.313 | 0.119 | -0.021 | 0.921 |
Discussion
To our knowledge, this study provides the first
evidence of differential DNA methylation patterns in the
GRIN2B promoter region among patients with BD. The present
findings not only demonstrate the association between GRIN2B
methylation status and BD diagnosis but also reveal clinically
meaningful correlations with comorbid anxiety symptoms and sleep
disturbances in this population.
The present analysis revealed differential DNA
methylation patterns at specific CpG sites within the GRIN2B
promoter region between patients with BD and HCs. Among the 12
analyzed CpG loci, six exhibited methylation alterations: CpG3,
CpG5, CpG10 and CpG12 showed hypermethylation, whereas CpG1 and
CpG7 displayed hypomethylation. Notably, after adjusting for
covariates through logistic regression, only the hypermethylation
patterns at CpG3, CpG5, CpG10 and CpG12 maintained statistical
significance, suggesting that site-specific epigenetic
modifications may be particularly relevant to BD pathophysiology.
The GRIN2B gene encodes a critical subunit of the NMDAR
complex and DNA methylation-dependent transcriptional modulation
may alter NMDAR subunit composition, potentially disrupting
synaptic plasticity in prefrontal-temporal-limbic circuits. The
functional impact of BD-associated GRIN2B polymorphisms
identified in German-Jewish (45)
and Chinese Han populations (46)
could be modulated by the local methylation status. Notably, the
clinical relevance of these mechanisms is underscored by prior
findings that the GRIN2B genotype predicts psychotic symptom
severity and relapse frequency in patients with BD (33), suggesting methylation-mediated
expression changes may similarly impact the disease course.
Intriguingly, the therapeutic efficacy of quetiapine in BD may
involve GRIN2B-related pathways, as molecular docking
studies suggest its interaction with neuroactive ligand-receptor
systems regulated by GRIN2B (47). Hypermethylation-induced
GRIN2B downregulation could reduce NMDAR density in key
brain regions (e.g., prefrontal cortex and hippocampus) (48) creating a neurobiological state
amenable to mood stabilizer modulation. While the present findings
align with gene-environment interaction models proposing
methylation as a dynamic interface between genetic predisposition
and environmental stressors, three critical considerations emerge:
First, the observed methylation differences may represent either
compensatory adaptations or pathogenic processes. Second,
glutamatergic dysfunction (49)
could serve as both precursor and consequence of neurodevelopmental
alterations in BD; and third, the functional consequences of
site-specific methylation require validation through
allele-specific expression analyses.
In the present study, it was also found that DNA
methylation levels of GRIN2B were associated with anxiety
and insomnia symptoms in BD, independent of age, gender, education
or depression severity. This epigenetic alteration may contribute
to BD-related psychopathology by suppressing GRIN2B
transcription, thereby reducing the availability of functional NR2B
subunits-a critical component of NMDARs implicated in synaptic
plasticity and glutamatergic signaling (50), diminished NR2B activity could
impair glutamate-mediated neurotransmission in limbic circuits
(e.g., prefrontal cortex-amygdala connectivity and hippocampal
networks), which are essential for emotion regulation and stress
adaptation (34,51-53).
The association between GRIN2B methylation
levels and anxiety symptoms may stem from dysregulated
NMDAR-dependent excitatory-inhibitory balance. Specifically,
hippocampal NMDARs are critical to behavioral inhibitory systems
(40), and NR2B subunit
deficiency-induced functional impairment could exacerbate
anxiety-related neural hyperactivity. For instance, preclinical
studies demonstrate that environmental stressors [e.g., benzo (a)
pyrene exposure or high-fat diets] induce anxiety-like behaviors in
tandem with elevated GRIN2B methylation in brain regions
governing fear responses (34,54)
epigenetic silencing likely attenuates NMDAR-mediated synaptic
potentiation in the ventral hippocampus and anterior cingulate
cortex-key regions where NR2B modulates anxiety-like phenotypes
(52,53,55),
ketamine's rapid anxiolytic effects-mediated by NMDAR antagonism
(56)-further support the
hypothesis that GRIN2B methylation-driven NMDAR hypofunction
could perpetuate anxiety states in BD.
Insomnia is a hallmark symptom of anti-NMDAR
encephalitis, with clinical evidence underscoring the critical role
of NMDAR dysfunction in sleep architecture disruption (57), aberrant GRIN2B methylation
may perturb sleep-wake regulation through two interconnected
pathways: i) Diminished NR2B expression in the hypothalamic SCN,
where NR2B-containing NMDARs are essential for circadian rhythm
entrainment (15,58); and ii) impaired Non-Rapid Eye
Movement (NREM) sleep homeostasis caused by reduced NMDAR-mediated
excitability in the lateral preoptic hypothalamus, a key region for
sleep initiation and maintenance (59). The findings of the present study
align with chronic sleep deprivation studies in young mice
demonstrating reduced NR2B levels (35), suggesting that GRIN2B
aberrant methylation may exacerbate insomnia by impairing
NMDAR-dependent synaptic adaptation to sleep pressure.
Additionally, selective NR2B antagonism disrupts cortical gamma
oscillations during REM sleep (60), indicating that GRIN2B
epigenetic silencing could destabilize sleep architecture through
dysregulated glutamatergic neurotransmitter interactions.
While the present study yielded promising results,
several critical limitations must be acknowledged. First, the
conclusions may be constrained by the relatively small sample size,
which reduces statistical power to detect robust epigenetic
associations. Given the established clinical and biological
heterogeneity of BD, the limited sample of the present study may
not adequately represent the full spectrum of disease subtypes.
These factors, combined with the cross-sectional design, preclude
causal inferences; it cannot be determined whether the observed
GRIN2B methylation alterations are causes or consequences of
BD pathophysiology. Second, the reliance on peripheral blood
methylation profiles raises questions about their biological
relevance to brain processes. While blood-based biomarkers offer
clinical practicality, it remains elusive whether these patterns
mirror those in the central nervous system. Future validation
should incorporate postmortem brain tissue analyses or emerging
cerebrospinal fluid-based epigenetic profiling techniques. Third,
the present study relied on retrospective data collection and
self-reported medical histories, which may have resulted in the
underreporting of comorbidities. This could lead to unintentional
inclusion of patients with undiagnosed conditions that met
exclusion criteria, potentially confounding the observed
methylation patterns. Fourth, while cohort homogeneity was improved
by exclusively recruiting patients with BD in the depressive phase,
this design limits the generalizability of findings to other
disease phases (e.g., manic or euthymic states). Epigenetic markers
may exhibit phase-dependent fluctuations, implying the results
could reflect state-specific alterations rather than trait
characteristics of BD. Fifth, the lack of integrated
single-nucleotide polymorphism analyses and gene expression data
limits our ability to elucidate gene-environment interactions
underlying the observed methylation changes. Most importantly,
these preliminary findings require replication in larger,
independent cohorts with longitudinal designs to establish clinical
generalizability. Future investigations should prioritize
multicenter collaborations, incorporate pharmaco-epigenetic
analyses and employ multi-omics approaches to comprehensively
characterize GRIN2B methylation dynamics in BD progression,
and explicitly address phase-specific biomarker variations through
cross-state comparisons.
In conclusion, the present study demonstrated for
the first time that DNA hypermethylation in the GRIN2B
promoter region is associated with BD and may play a role in
mediating the development of anxiety and insomnia symptoms in
patients with BD. These findings underscore the importance of
gene-environment interactions in BD and advance a better
understanding of its complex etiology. Early intervention
strategies targeting these mechanisms could improve timely
diagnosis, symptom management and long-term outcomes in BD. Further
studies are warranted to validate GRIN2B methylation as both
a diagnostic biomarker and therapeutic target for BD.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the Natural
Science Foundation of Xinjiang Uygur Autonomous Region (grant no.
2022D01C606) and the Tianshan Innovation Team Plan of Xinjiang
Uygur Autonomous Region (grant no. 2022D14011).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to ethical issues
involving the participants' data and privacy but may be requested
from the corresponding author.
Authors' contributions
SZ and HY conducted and designed the study. HY and
YW collected the data. HY analyzed the data and drafted the
manuscript. SZ revised the manuscript. SZ and YW have independently
checked and verified the authenticity of the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Human Research and
Ethics Committee of Xinjiang Uygur Autonomous Region People's
Hospital (Urumqi, China; approval no. KY2023020968). This study
adhered to the guidance listed in the latest version of the
Declaration of Helsinki. All subjects volunteered to participate in
the study provided written informed consent prior to the study. The
participants were also informed that they could withdraw from the
study at any time without any reason or consequence.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2019 Mental Disorders Collaborators:
Global, regional, and national burden of 12 mental disorders in 204
countries and territories, 1990-2019: A systematic analysis for the
global burden of disease study 2019. Lancet Psychiatry. 9:137–150.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grande I, Berk M, Birmaher B and Vieta E:
Bipolar disorder. Lancet. 387:1561–1572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pavlova B, Perlis RH, Alda M and Uher R:
Lifetime prevalence of anxiety disorders in people with bipolar
disorder: A systematic review and meta-analysis. Lancet Psychiatry.
2:710–717. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lopes FL, Zhu K, Purves KL, Song C, Ahn K,
Hou L, Akula N, Kassem L, Bergen SE, Landen M, et al: Polygenic
risk for anxiety influences anxiety comorbidity and suicidal
behavior in bipolar disorder. Transl Psychiatry.
10(298)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gamage N, Senanayake S, Kumbukage M,
Mendis J and Jayasekara A: The prevalence of anxiety and its
association with the quality of life and illness severity among
bipolar affective disorder patients in a developing country. Asian
J Psychiatr. 52(102044)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buckley V, Young AH and Smith P: Child and
adolescent anxiety as a risk factor for bipolar disorder: A
systematic review of longitudinal studies. Bipolar Disord.
25:278–288. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oakes DJ, Pearce HA, Roberts C, Gehrman
PG, Lewis C, Jones I and Lewis KJS: Associations between comorbid
anxiety and sleep disturbance in people with bipolar disorder:
Findings from actigraphy and subjective sleep measures. J Affect
Disord. 309:165–171. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi J, Kang J, Kim T and Nehs CJ: Sleep,
mood disorders, and the ketogenic diet: Potential therapeutic
targets for bipolar disorder and schizophrenia. Front Psychiatry.
15(1358578)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Uher R, Pavlova B, Najafi S, Adepalli N,
Ross B, Howes VE, Freeman K, Parker R, Propper L and Palaniyappan
L: Antecedents of major depressive, bipolar, and psychotic
disorders: A systematic review and meta-analysis of prospective
studies. Neurosci Biobehav Rev. 160(105625)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ghasemi M, Phillips C, Trillo L, De Miguel
Z, Das D and Salehi A: The role of NMDA receptors in the
pathophysiology and treatment of mood disorders. Neurosci Biobehav
Rev. 47:336–358. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang YP, Shimizu E, Dube GR, Rampon C,
Kerchner GA, Zhuo M, Liu G and Tsien JZ: Genetic enhancement of
learning and memory in mice. Nature. 401:63–69. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Carrier J, Semba K, Deurveilher S, Drogos
L, Cyr-Cronier J, Lord C and Sekerovick Z: Sex differences in
age-related changes in the sleep-wake cycle. Front Neuroendocrinol.
47:66–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakamura TJ, Nakamura W, Yamazaki S, Kudo
T, Cutler T, Colwell CS and Block GD: Age-related decline in
circadian output. J Neurosci. 31:10201–10205. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Biello SM, Bonsall DR, Atkinson LA,
Molyneux PC, Harrington ME and Lall GS: Alterations in
glutamatergic signaling contribute to the decline of circadian
photoentrainment in aged mice. Neurobiol Aging. 66:75–84.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Steponenaite A, Biello SM and Lall GS:
Aging clocks: Disrupted circadian rhythms. Aging (Albany NY).
10:3065–3066. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cevik OS, Cevik K, Temel GO and Sahin L:
Maternal separation increased memory function and anxiety without
effects of environmental enrichment in male rats. Behav Brain Res.
441(114280)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Healey K, Waters RC, Knight SG, Wandling
GM, Hall NI, Jones BN, Shobande MJ, Melton JG, Pandey SC,
Swartzwelder HS, et al: Adolescent intermittent ethanol exposure
alters adult exploratory and affective behaviors, and cerebellar
Grin2b expression in C57BL/6J mice. Drug Alcohol Depend.
253(111026)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shirvani-Farsani Z, Maloum Z,
Bagheri-Hosseinabadi Z, Vilor-Tejedor N and Sadeghi I: DNA
methylation signature as a biomarker of major neuropsychiatric
disorders. J Psychiatr Res. 141:34–49. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Papanicolau-Sengos A and Aldape K: DNA
methylation profiling: An emerging paradigm for cancer diagnosis.
Annu Rev Pathol. 17:295–321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fries GR, Li Q, McAlpin B, Rein T,
Walss-Bass C, Soares JC and Quevedo J: The role of DNA methylation
in the pathophysiology and treatment of bipolar disorder. Neurosci
Biobehav Rev. 68:474–488. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goud AC, Etain B, Bellivier F and
Marie-Claire C: DNA methylation as a biomarker of treatment
response variability in serious mental illnesses: A systematic
review focused on bipolar disorder, schizophrenia, and major
depressive disorder. Int J Mol Sci. 19(3026)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bundo M, Ueda J, Nakachi Y, Kasai K, Kato
T and Iwamoto K: Decreased DNA methylation at promoters and
gene-specific neuronal hypermethylation in the prefrontal cortex of
patients with bipolar disorder. Mol Psychiatry. 26:3407–3418.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ceylan D, Scola G, Tunca Z,
Isaacs-Trepanier C, Can G, Andreazza AC, Young LT and Özerdem A:
DNA redox modulations and global DNA methylation in bipolar
disorder: Effects of sex, smoking and illness state. Psychiatry
Res. 261:589–596. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Carrard A, Salzmann A, Malafosse A and
Karege F: Increased DNA methylation status of the serotonin
receptor 5HTR1A gene promoter in schizophrenia and bipolar
disorder. J Affect Disord. 132:450–453. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jeremian R, Chen YA, De Luca V, Vincent
JB, Kennedy JL, Zai CC and Strauss J: Investigation of correlations
between DNA methylation, suicidal behavior and aging. Bipolar
Disord. 19:32–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lima C, Suchting R, Scaini G, Cuellar VA,
Favero-Campbell AD, Walss-Bass C, Soares JC, Quevedo J and Fries
GR: Epigenetic GrimAge acceleration and cognitive impairment in
bipolar disorder. Eur Neuropsychopharmacol. 62:10–21.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shi X, Li M, Yao J, Li MD and Yang Z:
Alcohol drinking, DNA methylation and psychiatric disorders: A
multi-omics Mendelian randomization study to investigate causal
pathways. Addiction. 119:1226–1237. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pierconti F, Rossi ED, Cenci T, Carlino A,
Fiorentino V, Totaro A, Sacco E, Palermo G, Iacovelli R, Larocca
LM, et al: DNA methylation analysis in urinary samples: A useful
method to predict the risk of neoplastic recurrence in patients
with urothelial carcinoma of the bladder in the high-risk group.
Cancer Cytopathol. 131:158–164. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wei W, Fan P, Zhang Z, Wu D, Liu J, Wang
L, Duan X, Zhang X and Ding D: A urine-based liquid biopsy for
detection of upper tract urothelial carcinoma: A self-matched
study. BMC Cancer. 24(1180)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu JH, Bo HH, Liu BP and Jia CX: The
associations between DNA methylation and depression: A systematic
review and meta-analysis. J Affect Disord. 327:439–450.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Srivastava A, Dada O, Qian J, Al-Chalabi
N, Fatemi AB, Gerretsen P, Graff A and De Luca V: Epigenetics of
schizophrenia. Psychiatry Res. 305(114218)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gaynor SC, Breen ME, Monson ET, de Klerk
K, Parsons M, DeLuca AP, Scheetz TE, Zandi PP, Potash JB and
Willour VL: A targeted sequencing study of glutamatergic candidate
genes in suicide attempters with bipolar disorder. Am J Med Genet B
Neuropsychiatr Genet. 171:1080–1087. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dalvie S, Horn N, Nossek C, van der Merwe
L, Stein DJ and Ramesar R: Psychosis and relapse in bipolar
disorder are related to GRM3, DAOA, and GRIN2B genotype. Afr J
Psychiatry (Johannesbg). 13:297–301. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang W, Tian F, Zheng J, Li S and Qiang
M: Chronic administration of Benzo(a)pyrene induces memory
impairment and anxiety-like behavior and increases of NR2B DNA
methylation. PLoS One. 11(e0149574)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Xiao X, Wang L, Wang Q, Liang R,
Zheng C, Yang J and Ming D: Comparison effects of chronic sleep
deprivation on juvenile and young adult mice. J Sleep Res.
31(e13399)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Horvath S: DNA methylation age of human
tissues and cell types. Genome Biol. 14(R115)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Johansson A, Enroth S and Gyllensten U:
Continuous aging of the human DNA methylome throughout the human
lifespan. PLoS One. 8(e67378)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hannum G, Guinney J, Zhao L, Zhang L,
Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, et al:
Genome-wide methylation profiles reveal quantitative views of human
aging rates. Mol Cell. 49:359–367. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
First M, Spitze R, Gibbon M and Williams
J: Structured clinical interview for DSM-IV-TR axis I disorders,
research version, patient edition. (SCID-I/P). New York: Biometrics
Research, New York State Psychiatric Institute, 2002.
|
|
40
|
HAMILTON M: A rating scale for depression.
J Neurol Neurosurg Psychiatry. 23:56–62. 1960.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Young RC, Biggs JT, Ziegler VE and Meyer
DA: A rating scale for mania: Reliability, validity and
sensitivity. Brit J Psychiatry. 133:429–435. 1978.PubMed/NCBI View Article : Google Scholar
|
|
42
|
APA. Diagnostic and Statistical Manual of
Mental Disorders, Text Revision (DSM-IV-TR), 4th Edn. Washington,
DC: American psychiatric association; 1994.
|
|
43
|
HAMILTON M: The assessment of anxiety
states by rating. Br J Med Psychol. 32:50–55. 1959.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Buysse DJ, Reynolds CR III, Monk TH,
Berman SR and Kupfer DJ: The pittsburgh sleep quality index: A new
instrument for psychiatric practice and research. Psychiatry Res.
28:193–213. 1989.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Avramopoulos D, Lasseter VK, Fallin MD,
Wolyniec PS, McGrath JA, Nestadt G, Valle D and Pulver AE: Stage II
follow-up on a linkage scan for bipolar disorder in the Ashkenazim
provides suggestive evidence for chromosome 12p and the GRIN2B
gene. Genet Med. 9:745–751. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhao Q, Che R, Zhang Z, Wang P, Li J, Li
Y, Huang K, Tang W, Feng G, Lindpaintner K, et al: Positive
association between GRIN2B gene and bipolar disorder in the Chinese
Han Population. Psychiatry Res. 185:290–292. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li C, Tian H, Li R, Jia F, Wang L, Ma X,
Yang L, Zhang Q, Zhang Y, Yao K and Zhuo C: Molecular mechanisms of
quetiapine bidirectional regulation of bipolar depression and mania
based on network pharmacology and molecular docking: Evidence from
computational biology. J Affect Disord. 355:528–539.
2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Weiss F, Caruso V, De Rosa U, Beatino MF,
Barbuti M, Nicoletti F and Perugi G: The role of NMDA receptors in
bipolar disorder: A systematic review. Bipolar Disord. 25:624–636.
2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Beneyto M and Meador-Woodruff JH:
Lamina-specific abnormalities of NMDA receptor-associated
postsynaptic protein transcripts in the prefrontal cortex in
schizophrenia and bipolar disorder. Neuropsychopharmacology.
33:2175–2186. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Riaza BC, Perez-Rodriguez MM,
Vaquero-Lorenzo C and Baca-Garcia E: New perspectives in glutamate
and anxiety. Pharmacol Biochem Behav. 100:752–774. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bannerman DM, Sprengel R, Sanderson DJ,
McHugh SB, Rawlins JN, Monyer H and Seeburg PH: Hippocampal
synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci.
15:181–192. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guo H, Hu WC, Xian H, Shi YX, Liu YY, Ma
SB, Pan KQ, Wu SX, Xu LY, Luo C and Xie RG: CCL2 potentiates
inflammation pain and related anxiety-like behavior through NMDA
signaling in anterior cingulate cortex. Mol Neurobiol.
61:4976–4991. 2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ren D, Li JN, Qiu XT, Wan FP, Wu ZY, Fan
BY, Zhang MM, Chen T, Li H, Bai Y and Li YQ: Anterior cingulate
cortex mediates hyperalgesia and anxiety induced by chronic
pancreatitis in rats. Neurosci Bull. 38:342–358. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yan Z, Jiao F, Yan X and Ou H: Maternal
chronic folate supplementation ameliorates behavior disorders
induced by prenatal high-fat diet through methylation alteration of
BDNF and Grin2b in offspring hippocampus. Mol Nutr Food Res 61:
doi.org/10.1002/mnfr.201700461,
2017.
|
|
55
|
Barkus C, McHugh SB, Sprengel R, Seeburg
PH, Rawlins JN and Bannerman DM: Hippocampal NMDA receptors and
anxiety: At the interface between cognition and emotion. Eur J
Pharmacol. 626:49–56. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tully JL, Dahlen AD, Haggarty CJ, Schioth
HB and Brooks S: Ketamine treatment for refractory anxiety: A
systematic review. Br J Clin Pharmacol. 88:4412–4426.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Arino H, Munoz-Lopetegi A,
Martinez-Hernandez E, Armangue T, Rosa-Justicia M, Escudero D,
Matos N, Graus F, Sugranyes G, Castro-Fornieles J, et al: Sleep
disorders in anti-NMDAR encephalitis. Neurology. 95:e671–e684.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Burgdorf JS, Vitaterna MH, Olker CJ, Song
EJ, Christian EP, Sorensen L, Turek FW, Madsen TM, Khan MA, Kroes
RA and Moskal JR: NMDAR activation regulates the daily rhythms of
sleep and mood. Sleep. 42(zsz135)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Miracca G, Anuncibay-Soto B, Tossell K,
Yustos R, Vyssotski AL, Franks NP and Wisden W: NMDA receptors in
the lateral preoptic hypothalamus are essential for sustaining NREM
and REM sleep. J Neurosci. 42:5389–5409. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kocsis B: State-dependent increase of
cortical gamma activity during REM sleep after selective blockade
of NR2B subunit containing NMDA receptors. Sleep. 35:1011–1016.
2012.PubMed/NCBI View Article : Google Scholar
|