The tissue kallikrein-kinin system (KKS) is an
endogenous multiprotein metabolic cascade which is implicated in a
plethora of biological processes such as inflammation,
vasodi-lation, blood coagulation, fibrinolysis, blood pressure
control, vascular permeability, cardioprotection, smooth muscle
contraction, electrolyte balance and pain induction. Activation of
KKS leads to synthesis of the vasoactive peptides kinins by
enzymatic hydrolysis of precursor kininogens including high
molecular weight kininogen (HMWK) and low molecular weight
kininogen (LMWK) (1,2). Kininogens are physiological
substrates for proteolytic cleavage by a family of serine
prote-ases consisting of kallikreins (KLKs) originating from plasma
(pKLK) and tissue (tKLK) (3).
Human plasma kallikrein (KLKB1) is a serine protease synthesized
predominantly in the liver that possesses a high affinity binding
site for HMWK, which is cleaved to produce the nonapeptide
bradykinin. Human tissue kallikrein (KLK1) is a serine protease of
the S1 serine protease superfamily which cleaves LMWK to produce
the decapeptide Lys-bradykinin (kallidin) that is further processed
to bradykinin by a second aminopeptidase cleavage. Bradykinin is
the basic vasoactive peptide of the KKS involved in the regulation
of blood pressure as well as flow. Bradykinin-related peptides bind
to B1 and B2 bradykinin receptors in order to activate a number of
downstream targets such as nitric oxide (NO), cGMP, prostacyclin
and cAMP, which in turn induce numerous biological processes
implicated in angiogenesis by stimulation of vascular endothelial
growth factor (VEGF) formation through binding to B2 receptor,
increase of vascular permeability, vasodilation, smooth muscle
contraction/relaxation, inflammation and pain (4–8).
In addition, KLK1 protease showing both trypsin- and
chymotrypsin-like specificity, appears to have many physiological
protein substrates including pro-insulin, pro-renin, low-density
lipoprotein, and the matrix metalloproteinases (MMPs)
pro-gelatinase (MMP-2) and pro-collagenase (MMP-9) (9,10).
Human mesenchymal stem cells (MSCs) are multipotent
fibroblast-like somatic cells with the ability to self-renew,
proliferate and differentiate in order to give rise to tissue- or
organ-specific cells of the mesodermal lineage (e.g., osteoblasts,
chondrocytes, adipocytes, stroma cells, skeletal myoblasts and
endothelial cells). MSCs are a heterogeneous subset of stromal stem
cells that can be isolated from many different adult or fetal
tissue sources including bone marrow, adipose tissue, umbilical
cord blood, amniotic fluid, synovial fluid, peripheral blood,
dermis, liver, skin and skeletal muscle (11–14). As determined by the International
Society for Cellular Therapy (ISCT), human MSCs must meet the
following minimum criteria: adherence to tissue culture plastic
under standard culture conditions, expression of cell surface
molecules CD105, CD73 and CD90 and lack of expression of CD45,
CD34, CD14 or CD11b, CD79α or CD19 and HLA class II and capability
of differentiating into adipocytes, osteocytes and chondrocytes
under standard experimental conditions in vitro (15).
Another stem cell population which has been proposed
as remarkable candidate for stem cell therapy is human endothelial
progenitor cells (EPCs). EPCs are precursor cells that have the
potential to differentiate into mature endothelial cells and can be
isolated from bone marrow aspirate or peripheral blood of adult
organisms. EPCs participate in the processes of postnatal formation
of new blood vessels and recovery of damaged tissues by
incorporating into the vasculature and by secreting vasculogenic
cytokines and proangiogenic factors such as VEGF, angiopoietin-1
(Ang1), hepatocyte growth factor (HGF), platelet-derived growth
factor (PDGF), monocyte chemoattractant protein-1 (MCP-1), and
macrophage inflammatory protein-1 (MIP-1) (16–20). Vasculogenic cytokines recruit EPCs
to the process of healing in response to hypoxia or ischemia,
whereas proangiogenic cytokines regulate EPC mobilization, homing,
proliferation, and differentiation. The angiogenic potency of EPCs
is also demonstrated through their tube formation capacity in in
vitro assays or when injected to murine models. EPCs also
contribute to neovascularization and tissue repair of
musculoskeletal and neural tissue including the bone and spinal
cord. Transplantation of EPCs has been used to treat ischemic
diseases in animal models and clinical trials (20–22).
Key properties of human MSCs are their
immunomodulatory capability and their marked propensity to migrate
towards sites of injury or inflammation (tropism). Due to these
special characteristics, MSCs have been highlighted as promising
tools for clinical use in regenerative medicine as well as targeted
cell therapy of various diseases including cardiovascular,
cerebrovascular, renal, autoimmune disorders and cancer (13,23,24). MSCs of various origin can be
readily extracted from adult tissues and expanded in vitro
without the loss of their potential for clinical applications or
differentiation into multiple cell lineages (14,25).
One of the most intriguing features of MSCs is that
they can interact with cells of both the innate and adaptive immune
systems and modulate their effector functions by secreting several
cytokines. Interleukins 10 (IL-10) and 8 (IL-8) and transforming
growth factor-β (TGF-β) produced by MSCs lead to repression of
immune responses and promotion of tissue healing. MSC-mediated
immunomodulation results in MSC escape from host immunological
recognition and rejection in allogeneic injection due to lack of
major histocompatibility complex MHC-II and only minimal MHC-I
protein expression (13,24,26).
The other crucial feature of MSCs is that they can
physiologically perfuse into the peripheral blood and migrate to
injured or inflamed tissues (tropism), where they can inhibit the
release of pro-inflammatory cytokines and promote the survival of
damaged cells (24,27). MSC tropism is mediated through
paracrine signaling between the site of injury and corresponding
receptor expression on MSCs (23). For example, stromal cell-derived
factor-1 (SDF-1) is one of the main chemokines mediating the
mobilization and homing of stem cells to damaged tissues and was
found to improve repairing efficiency (28). These unique properties render MSCs
ideal vehicles for cellular gene transfer.
Interestingly, there is an MSC population that has
been particularly highlighted for its unique characteristics: The
MSCs derived from the Wharton’s Jelly (WJ-MSCs) - an anatomic
region within the umbilical cord. WJ-MSCs are primitive cells
categorized somewhere between embryonic stem cells (ESCs) and adult
stem cells. Due to their immunogenic and functional superiority to
other MSCs, a special mention of WJ-MSCs should be made. Similar to
ESCs and unlike adult MSCs, they are consistently positive for
pluripotency and self-renewal markers (29). Importantly, they are safer to use
since they do not form teratomas in vivo (in contrast to
ESCs) and sustain high proliferation rates for extended periods in
culture with no signs of transformation, in contrast to adult MSCs
that have been linked to transformation events as a result of
replicative senescence (30). The
most remarkable feature of WJ-MSCs is their hypo-immunogenic
profile (a key requirement for allogeneic transplantation) and
their capacity for immunomodulation (31). WJ-MSCs are capable of evading
immune recognition due to their lack of co-stimulatory molecule
expression, which is normally implicated in activation of T and B
cell responses and they can also suppress allogeneically stimulated
T cells to a greater extent than adult MSCs (32).
Clinical trials using human MSCs of various origin
as well as EPCs are currently underway to treat cardiovascular,
cerebrovascular, renal, intestinal and autoimmune diseases.
Accumulating evidence from a variety of animal
models of acute myocardial infarction (MI) injected or transplanted
with MSCs, has demonstrated that MSCs constitute promising
therapeutic tools for repairing and regenerating cardiac cells,
interrupting the progress of left ventricular remodeling following
acute MI and restoring heart structure and function by reducing
infarct size and enhancing angiogenesis and arteriogenesis in the
ischemic tissue (11,33–39). Their effects are attributed to
their special properties of homing to injured tissues,
self-proliferating and differentiating into cardiomyocytes in the
damaged area. Indeed, MSCs are able to differentiate into cells
that exhibit cardiomyocyte features in vitro, however, the
proportion of MSCs that differentiate into cardiomyocytes in
vivo and those that actually survive for a long period is very
small (40,41). As known, ischemia induces the
production of reactive oxygen species (ROS) and a number of
inflammatory molecules, such as tumor necrosis factor (TNF)-α,
intercellular adhesion molecule-1 (ICAM-1) and MCP-1 (42). After transplantation into the
body, MSCs exhibit paracrine activity by secreting various
cytokines including growth factors (e.g., VEGF) that produce
anti-inflammatory as well as reparative effects. These molecules
can decrease gene expression of inflammatory agents such as TNF-α
and IL-1β and IL-6 and they can also promote survival, growth, or
differentiation of other cells in the area of the MI, and this is
considered the major contribution of MSCs in treatment efficacy
(37,41,43,44). The functionally superior WJ-MSCs
transplanted by direct injection into the infarcted area of
myocardium could survive and differentiate into cardiomyocytes and
endothelial cells and also promoted recruitment and differentiation
of cardiac stem cells in a porcine model. In addition, WJ-MSC
transplantation was shown to reduce apoptosis and fibrosis, enhance
viable myocardium, and improve ventricular remodeling and function
(45). Scheduled and ongoing
clinical trials test the efficacy and safety of these cells in
patients with MI (e.g., clinicaltrials.gov; NCT01291329).
MSCs have been reported as having significant neural
differentiation potential in culture and being neurogenic after
transplantation in rodent models, therefore they have gained
interest in their potential usefulness in cell-based therapy
strategies for neurodegenerative diseases and traumatic injuries of
the nervous system (11,46,47). Indeed, prolonged cultured bone
marrow-derived MSCs can differentiate into neuron-like cells
(48). Transplantation of bone
marrow-derived MSCs to animal models of neurodegenerative disorders
including Parkinson’s disease and ischemic brain injury has been
reported to ameliorate functional deficits (49,50). The main challenge to stem cell
therapy of central nervous system (CNS) diseases is getting MSCs
into the CNS through the blood-brain barrier (51). When transplanted into the brain,
MSCs produce neurotrophic and growth factors that protect and
induce regeneration of damaged tissue. It has been shown that MSCs
can differentiate into neurons and glial cells. Additionally,
transplantation of MSCs enables the formation of new blood vessels,
thereby increasing blood flow in the ischemic region. It has been
shown that intravenous injection of umbilical cord blood-derived
MSCs to transgenic mice with Alzheimer’s disease results in a
decline of cerebral amyloid β (Aβ) peptide and an increase of this
peptide in blood plasma due to its excretion from the brain through
the blood-brain barrier, as well as a reduction of pro-inflammatory
responses in the brain and periphery (52–55). Moreover, WJ-MSCs have been used
for induction of neurons and glial cells (56) and they have been shown to promote
functional and morphologic recovery of peripheral nerves after
axonotmesis and neurotmesis injuries in a rat model (57). WJ-MSCs effectivity in patients
with chronic traumatic spinal cord injury is under clinical trial
(clinicaltrials.gov; NCT03003364).
Mounting evidence from ongoing or completed clinical
trials indicates that MSC therapy is feasible, safe, well
tolerated, and can effectively improve renal pathologies including
acute kidney injury (AKI) and chronic kidney disease (CKD),
diabetic nephropathy, focal segmental glomerulosclerosis (FSGS),
systemic lupus erythematous (SLE), and kidney transplantation.
Since regenerative capability of renal cells in humans is very
limited, damage in these cells usually lead to devastating
diseases. Numerous preclinical studies in various murine models
have paved the way for novel therapeutic strategies with the use of
MSCs and/or EPCs in a wide range of renal disorders both acute and
chronic (58–60).
MSCs have a renoprotective and regenerative action
on injured kidney tissues via paracrine mechanisms: anti-fibrotic,
anti-apoptotic, pro-angiogenic, proliferative, differentiative,
antioxidative, immunosuppressive and immunomodulatory. More
specifically, paracrine release of extracellular vesicles including
exosomes that contain genetic and protein material by MSCs has been
proposed to exert trophic and reparative effects, which can
activate mechanisms to ameliorate renal injury (21,60). It has been shown that implantation
of bone marrow-derived MSCs after ischemia/reperfusion
(I/R)-induced acute renal failure promotes restoring of renal
function and morphology, thereby implicating the great therapeutic
potential of MSCs in healing damaged kidneys (61–63). Administration of MSCs has
demonstrated significant reduction of intrarenal inflammatory
infiltrate, decreased fibrosis, and glomerulosclerosis in animal
models of CKD (59). Moreover, in
animal models of diabetic nephropathy, MSCs reduced
glomerulosclerosis and oxidative stress (64–66). Intrarenal delivery of MSCs and
EPCs in a porcine model of renovascular hypertension resulted in
decreased myocardial injury induced by renovascular hypertension as
well as decreased renal injury (67,68). In addition, the identification and
characterization of adult renal progenitor cells from rodents as
well as humans has provided further insights concerning stem cell
regenerative potential in renal tubular injuries (58,69,70). A meta-analysis of studies in
animal models by Papazova et al demonstrated that cell-based
therapy reduced development and progression of CKD by decreasing
urinary protein and urea associated with glomerulosclerosis and
interstitial fibrosis (71).
Although MSC delivery in in vivo models of FSGS has been
scarcely studied, results were promising as MSCs were shown to
stabilize and attenuate the progression of FSGS (72,73). Studies have shown that allogeneic
bone marrow or umbilical cord-derived MSC transplantation results
in amelioration of disease and could reverse multiorgan dysfunction
in SLE (74,75). Treatment with MSCs exhibiting
anti-inflammatory and immunomodulatory properties had either
beneficial or no adverse effect on autoimmune lupus nephritis (the
major clinical manifestation of SLE) as well as inflammatory bowel
disease (IBD), but more research as to whether MSCs could actually
benefit these patients is still underway (76,77). Furthermore, WJ-MSCs were shown to
improve renal function in a xenogeneic mouse model of acute renal
injury by increasing proliferation and decreasing apoptosis of
renal tubular cells, a function mediated through the mitochondrial
pathway, and through the increase of Akt phosphorylation (78). WJ-MSCs are being clinically tested
in patients with diabetic nephropathy (clinicaltrials.gov; NCT03288571).
EPCs are reduced in number and impaired in
angiogenic function in patients with atherosclerosis,
cardiovascular and chronic renal diseases. Preclinical studies have
shown that EPCs are mobilized from the bone marrow to peripheral
blood in response to VEGF or other chemotactic molecules, home in
at injured or inflamed tissues and differentiate into
vessel-forming endothelial cells and/or regulate pre-existing
endothelial cells (19,79–81). Moreover, EPCs attenuate vascular
inflammation and improve left ventricular function both in
vitro and in vivo, thereby being regarded as promising
tools for post-MI therapy. Indeed, post-MI implantation of EPCs
into ischemic myocardium of animal models can home to sites of
injury and enhance recovery. The number and function of circulating
EPCs has been inversely correlated with cardiovascular disease risk
as a potential biomarker for patients with hypertension and
coronary artery disease (18,80,82–86). Furthermore, EPCs have been
proposed as useful tools in cell-based treatment of renal and
ischemic cerebrovascular diseases. Studies have shown that
mobilization of EPCs contributes to endothelial repair in the
kidney immediately after I/R (87,88). EPCs have been also shown to
decrease neuronal apoptosis and promote the proliferation and
migration of neural stem cells by repairing vascular endothelial
cells and inducing neo-vascularization in animal models of cerebral
ischemia. Growth factor (e.g., VEGF) secretion by EPCs contributes
to post-stroke angiogenesis and neurogenesis, thereby
reconstructing the functions and structures of vascular and neural
networks (22,89,90).
Stem cell therapy using naïve MSCs and/or EPCs for
tissue regeneration confronts many challenges regarding stem cell
viability, vitality and functionality. After extensive debate on
these issues, research advances could finally provide safer
applicable solutions.
Genetic modification of human stem or progenitor
cells (e.g., MSCs and/or EPCs) for targeted delivery of specific
therapeutic agents or genes has been proven to be a very
significant advancement in regenerative medicine, since it can
improve viability, proliferative capability and metabolic features
of these cells which are sensitive to the hypoxic and inflammatory
environment in ischemic tissue. For example, MSCs overexpressing
the anti-apoptotic gene Akt1 (Akt-MSCs) were shown to
be more resistant to apoptosis in vitro and in vivo
(91). Moreover, the efficacy of
MSCs for clinical use can be optimized by pre-treatment with drugs,
cytokines, and growth factors (92,93).
MSCs can be genetically modified by viral and
non-viral methods. Non-viral physical and chemical methods of gene
transfer are able to deliver larger transgenes than viral methods,
but their main drawback is the low transfection efficiency and
transient gene expression (94).
MSCs can be efficiently transduced with viral vectors such as
lentiviruses, retroviruses, baculoviruses and adenoviruses without
affecting their stem cell properties. Viruses can be useful as
delivery vectors after being considerably modified in order to
become replication incompetent with attenuated cytopathic effects
and immunogenicity. Viral vectors are particularly appealing
because they can enable high transduction efficiency and, depending
on the type of virus used, can deliver long-term stable transgene
expression. The safety of cell-based therapy with the use of viral
vectors is a crucial issue that has not been resolved yet, but
advances in vector design have helped towards this direction
(23,95). For example, MSCs genetically
modified to express VEGF have been shown to enhance the
cardioprotective effects of MSCs followed by angiogenesis effects
for the treatment of acute MI, whereas Akt gene- or heme
oxygenase-1 (HO-1) gene-modified MSCs have been shown to
dramatically improve ischemic cardiac function and MSC viability
and prevent ventricular remodeling and apoptosis of cardiomyocytes
and endothelial cells, thus restoring the function of infarcted
hearts (91,96–98). In addition, MSCs genetically
modified with HGF or VEGF ameliorated acute renal damage,
inflammation and apoptosis (99,100).
The KKS through its components is a crucial
regulator of homeostasis of the cardiovascular system throughout
the life of an individual and has been implicated in blood pressure
regulation (vasodilation) as well as pathogenesis of hypertension
(6,101). Since the discovery of tissue
KLK1 localization in cardiac and vascular tissues (102–104), multiple experimental studies
have investigated its role and potential therapeutic application
both in vitro and in vivo. Tissue KLK1 has been
demonstrated to exert pleiotropic beneficial effects in
cardiovascular system function by reducing hypertension,
attenuating cardiac inflammation and myocardial fibrosis,
increasing NO formation, restoring coronary blood flow, decreasing
infarct size, promoting neo-vascularization and capillary density
and preventing restenosis after acute MI through the VEGF and kinin
B2 receptor-Akt-glycogen synthase kinase (GSK)-3β signaling
pathways (105–110). Many of these beneficial effects
of KLK1 are mediated by the activation of NO signaling pathways,
which are responsible for a decrease in oxidative stress in animal
models. In vivo studies with gene delivery using adenoviral
vectors that contained the human KLK1 gene or with KLK1
protein infusion have shown that KLK1 reduces cardiac inflammation,
hypertrophy, fibrosis and apoptosis of cardiomyocytes in animal
models of MI (105–108,111–113). In vitro studies on
cardiomyocytes and endothelial cells showed that tissue KLK1
expression inhibited hypoxia-induced ROS formation as well as
cardiomyocyte apoptosis via activation of Akt-mediated signaling
cascades (Akt-GSK-3β and Akt-Bad-14-3-3) (4,108,109,114). Moreover, KLK1 gene
delivery increases the population of cardiac progenitor cells
(CPCs) and promotes viability, increases the regional blood flow
and neo-vascularization in the peri-infarct myocardium (115). Adenovirus-mediated KLK1
gene delivery in rodent models was also found to induce endogenous
angiogenesis in response to ischemia, and to reduce neointima
formation in injured vessels or after balloon angioplasty via
activation of Akt and NO-cGMP signaling pathways (116–119). These data suggest that
KLK1 gene therapy might be applicable to peripheral
occlusive vascular diseases.
Engineered MSCs can be used as vehicles to deliver
therapeutic agents such as tissue KLK1 to injured end-organs. It
has been shown that MSCs transduced with adenovirus containing the
human tissue KLK1 gene (KLK1-MSCs) acquire improved
properties that augment their protective role in cardiovascular
diseases. KLK1-MSCs secrete KLK1 which may contribute to the
reduction in myocardial fibrosis via proteolytic activation of
pro-MMP-2 and -9. MMPs are known to degrade the physiological
collagen scaffold of the myocardium and other extracellular matrix
(ECM) proteins. KLK1-MSCs also express higher levels of VEGF
and VEGF-R compared to unmodified MSCs (39,107,120,121). The upregulation of VEGF and its
receptor could partly account for KLK1-induced neo-vascularization
in the infarct myocardium (107). The in vivo pro-angiogenic
effects of KLK1-MSCs exhibiting augmented VEGF secretion
were additionally confirmed in vitro in cultured endothelial
cells wherein a significant increase of proliferation, migration
and tube formation was observed (39). KLK1 can also inhibit collagen
synthesis and promote collagen breakdown. Notably, administration
of KLK1-MSCs to rats reduces cardiac collagen deposition and
cardiomyocyte hypertrophy (39,122). Moreover, KLK1-MSCs show
reduced caspase-3 activity compared to controls and their
administration to rats decreased myocardial apoptosis after MI.
Cultured KLK1-MSCs are more resistant to hypoxia-induced
apoptosis compared to control MSCs and this resistance possibly
enables engraftment of KLK1-MSCs to the infarct area in
larger amount than control MSCs. Administration of KLK1-MSCs
to rats also resulted in significant decrease of inflammatory cell
(neutrophil and monocyte/macrophage) accumulation in the myocardium
and parallel downregulation of TNF-α, ICAM-1 and MCP-1 after MI
(39). It has also been suggested
that KLK1-MSCs could provide significant cardioprotection
possibly due to the ability of KLK1 to activate the kinin B2
receptor to either form kinins or not, thereby attenuating
myocardial damage through Akt signaling and NO production (39,106). Taken together, the
aforementioned findings converge to the notion that MSCs modified
with human tissue KLK1 gene constitute appealing
therapeutics with multifaceted potential in cell-based therapy of
myocardial ischemia.
The renal KKS is involved in electrolyte and water
homeostasis, blood pressure regulation and inflammation.
Historically, tissue KLK1 was discovered in human urine at the
beginning of the twentieth century as a substance exerting
hypotensive action. KLK1 is localized in the collecting segment of
the renal distal tubule and its release into the tubules can be
induced by the electrolyte balance (low sodium levels, high
potassium levels) and antidiuretic hormone (114,124). KLK1 renal excretion in urine is
decreased in hypertensive rodents and humans to an extent that is
proportional to the severity of renal failure. This decrease might
result from a decrease in kinin generation (e.g., bradykinin) in
hypertensive conditions, since kininogen levels and kinin-forming
factors are reduced in essential and malignant hypertension. It has
been suggested that the role of renal bradykinin is to excrete the
excess sodium. Therefore, decrease in renal bradykinin generation
may lead to sodium accumulation in the body which in turn could
result in the development of hypertension (124–126).
Several studies have shown that KLK1 improves renal
function by increasing glomerular filtration rate and renal blood
flow via its anti-inflammatory, anti-oxidative, anti-fibrotic and
anti-apoptotic actions in animal models of renal injury. For
example, KLK1 gene delivery using an adenovirus vector or
KLK1 protein infusion in hypertensive Dahl salt-sensitive rats has
been shown to attenuate renal dysfunction, induce NO production and
reverse the process of renal inflammation and fibrosis in
bradykinin B2 receptor-mediated manner (127–131). Liu et al confirmed the
previous findings by blocking endogenous KLK1 activity in a rat
model of CKD which resulted in increased inflammatory cell
(macrophages/monocytes) infiltration and myofibroblast and collagen
deposition in kidneys (132).
Specifically, endogenous KLK1 was shown to inhibit angiotensin
II-induced ROS and superoxide formation as well as renal NADH
oxidase activity through NO production in deoxycorticosterone
acetate (DOCA)-salt hypertensive rats, which exhibit high renal
KLK1 levels. Moreover, KLK1 significantly increased MMP-2 activity
and inhibited synthesis of tissue inhibitor of MMP-2 (TIMP-2) as
well as plasminogen activator inhibitor-1 (PAI-1), thereby
promoting degradation of ECM protein components (e.g., collagen I
and fibronectin) as demonstrated both in vivo and in
cultured renal cells (63,132,133).
Most likely, KLK1 exerts its anti-fibrotic effect by increasing ECM
degradation which leads to a decrease of mesenchymal fibroblast
accumulation in the interstitium of the cortex and medulla.
Moreover, KLK1 was demonstrated to decrease renal hypertrophy,
namely kidney weight, glomerular size, and proliferation of
epithelial tubular cells. KLK1 protein infusion in rats also
promoted the recovery of gentamicin-induced nephrotoxicity by
inhibiting apoptosis and caspase-3 activity and increasing Akt
phosphorylation in proximal tubular renal cells. Therefore,
endogenous tissue KLK1 can attenuate and reverse renal injury by
reducing oxidative stress, apoptosis, inflammation and fibrosis
in vivo through activation of bradykinin B2 receptor
(63,114,133–135). Overall, these studies outline
the beneficial role of tissue KLK1 in the preservation of kidney
structure and function by promoting tissue repair and regeneration
in AKI and modulating the progression of CKD.
The KKS is capable of dilating cerebral arterial
vessels partly because of the release of endothelium-derived
relaxing factor NO which plays a complex role in cerebral ischemia.
Ischemic conditions trigger an excessive activation of neuronal NO
synthase (NOS), which results in production of NO that is toxic to
surrounding neurons, but critical in maintaining cerebral blood
flow and reducing infarct volume. The KKS through participation in
NOS activation and following NO formation is implicated in
endothelial cell function in the setting of ischemic stroke
(139).
Numerous studies have outlined the pleiotropic
beneficial effects of tissue KLK1 protease, component of the KKS,
in the protection of cardiovascular, renal and central nervous
systems from tissue injury. Genetic modification of stem cells or
progenitor cells with KLK1 gene enhances their viability and
proliferative, migratory and functional properties, thus increasing
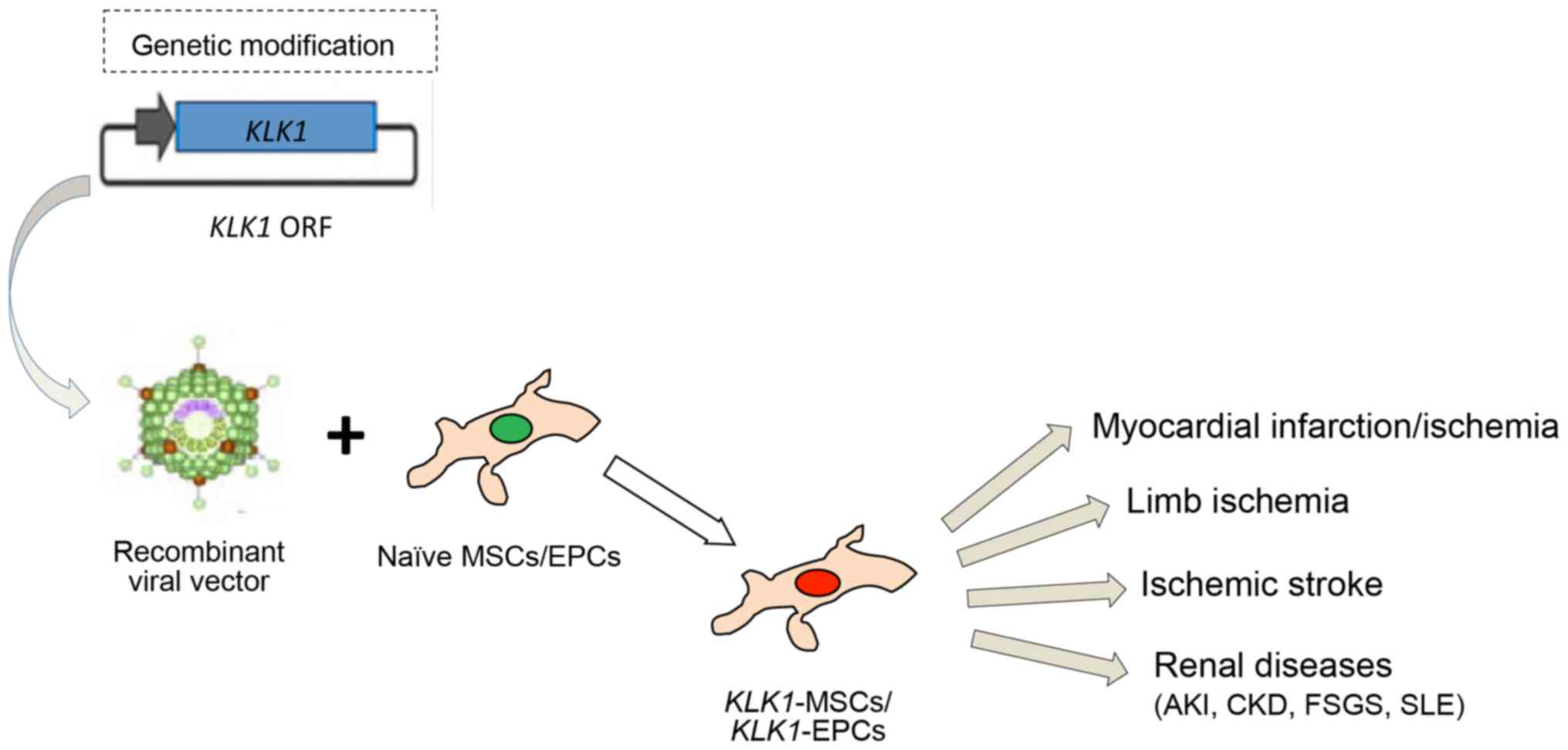
their tissue healing effects in various human diseases (Fig. 1). Engraftment of KLK1-MSCs
and/or KLK1-EPCs into animal models provided advanced
protection against vascular and organ damage. Aforementioned
findings reveal the KLK1 relevance to human diseases and pave the
way for further research on the potential therapeutic perspectives
of KLK1-MSCs and/or KLK1-EPCs that could lead to the
translation of preclinical studies into effective and safe targeted
therapies for cardiovascular, cerebrovascular and renal
diseases.
The present study was supported by IKY Fellowships
of Excellence for Postgraduate Studies in Greece - SIEMENS Program
(2016–2017).
|
1
|
Moreau ME, Garbacki N, Molinaro G, Brown
NJ, Marceau F and Adam A: The kallikrein-kinin system: Current and
future pharmacological targets. J Pharmacol Sci. 99:6–38. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kashuba E, Bailey J, Allsup D and Cawkwell
L: The kinin-kallikrein system: Physiological roles,
pathophysiology and its relationship to cancer biomarkers.
Biomarkers. 18:279–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourdet B, Pécher C, Minville V, Jaafar A,
Allard J, Blaes N, Girolami JP and Tack I: Distribution and
expression of B2-kinin receptor on human leukocyte subsets in young
adults and elderly using flow cytometry. Neuropeptides. 44:155–161.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chao J, Bledsoe G, Yin H and Chao L: The
tissue kallikrein-kinin system protects against cardiovascular and
renal diseases and ischemic stroke independently of blood pressure
reduction. Biol Chem. 387:665–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emami N and Diamandis EP: New insights
into the functional mechanisms and clinical applications of the
kallikrein-related peptidase family. Mol Oncol. 1:269–287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bryant JW and Shariat-Madar Z: Human
plasma kallikrein-kinin system: Physiological and biochemical
parameters. Cardiovasc Hematol Agents Med Chem. 7:234–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hillmeister P and Persson PB: The
kallikrein-kinin system. Acta Physiol (Oxf). 206:215–219. 2012.
View Article : Google Scholar
|
|
8
|
Björkqvist J, Jämsä A and Renné T: Plasma
kallikrein: The bradykinin-producing enzyme. Thromb Haemost.
110:399–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borgoño CA and Diamandis EP: The emerging
roles of human tissue kallikreins in cancer. Nat Rev Cancer.
4:876–890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sotiropoulou G, Pampalakis G and Diamandis
EP: Functional roles of human kallikrein-related peptidases. J Biol
Chem. 284:32989–32994. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee KD: Applications of mesenchymal stem
cells: An updated review. Chang Gung Med J. 31:228–236.
2008.PubMed/NCBI
|
|
12
|
Christodoulou I, Kolisis FN,
Papaevangeliou D and Zoumpourlis V: Comparative evaluation of human
mesenchymal stem cells of fetal (Wharton’s jelly) and adult
(adipose tissue) origin during prolonged in vitro expansion:
Considerations for cytotherapy. Stem Cells Int. 2013:2461342013.
View Article : Google Scholar
|
|
13
|
Shafei AE, Ali MA, Ghanem HG, Shehata AI,
Abdelgawad AA, Handal HR, Talaat KA, Ashaal AE and El-Shal AS:
Mesenchymal stem cells therapy: A promising cell based therapy for
treatment of myocardial infraction. J Gene Med. 19:e29952017.
View Article : Google Scholar
|
|
14
|
Rhee KJ, Lee JI and Eom YW: Mesenchymal
stem cell-mediated effects of tumor support or suppression. Int J
Mol Sci. 16:30015–30033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rehman J, Li J, Orschell CM and March KL:
Peripheral blood ‘endothelial progenitor cells’ are derived from
monocyte/macrophages and secrete angiogenic growth factors.
Circulation. 107:1164–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hristov M and Weber C: Endothelial
progenitor cells: Characterization, pathophysiology, and possible
clinical relevance. J Cell Mol Med. 8:498–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kränkel N, Lüscher TF and Landmesser U:
‘Endothelial progenitor cells’ as a therapeutic strategy in
cardiovascular disease. Curr Vasc Pharmacol. 10:107–124. 2012.
View Article : Google Scholar
|
|
19
|
Fu SS, Li FJ, Wang YY, You AB, Qie YL,
Meng X, Li JR, Li BC, Zhang Y and Da Li Q: Kallikrein gene-modified
EPCs induce angiogenesis in rats with ischemic hindlimb and
correlate with integrin αvβ3 expression. PLoS One. 8:e730352013.
View Article : Google Scholar
|
|
20
|
Kamei N, Atesok K and Ochi M: The use of
endothelial progenitor cells for the regeneration of
musculoskeletal and neural tissues. Stem Cells Int.
2017:19608042017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hickson LJ, Eirin A and Lerman LO:
Challenges and opportunities for stem cell therapy in patients with
chronic kidney disease. Kidney Int. 89:767–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao S, Luo C, Cao B, Hu H, Wang S, Yue H,
Chen L and Zhou Z: Endothelial progenitor cells for ischemic
stroke: Update on basic research and application. Stem Cells Int.
2017:21934322017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sage EK, Thakrar RM and Janes SM:
Genetically modified mesenchymal stromal cells in cancer therapy.
Cytotherapy. 18:1435–1445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View Article : Google Scholar
|
|
25
|
Mishra PJ, Mishra PJ, Glod JW and Banerjee
D: Mesenchymal stem cells: Flip side of the coin. Cancer Res.
69:1255–1258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye Z, Wang Y, Xie HY and Zheng SS:
Immunosuppressive effects of rat mesenchymal stem cells:
Involvement of CD4+CD25+ regulatory T cells.
Hepatobiliary Pancreat Dis Int. 7:608–614. 2008.PubMed/NCBI
|
|
27
|
Borlongan CV: Bone marrow stem cell
mobilization in stroke: A ‘bonehead’ may be good after all!
Leukemia. 25:1674–1686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kortesidis A, Zannettino A, Isenmann S,
Shi S, Lapidot T and Gronthos S: Stromal-derived factor-1 promotes
the growth, survival, and development of human bone marrow stromal
stem cells. Blood. 105:3793–3801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fong CY, Richards M, Manasi N, Biswas A
and Bongso A: Comparative growth behaviour and characterization of
stem cells from human Wharton’s jelly. Reprod Biomed Online.
15:708–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fong CY, Chak LL, Biswas A, Tan JH,
Gauthaman K, Chan WK and Bongso A: Human Wharton’s jelly stem cells
have unique transcriptome profiles compared to human embryonic stem
cells and other mesenchymal stem cells. Stem Cell Rev. 7:1–16.
2011. View Article : Google Scholar
|
|
31
|
Weiss ML, Anderson C, Medicetty S,
Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D and McIntosh KR:
Immune properties of human umbilical cord Wharton’s jelly-derived
cells. Stem Cells. 26:2865–2874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prasanna SJ and Jahnavi VS: Wharton’s
jelly mesenchymal stem cells as off-the-shelf cellular
therapeutics: A closer look into their regenerative and
immunomodulatory properties. Open Tissue Eng Regen Med J. 4:28–38.
2011. View Article : Google Scholar
|
|
33
|
Yoon J, Min BG, Kim Y-H, Shim WJ, Ro YM
and Lim D-S: Differentiation, engraftment and functional effects of
pre-treated mesenchymal stem cells in a rat myocardial infarct
model. Acta Cardiol. 60:277–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang J, Xie Q, Pan G, Wang J and Wang M:
Mesenchymal stem cells participate in angiogenesis and improve
heart function in rat model of myocardial ischemia with
reperfusion. Eur J Cardiothorac Surg. 30:353–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wolf D, Reinhard A, Krause U, Seckinger A,
Katus HA, Kuecherer H and Hansen A: Stem cell therapy improves
myocardial perfusion and cardiac synchronicity: New application for
echocardiography. J Am Soc Echocardiogr. 20:512–520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J, Zhou W, Zheng W, Ma Y, Lin L, Tang
T, Liu J, Yu J, Zhou X and Hu J: Effects of myocardial
transplantation of marrow mesenchymal stem cells transfected with
vascular endothelial growth factor for the improvement of heart
function and angiogenesis after myocardial infarction. Cardiology.
107:17–29. 2007. View Article : Google Scholar
|
|
37
|
Guo J, Lin GS, Bao CY, Hu ZM and Hu MY:
Anti-inflammation role for mesenchymal stem cells transplantation
in myocardial infarction. Inflammation. 30:97–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu X, Xu Z, Xu Y and Cui G: Effects of
mesenchymal stem cell transplantation on extracellular matrix after
myocardial infarction in rats. Coron Artery Dis. 16:245–255. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao L, Bledsoe G, Yin H, Shen B, Chao L
and Chao J: Tissue kallikrein-modified mesenchymal stem cells
provide enhanced protection against ischemic cardiac injury after
myocardial infarction. Circ J. 77:2134–2144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amado LC, Saliaris AP, Schuleri KH, St
John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart
G, et al: Cardiac repair with intramyocardial injection of
allogeneic mesenchymal stem cells after myocardial infarction. Proc
Natl Acad Sci USA. 102:11474–11479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goradel NH, Hoor FG, Negahdari B,
Malekshahi ZV, Hashemzehi M, Masoudifar A and Mirzaei H: Stem cell
therapy: A new therapeutic option for cardiovascular diseases. J
Cell Biochem. 119:95–104. 2018. View Article : Google Scholar
|
|
42
|
Chen XL, Zhang Q, Zhao R and Medford RM:
Superoxide, H2O2, and iron are required for
TNF-alpha-induced MCP-1 gene expression in endothelial cells: Role
of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol.
286:H1001–H1007. 2004. View Article : Google Scholar
|
|
43
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi SH, Jung SY, Kwon SM and Baek SH:
Perspectives on stem cell therapy for cardiac regeneration.
Advances and challenges. Circ J. 76:1307–1312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Liu XC, Yang L, Zhu DL, Zhang YD,
Chen Y and Zhang HY: Wharton’s jelly-derived mesenchymal stem cells
promote myocardial regeneration and cardiac repair after miniswine
acute myocardial infarction. Coron Artery Dis. 24:549–558. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Deng J, Petersen BE, Steindler DA,
Jorgensen ML and Laywell ED: Mesenchymal stem cells spontaneously
express neural proteins in culture and are neurogenic after
transplantation. Stem Cells. 24:1054–1064. 2006. View Article : Google Scholar
|
|
47
|
Tropel P, Platet N, Platel JC, Noël D,
Albrieux M, Benabid AL and Berger F: Functional neuronal
differentiation of bone marrow-derived mesenchymal stem cells. Stem
Cells. 24:2868–2876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tseng PY, Chen CJ, Sheu CC, Yu CW and
Huang YS: Spontaneous differentiation of adult rat marrow stromal
cells in a long-term culture. J Vet Med Sci. 69:95–102. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dezawa M, Hoshino M and Ide C: Treatment
of neurodegenerative diseases using adult bone marrow stromal
cell-derived neurons. Expert Opin Biol Ther. 5:427–435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim EJ, Kim N and Cho SG: The potential
use of mesenchymal stem cells in hematopoietic stem cell
transplantation. Exp Mol Med. 45:e22013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aleynik A, Gernavage KM, Mourad YS,
Sherman LS, Liu K, Gubenko YA and Rameshwar P: Stem cell delivery
of therapies for brain disorders. Clin Transl Med. 3:242014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nikolic WV, Hou H, Town T, Zhu Y, Giunta
B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, et al:
Peripherally administered human umbilical cord blood cells reduce
parenchymal and vascular β-amyloid deposits in Alzheimer mice. Stem
Cells Dev. 17:423–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tanna T and Sachan V: Mesenchymal stem
cells: Potential in treatment of neurodegenerative diseases. Curr
Stem Cell Res Ther. 9:513–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galieva LR, Mukhamedshina YO, Arkhipova SS
and Rizvanov AA: Human umbilical cord blood cell transplantation in
neuroregenerative strategies. Front Pharmacol. 8:6282017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee NK, Na DL and Chang JW: Killing two
birds with one stone: The multifunctional roles of mesenchymal stem
cells in the treatment of neurodegenerative and muscle diseases.
Histol Histopathol. Nov 30–2017.Epub ahead of print. PubMed/NCBI
|
|
56
|
Gärtner A, Pereira T, Gomes R, Luís AL,
França ML, Geuna S, Armada-da-Silva P and Maurício AC: Mesenchymal
stem cells from extra-embryonic tissues for tissue engineering -
regeneration of the peripheral nerve. Advances in Biomaterials
Science and Biomedical Applications. Pignatello R: InTech; 2013,
View Article : Google Scholar
|
|
57
|
Ribeiro J, Gartner A, Pereira T, Gomes R,
Lopes MA, Gonçalves C, Varejão A, Luís AL and Maurício AC:
Perspectives of employing mesenchymal stem cells from the Wharton’s
jelly of the umbilical cord for peripheral nerve repair. Int Rev
Neurobiol. 108:79–120. 2013. View Article : Google Scholar
|
|
58
|
Chambers BE and Wingert RA: Renal
progenitors: Roles in kidney disease and regeneration. World J Stem
Cells. 8:367–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Peired AJ, Sisti A and Romagnani P:
Mesenchymal stem cell-based therapy for kidney disease: A review of
clinical evidence. Stem Cells Int. 2016:47986392016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Aghajani Nargesi A, Lerman LO and Eirin A:
Mesenchymal stem cell-derived extracellular vesicles for kidney
repair: Current status and looming challenges. Stem Cell Res Ther.
8:2732017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tögel F, Hu Z, Weiss K, Isaac J, Lange C
and Westenfelder C: Administered mesenchymal stem cells protect
against ischemic acute renal failure through
differentiation-independent mechanisms. Am J Physiol Renal Physiol.
289:F31–F42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lange C, Tögel F, Ittrich H, Clayton F,
Nolte-Ernsting C, Zander AR and Westenfelder C: Administered
mesenchymal stem cells enhance recovery from
ischemia/reperfusion-induced acute renal failure in rats. Kidney
Int. 68:1613–1617. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chao J, Bledsoe G and Chao L:
Kallikrein-kinin in stem cell therapy. World J Stem Cells.
6:448–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ezquer F, Ezquer M, Simon V, Pardo F,
Yañez A, Carpio D and Conget P: Endovenous administration of
bone-marrow-derived multipotent mesenchymal stromal cells prevents
renal failure in diabetic mice. Biol Blood Marrow Transplant.
15:1354–1365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fang Y, Tian X, Bai S, Fan J, Hou W, Tong
H and Li D: Autologous transplantation of adipose-derived
mesenchymal stem cells ameliorates streptozotocin-induced diabetic
nephropathy in rats by inhibiting oxidative stress,
pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int
J Mol Med. 30:85–92. 2012.PubMed/NCBI
|
|
66
|
Castiglione RC, Maron-Gutierrez T, Barbosa
CM, Ornellas FM, Barreira AL, Dibarros CB, Vasconcelos-dos-Santos
A, Paredes BD, Pascarelli BM, Diaz BL, et al: Bone marrow-derived
mononuclear cells promote improvement in glomerular function in
rats with early diabetic nephropathy. Cell Physiol Biochem.
32:699–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu XY, Urbieta-Caceres V, Krier JD,
Textor SC, Lerman A and Lerman LO: Mesenchymal stem cells and
endothelial progenitor cells decrease renal injury in experimental
swine renal artery stenosis through different mechanisms. Stem
Cells. 31:117–125. 2013. View Article : Google Scholar
|
|
68
|
Eirin A, Zhu XY, Krier JD, Tang H, Jordan
KL, Grande JP, Lerman A, Textor SC and Lerman LO: Adipose
tissue-derived mesenchymal stem cells improve revascularization
outcomes to restore renal function in swine atherosclerotic renal
artery stenosis. Stem Cells. 30:1030–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bussolati B, Bruno S, Grange C,
Buttiglieri S, Deregibus MC, Cantino D and Camussi G: Isolation of
renal progenitor cells from adult human kidney. Am J Pathol.
166:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Angelotti ML, Ronconi E, Ballerini L,
Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M,
Rotondi M, et al: Characterization of renal progenitors committed
toward tubular lineage and their regenerative potential in renal
tubular injury. Stem Cells. 30:1714–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Papazova DA, Oosterhuis NR, Gremmels H,
van Koppen A, Joles JA and Verhaar MC: Cell-based therapies for
experimental chronic kidney disease: A systematic review and
meta-analysis. Dis Model Mech. 8:281–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ma H, Wu Y, Xu Y, Sun L and Zhang X: Human
umbilical mesenchymal stem cells attenuate the progression of focal
segmental glomerulosclerosis. Am J Med Sci. 346:486–493. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Belingheri M, Lazzari L, Parazzi V,
Groppali E, Biagi E, Gaipa G, Giordano R, Rastaldi MP, Croci D,
Biondi A, et al: Allogeneic mesenchymal stem cell infusion for the
stabilization of focal segmental glomerulosclerosis. Biologicals.
41:439–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sun L, Akiyama K, Zhang H, Yamaza T, Hou
Y, Zhao S, Xu T, Le A and Shi S: Mesenchymal stem cell
transplantation reverses multiorgan dysfunction in systemic lupus
erythematosus mice and humans. Stem Cells. 27:1421–1432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun L, Wang D, Liang J, Zhang H, Feng X,
Wang H, Hua B, Liu B, Ye S, Hu X, et al: Umbilical cord mesenchymal
stem cell transplantation in severe and refractory systemic lupus
erythematosus. Arthritis Rheum. 62:2467–2475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Munir H and McGettrick HM: Mesenchymal
stem cell therapy for autoimmune disease: Risks and rewards. Stem
Cells Dev. 24:2091–2100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Flores AI, Gómez-Gómez GJ, Masedo-González
Á and Martínez-Montiel MP: Stem cell therapy in inflammatory bowel
disease: A promising therapeutic strategy? World J Stem Cells.
7:343–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fang TC, Pang CY, Chiu SC, Ding DC and
Tsai RK: Renoprotective effect of human umbilical cord-derived
mesenchymal stem cells in immunodeficient mice suffering from acute
kidney injury. PLoS One. 7:e465042012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Werner N and Nickenig G: Endothelial
progenitor cells in health and atherosclerotic disease. Ann Med.
39:82–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jujo K, Ii M and Losordo DW: Endothelial
progenitor cells in neovascularization of infarcted myocardium. J
Mol Cell Cardiol. 45:530–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh
W, Sung J, Jeon ES, Oh HY and Kim DK: Decreased number and impaired
angiogenic function of endothelial progenitor cells in patients
with chronic renal failure. Arterioscler Thromb Vasc Biol.
24:1246–1252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Schuh A, Liehn EA, Sasse A, Hristov M,
Sobota R, Kelm M, Merx MW and Weber C: Transplantation of
endothelial progenitor cells improves neovascularization and left
ventricular function after myocardial infarction in a rat model.
Basic Res Cardiol. 103:69–77. 2008. View Article : Google Scholar
|
|
84
|
Umemura T and Higashi Y: Endothelial
progenitor cells: Therapeutic target for cardiovascular diseases. J
Pharmacol Sci. 108:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yao Y, Sheng Z, Li Y, Yan F, Fu C, Li Y,
Ma G, Liu N, Chao J and Chao L: Tissue kallikrein promotes cardiac
neovascularization by enhancing endothelial progenitor cell
functional capacity. Hum Gene Ther. 23:859–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Simard T, Jung RG, Motazedian P, Di Santo
P, Ramirez FD, Russo JJ, Labinaz A, Yousef A, Anantharam B,
Pourdjabbar A and Hibbert B: Progenitor cells for arterial repair:
Incremental advancements towards therapeutic reality. Stem Cells
Int. 2017:82704982017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kwon O, Miller S, Li N, Khan A, Kadry Z
and Uemura T: Bone marrow-derived endothelial progenitor cells and
endothelial cells may contribute to endothelial repair in the
kidney immediately after ischemia-reperfusion. J Histochem
Cytochem. 58:687–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Patschan D, Krupincza K, Patschan S, Zhang
Z, Hamby C and Goligorsky MS: Dynamics of mobilization and homing
of endothelial progenitor cells after acute renal ischemia:
Modulation by ischemic preconditioning. Am J Physiol Renal Physiol.
291:F176–F185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rosell A, Morancho A, Navarro-Sobrino M,
Martínez-Saez E, Hernández-Guillamon M, Lope-Piedrafita S, Barceló
V, Borrás F, Penalba A, García-Bonilla L and Montaner J: Factors
secreted by endothelial progenitor cells enhance neurorepair
responses after cerebral ischemia in mice. PLoS One. 8:e732442013.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li YF, Ren LN, Guo G, Cannella LA,
Chernaya V, Samuel S, Liu SX, Wang H and Yang XF: Endothelial
progenitor cells in ischemic stroke: An exploration from hypothesis
to therapy. J Hematol Oncol. 8:332015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mangi AA, Noiseux N, Kong D, He H, Rezvani
M, Ingwall JS and Dzau VJ: Mesenchymal stem cells modified with Akt
prevent remodeling and restore performance of infarcted hearts. Nat
Med. 9:1195–1201. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dzau VJ, Gnecchi M and Pachori AS:
Enhancing stem cell therapy through genetic modification. J Am Coll
Cardiol. 46:1351–1353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mastri M, Lin H and Lee T: Enhancing the
efficacy of mesenchymal stem cell therapy. World J Stem Cells.
6:82–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Park JS, Suryaprakash S, Lao YH and Leong
KW: Engineering mesenchymal stem cells for regenerative medicine
and drug delivery. Methods. 84:3–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Moradian Tehrani R, Verdi J, Noureddini M,
Salehi R, Salarinia R, Mosalaei M, Simonian M, Alani B, Ghiasi MR,
Jaafari MR, et al: Mesenchymal stem cells: A new platform for
targeting suicide genes in cancer. J Cell Physiol. July
13–2017.Epub ahead of print. PubMed/NCBI
|
|
96
|
Tang YL, Tang Y, Zhang YC, Qian K, Shen L
and Phillips MI: Improved graft mesenchymal stem cell survival in
ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J
Am Coll Cardiol. 46:1339–1350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Matsumoto R, Omura T, Yoshiyama M, Hayashi
T, Inamoto S, Koh KR, Ohta K, Izumi Y, Nakamura Y, Akioka K, et al:
Vascular endothelial growth factor-expressing mesenchymal stem cell
transplantation for the treatment of acute myocardial infarction.
Arterioscler Thromb Vasc Biol. 25:1168–1173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gnecchi M, He H, Melo LG, Noiseaux N,
Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ and Ingwall JS:
Early beneficial effects of bone marrow-derived mesenchymal stem
cells overexpressing Akt on cardiac metabolism after myocardial
infarction. Stem Cells. 27:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye
S, Peng X, Li W and Xu W: Hepatocyte growth factor modification
promotes the amelioration effects of human umbilical cord
mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev.
20:103–113. 2011. View Article : Google Scholar
|
|
100
|
Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y,
Liu CY, Fu LL, Liu DM, Liu HQ and Mei CL: VEGF-modified human
embryonic mesenchymal stem cell implantation enhances protection
against cisplatin-induced acute kidney injury. Am J Physiol Renal
Physiol. 300:F207–F218. 2011. View Article : Google Scholar
|
|
101
|
Regoli D, Plante GE and Gobeil F Jr:
Impact of kinins in the treatment of cardiovascular diseases.
Pharmacol Ther. 135:94–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Xiong W, Chen LM, Woodley-Miller C, Simson
JA and Chao J: Identification, purification, and localization of
tissue kallikrein in rat heart. Biochem J. 267:639–646. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nolly H, Carbini LA, Scicli G, Carretero
OA and Scicli AG: A local kallikrein-kinin system is present in rat
hearts. Hypertension. 23:919–923. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wolf WC, Harley RA, Sluce D, Chao L and
Chao J: Localization and expression of tissue kallikrein and
kallistatin in human blood vessels. J Histochem Cytochem.
47:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Agata J, Chao L and Chao J: Kallikrein
gene delivery improves cardiac reserve and attenuates remodeling
after myocardial infarction. Hypertension. 40:653–659. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yao YY, Yin H, Shen B, Chao L and Chao J:
Tissue kallikrein infusion prevents cardiomyocyte apoptosis,
inflammation and ventricular remodeling after myocardial
infarction. Regul Pept. 140:12–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yao YY, Yin H, Shen B, Smith RS Jr, Liu Y,
Gao L, Chao L and Chao J: Tissue kallikrein promotes
neovascularization and improves cardiac function by the
Akt-glycogen synthase kinase-3beta pathway. Cardiovasc Res.
80:354–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yin H, Chao L and Chao J: Kallikrein/kinin
protects against myocardial apoptosis after ischemia/reperfusion
via Akt-glycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling
pathways. J Biol Chem. 280:8022–8030. 2005. View Article : Google Scholar
|
|
109
|
Westermann D, Schultheiss HP and Tschöpe
C: New perspective on the tissue kallikrein-kinin system in
myocardial infarction: Role of angiogenesis and cardiac
regeneration. Int Immunopharmacol. 8:148–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yin H, Chao L and Chao J: Nitric oxide
mediates cardiac protection of tissue kallikrein by reducing
inflammation and ventricular remodeling after myocardial
ischemia/reperfusion. Life Sci. 82:156–165. 2008. View Article : Google Scholar
|
|
111
|
Yayama K, Wang C, Chao L and Chao J:
Kallikrein gene delivery attenuates hypertension and cardiac
hypertrophy and enhances renal function in Goldblatt hypertensive
rats. Hypertension. 31:1104–1110. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wolf WC, Yoshida H, Agata J, Chao L and
Chao J: Human tissue kallikrein gene delivery attenuates
hypertension, renal injury, and cardiac remodeling in chronic renal
failure. Kidney Int. 58:730–739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bledsoe G, Chao L and Chao J: Kallikrein
gene delivery attenuates cardiac remodeling and promotes
neovascularization in spontaneously hypertensive rats. Am J Physiol
Heart Circ Physiol. 285:H1479–H1488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chao J, Shen B, Gao L, Xia CF, Bledsoe G
and Chao L: Tissue kallikrein in cardiovascular, cerebrovascular
and renal diseases and skin wound healing. Biol Chem. 391:345–355.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Spillmann F, Graiani G, Van Linthout S,
Meloni M, Campesi I, Lagrasta C, Westermann D, Tschöpe C, Quaini F,
Emanueli C and Madeddu P: Regional and global protective effects of
tissue kallikrein gene delivery to the peri-infarct myocardium.
Regen Med. 1:235–254. 2006. View Article : Google Scholar
|
|
116
|
Emanueli C, Minasi A, Zacheo A, Chao J,
Chao L, Salis MB, Straino S, Tozzi MG, Smith R, Gaspa L, et al:
Local delivery of human tissue kallikrein gene accelerates
spontaneous angiogenesis in mouse model of hindlimb ischemia.
Circulation. 103:125–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Emanueli C and Madeddu P: Angiogenesis
therapy with human tissue kallikrein for the treatment of ischemic
diseases. Arch Mal Coeur Vaiss. 97:679–687. 2004.PubMed/NCBI
|
|
118
|
Murakami H, Miao RQ, Chao L and Chao J:
Adenovirus-mediated kallikrein gene transfer inhibits neointima
formation via increased production of nitric oxide in rat artery.
Immunopharmacology. 44:137–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Murakami H, Yayama K, Miao RQ, Wang C,
Chao L and Chao J: Kallikrein gene delivery inhibits vascular
smooth muscle cell growth and neointima formation in the rat artery
after balloon angioplasty. Hypertension. 34:164–170. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hagiwara M, Shen B, Chao L and Chao J:
Kallikrein-modified mesenchymal stem cell implantation provides
enhanced protection against acute ischemic kidney injury by
inhibiting apoptosis and inflammation. Hum Gene Ther. 19:807–819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
González A, Ravassa S, Beaumont J, López B
and Díez J: New targets to treat the structural remodeling of the
myocardium. J Am Coll Cardiol. 58:1833–1843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tschöpe C, Walther T, Königer J, Spillmann
F, Westermann D, Escher F, Pauschinger M, Pesquero JB, Bader M,
Schultheiss HP and Noutsias M: Prevention of cardiac fibrosis and
left ventricular dysfunction in diabetic cardiomyopathy in rats by
transgenic expression of the human tissue kallikrein gene. FASEB J.
18:828–835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yao Y, Sheng Z, Li Y, Fu C, Ma G, Liu N,
Chao J and Chao L: Tissue kallikrein-modified human endothelial
progenitor cell implantation improves cardiac function via enhanced
activation of akt and increased angiogenesis. Lab Invest.
93:577–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Naicker S, Naidoo S, Ramsaroop R, Moodley
D and Bhoola K: Tissue kallikrein and kinins in renal disease.
Immunopharmacology. 44:183–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Katori M and Majima M: A missing link
between a high salt intake and blood pressure increase. J Pharmacol
Sci. 100:370–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sharma JN and Narayanan P: The
kallikrein-kinin pathways in hypertension and diabetes. Prog Drug
Res. 69:15–36. 2014.PubMed/NCBI
|
|
127
|
Uehara Y, Hirawa N, Kawabata Y, Suzuki T,
Ohshima N, Oka K, Ikeda T, Goto A, Toyo-oka T and Kizuki K:
Long-term infusion of kallikrein attenuates renal injury in Dahl
salt-sensitive rats. Hypertension. 24:770–778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chao J, Zhang JJ, Lin KF and Chao L:
Adenovirus-mediated kallikrein gene delivery reverses salt-induced
renal injury in Dahl salt-sensitive rats. Kidney Int. 54:1250–1260.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hirawa N, Uehara Y, Suzuki T, Kawabata Y,
Numabe A, Gomi T, Lkeda T, Kizuki K and Omata M: Regression of
glomerular injury by kallikrein infusion in Dahl salt-sensitive
rats is a bradykinin B2-receptor-mediated event. Nephron.
81:183–193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L
and Chao J: Reversal of renal fibrosis, inflammation, and
glomerular hypertrophy by kallikrein gene delivery. Hum Gene Ther.
17:545–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang JJ, Bledsoe G, Kato K, Chao L and
Chao J: Tissue kallikrein attenuates salt-induced renal fibrosis by
inhibition of oxidative stress. Kidney Int. 66:722–732. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liu Y, Bledsoe G, Hagiwara M, Yang ZR,
Shen B, Chao L and Chao J: Blockade of endogenous tissue kallikrein
aggravates renal injury by enhancing oxidative stress and
inhibiting matrix degradation. Am J Physiol Renal Physiol.
298:F1033–F1040. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xia CF, Bledsoe G, Chao L and Chao J:
Kallikrein gene transfer reduces renal fibrosis, hypertrophy, and
proliferation in DOCA-salt hypertensive rats. Am J Physiol Renal
Physiol. 289:F622–F631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Schanstra JP, Neau E, Drogoz P, Arevalo
Gomez MA, Lopez Novoa JM, Calise D, Pecher C, Bader M, Girolami JP
and Bascands JL: In vivo bradykinin B2 receptor activation reduces
renal fibrosis. J Clin Invest. 110:371–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bledsoe G, Shen B, Yao YY, Hagiwara M,
Mizell B, Teuton M, Grass D, Chao L and Chao J: Role of tissue
kallikrein in prevention and recovery of gentamicin-induced renal
injury. Toxicol Sci. 102:433–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Dellalibera-Joviliano R, Reis ML and
Donadi EA: Kinin system in lupus nephritis. Int Immunopharmacol.
1:1889–1896. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Liu K, Li QZ, Delgado-Vega AM, Abelson AK,
Sánchez E, Kelly JA, Li L, Liu Y, Zhou J, Yan M, et al Profile
Study Group; Italian Collaborative Group; German Collaborative
Group; Spanish Collaborative Group; Argentinian Collaborative
Group; SLEGEN Consortium: Kallikrein genes are associated with
lupus and glomerular basement membrane-specific antibody-induced
nephritis in mice and humans. J Clin Invest. 119:911–923. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li Y, Raman I, Du Y, Yan M, Min S, Yang J,
Fang X, Li W, Lu J, Zhou XJ, et al: Kallikrein transduced
mesenchymal stem cells protect against anti-GBM disease and lupus
nephritis by ameliorating inflammation and oxidative stress. PLoS
One. 8:e677902013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xia CF, Yin H, Borlongan CV, Chao L and
Chao J: Kallikrein gene transfer protects against ischemic stroke
by promoting glial cell migration and inhibiting apoptosis.
Hypertension. 43:452–459. 2004. View Article : Google Scholar
|
|
140
|
Zhang JJ, Chao L, Chao J, Chu Y and
Heistad DD: Adenovirus-mediated kallikrein gene delivery reduces
aortic thickening and stroke-induced death rate in Dahl
salt-sensitive rats. Stroke. 30:1925–1931; discussion 1931-1932.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Xia CF, Yin H, Yao YY, Borlongan CV, Chao
L and Chao J: Kallikrein protects against ischemic stroke by
inhibiting apoptosis and inflammation and promoting angiogenesis
and neurogenesis. Hum Gene Ther. 17:206–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chao J and Chao L: Experimental therapy
with tissue kallikrein against cerebral ischemia. Front Biosci.
11:1323–1327. 2006. View
Article : Google Scholar
|
|
143
|
Kizuki K, Iwadate H and Ookubo R:
Growth-stimulating effect of kallikrein on rat neural stem cells -
II. Immunocytochemical analysis and specificity of the enzyme for
neural stem cells. Yakugaku Zasshi. 127:919–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Liu L, Liu H, Yang F, Chen G, Zhou H, Tang
M, Zhang R and Dong Q: Tissue kallikrein protects cortical neurons
against hypoxia/reoxygenation injury via the ERK1/2 pathway.
Biochem Biophys Res Commun. 407:283–287. 2011. View Article : Google Scholar : PubMed/NCBI
|