Introduction
Hepatitis C virus (HCV) is prevalent worldwide, with
nearly 180 million infected individuals all carrying a risk of
hepatocellular carcinoma (HCC) at later stages of the disease
(1,2). Interferon (IFN)-based therapies are
effective in 80% of patients infected with the HCV2 and 3 genotypes
and also in 50% of patients with the HCV1b genotype. However, IFN
therapy has several limitations, including medical and physical
contra-indications, adverse events and high cost (1–4).
HCV1b, the most frequently observed strain in Japan, is also a
common strain in the US (3,4).
Certain HCV patients show a spontaneous clearance of
the virus along with acquisition of specific immunity, which
encourages hopes of developing a clinically effective vaccine
(5–7). However, the development of either
prophylactic or therapeutic HCV vaccines is expected to be very
difficult, since HCVs are very heterogeneous and their antigens are
highly mutable (6–8). Indeed, in regards to a sustained
viral response (SVR), no clinical benefit has yet been reported
from HCV vaccines for either IFN-naive or IFN-resistant patients in
recent clinical trials, including our own, in spite of successful
immunological responses in a substantial number of patients
(9–13). However, we recently identified a
decrease in α-fetoprotein (AFP), a biomarker for HCC, in a
percentage of vaccinated patients who showed elevated AFP levels
prior to vaccination (12). These
results suggest that the HCV vaccine is effective as a cancer
prophylaxis in chronic hepatitis (CH) and liver cirrhosis (LC)
patients. Subsequently, we report the results of a follow-up study
of cancer prophylaxis in patients who had received a prolonged
course of peptide vaccinations at our university.
Materials and methods
Patients
Patients received the HCV-derived peptides under one
of two recent Phase I clinical studies held at the Kurume
University Hospital; one study was conducted with
HLA-A24+ patients (11)
and the other with patients bearing multiple HLA-class I alleles
(HLA-A2, -A3, -A11, -A24 -A26, -A31 or -A33) (12). The inclusion criteria were as
follows: i) persistent HCV infection confirmed by serological
HCV-RNA tests; ii) diagnosis of CH or LC; iii) non-response to
previous IFN-based treatment or refusal to receive such treatment;
iv) no detectable HCC at the time of entry into the study; v)
positive status for one of the following alleles: HLA-A2, -A3,
-A11, -A24, -A26, -A31 or -A33; vi) an Eastern Cooperative Oncology
Group performance status of 0-1, age between 20 and 75 years and
adequate hematological function (white blood cell count ≥2,400/μl,
hemoglobin level ≥8.0 g/dl and platelet counts ≥50,000/μl), renal
function (serum creatinine ≤1.4 g/dl) and hepatic function (total
bilirubin <2.5 mg/dl); and vii) negative status for hepatitis B
antigens. The protocols for both series were approved by the
Institutional Ethics Review Boards of Kurume University, and
complete written informed consent was obtained from all patients at
the time of enrollment. A total of 40 patients entered one of the
two protocols at our institution between November 2003 and November
2008. In addition, the 39 patients who received more than six
vaccinations were included in this follow-up study.
Peptides and vaccination
For the first protocol, four peptides capable of
inducing both cytotoxic T lymphocyte (CTL) and humoral responses
(IgG) in HLA-A24+ patients were provided for vaccination
as previously reported (11).
These four peptides were derived from well-conserved regions of
HCV1b as follows: protein E1-derived peptide from positions 213 to
221 (E1 213-221); protein E2-derived peptide from positions 488 to
496 (E2 488-496); non-structural region 3-derived peptide from
positions 1081 to 1090 (NS3 1081-1090); and non-structural region
5A-derived peptide from positions 2132 to 2140 (NS5A 2132-2140).
For the second protocol, we used a peptide for vaccination derived
from HCV core protein; this peptide was capable of inducing both
CTL and humoral responses (IgG) in nearly all HCV patients with
different HLA-class IA alleles in Japan (12,14).
This peptide originated from the HCV core protein at positions 35
to 44 (C 35-44), a well-known HLA-A2-restricted CTL epitope
(15).
These five peptides were prepared under conditions
of Good Manufacturing Practice by the American Peptide Company (San
Diego, CA, USA). The peptide emulsion was injected into the
subcutaneous region of the side of the abdomen or upper arm every 2
weeks from the 1st to the 24th vaccination, every 3 weeks from the
25th to the 48th vaccination and every 4 weeks thereafter. The
first cycle consisted of six vaccinations; a second cycle of six
vaccinations was conducted with patient consent in cases lacking
any signs of severe toxicity. When patients wished to continue with
the course of vaccinations, the series was extended, unless either
disease progression or severe toxicity was observed.
Humoral responses to peptides
Peptide-specific IgG levels in the blood samples
were measured using Luminex® systems as reported
previously (11). Briefly, diluted
plasma samples were incubated with peptide-coated microspheres.
After the microspheres were washed, they were incubated with
various antibodies (anti-human-IgG, -IgA, -IgM, -IgE, -IgG1, -IgG2,
-IgG3 and -IgG4; purchased from Vector, Bethyl, Vector, Biosource,
The Binding Site Ltd., Cappel, Cappel and The Binding Site Ltd.,
respectively). After being washed, the microspheres bound to each
antibody were reacted with biotin-labeled detection antibody (Zymed
or Cappel) and the corresponding R-phycoerythrin (Invitrogen)
antibody, and the antibody levels were detected by a Luminex system
as reported previously (11). All
pre- and post-vaccination samples were measured simultaneously in
order to avoid any possible biases associated with the in
vitro assay. IgG against recombinant HCV core protein was also
measured by means of a commercially available radioimmunoassay kit
(SRL Laboratory, Tokyo, Japan).
Clinical laboratory data
Clinical laboratory values (e.g., serum ALT and AFP
levels and blood platelet numbers) were measured by the Clinical
Laboratory Division of the Kurume University Hospital. Quantitation
of HCV-RNA, based on quantitative reverse transcription-polymerase
chain reaction (qRT-PCR), was performed by a clinical lab company
(SRL Laboratory).
Results
Patient characteristics and clinical
responses
Thirty-nine HCV-positive patients (33 CH and 6 LC)
who had received more than six rounds of HCV-derived peptide
vaccinations were included in the analysis (Tables I–III). All patients, with the exception of
1 (pt. 16, HCV2a), were infected with HCV1b. At the time of entry
into the study, 35 patients were non-responders to interferon-based
therapy, and the remaining 4 patients refused treatment. All 6 LC
patients had a history of HCC treatment, while 3 CH patients had
space-occupying hepatic lesions (SOLs) suspected of being cancerous
at the time they entered the study. The median frequency of
vaccination was 26 rounds (range 6–89), and the median duration of
the vaccination period was 16 months (range 2–69). No severe
toxicity was observed throughout the vaccination period, but grade
1 or 2 local inflammation at the injection site was observed in
most cases. Twelve patients were still receiving vaccinations at
the time of this writing (September 2009), and the course of
vaccinations had been terminated in the remaining 27 patients.
Eleven patients received IFN-based therapy combined with
vaccination followed by the vaccination alone, and 9 patients
received IFN-based therapy after the end of vaccination (Tables I–III).
 | Table I.Patient characteristics before
vaccination. |
Table I.
Patient characteristics before
vaccination.
| Patient | Age | Gender | Disease | Previous IFN | ALT | Plt | AFP | HCV-RNA |
|---|
| 1 | 38 | M | CH | - | 139 | 11 | 8 | 465 |
| 2 | 43 | M | CH (SOL) | IFN+RBV | 139 | 17 | 9 | 3,820 |
| 3 | 52 | F | LC (post HCC) | IFN+RBV | 105 | 9 | 173 | 651 |
| 4 | 65 | M | LC (post HCC) | IFN+RBV | 292 | 5 | 7 | 4,030 |
| 5 | 55 | M | CH | IFN+RBV | 119 | 9 | 11 | 4,290 |
| 6 | 42 | M | CH | IFN+RBV | 73 | 12 | 4 | 621 |
| 7 | 49 | M | CH | IFN+RBV | 48 | 13 | 3 | 1,010 |
| 8 | 53 | F | CH | IFN+RBV | 27 | 21 | 4 | 2,090 |
| 9 | 70 | F | CH | IFN+RBV | 38 | 12 | 12 | 2,200 |
| 10 | 66 | M | CH | IFN+RBV | 154 | 15 | 11 | 139 |
| 11 | 58 | F | CH | IFN+RBV | 51 | 11 | 17 | 4,590 |
| 12 | 50 | F | CH | IFN+RBV | 62 | 14 | 10 | 1,730 |
| 13 | 61 | F | CH | IFN+RBV | 77 | 11 | 33 | 91 |
| 14 | 51 | M | CH | IFN | 41 | 22 | 3 | 3,420 |
| 15 | 50 | M | CH | IFN+RBV | 206 | 11 | 74 | 500 |
| 16 | 71 | M | CH | IFN+RBV | 114 | 9 | 4 | 114 |
| 17 | 51 | M | CH | IFN | 60 | 14 | 5 | 892 |
| 18 | 60 | M | CH | IFN+RBV | 108 | 8 | 9 | 3,100 |
| 19 | 49 | F | CH | IFN+RBV | 129 | 16 | 44 | 2,480 |
| 20 | 38 | M | CH | IFN+RBV | 358 | 6 | 29 | 2,870 |
| 21 | 61 | F | LC (post HCC) | IFN+RBV | 53 | 9 | 131 | 1,670 |
| 22 | 58 | M | LC (post HCC) | RFA, IFN | 45 | 8 | 7 | 2,130 |
| 23 | 71 | M | CH | IFN | 46 | 13 | 2 | 4,520 |
| 24 | 70 | M | CH | IFN+RBV | 47 | 25 | 5 | 2,470 |
| 25 | 68 | M | CH | IFN+RBV | 37 | 17 | 4 | 2,260 |
| 26 | 64 | M | CH (SOL) | IFN+RBV | 60 | 13 | 13 | 3,630 |
| 27 | 70 | M | CH (SOL) | IFN+RBV | 51 | 24 | 4 | 591 |
| 28 | 62 | F | CH | - | 24 | 19 | 5 | 583 |
| 29 | 53 | F | CH | IFN | 34 | 23 | 4 | 4,370 |
| 30 | 63 | F | CH | - | 73 | 12 | 3 | 59 |
| 31 | 58 | F | CH | IFN+RBV | 66 | 10 | 8 | 2,790 |
| 32 | 63 | F | CH | IFN+RBV | 52 | 12 | 3 | 2,230 |
| 33 | 57 | F | CH | IFN+RBV | 55 | 11 | 9 | 2,340 |
| 34 | 58 | F | CH | IFN | 80 | 12 | 7 | 2,150 |
| 35 | 58 | F | CH | IFN+RBV | 47 | 12 | 11 | 16,000 |
| 36 | 61 | M | LC (post HCC) | IFN+RVB | 104 | 10 | 27 | 32,000 |
| 37 | 58 | M | CH | - | 83 | 15 | 6 | 20 |
| 38 | 60 | M | CH | IFN+RBV | 144 | 16 | 5 | 25,000 |
| 39 | 73 | M | LC (post HCC) | IFN+RBV | 42 | 12 | 66 | 3,200 |
 | Table III.Patient characteristics post
vaccination. |
Table III.
Patient characteristics post
vaccination.
| Patient | Post-vaccination
IFN | Total OP (m) | Onset of HCC
(m) |
|---|
| 1 | | | 69 |
| 2 | | | 67 |
| 3 | | | 66 |
| 4 | | + (49) | 64 |
| 5 | | + (46) | 63 |
| 6 | + | | 59 |
| 7 | + | | 57 |
| 8 | | | 56 |
| 9 | + | + (29) | 50 |
| 10 | + | | 49 |
| 11 | + | | 47 |
| 12 | | | 58 |
| 13 | | | 57 |
| 14 | + | | 50 |
| 15 | | | 64 |
| 16 | | | 62 |
| 17 | | | 61 |
| 18 | + | | 58 |
| 19 | | | 57 |
| 20 | | | 52 |
| 21 | + | + (18) | 39 |
| 22 | | | 28 |
| 23 | + | | 35 |
| 24 | | | 35 |
| 25 | | | 32 |
| 26 | | | 34 |
| 27 | | | 29 |
| 28 | | | 28 |
| 29 | | | 28 |
| 30 | | | 28 |
| 31 | | | 26 |
| 32 | | | 25 |
| 33 | | | 22 |
| 34 | | | 21 |
| 35 | | | 11 |
| 36 | | | 21 |
| 37 | | | 21 |
| 38 | | | 14 |
| 39 | | | 10 |
A significant decrease in ALT level (<70% ALT
level at the end of vaccination or at the time of last vaccination
vs. that before vaccination) was observed in 11 of 39 patients (7
of 28 patients treated with vaccination alone and 4 of 11 patients
treated with vaccination alone followed by combined IFN-based
therapy with vaccination). No significant decrease in platelet
number (<70% at the end of vaccination or at the time of last
vaccination vs. that before vaccination) was found in any of the 28
patients who received vaccination alone, but an increase (>130%)
was noted in 1 patient. By contrast, a decrease in platelet number
was observed in 4 of 11 patients with vaccination alone followed by
combined IFN therapy. A significant decrease in the AFP level was
found in 1 of 28 patients who received vaccination alone, whereas
it was observed in 4 of 9 patients with vaccination alone followed
by combined IFN therapy. HCV-RNA responders with >1 log decline
were not found among the 28 patients who received the vaccination
alone, while 4 of 11 patients who had received vaccination followed
by combination therapy, including 3 sustained viral responders
(SVR; pt. 1, 8 and 15), were HCV-RNA responders. As
post-vaccination treatment, 9 patients received IFN-based therapy
and only 1 (pt. 23) reached the status of SVR. The median
observation period of the 39 patients was 47 months (range
10–69).
Development of HCC
Under the circumstances described above, HCC became
detectable during the vaccination period in 2 of 3 CH patients (pt.
26 and 27) with SOLs prior to vaccination. By contrast, HCC was
undetectable throughout the vaccination period in the remaining 36
patients without a SOL prior to vaccination. However, HCC was
diagnosed in 4 of these 36 patients after the course of
vaccination, i.e., in 2 CH patients at 46 and 29 months after the
end of vaccination (pt. 5 and 9), and in 2 LC patients at 49 and 18
months after the end of vaccination (pt. 4 and 21). Three of these
4 patients received IFN-based therapy combined with vaccination
(pt. 5), or after the end of vaccination (pt. 9 and 21) and showed
no viral response (Tables
I–III).
Antibody responses
We measured the patient IgG responses to each of the
peptide vaccines in plasma samples before, during and at the end of
the vaccination period in all 39 patients. Moreover, when possible,
we measured the IgG responses in patients after the end of the
series of vaccinations (n=10). Representative results of all 39
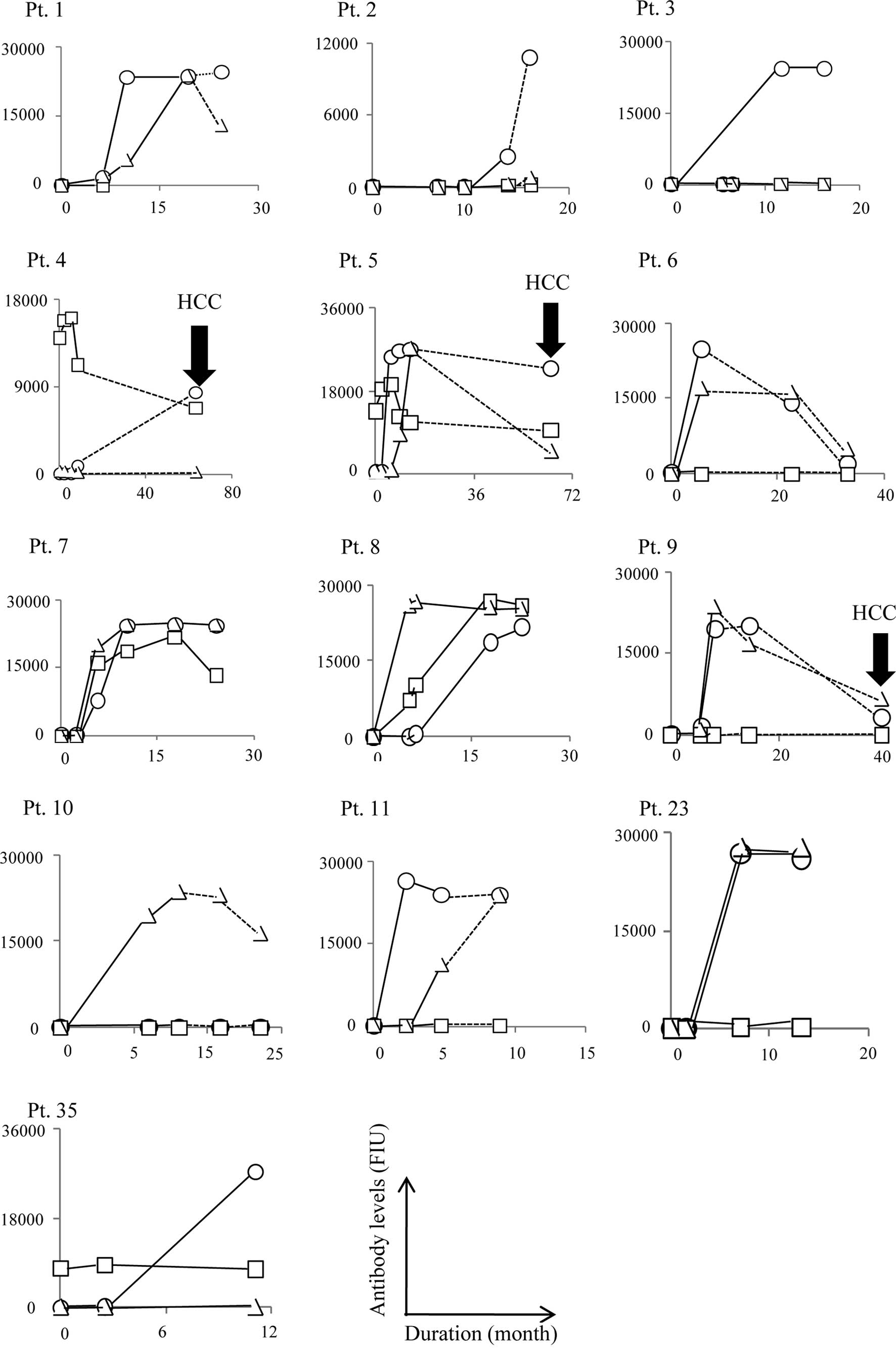
cases are shown in Fig. 1A–C. In
the first protocol for HLA-A24+ patients (n=13), an
increase in anti-peptide IgG to E1 213-221, E2 488-496, NS3
1081-1090 and NS5A 2132-2140 peptides at the end of the peptide
vaccination was observed in 0 of 7, 12 of 13, 4 of 13 and 9 of 13
patients who received the corresponding peptides, respectively
(Fig. 1A). An increase in IgG
reactive to at least one of the vaccination peptides was observed
in all 13 patients. The increased IgG reactive to the E2-488 or
NS5A-2132, but not to the NS3-1081 peptide, was sustained for 6, 6,
49, 46, 17, 9, 6 and 6 months when the samples after the end of
vaccination from pt. 1, 2, 4–6 and 9–11 were provided for
measurement.
In the second protocol for patients with different
HLA-A alleles, the C 35-44 peptide was used for vaccination in all
26 patients, and an increase in anti-peptide IgG at the end of the
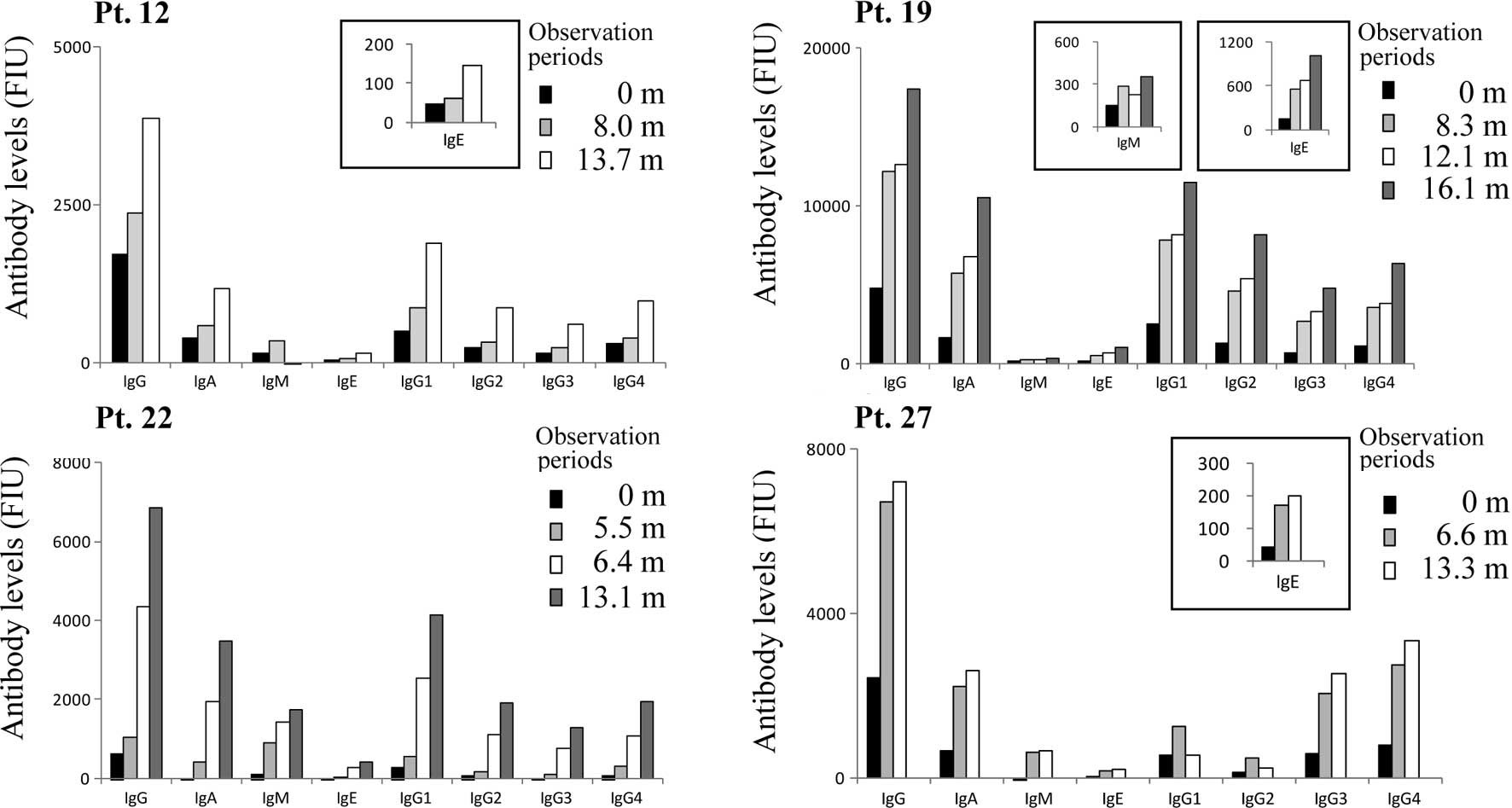
peptide vaccination was observed in 22 of these patients (Fig. 1B and C). Ig isotypes and IgG
subclasses of anti-C 35-44 peptide were also subjected to analysis
in order to address whether Th1- or Th2-type immune responses were
induced by peptide vaccination in the 26 patients who received C
35-44 peptide vaccinations. We found that all Ig isotypes (IgM,
IgA, IgG and IgE), as well as all IgG subclasses (IgG1 to IgG4),
were augmented by vaccination in all 22 patients exhibiting
elevated IgG. Four representative cases (pt. 12, 19, 22 and 27) are
shown in Fig. 2.
These results indicate that IgG responses to E2
488-496, NS5A 2132-2140 and C 35-44 peptides were boosted in the
majority of vaccinated patients, i.e., IgG responses to NS3
1081-1090 were boosted in half of the patients, whereas the IgG
response to the E1 213-221 peptide was not boosted in any of the
patients. These results are consistent with those reported
previously using samples from our Phase I study (11,12).
Measurement of cellular responses using
post-vaccination samples was not carried out in this study,
primarily due to the limited number of available peripheral blood
mononuclear cells, although an increase in cellular responses to at
least one of the vaccinated peptides during the Phase I studies was
observed in the majority of patients, as reported previously
(11,12).
Development of HCC and antibody
responses
We then addressed the relationship between the
development of HCC and patient immune responses.
HCC became detectable during the course of
vaccination in 2 of 3 CH patients (pt. 26 and 27) with SOLs prior
to vaccination. In these two patients, humoral responses to the
vaccinated peptides were well augmented in the post-vaccination
samples (Figs. 1 and 2). In the other patient (pt. 2) with a
SOL but without HCC, the humoral responses to the vaccinated
peptides were also augmented (Fig.
1).
HCC was undetectable in the remaining 36 patients
throughout the vaccination period, but it became detectable in 4
patients post-vaccination, i.e., in 2 CH patients at 46 and 29
months after the end of the vaccination (pt. 5 and 9), and in 2 LC
patients at 49 and 18 months after the end of the vaccination (pt.
4 and 21). The development of HCC in these four cases was
associated with a disappearance of vaccination-induced humoral
responses. Namely, the IgG boosting effect reactive to NS3-1081 and
NS5A-2132, but not to E2-488, disappeared in pt. 4 and 5 when HCC
became detectable 49 and 46 months after the end of vaccination,
respectively (Fig. 1A). The IgG
boosting effect reactive to NS5A-2132 and E2-488 also disappeared
in pt. 9 when HCC became detectable 29 months after the end of
vaccination. Similarly, the IgG boosting effect to the C-35 peptide
in pt. 21 disappeared by the time HCC developed, i.e., 18 months
after the end of the vaccination period (Fig. 1B).
The results presented above suggest an association
between HCC development and the weakening of boosted IgG responses
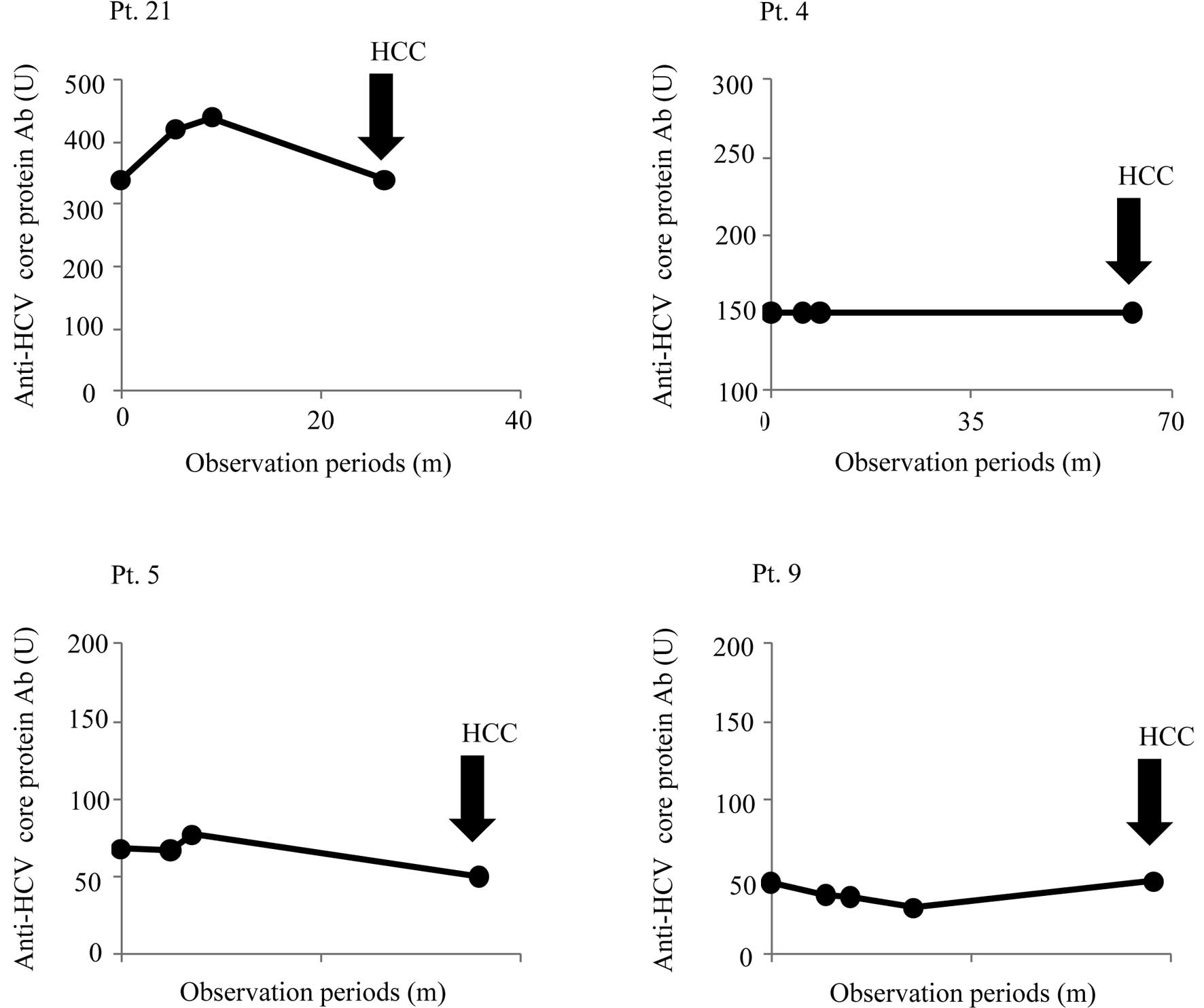
to the peptides used for vaccination. We then addressed whether IgG
responses to the HCV core protein had any association with HCC
development in pt. 21, who received core protein-derived peptide
C-35 (Fig. 3). The increase in IgG
levels in response to the HCV core protein was reduced to the
pre-vaccination baseline level at the time of HCC development. The
IgG responses in the remaining 3 patients who did not receive the
C-35 peptide were also measured. As expected, there was no
association in these 3 patients between IgG titers raised against
the HCV core protein and HCC development (Fig. 3).
Discussion
The reported annual occurrence rates of HCC in CH
patients, LC patients, non-responders and patients with a history
of HCC treatment vary largely from <1-5, 5–8, 1–7 and 10–20% per
year, respectively, depending on the population studied (5,16–22).
HCC has been shown to develop even in sustained viral responders at
a rate of <1–2% per year, depending on the population studied
(23,24). Only 39 vaccinated patients were
included in the present follow-up study; thus, information
regarding general HCC occurrence and recurrence rates at the Kurume
University Hospital could be useful for gaining a better
understanding of the results of this study. The 2-year occurrence
rate of HCC in non-vaccinated HCV1b+ patients with
platelet numbers of <130,000 per mm3 (stages F3 and
F4) (5) who failed to respond to
IFN-ribavirin therapy was 19%, while the 2-year recurrence rate in
non-vaccinated HCV1b+ patients after a history of HCC
treatment was 47.5% at Kurume University Hospital (Sata et
al, unpublished data). In this study, 16 and 6 patients were
matched to the former and latter group of patients, respectively. A
median observation period for these 22 patients after the
initiation of vaccination was 55 months (range 10–69). HCC was
undetectable in all of these 22 patients at least 30 months after
the initiation of peptide vaccination. However, HCC became
detectable 59, 58, 37 and 30 months after the initiation in pt. 4,
5, 9 and 21, respectively. With regard to post-vaccination, HCC
became detectable at 49, 46, 29 and 18 months in pt. 4, 5, 9 and
21, respectively. These results suggest that peptide vaccination
induced prophylaxis against HCC associated with HCV.
By contrast, HCC developed in 2 (pt. 26 and 27) of 3
CH patients with SOLs suspected of being cancerous at the start of
the vaccination period (Tables
I–III), suggesting that the
peptide vaccine has no prophylactic effects in patients with
pre-existing SOLs.
Our results suggest that the vaccination-induced
increase in peptide-specific IgG lasts for 6 months after the end
of a vaccination course. However, the duration of this response
appears to depend on the specific peptides used for vaccination, as
well as on the dose and frequency of vaccination. Thus, these
issues need to be further investigated in future clinical trials
with larger sample sizes. Moreover, HCC development was associated
with a reduction in boosted immune responses showing reactivity to
specific peptide vaccines, as well as to the corresponding core
protein. Therefore, IgG reactivity to peptides used for vaccination
may serve as a biomarker for predicting HCC development in
HCV-positive patients who have received a peptide vaccine. It
should be noted that only 4 patients were available for this aspect
of the present study and therefore, further investigation is
required in order to confirm our results. In addition, the
biological roles of the peptide antibodies remain unclear at the
present time and should be elucidated by future investigation.
Th1-type immune responses are thought to be involved
in chronic-phase liver damage (25,26).
Nelson et al found that interleukin-10 treatment resulted in
the normalization of ALT levels in 19 of 22 CH patients who had
been non-responders to INF-based treatment (27). Interleukin-10 promotes the
production of IgA, IgG1 and IgG3 (28,29).
We demonstrated an increase in all three of these Ig isotypes in
post-vaccination samples, suggesting that the Th2-type immune
response is indeed boosted by vaccination.
We also measured the CTL activity of PBMCs during
the course of vaccination; our results were reported elsewhere
(11,12). However, CTL activity was not
measured in the post-vaccination follow-up study primarily due to
the limited number of available samples.
In previously reported HCV vaccine trials (9–13),
as well as in the present follow-up study, there has yet to be a
sustained viral responder, regardless of the fact that successful
immune responses have been induced in a substantial number of
patients. The well-recognized difficulty of developing either
prophylactic or therapeutic HCV vaccines largely results from viral
heterogeneity and mutability (6–8). In
addition, many studies have demonstrated suppressed or imbalanced
immunity in CH or LC patients with HCV (25,26,30).
Previous and present results, when taken together, suggest that
developing an HCV vaccine to induce SVR as a primary endpoint will
not be feasible until novel scientific breakthroughs in this field
have been made. However, HCV peptide vaccines may augment both
cellular and humoral responses against HCV-derived peptides in a
large percentage of patients. This potential of peptide vaccines
provides some hope that boosted specific immunity eliminates
cancerous liver cells infected with HCV, which in turn may result
in prevention or delay of HCC development associated with HCV.
Indeed, the results of the present study support this hypothesis.
Further clinical studies are required to confirm whether or not
peptide vaccination using CTL epitopes derived from HCV protein are
effective for prophylaxis against HCC in HCV-positive patients
without SOL.
Acknowledgements
This study was supported in part by
Grants-in-Aid from the Ministry of Education, Science, Sports and
Culture of Japan to the Research Center of Innovative Cancer
Therapy of the 21st Century Center of Excellence (COE) Program for
Medical Science, and to Toshi-area Research.
References
|
1.
|
World Health Organization (WHO) Hepatitis
C. 2006, Available at: http://www.who.int/vaccine_research/diseases/viral_cancers/en/index2.html#disease%20burdenuri.
|
|
2.
|
Chander G, Sulkowski MS, Jenckes MW,
Torbenson MS, Herlong HF, Bass EB and Gebo KA: Treatment of chronic
hepatitis C: a systematic review. Hepatology. 36:S135–S144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kato N, Hijikata M, Ootsuyama Y, Nakagawa
M, Ohkoshi S and Sugimura T: Molecular cloning of the human
hepatitis C virus genome from Japanese patients with non-A, non-B
hepatitis. Proc Natl Acad Sci USA. 87:9524–9528. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Blatt LM, Mutchnick MG, Tong MJ, et al:
Assessment of hepatitis C virus RNA and genotype from 6807 patients
with chronic hepatitis C in the United States. J Viral Hepat.
7:196–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yoshida H, Shiratori Y, Moriyama M, et al:
Interferon therapy reduces the risk for hepatocellular carcinoma:
national surveillance program of cirrhotic and noncirrhotic
patients with chronic hepatitis C in Japan. IHIT Study Group.
Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann
Intern Med. 131:174–181. 1999. View Article : Google Scholar
|
|
6.
|
Houghton M and Abrignani S: Prospects for
a vaccine against the hepatitis C virus. Nature. 436:961–966. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Strickland GT, EI-Kamary SS, Klenerman P
and Nicosia A: Hepatitis C vaccine: supply and demand. Lancet
Infect Dis. 8:379–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bowen DG and Walker CM: Mutational escape
from CD8+ T cell immunity: HCV evolution, from chimpanzees to man.
J Exp Med. 201:1709–1714. 2005.
|
|
9.
|
Schlaphoff V, Klade CS, Jilma B, et al:
Functional and phenotypic characterization of
peptide-vaccine-induced HCV-specific CD8+ T cells in healthy
individuals and chronic hepatitis C patients. Vaccine.
25:6793–6806. 2007.PubMed/NCBI
|
|
10.
|
Klade CS, Wedemeyer H, Berg T, et al:
Therapeutic vaccination of chronic hepatitis C nonresponder
patients with the peptide vaccine IC41. Gastroenterology.
134:1385–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yutani S, Yamada A, Yoshida K, et al:
Phase I clinical study of a personalized peptide vaccination for
patients infected with hepatitis C virus (HCV) 1b who failed to
respond to interferon-based therapy. Vaccine. 25:7429–7435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yutani S, Komatsu N, Shichijo S, et al:
Phase I clinical study of a peptide vaccination for hepatitis C
virus-infected patients with different HLA-class I-A alleles.
Cancer Science. 100:1935–1942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Leroux-Roels G, Batens AH, Desombere I, et
al: Immunogenicity and tolerability of intradermal administration
of an HCV E1-based vaccine candidate in healthy volunteers and
patients with resolved or ongoing chronic HCV infection. Hum
Vaccin. 1:61–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Niu Y, Terasaki Y, Komatsu N, et al:
Identification of peptides applicable as vaccines for
HLA-A26-positive cancer patients. Cancer Science. 100:2167–2174.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cerny A, McHutchison JG, Pasquinelli C, et
al: Cytotoxic T lymphocyte response to hepatitis C virus-derived
peptides containing the HLA A2.1 binding motif. J Clin Invest.
95:521–530. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kiyosawa K, Umemura T, Ichijo T, et al:
Hepatocellular carcinoma: recent trends in Japan. Gastroenterology.
127:S17–S26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Taura K, Ikai I, Hatano E, et al:
Influence of coexisting cirrhosis on outcomes after partial hepatic
resection for hepatocellular carcinoma fulfilling the Milan
criteria: an analysis of 293 patients. Surgery. 142:685–694. 2007.
View Article : Google Scholar
|
|
18.
|
Shiratori Y, Shiina S, Teratani T, et al:
Interferon therapy after tumor ablation improves prognosis in
patients with hepatocellular carcinoma associated with hepatitis C
virus. Ann Intern Med. 138:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kubo M, Sakaguchi Y, Chung H, et al:
Long-term interferon maintenance therapy improves survival in
patients with HCV-related hepatocellular carcinoma after curative
radiofrequency ablation. A matched case-control study. Oncology.
72:132–138. 2007. View Article : Google Scholar
|
|
20.
|
Kim JH, Han KH, Lee KS, et al: Efficacy
and long term follow up of combination therapy with interferon
alpha and ribavirin for chronic hepatitis C in Korea. Yonsei
Medical Journal. 47:793–798. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Arase Y, Ikeda K, Suzuki F, et al: Long
term outcome after interferon therapy in elderly patients with
chronic hepatitis C. Intervirology. 50:16–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ikeda K, Saitoh S, Arase Y, et al: Effect
of interferon therapy on hepatocellular carcinogenesis in patients
with chronic hepatitis type C: a long-term observation study of
1,643 patients using statistical bias correction with proportional
hazard analysis. Hepatology. 29:1124–1130. 1999. View Article : Google Scholar
|
|
23.
|
Toyoda H, Kumada T, Tokuda A, et al:
Long-term follow-up of sustained responders to interferon therapy
in patients with chronic hepatitis C. J Viral Hepatitis. 7:414–419.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Okanoue T, Itoh Y, Minami M, et al:
Interferon therapy lowers the rate of progression to hepatocellular
carcinoma in chronic hepatitis C but not significantly in an
advanced stage: a retrospective study in 1148 patients. J Hepatol.
30:643–649. 1999. View Article : Google Scholar
|
|
25.
|
Bertoketti A, Bertoletti A, D’Elios MM, et
al: Different cytokine profiles of intrahepatic T cells in chronic
hepatitis B and hepatitis C virus infections. Gastroenterology.
112:193–199. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
McGuinness PH, Painter D, Davies S and
McCaughan GW: Increases in intrahepatic CD68 positive cells, MAC387
positive cells and proinflammatory cytokines (particularly
interleukin 18) in chronic hepatitis C infection. Gut. 46:260–269.
2000. View Article : Google Scholar
|
|
27.
|
Nelson DR, Lauwers GY, Lau JY and Davis
GL: Interleukin 10 treatment reduces fibrosis in patients with
chronic hepatitis C: a pilot trial of interferon nonresponders.
Gastroenterology. 118:655–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Brière F, Servet-Delprat C, Bridon JM,
Saint-Remy JM and Banchereau J: Human interleukin 10 induces naive
surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3.
J Exp Med. 179:757–762. 1994.PubMed/NCBI
|
|
29.
|
Defrance T, Vanbervliet B, Brière F, et
al: Interleukin 10 and transforming growth factor beta cooperate to
induce anti-CD40-activated naive human B cells to secrete
immunoglobulin A. J Exp Med. 175:671–682. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Rushbrook SM, Ward SM, Unitt E, et al:
Regulatory T cells suppress in vitro proliferation of
virus-specific CD8+ T cells during persistent hepatitis C virus
infection. J Virol. 79:7852–7859. 2005.
|