Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are
the two major forms of chronic inflammatory bowel disease (IBD).
Increasing epidemiological data have suggested a link between
vitamin D deficiency and the incidence of IBD, and vitamin D
deficiency has been shown to be prevalent in patients with IBD
(1–3). As the vitamin D receptor (VDR) is
highly expressed in intestinal epithelial cells (IECs), the vitamin
D/VDR signaling pathway may play a key role in the pathogenesis of
IBD. Moreover, it has been reported that VDR gene polymorphisms are
associated with the incidence of IBD (4,5)
and VDR knockout mice have been shown to have a compromised mucosal
barrier, leading to increased susceptibility to mucosal damage and
an increased risk of developing IBD (6). These data suggest that vitamin D
and/or VDR serve as an environmental and/or genetic factor in the
pathogenesis of IBD.
The intestinal epithelial barrier plays an important
role in the development of colitis, which consists of a monolayer
of epithelial cells and intercellular junctions between adjacent
cells that seal the paracellular gap (7). The barrier regulates intestinal
permeability between the lumen and mucosal layer, protecting
mucosal immune cells from coming into contact with gut microbiome,
harmful solutes, toxins and luminal antigens (8–11).
The compromise or disruption of the intestinal barrier function
results in intestinal inflammation. The integrity of the intestinal
epithelial barrier is preserved by thousands of IECs. The aberrant
apoptosis of IECs has been proven to be a major pathophysiological
mechanism of increased gut permeability and inflammation (12–15). Previous studies have reported
increased IEC apoptosis in patients with UC and CD, as well as in
murine models of colitis (12–15). The excessive apoptosis of IECs
causes the disruption of the epithelial barrier, leading to
increased intestinal permeability and the subsequent invasion of
pro-inflammatory substances (13–15). Those substances can induce the
production of inflammatory cytokines and chemokines in the colonic
lamina propria. This vicious series of intestinal events promotes
the development of colonic inflammation and eventually results in
IBD.
1,25-Dihydroxyvitamin D3 (calcitriol) is
the active form of vitamin D and binds with VDR. Apart from its
classical calcium-regulating effect, vitamin D serves as a potent
regulator of multiple biological activities, including
antimicrobial activities, the inhibition of apoptosis and
immunomodulatory functions (16).
Recently, it was suggested that VDR transgenic mice exhibit less
colitis than wild-type mice, indicating the protective role of VDR
in the development of intestinal inflammation (13). Thus, we hypothesized that
treatment with vitamin D may attenuate the severity of
2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis by
inhibiting IEC apoptosis.
Materials and methods
Human biopsies
Human biopsies were collected through endoscopic
examination from patients with UC and CD at the Shengjing Hospital
of China Medical University between January 2012 and December 2012.
Non-IBD control subjects were selected from subjects who underwent
endoscopic examination for the elimination of other bowel diseases
and were proven to be free of gut-related diseases. Written
informed consent was obtained from each subject prior to enrollment
in the study.
Induction of acute colitis
Male adult C57BL6/J mice, weighing 20–25 g, were
supplied by the Center of Experimental Animals of China Medical
University, Shenyang, China. All animal procedures were reviewed
and approved by the Laboratory Animal Ethics Committee of China
Medical University. The mice were anaesthetized by an
intraperitoneal (i.p.) injection of cocktail anesthetics [ketamine
(ketavest 100 mg/ml); Pfizer, New York, NY, USA) and [xylazine
(Rompun 2%); Bayer HealthCare, Leverkusen, Germany). The mice were
treated with 100 mg/kg TNBS (Sigma-Aldrich, St. Louis, MO, USA)
dissolved in 50% ethanol by intrarectal injection with an 18-gauge
stainless steel gavage needle. The control group was treated with
50% ethanol without TNBS, as previoulsy described (17).
Treatment with vitamin D or the
vehicle
The TNBS and control groups were randomly divided
into 2 groups, respectively. One group was treated with the vitamin
D analog, paricalcitol (Sigma-Aldrich), dissolved in propylene
glycol:ethanol, 90:10 at 0.5 μg/kg body weight, while the
other group was administered the dissolvent only. Paricalcitol or
the vehicle were administered through i.p. injection 30 min before,
1, 3 and 5 days after the TNBS injection.
Reverse transcription-quantitative PCR
(RT-qPCR)
The mice were sacrificed 48 h after the TNBS
injection. A 2-cm section of the colon was cut from each mouse and
the colonic mucosa was harvested. Total RNA was isolated using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA
was synthesized from 3 μg of total RNA in a 20 μl
reaction system using M-MLV reverse transcriptase (Invitrogen) and
random primers. Quantitative (real-time) PCR (qPCR) was performed
on a Roche 480 Real-Time PCR system using SYBR-Green PCR reagent
kits (Clontech Laboratories Inc., Mountain View, CA, USA). The
relative amounts of mRNA were calculated using the
2−ΔΔCt formula. β-2 microglobulin (B2M) was used as an
internal control. The sequences of the primers used for PCR are
provided in Table I.
 | Table IPrimers used in this study for
PCR. |
Table I
Primers used in this study for
PCR.
| Primer name | Forward (5′→3′) | Reverse (3′→5′) |
|---|
| Human VDR |
ACCTGGTCAGTTACAGCATC |
ACTGACGCGGTACTTGTAGT |
| Human B2M |
TGGGTTTCATCCATCCGACA |
ACGGCAGGCATACTCATCTT |
| Mouse IL-1β |
AATGAAAGACGGCACACCCA |
TGCTTGTGAGGTGCTGATGT |
| Mouse IL-6 |
CCTCTGGTCTTCTGGAGTACC |
ACTCCTTCTGTGACTCCAGC |
| Mouse TNF-α |
ATGAGCACAGAAAGCATGA |
AGTAGACAGAAGAGCGTGGT |
| Mouse IFN-γ |
TTCTTCAGCAACAGCAAGGC |
TCAGCAGCGACTCCTTTTCC |
| Mouse MCP-1 |
GCTCAGCCAGATGCAGTTAA |
TCTTGAGCTTGGTGACAAAAACT |
| Mouse IL-17 |
TCTCCACCGCAATGAAGACC |
CACACCCACCAGCATCTTCT |
| Mouse IL-23 p19 |
GCTGTGCCTAGGAGTAGCAG |
TGGCTGTTGTCCTTGAGTCC |
| Mouse B2M |
CGGCCTGTATGCTATCCAGA |
GGGTGAATTCAGTGTGAGCC |
Western blot analysis
The colonic mucosa lysates were separated by
SDS-PAGE, and the proteins were transferred electrophoretically
onto polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). We used ImageJ software (National Institutes of Health,
Bethesda, MD, USA) to quantify the density of the bands normalized
to that of β-actin. The antibodies used in the present study
included: anti-VDR (C20; 1:2,000; Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA), anti-β-actin (A5316; 1:10,000;
Sigma-Aldrich), anti-p53 (9282; 1:3,000; Cell Signaling Technology,
Beverly, MA, USA), anti-p53 upregulated modulator of apoptosis
(PUMA; 7467; 1:3,000; Cell Signaling Technology) and anti-caspase 3
(9662; 1:1,000; Cell Signaling Technology) antibodies.
Hematoxylin and eosin staining
The whole colons were harvested on day 4 after the
TNBS injection. The colon morphology was recorded and scored
according to a macroscopic scoring system (18). The distal colon were fixed
overnight with 4% formaldehyde in PBS (pH 7.4), dehydrated with
graded alcohol, placed in xylene for 1 h and then embedded in
paraffin at 60°C. Sections of the colon (4 μm) were stained
with hematoxylin and eosin. Colon sections from all the groups were
examined for any histological changes. From each section, 20 random
spots were examined under a microscope (Leica DFC425; Leica
Microsystems, Heerbrugg, Switzerland) at a magnification of x100.
Microscopic scoring was performed for each spot according to a
scoring system (18)
independently by two pathologists who were blinded to the group
design.
Measurement of serum 25-hydroxyvitamin
D
The human serum 25-hydroxyvitamin D concentration
(nmol/l) was measured using a commercial 25-hydroxyvitamin D EIA
kit (Immunodiagnostic Systems PLC, Boldon, Tyne & Wear, UK)
according to the manufacturer’s instructions.
FITC-dextran intestinal permeability
The mice were denied access to food, but were
allowed to drink water for 4 h before gavage. FITC-4 kDa dextran
(50 mg/ml) (Sigma-Aldrich) was administered by gavage at a dose of
4 μl/g body weight through an 18-guage stainless steel
gavage needle. Blood serum was collected 3 h later. The blood serum
was then placed at 150 μl/well into a 96-well plate and
analyzed using a Synergy HT microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Results
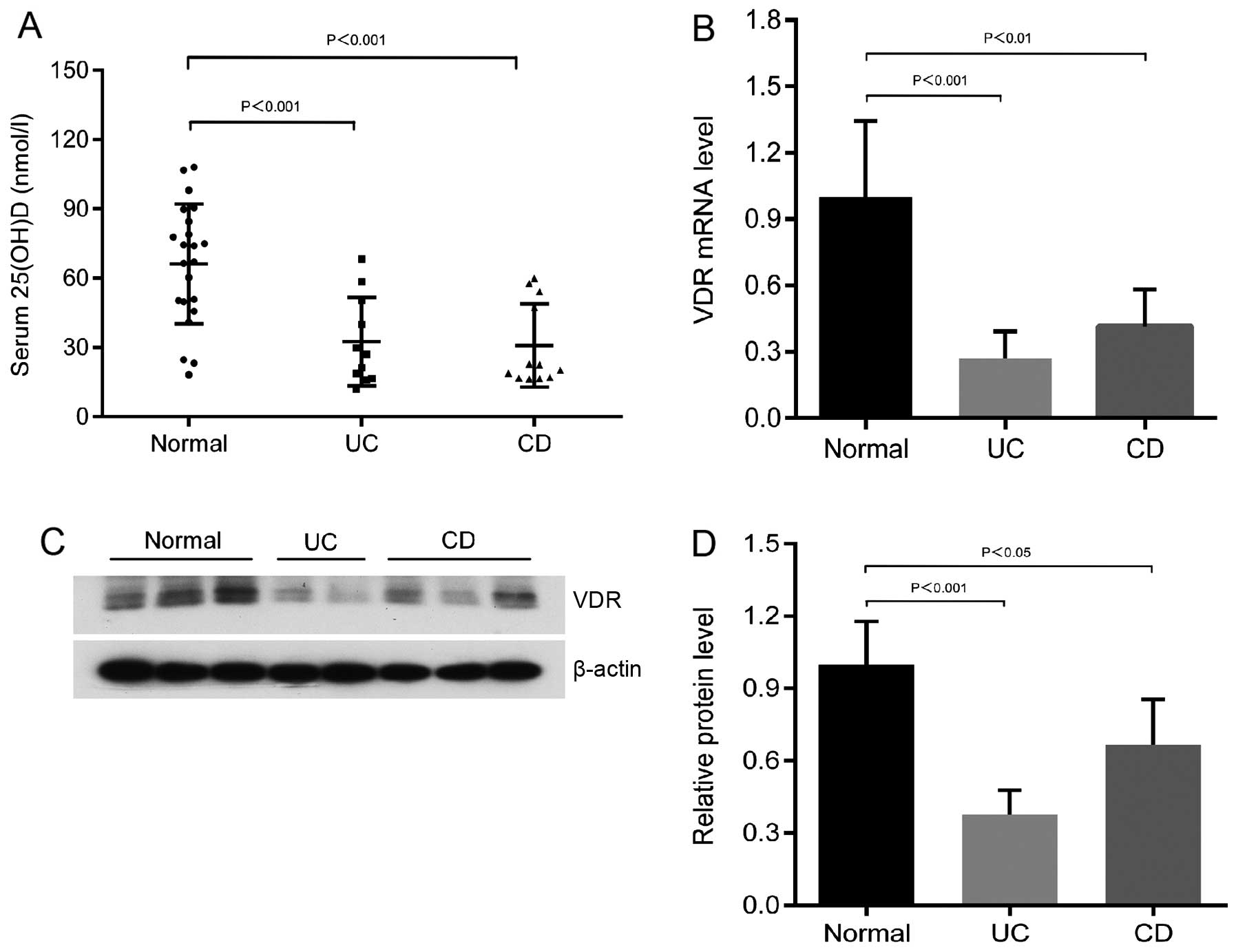
Low 25-hydroxyvitamin D levels and
reduced VDR expression in patients with UC and CD compared to the
normal controls
The average serum 25-hydroxyvitamin D levels in the
patients with UC and CD were significantly lower than those of the
normal controls (Fig. 1A). The
25-hydroxyvitamin D levels of the majority of patients with IBD
were in the vitamin D deficiency range (<50 nmol/l; Fig. 1A). In addition, the mRNA
expression of VDR was markedly decreased in the patients with UC
and CD compared to the normal subjects (n>6; Fig. 1B). Western blot analyses with
anti-VDR antibody revealed the decreased expression of the colonic
VDR protein level in the patients with UC and CD (Fig. 1C and D). These data from human
subjects indicate that a low vitamin D and VDR protein expression
may be a pathological factor for the development of colitis.
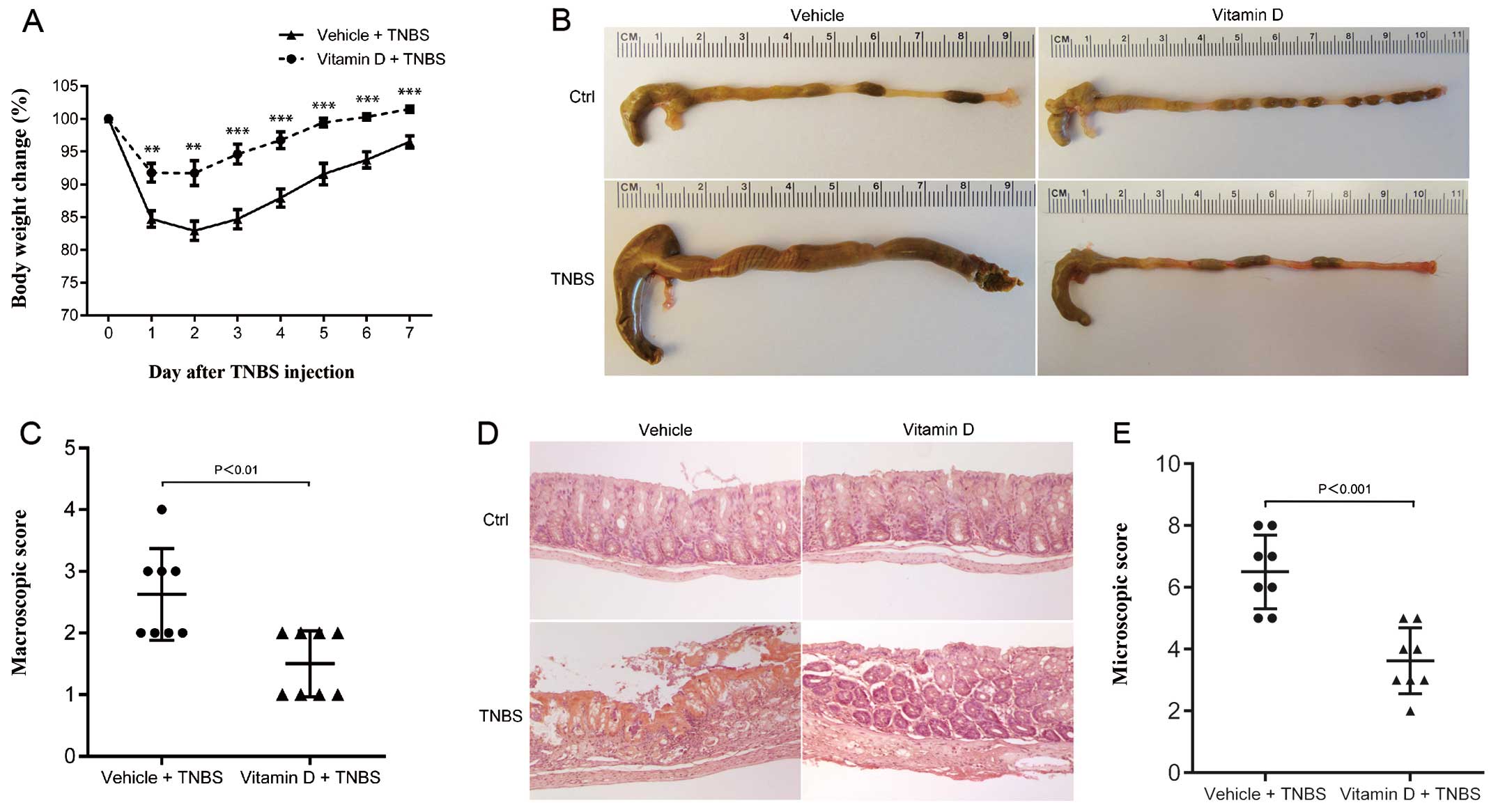
Administration of vitamin D ameliorates
TNBS-induced colitis
We used a mouse model of TNBS-induced colitis to
mimic the pathological process of IBD. Paricalcitol, a vitamin D
analog, was administrated to investigate the protective role of the
vitamin D/VDR signaling pathway. Paricalcitol has been proven to
exert the same curative effect as calcitriol, but it produces less
side-effects, including less hypercalcemia (19,20). After the TNBS injection, the body
weight of the mice in both the vitamin D-treated group (VD) and the
vehicle-treated group (VE) decreased over time, but the mice in the
VD group only developed mild colitis with minor weight loss
(Fig. 2A). In the gross
observation, the colons of the mice in the VD group were much
closer to normal than those of the mice in the VE group (Fig. 2B). Microscopic examination
revealed severe hemorrhage, inflammatory cell infiltration and the
breakdown of the normal intestinal tissue barrier in the mice in
the VE group, while the mice in the VD group presented minimal
histological damage (Fig. 2D).
The mice in the VE group also had a higher macroscopic score
(Fig. 2C) and histological score
(Fig. 2E) than the mice in the VD
group. These results provide evidence that TNBS-induced colitis may
be substantially suppressed by the administration of vitamin D.
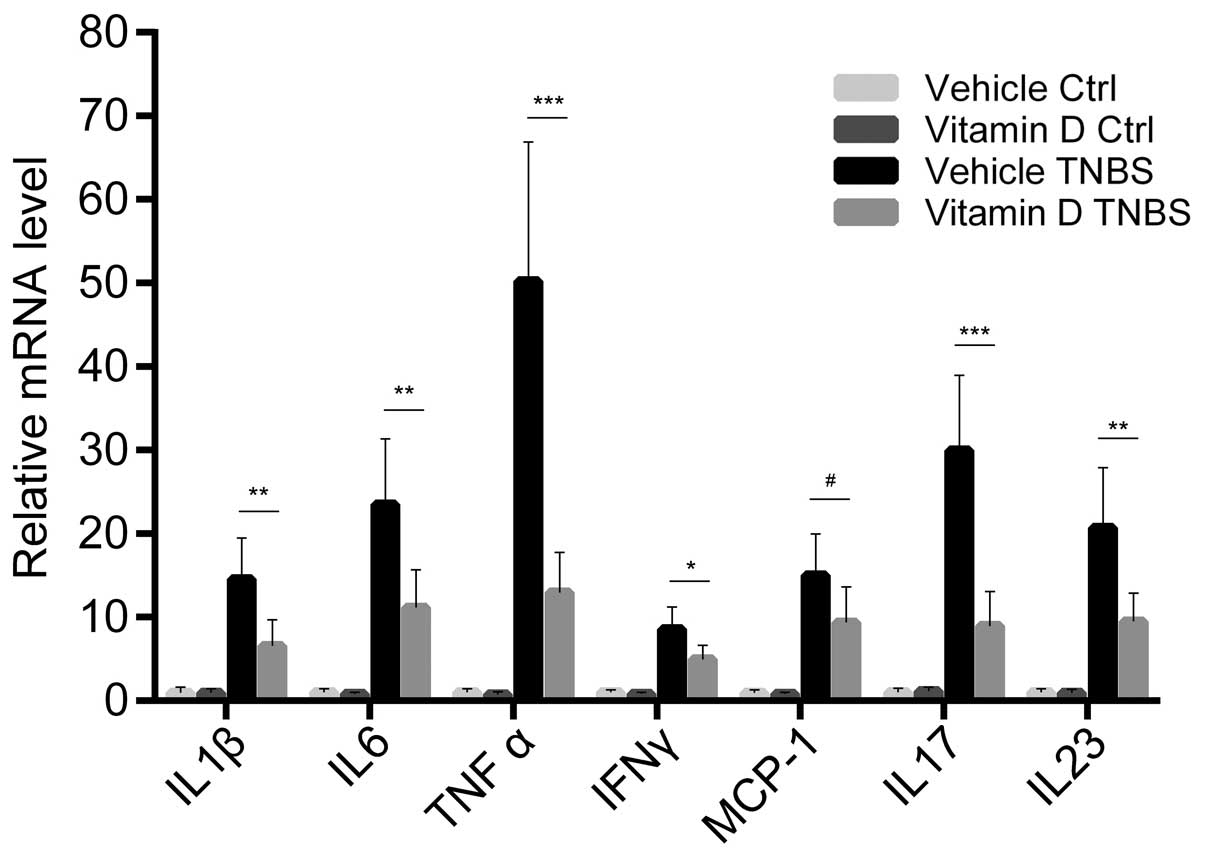
Administration of vitamin D downregulates
the expression of pro-inflammatory cytokines and chemokines
We further examined the expression of
pro-inflammatory cytokines and chemokines in the colonic mucosa.
The mRNA expression of cytokines and chemokines was markedly
increased by the TNBS injection, while paricalcitol markedly
reversed this increase in the expression of the majority of
cytokines. This tendency was most obvious with the expression of
tumor necrosis factor (TNF)-α and interleukin (IL)-17, two
important cytokines associated with the Th1 and Th17 response,
respectively. However, monocyte chemotactic protein-1 (MCP-1) was
the only chemokine which showed no statistically significant
differences in its expression between the VD and VE group (Fig. 3). These result prove that the
administration of vitamin D has a direct inhibitory effect on the
inflammatory status during the development of colitis.
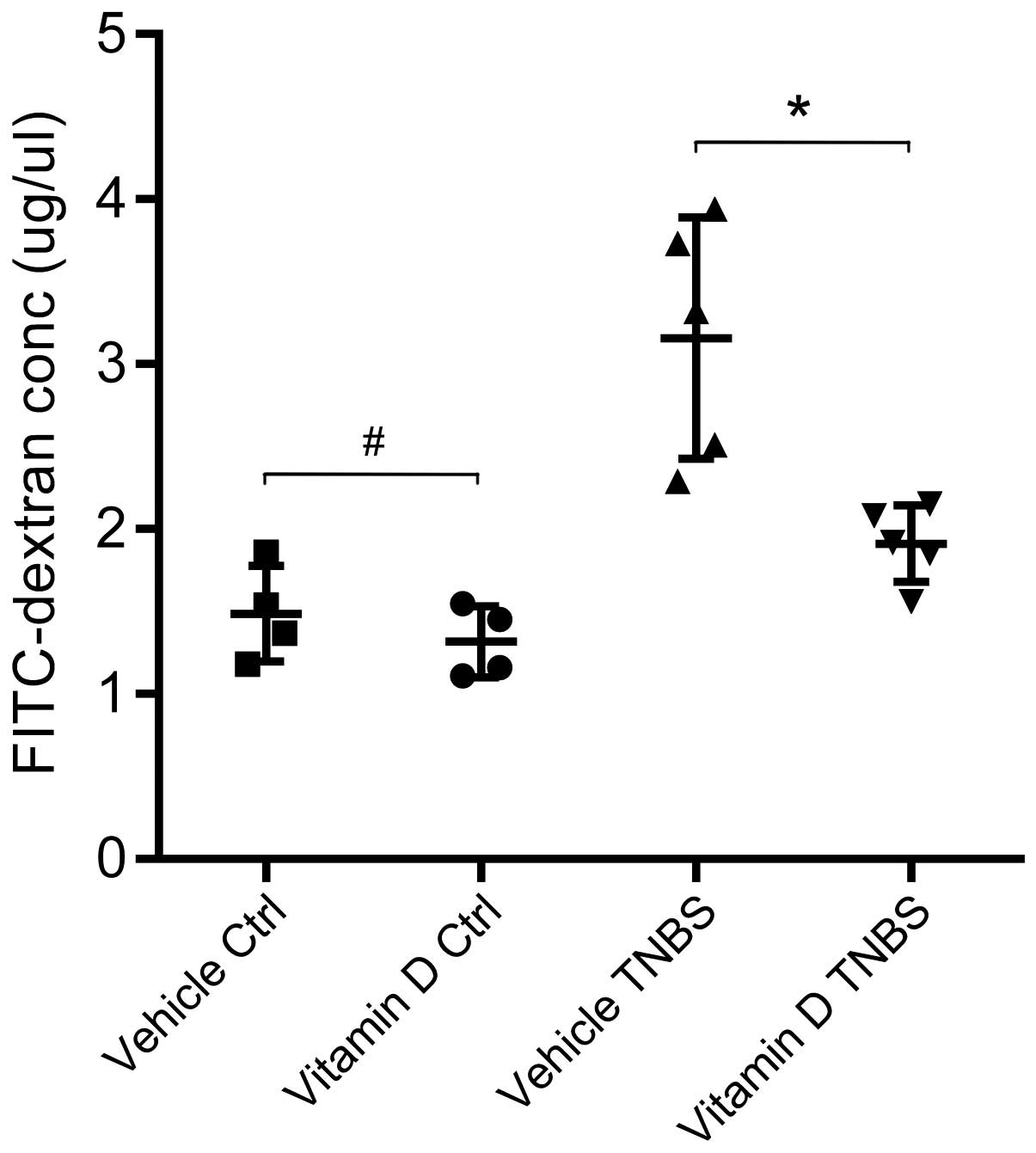
Administration of vitamin D protects
against barrier disruption and inhibits the increase in intestinal
pe rmeability
We evaluated intestinal permeability following the
administration of vitamin D in our mouse model of TNBS-induced
colitis. After the TNBS injection, the permeability of the
intestinal barrier increased in both the VD and VE group, while the
serum concentration of FITC-4-kDa dextran in the mice in the VD
group was exclusively lower than that of the mice in the VE group.
In other words, the administration of vitamin D protects the
integrity of the intestinal barrier and, thus, inhibits the
increase in intestinal permeability (Fig. 4).
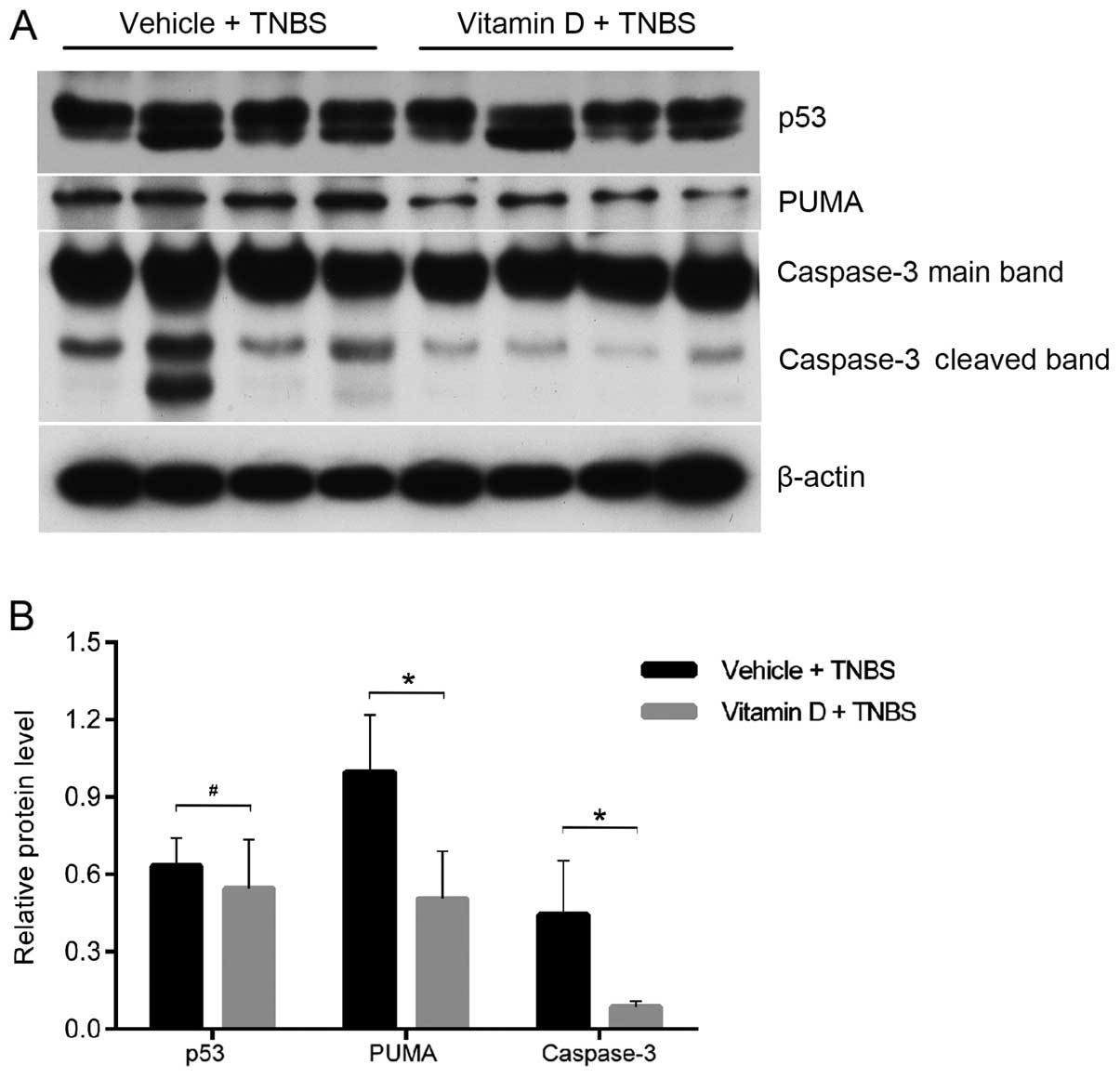
Administration of vitamin D inhibits
intestinal epithelial apoptosis by suppressing the induction of the
PUMA apoptotic pathway
In order to further disclose the mechanisms
responsible for the protective effects of vitamin D, we analyzed
the expression of p53 and PUMA, two upstream pro-apoptotic proteins
of caspase-3. They independently mediate the apoptosis of IECs in
patients and mice with colitis (15). Using western blot analysis, we
found that the cleavage of caspase-3 was markedly attenuated by
treatment with paricalcitol (Fig.
5A). PUMA expression was also decreased in the mice in the VD
group; however, no differences were observed in the p53 protein
level (Fig. 5). These findings
suggest that enhanced vitamin D/VDR signaling inhibits IEC
apoptosis by downregulating PUMA expression.
Discussion
Epidemiological evidence suggests a link between
vitamin D deficiency and an increased risk of developing IBD
(1–3). The incidence of IBD is parallel to
exposure to sunshine, with the highest incidence around the north
pole and the lowest along the equator (21,22). Other studies have suggested a link
between VDR gene polymorphisms and the risk of developing IBD
(4,5). TaqI, BsmI, FokI
and ApaI are the four VDR polymorphisms associated with the
higher incidence of IBD, despite variability among different races
and populations (4,5,23).
Based on the population of northeast China in the present study, we
obtained a similar finding, namely that patients with IBD had a
worse vitamin D status and lower VDR expression than the normal
controls. These data confirm the role of the vitamin D/VDR pathway
in the development of gut inflammation, and provide valuable
insight into the genetic therapy of IBD.
VDR is highly expressed in the intestine. The
classical function of VDR in the small intestine is to regulate the
transportation and absorption of calcium and maintain calcium
homeostasis. However, the function of VDR in the colon remains to
be illustrated. Kong et al (6) first reported that vitamin D
deficiency compromises the mucosal barrier, leading to increased
susceptibility to mucosal damage and an increased risk of
developing IBD. Recently, another study suggested that epithelial
VDR signaling plays an important role in the homeostasis of luminal
microorganisms, antigens and the body (13). VDR is also expressed in immune
cells (24). The endogenous serum
metabolite of vitamin D, calcitriol, is considered a true steroid
hormone, and similar to other glucocorticoids and gonadal hormones,
may exert several immunomodulatory effects (24–26). Accumulating evidence indicates an
important role of vitamin D in reducing the risk of developing
several chronic inflammatory or autoimmune conditions, such as
multiple sclerosis, type 1 diabetes and rheumatoid arthritis
(24–26). Moreover, vitamin D/VDR pathway
dysfunction has been shown to promote the development of
inflammation in IL-10 knockout mice, a model of IBD (27). These laboratory data provide a
therapeutic foundation for enhancing vitamin D/VDR signaling to
inhibit intestinal inflammation.
Clinical studies have revealed that vitamin D
supplementation can deter the pathological process of IBD and
relieve the symptoms (reviewed in 28); however, the mechanisms
responsible for this effect have not yet been fully elucidated. In
this study, we found that the vitamin D analog, paricalcitol,
substantially alleviated the severity of colitis induced by TNBS, a
model of Th1-mediated colitis. The effects of paricalcitol were, at
least in part, mediated through the inhibition of the apoptosis of
IECs.
PUMA is a key mediator of IEC apoptosis in IBD
(15). PUMA is a pro-apoptotic
Bcl-2 family member that interacts with anti-apoptotic Bcl-2 family
members to activate Bax and/or Bak. This activation induces
mitochondrial apoptosis and eventually leads to cell death through
the caspase cascade (29–31). Excess epithelial cell apoptosis
causes the focal disruption of the intestinal mucosal barrier,
leading to the invasion of luminal pathogens and increased
intestinal permeability (6). This
study demonstrated that the vitamin D analog, paricalcitol,
inhibited the activation of PUMA in IECs after the TNBS injection,
and therefore, maintained the integrity of the intestinal
epithelial barrier. An enhanced epithelial barrier can prevent
luminal microorganisms and antigens from invading. In this way, it
attenuates the release of pro-inflammatory cytokines and chemokines
and relieves inflammatory responses in the colon. This may be one
of the pivotal mechanisms through which vitamin D inhibits the
development of intestinal inflammation.
In conclusion, this study provides evidence that
vitamin D attenuates the development of colitis by inhibiting the
apoptosis of IECs. The mechanisms involved include the
downregulation of PUMA expression. The present study may shed new
light on the curative mechanisms of vitamin D in patients with
IBD.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81271938) and the Outstanding
Scientific Fund of Shengjing Hospital.
References
|
1
|
Tan B, Li P, Lv H, et al: Vitamin D Levels
and bone metabolism in Chinese adult patients with inflammatory
bowel disease. J Dig Dis. 15:116–123. 2014. View Article : Google Scholar
|
|
2
|
Levin AD, Wadhera V, Leach ST, et al:
Vitamin D deficiency in children with inflammatory bowel disease.
Dig Dis Sci. 56:830–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Wang ZT, Hu JJ, Fan R, Zhou J and
Zhong J: Polymorphisms of the vitamin D receptor gene and the risk
of inflammatory bowel disease: a meta-analysis. Genet Mol Res.
13:2598–2610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue LN, Xu KQ, Zhang W, Wang Q, Wu J and
Wang XY: Associations between vitamin D receptor polymorphisms and
susceptibility to ulcerative colitis and Crohn’s disease: a
meta-analysis. Inflamm Bowel Dis. 19:54–60. 2013. View Article : Google Scholar
|
|
6
|
Kong J, Zhang ZY, Musch MW, et al: Novel
role of the vitamin D receptor in maintaining the integrity of the
intestinal mucosal barrier. Am J Physiol Gastrointest Liver
Physiol. 294:208–216. 2008. View Article : Google Scholar
|
|
7
|
Peterson LW and Artis D: Intestinal
epithelial cells: regulators of barrier function and immune
homeostasis. Nat Rev Immunol. 14:141–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fasano A and Shea-Donohue T: Mechanisms of
disease: the role of intestinal barrier function in the
pathogenesis of gastrointestinal autoimmune diseases. Nat Clin
Pract Gastroenterol Hepatol. 2:416–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibson PR: Increased gut permeability in
Crohn’s disease: is TNF the link? Gut. 53:1724–1725. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watson AJ, Chu S, Sieck L, et al:
Epithelial barrier function in vivo is sustained despite gaps in
epithelial layers. Gastroenterology. 129:902–912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abraham C and Cho JH: Inflammatory bowel
disease. N Engl J Med. 361:2066–2078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su L, Nalle SC, Shen L, et al: TNFR2
activates MLCK-dependent tight junction dysregulation to cause
apoptosis-mediated barrier loss and experimental colitis.
Gastroenterology. 145:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Chen Y, Golan MA, et al: Intestinal
epithelial vitamin D receptor signaling inhibits experimental
colitis. J Clin Invest. 123:3983–3996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dirisina R, Katzman RB, Goretsky T, et al:
p53 and PUMA independently regulate apoptosis of intestinal
epithelial cells in patients and mice with colitis.
Gastroenterology. 141:1036–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu W, Wu B, Wang X, et al: PUMA-mediated
intestinal epithelial apoptosis contributes to ulcerative colitis
in humans and mice. J Clin Invest. 121:1722–1732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouillon R, Carmeliet G, Verlinden L, et
al: Vitamin D and human health: lessons from vitamin D receptor
null mice. Endocr Rev. 29:726–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Appleyard CB and Wallace JL: Reactivation
of hapten-induced colitis and its prevention by anti-inflammatory
drugs. Am J Physiol. 269:G119–G125. 1995.PubMed/NCBI
|
|
19
|
Cheng S and Coyne D: Paricalcitol capsules
for the control of secondary hyperparathyroidism in chronic kidney
disease. Expert Opin Pharmacother. 7:617–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greenbaum LA, Benador N, Goldstein SL, et
al: Intravenous paricalcitol for treatment of secondary
hyperparathyroidism in children on hemodialysis. Am J Kidney Dis.
49:814–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harries AD, Brown R, Heatley RV, Williams
LA, Woodhead S and Rhodes J: Vitamin D status in Crohn’s disease:
association with nutrition and disease activity. Gut. 26:1197–1203.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moum B, Aadland E, Ekbom A and Vatn MH:
Seasonal variations in the onset of ulcerative colitis. Gut.
38:376–378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naderi N, Farnood A, Habibi M, et al:
Association of vitamin D receptor gene polymorphisms in Iranian
patients with inflammatory bowel disease. J Gastroenterol Hepatol.
23:1816–1822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cutolo M, Paolino S, Sulli A, Smith V,
Pizzorni C and Seriolo B: Vitamin D, steroid hormones, and
autoimmunity. Ann NY Acad Sci. 1317:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cantorna MT, McDaniel K, Bora S, Chen J
and James J: Vitamin D, immune regulation, the microbiota, and
inflammatory bowel disease. Exp Biol Med. 239:1524–1530. 2014.
View Article : Google Scholar
|
|
26
|
Cantorna MT and Mahon BD: Mounting
evidence for vitamin D as an environmental factor affecting
autoimmune disease prevalence. Exp Biol Med (Maywood).
229:1136–1142. 2004.
|
|
27
|
Froicu M, Zhu Y and Cantorna MT: Vitamin D
receptor is required to control gastrointestinal immunity in IL-10
knockout mice. Immunology. 117:310–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicholson I, Dalzell AM and El-Matary W:
Vitamin D as a therapy for colitis: a systematic review. J Crohns
Colitis. 6:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han J, Flemington C, Houghton AB, et al:
Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by
diverse cell death and survival signals. Proc Natl Acad Sci USA.
98:11318–11323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J and Zhang L: PUMA, a potent killer
with or without p53. Oncogene. 27:S71–S83. 2008. View Article : Google Scholar
|
|
31
|
Edelblum KL, Yan F, Yamaoka T and Polk DB:
Regulation of apoptosis during homeostasis and disease in the
intestinal epithelium. Inflamm Bowel Dis. 12:413–424. 2006.
View Article : Google Scholar : PubMed/NCBI
|