Introduction
Breast cancer is the most frequent type of tumor
occurring in women globally and its incidence has recently
increased (1). Epidemiological
investigations have demonstrated an association between the
incidence of breast cancer and dietary habits. For example, a
high-fat diet has been shown to increase the risk of breast cancer
(2,3). Notably, polyunsaturated fatty acids
(PUFAs) are highly associated with mammary carcinogenesis. For
example, n-3 fatty acids, such as eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA) have been shown to suppress the growth
of breast cancer in vitro and in vivo (4-6).
In contrast to these findings, n-6 fatty acids, such as linoleic
acid (LA) and arachidonic acid (AA) have been shown to promote the
development of breast cancer (7,8). The
association between fatty acids and carcinogenesis thus needs to be
clarified, in order to establish new dietary habits which may
prevent cancers. The effects of n-9 fatty acids on breast
carcinogenesis are not yet well understood.
Mead acid (MA) is a 20:3 n-9 fatty acid
(5,8,11-eicosatorienoic acid) that was characterized by Mead and
Slaton (9). MA can be found in
minor quantities in the plasma and tissues of adult mammals and is
synthesized from oleic acid (OA;18:1 n-9) by elongation and
desaturation when essential n-3 and n-6 PUFAs are deficient
(10,11).
The anticancer effects of MA were previously
investigated against luminal type A breast cancer (12). MA was found to suppress the growth
of breast cancer cells (KPL-1) in vitro. The dietary
administration of MA was also shown to suppress the growth of
transplanted KPL-1 tumors in nude mice (12). In another previous in vivo
study using female rats, breast cancer was induced by the
carcinogen, N-methyl-N-nitrosourea (MNU), and MA was
shown to suppress the growth of breast tumor xenografts (13).
In addition to the present study, to the best of our
knowledge, only one previous study has been conducted to date
reporting that MA exerts an anticancer effect against breast cancer
(14). Heyd and Eynard
demonstrated that MA suppressed the proliferation of the breast
cancer cell line, MCF-7(14). In
their study, they further examined the influence of MA on the
bladder cancer cell line, T-24, and on the colon cancer cell line,
HRT-18. Unlike the data obtained with MCF-7 cells, MA was shown to
promote the growth of HRT-18 cells. In the presence of a high cell
density, MA increased the proliferation of T-24 cells (14). Opposite findings were noted
(decreased proliferation) when the cell density was low (14).
MA has been shown to exert various effects depending
on the cancer type. However, the ability of MA to affect the
prevention of carcinogenesis is not yet well understood. To further
investigate the anticancer effects and mechanisms of MA, which
could lead to new practical applications, such as a novel
therapeutic agent, dietary habits and functional foods, additional
studies with different models are required. In the present study,
the anticancer effects of MA were investigated in a rat model of
7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer, as
previously described (15).
Materials and methods
Diet
The experimental diets contained the same amounts of
nutrients, but included different fatty acid compositions (Table I). In brief, the MA and control
(CTR) diets were modifications of the AIN-76 diet. The MA diet
contained 5 or 10% SUNTGM33, which in turn contains 48% MA
(12,13). SUNTGM33 is a microbial oil obtained
by fungal fermentation (16). The
CTR diets contained 5 or 10% olive oil (Nakalai Tesque), which
contained 74.7% oleic acid (OA). OA is a precursor of MA. The
composition of SUNTGM33 and olive oil have been described in our
previous studies (12,13). The experimental diets contained 2.4
or 4.8% MA, while the CTR diet did not contain MA. The
concentration of MA in the experimental diet was consistent with
our previous studies (12,13). The highest concentration of MA
(4.8%) in the diet contained almost the same amount of MA, which
was considered the upper limit on the blend material level for the
diet used in rat feeding studies.
 | Table IComposition of the experimental
diets. |
Table I
Composition of the experimental
diets.
| | 2.4% MA
experimental group | 4.8% MA
experimental group |
|---|
| Component | CTR diet | MA diet | CTR diet | MA diet |
|---|
| Gasein | 20 | 20 | 20 | 20 |
| DL-methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Cornstarch | 43 | 43 | 38 | 38 |
| α-Cornstarch | 12 | 12 | 12 | 12 |
| Sucrose | 10 | 10 | 10 | 10 |
| Cellulose | 5 | 5 | 5 | 5 |
| AIN-76 mineral
mix | 3.5 | 3.5 | 3.5 | 3.5 |
| AIN-76 vitamin
mix | 1 | 1 | 1 | 1 |
| Choline
bitartrate | 0.2 | 0.2 | 0.2 | 0.2 |
| SUNTGM33 | 0 | 5 | 0 | 10 |
| Olive oil | 5 | 0 | 10 | 0 |
Carcinogen
DMBA was obtained in powder form from Eastman
Chemical. Prior to its use, DMBA was dissolved in sesame oil at
120˚C (DMBA 1,000 mg/50 ml sesame oil). A single dose of 80 mg/kg
body weight was administered orally (17). The same amount of sesame oil
without DMBA was administered to the animals in the CTR group.
Animals and experimental
procedures
The study protocol and animal procedures were
approved by the Animal Care and Use Committee of Kansai Medical
University, Hirakata, Osaka, Japan (permit no. 13-060). Throughout
the experiments, the animals were housed and treated in accordance
with the Guidelines for Animal Experimentation of Kansai Medical
University. In the present study, the following criteria for humane
endpoints were also used (NIH guidelines for endpoints in animal
study proposals): i) A tumor burden >10% of the animal body
weight; ii) the tumor should not exceed 40 mm in any one dimension;
iii) tumors that become ulcerated, necrotic or infected; iv) tumors
that interfere with the eating ability or impair the ambulation of
the animals.
In brief, 86 female Sprague-Dawley rats [Crl:CD(SD),
6 weeks old] were purchased from Charles River Laboratories Japan.
They were housed in groups of 4 or 5 in plastic cages with paper
bedding (Paper Clean; Japan SLC, Inc.) in a specific pathogen-free
environment maintained at 22±2˚C and at 60±10% relative humidity
with a 12-h light/dark cycle (lights on at 8:00 a.m. and lights off
at 8:00 p.m.). In the experiment with 2.4% MA, the rats were
randomly divided into 4 groups as follows: The CTR + sesame oil
(n=5), CTR + DMBA (n=13), 2.4% MA + sesame oil (n=5) and 2.4% MA +
DMBA (n=13) groups. In the experiment with 4.8% MA, the rats were
divided into 4 groups as follows: CTR + sesame oil (n=10), 4.8% MA
+ sesame oil (n=10), CTR + DMBA (n=15) and 4.8% MA + DMBA (n=15)
groups.
Fresh sterilized stocks of the pellet diet were
provided to the animals twice a week starting at 6 weeks of age.
The previous pellets were discarded to minimize the ingestion of
oxidized fatty acids. The animals in the experimental groups
received DMBA, whereas the animals in the CTR groups received
sesame oil at 7 weeks of age and all animals remained on the same
diets for the remaining duration of the experiments (until 19 weeks
of age). The experimental diets and water were available freely.
During the dosing period, the dose of the diet ingested, body
weight and tumor volume were measured once a week. The tumor volume
was calculated using the standard formula: Width2 x
length x 0.5. The tumor volume measurement was used for monitoring
of tumor incidence and growth. Prior to sacrifice, all rats were
anesthetized with isoflurane (Wako Pure Chemical Industries, Ltd.).
Before necropsy, the isoflurane was made to soak into paper and was
put in closed chamber. Subsequently, rats were anesthetized in a
pervasive chamber of isoflurane which was vaporized. A total of 4%
of isoflurane was used for the induction of anesthesia and blood
was sampled by inferior vena cava puncture. Subsequently, the
animals were sacrificed by exsanguination and aortic transection.
At necropsy, all organs were examined macroscopically and the
breast tissue and tumors were examined histologically. The tissues
were fixed in 10% neutral-buffered formalin, embedded in paraffin
and finally stained with hematoxylin and eosin (Wako Pure Chemical
Industries, Ltd.). Cell kinetics were also assessed. The serum
samples and the sections of the non-tumor breast tissues were used
for fatty acid analysis. During the examination of the animals
receiving the 4.8% MA diet, fatty acid analysis was not carried
out.
Cell kinetics
The cell kinetics (cell proliferation activity and
apoptosis) in the 6 largest DMBA-induced tumors were evaluated. The
cell proliferative activity was evaluated using anti-Ki-67 antibody
(cat. no. 418071, prediluted, clone SP6, Nichirei Biosciences). The
incubation condition was 1 h at room temperature. The induction of
apoptosis was evaluated by the anti-phospho-histone H2A.X (γ-H2A.X)
antibody (cat. no. 2577S, x100, clone Ser139, 1:100; Cell Signaling
Technology, Inc.), an immunomarker of the DNA damage response. The
incubation condition was 1 h at room temperature.
Immunohistochemical analysis was performed with the Histofine
MAX-PO for rats kit (Nichirei Biosciences) according to the
manufacturer's protocol. Each slide was scanned with a
high-resolution digital scanner (NanoZoomer 2.0 Digital Pathology;
Hamamatsu Photonics) to prepare the digital images (NDPI image).
The NDPI image files were opened in color mode with the NDP.view
software (Hamamatsu Photonics). The images were converted to the
JPEG files (magnification, x400) in 5 randomly selected areas
within each tumor and were analyzed by immunohistochemical
staining, as previously described (12,18,19).
The Ki-67 and γ-H2A.X labeling indices were assessed by positive
cells/1,000 cells as an index of cell kinetics.
Fatty acid analysis of serum and
mammary tissue
The fatty acid composition of the total phospholipid
fraction of serum was determined and mammary gland samples were
extracted using the method described in the study by Bligh and Dyer
(20). The total phospholipid
fraction was separated by thin-layer chromatography. The compound
1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (Avanti Polar
Lipids, Inc.) was added as an internal standard. Total phospholipid
fractions were transmethylated with HCL-methanol and subsequently,
the fatty acid composition was analyzed by gas chromatography
(GC-2014, Shimadzu Corporation) with a capillary column DB-225
(0.25 mm x 30 m x 0.25 µm) (J&M Scientific, Folsom). The system
was controlled with the gas chromatography software (GC solution;
Shimadzu Corporation). The fatty acid composition of the total
lipid fraction of the non-tumor mammary gland was determined.
Frozen tissues were thawed, minced and homogenized 3 times for 10
sec in 8 ml chloroform-methanol (2:1) by a polytron homogenizer
(Kinematica). The fatty acid analysis of the total lipid content in
the breast tissues was performed by a similar method as that
described in the analysis of the serum and mammary glands, with the
exception of excluding the separation step performed by thin-layer
chromatography (9,10,20).
Statistical analysis
The values are expressed as the means ± standard
error of the mean. The parameters body weight, tumor volume, tumor
weight, fatty acid composition and the percentage of Ki-67-positive
and γ-H2A.X-positive cells among the groups were analyzed using the
Student's t-test. The incidence of breast cancer was analyzed using
a χ2 test.
Results
Host animals
During the dosing period, the daily dose of food
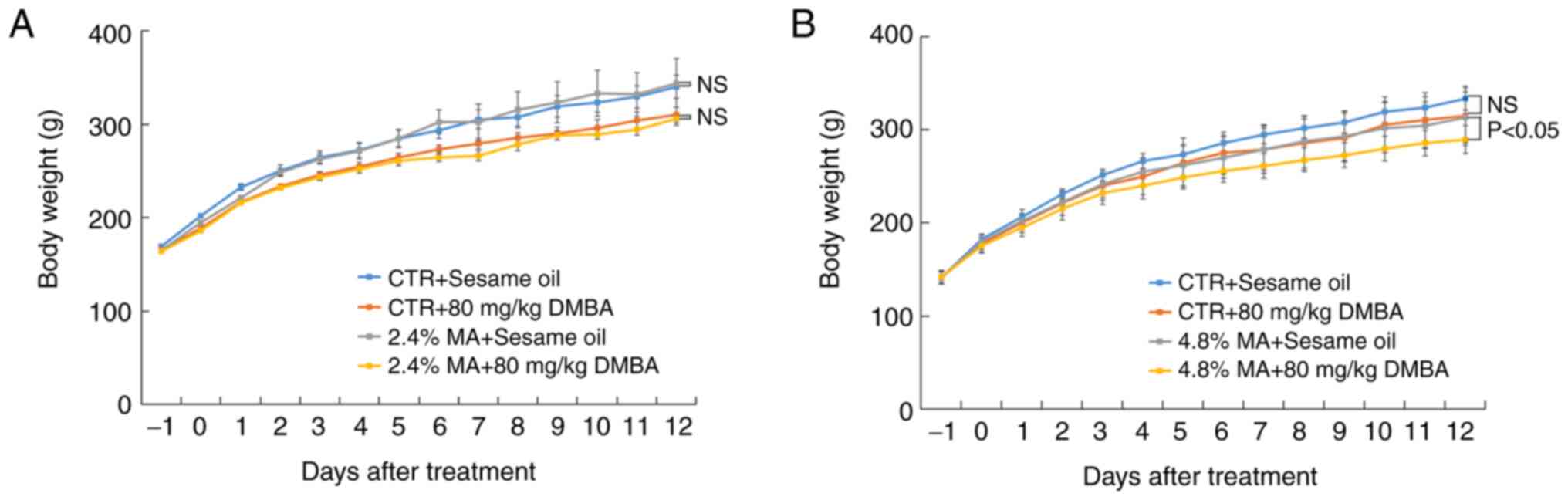
ingestion was compatible among the different groups. The parameter
body weight did not reveal significant differences when the 2.4% MA
diet was used, while in the 4.8% MA diet experimental protocol, the
body weight in the group administered the 4.8% MA diet and exposed
to DMBA was significantly decreased compared with that of the group
administered the 4.8% MA diet and given sesame oil (Fig. 1).
Mammary carcinogenesis
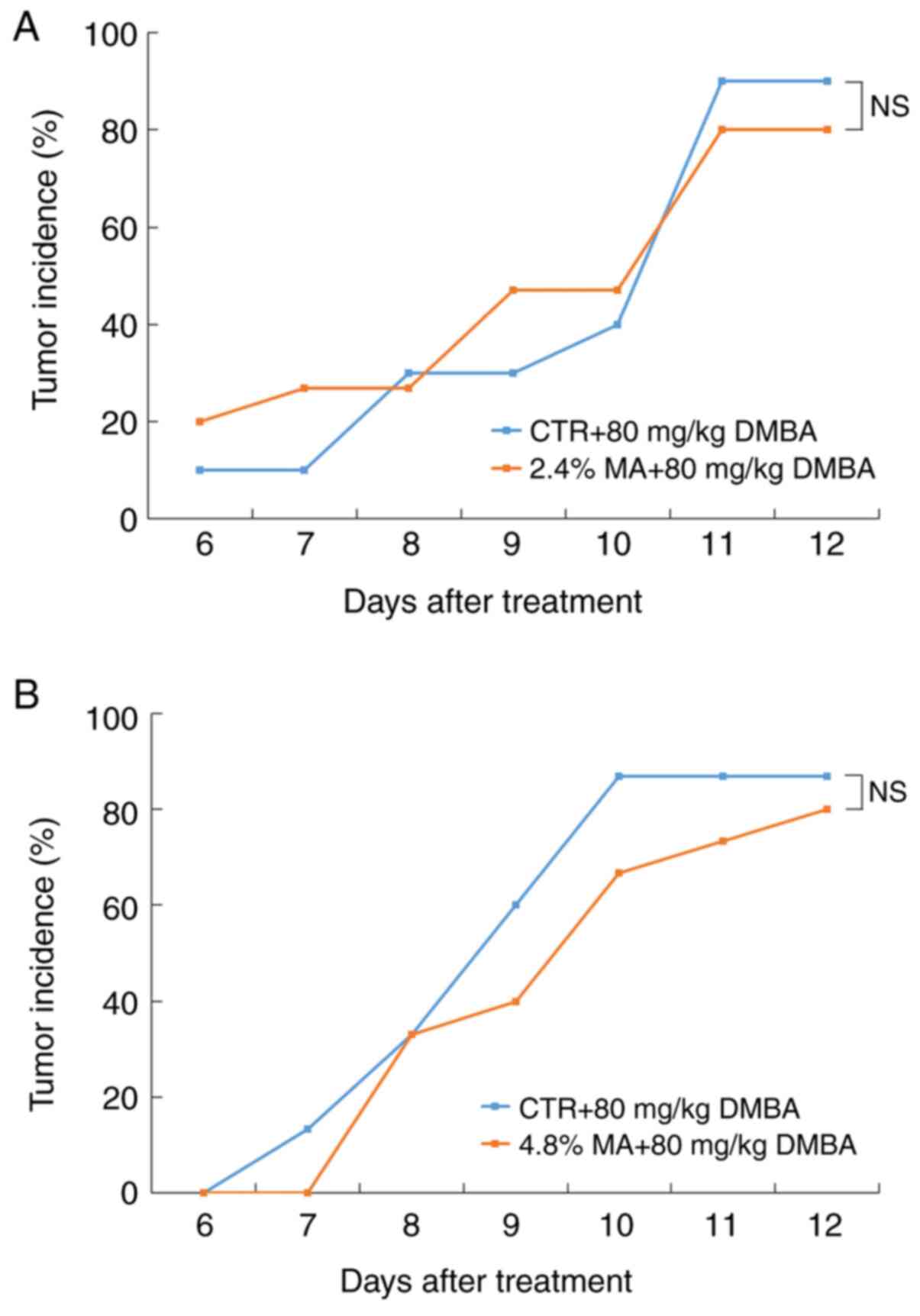
All mammary tumors were examined and confirmed
histologically as mammary cancers. At the end of the experimental
period, although the tumor incidence in both the 2.4 and 4.8% MA
diet groups was lower than that noted in the CTR diet groups, the
differences were not significant (Fig.
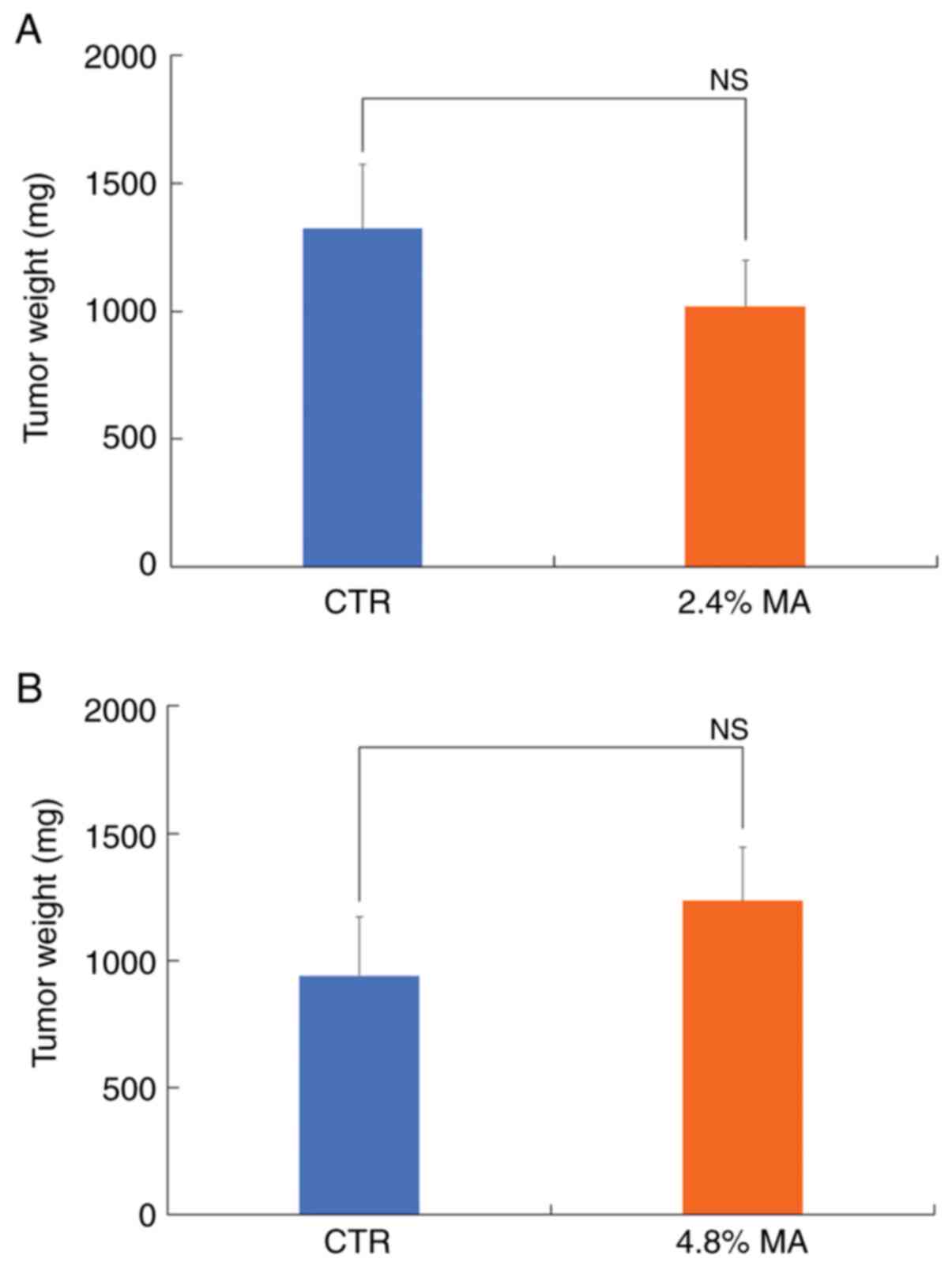
2). The mean values of DMBA-induced breast tumor weight in the
CTR diet and 2.4% MA diet groups were 1,323±251.4 mg and
1,019.3±178.6 mg, respectively, while the final average breast
tumor weight in the CTR diet and 4.8% MA diet groups was
941.3±231.4 mg and 1,235.6±208.4 mg, respectively. Neither of these
groups demonstrated a significant difference (Table II and Fig. 3). The mean values for tumor volume
in the CTR diet and 2.4% MA diet groups were 1,582±451
mm3 (maximum diameter, 29 mm) and 1,520 ± 397
mm3 (maximum diameter, 31 mm), respectively, while the
final average values for tumor volume in the CTR diet and 4.8% MA
diet groups were 633±1301 mm3 (maximum diameter, 27 mm)
and 930±2,418 mm3 (maximum diameter, 32 mm),
respectively. Neither of these groups demonstrated a significant
difference. The largest volume in the CTR diet and 2.4% diet groups
were 7,406 mm3 (28 mm in diameter) and 10,478
mm3 (31 mm in diameter), respectively, while the largest
tumor volumes in the CTR diet group and 4.8% MA diet groups were
5,832 mm3 (27 mm in diameter) and 10,240 mm3
(32 mm in diameter) respectively. The mean values of DMBA-induced
breast tumor multiplicity in the CTR diet and 2.4% MA diet groups
were 5.0±1.3 and 4.1±0.3, respectively, while the final average
breast tumor multiplicity values in the CTR diet and 4.8% MA diet
groups were 2.3±0.7 and 2.0±0.3, respectively. Neither of these
groups demonstrated a significant difference (data not shown).
 | Table IIWeight of DMBA-induced breast cancer
(mg). |
Table II
Weight of DMBA-induced breast cancer
(mg).
| | 2.4% MA
experimental group | 4.8% MA
experimental group |
|---|
| | CTR diet | MA diet | CTR diet | MA diet |
|---|
| Tumor weight | 1,323±251.4 | 1,019.3±178.6 | 941.3±231.4 | 1,235.6±208.4 |
| Significance | NS | NS |
In the groups in which DMBA was not administered,
the presence of breast tumors was not observed, in the presence of
either the CTR or MA diet in both experimental settings. No
conspicuous morphological differences were noted between the CTR
diet and the MA diet groups. No lymph node metastasis was noted in
any animal.
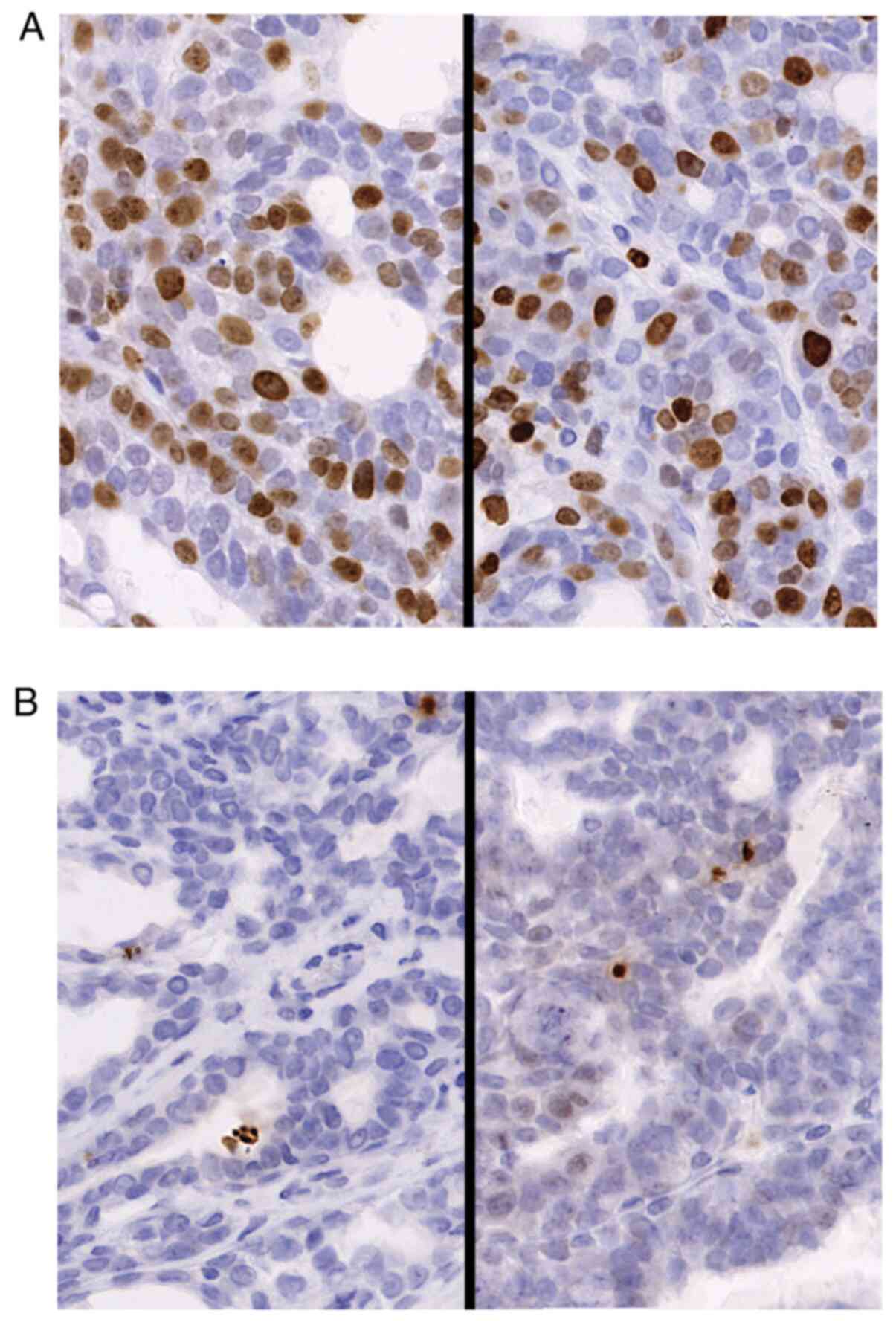
Proliferation and apoptotic ratio of
DMBA-induced breast cancer
The percentages of Ki-67-positive cells and
γ-H2A.X-positive cells from the CTR diet and MA diet groups were
compared with regard to the ratio of proliferative cells and the
apoptotic cell number. The proliferative cell number and apoptotic
cell ratio are presented in Table
III and Fig. 4. Although the
MA diet exhibited a tendency to suppress cancer cell proliferation,
the differences observed were not significant. In both experimental
settings with the 2.4% MA and 4.8% MA diet, the ratio of apoptotic
cells was exactly the same.
 | Table IIICell kinetics of DMBA-induced breast
cancer (%). |
Table III
Cell kinetics of DMBA-induced breast
cancer (%).
| | 2.4% MA
experimental group | 4.8% MA
experimental group |
|---|
| Examination | CTR diet | MA diet | CTR diet | MA diet |
|---|
| Ki-67 LI | 46.5±8.6 | 35.1±3.8 | 33.2±3.3 | 26.0±3.5 |
| γH2A.X LI | 0.7±0.3 | 0.6±0.2 | 0.7±0.3 | 0.6±0.2 |
| Significance | NS | NS |
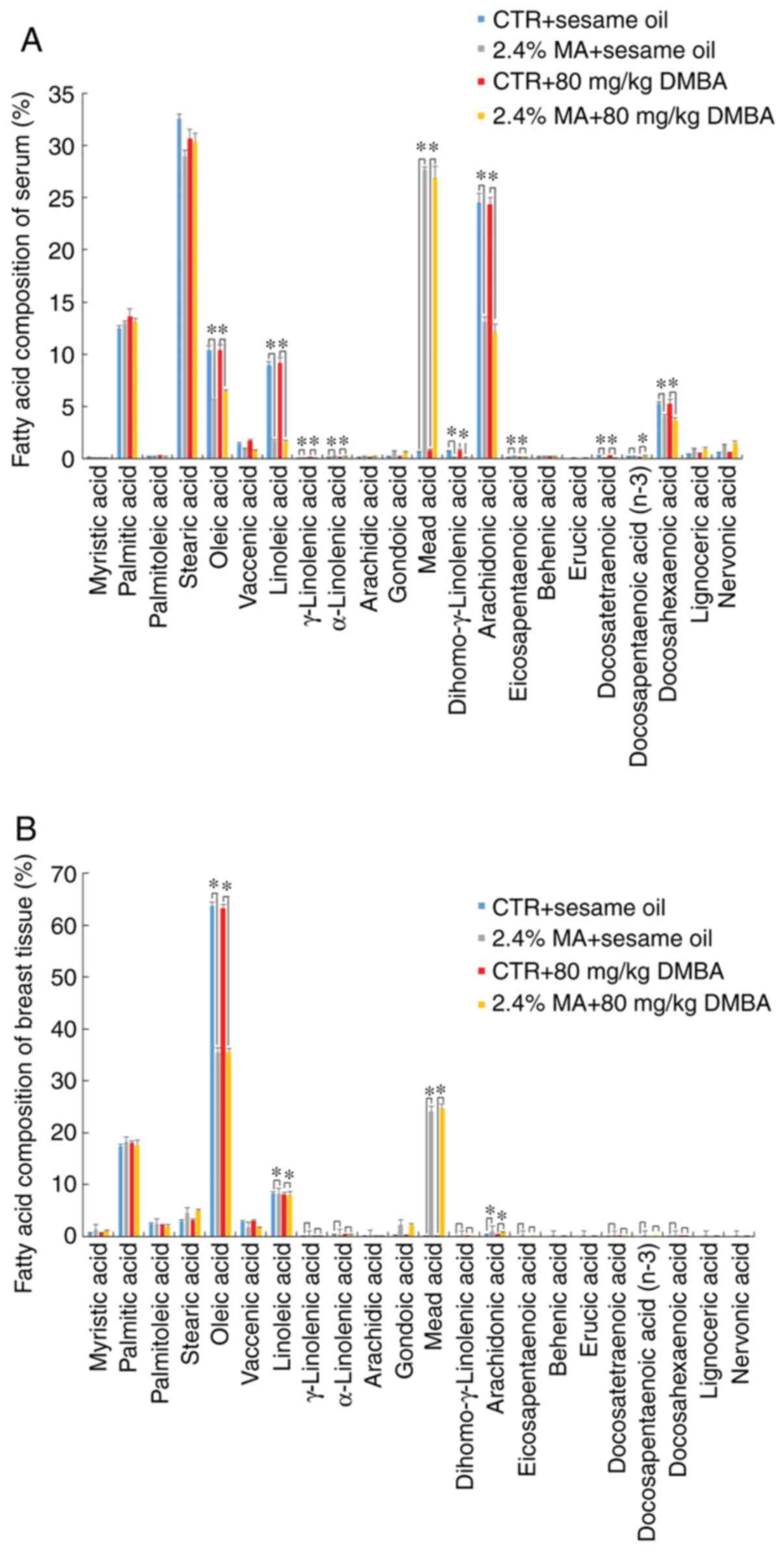
Fatty acid composition of serum and
mammary tissue
The different diet groups exhibited different fatty
acid compositions in serum and mammary tissues, reflecting the
content of the respective diets. Exposure to DMBA did not affect
the fatty acid composition. The n-3, n-6 and n-9 (MA) fatty acid
composition levels in the serum of the animals receiving the 2.4%
MA diet were significantly increased compared with those noted in
the CTR diet group, whereas the concentrations of OA, LA, AA and
DHA were significantly decreased compared with those noted in the
CTR + sesame oil group (Fig. 5A).
The levels of OA and LA in the non-tumor mammary gland were
significantly decreased and the level of AA in non-tumor mammary
gland was significantly increased in the MA group compared with
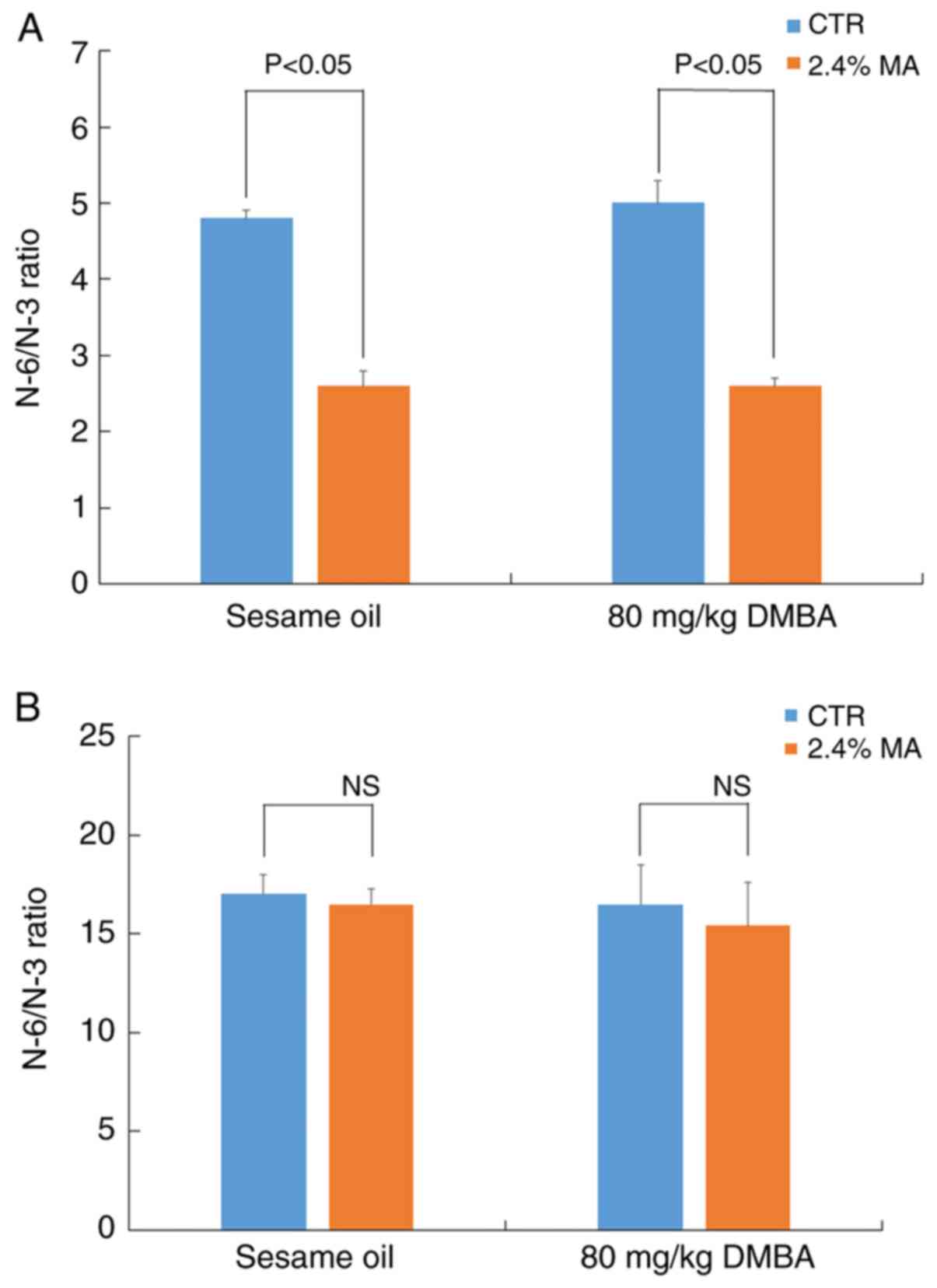
those noted in the CTR + sesame oil group (Fig. 5B). The changes in serum fatty acid
composition resulted in a significant decrease in the N-6/N-3 ratio
in the 2.4% MA diet group (Fig.
6A). However, the N-6/N-3 ratio noted in the non-tumor breast
tissue did not exhibit any marked changes (Fig. 6B).
Discussion
It is well known that there are a number of risk
factors for breast cancer (e.g., an advanced age or viral
infection) (21). The dietary
quality and habits are also one of the factors affecting breast
cancer (e.g., the consumption of red meat, ultra-processed sugary
products, sulforaphane, vitamin D, calcium, soy isoflavone)
(18,22-25). Recently, the influence of the quality of daily foods
on breast cancer has attracted considerable attention. For example,
Lo et al reported that the consumption of red meat increases
the risk of developing breast cancer (22). A large-scale cohort study carried
out in France revealed that the intake of ultra-processed sugary
products was associated with the incidence of breast cancers
(23). By contrast, sulforaphane
extracted from broccoli, vitamin D, calcium and soy isoflavone have
been reported to function as possible cancer-preventive agents
(18,24,25).
Therefore, it seems that the constituents of foods consumed on a
daily basis play a role in breast carcinogenesis.
The present study investigated the concentration of
fatty acids and its influence on breast cancer. Previous studies
have examined the influence of fatty acid composition on breast
carcinogenesis. The majority of previous studies have focused on
n-3 PUFA and/or n-6 PUFA. The effects of n-9 PUFA were previously
examined against breast cancer and the data indicated that MA
inhibited the growth of luminal A type breast cancer by suppressing
the expression of VEGFR. In addition, MA inhibited the growth of
transplanted luminal A type breast cancer cells in nude mice and
their metastasis to the lymph nodes (12). MA also inhibited the formation of
MNU-induced breast cancer in rats (13). Based on these data, MA appeared to
be beneficial for the suppression of breast cancer. However, it has
been reported that the effects of MA vary depending on the target
cells (14). Heyd and Eynard
examined the effects of MA on 3 different cancer cell lines (T-24;
bladder cancer cell line, MCF-7; breast cancer cell line and
HRT-18; colon cancer cell line) (14). In their study, MA suppressed the
cell proliferation of MCF-7, but promoted the growth of HRT-18
cells. When the cells were seeded at a high density, MA increased
the cell proliferation of T-24 cells, while the opposite results
were noted at a low cell density. In their study, MA treatment also
increased the expression levels of E-cadherin in the MCF-7 cell
line (14). However, E-cadherin
expression levels has not been found to be altered in the breast
cancer cell line, KPL-1(12).
Moreover, our previous studies indicated that MA inhibited the
expression levels of VEGFR, but did not affect angiogenesis
(12,13). By contrast, Hamazaki et al
measured angiogenesis by a co-culture system using umbilical vein
endothelial cells and human diploid fibroblasts with or without MA
and reported that MA inhibited angiogenesis (26). Moreover, Eynard et al
reported that MA inhibited the expression of E-cadherin and
stimulated the growth of squamous cell carcinoma (27).
The effects of MA on different cancer types vary
greatly and only 4 studies have been previously published examining
the association between MA and cancer cell progression (12-14,27).
In vivo studies were performed using two carcinogens, which
resulted in the induction of breast cancer formation via different
mechanisms of action. MNU is a direct-acting alkylating agent that
interacts with DNA and yields a variety of conversion products
(28). These products induce
breast cancer by causing DNA damage, DNA methylation and several
genetic abnormalities. DMBA was the carcinogen used in the present
study that could induce cancer progression through the formation of
DNA adducts and DNA damage (29,30).
In the present study, the MA diet did not suppress
the incidence of breast cancer, although the Ki-67 labeling index
was lower in the MA groups compared with that of the CTR diet
group. The N-6/N-3 ratio in serum in the MA diet group indicated a
significant decrease compared with that in the CTR diet group,
whereas significant changes were not detected in the breast tissues
in both groups. However, it has been previously reported that a
lower ratio N-6/N-3 in the serum is associated with a lower
incidence of breast cancer in humans (31). The reason for the discrepancy
between these studies is not clear; however, it may be associated
with the use of the two different carcinogens, MNU and DMBA.
In conclusion, the present study reported that the
parameters tumor incidence, Ki-67 labeling index and
γ-H2A.X-labeling index were not significantly affected by the
specific MA diets in female Sprague-Dawley rats with DMBA-induced
breast cancer. To further clarify the effects of MA on breast
carcinogenesis, further investigations with different experimental
breast cancer models are thus recommended.
Acknowledgements
The authors would like to thank Dr Robert R.
Maronpot, Maronpot Consulting, LLC, for his excellent scientific
advice and English grammar editing.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YK, MYo, ATs and KY made substantial contributions
to the conception and design of the study. YK, MYo, YM, TY, MYu, CK
and ATa were involved in data acquisition, data analysis and
interpretation. YK and KH were involved in fatty acid analysis. ATs
and KY drafted the article or critically revised it for important
intellectual content. All authors gave the final approval of the
version to be published and all author agree to be accountable for
all aspects of the work to ensure that questions regarding the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study protocol and animal procedures were
approved by the Animal Care and Use Committee of Kansai Medical
University, Hirakata, Osaka, Japan (permit no. 13-060).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colditz G and Chia KS: Invasive breast
carcinoma: Introduction and general features. In: World Health
Organization, Pathology & Genetics. Tumours of the Breast.
Lakhani SR, Ellis IO, Schnitt ST, Tan PH and van de Vijver MC
(eds). IARC Press, Lyon, pp14-31, 2012.
|
|
2
|
Adami HO, Signorello LB and Trichopulos D:
Towards an understanding of breast cancer etiology. Semin Cancer
Biol. 8:225–262. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Freedman LS, Kipnis V, Schatzkin A and
Potischman N: Methods of epidemiology: Evaluating the fat-breast
cancer hypothesis-comparing dietary instruments and other
developments. Cancer. 14:69–74. 2002.
|
|
4
|
Senzaki H, Tsubura A and Takada H: Effect
of eicosapentaenoic acid on the suppression of growth and
metastasis of human breast cancer cells in vivo and in vitro. World
Rev Nutr Diet. 88:117–125. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yamamoto D, Kiyozuka Y, Adachi Y, Takada
H, Hiroki K and Tsubura A: Synergistic action of apoptosis induced
by eicosapentaenoic acid and TNP-470 on human breast cancer cells.
Breast Cancer Res Treat. 55:149–169. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
VanderSluis L, Mazurak VC, Damaraju S and
Field CJ: Determination of relative efficacy of eicosapentaenoic
acid and docosahexaenoic acid for anti-cancer effects in human
breast cancer models. Int J Mol Sci. 18(2607)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Senzaki H, Iwamoto S, Ogura E, Kiyozuka Y,
Arita S, Kurebayashi J, Takada H, Hiroki K and Tubura A: Dietary
effects of fatty acids on growth and metastasis of KPL-1 human
breast cancer cells in vivo and in vitro. Anticancer Res.
18:1621–1627. 1998.PubMed/NCBI
|
|
8
|
Chung NW, Wu CT, Chen DR, Yeh CY and Lin
C: Hight levels of arachidonic acid and peroxisome
proliferator-activated receptor-alpha in breast cancer tissues and
associated with promoting cancer cell proliferation. J Nutr
Biochem. 24:274–281. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mead JF and Slaton WH Jr: Metabolism of
essential fatty acids. III. Isolation of 5,8,11-eicosatrienoic acid
from fat-deficient rats. J Biol Chem. 219:705–709. 1956.PubMed/NCBI
|
|
10
|
Fulco AJ and Mead JF: Metabolism of
essential fatty acids VIII. Origin of 5,8,11-eicosatrienoic acid in
the fat-deficient rat. J Biol Chem. 234:1411–1416. 1959.PubMed/NCBI
|
|
11
|
Ichi I, Kono N, Arita Y, Haga S, Arisawa
K, Yamano M, Nagase M, Fujiwara Y and Arai H: Identification of
genes and pathways involved in the synthesis of Mead acid (20:3
n-9), an indicator of essential fatty acid deficiency. Biochim
Biophys Acta. 1841:204–213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kinoshita Y, Yoshizawa K, Hamazaki K,
Emoto Y, Yuri T, Yuki M, Shikata N, Kawashima H and Tsubura A: Mead
acid inhibits the growth of KPL-1 human breast cancer cells in
vitro and in vivo. Oncol Rep. 32:1385–1394.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kinoshita Y, Yoshizawa K, Hamazaki K,
Emoto Y, Yuri T, Yuki M, Kawashima H, Shikata N and Tsubura A:
Dietary effects of mead acid on
N-methyl-N-nitrosourea-induced mammary cancers in
female Sprague-Dawly rats. Biomed Rep. 4:33–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heyd VL and Eynard AR: Effects of
eicosatrienoic acid (20:3 n-9, Mead's acid) on some
premalignant-related properties of three human cancer cell lines.
Prostaglandins Other Lipid Mediat. 71:177–188. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsubura A, Izuno Y, Shoji T, Kusunose N
and Morii S: Influence of strain and sex on the local development
of mammary tumors induced by direct application of DMBA powder to
rat mammary glands. Acta Pathol Jpn. 40:9–13. 1990.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sakuradani E, Kameda N, Hirano Y,
Nishihara M, Kawsashima H, Akimoto K, Higashiyama K, Ogawa J and
Shimizu S: Production of 5,8,11-eicosatrienoic acid by a delta5 and
delta6 desaturation activity-enhanced mutant derived from a delta12
desaturation activity-defective mutant of Mortierella alpine 1S-4.
Appl Microbiol Biotecnol. 60:281–287. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kawamura I, Mizota T, Kondo N, Shimomura K
and Kohsaka M: Antitumor effects of droloxifene, a new antiestrogen
drug, against 7,12-dimethylbenz (a) anthracence-induced mammary
tumor in rats. Japan J Pharmacol. 57:215–224. 1991.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kanematsu S, Yoshida K, Uehara N, Miki H,
Sasaki T, Kuro M, Lai YC, Kimura A, Yuri T and Tsubura A:
Sulforaphane inhibits the growth of KPL-1 human breast cancer cells
in vitro and suppress the growth and metastasis of
orthotopically transplanted KPL-1 cells in female athymic mice.
Oncol Rep. 26:603–608. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rodon CE, Weyemi U, Parekh PR, Huang D,
Burrell AS and Bonner WH: γH2A.X and other histone
post-translational modifications in the clinic. Biochim Biophys
Acta. 1819:743–756. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
3:911–917. 1959.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Bruzzi P, Green SB, Byar DP, Brinton LA
and Schairer C: Estimating the population attributable risk for
multiple risk factors using case-control data. Am J Epidemiol.
122:904–914. 1985.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lo JJ, Park YM, Sinha R and Sandler DP:
Association between meet consumption and risk of breast cancer:
Findings from the Sister Study. Int J Cancer. 146:2156–2165.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fiolet T, Srour B, Sellem L, Kesse-Guyot
E, Allès B, Mèjean C, Deschasaux M, Fassier P, Latino-Martel P,
Beslay M, et al: Consumption of ultra-processed foods and cancer
risk: Results from NutriNet-Sanè prospective cohort. BMJ.
360(k322)2018.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Chen P, Hu P, Xie D, Wang F and Wang H:
Meta-analysis of vitamin D, calcium and the prevention of breast
cancer. Breast Cancer Res Treat. 121:469–477. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Moorehead RA: Rodent models assessing
mammary tumor prevention by soy or soy isofravones. Genes (Basel).
10(566)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hamazaki T, Nagasawa Y, Hamazaki K and
Itomura M: Inhibitory effect of 5,8,11-eicosatorienoic acid on
angiogenesis. Prostaglandins Leukot Essent Fatty Acids. 86:221–224.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Eynard AR, Jiang WG and Mansel RE:
Eicosatrienoic acid (20:3 n-9) inhibits the expression of
E-cadherin and desmoglein in human squamous cell carcinoma in
vitro. Prostaglandins Leukot Essent Fatty Acids. 59:371–377.
1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tsubura A, Yoshizawa K and Sasaki T:
Methylnitrosourea. In Waxler P (Ed.), Encyclopedia of Toxicology,
3rd edition. Elsevier Inc., Academic press vol. 3, pp. 321-323,
2014.
|
|
29
|
Watabe T, Ishizuka T, Isobe M and Ozawa N:
A 7-hydoroxymethyl sulfate ester as an active metabolite of 7,
12-dimethyl[alpha]anthracene. Science. 215:403–405. 1982.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee HJ, Lee YJ, Kang CM, Bae S, Jeoung D,
Jang JJ, Lee SS, Cho CK and Lee YS: Differential gene signatures in
rat mammary tumors induced by DMBA and those induced by
tractionated gamma radiation. Radiat Res. 170:579–590.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Okuyama H, Kobayashi T and Watanabe S:
Dietary fatty acids-the N-6/N-3 balance and chronic elderly
diseases. Excess the linoleic acid and relative N-3 deficiency
syndrome seen in Japan. Prog Lipid Res. 35:409–457. 1996.PubMed/NCBI View Article : Google Scholar
|