Introduction
Reactive oxygen species (ROS) are derived from the
metabolism of molecular oxygen and chemically reactive molecules
containing oxygen, such as superoxide anion radicals
(O2−), hydrogen peroxide
(H2O2) and hydroxyl radicals
(−OH). Under normal cellular conditions, ROS are
primarily generated by mitochondrial respiratory metabolism and are
then efficiently neutralized by cellular antioxidant defense
mechanisms such as superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPx) (1–3).
However, the excessive generation of ROS during environmental
stress results in significant oxidative damage to lipids, DNA and
proteins (4). Such ROS-dependent
damage can lead to dysfunction or cell death. Consequently, ROS
have been implicated in the aging process, carcinogenesis,
rheumatoid arthritis and inflammation (5). Excessive ROS are particularly
deleterious in the pancreas, and their levels correlate with the
loss of β-cell mass, islet destruction and dysfunction in diabetes
(6).
Diabetes is a group of metabolic diseases in which a
person has high blood sugar caused either by defective insulin
production, insufficient insulin activity or both (7). Among the subtypes, type 1 diabetes
is due to an absolute deficiency of insulin secretion resulting
from the loss of β-cells upon autoimmune attack or oxidative stress
(8). The major effects of insulin
include the promotion of glucose uptake, the stimulation of
glycogen synthesis in the liver and muscle, triglyceride formation
and storage in adipocytes and an increase in protein synthesis
(9,10). Insufficient insulin secretion and
its downstream consequences thus lead to high blood sugar levels.
Prolonged insulin dysfunction results in the progressive
development of specific complications, including retinopathy with
potential blindness, nephropathy that may lead to renal failure,
neuropathy with risk of foot ulcers, limb amputation and
cardiovascular disease (11). In
modern medicine, there are no effective curative therapies for
diabetes mellitus (12). In
addition, current anti-diabetic therapies, such as insulin
injection and hypoglycemic agents, usually have adverse
side-effects and decreased efficacy over time (13,14). They can also be relatively
ineffective against certain long-term diabetic complications and
are associated with a high cost for patients and the health care
industry (15,16). Therefore, the development of
anti-diabetic natural products would be a promising solution for
patients confronted with the negative side-effects of current
anti-diabetic therapies (17).
In traditional medicine, several medicinal plants or
their extracts are widely used in a number of countries for the
treatment of disease. Morus alba (M. alba), the
mulberry plant, belongs to this class of well-known natural
medicinal species. M. alba belongs to the family Moraceae
and the genus Morus and is a perennial, fast-growing woody
plant that has a short proliferation period (18). Usually, 10–16 species of the genus
Morus are found in subtropical, warm and temperate regions
of Asia, Africa and North America. The roots and leaves of M.
alba are used in Chinese traditional medicine for their various
health benefits and for treating ailments. In recent studies, the
antioxidant activity of parts of the plant, such as the roots and
leaves in different model systems have also been documented
(19–22). However, the potential effects of
mulberry extracts, (the actual fruit of M. alba), in the
treatment of diabetes are currently unclear.
To this end, in this study, we aimed to assess the
effects of mulberry extracts on H2O2-induced
oxidative injury in insulin-producing pancreatic MIN6N β-cells and
to determine whether the extracts may be used in the treatment of
type 1 diabetes.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin (PS) and trypsin-EDTA
were purchased from Gibco (Grand Island, NY, USA).
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCF-DA)
was obtained from Molecular Probes (Carlsbad, CA, USA).
2,2-Diphenyl-1-picrylhydrazyl (DPPH), H2O2
and Hoechst 33342 were purchased from Sigma Biochemical (St. Louis,
MO, USA). All other chemicals were of analytical grade.
Preparation of fractionated extracts
An ethanol extract of the mulberry fruits (15 kg)
was acquired by extraction with 70% ethanol (36 liters) for 24 h at
room temperature 3 times. The solvent was filtered and evaporated
under a vacuum to afford 2,200 g crude ethanol extracts (yield,
14.6%). The lyophilized crude ethanol extracts were successively
fractionated with ethyl-acetate (EtOAc, MAE extract), n-butanol
(BuOH, MAB extract) and water (H2O, MAH extract). Each
fraction was evaporated in vacuo. The yields of the extracts
and the fractions were as follows: 1.59% (EtOAc fraction), 10.23%
(BuOH fraction) and 83.63% (H2O fraction).
Cell culture
Mouse insulin-producing MIN6N pancreatic β-cells
were derived from a mouse pancreatic islet cell line. The MIN6N
β-cells were provided by Professor H.Y. Kwon (College of Medicine,
Hallym University, Chuncheon, Korea). The cells were cultured in
DMEM (11 mM glucose; Gibco) supplemented with 10% inactivated FBS
and 1% PS and maintained at 37°C in a humidified 5% CO2
incubator. The cells were cultured up to approximately 85%
confluence and harvested with 0.25% trypsin-EDTA. The cells were
harvested and subcultured for an additional 48 h in DMEM. The cells
were maintained in these culture conditions for all the
experiments.
DPPH radical scavenging activity
The effects of the fractionated extracts (MAB, MAE
and MAH) on DPPH radicals was estimated according to the Blois
method (1). MAB, MAE and MAH were
dissolved in EtOH over a concentration range of 10 to 500 μg/ml.
Each sample solution (400 μl each) was mixed with DPPH solution
(600 μl) and incubated for 30 min. to allow reactions. The
absorbance of the resulting solution was measured at 520 nm using
an ELISA microplate reader (Model 550; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Measurement of cytotoxicity
The cytotoxic effects of the fractionated extracts
in pancreatic MIN6N β-cells were evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, which is based on the reduction of a tetrazolium salt by
mitochondrial dehydrogenase in viable cells (23). The MIN6N β-cells were seeded in
12-well plates and incubated for 24 h. The cells were then treated
with the fractionated extracts and incubated for 20 h at 37°C; 500
μg/ml MTT working solution were then added to each well followed by
incubation for 2.5 h at 37°C. The violet formazan crystal in each
well was dissolved in isopropyl alcohol and the absorbance of each
well was measured at 570 nm using an ELISA microplate reader (model
550; Bio-Rad Laboratories, Inc.).
Analysis of the protective effects of
mulberry extracts
To investigate the protective effects of the
fractionated extracts against H2O2-induced
cell death, the cells were first incubated with DMEM containing
0.5% FBS for 24 h. Following this, the indicated dose of the
fractionated extract was added for 20 h, prior to treatment with
0.7 mM H2O2 for 4 h. Cell viability was
measured by MTT assay. In brief, 500 μg/ml MTT solution were added
to each well followed by incubation for 2.5 h at 37°C. The
absorbance of each well was then measured at 570 nm using an ELISA
microplate reader (model 550; Bio-Rad Laboratories, Inc.).
Intracellular ROS scavenging activity and
image analysis
To determine the effects of fractionated extracts on
H2O2-induced intracellular ROS generation,
the MIN6N β-cells were seeded in 12-well plates the day prior to
treatment with the extracts. The β-cells were treated with various
concentrations of fractionated extracts for 20 h, followed by the
addition of 0.7 mM H2O2 to each well. After 4
h of incubation, 5 μM H2DCF-DA solution in
phosphate-buffered saline (PBS, pH 7.38) was added and the
fluorescence was measured at excitation and emission wavelengths of
485 and 535 nm, respectively, using a microplate
spectrofluorometer. For image analysis of the production of
intracellular ROS, the MIN6N β-cells were seeded in cover
slip-loaded 12-well plates, which were then treated with various
concentrations of fractionated extracts for 20 h, followed by the
addition of 0.7 mM H2O2 to each well. After 4
h of incubation, H2DCF-DA solution was added to each
well of the plate, which was incubated for 2 h at 37°C. Images of
the stained cells were collected using a fluorescence microscope
(Nikon, Tokyo, Japan).
Lipid peroxidation inhibitory
activity
Lipid peroxidation was assayed using the
thiobarbituric acid (TBA) reaction as previously described
(24). In brief, the cells were
seeded in 12-well plates and incubated for 24 h. Subsequently, the
cells were treated with various concentrations of fractionated
extracts for 20 h, followed by the addition of 0.7 mM
H2O2 to each well. After 4 h of incubation,
the cells were washed with cold PBS, harvested with 0.25%
trypsin-EDTA and homogenized in cold 1.15% potassium chloride
(KCl). A 100-ml aliquot of homogenized cells was then mixed with
0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid
(pH 3.6) and 1.5 ml of 0.8% TBA. The solution was then heated to
95°C for 2 h. After cooling at room temperature, an BuOH/pyridine
mixture (15:1, v/v) was added and the mixture was shaken for 5 min
and then centrifuged at 1,000 × for 10 min. The supernatant was
isolated and the absorbance was measured at 535 nm.
Image analysis of DNA fragmentation
The MIN6N β-cells were labeled using the
cell-permeable, DNA-specific fluorescent dye, Hoechst 33342 as
previously described (25). Cells
with homogeneously stained nuclei were considered viable, whereas
the presence of chromatin fragmentation was indicative of
H2O2-induced apoptosis (26). The MIN6N β-cells were seeded in
12-well plates. Twenty-four hours later, the cells were treated
with various concentrations of fractionated extracts for 20 h,
followed by further incubation for 4 h prior to exposure to 0.7 mM
H2O2. Hoechst 33342 working solution was then
added to each well, followed by 15 min of incubation at room
temperature. Images of the stained cells were collected using a
fluorescence microscope (Nikon), in order to examine the degree of
DNA fragmentation.
Statistical analysis
All measurements are from at least 3 independent
experiments and the values are expressed as the means ± SD.
Statistical analysis was performed using the Student’s t-test. A
value of p<0.05 was considered to indicate a statistically
significant difference.
Results
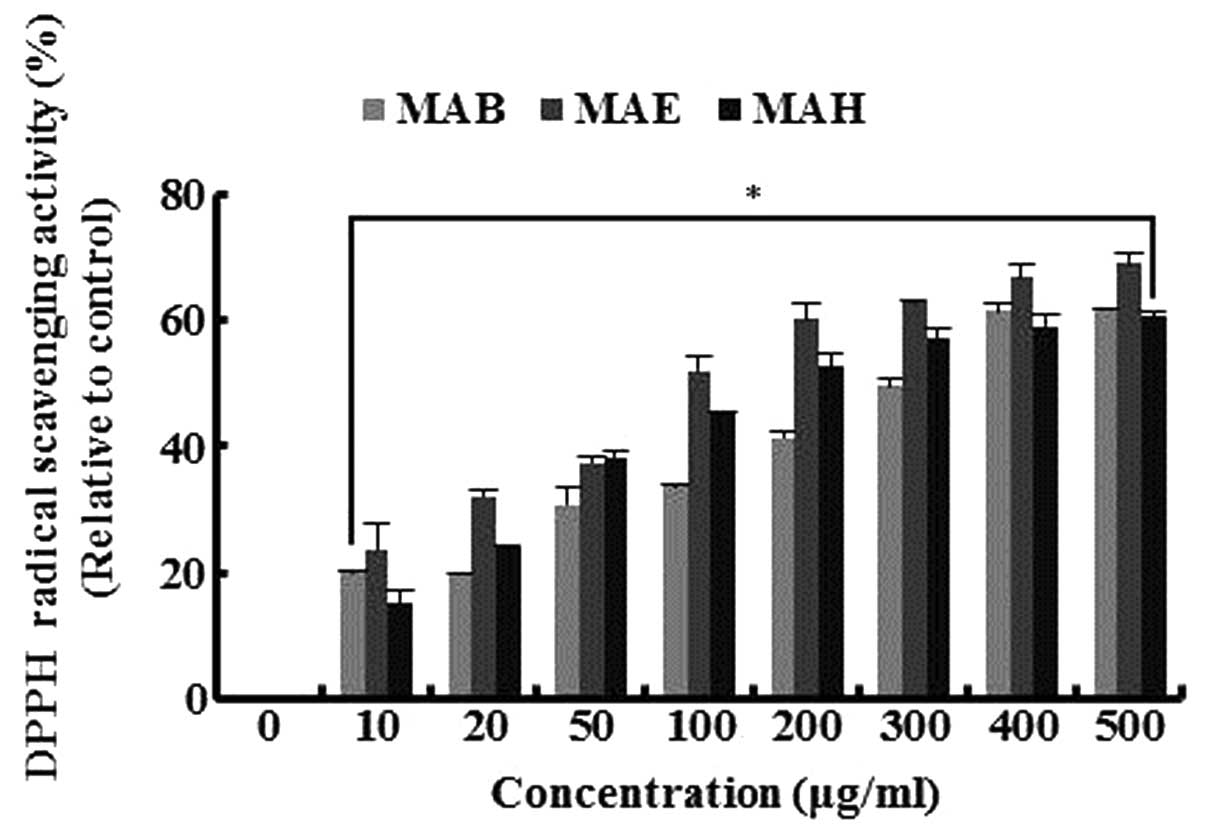
DPPH radical scavenging activity of
fractionated extracts
To determine the radical scavenging activity of the
fractionated extracts, we measured scavenged DPPH radicals in the
presence or absence of fractionated extracts (MAB, MAE and MAH). As
shown Fig. 1, the extracts
scavenged the DPPH radicals in a dose-dependent manner, with a 400
μg/ml dose of each extract inducing a reduction in DPPH radicals
between 59 and 67%. MAE exhibited the most potent DPPH radical
scavenger activity, inducing reductions of 51, 67 and 69% at
concentrations of 100, 400 and 500 μg/ml, respectively. These
results suggest that fractionated mulberry fruit extracts have good
antioxidant properties.
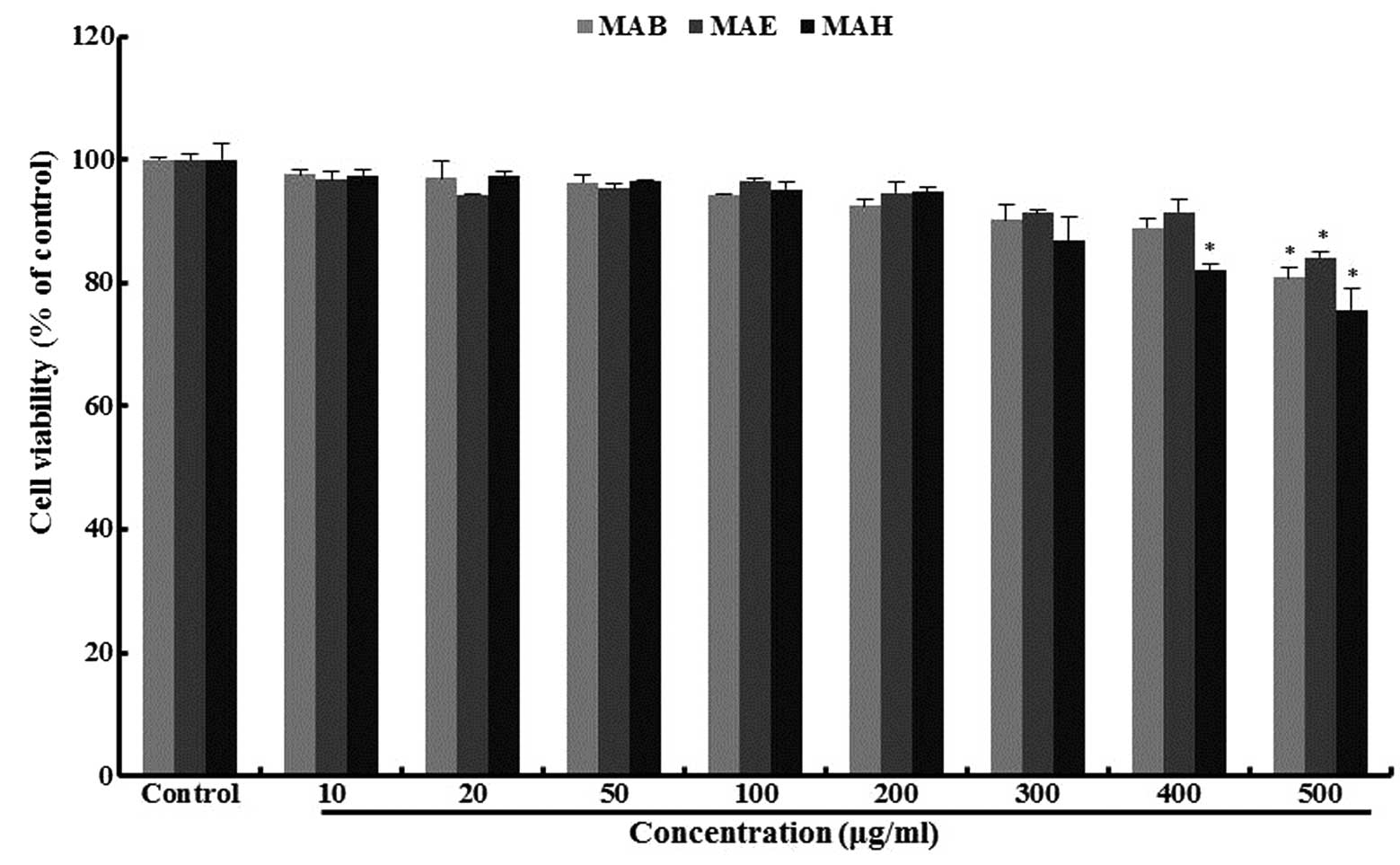
Cytotoxicity of fractionated extracts of
mulberry in pancreatic β-cells
To determine the range of doses over which
fractionated extracts of mulberry were non-toxic, MIN6N β-cell
viability was measured by MTT assay (Fig. 2). Cell viability was >90% in
the presence of all extracts, up to a concentration of 200 μg/ml
and in the case of MAE, viability was still above this value (92%)
at a 400 μg/ml dose.
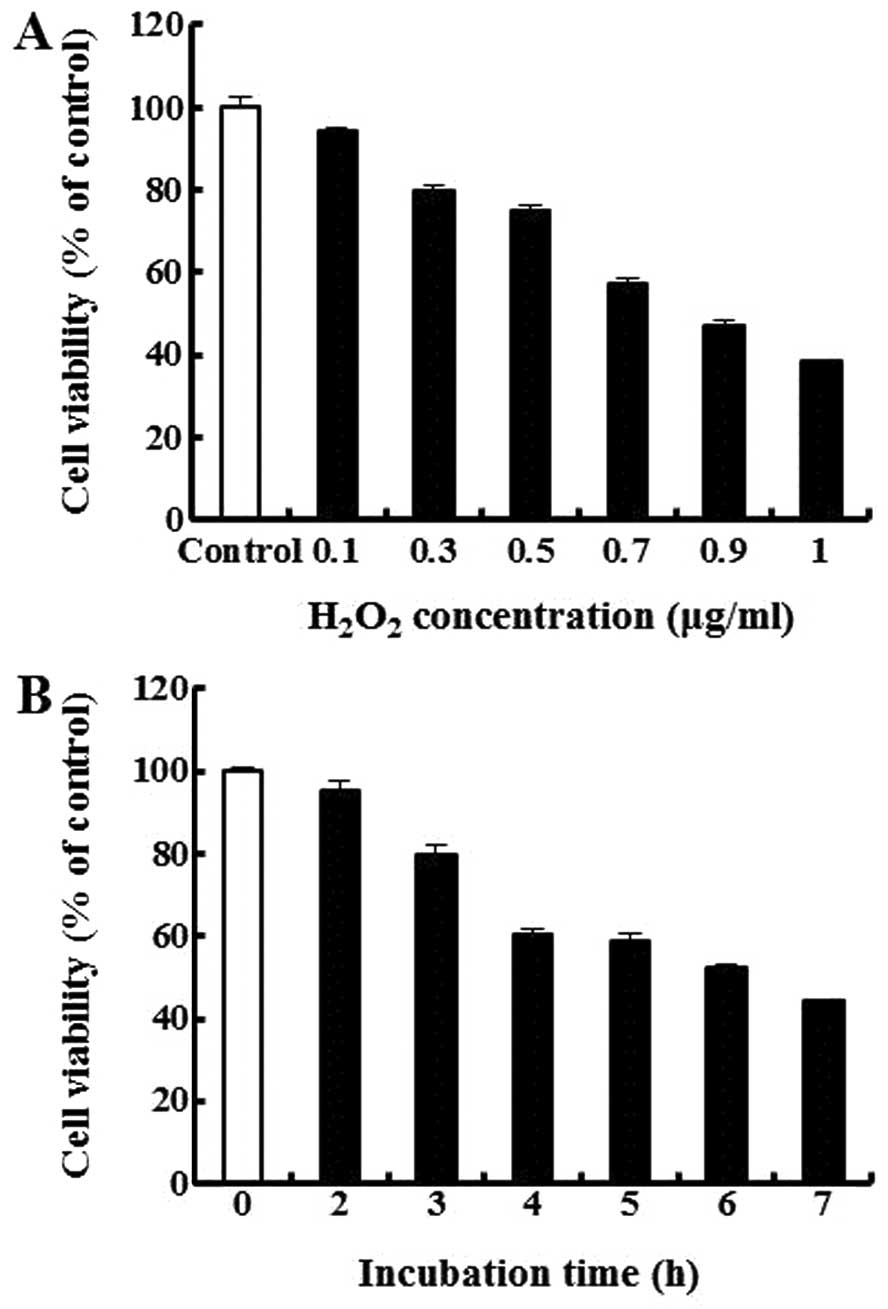
Cytotoxicity of
H2O2 in the MIN6N β-cells
To measure the cytotoxicity of
H2O2, we treated the cells with various doses
of H2O2 for 4 h. As shown in Fig. 3A, the concentrations of
H2O2 at ≥0.7 mM markedly reduced cell
viability, with cell death reaching approximately 57% at 0.7 mM
H2O2. This dose of H2O2
also induced cell death in a time-dependent manner (Fig. 3B). We thus selected 0.7 mM
H2O2 and an exposure time of 4 h for the
subsequent experiments.
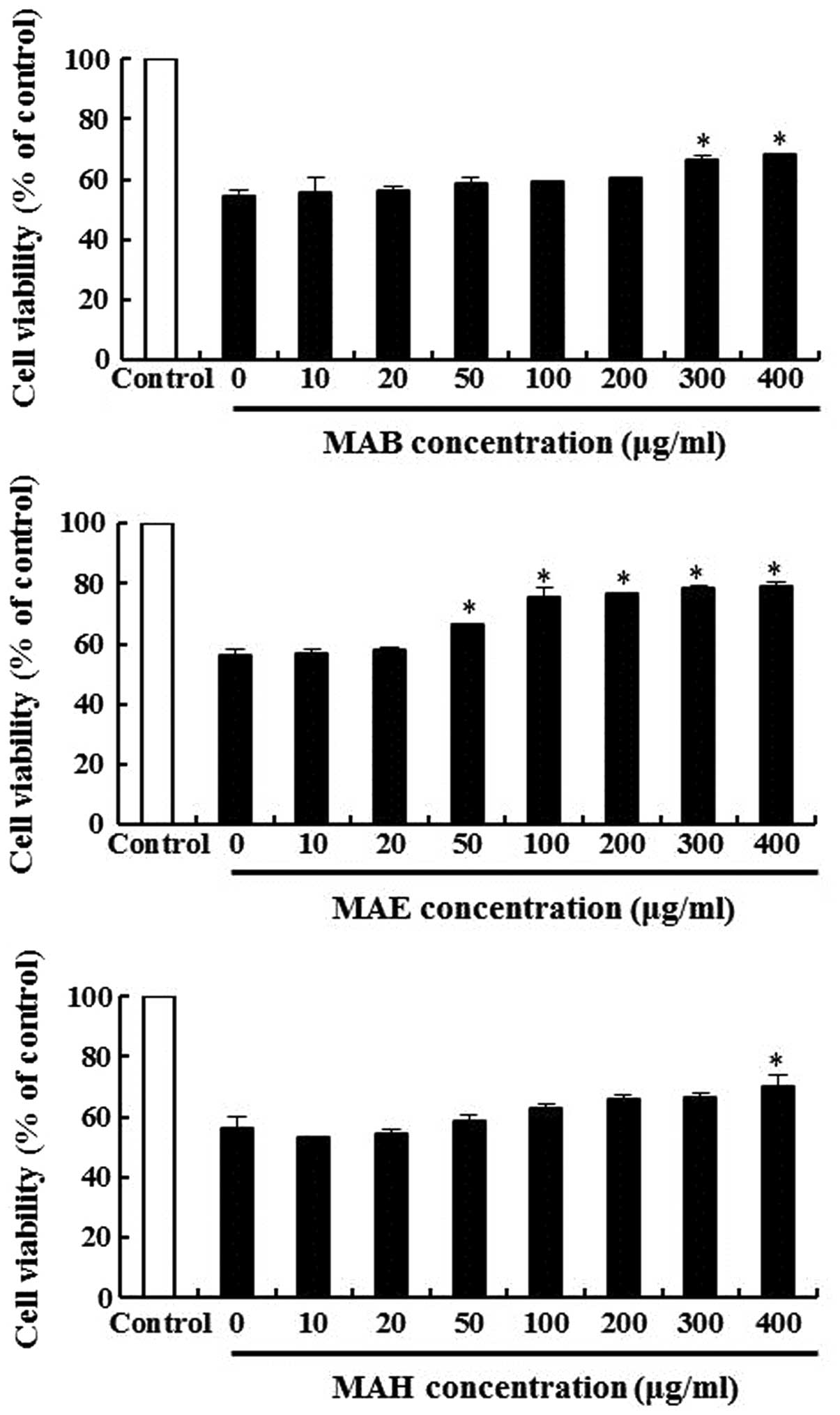
Protective effects of fractionated
mulberry extracts in MIN6N β-cells
The protective effects of MAB, MAE and MAH in the
H2O2-treated MIN6N β-cells were measured by
MTT assay. The cells were pre-treated with various concentrations
of MAB, MAE and MAH for 20 h, followed by treatment with
H2O2 for 4 h. In these experiments, cell
viability decreased to approximately 55% following treatment with
H2O2 in the controls. Pre-treatment with 100
μg/ml MAB, MAE and MAH restored cell viability to 62, 75 and 63% of
the control (Fig. 4),
respectively. MAE was particularly effective in preventing
H2O2-induced cell death. Thus, we conclude
that all 3 extracts are capable of preventing cell death induced by
oxidative stress.
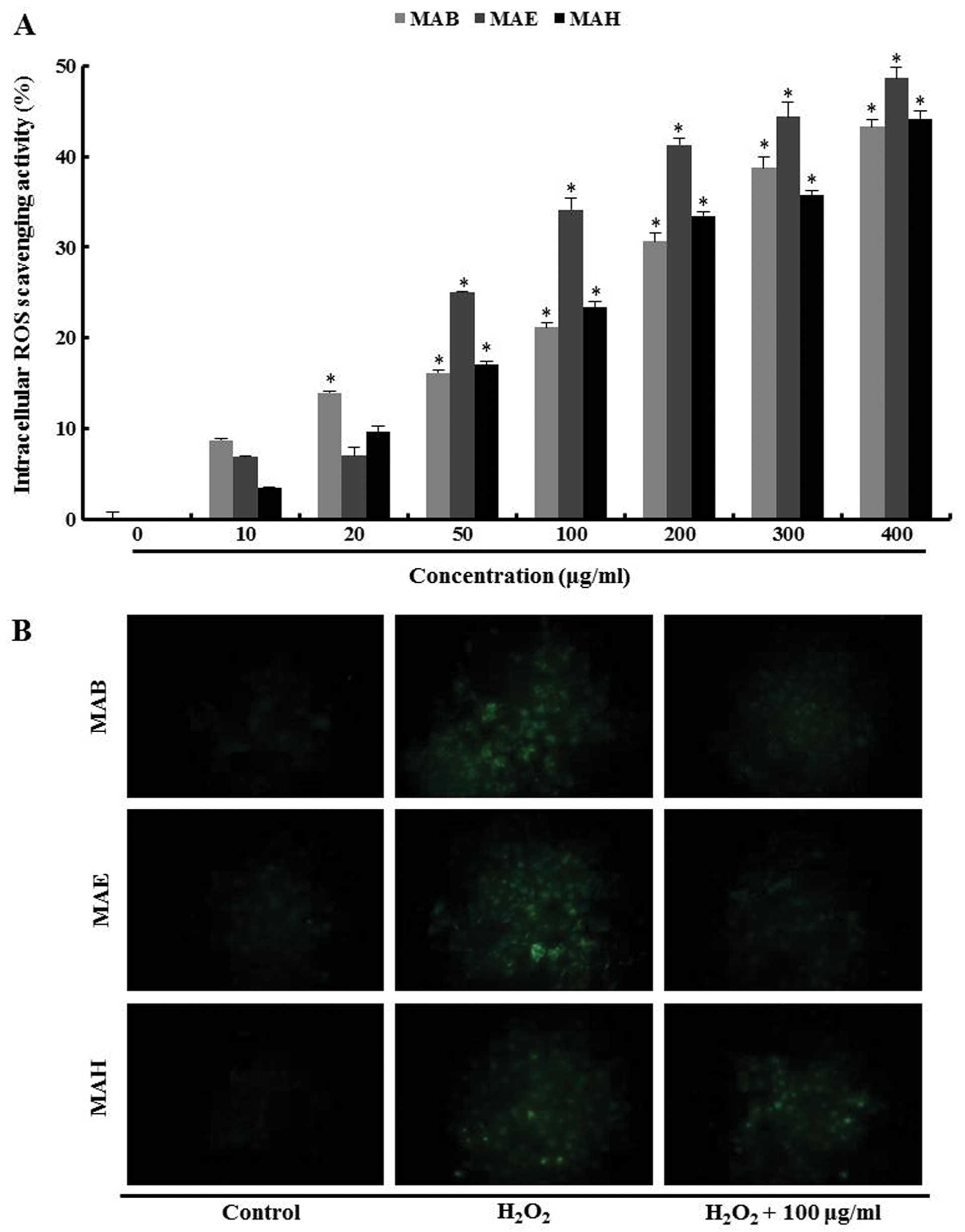
Intracellular ROS scavenging activity of
fractionated mulberry extracts
Intracellular ROS levels in the
H2O2-treated MIN6N β-cells were determined
using the ROS-sensitive fluorescent probe, H2DCF-DA,
which is cleaved by intracellular esterases into its
non-fluorescent form, DCFH. This form, which is no longer membrane
permeable, can be further oxidized by H2O2 to
its fluorescent form, DCF (27).
MAB, MAE and MAH were able to scavenge intracellular ROS in a
dose-dependent manner. Consistent with its protective effects on
cell viability, MAE was a much more effective suppressor of
intracellular ROS compared with the other fractions. The ROS
scavenging activity increased up to approximately 34% with 100
μg/ml MAE compared with the cells treated only with
H2O2 (Fig.
5A).
In the fluorescent microscopic images, the
fluorescence intensity of the H2DCF-DA stain was
enhanced in the H2O2-treated MIN6N β-cells.
As a result, the fractionated mulberry extracts reduced the green
fluorescence intensity, compared with the cells treated only with
H2O2, indicating a reduction in ROS
generation. Among the fractionated extracts, MAB and MAE markedly
decreased the intracellular fluorescence intensity at 100 μg/ml
(Fig. 5B). These data suggest
that MAB and MAE possessed greater ROS scavenging activity than
MAH.
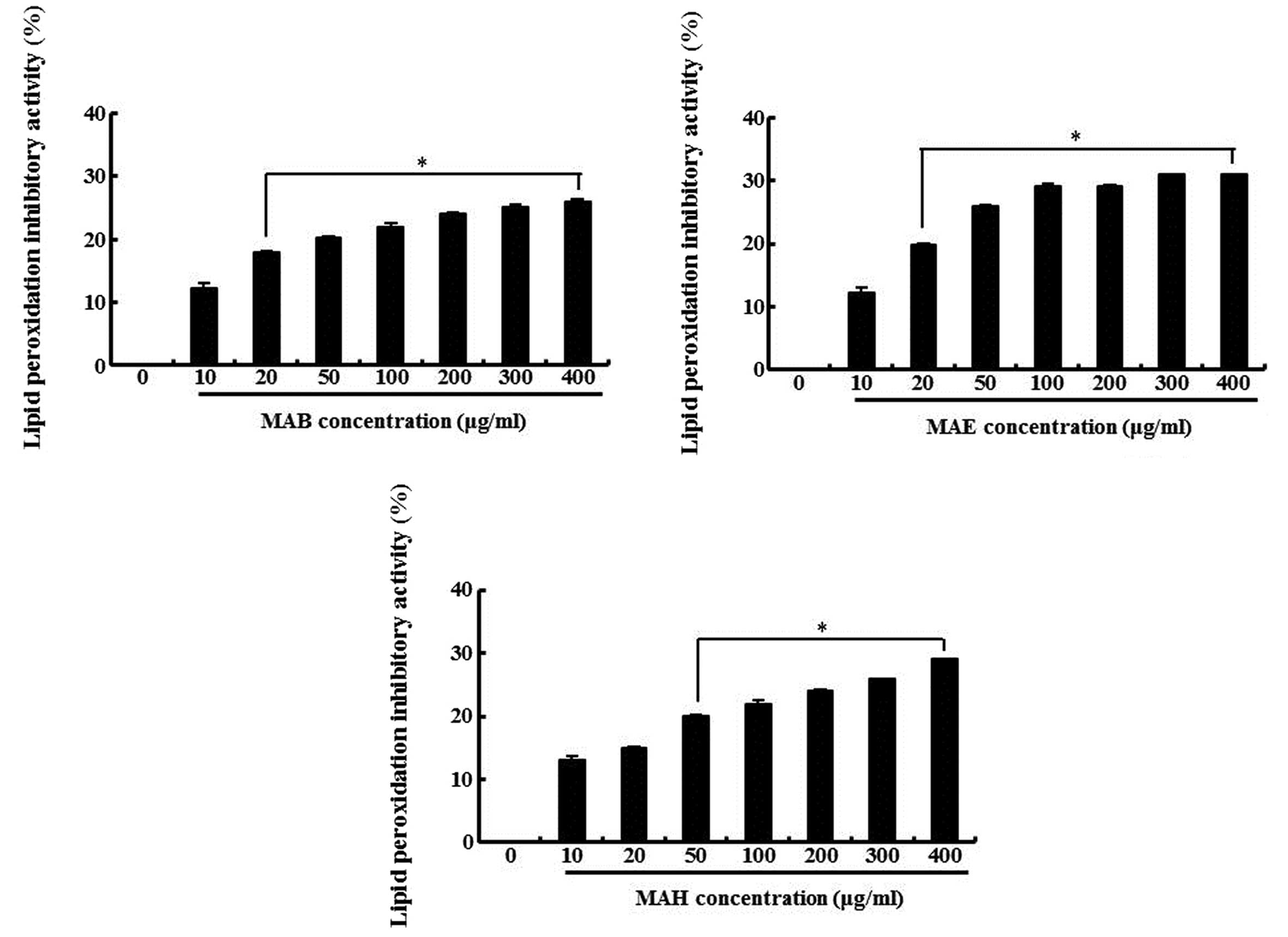
Inhibitory effect of fractionated
mulberry extracts on lipid peroxidation in MIN6N β-cells
Based on the above results, we hypothesized that the
antioxidant properties of the extracts would reduce ROS-induced
cellular damage, such as lipid peroxidation and DNA
fragmentation.
The inhibitory effect of the fractionated extracts
on lipid peroxidation in H2O2-treated MIN6N
β-cells was determined by measuring TBA reactive substances
(TBARS), lipid peroxidation products, as previously described
(28). As shown in Fig. 6, the cells exposed to
H2O2 showed an increase in lipid peroxidation
as indicated by the generation of TBARS, whereas, the fractionated
extracts inhibited lipid peroxidation. The inhibitory effect of 100
μg/ml MAB, MAE and MAH was 22, 29 and 22%, respectively compared
with the cells treated only with H2O2. These
results indicate that the ability of the fractionated extracts to
block on H2O2-induced TBARS formation may be
due to their intracellular ROS scavenging activity.
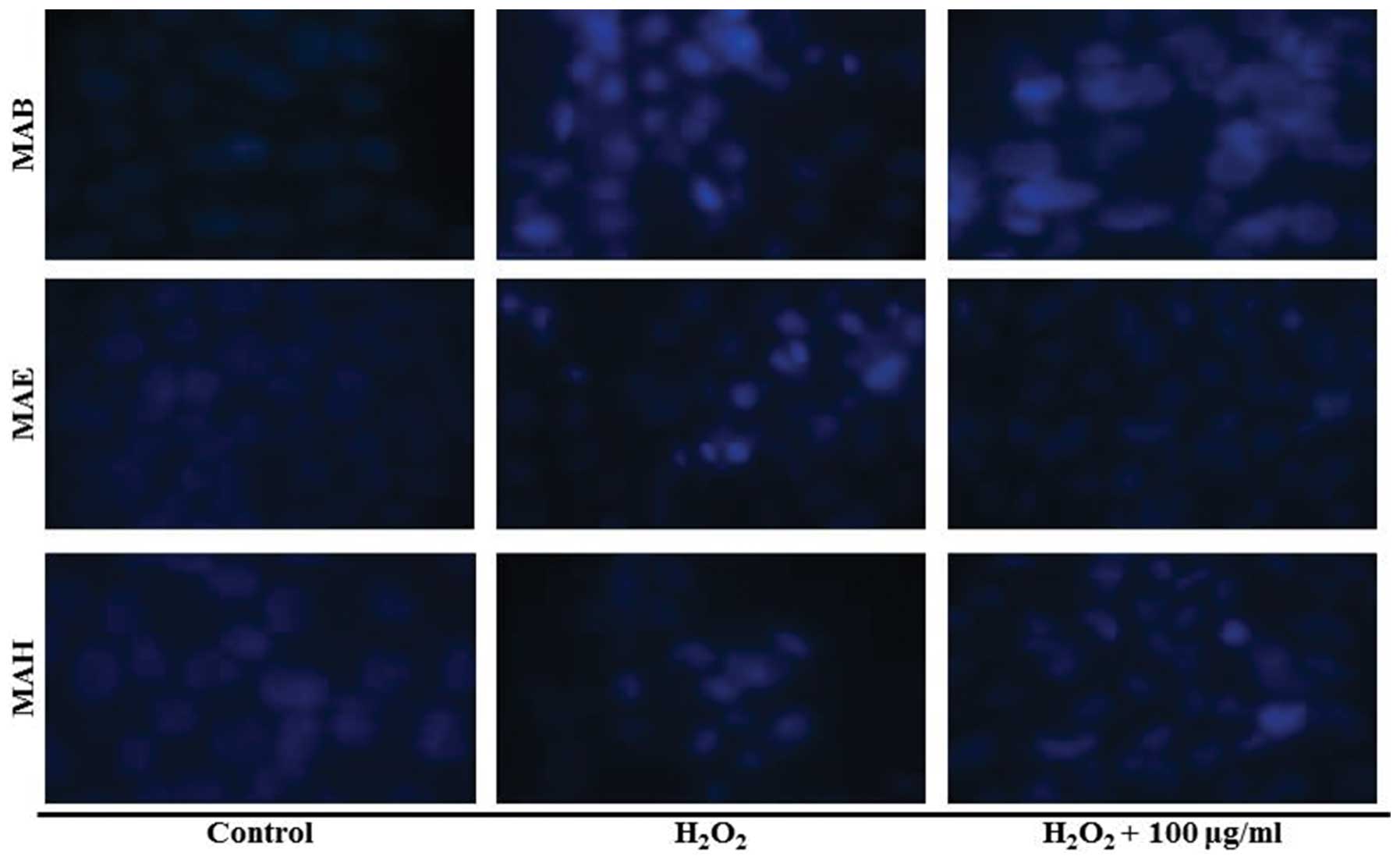
Effect of fractionated mulberry extracts
on DNA fragmentation in MIN6N β-cells
In order to confirm the DNA fragmentation induced by
H2O2 in MIN6N β-cells, we stained their DNA
with Hoechst 33342. The microscopic images in presented Fig. 7 illustrate that treatment the of
the cells with H2O2 induced DNA
fragmentation, a characteristic feature of apoptosis. However,
pre-treatment with 100 μg/ml of each fractionated extract decreased
the level of DNA fragmentation when compared with the cells treated
with H2O2 alone. These results confirmed that
MAB, MAE and MAH inhibited oxidative stress-induced DNA
fragmentation in MIN6N β-cells.
Discussion
ROS production contributes to metabolic disorders
that accompany diabetes (29).
Although the body has many natural defense systems to guard against
the deleterious effects of ROS, a combination of aging and
lifestyle choices can exacerbate their effects. Therefore,
preventing the formation of ROS or scavenging intracellular ROS may
be a useful treatment for several metabolic disorders.
In this regard, there is an increasing demand for
alternative, safe and low-cost materials/substances with
antioxidant properties isolated from natural sources. Some
medicinal plants, including Vaccinium spp. and Rubus
coreanus, both produce red pigments that are composed of
anthocyanin derivatives and polyphenols, which are compounds with
antioxidant activity (30–34).
Although M. alba is mainly used as a food
supply to raise silkworms, its leaves and branches have been used
in medicine to treat fever, liver injury and diabetes (35–38). However, the plant also produces
compounds with potential antioxidant properties, and in this study,
to our knowledge, we demonstrate for the first time that this can
be exploited for protection against oxidative stress-induced death
of pancreatic cells.
Specifically, we demonstrated that fractionated
mulberry extracts (MAB, MAE and MAH) decreased intracellular DPPH
radicals in H2O2-treated MIN6N β-cells and
that treament with all these extracts effectively attenuated
H2O2-induced β-cell death. The extracts also
reduced H2O2-induced intracellular ROS and
blocked H2O2-induced cell death by inhibiting
lipid peroxidation and DNA fragmentation.
In conclusion, we demonstrate that mulberry extracts
protect pancreatic β-cells against oxidate stress induced by
H2O2 by limiting ROS production, which
suggests that such extracts may prove useful in the treatment of
diabetes. Finally, we suggest that additional studies are required
to further define the mechanisms by which mulberry extracts
attenuate oxidative stress and exert anti-diabetic effects in
pancreatic β-cells.
Acknowledgements
This study was supported by the High Value-added
Food Technology Development Program, Ministry of Agriculture, Food
and Rural Affairs, Republic of Korea.
References
|
1
|
Blois MS: Free Radicals in Biological
Systems. Science. 132:306–307. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diplock AT, Charleux JL, Crozier-Willi G,
et al: Functional food science and defence against reactive
oxidative species. Br J Nutr. 80(Suppl 1): S77–S112. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalton TP, Shertzer HG and Puga A:
Regulation of gene expression by reactive oxygen. Annu Rev
Pharmacol Toxicol. 39:67–101. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blumberg J: Use of biomarkers of oxidative
stress in research studies. J Nutr. 134:3188S–3189S.
2004.PubMed/NCBI
|
|
5
|
Lizard G, Fournel S, Genestier L, et al:
Kinetics of plasma membrane and mitochondrial alterations in cells
undergoing apoptosis. Cytometry. 21:275–283. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Lee JS, Kim YR, et al: Hispidin
isolated from Phellinus linteus protects against hydrogen
peroxide-induced oxidative stress in pancreatic MIN6N β-cells. J
Med Food. 14:1431–1438. 2011.
|
|
7
|
Maritim AC, Sanders RA and Watkins JB III:
Diabetes, oxidative stress, and antioxidants: a review. J Biochem
Mol Toxicol. 17:24–38. 2003. View Article : Google Scholar
|
|
8
|
Zhong J, Rao X, Xu JF, Yang P and Wang CY:
The role of endoplasmic reticulum stress in autoimmune-mediated
beta-cell destruction in type 1 diabetes. Exp Diabetes Res.
2012:2389802012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bach JF: Insulin-dependent diabetes
mellitus as an autoimmune disease. Endocr Rev. 15:516–542. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuoka T, Kajimoto Y, Watada H, et al:
Glycation-dependent, reactive oxygen species-mediated suppression
of the insulin gene promoter activity in HIT cells. J Clin Invest.
99:144–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen DA, Yaqoob MM and Harwood SM:
Mechanisms of high glucose-induced apoptosis and its relationship
to diabetic complications. J Nutr Biochem. 16:705–713. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis KL, Tangirala M, Meyers JL and Wei
W: Real-world comparative outcomes of US type 2 diabetes patients
initiating analog basal insulin therapy. Curr Med Res Opin.
9:1083–1091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rolo AP and Palmeira CM: Diabetes and
mitochondrial function: role of hyperglycemia and oxidative stress.
Toxicol Appl Pharmacol. 212:167–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giugliano D, Ceriello A and Paolisso G:
Oxidative stress and diabetic vascular complications. Diabetes
Care. 19:257–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samyshkin Y, Guillermin AL, Best JH,
Brunell SC and Lloyd A: Long-term cost-utility analysis of
exenatide once weekly versus insulin glargine for the treatment of
type 2 diabetes patients in the US. J Med Econ. 15(Suppl 2):
S6–S13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koster I, Schubert I and Huppertz E:
Follow up of the CoDiM-Study: Cost of diabetes mellitus 2000–2009.
Dtsch Med Wochenschr. 137:1013–1016. 2012.(In German).
|
|
17
|
Riblett B: Diabetes mellitus: the
undiscussed side effect: sexual dysfunction. RN (For Managers).
46:40–41. 1983.PubMed/NCBI
|
|
18
|
Pan G and Lou C: Isolation of an
1-aminocyclopropane-1-carboxylate oxidase gene from mulberry
(Morus alba L.) and analysis of the function of this gene in
plant development and stresses response. J Plant Physiol.
165:1204–1213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim GN, Kwon YI and Jang HD: Mulberry leaf
extract reduces postprandial hyperglycemia with few side effects by
inhibiting a-glucosidase in normal rats. J Med Food. 14:712–717.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon HJ, Chung JY, Kim JY and Kwon O:
Comparison of 1-deoxynojirimycin and aqueous mulberry leaf extract
with emphasis on postprandial hypoglycemic effects: in vivo and in
vitro studies. J Agric Food Chem. 59:3014–3019. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng ZP, Cheng KW, Zhu Q, Wang XC, Lin ZX
and Wang M: Tyrosinase inhibitory constituents from the roots of
Morus nigra: a structure-activity relationship study. J
Agric Food Chem. 58:5368–5373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin YS, Lee MJ, Han W, Heo SI, Sohn SI and
Wang MH: Antioxidant effects and hepatoprotective activity of
2,5-dihydroxy-4,3′-di(beta-d-glucopyranosyloxy)-trans-stilbene from
Morus bombycis Koidzumi roots on CCl4-induced liver damage.
Free Radic Res. 40:986–992. 2006.PubMed/NCBI
|
|
23
|
Jang JS, Lee JS, Lee JH, et al: Hispidin
produced from Phellinus linteus protects pancreatic
beta-cells from damage by hydrogen peroxide. Arch Pharm Res.
33:853–861. 2010.
|
|
24
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang KA, Kim JS, Zhang R, et al: KIOM-4
protects against oxidative stress-induced mitochondrial damage in
pancreatic β-cells via its antioxidant effects. Evid Based
Complement Alternat Med. 2011:9786822011.PubMed/NCBI
|
|
26
|
Gschwind M and Huber G: Apoptotic cell
death induced by beta-amyloid 1–42 peptide is cell type dependent.
J Neurochem. 65:292–300. 1995.
|
|
27
|
Ortega-Camarillo C, Guzman-Grenfell AM,
Garcia-Macedo R, et al: Hyperglycemia induces apoptosis and p53
mobilization to mitochondria in RINm5F cells. Mol Cell Biochem.
281:163–171. 2006. View Article : Google Scholar
|
|
28
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Are oxidative stress-activated signaling pathways
mediators of insulin resistance and beta-cell dysfunction?
Diabetes. 52:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang R, Kim JS, Kang KA, Piao MJ, Kim KC
and Hyun JW: Protective mechanism of KIOM-4 in
streptozotocin-induced pancreatic β-cells damage is involved in the
inhibition of endoplasmic reticulum stress. Evid Based Complement
Alternat Med. 2011:2319382011.PubMed/NCBI
|
|
30
|
Patel KD, Scarano FJ, Kondo M, Hurta RA
and Neto CC: Proanthocyanidin-rich extracts from cranberry fruit
(Vaccinium macrocarpon Ait.) selectively inhibit the growth
of human pathogenic fungi Candida spp and Cryptococcus
neoformans. J Agric Food Chem. 59:12864–12873. 2011.PubMed/NCBI
|
|
31
|
Kim TG, Kang SY, Jung KK, et al: Antiviral
activities of extracts isolated from Terminalis chebula
Retz., Sanguisorba officinalis L, Rubus coreanus Miq
and Rheum palmatum L against hepatitis B virus. Phytother
Res. 15:718–720. 2001.PubMed/NCBI
|
|
32
|
Ku CS and Mun SP: Antioxidant activities
of ethanol extracts from seeds in fresh Bokbunja (Rubus coreanus
Miq.) and wine processing waste. Bioresour Technol.
99:4503–4509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang HM, Oh SM, Lim SS, Shin HK, Oh YS and
Kim JK: Antiinflammatory activities of Rubus coreanus depend
on the degree of fruit ripening. Phytother Res. 22:102–107.
2008.
|
|
34
|
Im SE, Nam TG, Lee H, et al: Anthocyanins
in the ripe fruits of Rubus coreanus Miquel and their
protective effect on neuronal PC-12 cells. Food Chem. 139:604–610.
2013.PubMed/NCBI
|
|
35
|
Liu JD: Study of the etiology of low-fever
among female silk-weaving workers: extrinsic allergic alveolitis
due to mulberry silk dust exposure. Zhonghua Yu Fang Yi Xue Za Zhi.
19:354–357. 1985.(In Chinese).
|
|
36
|
Yang TP, Lee HJ, Ou TT, Chang YJ and Wang
CJ: Mulberry leaf polyphenol extract induced apoptosis involving
regulation of adenosine monophosphate-activated protein
kinase/fatty acid synthase in a p53-negative hepatocellular
carcinoma cell. J Agric Food Chem. Jun 26–2012.(Epub ahead of
print).
|
|
37
|
Kaewkaen P, Tong-Un T, Wattanathorn J,
Muchimapura S, Kaewrueng W and Wongcharoenwanakit S: Mulberry fruit
extract protects against memory impairment and hippocampal damage
in animal model of vascular dementia. Evid Based Complement
Alternat Med. 2012:2635202012.PubMed/NCBI
|
|
38
|
Kaneto H, Fujii J, Myint T, et al:
Reducing sugars trigger oxidative modification and apoptosis in
pancreatic beta-cells by provoking oxidative stress through the
glycation reaction. Biochem J. 320:855–863. 1996.PubMed/NCBI
|